Abstract
Obstructive sleep apnea (OSA) is associated with multiple chronic comorbidities with treatments including continuous positive airway pressure (CPAP), upper airway surgery (UAS), and hypoglossal nerve stimulation (HNS). Given the complexity of the condition and multiple treatment options, there is an ongoing debate to determine the best management. O’Connor-Reina et al. recently published a paper titled “Risk of diabetes in patients with sleep apnea: comparison of surgery versus CPAP in a long-term follow-up study.” In their study, the authors stated that OSA patients who received surgery had a 50% less chance of developing diabetes compared to patients who only received CPAP treatment. However, we would like to point out some limitations that warrant attention and caution interpretation of the findings by physicians and patients.
Keywords: Obstructive sleep apnea, Continuous positive airway pressure (CPAP), Upper airway surgery, Diabetes, Big data
Opening
A recent article was published in the Journal of Otolaryngology—Head & Neck Surgery titled “Risk of diabetes in patients with sleep apnea: comparison of surgery versus CPAP in a long-term follow-up study” by O’Connor-Reina et al., highlighting a strong interest in the potential benefits of upper airway surgeries (UAS) in reducing risk of diabetes in patients with obstructive sleep apnea (OSA) [1]. This study utilized a federated de-identified database, TriNetX, that compared the rates of new-onset diabetes and mortality in OSA patients treated with UAS to continuous positive airway pressure (CPAP). The idea behind this study was interesting and contributed to the ongoing discourse on the different treatments of OSA. To further this discussion, we would like to point out some limitations that warrant attention and caution interpretation of the findings by physicians and patients.
Selection bias
First, it is possible that the findings were confounded by selection bias: i.e. patients in the CPAP cohort had significantly more comorbidities than those who underwent UAS, and were therefore more likely to also develop diabetes. As seen in Table 4 of the study, 33.20% of the CPAP group suffered from obesity compared to 18.00% in the UAS cohort and 49.60% of CPAP patients had hypertension compared to 16.50% of the surgery group. This was a consistent trend for all baseline comorbidities in the study. Even though the study mentioned the significant “differences between age, sex and the presence of comorbidity between both cohorts before matching,” this point may need further emphasis. Risk factors for developing diabetes include obesity, a sedentary lifestyle, and lack of physical activity [2–4]. Furthermore, a sedentary lifestyle and inactivity are also correlated to cardiovascular co-morbidities such as hypertension and dyslipidemia [5]. These are important risk factors that should be considered in propensity score matching while building cohorts. The authors noted that all cohorts were matched for age, sex, and co-morbidities, but the large difference in co-morbidities in the CPAP group indicated that these patients likely had other co-morbidities and behavioral factors that were not considered [6]. While authors matched for many relevant comorbidities, the results may still be biased due to the nature of the retrospective study.
Secondly, we agree with the authors that for a multi-year retrospective study, it is imperative to account for follow-up time in selecting cohorts. O’Connor-Reina et al. ensured that all patients had 5-year follow-up from the date of OSA diagnosis, but we believe that selecting the index event as the diagnosis date of OSA, instead of time of CPAP initiation or surgery date, was another limitation. Index events describe the initial occurrence or presentation of a medical condition. It marks the beginning of a diagnosis or treatment and is different for each patient [7, 8]. Since O’Connor-Reina et al. included data “obtained from up to 20 years ago,” a patient diagnosed with OSA in 2003 may have contributed data to this study during 2003–2008. However, it is possible that they didn’t receive UAS until 2020. Therefore, they would have been incorrectly included in the surgical cohort in this study. As such, we suggest the index date should be set to the date of procedure to compare the efficacy of two treatments [9, 10].
