Abstract
Background
Acupuncture, with many categories such as traditional acupuncture, electroacupuncture, laser acupuncture, and acupoint injection, has been shown to be relatively safe with few adverse effects. It is accessible and inexpensive, at least in China, and is likely to be widely used there for psychotic symptoms.
Objectives
To review the effects of acupuncture, alone or in combination treatments compared with placebo (or no treatment) or any other treatments for people with schizophrenia or related psychoses.
Search methods
We searched Cochrane Schizophrenia Group’s Trials Register (February 2012), which is based on regular searches of CINAHL, BIOSIS, AMED, EMBASE, PubMed, MEDLINE, PsycINFO and clinical trials registries. We also inspected references of identified studies and contacted relevant authors for additional information.
Selection criteria
We included all relevant randomised controlled trials involving people with schizophrenia‐like illnesses, comparing acupuncture added to standard dose antipsychotics with standard dose antipsychotics alone, acupuncture added to low dose antipsychotics with standard dose antipsychotics, acupuncture with antipsychotics, acupuncture added to Traditional Chinese Medicine (TCM) drug with TCM drug, acupuncture with TCM drug, electric acupuncture convulsive therapy with electroconvulsive therapy.
Data collection and analysis
We reliably extracted data from all included studies, discussed any disagreement, documented decisions and contacted authors of studies when necessary. We analysed binary outcomes using a standard estimation of risk ratio (RR) and its 95% confidence interval (CI). For continuous data, we calculated mean differences with 95% CI. For homogeneous data we used fixed‐effect model. We assessed risk of bias for included studies and created 'Summary of findings' tables using GRADE.
Main results
After an update search in 2012 the review now includes 30 studies testing different forms of acupuncture across six different comparisons. All studies were at moderate risk of bias.
When acupuncture plus standard antipsychotic treatment was compared with standard antipsychotic treatment alone, people were at less risk of being 'not improved' (n = 244, 3 RCTs, medium‐term RR 0.40 CI 0.28 to 0.57, very low quality evidence). Mental state findings were mostly consistent with this finding as was time in hospital (n = 120, 1 RCT, days MD ‐16.00 CI ‐19.54 to ‐12.46, moderate quality evidence). If anything, adverse effects were less for the acupuncture group (e.g. central nervous system, insomnia, short‐term, n = 202, 3 RCTs, RR 0.30 CI 0.11 to 0.83, low quality evidence).
When acupuncture was added to low dose antipsychotics and this was compared with standard dose antipsychotic drugs, relapse was less in the experimental group (n = 170, 1 RCT, long‐term RR 0.57 CI 0.37 to 0.89, very low quality evidence) but there was no difference for the outcome of 'not improved'. Again, mental state findings were mostly consistent with the latter. Incidences of extrapyramidal symptoms ‐ akathisia, were less for those in the acupuncture added to low dose antipsychotics group (n = 180, 1 RCT, short‐term RR 0.03 CI 0.00 to 0.49, low quality evidence) ‐ as dry mouth, blurred vision and tachycardia.
When acupuncture was compared with antipsychotic drugs of known efficacy in standard doses, there were equivocal data for outcomes such as 'not improved' using different global state criteria. Traditional acupuncture added to TCM drug had benefit over use of TCM drug alone (n = 360, 2 RCTs, RR no clinically important change 0.11 CI 0.02 to 0.59, low quality evidence), but when traditional acupuncture was compared with TCM drug directly there was no significant difference in the short‐term. However, we found that participants given electroacupuncture were significantly less likely to experience a worsening in global state (n = 88, 1 RCT, short‐term RR 0.52 CI 0.34 to 0.80, low quality evidence).
In the one study that compared electric acupuncture convulsive therapy with electroconvulsive therapy there were significantly different rates of spinal fracture between the groups (n = 68, 1 RCT, short‐term RR 0.33 CI 0.14 to 0.81, low quality evidence). Attrition in all studies was minimal. No studies reported death, engagement with services, satisfaction with treatment, quality of life, or economic outcomes.
Authors' conclusions
Limited evidence suggests that acupuncture may have some antipsychotic effects as measured on global and mental state with few adverse effects. Better designed large studies are needed to fully and fairly test the effects of acupuncture for people with schizophrenia.
Plain language summary
Acupuncture for schizophrenia
Although acupuncture or Traditional Chinese Medicine has been practised for over 2000 years in China and the Far East, especially in Korea and Japan, it is a relatively new form of treament for physical and psychological conditions in the West. Acupuncture inserts needles into the skin to stimulate specific points of the body (acupoints). The aim is to achieve balance and harmony of the body.
Schizophrenia is a serious mental illness and is usually treated using antipsychotic medication. However, although effective, antipsychotic medication can cause side‐effects (such as sleepiness, weight gain and even dribbling). Acupuncture has been shown to have very few negative effects on the individual and could be more socially acceptable and tolerable for people with mental health problems. Acupuncture may also be less expensive than drugs made by pharmaceutical companies, so reducing costs to individuals and health services.
This reviews looks at the effectiveness of various types of acupuncture as treatment for people with schizophrenia. An update search for studies was carried out in 2012 and found 30 studies that randomised participants who were receiving antipsychotic medication to receive additional acupuncture or standard care.
Although some of the studies did favour acupuncture when combined with antipsychotics, the information available was small scale and rated to be very low or low quality by the review authors, so not completely provable and valid. Depression was reduced when combining acupuncture with antipsychotic medication, but again this finding came from small‐scale research, so cannot be clearly shown to be true. The review concludes that people with mental health problems, policy makers and health professionals need much better evidence in order to establish if there are any potential benefits to acupuncture.
This means that the question of whether acupuncture is of benefit to people, and whether it is of greater benefit than antipsychotic medication, remains unanswered. There is not enough information to establish that acupuncture is of benefit or harm to people with mental health problems.
Benjamin Gray, Service User and Service User Expert, Rethink Mental Illness.
Summary of findings
Summary of findings for the main comparison. ACUPUNCTURE added to STANDARD DOSE ANTIPSYCHOTICS versus STANDARD DOSE ANTIPSYCHOTICS for schizophrenia.
| ACUPUNCTURE added to STANDARD DOSE ANTIPSYCHOTICS versus STANDARD DOSE ANTIPSYCHOTICS for schizophrenia | ||||||
| Patient or population: patients with schizophrenia Intervention: ACUPUNCTURE added to STANDARD DOSE ANTIPSYCHOTICS versus STANDARD DOSE ANTIPSYCHOTICS | ||||||
| Outcomes | Illustrative comparative risks* (95% CI) | Relative effect (95% CI) | No of Participants (studies) | Quality of the evidence (GRADE) | Comments | |
| Assumed risk | Corresponding risk | |||||
| Control | ACUPUNCTURE added to STANDARD DOSE ANTIPSYCHOTICS versus STANDARD DOSE ANTIPSYCHOTICS | |||||
| Global state: Not improved, endpoint ‐ medium‐term (various similar criteria) | 557 per 1000 | 223 per 1000 (156 to 318) | RR 0.4 (0.28 to 0.57) | 244 (3 study) | ⊕⊝⊝⊝ very low1,2 | Relapse was not reported but 'no clinically important change in global state' was reported and another outcome without clear duration indicated no difference between two comparison groups. |
| Mental state: PANSS (not improved, reduced rated < 25%, short‐term) | 432 per 1000 | 281 per 1000 (194 to 406) | RR 0.65 (0.45 to 0.94) | 197 (3 studies) | ⊕⊝⊝⊝ very low1,2 | Data from PANSS were equivocal because of different criteria. Other mental state findings were mostly consistent with this finding. |
| Behaviour: Leaving the study early (short‐term) | 7 per 1000 | 9 per 1000 (2 to 38) | RR 1.33 (0.33 to 5.45) | 870 (10 studies) | ⊕⊝⊝⊝ very low2,3,4 | Similar outcomes at medium‐term. |
| Service outcomes: Time in hospital (days) | The mean service outcomes: time in hospital (days) in the intervention groups was 16 lower (19.54 to 12.46 lower) | 120 (1 study) | ⊕⊕⊕⊝ moderate2 | 0nly one study reported service outcomes ‐ time in hospital. | ||
| Adverse effects: Central Nervous System ‐ insomnia (short‐term) | 131 per 1000 | 39 per 1000 (14 to 109) | RR 0.30 (0.11 to 0.83) | 202 (3 studies) | ⊕⊕⊝⊝ low2,3 | Only insomnia rate indicated difference between two compared groups. |
| Quality of life: No clinically important change in quality of life | See comment | See comment | Not estimable | ‐ | See comment | Not reported. |
| Economic outcomes: Cost of care | See comment | See comment | Not estimable | ‐ | See comment | Not reported. |
| *The basis for the assumed risk (e.g. the median control group risk across studies) is provided in footnotes. The corresponding risk (and its 95% confidence interval) is based on the assumed risk in the comparison group and the relative effect of the intervention (and its 95% CI). CI: Confidence interval; RR: Risk ratio; | ||||||
| GRADE Working Group grades of evidence High quality: Further research is very unlikely to change our confidence in the estimate of effect. Moderate quality: Further research is likely to have an important impact on our confidence in the estimate of effect and may change the estimate. Low quality: Further research is very likely to have an important impact on our confidence in the estimate of effect and is likely to change the estimate. Very low quality: We are very uncertain about the estimate. | ||||||
1 Risk of bias: rated 'very serious' ‐ Unblinding of participants and personnel and incomplete outcome data. 2 Imprecision: rated 'serious' ‐ Few participants and/or few events. 3 Risk of bias: rated 'serious' ‐ Unblinding of participants and personnel. 4 Publication bias: rated 'strong suspected' ‐ One author worked for drug industry.
Summary of findings 2. ACUPUNCTURE added to LOW DOSE ANTIPSYCHOTICS versus STANDARD DOSE ANTIPSYCHOTICS for schizophrenia.
| ACUPUNCTURE added to LOW DOSE ANTIPSYCHOTICS versus STANDARD DOSE ANTIPSYCHOTICS for schizophrenia | ||||||
| Patient or population: patients with schizophrenia Intervention: ACUPUNCTURE added to LOW DOSE ANTIPSYCHOTICS versus STANDARD DOSE ANTIPSYCHOTICS | ||||||
| Outcomes | Illustrative comparative risks* (95% CI) | Relative effect (95% CI) | No of Participants (studies) | Quality of the evidence (GRADE) | Comments | |
| Assumed risk | Corresponding risk | |||||
| Control | ACUPUNCTURE added to LOW DOSE ANTIPSYCHOTICS versus STANDARD DOSE ANTIPSYCHOTICS | |||||
| Global state: Relapse (follow‐up, long‐term) | 431 per 1000 | 246 per 1000 (159 to 383) | RR 0.57 (0.37 to 0.89) | 170 (1 study) | ⊕⊝⊝⊝ very low1,2,3 | Only one study reported this outcome. Other global state findings reported no difference between the two comparison groups. |
| Mental state: BPRS (not improved, reduced rate < 30%, short‐term) | 133 per 1000 | 100 per 1000 (24 to 409) | RR 0.75 (0.18 to 3.07) | 60 (1 study) | ⊕⊝⊝⊝ very low4,5 | Though measured with different criteria of 'no clinically important change in general mental state', there was no difference between the two comparison groups at short‐term and mostly similar results from other mental state findings. |
| Behaviour: Leaving the study early (short‐term) | 19 per 1000 | 16 per 1000 (6 to 45) | RR 0.81 (0.29 to 2.29) | 662 (8 studies) | ⊕⊝⊝⊝ very low2,3,4 | |
| Service outcomes: Hospitalisation | See comment | See comment | Not estimable | ‐ | See comment | Not reported. |
| Adverse effects: Extrapyramidal symptoms ‐ specific ‐ akathisia (short‐term) | 185 per 1000 | 6 per 1000 (0 to 91) | RR 0.03 (0 to 0.49) | 180 (1 study) | ⊕⊕⊝⊝ low2,4 | Similar to the akathisia data, acupuncture added to low dose antipsychotics reduced participants experiencing dry mouth, blurred vision, tachycardia at short‐term. Only one study focused on acupoint catgut treatment relative adverse effects but did not find skin infection in either groups. |
| Quality of life: No clinically important change in quality of life | See comment | See comment | Not estimable | ‐ | See comment | Not reported. |
| Economic outcomes: Cost of care | See comment | See comment | Not estimable | ‐ | See comment | Not reported. |
| *The basis for the assumed risk (e.g. the median control group risk across studies) is provided in footnotes. The corresponding risk (and its 95% confidence interval) is based on the assumed risk in the comparison group and the relative effect of the intervention (and its 95% CI). CI: Confidence interval; RR: Risk ratio; | ||||||
| GRADE Working Group grades of evidence High quality: Further research is very unlikely to change our confidence in the estimate of effect. Moderate quality: Further research is likely to have an important impact on our confidence in the estimate of effect and may change the estimate. Low quality: Further research is very likely to have an important impact on our confidence in the estimate of effect and is likely to change the estimate. Very low quality: We are very uncertain about the estimate. | ||||||
1 Risk of bias: rated 'very serious' ‐ Unblinding of participants and personnel and incomplete outcome data. 2 Imprecision: rated 'serious' ‐ Few participants and/or few events. 3 Publication bias: rated 'strongly suspected' ‐ There was some difference between two references of the same study. 4 Risk of bias: rated 'serious' ‐ Unblinding of participants and personnel. 5 Imprecision: rated 'very serious' ‐ Few participants and/or few events and wide confidence intervals.
Summary of findings 3. ACUPUNCTURE versus STANDARD DOSE ANTIPSYCHOTICS for schizophrenia.
| ACUPUNCTURE versus STANDARD DOSE ANTIPSYCHOTICS for schizophrenia | ||||||
| Patient or population: patients with schizophrenia Intervention: ACUPUNCTURE versus ANTIPSYCHOTICS | ||||||
| Outcomes | Illustrative comparative risks* (95% CI) | Relative effect (95% CI) | No of Participants (studies) | Quality of the evidence (GRADE) | Comments | |
| Assumed risk | Corresponding risk | |||||
| Control | ACUPUNCTURE versus ANTIPSYCHOTICS | |||||
| Global state: Not improved (talk, behaviour and expression still existed as before; the reduction of BPRS < 29%), endpoint (short‐term) | 317 per 1000 | 104 per 1000 (60 to 187) | RR 0.33 (0.19 to 0.59) | 240 (2 studies) | ⊕⊝⊝⊝ very low1,2 | Relapse was not reported but 'no clinically important change in global state' was reported and another three outcomes with different but similar criteria indicated no difference between the two comparison groups in the short‐term. |
| Mental state: BPRS, endpoint (high score = worse, short‐term) ‐ traditional acupuncture | The mean mental state: BPRS, endpoint (high score = worse, short‐term) ‐ traditional acupuncture in the intervention groups was 11.56 lower (16.36 to 6.76 lower) | 32 (1 study) | ⊕⊝⊝⊝ very low1,2 | The outcome of 'no clinically important change in general mental state' was not reported but average endpoint BPRS data were reported as was the SAPS score. There was no difference between the two comparison groups in the short‐term using either score. | ||
| Behaviour: Leaving the study early (short‐term) | See comment | See comment | Not estimable | 421 (6 studies) | ⊕⊕⊝⊝ low1,3 | No participants left each compared group early. |
| Service outcomes: Hospitalisation | See comment | See comment | Not estimable | ‐ | See comment | Not reported. |
| Adverse effects: Extrapyramidal symptoms (short‐term) | 800 per 1000 | 40 per 1000 (0 to 664) | RR 0.05 (0 to 0.83) | 21 (1 study) | ⊕⊕⊝⊝ low1,3 | One study reported laser acupuncture relative adverse effects ‐ numbness over ear, upper extremity and chest on the treated side, but no difference between the two comparison groups. |
| Quality of life: No clinically important change in quality of life | See comment | See comment | Not estimable | ‐ | See comment | Not reported. |
| Economic outcomes: Cost of care | See comment | See comment | Not estimable | ‐ | See comment | Not reported. |
| *The basis for the assumed risk (e.g. the median control group risk across studies) is provided in footnotes. The corresponding risk (and its 95% confidence interval) is based on the assumed risk in the comparison group and the relative effect of the intervention (and its 95% CI). CI: Confidence interval; RR: Risk ratio; | ||||||
| GRADE Working Group grades of evidence High quality: Further research is very unlikely to change our confidence in the estimate of effect. Moderate quality: Further research is likely to have an important impact on our confidence in the estimate of effect and may change the estimate. Low quality: Further research is very likely to have an important impact on our confidence in the estimate of effect and is likely to change the estimate. Very low quality: We are very uncertain about the estimate. | ||||||
1 Risk of bias: rated 'serious' ‐ Unblinding of participants and personnel. 2 Imprecision: rated 'very serious' ‐ Few participants and/or few events and wide confidence intervals. 3 Imprecision: rated 'serious' ‐ Few participants and/or few events.
Summary of findings 4. ACUPUNCTURE added to TCM DRUG versus TCM DRUG for schizophrenia.
| ACUPUNCTURE plus TCM DRUG versus TCM DRUG for schizophrenia | ||||||
| Patient or population: patients with schizophrenia Intervention: ACUPUNCTURE added to TCM DRUG versus TCM DRUG | ||||||
| Outcomes | Illustrative comparative risks* (95% CI) | Relative effect (95% CI) | No of Participants (studies) | Quality of the evidence (GRADE) | Comments | |
| Assumed risk | Corresponding risk | |||||
| Control | ACUPUNCTURE added to TCM DRUG versus TCM DRUG | |||||
| Global state: Not improved (talk, behaviour and expression still existed as before, the reduction of BPRS < 29%), endpoint (short‐term) | 72 per 1000 | 8 per 1000 (1 to 43) | RR 0.11 (0.02 to 0.59) | 360 (2 studies) | ⊕⊕⊝⊝ low1,2 | Relapse was not reported but 'no clinically important change in global state' was reported and the other outcome with different criteria of 'no clinically important change in global state' indicated no difference between the two comparison groups in the short‐term. |
| Mental state: No clinically important change in mental state | See comment | See comment | Not estimable | ‐ | See comment | Not reported. |
| Behaviour: Leaving the study early (short‐term) | See comment | See comment | Not estimable | 454 (3 studies) | ⊕⊕⊝⊝ low1,2 | No participants left either group early. |
| Service outcomes: Hospitalisation | See comment | See comment | Not estimable | ‐ | See comment | Not reported. |
| Adverse effects: Clinically important general adverse effects | See comment | See comment | Not estimable | 0 (0) | See comment | Three studies reported this outcome, however, two studies only reported one group's data and the other one study did not report the data. |
| Quality of life: No clinically important change in quality of life | See comment | See comment | Not estimable | ‐ | See comment | Not reported. |
| Economic outcomes: Cost of care | See comment | See comment | Not estimable | ‐ | See comment | Not reported. |
| *The basis for the assumed risk (e.g. the median control group risk across studies) is provided in footnotes. The corresponding risk (and its 95% confidence interval) is based on the assumed risk in the comparison group and the relative effect of the intervention (and its 95% CI). CI: Confidence interval; RR: Risk ratio; | ||||||
| GRADE Working Group grades of evidence High quality: Further research is very unlikely to change our confidence in the estimate of effect. Moderate quality: Further research is likely to have an important impact on our confidence in the estimate of effect and may change the estimate. Low quality: Further research is very likely to have an important impact on our confidence in the estimate of effect and is likely to change the estimate. Very low quality: We are very uncertain about the estimate. | ||||||
1 Risk of bias: rated 'serious' ‐ Unblinding of participants and personnel. 2 Imprecision: rated 'serious' ‐ Few participants and/or few events.
