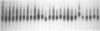Abstract
Fragile X syndrome results from the unstable expansion of a CGG repeat within the FMR1 gene. Three classes of FMR1 alleles have been identified, normal alleles with 6-60 repeats, premutations with 60-200 repeats, and full mutations with > 230 repeats. Premutations are exquisitely unstable upon transmission. Normal alleles, while generally stable upon transmission, are thought to have different intrinsic mutation frequencies, such that some normal alleles may be predisposed towards expansion while others may be more resistant to such change. One variable that may account for this difference is the occurrence of one or more AGG triplets punctuating the normal CGG repeat. The AGG interruptions lead to alleles that have equivalent overall length but different lengths of perfect repeats. To test the influence of the length of perfect repeats on stability, we examined the CGG repeat of single sorted sperm from two males, each with 39 total repeats, but distinct AGG interruption patterns. Sorted sperm of each donor showed -15% variation in repeat length, consistent with previous studies of sorted sperm at other triplet repeat loci. However, when discounting the majority variation of +/-1 repeat, the male with 29 perfect repeats showed 3% expansion changes while the donor with only 19 perfect repeats had none (< 0.9%). Moreover, > 90% of all variant sperm, including all those observed with expansions, showed expansion or contraction of the 3' end of the repeat array. These data are consistent with the hypothesis that perfect repeat tracts influence the repeat stability and that changes of the FMR1 repeat exhibit polarity.
Full text
PDF



Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Ashley C. T., Jr, Warren S. T. Trinucleotide repeat expansion and human disease. Annu Rev Genet. 1995;29:703–728. doi: 10.1146/annurev.ge.29.120195.003415. [DOI] [PubMed] [Google Scholar]
- Ashley C. T., Jr, Wilkinson K. D., Reines D., Warren S. T. FMR1 protein: conserved RNP family domains and selective RNA binding. Science. 1993 Oct 22;262(5133):563–566. doi: 10.1126/science.7692601. [DOI] [PubMed] [Google Scholar]
- Ashley C. T., Sutcliffe J. S., Kunst C. B., Leiner H. A., Eichler E. E., Nelson D. L., Warren S. T. Human and murine FMR-1: alternative splicing and translational initiation downstream of the CGG-repeat. Nat Genet. 1993 Jul;4(3):244–251. doi: 10.1038/ng0793-244. [DOI] [PubMed] [Google Scholar]
- Chung M. Y., Ranum L. P., Duvick L. A., Servadio A., Zoghbi H. Y., Orr H. T. Evidence for a mechanism predisposing to intergenerational CAG repeat instability in spinocerebellar ataxia type I. Nat Genet. 1993 Nov;5(3):254–258. doi: 10.1038/ng1193-254. [DOI] [PubMed] [Google Scholar]
- Eberhart D. E., Malter H. E., Feng Y., Warren S. T. The fragile X mental retardation protein is a ribonucleoprotein containing both nuclear localization and nuclear export signals. Hum Mol Genet. 1996 Aug;5(8):1083–1091. doi: 10.1093/hmg/5.8.1083. [DOI] [PubMed] [Google Scholar]
- Eichler E. E., Holden J. J., Popovich B. W., Reiss A. L., Snow K., Thibodeau S. N., Richards C. S., Ward P. A., Nelson D. L. Length of uninterrupted CGG repeats determines instability in the FMR1 gene. Nat Genet. 1994 Sep;8(1):88–94. doi: 10.1038/ng0994-88. [DOI] [PubMed] [Google Scholar]