Coding and cohort queries
The ICD-10-PCS codes for Upper Airway Surgeries listed in Table 1 in the O'Connor-Reina study may nonspecifically represent surgeries for OSA or other diagnoses. Upper airway surgeries have many indications in both children and adults, including chronic bacterial tonsillitis, chronic ear infections, etc. [11–13]. Therefore, it is difficult to be certain that documented upper airway surgeries were intended to treat OSA and not other conditions, as the authors noted in the limitations section. One way to minimize this confounding variable is to set a time relation by linking the diagnosis code of OSA within the same day that patients receive UAS treatments. This way, the specificity of the targeted patient population can be improved.
In reviewing the diagnosis codes utilized to build cohorts, we noticed that the cohorts may not be entirely composed of patients of interest. When capturing patients who were prescribed CPAP, the authors used the codes ICD-10-PCS 5A09357, ICD-10-PCS 5A09457, and ICD-10-PCS 5A09557, noting that two of the codes mandate continuous use for more than 24 h which would not be home CPAP patients (Table 1). In the United States, ICD-10-PCS codes are used only to classify procedures performed in an inpatient setting [14]. Inpatient CPAP treatment is indicated for respiratory distress syndrome and respiratory failure, suggesting a sicker patient population [15–18]. Since O’Connor-Reina et al. utilized the Global Collaborative Network in TriNetX, which captures patients globally, this study likely included hospitalized patients with serious conditions. Even though OSA treatment using CPAP therapy may require hospital titration, solely using ICD-10-PCS codes may exclude the general OSA patients using CPAP at home [19]. This has significant implications on the analysis of comorbidities and mortality. On this note, studies have used CPT codes that more appropriately characterizes regular CPAP use because they require physicians to order a CPAP machine and perform face-to-face patient care like mask fitting, titration pressure, and instruction on how to use the machine [20, 21]. That said, we recognize the challenges in gathering the correct codes for CPAP treatment. Since there is no unifying guideline delineating the coding differences across multiple countries, it is difficult to perfectly identify patients receiving CPAP treatment for OSA in a global retrospective study. We also acknowledge that ICD-10-PCS codes are also primarily used in Europe, while CPT codes are the standard for billing in the United States. As there is no translation for CPT into PCS codes for US data, researchers should be careful in choosing the appropriate codes for their study.
Table 1.
ICD code for CPAP use. Reproduced from O’Connor-Reina et al. [1]
| ICD code | Continuous positive airway pressure use |
|---|---|
| 5A09357 | Assistance with respiratory ventilation, less than 24 consecutive hours, continuous positive airway pressure |
| 5A09457 | Assistance with respiratory ventilation, 24–96 consecutive hours, continuous positive airway pressure |
| 5A09557 | Assistance with respiratory ventilation, greater than 96 consecutive hours, continuous positive airway pressure |
OSA treatment efficacy and TriNetX limitations
One of the advantages of large retrospective databases like TriNetX is its ability to provide a bigger sample size, allowing researchers to study rare diseases on a large scale. However, one of their drawbacks is the dependence on diagnosis and procedural codes. As the the authors pointed out in the limitations section, TriNetX “records for CPAP did not include data for the adherence and acceptance of this therapy.” Studies have shown short term CPAP treatment or poor CPAP adherence does not result in decreased diabetic rates, while CPAP adherence and consistent long term CPAP treatments are associated with decreased risk of diabetes [22–26]. Therefore, without a means to accurately measure patient compliance, the results might be biased. Despite a large difference in risk development of new diabetes diagnosis between CPAP and UAS, readers should take cautions when concluding that surgery has a clinically significant role.