Summary of findings 5. ACUPUNCTURE versus TCM DRUG for schizophrenia.
| ACUPUNCTURE versus TCM DRUG for schizophrenia | ||||||
| Patient or population: patients with schizophrenia Intervention: ACUPUNCTURE versus TCM DRUG | ||||||
| Outcomes | Illustrative comparative risks* (95% CI) | Relative effect (95% CI) | No of Participants (studies) | Quality of the evidence (GRADE) | Comments | |
| Assumed risk | Corresponding risk | |||||
| Control | ACUPUNCTURE versus TCM DRUG | |||||
| Global state: Not improved, endpoint (short‐term) ‐ Not improved (no change in symptoms) ‐ Electroacupuncture | 711 per 1000 | 370 per 1000 (242 to 569) | RR 0.52 (0.34 to 0.80) | 88 (1 study) | ⊕⊕⊝⊝ low1,2 | Relapse was not reported but 'no clinically important change in global state' was reported and other outcomes with different criteria of 'no clinically important change in global state' indicated no difference between the two comparison groups in the short‐term. |
| Mental state: No clinically important change in general mental state | See comment | See comment | Not estimable | ‐ | See comment | Not reported. |
| Behaviour: Leaving the study early (short‐term) | See comment | See comment | Not estimable | 328 (3 studies) | See comment | No participants left either group early. |
| Service outcomes: Hospitalisation | See comment | See comment | Not estimable | ‐ | See comment | Not reported. |
| Adverse effects: Clinically important general adverse effects | See comment | See comment | Not estimable | ‐ | See comment | Three studies reported this outcome, however, two studies only reported one group's data and the other one study did not report the data. |
| Quality of life: No clinically important change in quality of life | See comment | See comment | Not estimable | ‐ | See comment | Not reported. |
| Economic outcomes: Cost of care | See comment | See comment | Not estimable | ‐ | See comment | Not reported. |
| *The basis for the assumed risk (e.g. the median control group risk across studies) is provided in footnotes. The corresponding risk (and its 95% confidence interval) is based on the assumed risk in the comparison group and the relative effect of the intervention (and its 95% CI). CI: Confidence interval; RR: Risk ratio; | ||||||
| GRADE Working Group grades of evidence High quality: Further research is very unlikely to change our confidence in the estimate of effect. Moderate quality: Further research is likely to have an important impact on our confidence in the estimate of effect and may change the estimate. Low quality: Further research is very likely to have an important impact on our confidence in the estimate of effect and is likely to change the estimate. Very low quality: We are very uncertain about the estimate. | ||||||
1 Risk of bias: rated 'serious' ‐‐ Unblinding of participants and personnel. 2 Impression: rated 'serious' ‐ Few participants and/or few evens.
Summary of findings 6. ELECTRIC ACUPUNCTURE CONVULSIVE THERAPY versus ELECTROCONVULSIVE THERAPY for schizophrenia.
| ELECTRIC ACUPUNCTURE CONVULSIVE THERAPY versus ELECTRIC CONVULSIVE THERAPY for schizophrenia | ||||||
| Patient or population: patients with schizophrenia Intervention: ELECTRIC ACUPUNCTURE CONVULSIVE THERAPY versus ELECTRIC CONVULSIVE THERAPY | ||||||
| Outcomes | Illustrative comparative risks* (95% CI) | Relative effect (95% CI) | No of Participants (studies) | Quality of the evidence (GRADE) | Comments | |
| Assumed risk | Corresponding risk | |||||
| Control | ELECTRIC ACUPUNCTURE CONVULSIVE THERAPY versus ELECTRIC CONVULSIVE THERAPY | |||||
| Global state: Relapse | See comment | See comment | Not estimable | ‐ | See comment | Not reported. |
| Mental state: No clinically important change in general mental state | See comment | See comment | Not estimable | ‐ | See comment | Not reported. |
| Behaviour: Leaving the study early (short‐term) | See comment | See comment | Not estimable | 68 (1 study) | ⊕⊕⊝⊝ low1,2 | No participants in either group left early. |
| Service outcomes: Hospitalisation | See comment | See comment | Not estimable | ‐ | See comment | Not reported. |
| Adverse effects: Spinal fracture (short‐term) | 441 per 1000 | 146 per 1000 (62 to 357) | RR 0.33 (0.14 to 0.81) | 68 (1 study) | ⊕⊕⊝⊝ low1,2 | Data from a single study where there was no difference in back pain between the two groups. |
| Quality of life: No clinically important change in quality of life | See comment | See comment | Not estimable | ‐ | See comment | Not reported. |
| Economic outcomes: Cost of care | See comment | See comment | Not estimable | ‐ | See comment | Not reported. |
| *The basis for the assumed risk (e.g. the median control group risk across studies) is provided in footnotes. The corresponding risk (and its 95% confidence interval) is based on the assumed risk in the comparison group and the relative effect of the intervention (and its 95% CI). CI: Confidence interval; RR: Risk ratio; | ||||||
| GRADE Working Group grades of evidence High quality: Further research is very unlikely to change our confidence in the estimate of effect. Moderate quality: Further research is likely to have an important impact on our confidence in the estimate of effect and may change the estimate. Low quality: Further research is very likely to have an important impact on our confidence in the estimate of effect and is likely to change the estimate. Very low quality: We are very uncertain about the estimate. | ||||||
1 Risk of bias; rated 'serious' ‐ Unblinding of participants and personnel. 2 Imprecision: rated 'serious' ‐ Few participants and few events.
Background
Description of the condition
Schizophrenia affects 1% of people and therefore, one fifth of people with schizophrenia are of Chinese origin. In Chinese health care, theories for the aetiology, pathology and treatment of schizophrenia are different to those of Western medicine. Conventional medicine diagnoses schizophrenia primarily by operationalised criteria such as the Diagnostic and Statistical Manual (DSM‐IV) or the International Classification of Diseases (ICD10). Chinese medicine uses different methodology to diagnose mental health disorders such as schizophrenia by pattern differentiation. The four diagnostic methods (inspection, listening/smelling, inquiry and palpation) are the way by which patient information is obtained (Xu 1991). This information is then analysed using one or several diagnostic models (Zang Fu theory, Eight Principles [yin/yang, interior/exterior, excess/deficiency, hot/cold], Four Division Pattern [Wei Qi Ying Xue ], Six Division Pattern, Five Elements Pattern [wood, fire, earth, metal, and water], Pattern of Qi, Blood, and Body Fluids, Three Burners Pattern) to arrive at a diagnosis. Thus, two people diagnosed with DSM‐IV schizophrenia could nevertheless, from a Chinese medicine perspective, have different sub‐types or patterns and therefore require different treatment. The Psychosis Professional Committee of Chinese Integrative Medicine Association drafted the Standard of Integrative Medicine Syndrome Type of Schizophrenia. There are six main patterns which fall within the disease category of Dian Kuang/withdrawal mania which can also encompass schizophrenia. The six types are: 1. Internal disturbance of pyrophlegm; 2. Internal retention of phlegm and dampness; 3. Qi stagnation and blood stasis; 4. Yin deficiency and fire excess; 5. Yang deficiency; 6.Other miscellaneous types (The Psychosis Professional Committee 1988; Guo 2010; Zhang 1996).
Description of the intervention
The illness often causes distortions of perception, thinking, behaving and even movement and, although antipsychotic drugs have been the mainstay of treatment of schizophrenia since the early 1950s, these treatments still leave many with residual symptoms and disabling adverse effects. Acupuncture (Figure 1) has been shown to have few adverse effects (Ernst 2001; MacPherson 2001) and may be more socially acceptable, tolerable and inexpensive than the more conventional drugs available from the pharmaceutical industry. Chinese medicine, which includes acupuncture and is also referred to as Traditional Chinese Medicine (TCM), has been used to treat 'schizophrenia‐like illnesses' (i.e. Dian Kuang/withdrawal mania) for over 2000 years (Ming 2001). Acupuncture is practiced as an accepted healthcare model in China, Korea and Japan, although the methods used in each country are distinct (Kaptchuk 2002). There are many categories of acupuncture such as traditional acupuncture, electroacupuncture, laser acupuncture, and acupoint injection.
1.
Acupuncture
How the intervention might work
Acupuncture involves the stimulation of specific points (acupoints). The internal diseases are treated using external applications by dredging meridians, regulating Yin and Yang, reinforcing the healthy Qi and eliminating the pathogenic factors. Though acupuncture has a long history, any mechanism for treating schizophrenia is still unclear (Shi 2010; Xu 2010). Acupuncture may affect the central nervous functions of the cerebral cortex through recuperating the Qi, blood, dredging meridians and then adjusting the nervous system's functions and endocrine level (Wang 2007). There is, however, not enough modern neural physiological and biochemical research, imaging or animal studies to support this (Xu 2010).
Why it is important to do this review
Acupuncture has been used to treat schizophrenia for many years and now it is considered as one of the most popular types of complementary and alternative medicine (CAM) in Western health care. We wish to update past work, undertake a review that is systematic and quantify the effects of this inexpensive treatment.
Objectives
To review the effects of acupuncture for people with schizophrenia and related psychoses, evaluating acupuncture alone or in combination regimens compared with placebo (or no treatment), or any other treatments.
Methods
Criteria for considering studies for this review
Types of studies
We included all relevant randomised controlled trials. We planned that if a trial was described as 'double‐blind' but it was implied that the study was randomised, these trials would have been included in a sensitivity analysis. If there was no substantive difference within primary outcomes (see 'Types of outcome measures') when these 'implied randomisation' studies were added, then they would have been included in the final analysis. If there was a substantive difference, only clearly randomised trials would have been utilised and the results of the sensitivity analysis described in the text. Quasi‐randomised studies, such as those allocating by using alternate days of the week, were excluded.
Types of participants
We included patients with schizophrenia, schizophreniform psychosis and schizophrenia‐like illnesses, any age, diagnosed by any criteria.
Types of interventions
1. All categories of acupuncture
Administered solely or in conjunction with any other treatments, versus:
2. Placebo (sham acupuncture) or no treatment
3. Any other treatments
We do recognise that there are other possible combinations of treatments that could be included in this review but this (2012) update focused on only the above comparisons.
For previous type of interventions please see Appendix 1.
Types of outcome measures
Outcomes were divided into short‐term (less than three months), medium‐term (three to 12 months) and long‐term (more than one year).
Primary outcomes
1. Global state
1.1 Relapse
2. Mental state
2.2 No clinically important change in general mental state ‐ as defined by each of the studies
3. Service outcomes
3.1 Hospitalisation
4. Adverse effects
4.1 Clinically important general adverse effects
Secondary outcomes
1. Death ‐ suicide or natural causes
2. Global state
2.1 No clinically important change in global state ‐ as defined by each of the studies 2.2 Average endpoint global state score 2.3 Average change in global state scores
3. Mental state
3.1 No change in general mental state 3.2 Average endpoint general mental state score 3.3 Average change in general mental state scores 3.4 No clinically important change in specific symptoms ‐ as defined by each of the studies 3.5 No change in specific symptoms 3.6 Average endpoint specific symptom score 3.7 Average change in specific symptom scores
4. Behaviour
4.1 Leaving the study early 4.2 No clinically important change in general behaviour ‐ as defined by each of the studies 4.3 No change in general behaviour 4.4 Average endpoint general behaviour score 4.5 Average change in general behaviour scores 4.6 No clinically important change in specific aspects of behaviour 4.7 No change in specific aspects of behaviour 4.8 Average endpoint specific aspects of behaviour 4.9 Average change in specific aspects of behaviour
5. Service outcomes
5.1 Time to hospitalisation
6. Adverse effects
6.1 Any general adverse effects 6.2 Average endpoint general adverse effect score 6.3 Average change in general adverse effect scores 6.4 No clinically important change in specific adverse effects ‐ as defined by each of the studies 6.5 No change in specific adverse effects 6.6 Average endpoint specific adverse effects 6.7 Average change in specific adverse effects
7. Engagement with services
7.1 No clinically important engagement ‐ as defined by each of the studies 7.2 No engagement 7.3 Average endpoint engagement score 7.4 Average change in engagement scores
8. Satisfaction with treatment
8.1 Recipient of care not satisfied with treatment 8.2 Recipient of care average satisfaction score 8.3 Recipient of care average change in satisfaction scores
9. Quality of life
9.1 No clinically important change in quality of life ‐ as defined by each of the studies 9.2 No change in quality of life 9.3 Average endpoint quality of life score 9.4 Average change in quality of life scores
10. Economic outcomes
10.1 Costs of care
11. 'Summary of findings' table
We used the GRADE approach to interpret findings (Schünemann 2008) and used the GRADE profiler to import data from Review Manager (RevMan) to create 'Summary of findings' tables. These tables provide outcome‐specific information concerning the overall quality of evidence from each included study in the comparison, the magnitude of effect of the interventions examined, and the sum of available data on all outcomes we rated as important to patient‐care and decision making. We selected the following main outcomes for inclusion in the 'Summary of findings' table.
1. Global state: relapse. 2. Mental state: no clinically important change in general mental state ‐ as defined by each of the studies. 3. Behaviour: leaving the study early. 4. Service outcomes: hospitalisation. 5. Adverse effects: clinically important general adverse effects. 6. Quality of life: no clinically important change in quality of life ‐ as defined by each of the studies. 7. Economic outcomes: costs of care.
Search methods for identification of studies
Electronic searches
1. Cochrane Schizophrenia Group’s Trials Register
The Trials Search Co‐ordinator (TSC) searched the Cochrane Schizophrenia Group’s Registry of Trials (February 2012) using the following search strategies:
(*acup* or *moxibustion*) in Title Field of REFERENCE or (*acupuncture* or *moxibustion*) in Intervention Field of STUDY
The Cochrane Schizophrenia Group’s Registry of Trials is compiled by systematic searches of major resources (including AMED, BIOSIS, CINAHL, EMBASE, MEDLINE, PsycINFO, PubMed, and registries of clinical trials) and their monthly updates, handsearches, grey literature, and conference proceedings (see Group Module). There is no language, date, document type, or publication status limitations for inclusion of records into the register.
For previous searches, see Appendix 2.
Searching other resources
1. Reference searching
We inspected references of all identified studies for further relevant studies.
2. Personal contact
We contacted the first author of each included study for information regarding unpublished trials.
Data collection and analysis
Methods used in data collection and analysis for this 2012 update are below; for previous methods, please see Appendix 3.
Selection of studies
For this 2012 update, review author XS independently inspected citations from the new electronic search and identified relevant abstracts. XS also inspected full articles of the abstracts meeting inclusion criteria. JX carried out the reliability check of all citations from the new electronic search. Where disputes arose, we resolved disagreements by discussion or, if doubt remained, by acquiring the full article for more detailed scrutiny. If doubt still remained, we added these trials to the list of those awaiting assessment pending acquisition of further information and we contacted the authors of studies. For details of previous author contributions in study selection see Acknowledgements.
Data extraction and management
1. Extraction
For this 2012 update, XS extracted data from all included studies. In addition, to ensure reliability, JX independently extracted data from a random sample of these studies, comprising 10% of the total. We discussed disagreements, documented our decisions and, if necessary, contracted authors of studies for clarification. With any remaining problems, CEA helped clarify issues, although he could not translate the Mandarin text. When uncertainty persisted, we allocated the trial to Studies awaiting classification. Data presented only in graphs and figures were extracted whenever possible, but included only if two review authors independently extracted the same results. In order to obtain missing information or for clarification, whenever necessary we contacted authors through an open‐ended request . If studies were multi‐centre, where possible, we extracted data relevant to each component centre separately.
2. Management
2.1 Forms
We extracted data onto standard, simple forms.
2.2 Scale‐derived data
We included continuous data from rating scales only if: a. the psychometric properties of the measuring instrument have been described in a peer‐reviewed journal (Marshall 2000); and b. the measuring instrument has not been written or modified by one of the trialists for that particular trial.
Ideally, the measuring instrument should either be i. a self‐report or ii. completed by an independent rater or relative (not the therapist). We realise that this is not often reported clearly; we have noted whether or not this is the case in Description of studies.
2.3 Endpoint versus change data
There are advantages of both endpoint and change data. Change data can remove a component of between‐person variability from the analysis. On the other hand, calculation of change needs two assessments (baseline and endpoint), which can be difficult in unstable and difficult to measure conditions such as schizophrenia. We decided primarily to use endpoint data, and only use change data if the former were not available. We combined endpoint and change data in the analysis as we used mean differences (MD) rather than standardised mean differences throughout (Higgins 2011, Chapter 9.4.5.2).
Previous version of this review did not combine endpoint and change data in the analysis (see Appendix 3).
2.4 Skewed data
Continuous data on clinical and social outcomes are often not normally distributed. To avoid the pitfall of applying parametric tests to non‐parametric data, we aimed to apply the following standards to all data before inclusion: a) standard deviations (SDs) and means are reported in the paper or obtainable from the authors; b) when a scale starts from the finite number zero, the SD, when multiplied by two, is less than the mean (as otherwise the mean is unlikely to be an appropriate measure of the centre of the distribution, (Altman 1996); c) if a scale started from a positive value (such as the Positive and Negative Syndrome Scale (PANSS), (Kay 1986)), which can have values from 30 to 210), the calculation described above will be modified to take the scale starting point into account. In these cases skew is present if 2 SD > (S‐S min), where S is the mean score and 'S min' is the minimum score.
Endpoint scores on scales often have a finite start and end point and these rules can be applied. Skewed data pose less of a problem when looking at means if the sample size is large (>200) and we will enter these into the syntheses. We presented skewed endpoint data from studies of less than 200 participants as 'Other data' in the Data & analyses rather than enter such data in analyses. When continuous data are presented on a scale that includes a possibility of negative values (such as change data), it is difficult to tell whether data are skewed or not. We planned to present and enter change data into analyses.
2.5 Common measure
To facilitate comparison between trials, we intended to convert variables that can be reported in different metrics, such as days in hospital (mean days per year, per week or per month) to a common metric (e.g. mean days per month).
2.6 Conversion of continuous to binary
Where possible, we made efforts to convert outcome measures to dichotomous data. This can be done by identifying cut‐off points on rating scales and dividing participants accordingly into 'clinically improved' or 'not clinically improved'. It is generally assumed that if there is a 50% reduction in a scale‐derived score such as the Brief Psychiatric Rating Scale (BPRS, Overall 1962) or the Positive and Negative Syndrome Scale (PANSS, Kay 1986), this could be considered as a clinically significant response (Leucht 2005; Leucht 2005a). If data based on these thresholds were not available, we used the primary cut‐off presented by the original authors.
2.7 Direction of graphs
Where possible, we entered data in such a way that the area to the left of the line of no effect indicated a favourable outcome for acupuncture. If we had to enter data so the area to the left of the line indicated a favourable outcome for the control treatment, this was noted in the relevant graphs.
Assessment of risk of bias in included studies
For this 2012 update, XS worked independently by using criteria described in the Cochrane Handbook for Systematic Reviews of Interventions (Higgins 2011) to assess trial quality. This new set of criteria is based on evidence of associations between overestimate of effect and high risk of bias of the article such as sequence generation, allocation concealment, blinding, incomplete outcome data and selective reporting. JX assessed risk of bias from a random sample of these studies, comprising 10% of the total.
Any disagreements between raters were resolved through discussion with CEA. Where inadequate details of randomisation and other characteristics of trials were provided, we contacted authors of the studies in order to obtain further information. All disagreements in quality assessment were recorded and reported. If disputes arose as to which quality category a trial was to be rated, again, we resolved these through discussion.
We have noted the level of risk of bias in both the text of the review and in the 'Summary of findings' tables.
The previous version of this review used a different, less well‐developed, means of categorising risk of bias (see Appendix 3).
Measures of treatment effect
1. Binary data
For binary outcomes we calculated a standard estimation of the risk ratio (RR) and its 95% confidence interval (CI). It has been shown that RR is more intuitive (Boissel 1999) than odds ratios and that odds ratios tend to be interpreted as RR by clinicians (Deeks 2000). The Number Needed to Treat/Harm (NNT/H) statistic with its confidence intervals is intuitively attractive to clinicians but is problematic both in its accurate calculation in meta‐analyses and interpretation (Hutton 2009). For binary data presented in the 'Summary of findings' tables, where possible, we calculated illustrative comparative risks.
2. Continuous data
For continuous outcomes we estimated mean difference (MD) between groups. We would prefer not to calculate effect size measures (standardised mean difference (SMD)). However, if scales of very considerable similarity were used, we presumed there was a small difference in measurement, and we would have calculated effect size and transformed the effect back to the units of one or more of the specific instruments.