- Fu Y. H., Kuhl D. P., Pizzuti A., Pieretti M., Sutcliffe J. S., Richards S., Verkerk A. J., Holden J. J., Fenwick R. G., Jr, Warren S. T. Variation of the CGG repeat at the fragile X site results in genetic instability: resolution of the Sherman paradox. Cell. 1991 Dec 20;67(6):1047–1058. doi: 10.1016/0092-8674(91)90283-5. [DOI] [PubMed] [Google Scholar]
- Heitz D., Devys D., Imbert G., Kretz C., Mandel J. L. Inheritance of the fragile X syndrome: size of the fragile X premutation is a major determinant of the transition to full mutation. J Med Genet. 1992 Nov;29(11):794–801. doi: 10.1136/jmg.29.11.794. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hirst M. C., Grewal P. K., Davies K. E. Precursor arrays for triplet repeat expansion at the fragile X locus. Hum Mol Genet. 1994 Sep;3(9):1553–1560. doi: 10.1093/hmg/3.9.1553. [DOI] [PubMed] [Google Scholar]
- Khandjian E. W., Corbin F., Woerly S., Rousseau F. The fragile X mental retardation protein is associated with ribosomes. Nat Genet. 1996 Jan;12(1):91–93. doi: 10.1038/ng0196-91. [DOI] [PubMed] [Google Scholar]
- Kremer E. J., Pritchard M., Lynch M., Yu S., Holman K., Baker E., Warren S. T., Schlessinger D., Sutherland G. R., Richards R. I. Mapping of DNA instability at the fragile X to a trinucleotide repeat sequence p(CCG)n. Science. 1991 Jun 21;252(5013):1711–1714. doi: 10.1126/science.1675488. [DOI] [PubMed] [Google Scholar]
- Kunst C. B., Warren S. T. Cryptic and polar variation of the fragile X repeat could result in predisposing normal alleles. Cell. 1994 Jun 17;77(6):853–861. doi: 10.1016/0092-8674(94)90134-1. [DOI] [PubMed] [Google Scholar]
- Kunst C. B., Zerylnick C., Karickhoff L., Eichler E., Bullard J., Chalifoux M., Holden J. J., Torroni A., Nelson D. L., Warren S. T. FMR1 in global populations. Am J Hum Genet. 1996 Mar;58(3):513–522. [PMC free article] [PubMed] [Google Scholar]
- Leeflang E. P., Zhang L., Tavaré S., Hubert R., Srinidhi J., MacDonald M. E., Myers R. H., de Young M., Wexler N. S., Gusella J. F. Single sperm analysis of the trinucleotide repeats in the Huntington's disease gene: quantification of the mutation frequency spectrum. Hum Mol Genet. 1995 Sep;4(9):1519–1526. doi: 10.1093/hmg/4.9.1519. [DOI] [PubMed] [Google Scholar]
- Monckton D. G., Wong L. J., Ashizawa T., Caskey C. T. Somatic mosaicism, germline expansions, germline reversions and intergenerational reductions in myotonic dystrophy males: small pool PCR analyses. Hum Mol Genet. 1995 Jan;4(1):1–8. doi: 10.1093/hmg/4.1.1. [DOI] [PubMed] [Google Scholar]
- Mornet E., Chateau C., Hirst M. C., Thepot F., Taillandier A., Cibois O., Serre J. L. Analysis of germline variation at the FMR1 CGG repeat shows variation in the normal-premutated borderline range. Hum Mol Genet. 1996 Jun;5(6):821–825. doi: 10.1093/hmg/5.6.821. [DOI] [PubMed] [Google Scholar]
- Nolin S. L., Lewis F. A., 3rd, Ye L. L., Houck G. E., Jr, Glicksman A. E., Limprasert P., Li S. Y., Zhong N., Ashley A. E., Feingold E. Familial transmission of the FMR1 CGG repeat. Am J Hum Genet. 1996 Dec;59(6):1252–1261. [PMC free article] [PubMed] [Google Scholar]
- Pieretti M., Zhang F. P., Fu Y. H., Warren S. T., Oostra B. A., Caskey C. T., Nelson D. L. Absence of expression of the FMR-1 gene in fragile X syndrome. Cell. 1991 Aug 23;66(4):817–822. doi: 10.1016/0092-8674(91)90125-i. [DOI] [PubMed] [Google Scholar]