Updated risk of diabetes methods and results
We developed more comprehensive and balanced cohorts to study the risk of diabetes in patients with OSA. The TriNetX database was queried to identify OSA patients over 18 years of age (ICD-10 G47.30 and G47.33). To ensure surgeries were indicated for OSA, patients with head and neck neoplasms were excluded. Patients with a BMI ≥ 35 kg/m2 were recommended to undergo weight loss surgery prior to UAS [27]. Thus, these patients were also excluded from the study. Two cohorts were built based on the ICD and CPT codes to ensure comprehensive coverage of CPAP use or UAS (Table 2). In the CPAP cohort, patients with any head and neck surgeries were excluded. Likewise, any patients in the UAS cohort who received CPAP after the procedure were excluded. Only patients with at least 5 years of follow-up after treatment date were included in the study. To balance for confounding variables, 1:1 propensity score matching was performed on patient demographics (age, sex, race, ethnicity) and co-morbidities (Tables 3 and 4). Baseline characteristic comparison and relative risk analysis were performed. Kaplan–Meier analysis was used to estimate 5-year “survival rate” of not developing diabetes. We would caution the interpretation of this analysis as it does not represent survival, but instead depicts developing the outcome of interest—in this case, developing diabetes [28].
Table 2.
Updated ICD-10 and CPT codes of CPAP and UAS
| Procedures | ICD-10 or CPT code |
|---|---|
| CPAP | CPT 94660, ICD-10-PCS 5A09357, HCPCS A7034, SNOMED 47545007 |
| Upper airway surgeries | CPT 42145, 42299, 42140, 42281, 21685, 42821, 42836, 42892, 42950, 42826, 21,199, 42870, 41120, 42831; ICD-10-PCS 0CQ3, 0CQM0ZZ, 0CQ33ZZ, 0CQN, 0CQN0ZZ, 0CQ30ZZ, 0CTNXZZ, 0CTN0ZZ, 0C570ZZ, 0CTN, 0CU3, 0CU2, 0NQV3ZZ, 0NSX0ZZ, 0NSX04Z, 0NQT3ZZ, 0NQV0ZZ, 0NQTXZZ, 0CBPXZZ, 0CBP3ZZ, 0CBP0ZZ, 0C573ZZ, 0C57XZZ, 0NQT0ZZ, 0NQVXZZ, 0CQ3XZZ |
Table 3.
Diagnosis characteristics used in propensity score match between CPAP and UAS cohorts
| Characteristics | ICD-10 code |
|---|---|
| Diabetes mellitus | E08-E13 |
| Tobacco use | Z72.0 |
| Overweight, obesity and other hyperalimentation | E65-E68 |
| Other chronic obstructive pulmonary disease | J44 |
| Diseases of the nervous system | G00-G99 |
| Cerebrovascular diseases | I60-I69 |
| Other forms of heart disease | I30-I5A |
| Ischemic heart diseases | I20-I25 |
| Other and unspecified disorders of the circulatory system | I95-I99 |
| Diseases of veins, lymphatic vessels and lymph nodes, not elsewhere classified | I80-I89 |
| Diseases of arteries, arterioles and capillaries | I70-I79 |
| Pulmonary heart disease and diseases of pulmonary circulation | I26-I28 |
| Chronic rheumatic heart diseases | I05-I09 |
| Acute rheumatic fever | I00-I02 |
| Hypertensive diseases | I10-I16 |
| Diseases of the digestive system | K00-K95 |
| Mental, Behavioral and Neurodevelopmental disorders | F01-F99 |
| Diseases of the genitourinary system | N00-N99 |
| Malignant neoplasms of eye, brain and other parts of central nervous system | C69-C72 |
| Epilepsy and recurrent seizures | G40 |
Table 4.