Unit of analysis issues
1. Cluster trials
Studies increasingly employ 'cluster randomisation' (such as randomisation by clinician or practice), but analysis and pooling of clustered data poses problems. Authors often fail to account for intra‐class correlation in clustered studies, leading to a 'unit of analysis' error (Divine 1992) whereby P values are spuriously low, confidence intervals unduly narrow and statistical significance overestimated. This causes type I errors (Bland 1997; Gulliford 1999).
If cluster trials where clustering was not accounted for in primary studies had been included, we would have presented data in a table, with a (*) symbol to indicate the presence of a probable unit of analysis error. We would have contacted the first authors of studies to obtain intra‐class correlation coefficients (ICCs) for their clustered data and to adjust for this by using accepted methods (Gulliford 1999). If clustering had been incorporated into the analysis of primary studies, we would have presented these data as if from a non‐cluster randomised study, but adjusted for the clustering effect.
We have sought statistical advice and have been advised that the binary data as presented in a report should be divided by a 'design effect'. This is calculated using the mean number of participants per cluster (m) and the ICC [Design effect = 1+(m‐1)*ICC] (Donner 2002). If the ICC had not been reported, it would have been assumed to be 0.1 (Ukoumunne 1999).
If cluster studies have been appropriately analysed taking into account ICCs and relevant data documented in the report, synthesis with other studies would have been possible using the generic inverse variance technique.
2. Cross‐over trials
A major concern of cross‐over trials is the carry‐over effect. It occurs if an effect (e.g. pharmacological, physiological or psychological) of the treatment in the first phase is carried over to the second phase. As a consequence, on entry to the second phase, the participants can differ systematically from their initial state despite a washout phase. For the same reason cross‐over trials are not appropriate if the condition of interest is unstable (Elbourne 2002). As both effects are very likely in severe mental illness, if we had identified any cross‐over trials, we would only have used data from the first phase of cross‐over studies.
3. Studies with multiple treatment groups
Where a study involves more than two treatment arms, if relevant, we would have presented the additional treatment arms in comparisons. If data were binary we would simply add these and combine within the two‐by‐two table. If data were continuous we would have combined data following the formula in section 7.7.3.8 (Combining groups) of the Handbook (Higgins 2011). Where the additional treatment arms were not relevant, we did not reproduce these data.
Dealing with missing data
1. Overall loss of credibility
At some degree of loss of follow‐up, data must lose credibility (Xia 2009). We chose that, if for any particular outcome, should more than 50% of data be unaccounted for, we would not reproduce these data or use them within analyses, (except for the outcome 'leaving the study early'). If, however, more than 50% of those in one arm of a study were lost, but the total loss was less than 50%, we would mark such data with (*) to indicate that such a result may well be prone to bias.
The previous version of this review excluded data from studies where more than 50% of participants in any group were lost to follow‐up (see Appendix 3).
2. Binary
In the case where attrition for a binary outcome was between 0% and 50% and where these data were not clearly described, we presented data on a 'once‐randomised‐always‐analyse' basis (an intention‐to‐treat analysis). Those leaving the study early were all assumed to have the same rates of negative outcome as those who completed, with the exception of the outcome of death and adverse effects. For these outcomes, the rate of those who stayed in the study ‐ in that particular arm of the trial ‐ was used for those who did not. If the above assumptions had been used for the primary outcomes we would have undertaken a sensitivity analysis to test how prone the primary outcomes were to change when data only from people who completed the study to that point were compared to the intention‐to‐treat analysis using the above assumptions.
3. Continuous
3.1 Attrition
In the case where attrition for a continuous outcome was between 0% and 50%, and data only from people who completed the study to that point were reported, we reproduced these.
3.2 Standard deviations
When standard deviations were not reported, we first tried to obtain the missing values from the authors. When not available, where there are missing measures of variance for continuous data, but an exact standard error (SE) and confidence intervals are available for group means, and either a P value or T value available for differences in mean, we could calculate them according to the rules described in the Handbook (Higgins 2011): When only the SE is reported, standard deviations (SDs) are calculated by the formula SD = SE * square root (n). Chapters 7.7.3 and 16.1.3 of the Handbook (Higgins 2011) present detailed formulae for estimating SDs from P values, T or F values, confidence intervals, ranges or other statistics. If these formulae do not apply, we can calculate the SDs according to a validated imputation method which is based on the SDs of the other included studies (Furukawa 2006). Although some of these imputation strategies can introduce error, the alternative would be to exclude a given study’s outcome and thus to lose information. If possible, we planned to examine the validity of the imputations in a sensitivity analysis excluding imputed values.
3.3 Last observation carried forward
We anticipated that in some studies the method of last observation carried forward (LOCF) would be employed within the study report. As with all methods of imputation to deal with missing data, LOCF introduces uncertainty about the reliability of the results (Leucht 2007). Therefore, if LOCF data had been used in the trial, if less than 50% of the data had been assumed, we would have reproduced these data and indicated that they were the product of LOCF assumptions.
Assessment of heterogeneity
1. Clinical heterogeneity
We considered all included studies initially, without seeing comparison data, to judge clinical heterogeneity. We simply inspected all studies for clearly outlying people or situations which we had not predicted would arise. When such situations or participant groups arose, we fully discussed these.
2. Methodological heterogeneity
We considered all included studies initially, without seeing comparison data, to judge methodological heterogeneity. We simply inspected all studies for clearly outlying methods which we had not predicted would arise. When such methodological outliers arose, we fully discussed these.
3. Statistical heterogeneity
3.1 Visual inspection
We visually inspected graphs to investigate the possibility of statistical heterogeneity.
3.2 Employing the I2 statistic
We investigated heterogeneity between studies by considering the I2 method alongside the Chi2 P value. The I2 provides an estimate of the percentage of inconsistency thought to be due to chance (Higgins 2003). The importance of the observed value of I2 depends on i. magnitude and direction of effects and ii. strength of evidence for heterogeneity (e.g. P value from Chi2 test, or a confidence interval for I2). An I2 estimate greater than or equal to around 50% accompanied by a statistically significant Chi2 statistic was interpreted as evidence of substantial levels of heterogeneity (Higgins 2011). When substantial levels of heterogeneity were found in the primary outcome, we explored reasons for heterogeneity (Subgroup analysis and investigation of heterogeneity).
The previous version of this review used a different, less‐well developed approach to deal with heterogeneity (see Appendix 3).
Assessment of reporting biases
Reporting biases arise when the dissemination of research findings is influenced by the nature and direction of results (Egger 1997). These are described in Section 10 of the Handbook (Higgins 2011). We are aware that funnel plots may be useful in investigating reporting biases but are of limited power to detect small‐study effects. We did not use funnel plots for outcomes where there were 10 or fewer studies, or where all studies were of similar sizes. In other cases, where funnel plots are possible, we sought statistical advice in their interpretation.
The previous version of this review used a different, less‐well developed approach to assess the reporting biases (see Appendix 3).
Data synthesis
We understand that there is no closed argument for preference for use of fixed‐effect or random‐effects models. The random‐effects method incorporates an assumption that the different studies are estimating different, yet related, intervention effects. This often seems to be true to us and the random‐effects model takes into account differences between studies even if there is no statistically significant heterogeneity. There is, however, a disadvantage to the random‐effects model: it puts added weight onto small studies, which often are the most biased ones. Depending on the direction of effect, these studies can either inflate or deflate the effect size. We chose the fixed‐effect model for all analyses. we only used random‐effects model when heterogeneity was present.
Subgroup analysis and investigation of heterogeneity
1. Subgroup analyses
1.1 Clinical state, stage or problem
We proposed to undertake this review and provide an overview of the effects of acupuncture for people with schizophrenia in general. In addition, however, if possible, we tried to report data on subgroups of people in the same clinical state, stage and with similar problems.
2. Investigation of heterogeneity
If inconsistency was high, we have reported this. First, we investigated whether data had been entered correctly. Second, if data were correct, we visually inspected the graph and successively removed outlying studies to see if homogeneity was restored. For this review, we decided that should this occur with data contributing to the summary finding of no more than around 10% of the total weighting, we would present data. If not, then we did not pool data and discussed issues. We know of no supporting research for this 10% cut‐off, but we use prediction intervals as an alternative to this unsatisfactory state.
When unanticipated clinical or methodological heterogeneity was obvious, we simply stated hypotheses regarding these for future reviews or versions of this review. We do not anticipate undertaking analyses relating to these.
Sensitivity analysis
1. Implication of randomisation
Had we found trials which were described in some way so as to imply randomisation, we would have included these in a sensitivity analysis. For the primary outcomes we would have included these studies and if there was no substantive difference when the implied randomised studies were added to those with better description of randomisation, then we would have entered all data from these studies.
2. Assumptions for lost binary data
Where assumptions had to be made regarding people lost to follow‐up (see Dealing with missing data), we compared the findings of the primary outcomes when we used our assumption/s and when we used data only from people who completed the study to that point. If there was a substantial difference, we reported results and discussed them, but continued to employ our assumption.
Where assumptions had to be made regarding missing SDs data (see Dealing with missing data), if possible, we compared the findings of outcomes when we used our assumption/s and when we used data only from people who completed the study to that point. If there was a substantial difference, we reported results and discussed them, but continued to employ our assumption.
3. Risk of bias
We analysed the effects of excluding trials that were judged to be at high risk of bias across one or more of the domains of randomisation (implied as randomised with no further details available): allocation concealment, blinding and outcome reporting for the meta‐analysis of the primary outcome. If the exclusion of trials at high risk of bias did not substantially alter the direction of effect or the precision of the effect estimates, then we included data from these trials in the analysis.
4. Imputed values
If we had found cluster randomised trials we would have undertaken a sensitivity analysis to assess the effects of including data from trials where we used imputed values for ICC in calculating the design effect in cluster randomised trials.
If we had undertaken sensitivity analysis and noted substantial differences in the direction or precision of effect estimates in any of the sensitivity analyses listed above, we would not have pooled data from the excluded trials with the other trials contributing to the outcome, but would have presented them separately.
Results
Description of studies
For substantive descriptions of studies please see Characteristics of included studies, Characteristics of excluded studies and Characteristics of studies awaiting classification.
Previous version of description of studies please see Appendix 4.
Results of the search
The update searches in 2012 yielded references to 49 reports including all the reports in the previous version (Figure 2). We selected 39 studies with 47 reports for further inspection of full papers excluding 2 records for one not relating to acupuncture and one not relating with schizophrenia after initial appraisal. We were able to include 30 studies with 36 reports.
2.
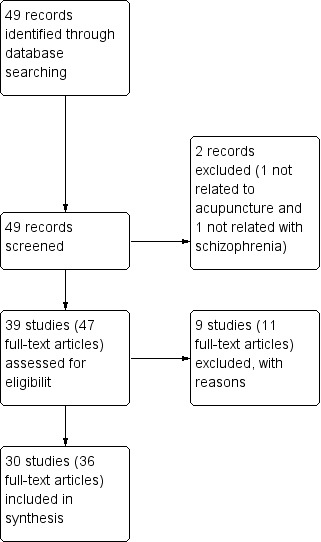
Study flow diagram.
Included studies
We included thirty studies.
1. Allocation
All included studies were randomised controlled trials. Only 10 of the included studies described the randomisation method. Three studies used a random sampling method (acupoint cat ‐ Sun 2005; electro ‐ Chen 2008; electro ‐ Zhang 2001); three were randomised by using a random allocation table (traditional ‐ Liu 2010; traditional ‐ Wang 2006; traditional ‐ Xu 2004); two drew lots (acupoint inj ‐ Wang 2000; electro ‐ Yao 2006); one study used the SAS program ‐ this was the only trial that described allocation concealment by using opaque sealed envelopes (electro ‐ Cheng 2009) and one used the coin‐tossing method (electro ‐ Cui 2000). Five studies were assessor‐blinded (acupoint inj ‐ Pan 2002; acupoint inj ‐ Wang 2000; electro ‐ Xiong 2010; electro ‐ Zhou 1997; laser ‐ Zhang 1991); two were patient‐ and assessor‐blinded (electro ‐ Cheng 2009; traditional ‐ Bouhlel 2011); and we could not ascertain how blinding was achieved in traditional ‐ Liu 2010, although it was reported as single blind.
2. Study duration
Study duration varied from two weeks (acupoint inj ‐ Wang 2000) to six months (acupoint inj ‐ Yang 2000). The majority of studies were short‐term. Five of all included studies were medium‐term (acupoint inj ‐ Yang 2000; electro ‐ Chen 2008; traditional ‐ Liu 2010; traditional ‐ Luo 2006; traditional ‐ Tang 2005) and the duration of laser ‐ Ma 1999 was unclear. acupoint inj ‐ Pan 2002 had a two‐year follow‐up and electro ‐ Yao 2006 a six‐month follow‐up.
3. Setting
Twenty‐two studies were hospital‐based with inpatients. Two studies, electro ‐ Chen 2006 and traditional ‐ Ma 2008, were undertaken with both inpatients and outpatients. Two studies were undertaken with outpatients (traditional ‐ Zhao 2005a; traditional ‐ Zhao 2005b). The settings of the four remaining studies were not mentioned (acupoint inj ‐ Yang 2000; laser ‐ Liu 1986; traditional ‐ Luo 2006; EACT ‐ Xue 1987).
4. Country
All included studies were undertaken in China except one traditional ‐ Bouhlel 2011, which took place in Tunisia.
5. Participants
It was reported in all studies that the participants suffered from schizophrenia. Seventeen adopted the standards of Chinese Classification of Mental Disorder such as second edition (CCMD‐2, acupoint inj ‐ Yang 2000; laser ‐ Ma 1999), second edition revision (CCMD‐2‐R, acupoint inj ‐ Pan 2002; acupoint cat ‐ Wang 1997; acupoint inj ‐ Wang 2000; traditional ‐ Wang 2006; electro ‐ Cui 2000; electro ‐ Zhang 1993; traditional ‐ Xu 2004) or third edition (CCMD‐3, acupoint cat ‐ Sun 2005; electro ‐ Chen 2006; electro ‐ Ding 2005; electro ‐ Xiong 2010; electro ‐ Yao 2006; traditional ‐ Liu 2010; traditional ‐ Luo 2006; traditional ‐ Tang 2005). Two studies diagnosed schizophrenia according to DSM IV (electro ‐ Cheng 2009; traditional ‐ Bouhlel 2011). Two studies diagnosed schizophrenia with both CCMD‐3 and Andreasen's diagnosis standards (electro ‐ Chen 2008; electro ‐ Wang 2005); two with both standards of CCMD‐2‐R and DSM III (laser ‐ Zhang 1991) or CCMD‐2‐R and ICD‐10 (electro ‐ Zhang 2001); three with both standards of TCM and CCMD‐2‐R (traditional ‐ Zhao 2005a; traditional ‐ Zhao 2005b) or TCM and CCMD‐3 (traditional ‐ Ma 2008). Only one study (electro ‐ Zhou 1997) adopted three standards ‐ DSM III, CCMD and TCM. The other three studies did not mention the diagnostic standard (EACT ‐ Xue 1987; electro ‐ Zhang 1987; laser ‐ Liu 1986).
The age of participants of 15 studies ranged from 15 to 67 years. Three studies did not definitely describe the age range (traditional ‐ Tang 2005; traditional ‐ Zhao 2005a; traditional ‐ Zhao 2005b). The other 12 studies reported mean age. The majority of studies included both women and men except electro ‐ Ding 2005 and traditional ‐ Xu 2004 (only men). acupoint inj ‐ Pan 2002 and electro ‐ Zhang 1993 did not describe gender. All studies except traditional ‐ Tang 2005 reported the history of illness.
6. Study size
The number of participants ranged from 31 to 300; acupoint cat ‐ Wang 1997, traditional ‐ Zhao 2005a and traditional ‐ Zhao 2005b were over 200 participants. The size of electro ‐ Zhang 1993 was unclear.
7. Interventions
We found six categories of acupuncture were used whether alone or in combination regimens: traditional acupuncture, electroacupuncture, acupoint injection, laser acupuncture, acupoint catgut treatment and electric acupuncture convulsive therapy. Traditional acupuncture included acupuncture manipulation and moxibustion. Acupuncture manipulation refers to an operation method to prevent disease by using different needles or non‐needle way to stimulate the specific points (acupoints) with certain practices or methods. Moxibustion not only refers to burning, smoking and ironing body surface mainly using moxa but also refers to any external treatment of non‐fire source (Shi 2007). Electroacupuncture refers to a combination method of needles and electrical stimulation to prevent disease that connects needles with trace current which are close to the human bio‐electricity after inserting needles into acupoints and getting the feeling of 'DeQi' (Shi 2007). Acupoint injection, guiding by the basic theory of Chinese medicine, is a treatment method with synergistic effects of acupuncture manipulation and drugs, which is to inject drugs into relative acupoint or special point (Tang 2010). Laser acupuncture, guiding by the basic theory of Chinese medicine, is a treatment method of preventing disease, treating disease, and health care to stimulate acupoints effectively using low‐intensity laser beam to irradiate acupoints directly, focus on acupoints or with beam expander (Fan 2010). Acupoint catgut treatment, guiding by the basic theory of Chinese medicine, is an external treatment method of treating diseases with catgut's stimulation effects on acupoints by using different types of catgut to embed acupoints selectively (Meng 2012). Electric acupuncture convulsive therapy, deriving from acupuncture, is a treatment method for mental disorders by using subconvulsive stimulating currents and with the electrodes in acupoints (Baihui and Renzhong) (Xue 1985).
7.1 Acupuncture added to standard dose antipsychotics
7.1.1 Acupuncture
The categories of acupuncture were traditional acupuncture (traditional ‐ Bouhlel 2011; traditional ‐ Liu 2010; traditional ‐ Luo 2006; traditional ‐ Ma 2008; traditional ‐ Tang 2005); electroacupuncture (electro ‐ Chen 2006; electro ‐ Chen 2008; electro ‐ Cheng 2009; electro ‐ Ding 2005; electro ‐ Wang 2005; electro ‐ Yao 2006; electro ‐ Zhang 1993; electro ‐ Zhang 2001); acupoint injection (acupoint inj ‐ Wang 2000; acupoint inj ‐ Yang 2000); laser acupuncture (laser ‐ Ma 1999); and acupoint catgut treatment (acupoint cat ‐ Wang 1997). None of the above acupuncture interventions were the same.
7.1.2 Antipsychotics
Five studies used risperidone and the dose of four of these studies (electro ‐ Wang 2005; traditional ‐ Liu 2010; traditional ‐ Luo 2006; traditional ‐ Ma 2008) ranged from 2 to 6 mg/d. electro ‐ Cheng 2009 used the average dosage of 5.15 ± 0.46 mg/d. One study, electro ‐ Chen 2008, used aripiprazole (average dosage 18.4 ± 6.2 mg/d); one, electro ‐ Yao 2006, used clozapine (total dosage 200 to 300 mg/d); and one, laser ‐ Ma 1999, chlorpromazine (average dosage 395 ± 55 mg/d). The other three studies did not describe more details of antipsychotics (acupoint cat ‐ Wang 1997; electro ‐ Zhang 2001; traditional ‐ Bouhlel 2011). The remaining six studies reported that people remained on previous antipsychotics treatment (acupoint inj ‐ Wang 2000; acupoint inj ‐ Yang 2000; electro ‐ Chen 2006; electro ‐ Ding 2005; electro ‐ Zhang 1993; traditional ‐ Tang 2005).
7.2 Acupuncture added to low dose antipsychotics
7.2.1 Acupuncture
The categories of acupuncture were traditional acupuncture (traditional ‐ Wang 2006; traditional ‐ Xu 2004), electroacupuncture (electro ‐ Cui 2000; electro ‐ Xiong 2010; electro ‐ Zhou 1997), acupoint injection (acupoint inj ‐ Pan 2002), laser acupuncture (laser ‐ Zhang 1991) and acupoint catgut treatment (acupoint cat ‐ Sun 2005). None of them were identical.