- Richards R. I., Holman K., Friend K., Kremer E., Hillen D., Staples A., Brown W. T., Goonewardena P., Tarleton J., Schwartz C. Evidence of founder chromosomes in fragile X syndrome. Nat Genet. 1992 Jul;1(4):257–260. doi: 10.1038/ng0792-257. [DOI] [PubMed] [Google Scholar]
- Richards R. I., Sutherland G. R. Simple repeat DNA is not replicated simply. Nat Genet. 1994 Feb;6(2):114–116. doi: 10.1038/ng0294-114. [DOI] [PubMed] [Google Scholar]
- Rousseau F., Heitz D., Biancalana V., Blumenfeld S., Kretz C., Boué J., Tommerup N., Van Der Hagen C., DeLozier-Blanchet C., Croquette M. F. Direct diagnosis by DNA analysis of the fragile X syndrome of mental retardation. N Engl J Med. 1991 Dec 12;325(24):1673–1681. doi: 10.1056/NEJM199112123252401. [DOI] [PubMed] [Google Scholar]
- Snow K., Doud L. K., Hagerman R., Pergolizzi R. G., Erster S. H., Thibodeau S. N. Analysis of a CGG sequence at the FMR-1 locus in fragile X families and in the general population. Am J Hum Genet. 1993 Dec;53(6):1217–1228. [PMC free article] [PubMed] [Google Scholar]
- Snow K., Tester D. J., Kruckeberg K. E., Schaid D. J., Thibodeau S. N. Sequence analysis of the fragile X trinucleotide repeat: implications for the origin of the fragile X mutation. Hum Mol Genet. 1994 Sep;3(9):1543–1551. doi: 10.1093/hmg/3.9.1543. [DOI] [PubMed] [Google Scholar]
- Sutcliffe J. S., Nelson D. L., Zhang F., Pieretti M., Caskey C. T., Saxe D., Warren S. T. DNA methylation represses FMR-1 transcription in fragile X syndrome. Hum Mol Genet. 1992 Sep;1(6):397–400. doi: 10.1093/hmg/1.6.397. [DOI] [PubMed] [Google Scholar]
- Verkerk A. J., Pieretti M., Sutcliffe J. S., Fu Y. H., Kuhl D. P., Pizzuti A., Reiner O., Richards S., Victoria M. F., Zhang F. P. Identification of a gene (FMR-1) containing a CGG repeat coincident with a breakpoint cluster region exhibiting length variation in fragile X syndrome. Cell. 1991 May 31;65(5):905–914. doi: 10.1016/0092-8674(91)90397-h. [DOI] [PubMed] [Google Scholar]
- Warren S. T., Ashley C. T., Jr Triplet repeat expansion mutations: the example of fragile X syndrome. Annu Rev Neurosci. 1995;18:77–99. doi: 10.1146/annurev.ne.18.030195.000453. [DOI] [PubMed] [Google Scholar]
- Warren S. T., Nelson D. L. Advances in molecular analysis of fragile X syndrome. JAMA. 1994 Feb 16;271(7):536–542. [PubMed] [Google Scholar]
- Warren S. T. The expanding world of trinucleotide repeats. Science. 1996 Mar 8;271(5254):1374–1375. doi: 10.1126/science.271.5254.1374. [DOI] [PubMed] [Google Scholar]
- Zhang L., Fischbeck K. H., Arnheim N. CAG repeat length variation in sperm from a patient with Kennedy's disease. Hum Mol Genet. 1995 Feb;4(2):303–305. doi: 10.1093/hmg/4.2.303. [DOI] [PubMed] [Google Scholar]
- Zhang L., Leeflang E. P., Yu J., Arnheim N. Studying human mutations by sperm typing: instability of CAG trinucleotide repeats in the human androgen receptor gene. Nat Genet. 1994 Aug;7(4):531–535. doi: 10.1038/ng0894-531. [DOI] [PubMed] [Google Scholar]
- Zhong N., Yang W., Dobkin C., Brown W. T. Fragile X gene instability: anchoring AGGs and linked microsatellites. Am J Hum Genet. 1995 Aug;57(2):351–361. [PMC free article] [PubMed] [Google Scholar]