Demographic and clinical characteristics of the study population (n = 65,881)
| Before matching | After matching | |||||
|---|---|---|---|---|---|---|
| Continuous positive airway pressure (n = 59,787) | Upper airway surgery (n = 9224) | P-Value | Continuous positive airway pressure (n = 6651) | Upper airway surgery (n = 6651) | P-Value | |
| Age | 61.0 ± 12.7 | 42.9 ± 13.6 | < 0.001 | 46.3 ± 13.2 | 46.4 ± 12.4 | 0.5 |
| Sex | ||||||
| Male | 33,253 (55.8%) | 4814 (57.8%) | 0.001 | 3901 (58.7%) | 3849 (57.9%) | 0.4 |
| Female | 26,285 (44.1%) | 3508 (42.1%) | 0.001 | 2750 (41.3%) | 2801 (42.1%) | 0.4 |
| Unknown | 31 (0.1%) | 10 (0.1%) | 0.02 | 0 | 10 (0.2%) | 0.002 |
| Race | ||||||
| White | 41,427 (69.5%) | 5247 (63.0%) | < 0.001 | 4247 (63.9%) | 4246 (63.8%) | 1.0 |
| Black or African American | 8322 (14.0%) | 1362 (16.4%) | < 0.001 | 1014 (15.2%) | 1034 (15.5%) | 0.6 |
| Asian | 353 (0.6%) | 141 (1.7%) | < 0.001 | 89 (1.3%) | 96 (1.4%) | 0.6 |
| American Indian or Alaska Native | 244 (0.4%) | 35 (0.4%) | 1.0 | 33 (0.5%) | 32 (0.5%) | 0.9 |
| Native Hawaiian or Other Pacific Islander | 52 (0.1%) | 12 (0.1%) | 0.1 | 10 (0.2%) | 10 (0.2%) | 1 |
| Unknown | 9171 (15.4%) | 1526 (18.3%) | < 0.001 | 1261 (19.0%) | 1233 (18.5%) | 0.5 |
| Ethnicity | ||||||
| Not Hispanic or Latino | 46,411 (77.9%) | 5490 (66.0%) | < 0.001 | 4560 (68.6%) | 4577 (68.8%) | 0.8 |
| Hispanic or Latino | 2399 (4.0%) | 773 (9.3%) | < 0.001 | 507 (7.6%) | 519 (7.8%) | 0.7 |
| Unknown | 10,759 (18.1%) | 2060 (24.8%) | < 0.001 | 1584 (23.8%) | 1555 (23.4%) | 0.6 |
| Comorbidities | ||||||
| Diabetes mellitus | 29,077 (48.8%) | 1255 (15.1%) | < 0.001 | 1195 (18.0%) | 1227 (18.4%) | 0.5 |
| Tobacco use | 3045 (5.1%) | 190 (2.3%) | < 0.001 | 160 (2.4%) | 177 (2.7%) | 0.3 |
| Overweight, obesity and other hyperalimentation | 39,864 (66.9%) | 3183 (38.2%) | < 0.001 | 2927 (44.0%) | 2885 (43.4%) | 0.5 |
| Other chronic obstructive pulmonary disease | 16,460 (27.6%) | 429 (5.2%) | < 0.001 | 405 (6.1%) | 427 (6.4%) | 0.4 |
| Diseases of the nervous system | 54,858 (92.1%) | 7280 (87.5%) | < 0.001 | 5782 (86.9%) | 5763 (86.6%) | 0.6 |
| Cerebrovascular diseases | 11,177 (18.8%) | 344 (4.1%) | < 0.001 | 351 (5.3%) | 329 (4.9%) | 0.4 |
| Other forms of heart disease | 36,447 (61.2%) | 1636 (19.7%) | < 0.001 | 1588 (23.9%) | 1555 (23.4%) | 0.5 |
| Ischemic heart diseases | 23,472 (39.4%) | 645 (7.7%) | < 0.001 | 665 (10.0%) | 635 (9.5%) | 0.4 |