7.2.2 Low dose antipsychotics
Five studies (acupoint inj ‐ Pan 2002; electro ‐ Cui 2000; laser ‐ Zhang 1991; traditional ‐ Wang 2006; traditional ‐ Xu 2004) used chlorpromazine less than 300 mg/d. One study, electro ‐ Xiong 2010, reported the use of clozapine 100 to 150 mg/d, and another study, acupoint cat ‐ Sun 2005, used risperidone 1 to 2 mg/d. Finally, electro ‐ Zhou 1997 described the dosage of the antipsychotics was a reduction of ˜60% of their previous daily levels.
7.3 Acupuncture added to TCM drug
7.3.1 Acupuncture
Two studies used traditional acupuncture (traditional ‐ Zhao 2005a; traditional ‐ Zhao 2005b) and one study, electro ‐ Zhang 1987, used electroacupuncture.
7.3.2 TCM drug
Two studies used Fuyuankang capsule (traditional ‐ Zhao 2005a; traditional ‐ Zhao 2005b) and one study, electro ‐ Zhang 1987, traditional decoction of herbs ‐ Dang Gui Cheng Qi Tang.
7.4 Acupuncture
The categories of acupuncture were traditional acupuncture (traditional ‐ Wang 2006; traditional ‐ Zhao 2005a; traditional ‐ Zhao 2005b), electroacupuncture (electro ‐ Zhang 1987) and laser acupuncture (laser ‐ Liu 1986; laser ‐ Zhang 1991) with different acupoints.
7.5 Antipsychotics
Eight studies used risperidone ranging from 2 to 10 mg/d (acupoint cat ‐ Sun 2005; electro ‐ Cheng 2009; electro ‐ Wang 2005; traditional ‐ Liu 2010; traditional ‐ Luo 2006; traditional ‐ Ma 2008; traditional ‐ Zhao 2005a; traditional ‐ Zhao 2005b). Six studies used chlorpromazine (acupoint inj ‐ Pan 2002; electro ‐ Cui 2000; electro ‐ Zhang 1987; laser ‐ Liu 1986; laser ‐ Ma 1999; laser ‐ Zhang 1991). Three studies did not describe more details of antipsychotics used (acupoint cat ‐ Wang 1997; electro ‐ Zhang 2001; traditional ‐ Bouhlel 2011). Two studies reported using clozapine (electro ‐ Xiong 2010; electro ‐ Yao 2006), one study ‐ electro ‐ Chen 2008 ‐ used aripiprazole and the average dosage was 20.1 ± 4.3 mg/d. The remaining seven studies reported that people remained on previous antipsychotics treatment (acupoint inj ‐ Wang 2000; acupoint inj ‐ Yang 2000; electro ‐ Chen 2006; electro ‐ Ding 2005; electro ‐ Zhang 1993; electro ‐ Zhou 1997; traditional ‐ Tang 2005). One study ‐ traditional ‐ Wang 2006 ‐ reported using enough dosage antipsychotics, which was equal to chlorpromazine dosage ranged from 0.4 to 0.6 g/d) and traditional ‐ Xu 2004 reported maximum daily dosage was equivalent chlorpromazine dosage 0.4 to 0.7 g/d.
7.6 TCM drug
Two studies used Fuyuankang capsule (traditional ‐ Zhao 2005a; traditional ‐ Zhao 2005b) and one ‐ electro ‐ Zhang 1987 ‐ used traditional decoction of herbs ‐ Dang Gui Cheng Qi Tang.
7.7 Electric acupuncture convulsive therapy
Only one study, EACT ‐ Xue 1987, reported using acupoints Renzhong and Baihui with average electricity consumption 1.27 Joule.
7.8 Electric convulsive therapy
One study, EACT ‐ Xue 1987, reported using classic electroconvulsive therapy with average electricity consumption 34.97 Joule.
8. Outcomes
8.1 General remarks
Most outcomes of global state, behaviour and adverse effects were dichotomous and trials used a variety of scales. Two studies also reported adding medication outcomes, see Table 7. However some of the scale‐derived data were skewed data and difficult to understand.
1. Other outcomes authors reported.
| Global state: Adding medication | |||
| Study | Drug |
Events/Acupuncture group (n/N) |
Events/Control group (n/N) |
| electro ‐ Cui 2000 | Artane | 6/30 | 11/30 |
| Promethazine | 3/30 | 6/30 | |
| Propranolol | 2/30 | 4/30 | |
| Chlorpheniramine | 0/30 | 1/30 | |
| electro ‐ Wang 2005 | Propranolol | 7/40 | 7/35 |
| Artane | 6/40 | 9/35 | |
| Benzodiazepine drugs | 4/40 | 7/35 | |
| Mental state: Time to auditory hallucinations disappeared (days) | |||
| Study | Intervention | Mean | SD |
| laser ‐ Ma 1999 | Laser acupuncture added to standard dose antipsychotics | 19 | 4.1 |
| Standard dose antipsychotics | 31 | 4.4 | |
8.2 Outcomes scales from which it was possible to use data
8.2.1 Global state scales
8.2.1.1 Clinical Global Impression Scale ‐ CGI (Guy 1970) The CGI is a three‐item scale commonly used in studies on schizophrenia that enables clinicians to quantify severity of illness and overall clinical improvement. The items are: severity of illness, global improvement and efficacy index. A seven‐point scoring system is usually used with low scores indicating decreased severity and/or greater recovery. laser ‐ Zhang 1991 and electro ‐ Zhou 1997 reported CGI data.
8.2.2 Mental state scales
8.2.2.1 Brief Psychiatric Rating Scale ‐ BPRS (Overall 1962) The BPRS is an 18‐item scale measuring positive symptoms, general psychopathology and affective symptoms. The original scale has 16 items, but a revised 18‐item scale is commonly used. Scores can range from zero to 126. Each item is rated on a seven‐point scale, with high scores indicating more severe symptoms. Thirteen studies reported BPRS data (acupoint inj ‐ Pan 2002; acupoint inj ‐ Yang 2000; electro ‐ Cui 2000; electro ‐ Ding 2005; electro ‐ Xiong 2010; electro ‐ Zhang 1993; electro ‐ Zhou 1997; laser ‐ Ma 1999; laser ‐ Zhang 1991; traditional ‐ Liu 2010; traditional ‐ Luo 2006; traditional ‐ Wang 2006; traditional ‐ Xu 2004). One study, acupoint inj ‐ Wang 2000, only reported the data of the twelfth item of BPRS ‐ hallucinations.
8.2.2.2 Positive and Negative Syndrome Scale ‐ PANSS* (Kay 1986) This is a 30‐item scale, each of which can be defined on a seven point scoring system from absent to extreme. It has three sub‐scales for measuring the severity of general psychopathology, positive symptoms (PANSS‐P), and negative symptoms (PANSS‐N). A low score indicates lesser severity. Eight studies reported data using this scale (acupoint cat ‐ Sun 2005; electro ‐ Chen 2008; electro ‐ Cheng 2009; electro ‐ Ding 2005; electro ‐ Wang 2005; electro ‐ Yao 2006; traditional ‐ Bouhlel 2011; traditional ‐ Ma 2008).
8.2.2.3 Scale for the Assessment of Positive Symptoms ‐ SAPS (Andreasen 1982) This six‐point scale gives a global rating of positive symptoms such as delusions, hallucinations and disordered thinking. Higher scores indicate more symptoms. Five studies reported SAPS data (acupoint cat ‐ Sun 2005; electro ‐ Zhang 1993; traditional ‐ Bouhlel 2011; traditional ‐ Wang 2006; traditional ‐ Xu 2004).
8.2.2.4 Scale for the Assessment of Negative Symptoms ‐ SANS (Andreasen 1982) This scale allows a global rating of the following negative symptoms: alogia (impoverished thinking), affective blunting, avolition‐apathy, anhedonia‐asociality and attention impairment. Assessments are made on a six‐point scale (zero = not at all to five = severe). Higher scores indicate more symptoms. Data for this scale were reported by five studies (electro ‐ Zhang 1993; traditional ‐ Bouhlel 2011; traditional ‐ Luo 2006; traditional ‐ Wang 2006; traditional ‐ Xu 2004).
8.2.2.5 Hamilton Rating Scale for Depression ‐ HAMD (Hamilton 1967) This instrument is designed to be used only on patients already diagnosed as suffering from affective disorder of the depressive type. It is used for quantifying the results of an interview, and its value depends entirely on the skill of the interviewer in eliciting the necessary information. The scale contains 17 variables measured on either a five‐point or a three‐point rating scale, the latter being used where quantification of the variable is either difficult or impossible. Among the variables are: depressed mood, suicide, work and loss of interest, retardation, agitation, gastro‐intestinal symptoms, general somatic symptoms, hypochondriasis, loss of insight, and loss of weight. It is useful to have two raters independently scoring a patient at the same interview. The scores of the patient are obtained by summing the scores of the two physicians. A score of 11 is generally regarded as indicative of a diagnosis of mild depression, 14 to 17 mild to moderate depression and >17 moderate to severe depression. electro ‐ Chen 2006 and electro ‐ Zhang 2001 reported data of this scale.
8.2.2.6 Zung Self‐Rating Depression Scale ‐ SDS (Zung 1965) The Zung Self‐Rating Depression Scale is a 20‐item self‐rated scale that is widely used as a screening tool, covering affective, psychological and somatic symptoms associated with depression. The questionnaire takes approximately 10 minutes to complete and items are framed in terms of positive and negative statements. It can be effectively used in a variety of settings, including primary care, psychiatric clinics, drug trials and various research situations. Each item is scored on a Likert scale ranging from one to four. Most people with depression score between 50 and 69, while a score of 70 and above indicates severe depression. electro ‐ Zhang 2001 reported SDS data.
8.2.2.7 Psychotic symptom rating scale ‐ PSYRHS* (Haddock 1999) Psychotic Symptom Rating Scales is a 17‐item, five‐point scale to rate symptom scores (zero to four) with high scores indicating more severe symptoms. The scales consist of two subscales ‐ the auditory hallucinations subscale (AH) and delusions subscale (DS). The auditory hallucinations subscale is an 11‐item scale. The dimensions of auditory hallucinations are frequency, duration, location, loudness, beliefs re‐origin of voices, amount of negative content of voices, degree of negative content, amount of distress, intensity of distress, disruption to life caused by voices, controllability of voices. The total AH score ranges from zero to 44. The delusions subscale is a six‐item scale. The dimensions of delusions include amount of preoccupation with delusions, duration of preoccupation with delusions, conviction, amount of distress, intensity of distress and disruption to life caused by beliefs. The total DS score ranges from zero to 24. electro ‐ Cheng 2009 reported data of auditory hallucinations subscale (PSYRHS‐AH).
8.2.2.8 Specific Auditory Hallucination Scale ‐ SAHS* (Lu 2006) Specific Auditory Hallucination Scale derives from Scale for the Assessment of Positive Symptoms. The scale includes four items of SAPS. The four items are auditory hallucinations, voice commenting, voice conversing, global rating of severity of hallucinations. Higher scores indicate more symptoms. Only traditional ‐ Liu 2010 reported SAHS data.
8.2.3 Adverse effects scales
8.2.3.1 Treatment Emergent Symptom Scale/Form ‐ TESS/F (Guy 1976) This checklist assesses a variety of characteristics for each adverse event, including severity, relationship to the drug, temporal characteristics (timing after a dose, duration and pattern during the day), contributing factors, course and action taken to counteract the effect. Symptoms can be listed a priori or can be recorded as observed by the investigator. Fourteen studies used this scale (acupoint inj ‐ Pan 2002; acupoint cat ‐ Sun 2005; electro ‐ Chen 2006; electro ‐ Chen 2008; electro ‐ Cui 2000; electro ‐ Xiong 2010; electro ‐ Yao 2006; electro ‐ Zhang 1993; electro ‐ Zhou 1997; traditional ‐ Liu 2010; traditional ‐ Ma 2008;electro ‐ Wang 2005; traditional ‐ Wang 2006; traditional ‐ Xu 2004). electro ‐ Zhang 1993 only reported part of data and only six studies reported scores of this scale (acupoint inj ‐ Pan 2002; electro ‐ Xiong 2010; electro ‐ Yao 2006; electro ‐ Zhou 1997; traditional ‐ Wang 2006; traditional ‐ Xu 2004), three of them with skewed data (electro ‐ Xiong 2010; traditional ‐ Wang 2006; traditional ‐ Xu 2004).
8.2.3.2 Rating Scale for Extrapyramidal Side Effects ‐ RESES* (Simpson 1970) Rating scale for Extrapyramidal Side Effects is a 10‐item scale relating to extrapyramidal side effects. The score of each item rates symptom from zero to four. Zero means normal and high scores indicate severe side effects. The items are gait, arm dropping, shoulder shaking, elbow rigidity, wrist rigidity of fixation of position, pendulousness of legs, head dropping, glabella tap, tremor and salivation. Only one study, laser ‐ Zhang 1991, used this scale and reported mean, events number of each group and T‐value.
Outcomes with '*' are extra outcomes added in this 2012 update.
9. Missing outcomes
The included studies did not attempt to quantify outcomes of death, engagement with services, satisfaction with treatment, quality of life, economic outcomes.
Excluded studies
We excluded nine studies. Studies listed in the Characteristics of excluded studies had to be inspected in hard copy in order to make the final decision. One study (Sun 1994) was a case series. Two studies, Ma 2004 and Zhuge 1993, were not randomised. Xiong 2009 was quasi‐randomised. Participants of Zhong 1995 did not suffer from schizophrenia, schizophreniform psychosis or schizophrenia‐like illnesses. Participants of Luo 2006a were people with schizophrenia or mood disorders manic episode and outcomes were not reported by diagnostic group. Two studies, Ma 2002 and Xue 1985, had to be excluded because of the interventions. One study (Wu 2004) was excluded because some important data reported in this study were identical to those of another included study (acupoint inj ‐ Pan 2002) but the allocation numbers of the two groups, the acupoint choice method and the BPRS factor data were different. We could not acquire further details.
Awaiting assessment
One study (NCT01167348) awaits assessment as we have not acquired further details and were unable to confirm whether the trial has ended; we were unable to locate the full text of the study.
Ongoing
We know of no ongoing studies.
Risk of bias in included studies
We judged the risk of bias for the included studies and, overall, we judged that the risk of bias to be moderate (Figure 3; Figure 4).
3.
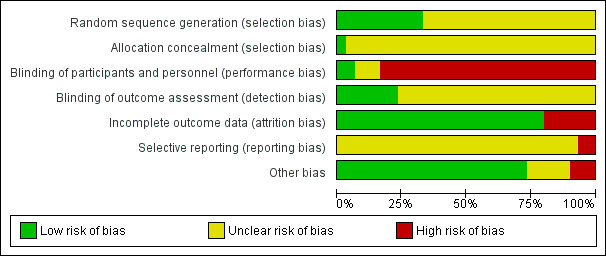
'Risk of bias' graph: review authors' judgements about each risk of bias item presented as percentages across all included studies.
4.
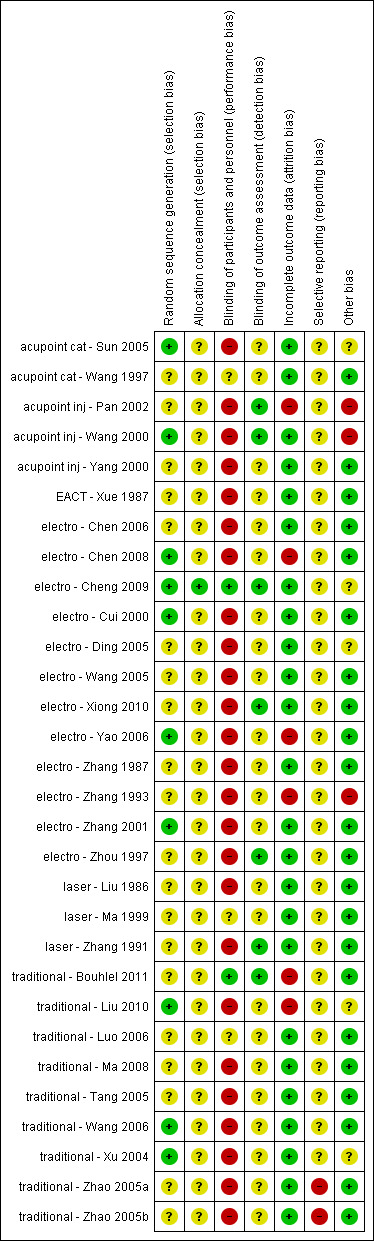
'Risk of bias' summary: review authors' judgements about each risk of bias item for each included study.
Allocation
All 30 included studies were stated to be randomised, but only 10 provided descriptions of the randomisation method used (acupoint cat ‐ Sun 2005; acupoint inj ‐ Wang 2000; electro ‐ Chen 2008; electro ‐ Cheng 2009; electro ‐ Cui 2000; electro ‐ Yao 2006; electro ‐ Zhang 2001; traditional ‐ Liu 2010; traditional ‐ Wang 2006; traditional ‐ Xu 2004) and one (electro ‐ Cheng 2009), also described allocation concealment. Two studies did not definitely report the number of participants in each group (electro ‐ Zhang 1993; traditional ‐ Bouhlel 2011). The number of participants in the remaining studies were evenly balanced in each group except acupoint inj ‐ Pan 2002 (105/65), electro ‐ Zhou 1997 (25/15), traditional ‐ Zhao 2005a (90/90/90/30), and traditional ‐ Zhao 2005b (90/90/90/30).
Blinding
Sham acupuncture is difficult to use and it is unclear if this is a successful method though assessor blinding is easier. Two studies stated that both patients and assessors were blinded (electro ‐ Cheng 2009; traditional ‐ Bouhlel 2011). Five studies were assessor‐blinded (acupoint inj ‐ Pan 2002; acupoint inj ‐ Wang 2000; electro ‐ Xiong 2010; electro ‐ Zhou 1997; laser ‐ Zhang 1991) and in one study, traditional ‐ Liu 2010, it was with unclear whether it was single blind. The remaining 22 studies did not report whether assessor blinding had been used but participants of 19 trials were unblinded and three trials did not report if they used a method by which participants were blinded (acupoint cat ‐ Wang 1997; laser ‐ Ma 1999; traditional ‐ Luo 2006).
Incomplete outcome data
Five studies reported incomplete outcome data ‐ totaling 71 participants (acupoint inj ‐ Pan 2002; electro ‐ Chen 2008; electro ‐ Yao 2006; traditional ‐ Bouhlel 2011; traditional ‐ Liu 2010) and for the participants of one study, electro ‐ Zhang 1993, it was unclear. Two studies used Intention‐to‐treat (ITT) method of analysis (electro ‐ Cheng 2009; traditional ‐ Xu 2004) and no participants of the other 23were lost to follow‐up.
Selective reporting
The protocols of 28 studies were not available, therefore we could not compare outcomes in the protocols with those in the published reports. Authors of another two studies recorded that BPRS was one of the outcomes but they only reported global state, rather than this measure (traditional ‐ Zhao 2005a; traditional ‐ Zhao 2005b).
Other potential sources of bias
The references of two studies, acupoint inj ‐ Pan 2002 (supported by government) and electro ‐ Zhang 1993, reported different data and one of the authors of acupoint inj ‐ Wang 2000 worked for the drug industry, hence these three studies have potential sources of bias. Three studies were supported by government or an institute (acupoint cat ‐ Sun 2005; electro ‐ Cheng 2009; traditional ‐ Liu 2010). Two studies (electro ‐ Ding 2005; traditional ‐ Xu 2004), only included men. We were unclear whether these five studies had 'other potential sources' of bias. Overall, 29 of 30 included studies in this update were from the People's Republic of China. It is unclear if this represents a racial or cultural bias when applied to other regions.
Effects of interventions
See: Table 1; Table 2; Table 3; Table 4; Table 5; Table 6
It is impossible to judge if different categories of acupuncture really have different mechanisms of action. We undertook subgroup analyses to investigate effects for the same outcome of various categories of acupuncture and, when heterogeneity was low, we synthesised data. We also added a table of adverse effects of acupuncture according to different categories (Table 8).
2. Adverse effects of acupuncture.
| Intervention | Term | Adverse effects |
Adverse effects/Intervention group (n/N) |
Study | |
| Traditional acupuncture | |||||
| Traditional acupuncture added to standard dose antipsychotics versus standard dose antipsychotics | Short term | Extrapyramidal symptoms | Overall | 1/30 | traditional ‐ Ma 2008 |
| Anticholinergic symptoms | Dry mouth | 3/30 | |||
| Cardiovascular | Dizziness | 3/30 | |||
| Tachycardia | 2/30 | ||||
| Medium term | Extrapyramidal symptoms | Overall | 5/47 | traditional ‐ Liu 2010 | |
| Central Nervous System | Anxiety | 2/47 | |||
| Insomnia | 3/47 | ||||
| Cardiovascular symptoms (or headache) | Dizziness or headache | 1/47 | |||
| Gastrointestinal system | Unspecifid gastrointestinal symptoms | 2/47 | |||
| Lab test | Liver function abnormal | 2/47 | |||
| Electroacupuncture | |||||
| Electroacupuncture (or added to TCM drug) versus TCM drug | Short term | Hold breath, facial cyanosis, arrhythmia, transient increase of blood pressure, injury of teeth, tongue and lips, epileptic attacks with strong stimulation | not reported | electro ‐ Zhang 1987 | |
| Electroacupuncture added to standard dose antipsychotics versus standard dose antipsychotics | Short term | Extrapyramidal symptoms | Overall | 18/73 | electro ‐ Chen 2006; electro ‐ Wang 2005 |
| Tremor | 5/40 | electro ‐ Wang 2005 | |||
| Akathisia | 6/40 | ||||
| Central Nervous System | Insomnia | 4/73 | electro ‐ Chen 2006; electro ‐ Wang 2005 | ||
| Anticholinergic symptoms | Dry mouth | 6/73 | |||
| Blurred vision | 4/73 | ||||
| Constipation | 3/73 | ||||
| Nausea and vomiting | 3/73 | ||||
| Cardiovascular symptoms | Dizziness | 3/73 | |||
| Tachycardia | 10/78 | electro ‐ Chen 2008; electro ‐ Yao 2006 | |||
| Metabolic system | Weight gain | 3/73 | electro ‐ Chen 2006; electro ‐ Wang 2005 | ||
| Lab test | Liver function abnormal | 4/118 | electro ‐ Chen 2006; electro ‐ Wang 2005; electro ‐ Yao 2006 | ||
| ECG abnormal (myocardial ischaemia) | 1/118 | ||||
| blood routine test abnormal (leukocyte change) | 4/118 | ||||
| Medium term | Extrapyramidal symptoms | Overall | 6/30 | electro ‐ Chen 2008 | |
| Myotonia | 1/30 | ||||
| Tremor | 2/30 | ||||
| Akathisia | 3/30 | ||||
| Central Nervous System | Insomnia | 1/30 | |||
| Headache | 2/30 | ||||
| Anticholinergic symptoms | Blurred vision | 1/30 | |||
| Sweating | 3/30 | ||||
| Electroacupuncture added low dose antipsychotics versus standard dose antipsychotics | Short term | Extrapyramidal symptoms | Overall | 5/30 | electro ‐ Cui 2000 |
| Central Nervous System | Sleepiness | 1/30 | |||
| Anticholinergic symptoms | Dry mouth | 4/30 | |||
| Blurred vision | 2/30 | ||||
| Cardiovascular symptoms | Tachycardia | 5/30 | |||
| Laser acupuncture | |||||
| Laser acupuncture versus standard dose antipsychotics | Short term | Numbness over the ear, upper extremity and chest on the treated side | 1/20 | laser ‐ Liu 1986 | |
| Sensation of heat at irradiated site and a feeling of plugged auditory canal | one third of participants of He‐Ne laser irradiation group | ||||
| Laser acupuncture added to low dose antipsychotics versus standard dose antipsychotics | Short term | Extrapyramidal symptoms | Overall | 7/10 | laser ‐ Zhang 1991 |
| Acupoint catgut treatment | |||||
| Acupoint catgut treatment added to standard dose antipsychotics versus standard dose antipsychotics | Short term | Local pain when eating | not reported | acupoint cat ‐ Wang 1997 | |
| Acupoint catgut treatment added to low dose antipsychotics versus standard dose antipsychotics | Short term | Cardiovascular symptoms | Dizziness | 2/88 | acupoint cat ‐ Sun 2005 |
| Tachycardia | 2/88 | ||||
| Electric acupuncture convulsive therapy | |||||
| Electric acupuncture convulsive therapy versus electroconvulsive therapy | Short term | Back pain | 2/34 | EACT ‐ Xue 1987 | |
| Spinal fracture | 5/34 | ||||
For the previous version of description of studies, please see Appendix 5.
We calculated risk ratios (RR) for dichotomous data and estimated mean differences (MD) for continuous data, with their respective 95% confidence intervals (CIs) throughout.
1. COMPARISON 1: ACUPUNCTURE added to STANDARD DOSE ANTIPSYCHOTICS versus STANDARD DOSE ANTIPSYCHOTICS
Seventeen studies (acupoint cat ‐ Wang 1997; acupoint inj ‐ Wang 2000; acupoint inj ‐ Yang 2000; electro ‐ Chen 2006; electro ‐ Chen 2008; electro ‐ Cheng 2009; electro ‐ Ding 2005; electro ‐ Wang 2005; electro ‐ Yao 2006; electro ‐ Zhang 1993; electro ‐ Zhang 2001; laser ‐ Ma 1999; traditional ‐ Liu 2010; traditional ‐ Luo 2006; traditional ‐ Ma 2008; traditional ‐ Tang 2005; traditional ‐ Bouhlel 2011) compared acupuncture added to standard dose antipsychotics with standard dose antipsychotics.
1.1 Global state
1.1.1 Not improved
The binary data of three studies with traditional acupuncture used three different criteria of 'not improved' at medium‐term but the criteria were similar. We found medium‐term global state data favoured acupuncture added to standard dose antipsychotics (n = 244, 3 RCTs, RR 0.40 CI 0.28 to 0.57). We, however, found a different result from one study with laser acupuncture where the criteria and duration of study were unclear (n = 120, 1 RCT, RR 0.20 CI 0.01 to 4.08, Analysis 1.1).
1.1. Analysis.
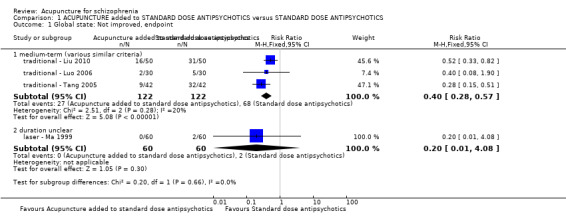
Comparison 1 ACUPUNCTURE added to STANDARD DOSE ANTIPSYCHOTICS versus STANDARD DOSE ANTIPSYCHOTICS, Outcome 1 Global state: Not improved, endpoint.
1.1.2 Adding medication
Only electro ‐ Wang 2005 reported using additional medication, which was not a pre‐stated outcome of interest for this review, but we have listed these data in Table 7.
1.2 Mental state
1.2.1 Brief Psychiatric Rating Scale (BPRS) endpoint score
We found short‐term BPRS scores of four studies ‐ two with traditional acupuncture, one with electroacupuncture and one with laser acupuncture ‐ significantly favoured acupuncture added to standard dose antipsychotics compared with standard dose antipsychotics alone (n = 327, 4 RCTs, MD ‐4.32 CI ‐5.28 to ‐3.36). Three studies reported medium‐term BPRS scores with considerable heterogeneity (I2 > 75%, Analysis 1.2). With the medium‐term subgroup analysis, according to different categories of acupuncture, we found a substantial heterogeneity between traditional acupuncture and the acupoint injection subgroup (I2 > 50%) and also between traditional ‐ Liu 2010 and traditional ‐ Luo 2006 in the same subgroup (I2 > 75%). With traditional acupuncture we found that those given traditional acupuncture added to standard dose antipsychotics had a significantly better rating in their mental state compared with the standard dose antipsychotics group (traditional ‐ Liu 2010: n = 96, 1 RCT, MD ‐4.87 CI ‐16.24 to ‐3.50; traditional ‐ Luo 2006: n = 60, 1 RCT, MD ‐11.35 CI ‐14.48 to ‐8.22). However, there was no difference between acupoint injection added to standard dose antipsychotics and standard dose antipsychotics alone (n = 64, 1 RCT, MD ‐0.70 CI ‐5.02 to 3.62, Analysis 1.3)
1.2. Analysis.
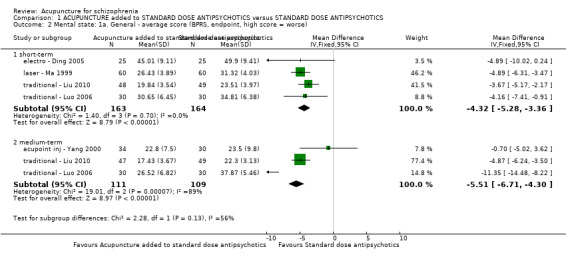
Comparison 1 ACUPUNCTURE added to STANDARD DOSE ANTIPSYCHOTICS versus STANDARD DOSE ANTIPSYCHOTICS, Outcome 2 Mental state: 1a. General ‐ average score (BPRS, endpoint, high score = worse).
1.3. Analysis.
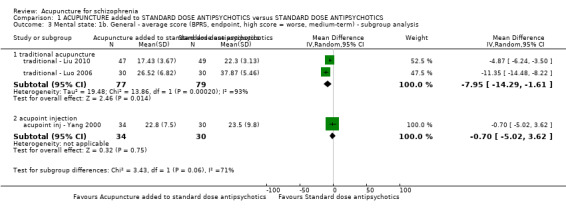
Comparison 1 ACUPUNCTURE added to STANDARD DOSE ANTIPSYCHOTICS versus STANDARD DOSE ANTIPSYCHOTICS, Outcome 3 Mental state: 1b. General ‐ average score (BPRS, endpoint, high score = worse, medium‐term) ‐ subgroup analysis.
1.2.2 Positive and Negative Syndrome Scale (PANSS) endpoint score
One study with traditional acupuncture and three studies with electroacupuncture reported short‐term PANSS scores without skewed data. Data indicated considerable heterogeneity (I2 > 75%) but, by the medium‐term, there was significant difference between acupuncture added to standard dose antipsychotics and standard dose antipsychotics alone group (n = 135, 2 RCTs, MD ‐3.79 CI ‐6.43 to ‐1.15, Analysis 1.4). With subgroup analysis, we found there was a considerable heterogeneity between these two categories of acupuncture (I2 > 75%). With traditional acupuncture there was only one study and this indicated no difference between traditional acupuncture added to standard dose antipsychotics and standard dose antipsychotics alone (n = 60, 1 RCT, MD 1.30 CI ‐1.56 to 4.16).
1.4. Analysis.
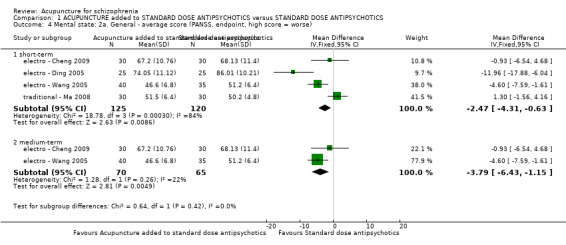
Comparison 1 ACUPUNCTURE added to STANDARD DOSE ANTIPSYCHOTICS versus STANDARD DOSE ANTIPSYCHOTICS, Outcome 4 Mental state: 2a. General ‐ average score (PANSS, endpoint, high score = worse).
The PANSS endpoint score from the three studies with electroacupuncture contained substantial heterogeneity (I2 > 50%, Analysis 1.5). Removing the study with results that were causing this heterogeneity, as judged by visual inspection (electro ‐ Ding 2005), eliminated this heterogeneity. We found short‐term data favoured electroacupuncture added to the standard dose antipsychotics group (n = 135, 2 RCTs, MD ‐3.79 CI ‐6.43 to ‐1.15, Analysis 1.6). Three studies also used binary data to assess PANSS at short‐term with the assertion that less than 25% reduction as 'not improved' (electro ‐ Chen 2008; electro ‐ Wang 2005; traditional ‐ Ma 2008). There was a difference between acupuncture added to standard dose antipsychotics and standard dose antipsychotics (n = 197, 3 RCTs, RR 0.65 CI 0.45 to 0.94). The electro ‐ Yao 2006 trial used binary data to assess PANSS at short‐term and medium‐term ‐ with assumption of less than 30% reduction as 'not improved'. There was no difference between electroacupuncture added to standard dose antipsychotics and standard dose antipsychotics alone in the short‐term (n = 90, 1 RCT, RR 0.91 CI 0.43 to 1.92) or by medium‐term (n = 90, 1 RCT, RR 0.69 CI 0.36 to 1.31, Analysis 1.8). Three studies reported skewed PANSS data at short‐term and one study provided skewed PANSS data at medium‐term (Analysis 1.7).
1.5. Analysis.
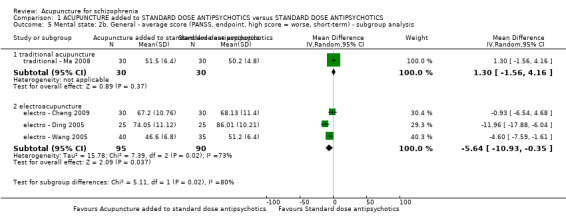
Comparison 1 ACUPUNCTURE added to STANDARD DOSE ANTIPSYCHOTICS versus STANDARD DOSE ANTIPSYCHOTICS, Outcome 5 Mental state: 2b. General ‐ average score (PANSS, endpoint, high score = worse, short‐term) ‐ subgroup analysis.
1.6. Analysis.
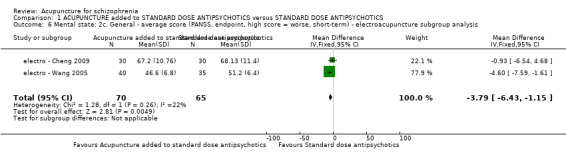
Comparison 1 ACUPUNCTURE added to STANDARD DOSE ANTIPSYCHOTICS versus STANDARD DOSE ANTIPSYCHOTICS, Outcome 6 Mental state: 2c. General ‐ average score (PANSS, endpoint, high score = worse, short‐term) ‐ electroacupuncture subgroup analysis.
1.8. Analysis.
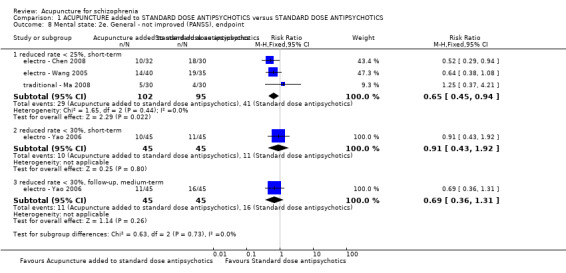
Comparison 1 ACUPUNCTURE added to STANDARD DOSE ANTIPSYCHOTICS versus STANDARD DOSE ANTIPSYCHOTICS, Outcome 8 Mental state: 2e. General ‐ not improved (PANSS), endpoint.
1.7. Analysis.
Comparison 1 ACUPUNCTURE added to STANDARD DOSE ANTIPSYCHOTICS versus STANDARD DOSE ANTIPSYCHOTICS, Outcome 7 Mental state: 2d. General ‐ average score (PANSS, endpoint, high score = worse) ‐ Skewed data.
| Mental state: 2d. General ‐ average score (PANSS, endpoint, high score = worse) ‐ Skewed data | |||||
|---|---|---|---|---|---|
| Study | Intervention | Mean | SD | N | Note |
| short‐term | |||||
| electro ‐ Chen 2008 | Electroacupuncture added to standard dose antipsychotics | 50.73 | 13.32 | 30 | Compared with the score before treatment P < 0.05 |
| electro ‐ Chen 2008 | Standard dose antipsychotics | 72.30 | 7.01 | 30 | |
| electro ‐ Yao 2006 | Electroacupuncture added to standard dose antipsychotics | 46.51 | 17.10 | 45 | Compared with the score before treatment P < 0.01 |
| electro ‐ Yao 2006 | Standard dose antipsychotics | 46.45 | 17.23 | 45 | Compared with the score before treatment P < 0.01 |
| traditional ‐ Bouhlel 2011 | Traditional acupuncture added to standard dose antipsychotics | 77.40 | 25.73 | 15 | P = 0.501 |
| traditional ‐ Bouhlel 2011 | Standard dose antipsychotics | 77.81 | 26.77 | 16 | |
| medium‐term | |||||
| electro ‐ Chen 2008 | Electroacupuncture added to standard dose antipsychotics | 32.02 | 11.21 | 30 | Compared with the score before treatment P < 0.01 |
| electro ‐ Chen 2008 | Standard dose antipsychotics | 52.10 | 10.32 | 30 | Compared with the score before treatment P < 0.01 |
1.2.3 Scale for the Assessment of Positive Symptoms (SAPS) endpoint score
Two studies, one with traditional acupuncture and one with electroacupuncture, reported SAPS scores (short‐term) but these were skewed continuous data (Analysis 1.9).
1.9. Analysis.
Comparison 1 ACUPUNCTURE added to STANDARD DOSE ANTIPSYCHOTICS versus STANDARD DOSE ANTIPSYCHOTICS, Outcome 9 Mental state: 3. Specific ‐ average score ‐ positive symptoms (SAPS, endpoint, high score = worse, short‐term) ‐ Skewed data.
| Mental state: 3. Specific ‐ average score ‐ positive symptoms (SAPS, endpoint, high score = worse, short‐term) ‐ Skewed data | |||||
|---|---|---|---|---|---|
| Study | Intervention | Mean | SD | N | Note |
| electro ‐ Zhang 1993 | Electroacupuncture added to standard dose antipsychotics | 6.71 | 6.15 | 38 | P < 0.01 |
| electro ‐ Zhang 1993 | Standard dose antipsychotics | 10.65 | 6.95 | 31 | |
| traditional ‐ Bouhlel 2011 | Traditional acupuncture added to standard dose antipsychotics | 42.00 | 28.69 | 15 | P = 0.539 |
| traditional ‐ Bouhlel 2011 | Standard dose antipsychotics | 46.75 | 25.05 | 16 | |
1.2.4 Scale for the Assessment of Negative Symptoms (SANS) endpoint score
The traditional ‐ Luo 2006 trial used the SANS to assess mental state. Those given traditional acupuncture added to standard dose antipsychotics had a significantly better rating in their negative symptoms compared with the standard dose antipsychotics group (short‐term, n = 60, 1 RCT, MD ‐7.66 CI ‐13.05 to ‐2.27; medium‐term, n = 60, 1 RCT, MD ‐12.35 CI ‐17.54 to ‐7.16, Analysis 1.10). Another two studies reported short‐term skewed SANS endpoint score data (Analysis 1.11).
1.10. Analysis.
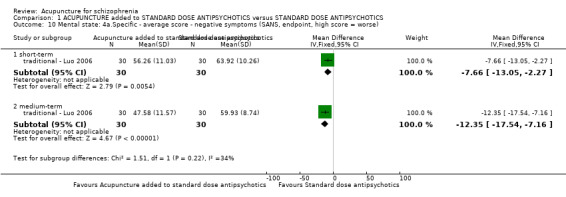
Comparison 1 ACUPUNCTURE added to STANDARD DOSE ANTIPSYCHOTICS versus STANDARD DOSE ANTIPSYCHOTICS, Outcome 10 Mental state: 4a.Specific ‐ average score ‐ negative symptoms (SANS, endpoint, high score = worse).