| Other and unspecified disorders of the circulatory system | 12,741 (21.4%) | 456 (5.5%) | < 0.001 | 415 (6.2%) | 416 (6.3%) | 1.0 |
| Diseases of veins, lymphatic vessels and lymph nodes | 15,401 (25.9%) | 683 (8.2%) | < 0.001 | 635 (9.5%) | 631 (9.5%) | 0.9 |
| Diseases of arteries, arterioles and capillaries | 15,873 (26.6%) | 519 (6.2%) | < 0.001 | 544 (8.2%) | 497 (7.5%) | 0.1 |
| Pulmonary heart disease and diseases of pulmonary circulation | 13,086 (22.0%) | 221 (2.7%) | < 0.001 | 252 (3.8%) | 217 (3.3%) | 0.1 |
| Chronic rheumatic heart diseases | 6295 (10.6%) | 102 (1.2%) | < 0.001 | 103 (1.5%) | 100 (1.5%) | 0.8 |
| Acute rheumatic fever | 218 (0.4%) | 10 (0.1%) | < 0.001 | 10 (0.2%) | 10 (0.2%) | 1 |
| Hypertensive diseases | 47,055 (79.0%) | 3130 (37.6%) | < 0.001 | 2996 (45.0%) | 2984 (44.9%) | 0.8 |
| Diseases of the digestive system | 45,097 (75.7%) | 4965 (59.7%) | < 0.001 | 4179 (62.8%) | 4151 (62.4%) | 0.6 |
| Mental, Behavioral and Neurodevelopmental disorders | 38,332 (64.3%) | 4016 (48.3%) | < 0.001 | 3461 (52.0%) | 3435 (51.6%) | 0.7 |
| Diseases of the genitourinary system | 41,119 (69.0%) | 3717 (44.7%) | < 0.001 | 3098 (46.6%) | 3144 (47.3%) | 0.4 |
| Malignant neoplasms of eye, brain and other parts of CNS | 235 (0.4%) | 21 (0.3%) | 0.05 | 21 (0.3%) | 18 (0.3%) | 0.6 |
| Epilepsy and recurrent seizures | 2661 (4.5%) | 258 (3.1%) | < 0.001 | 239 (3.6%) | 225 (3.4%) | 0.5 |
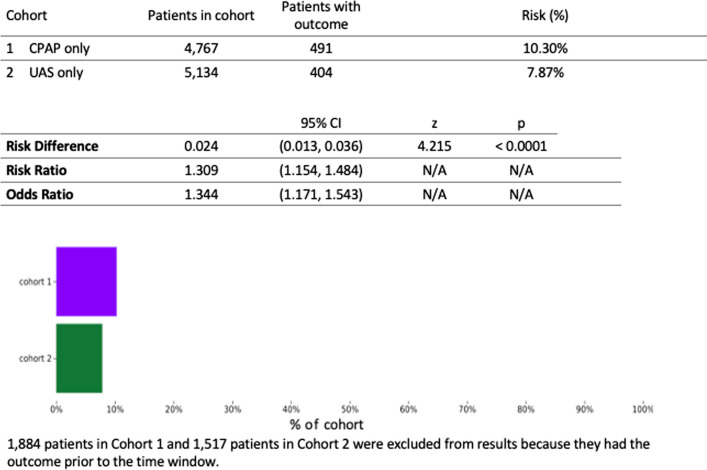
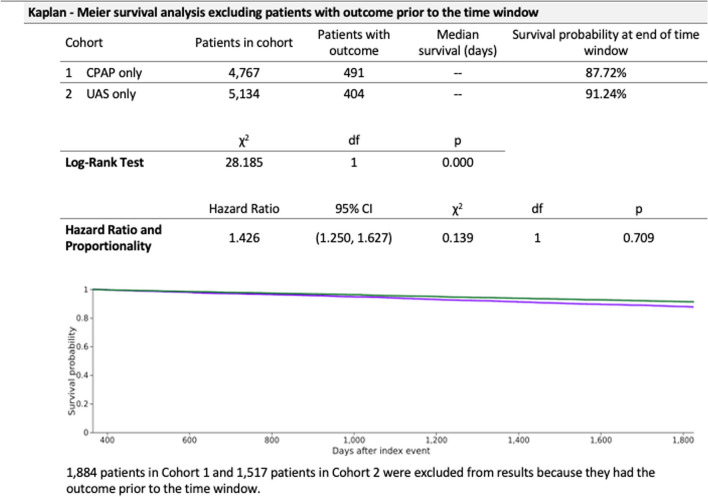
Of all patients greater than 18 years of age who had at least 5 years of follow-up after treatment initiation, there were 59,787 and 9224 patients in the CPAP and UAS cohorts, respectively. After 1:1 propensity score matching and excluding patients who did not satisfy inclusion criteria, there were 6651 patients in each cohort. The mean age at index was 46 years of age. Both groups included about 58% male with 64% white, 15% black, and 8% Hispanic. There were 491 (10.3%) patients in the CPAP group and 404 (7.9%) patients in the UAS group with new onset diabetes (Fig. 1). Within the CPAP group, the number of patients with a new diagnosis of diabetes after treatment in the UAS group was significantly lower than the CPAP group (risk ratio RR = 1.31, 95% confidence interval CI = [1.15, 1.48], p < 0.0001). Figure 2 shows a 5-year probability of not developing diabetes in CPAP vs UAS cohort (87.7% vs 91.2%, hazard ratio = 1.43, 95% CI = [1.25, 1.63]).