1.11. Analysis.
Comparison 1 ACUPUNCTURE added to STANDARD DOSE ANTIPSYCHOTICS versus STANDARD DOSE ANTIPSYCHOTICS, Outcome 11 Mental state: 4b. Specific ‐ average score ‐ negative symptoms (SANS, endpoint, high score = worse, short‐term) ‐ Skewed data.
| Mental state: 4b. Specific ‐ average score ‐ negative symptoms (SANS, endpoint, high score = worse, short‐term) ‐ Skewed data | |||||
|---|---|---|---|---|---|
| Study | Intervention | Mean | SD | N | Note |
| traditional acupuncture | |||||
| traditional ‐ Bouhlel 2011 | Traditional acupuncture added to standard dose antipsychotics | 55.60 | 24.71 | 15 | P = 0.406 |
| traditional ‐ Bouhlel 2011 | Standard dose antipsychotics | 52.81 | 30.88 | 16 | |
| electroacupuncture | |||||
| electro ‐ Zhang 1993 | Electroacupuncture added to standard dose antipsychotics | 24.97 | 20.86 | 38 | P < 0.005 |
| electro ‐ Zhang 1993 | Standard dose antipsychotics | 37.26 | 16.02 | 31 | |
1.2.5 Hamilton Rating Scale for Depression (HAMD) endpoint score
Only two studies with electroacupuncture used this scale for short‐term assessment. There was a considerable heterogeneity between these two studies (I2 = 75%). We found the scores of electroacupuncture added to the standard dose antipsychotics group were lower than the standard dose antipsychotics group (electro ‐ Chen 2006: n = 67, 1 RCT, MD ‐6.90 CI ‐9.31 to ‐4.49; electro ‐ Zhang 2001: n = 42, 1 RCT, MD ‐10.41 CI ‐12.81 to ‐8.01, Analysis 1.12). A similar result was found if converting continuous data to binary data but heterogeneity disappeared (n = 109, 2 RCTs, RR 0.17 CI 0.08 to 0.34, I2 = 0%, Analysis 1.13).
1.12. Analysis.
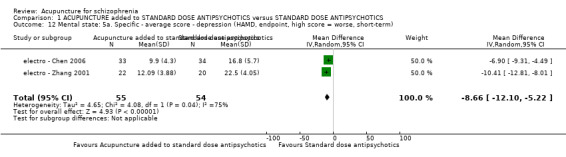
Comparison 1 ACUPUNCTURE added to STANDARD DOSE ANTIPSYCHOTICS versus STANDARD DOSE ANTIPSYCHOTICS, Outcome 12 Mental state: 5a. Specific ‐ average score ‐ depression (HAMD, endpoint, high score = worse, short‐term).
1.13. Analysis.
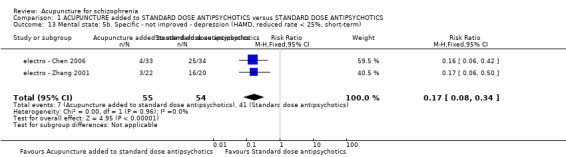
Comparison 1 ACUPUNCTURE added to STANDARD DOSE ANTIPSYCHOTICS versus STANDARD DOSE ANTIPSYCHOTICS, Outcome 13 Mental state: 5b. Specific ‐ not improved ‐ depression (HAMD, reduced rate < 25%, short‐term).
1.2.6 Self‐Rating Depression Scale (SDS) endpoint score
Continuous data of the only study to report relevant data indicated a significant difference between electroacupuncture added to standard dose antipsychotics compared with standard dose antipsychotics alone (short‐term, n = 42, 1 RCT, MD ‐24.25 CI ‐28.01 to ‐20.49, Analysis 1.16).
1.16. Analysis.
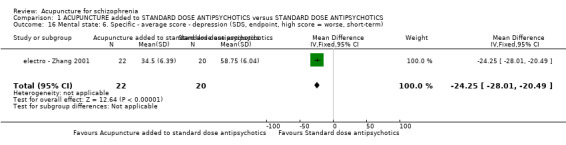
Comparison 1 ACUPUNCTURE added to STANDARD DOSE ANTIPSYCHOTICS versus STANDARD DOSE ANTIPSYCHOTICS, Outcome 16 Mental state: 6. Specific ‐ average score ‐ depression (SDS, endpoint, high score = worse, short‐term).
1.2.7 Psychotic Symptom Rating Scales Auditory Hallucination Subscale (PSYRAS‐AH) endpoint score
We found only one study, electro ‐ Cheng 2009, which used the PSYRAS‐AH subscale to assess specific symptoms. Participants given electroacupuncture added to standard dose antipsychotics had a lower score than those only given standard dose antipsychotics alone (short‐term, n = 60, 1 RCT, MD ‐2.17 CI ‐4.16 to ‐0.18, Analysis 1.15). Converting continuous data to binary found a similar result (n = 60, 1 RCT, RR 0.27 CI 0.14 to 0.52, Analysis 1.14).
1.15. Analysis.
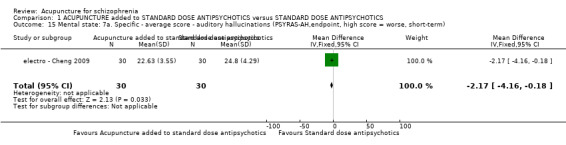
Comparison 1 ACUPUNCTURE added to STANDARD DOSE ANTIPSYCHOTICS versus STANDARD DOSE ANTIPSYCHOTICS, Outcome 15 Mental state: 7a. Specific ‐ average score ‐ auditory hallucinations (PSYRAS‐AH,endpoint, high score = worse, short‐term).
1.14. Analysis.
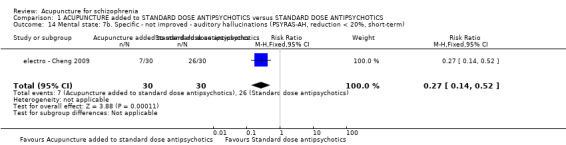
Comparison 1 ACUPUNCTURE added to STANDARD DOSE ANTIPSYCHOTICS versus STANDARD DOSE ANTIPSYCHOTICS, Outcome 14 Mental state: 7b. Specific ‐ not improved ‐ auditory hallucinations (PSYRAS‐AH, reduction < 20%, short‐term).
1.2.8 Auditory hallucinations not improved
acupoint cat ‐ Wang 1997, acupoint inj ‐ Yang 2000 and laser ‐ Ma 1999 reported whether people heard auditory hallucinations at different time periods using different criteria for 'not improved'. We found a significant difference between acupuncture added to standard dose antipsychotics group compared with the standard dose antipsychotics group when acupoint catgut treatment was used (short‐term, n = 216, 1 RCT, RR 0.24 CI 0.13 to 0.44) or acupoint injection (medium‐term, n = 64, 1 RCT, RR 0.32 CI 0.17 to 0.61), but not when comparing laser acupuncture added to standard dose antipsychotics with standard dose antipsychotics alone (n = 120, 1 RCT, RR 0.25 CI 0.03 to 2.17), though the duration of this study was unclear (Analysis 1.17).
1.17. Analysis.
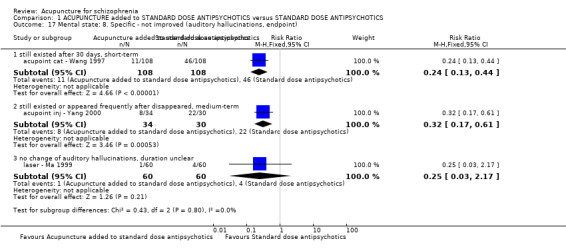
Comparison 1 ACUPUNCTURE added to STANDARD DOSE ANTIPSYCHOTICS versus STANDARD DOSE ANTIPSYCHOTICS, Outcome 17 Mental state: 8. Specific ‐ not improved (auditory hallucinations, endpoint).
1.2.9 Specific Auditory Hallucination Scale (SAHS) endpoint score
traditional ‐ Liu 2010 reported skewed continuous data from SAHS for the short‐ and medium‐term (Analysis 1.18).
1.18. Analysis.
Comparison 1 ACUPUNCTURE added to STANDARD DOSE ANTIPSYCHOTICS versus STANDARD DOSE ANTIPSYCHOTICS, Outcome 18 Mental state: 9. Specific ‐ average score ‐ auditory hallucinations (SAHS, endpoint, high score = worse) ‐ Skewed data.
| Mental state: 9. Specific ‐ average score ‐ auditory hallucinations (SAHS, endpoint, high score = worse) ‐ Skewed data | |||||
|---|---|---|---|---|---|
| Study | Intervention | Mean | SD | N | Note |
| short‐term | |||||
| traditional ‐ Liu 2010 | Traditional acupuncture added to standard dose antipsychotics | 9.26 | 5.37 | 48 | P < 0.05 |
| traditional ‐ Liu 2010 | Standard dose antipsychotics | 13.24 | 5.07 | 49 | |
| medium‐term | |||||
| traditional ‐ Liu 2010 | Traditional acupuncture added to standard dose antipsychotics | 5.12 | 3.64 | 47 | P < 0.01 |
| traditional ‐ Liu 2010 | Standard dose antipsychotics | 12.63 | 2.89 | 49 | |
1.2.10 Hallucinations
acupoint inj ‐ Wang 2000 reported data from the twelfth item of the BPRS. We found short‐term data were significantly different between acupoint injection added to standard dose antipsychotics and standard dose antipsychotics alone (n = 90, 1 RCT, MD ‐0.73 CI ‐1.28 to ‐0.18, Analysis 1.19).
1.19. Analysis.
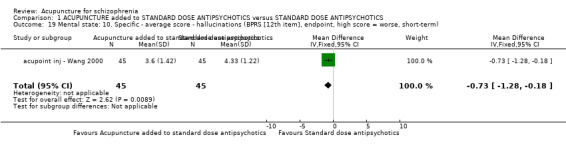
Comparison 1 ACUPUNCTURE added to STANDARD DOSE ANTIPSYCHOTICS versus STANDARD DOSE ANTIPSYCHOTICS, Outcome 19 Mental state: 10. Specific ‐ average score ‐ hallucinations (BPRS [12th item], endpoint, high score = worse, short‐term).
1.2.11 Time for auditory hallucinations to disappear
laser ‐ Ma 1999 reported this outcome. These data are listed using additional Table 7.
1.3 Behaviour
1.3.1 Leaving the study early
We found 10 studies with five different categories of acupuncture (all short‐term data). Only seven of 870 participants left the studies early. There was no difference between acupuncture added to standard dose antipsychotics group and standard dose antipsychotics alone (n = 870, 10 RCTs, RR 1.33 CI 0.33 to 5.45). Five of 370 participants with either acupoint injection, electroacupuncture or traditional acupuncture left the studies early by the medium‐term (n = 370, 5 RCTs, RR 3.58 CI 0.60 to 21.27, Analysis 1.20).
1.20. Analysis.
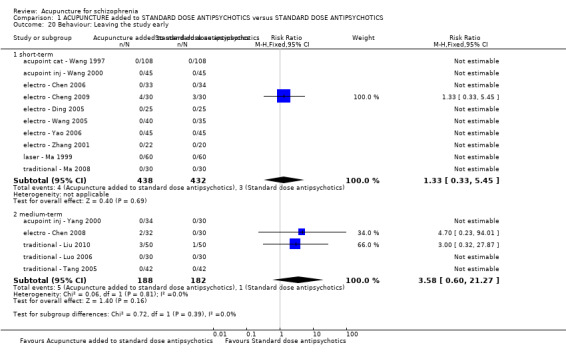
Comparison 1 ACUPUNCTURE added to STANDARD DOSE ANTIPSYCHOTICS versus STANDARD DOSE ANTIPSYCHOTICS, Outcome 20 Behaviour: Leaving the study early.
1.4 Service outcomes
1.4.1 Time in hospital
Only one study with laser acupuncture reported the outcome of time in hospital. Although this was not a pre‐stated outcome of interest for this review, it was the only service outcome and we analysed data and found the days in hospital of participants given laser acupuncture added to standard dose antipsychotics was less than the number of days of participants given standard dose antipsychotics alone (n = 120, 1 RCT, MD ‐16.00 CI ‐19.54 to ‐12.46, Analysis 1.21).
1.21. Analysis.
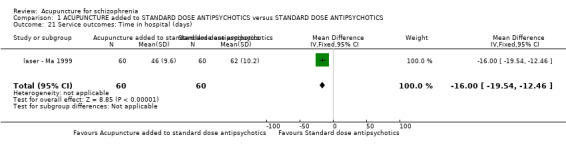
Comparison 1 ACUPUNCTURE added to STANDARD DOSE ANTIPSYCHOTICS versus STANDARD DOSE ANTIPSYCHOTICS, Outcome 21 Service outcomes: Time in hospital (days).
1.5 Adverse effects
1.5.1 Treatment Emergent Symptom Scale/Form (TESS) endpoint score
electro ‐ Yao 2006 reported TESS endpoint score at short‐term with result significantly favouring the electroacupuncture added to standard dose antipsychotics group (n = 90, 1 RCT, MD ‐2.80 CI ‐3.09 to ‐2.51, Analysis 1.22).
1.22. Analysis.
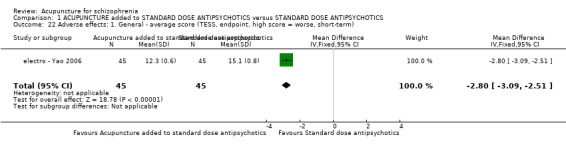
Comparison 1 ACUPUNCTURE added to STANDARD DOSE ANTIPSYCHOTICS versus STANDARD DOSE ANTIPSYCHOTICS, Outcome 22 Adverse effects: 1. General ‐ average score (TESS, endpoint, high score = worse, short‐term).
1.5.2 Extrapyramidal symptoms
Acupuncture added to standard dose antipsychotics did not reduce the incidence of extrapyramidal symptoms in the short‐term (n = 202, 3 RCTs, RR 0.66 CI 0.40 to 1.09). There was substantial heterogeneity between traditional acupuncture and electroacupuncture group (traditional acupuncture: n = 60, 1 RCT, RR 0.17 CI 0.02 to 1.30; electroacupuncture: n = 142, 2 RCTs, RR 0.79 CI 0.47 to 1.34, subgroup I2>50%). We found a similar result at medium‐term (n = 156, 2 RCTs, RR 0.62 CI 0.32 to 1.23). Further, electro ‐ Wang 2005 reported there was no difference of the rate of tremor (n = 75, 1 RCT, RR 0.73 CI 0.24 to 2.18) and akathisia (n = 75, 1 RCT, RR 0.58 CI 0.23 to 1.48) if electroacupuncture was added to standard dose antipsychotics group compared with standard dose antipsychotics alone in the short‐term. electro ‐ Chen 2008 reported no difference in rate of myotonia (n = 60, 1 RCT, RR 1.00 CI 0.07 to 15.26), tremor (n = 60, 1 RCT, RR 0.67 CI 0.12 to 3.71) and akathisia (n = 60, 1 RCT, RR 0.60 CI 0.16 to 2.29) when electroacupuncture was added to standard dose antipsychotics group compared with standard dose antipsychotics alone by the medium‐term (Analysis 1.23; Analysis 1.24).
1.23. Analysis.
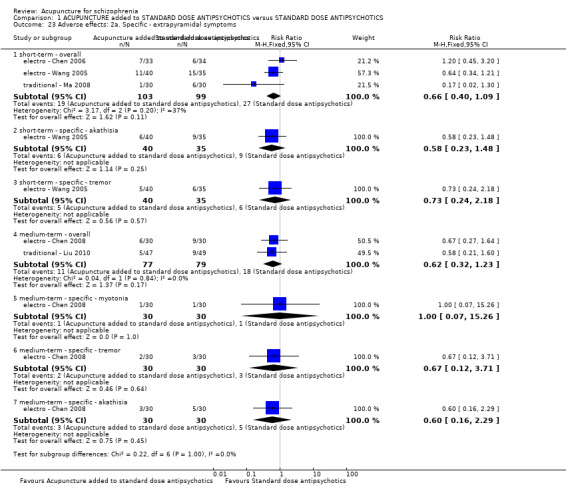
Comparison 1 ACUPUNCTURE added to STANDARD DOSE ANTIPSYCHOTICS versus STANDARD DOSE ANTIPSYCHOTICS, Outcome 23 Adverse effects: 2a. Specific ‐ extrapyramidal symptoms.
1.24. Analysis.
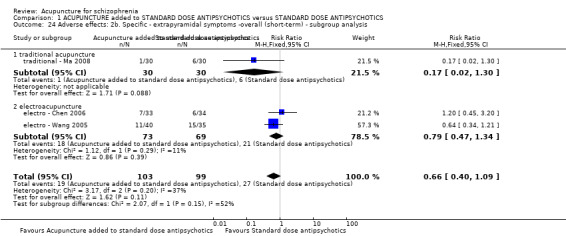
Comparison 1 ACUPUNCTURE added to STANDARD DOSE ANTIPSYCHOTICS versus STANDARD DOSE ANTIPSYCHOTICS, Outcome 24 Adverse effects: 2b. Specific ‐ extrapyramidal symptoms ‐overall (short‐term) ‐ subgroup analysis.
1.5.3 Central Nervous System
traditional ‐ Liu 2010 provided incidences of anxiety in the medium‐term and we found traditional acupuncture added to standard dose antipsychotics did not reduce the incidences compared with standard dose antipsychotics alone (n = 96, 1 RCT, RR 0.42 CI 0.09 to 2.05). Data from electro ‐ Chen 2006, electro ‐ Wang 2005 or traditional ‐ Ma 2008 indicated acupuncture added to standard dose antipsychotics reduced the frequency of insomnia compared with standard dose antipsychotics by the short‐term (n = 202, 3 RCTs, RR 0.30 CI 0.11 to 0.83), however, two studies reported no difference by the medium‐term (n = 156, 2 RCTs, RR 0.34 CI 0.12 to 1.02, Analysis 1.25). electro ‐ Chen 2008 reported no different for headache between electroacupuncture added to standard dose antipsychotics group compared with standard dose antipsychotics alone by the medium‐term (n = 60, 1 RCT, RR 3.00 CI 0.33 to 27.23, Analysis 1.25).
1.25. Analysis.
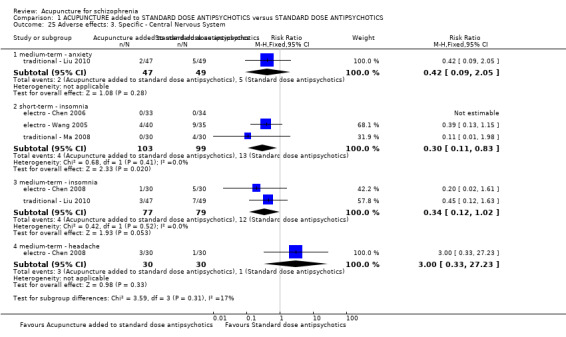
Comparison 1 ACUPUNCTURE added to STANDARD DOSE ANTIPSYCHOTICS versus STANDARD DOSE ANTIPSYCHOTICS, Outcome 25 Adverse effects: 3. Specific ‐ Central Nervous System.
1.5.4 Anticholinergic symptoms
We found no difference in dry mouth in the short‐term (n = 202, 3 RCTs, RR 1.18 CI 0.47 to 3.00), blurred vision (n = 202, 3 RCTs, short‐term RR 0.88 CI 0.24 to 3.24; n = 156, 2 RCTs, medium‐term RR 1.00 CI 0.15 to 6.64), sweating (n = 156, 2 RCTs, medium‐term RR 3.00 CI 0.13 to 70.83), constipation (n = 202, 3 RCTs, short‐term RR 0.88 CI 0.19 to 4.06) and nausea and vomiting (n = 202, 3 RCTs, short‐term RR 0.41 CI 0.12 to 1.32) when comparing acupuncture added to standard dose antipsychotics and standard dose antipsychotics alone (Analysis 1.26).
1.26. Analysis.
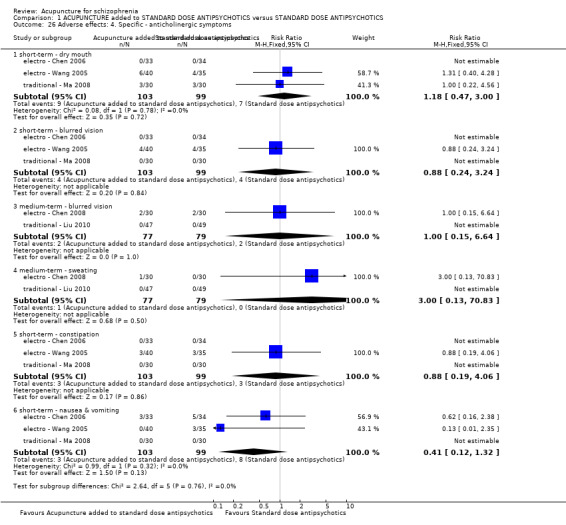
Comparison 1 ACUPUNCTURE added to STANDARD DOSE ANTIPSYCHOTICS versus STANDARD DOSE ANTIPSYCHOTICS, Outcome 26 Adverse effects: 4. Specific ‐ anticholinergic symptoms.