Fig. 1.
Risk analysis after excluding patients with the outcome (Diabetes) prior to time window
Fig. 2.
Kaplan Meier plot comparing outcome of diabetes after five years of follow up in both cohorts
Our results showed that with a comprehensive usage of codes and extensive co-morbidities matching, there is still a statistically significant reduction in the risk of developing new diabetes in the UAS cohort compared to CPAP group, though the absolute risk difference may not be as clinically relevant.
Conclusion
In conclusion, we feel strongly that there are limitations in the study published by O'Connor-Reina et al. which bias the comparison of UAS versus CPAP. We commend the authors for studying an important topic and we appreciate their consideration of the points we have made here. By facilitating a balanced discussion on this topic, we can advance the understanding and management of OSA.
Acknowledgments
We would like to acknowledge Caia Hypatia for their help on manuscript preparation for submission. We would also like to recognize the support from the TriNetX design team.
Abbreviations
- OSA
Obstructive sleep apnea
- CPAP
Continuous positive airway pressure
- UAS
Upper airway surgery
- HCOs
Health-care organizations
- CI
Confidence interval
- BMI
Body mass index
- ICD-10-PCS
International classification of diseases procedure coding system
- CPT
Current procedural terminology
Author contributions
Conception and design: NT, FJL, NG. Acquisition of data: T. Analysis and interpretation of data: all authors. Drafting the article: NT, BS, FJL. Critically revising the article: all authors. Reviewed submitted version of manuscript: all authors. Approved the final version of the manuscript on behalf of all authors: NG. Statistical analysis: NT. Administrative/technical/material support: FJL, NG. Study supervision: NG.
Funding
The project was supported by the National Center for Advancing Translational Sciences, National Institutes of Health (NIH), through Grant UL1 TR002014. The content is solely the responsibility of the authors and does not necessarily represent the official views of the NIH.
Availability of data and materials
The data that support the findings of this study are available from the corresponding author upon reasonable request.
Declarations
Ethics approval and consent to participate
As a de-identified federated network, research studies using TriNetX do not require ethical approval. To comply with legal frameworks and ethical guidelines guarding against data re-identification, the identity of participating HCOs and their individual contribution to each dataset are not disclosed. The TriNetX platform only uses aggregated counts and statistical summaries of de-identified information. No protected health Information or personal data is made available to the users of the platform.
Consent for publication
Not applicable.
Competing interests
The authors declare no competing interests.
Footnotes
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
References
- 1.O’Connor-Reina C, Alcala LR, Ignacio JM, et al. Risk of diabetes in patients with sleep apnea: comparison of surgery versus CPAP in a long-term follow-up study. J Otolaryngol Head Neck Surg. 2023;52(1):16. doi: 10.1186/s40463-022-00616-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Wu Y, Ding Y, Tanaka Y, Zhang W. Risk factors contributing to type 2 diabetes and recent advances in the treatment and prevention. Int J Med Sci. 2014;11(11):1185–1200. doi: 10.7150/ijms.10001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Zimmet P, Alberti KG, Shaw J. Global and societal implications of the diabetes epidemic. Nature. 2001;414(6865):782–787. doi: 10.1038/414782a. [DOI] [PubMed] [Google Scholar]
- 4.Hu FB, Manson JE, Stampfer MJ, et al. Diet, lifestyle, and the risk of type 2 diabetes mellitus in women. N Engl J Med. 2001;345(11):790–797. doi: 10.1056/NEJMoa010492. [DOI] [PubMed] [Google Scholar]
- 5.Park JH, Moon JH, Kim HJ, Kong MH, Oh YH. Sedentary lifestyle: overview of updated evidence of potential health risks. Korean J Fam Med. 2020;41(6):365–373. doi: 10.4082/kjfm.20.0165. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Jehan S, Auguste E, Pandi-Perumal SR, et al. Depression, obstructive sleep apnea and psychosocial health. Sleep Med Disord Int J. 2017;1(3):00012. [PMC free article] [PubMed] [Google Scholar]
- 7.Mann DL. Heart failure. Tex Heart Inst J. 2006;33(2):201–203. [PMC free article] [PubMed] [Google Scholar]
- 8.Prebay ZJ, Ostrovsky AM, Buck M, Chung PH. A TriNetX registry analysis of the need for second procedures following index anterior and posterior urethroplasty. J Clin Med. 2023;12(5):2055. doi: 10.3390/jcm12052055. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Hadi YB, Thakkar S, Shah-Khan M, Sohail AMAH, Mann R, Singh S. S1266 Roux-en-Y gastric bypass is associated with worse metabolic bone health compared to sleeve gastrectomy: propensity matched analysis of a large research network. Off J Am Coll Gastroenterol ACG. 2021;116:582. doi: 10.14309/01.ajg.0000778596.07584.c4. [DOI] [Google Scholar]
- 10.Menon N, Turcotte J, Patton C. Structural allograft versus synthetic interbody cage for anterior cervical discectomy and fusion: a comparison of 1-year outcomes from a national database. Glob Spine J. 2021;11(8):1215–1222. doi: 10.1177/2192568220942217. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Park K. Otitis media and tonsils–role of adenoidectomy in the treatment of chronic otitis media with effusion. Adv Otorhinolaryngol. 2011;72:160–163. doi: 10.1159/000324781. [DOI] [PubMed] [Google Scholar]
- 12.Burton MJ, Glasziou PP, Chong LY, Venekamp RP. Tonsillectomy or adenotonsillectomy versus non-surgical treatment for chronic/recurrent acute tonsillitis. Cochrane Database Syst Rev. 2014;2014(11):001802. doi: 10.1002/14651858.CD001802.pub3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Fairbanks DNF. Snoring: surgical versus nonsurgical management. Laryngoscope. 1984;94(9):1188–1192. doi: 10.1288/00005537-198409000-00011. [DOI] [PubMed] [Google Scholar]
- 14.ICD-10-PCS Official Guidelines for Coding and Reporting 2024.