1.5.5 Gastrointestinal system
People given traditional acupuncture added to standard dose antipsychotics had similar 'unspecified gastrointestinal symptoms' by the medium‐term (n = 96, 1 RCT, RR 0.52 CI 0.10 to 2.71), constipation (n = 202, 3 RCTs, RR 0.88 CI 0.19 to 4.06) and nausea and vomiting (n = 202, 3 RCTs, RR 0.41 CI 0.12 to 1.32) in the short‐term as those only given standard dose antipsychotics alone (Analysis 1.27).
1.27. Analysis.
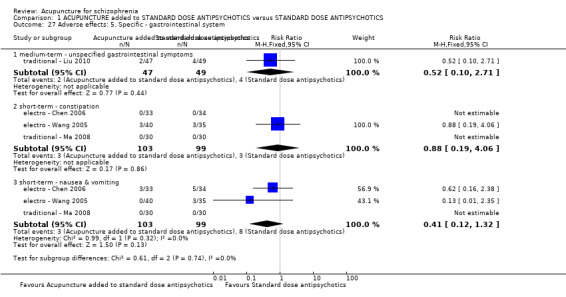
Comparison 1 ACUPUNCTURE added to STANDARD DOSE ANTIPSYCHOTICS versus STANDARD DOSE ANTIPSYCHOTICS, Outcome 27 Adverse effects: 5. Specific ‐ gastrointestinal system.
1.5.6 Cardiovascular symptoms
Acupuncture added to standard dose antipsychotics group did not reduce dizziness (n = 202, 3 RCTs, short‐term RR 0.94 CI 0.32 to 2.75), dizziness or headache (n = 96, 1 RCT, medium‐term RR 0.35 CI 0.04 to 3.22), or tachycardia (n = 217, 3 RCTs, short‐term RR 0.87 CI 0.42 to 1.79; n = 156, 2 RCTs, medium‐term RR 0.34 CI 0.04 to 3.20, Analysis 1.28).
1.28. Analysis.
Comparison 1 ACUPUNCTURE added to STANDARD DOSE ANTIPSYCHOTICS versus STANDARD DOSE ANTIPSYCHOTICS, Outcome 28 Adverse effects: 6. Specific ‐ cardiovascular symptoms (or headache).
1.5.7 Metabolic system
Comparing acupuncture added to standard dose antipsychotics group with standard dose antipsychotics group indicated substantial heterogeneity (I2 > 50%) but when undertaking subgroup analysis we found acupuncture added to standard dose antipsychotics did not change weight gain compared with standard dose antipsychotics alone in the short‐term ‐ traditional acupuncture (n = 60, 1 RCT, RR 0.14 CI 0.01 to 2.65) and electroacupuncture (n = 142, 2 RCTs, RR 6.15 CI 0.33 to 115.01). The same held for the findings for traditional acupuncture in the medium‐term (n = 96, 1 RCT, RR 0.21 CI 0.01 to 4.23, Analysis 1.29; Analysis 1.30).
1.29. Analysis.
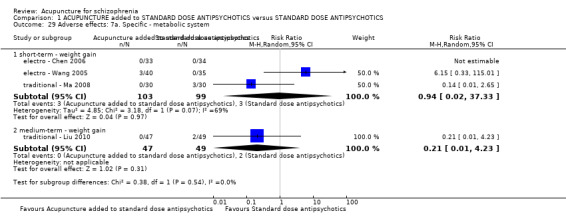
Comparison 1 ACUPUNCTURE added to STANDARD DOSE ANTIPSYCHOTICS versus STANDARD DOSE ANTIPSYCHOTICS, Outcome 29 Adverse effects: 7a. Specific ‐ metabolic system.
1.30. Analysis.
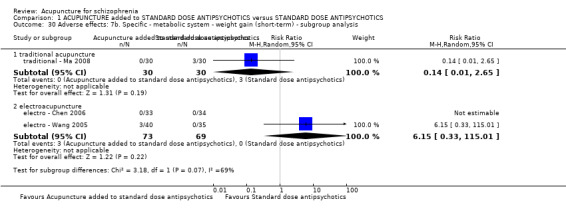
Comparison 1 ACUPUNCTURE added to STANDARD DOSE ANTIPSYCHOTICS versus STANDARD DOSE ANTIPSYCHOTICS, Outcome 30 Adverse effects: 7b. Specific ‐ metabolic system ‐ weight gain (short‐term) ‐ subgroup analysis.
1.5.8 Endocrine system
There was no difference in the rate of irregular menstruation (short‐term) between acupuncture added to standard dose antipsychotics group and standard dose antipsychotics alone (n = 127, 2 RCTs, RR 0.33 CI 0.01 to 7.87, Analysis 1.31).
1.31. Analysis.
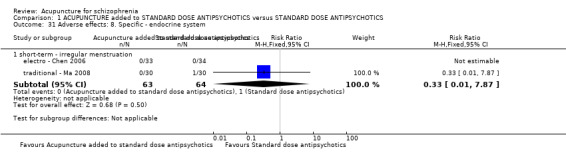
Comparison 1 ACUPUNCTURE added to STANDARD DOSE ANTIPSYCHOTICS versus STANDARD DOSE ANTIPSYCHOTICS, Outcome 31 Adverse effects: 8. Specific ‐ endocrine system.
1.5.9 Laboratory tests
Short‐term (n = 292, 4 RCTs, RR 1.01 CI 0.26 to 3.90) and medium‐term (n = 96, 1 RCT, RR 0.70 CI 0.12 to 3.98) data indicate acupuncture added to standard dose antipsychotics did not affect the rate of abnormal liver function tests. This also applied to ECG abnormalities (n = 292, 4 RCTs, RR 0.50 CI 0.05 to 5.32). Overall, participants allocated to acupuncture had no different rates of blood abnormalities in routine tests than those given only standard dose antipsychotics (n = 292, 4 RCTs, RR 1.33 CI 0.32 to 5.62, Analysis 1.32).
1.32. Analysis.
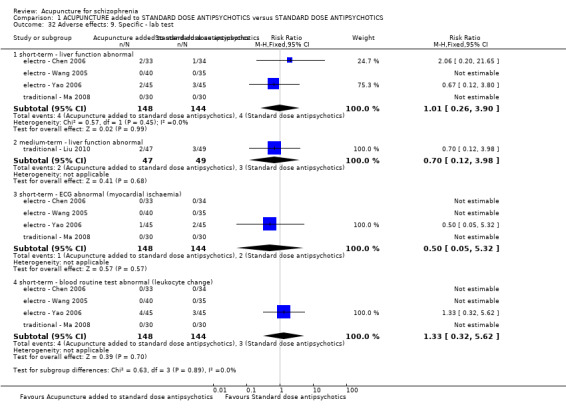
Comparison 1 ACUPUNCTURE added to STANDARD DOSE ANTIPSYCHOTICS versus STANDARD DOSE ANTIPSYCHOTICS, Outcome 32 Adverse effects: 9. Specific ‐ lab test.
2. COMPARISON 2: ACUPUNCTURE added to LOW DOSE ANTIPSYCHOTICS versus STANDARD DOSE ANTIPSYCHOTICS
Eight studies (acupoint cat ‐ Sun 2005; acupoint inj ‐ Pan 2002; electro ‐ Cui 2000; electro ‐ Xiong 2010; electro ‐ Zhou 1997; laser ‐ Zhang 1991; traditional ‐ Wang 2006; traditional ‐ Xu 2004) compared acupuncture added to low dose antipsychotics with standard dose antipsychotics.
2.1 Global state
2.1.1 Relapse
Only one study (acupoint inj ‐ Pan 2002) with acupoint injection reported binary data for 'relapse'. We found acupoint injection added to low dose antipsychotics group reduced the rate of relapse compared with standard dose antipsychotics alone at long‐term follow‐up (n = 170, 1 RCT, RR 0.57 CI 0.37 to 0.89, Analysis 2.1).
2.1. Analysis.
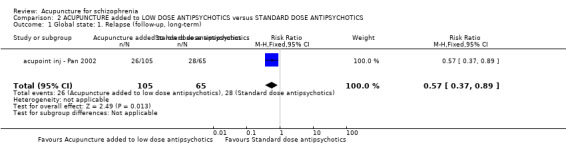
Comparison 2 ACUPUNCTURE added to LOW DOSE ANTIPSYCHOTICS versus STANDARD DOSE ANTIPSYCHOTICS, Outcome 1 Global state: 1. Relapse (follow‐up, long‐term).
2.1.2 Not improved
Four studies (acupoint cat ‐ Sun 2005; electro ‐ Zhou 1997; laser ‐ Zhang 1991; traditional ‐ Wang 2006), with traditional acupuncture, electroacupuncture, laser acupuncture or acupoint catgut treatment, used three different criteria of 'not improved' (all short‐term). We found global data did not significantly favour acupuncture added to low dose antipsychotics (n = 272, 4 RCTs, RR 0.83 CI 0.40 to 1.72, Analysis 2.2).
2.2. Analysis.
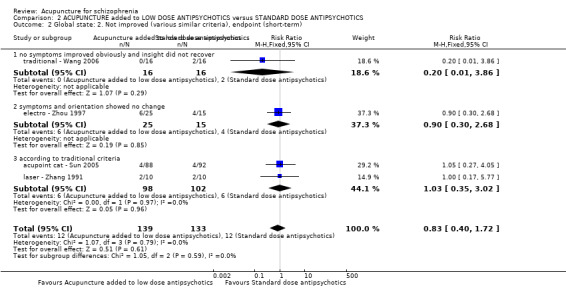
Comparison 2 ACUPUNCTURE added to LOW DOSE ANTIPSYCHOTICS versus STANDARD DOSE ANTIPSYCHOTICS, Outcome 2 Global state: 2. Not improved (various similar criteria), endpoint (short‐term).
2.1.3 Clinical Global Impression Scale (CGI) endpoint score
The CGI consists of three item ‐ severity of illness (SI), global improvement (GI) and efficacy index (EI). electro ‐ Zhou 1997 reported continuous data (short‐term) but data of CGI‐GI and CGI‐EI were skewed (Analysis 2.4; Analysis 2.5). We found no difference of CGI‐SI at short‐term between participants given electroacupuncture added to low dose antipsychotics and those given standard dose antipsychotics (n = 40, 1 RCT, MD ‐0.40 CI ‐1.08 to 0.28, Analysis 2.3).
2.4. Analysis.
Comparison 2 ACUPUNCTURE added to LOW DOSE ANTIPSYCHOTICS versus STANDARD DOSE ANTIPSYCHOTICS, Outcome 4 Global state: 3b. CGI ‐ average score ‐ CGI‐GI (endpoint, high score = worse, short‐term) ‐ Skewed data.
| Global state: 3b. CGI ‐ average score ‐ CGI‐GI (endpoint, high score = worse, short‐term) ‐ Skewed data | |||||
|---|---|---|---|---|---|
| Study | Intervention | Mean | SD | N | Note |
| electro ‐ Zhou 1997 | Electroacupuncture added to low dose antipsychotics | 2.2 | 1.1 | 25 | No significant difference |
| electro ‐ Zhou 1997 | Standard dose antipsychotics | 2.3 | 1.3 | 15 | |
2.5. Analysis.
Comparison 2 ACUPUNCTURE added to LOW DOSE ANTIPSYCHOTICS versus STANDARD DOSE ANTIPSYCHOTICS, Outcome 5 Global state: 3c. CGI ‐ average score ‐ CGI‐EI (endpoint, high score = worse, short‐term) ‐ Skewed data.
| Global state: 3c. CGI ‐ average score ‐ CGI‐EI (endpoint, high score = worse, short‐term) ‐ Skewed data | |||||
|---|---|---|---|---|---|
| Study | Intervention | Mean | SD | N | Note |
| electro ‐ Zhou 1997 | Electroacupuncture added to low dose antipsychotics | 2.5 | 1.3 | 25 | P < 0.05 |
| electro ‐ Zhou 1997 | Standard dose antipsychotics | 1.5 | 0.8 | 15 | |
2.3. Analysis.
Comparison 2 ACUPUNCTURE added to LOW DOSE ANTIPSYCHOTICS versus STANDARD DOSE ANTIPSYCHOTICS, Outcome 3 Global state: 3a. CGI ‐ average score ‐ CGI‐SI (endpoint, high score = worse, short‐term).
2.1.4 Adding medication
One study (electro ‐ Cui 2000) reported using adding medication, which was not the outcome we measured, but we listed it using additional table (Table 7).
2.2 Mental state
2.2.1 BPRS endpoint/change score
We found continuous data from BPRS for the short‐term were not significantly different between acupuncture added to low dose antipsychotics group and standard dose antipsychotics alone group but with considerable heterogeneity between each study (n = 332, 5 RCTs, MD ‐5.55 CI ‐14.40 to 3.29; I2 > 75%). If we removed the study that was causing this heterogeneity, as judged by visual inspection (traditional ‐ Wang 2006) and undertook a subgroup analysis, we found a similar conclusion (n = 300, 4 RCTs, MD ‐1.36 CI ‐3.13 to 0.41, I2 = 49%, subgroup I2 = 49.3%). However, it was different for the long‐term follow‐up (n = 137, 1 RCT, MD ‐4.87 CI ‐8.21 to ‐1.53, Analysis 2.6; Analysis 2.7). Three studies also used binary data to assess BPRS (short‐term) with no difference between acupuncture added to low dose antipsychotics and standard dose antipsychotics alone even with different reduction of BPRS as criteria (reduced rate < 30%: n = 60, 1 RCT, RR 0.75 CI 0.18 to 3.07; reduced rate < 25%: n = 170, 1 RCT, RR 0.62 CI 0.31 to 1.25; reduced rate ≤ 20%: n = 40, 1 RCT, RR 0.90 CI 0.30 to 2.68, Analysis 2.8). Only one study reported BPRS change scores (short‐term) and we found there was no difference between electroacupuncture added to low dose antipsychotics group and standard dose antipsychotics alone group (n = 60, 1 RCT, MD 0.81 CI ‐3.17 to 4.79, Analysis 2.9).
2.6. Analysis.
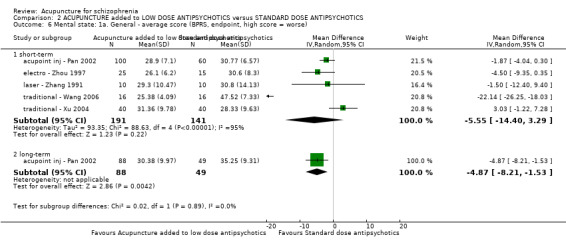
Comparison 2 ACUPUNCTURE added to LOW DOSE ANTIPSYCHOTICS versus STANDARD DOSE ANTIPSYCHOTICS, Outcome 6 Mental state: 1a. General ‐ average score (BPRS, endpoint, high score = worse).
2.7. Analysis.
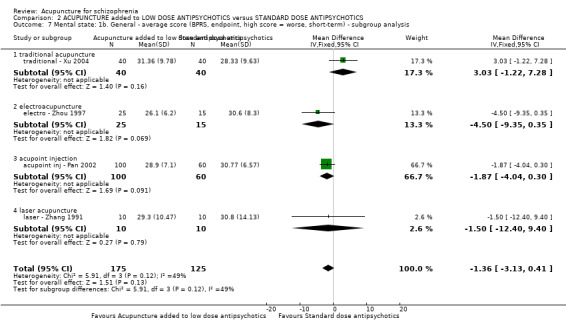
Comparison 2 ACUPUNCTURE added to LOW DOSE ANTIPSYCHOTICS versus STANDARD DOSE ANTIPSYCHOTICS, Outcome 7 Mental state: 1b. General ‐ average score (BPRS, endpoint, high score = worse, short‐term) ‐ subgroup analysis.
2.8. Analysis.
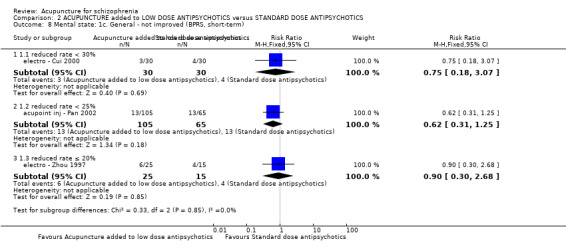
Comparison 2 ACUPUNCTURE added to LOW DOSE ANTIPSYCHOTICS versus STANDARD DOSE ANTIPSYCHOTICS, Outcome 8 Mental state: 1c. General ‐ not improved (BPRS, short‐term).
2.9. Analysis.
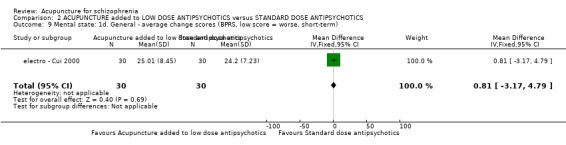
Comparison 2 ACUPUNCTURE added to LOW DOSE ANTIPSYCHOTICS versus STANDARD DOSE ANTIPSYCHOTICS, Outcome 9 Mental state: 1d. General ‐ average change scores (BPRS, low score = worse, short‐term).
2.2.2 PANSS endpoint score
The PANSS was used to assess symptoms by acupoint cat ‐ Sun 2005 and electro ‐ Xiong 2010. Data are skewed (Analysis 2.10). electro ‐ Xiong 2010 also used binary data to assess PANSS and found no difference between electroacupuncture added to low dose antipsychotics and standard dose alone antipsychotics (n = 80, 1 RCT, RR 1.22 CI 0.57 to 2.62, Analysis 2.11).
2.10. Analysis.
Comparison 2 ACUPUNCTURE added to LOW DOSE ANTIPSYCHOTICS versus STANDARD DOSE ANTIPSYCHOTICS, Outcome 10 Mental state: 2a. General ‐ Average score (PANSS, endpoint, high score = worse, short‐term) ‐ Skew data.
| Mental state: 2a. General ‐ Average score (PANSS, endpoint, high score = worse, short‐term) ‐ Skew data | |||||
|---|---|---|---|---|---|
| Study | Intervention | Mean | SD | N | Note |
| acupoint cat ‐ Sun 2005 | Acupoint catgut treatment added to low dose antipsychotics | 52.85 | 15.86 | 88 | P > 0.05 |
| acupoint cat ‐ Sun 2005 | Standard dose antipsychotics | 49.75 | 12.77 | 92 | |
| electro ‐ Xiong 2010 | Electroacupuncture added to low dose antipsychotics | 46.34 | 11.10 | 40 | Cmpared with score before treatment P < 0.01 |
| electro ‐ Xiong 2010 | Standard dose antipsychotics | 45.21 | 11.36 | 40 | Cmpared with score before treatment P < 0.01 |
2.11. Analysis.
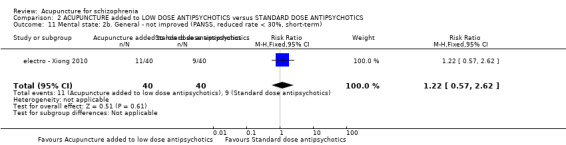
Comparison 2 ACUPUNCTURE added to LOW DOSE ANTIPSYCHOTICS versus STANDARD DOSE ANTIPSYCHOTICS, Outcome 11 Mental state: 2b. General ‐ not improved (PANSS, reduced rate < 30%, short‐term).
2.2.3 SAPS endpoint score
Three studies (acupoint cat ‐ Sun 2005; traditional ‐ Wang 2006; traditional ‐ Xu 2004) with traditional acupuncture or acupoint catgut treatment added to low dose antipsychotics reported this result in the short‐term. One study (traditional ‐ Xu 2004). reports skewed data (Analysis 2.13).The other two, using different categories, had considerable heterogeneity (I2 > 75%). Data from traditional ‐ Wang 2006 indicated significant difference between traditional acupuncture added to low dose antipsychotics group and standard dose antipsychotics group (n = 32, 1 RCT, MD ‐13.34 CI ‐16.94 to ‐9.74) but there was no difference between acupoint catgut treatment added to low dose antipsychotics group and standard dose antipsychotics alone group (n = 180, 1 RCT, MD 1.21 CI 0.96 to 1.46, Analysis 2.12).