- 15.Gupta S, Donn SM. Continuous positive airway pressure: to bubble or not to bubble? Clin Perinatol. 2016;43(4):647–659. doi: 10.1016/j.clp.2016.07.003. [DOI] [PubMed] [Google Scholar]
- 16.Polin RA, Sahni R. Newer experience with CPAP. Semin Neonatol. 2002;7(5):379–389. doi: 10.1053/siny.2002.0132. [DOI] [PubMed] [Google Scholar]
- 17.Tingting X, Danming Y, Xin C. Non-surgical treatment of obstructive sleep apnea syndrome. Eur Arch Oto-Rhino-Laryngol Off J Eur Fed Oto-Rhino-Laryngol Soc EUFOS Affil Ger Soc Oto-Rhino-Laryngol Head Neck Surg. 2018;275(2):335–346. doi: 10.1007/s00405-017-4818-y. [DOI] [PubMed] [Google Scholar]
- 18.Sullivan DR, Kim H, Gozalo PL, Bunker J, Teno JM. Trends in noninvasive and invasive mechanical ventilation among medicare beneficiaries at the end of life. JAMA Intern Med. 2021;181(1):93. doi: 10.1001/jamainternmed.2020.5640. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Fietze I, Laharnar N, Bargiotas P, et al. Management of obstructive sleep apnea in Europe—a 10-year follow-up. Sleep Med. 2022;97:64–72. doi: 10.1016/j.sleep.2022.06.001. [DOI] [PubMed] [Google Scholar]
- 20.Weaver EM, Maynard C, Yueh B. Survival of veterans with sleep apnea: continuous positive airway pressure versus surgery. Otolaryngol Neck Surg. 2004;130(6):659–665. doi: 10.1016/j.otohns.2003.12.012. [DOI] [PubMed] [Google Scholar]
- 21.Carr JB, Cancienne JM, Werner BC. Obstructive sleep apnea affects complication rates following knee arthroscopy but use of continuous positive airway pressure is not protective against complications. Knee Surg Sports Traumatol Arthrosc. 2019;27(2):534–540. doi: 10.1007/s00167-018-5144-7. [DOI] [PubMed] [Google Scholar]
- 22.Hoyos CM, Killick R, Yee BJ, Phillips CL, Grunstein RR, Liu PY. Cardiometabolic changes after continuous positive airway pressure for obstructive sleep apnoea: a randomised sham-controlled study. Thorax. 2012;67(12):1081–1089. doi: 10.1136/thoraxjnl-2011-201420. [DOI] [PubMed] [Google Scholar]
- 23.Muraki I, Wada H, Tanigawa T. Sleep apnea and type 2 diabetes. J Diabetes Investig. 2018;9(5):991–997. doi: 10.1111/jdi.12823. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Steiropoulos P, Papanas N, Nena E, et al. Markers of glycemic control and insulin resistance in non-diabetic patients with Obstructive Sleep Apnea Hypopnea Syndrome: Does adherence to CPAP treatment improve glycemic control? Sleep Med. 2009;10(8):887–891. doi: 10.1016/j.sleep.2008.10.004. [DOI] [PubMed] [Google Scholar]
- 25.Nena E, Steiropoulos P, Tzouvelekis A, et al. Reduction of serum retinol-binding protein-4 levels in nondiabetic obstructive sleep apnea patients under continuous positive airway pressure treatment. Respir Int Rev Thorac Dis. 2010;80(6):517–523. doi: 10.1159/000295903. [DOI] [PubMed] [Google Scholar]
- 26.Xu PH, Hui CKM, Lui MMS, Lam DCL, Fong DYT, Ip MSM. Incident type 2 diabetes in OSA and effect of CPAP treatment: a retrospective clinic cohort study. Chest. 2019;156(4):743–753. doi: 10.1016/j.chest.2019.04.130. [DOI] [PubMed] [Google Scholar]
- 27.Ashrafian H, le Roux CW, Rowland SP, et al. Metabolic surgery and obstructive sleep apnoea: the protective effects of bariatric procedures. Thorax. 2012;67(5):442–449. doi: 10.1136/thx.2010.151225. [DOI] [PubMed] [Google Scholar]
- 28.Understanding Kaplan-Meier Survival Results. TriNetX Help Center. 2023. https://support.trinetx.com/hc/en-us/articles/360003922793-Understanding-Kaplan-Meier-Survival-Results-.
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
The data that support the findings of this study are available from the corresponding author upon reasonable request.