2.13. Analysis.
Comparison 2 ACUPUNCTURE added to LOW DOSE ANTIPSYCHOTICS versus STANDARD DOSE ANTIPSYCHOTICS, Outcome 13 Mental state: 3b. Specific ‐ average score ‐ positive symptoms (SAPS, endpoint, high score = worse, short‐term) ‐ Skewed data.
| Mental state: 3b. Specific ‐ average score ‐ positive symptoms (SAPS, endpoint, high score = worse, short‐term) ‐ Skewed data | |||||
|---|---|---|---|---|---|
| Study | Intervention | Mean | SD | N | Note |
| traditional ‐ Xu 2004 | Traditional acupuncture added to low dose antipsychotics | 11.86 | 11.41 | 40 | No significant difference |
| traditional ‐ Xu 2004 | Standard dose antipsychotics | 11.12 | 9.87 | 40 | |
2.12. Analysis.
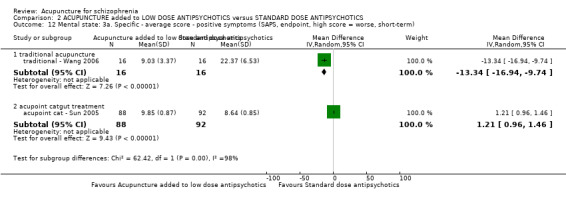
Comparison 2 ACUPUNCTURE added to LOW DOSE ANTIPSYCHOTICS versus STANDARD DOSE ANTIPSYCHOTICS, Outcome 12 Mental state: 3a. Specific ‐ average score ‐ positive symptoms (SAPS, endpoint, high score = worse, short‐term).
2.2.4 SANS endpoint score
traditional ‐ Xu 2004 reported no difference for SANS endpoint score (short‐term) between traditional acupuncture added to low dose antipsychotics group and standard dose antipsychotics alone group (n = 80, 1 RCT, MD 0.61 CI ‐3.30 to 4.52, Analysis 2.14). traditional ‐ Wang 2006 also reported data from SANS endpoint score but the data were skewed (Analysis 2.15).
2.14. Analysis.
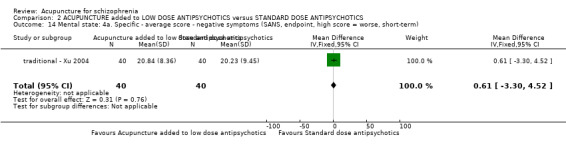
Comparison 2 ACUPUNCTURE added to LOW DOSE ANTIPSYCHOTICS versus STANDARD DOSE ANTIPSYCHOTICS, Outcome 14 Mental state: 4a. Specific ‐ average score ‐ negative symptoms (SANS, endpoint, high score = worse, short‐term).
2.15. Analysis.
Comparison 2 ACUPUNCTURE added to LOW DOSE ANTIPSYCHOTICS versus STANDARD DOSE ANTIPSYCHOTICS, Outcome 15 Mental state: 4b. Specific ‐ average score ‐ negative symptoms (SANS, endpoint, high score = worse, short‐term) ‐ Skewed data.
| Mental state: 4b. Specific ‐ average score ‐ negative symptoms (SANS, endpoint, high score = worse, short‐term) ‐ Skewed data | |||||
|---|---|---|---|---|---|
| Study | Intervention | Mean | SD | N | Note |
| traditional ‐ Wang 2006 | Traditional acupuncture added to low dose antipsychotics | 14.23 | 8.68 | 16 | P < 0.01 |
| traditional ‐ Wang 2006 | Standard dose antipsychotics | 30.56 | 13.31 | 16 | |
2.3 Behaviour
2.3.1 Leaving the study early
We found participants given acupuncture added to low dose antipsychotics were not less likely to drop out than those give standard dose antipsychotics at short‐term (n = 662, 8 RCT, RR 0.81 CI 0.29 to 2.29, Analysis 2.16).
2.16. Analysis.
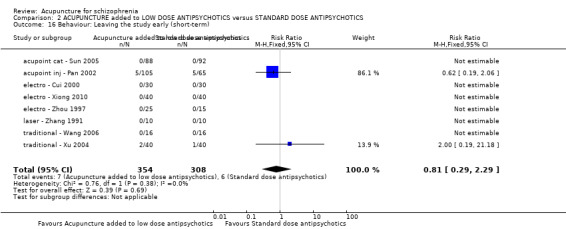
Comparison 2 ACUPUNCTURE added to LOW DOSE ANTIPSYCHOTICS versus STANDARD DOSE ANTIPSYCHOTICS, Outcome 16 Behaviour: Leaving the study early (short‐term).
2.4 Adverse effects
2.4.1 TESS endpoint score
Five studies (acupoint inj ‐ Pan 2002; electro ‐ Xiong 2010; electro ‐ Zhou 1997; traditional ‐ Wang 2006;traditional ‐ Xu 2004) reported short‐term TESS endpoint score but three sets of data were skewed (Analysis 2.18). The remaining two indicated significant differences between acupuncture added to low dose antipsychotics group and standard dose antipsychotics group (n = 200, 2 RCTs, MD ‐0.56 CI ‐0.86 to ‐0.26, Analysis 2.17).
2.18. Analysis.
Comparison 2 ACUPUNCTURE added to LOW DOSE ANTIPSYCHOTICS versus STANDARD DOSE ANTIPSYCHOTICS, Outcome 18 Adverse effects: 1b. General ‐ average score (TESS, endpoint, high score = worse, short‐term) ‐ Skewed data.
| Adverse effects: 1b. General ‐ average score (TESS, endpoint, high score = worse, short‐term) ‐ Skewed data | |||||
|---|---|---|---|---|---|
| Study | Intervention | Mean | SD | N | Note |
| electro ‐ Xiong 2010 | Electroacupuncture added to low dose antipsychotics | 5.08 | 4.56 | 40 | P < 0.01 |
| electro ‐ Xiong 2010 | Standard dose antipsychotics | 10.38 | 6.52 | 40 | |
| traditional ‐ Wang 2006 | Traditional acupuncture added to low dose antipsychotics | 2.57 | 3.28 | 16 | P < 0.01 |
| traditional ‐ Wang 2006 | Standard dose antipsychotics | 6.93 | 5.28 | 16 | |
| traditional ‐ Xu 2004 | Traditional acupuncture added to low dose antipsychotics | 2.65 | 3.47 | 40 | P < 0.01 |
| traditional ‐ Xu 2004 | Standard dose antipsychotics | 10.12 | 8.12 | 40 | |
2.17. Analysis.
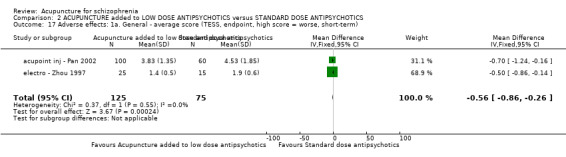
Comparison 2 ACUPUNCTURE added to LOW DOSE ANTIPSYCHOTICS versus STANDARD DOSE ANTIPSYCHOTICS, Outcome 17 Adverse effects: 1a. General ‐ average score (TESS, endpoint, high score = worse, short‐term).
2.4.2 Rating Scale for Extrapyramidal Side Effects (RESES) endpoint score
One study (laser ‐ Zhang 1991) reported RESES endpoint score but only reported mean and T‐value. We calculated SD according to the Cochrane Handbook for Systematic Reviews of Interventions (Higgins 2011) and data indicated that scores of acupuncture added to low dose antipsychotics group were lower than those in the standard dose antipsychotics group (n = 20, 1 RCT, MD ‐0.60 CI ‐0.73 to ‐0.47, Analysis 2.19).
2.19. Analysis.
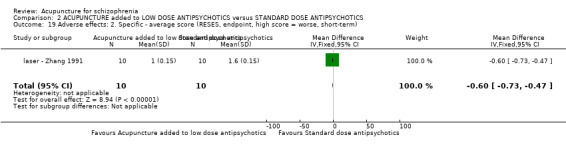
Comparison 2 ACUPUNCTURE added to LOW DOSE ANTIPSYCHOTICS versus STANDARD DOSE ANTIPSYCHOTICS, Outcome 19 Adverse effects: 2. Specific ‐ average score (RESES, endpoint, high score = worse, short‐term).
2.4.3 Extrapyramidal symptoms
Studies with three categories of acupuncture had considerable heterogeneity between subgroups in the short‐term (I2 > 75%). The incidences of extrapyramidal symptoms were less for those in the acupuncture group whether given acupoint catgut (n = 180, 1 RCT, RR 0.02 CI 0.00 to 0.35) or electroacupuncture (n = 60, 1 RCT, RR 0.36 CI 0.15 to 0.87) ‐ both added to low dose antipsychotics. Laser acupuncture, however, seemed not to make any difference in one small trial (n = 20, 1 RCT, RR 0.88 CI 0.53 to 1.46). There were no differences for short‐term incidences of myotonia (n = 180, 1 RCT, RR 0.21 CI 0.01 to 4.29) and tremor (n = 180, 1 RCT, RR 0.09 CI 0.01 to 1.69) but data indicated acupoint catgut treatment added to low dose antipsychotics reduced akathisia compared with standard dose antipsychotics group (short‐term, n = 180, 1 RCT, RR 0.03 CI 0.00 to 0.49, Analysis 2.20)
2.20. Analysis.
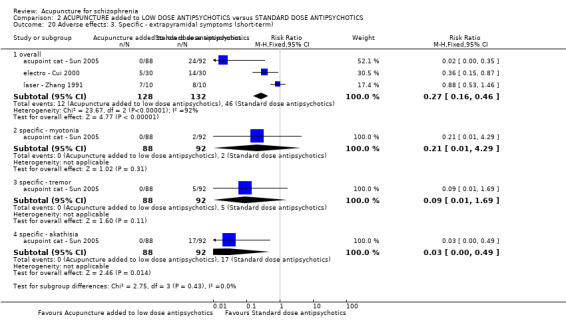
Comparison 2 ACUPUNCTURE added to LOW DOSE ANTIPSYCHOTICS versus STANDARD DOSE ANTIPSYCHOTICS, Outcome 20 Adverse effects: 3. Specific ‐ extrapyramidal symptoms (short‐term).
2.4.4 Central Nervous System
acupoint cat ‐ Sun 2005 reported "activity‐increasing adverse effects". We found acupoint catgut treatment added to low dose antipsychotics group could not reduce the event rate compared with standard antipsychotics group in the short‐term (n = 180, 1 RCT, RR 0.15 CI 0.01 to 2.85). acupoint cat ‐ Sun 2005 also reported short‐term insomnia. There was no difference for insomnia between acupoint catgut treatment added to low dose antipsychotics group and standard dose antipsychotics alone (n = 180, 1 RCT, RR 0.07 CI 0.00 to 1.20). We did not find a clear difference for sleeplessness (n = 240, 2 RCTs, RR 0.33 CI 0.04 to 3.03, Analysis 2.21)
2.21. Analysis.
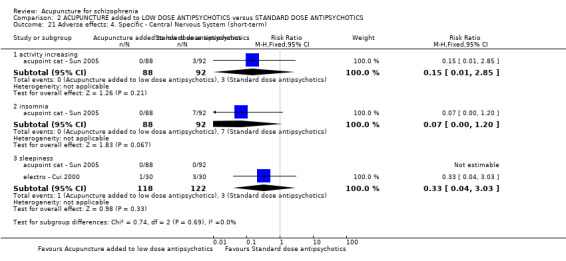
Comparison 2 ACUPUNCTURE added to LOW DOSE ANTIPSYCHOTICS versus STANDARD DOSE ANTIPSYCHOTICS, Outcome 21 Adverse effects: 4. Specific ‐ Central Nervous System (short‐term).
2.4.5 Anticholinergic symptoms
In the short‐term, data for dry mouth favoured acupuncture (n = 240, 2 RCTs, RR 0.22 CI 0.09 to 0.58). Nasal congestion (short‐term) was no different between groups (n = 180, 1 RCT, RR 0.09 CI 0.01 to 1.69). Two studies indicated that acupuncture added to low dose antipsychotics could reduce blurred vision (short‐term, n = 240, 2 RCTs, RR 0.22 CI 0.06 to 0.83). There was no difference in constipation (n = 180, 1 RCT, short‐term RR 0.07 CI 0.00 to 1.20, Analysis 2.22)
2.22. Analysis.
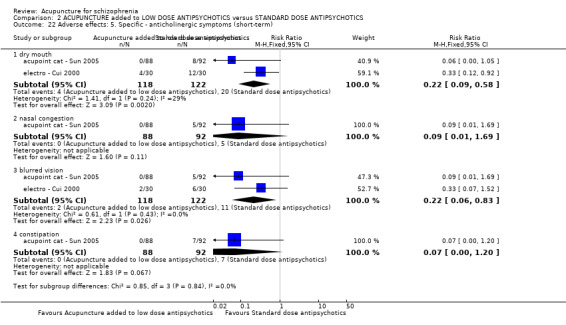
Comparison 2 ACUPUNCTURE added to LOW DOSE ANTIPSYCHOTICS versus STANDARD DOSE ANTIPSYCHOTICS, Outcome 22 Adverse effects: 5. Specific ‐ anticholinergic symptoms (short‐term).
2.4.6 Cardiovascular symptoms
Short‐term data indicate that there was no difference for dizziness between the two groups ‐ acupoint catgut treatment added to low dose antipsychotics group and standard dose antipsychotics alone group (n = 180, 1 RCT, RR 0.70 CI 0.12 to 4.07). We found participants given acupuncture added to low dose antipsychotics had less tachycardia than people given standard dose antipsychotics alone (n = 240, 2 RCTs, RR 0.25 CI 0.11 to 0.53, Analysis 2.23).
2.23. Analysis.
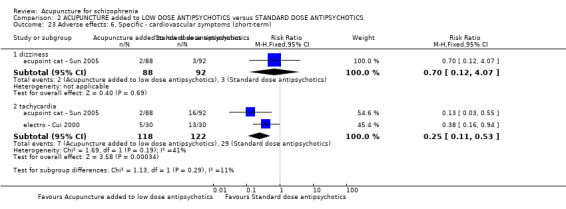
Comparison 2 ACUPUNCTURE added to LOW DOSE ANTIPSYCHOTICS versus STANDARD DOSE ANTIPSYCHOTICS, Outcome 23 Adverse effects: 6. Specific ‐ cardiovascular symptoms (short‐term).
2.4.7 Skin infection
No study found skin infection (Analysis 2.24).
2.24. Analysis.

Comparison 2 ACUPUNCTURE added to LOW DOSE ANTIPSYCHOTICS versus STANDARD DOSE ANTIPSYCHOTICS, Outcome 24 Adverse effects: 7. Specific ‐ skin infection (short‐term).
3. COMPARISON 3: ACUPUNCTURE versus STANDARD DOSE ANTIPSYCHOTICS
Six studies (electro ‐ Zhang 1987; laser ‐ Liu 1986; laser ‐ Zhang 1991; traditional ‐ Wang 2006; traditional ‐ Zhao 2005a; traditional ‐ Zhao 2005b) compared acupuncture with standard dose antipsychotics.
3.1Global state
3.1.1 Not improved
Five studies (electro ‐ Zhang 1987; laser ‐ Zhang 1991; traditional ‐ Wang 2006; traditional ‐ Zhao 2005a; traditional ‐ Zhao 2005b) with a duration of less than three months are included in this comparison. Three (traditional ‐ Wang 2006; traditional ‐ Zhao 2005a; traditional ‐ Zhao 2005b) used traditional acupuncture, one electroacupuncture and the other laser acupuncture. Although studies all reported 'clinically important change' in global state, the criteria of 'not improved' were different except for traditional ‐ Zhao 2005a and traditional ‐ Zhao 2005b. We found no difference between acupuncture and standard dose antipsychotics which used similar criteria (n = 141, 3 RCTs, RR 0.88 CI 0.53 to 1.48). However, the other two trials, traditional ‐ Zhao 2005a and traditional ‐ Zhao 2005b, with identical criteria, indicated a difference between two groups (n = 240, 2 RCTs, RR 0.33 CI 0.19 to 0.59, Analysis 3.1)
3.1. Analysis.
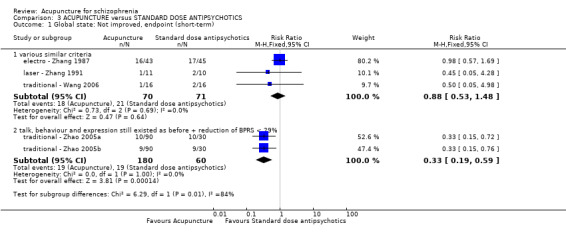
Comparison 3 ACUPUNCTURE versus STANDARD DOSE ANTIPSYCHOTICS, Outcome 1 Global state: Not improved, endpoint (short‐term).
3.2 Mental state
All included studies were with short‐term.
3.2.1 BPRS endpoint score
Only two studies reported BPRS. Continuous data favoured traditional acupuncture (n = 32, 1 RCT, MD ‐11.56 CI ‐16.36 to ‐6.76) but other continuous data indicated no difference between laser acupuncture and standard dose antipsychotics (n = 21, 1 RCT, MD ‐1.07 CI ‐11.19 to 9.05). There is substantial heterogeneity between two studies (I2 > 50%, Analysis 3.2).
3.2. Analysis.
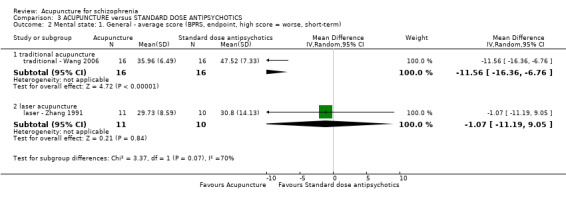
Comparison 3 ACUPUNCTURE versus STANDARD DOSE ANTIPSYCHOTICS, Outcome 2 Mental state: 1. General ‐ average score (BPRS, endpoint, high score = worse, short‐term).
3.2.2 SAPS endpoint score
Only traditional ‐ Wang 2006 reported SAPS. We found scores of participants given traditional acupuncture were significantly less than those given antipsychotics (n = 32, 1 RCT, MD ‐5.24 CI ‐9.06 to ‐1.42, Analysis 3.3).
3.3. Analysis.
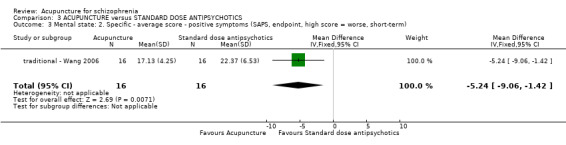
Comparison 3 ACUPUNCTURE versus STANDARD DOSE ANTIPSYCHOTICS, Outcome 3 Mental state: 2. Specific ‐ average score ‐ positive symptoms (SAPS, endpoint, high score = worse, short‐term).
3.2.3 SANS endpoint score
traditional ‐ Wang 2006 indicated no difference between traditional acupuncture and antipsychotics (n = 32, 1 RCT, MD ‐7.92 CI ‐17.01 to 1.17, Analysis 3.4).
3.4. Analysis.
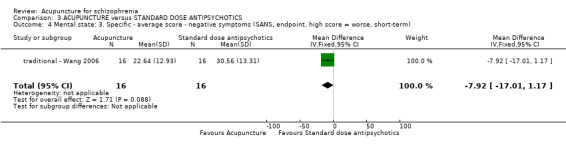
Comparison 3 ACUPUNCTURE versus STANDARD DOSE ANTIPSYCHOTICS, Outcome 4 Mental state: 3. Specific ‐ average score ‐ negative symptoms (SANS, endpoint, high score = worse, short‐term).
3.3 Behaviour
We included six studies and there were no participants who left the studies early in each group in the short‐term (Analysis 3.5).
3.5. Analysis.
Comparison 3 ACUPUNCTURE versus STANDARD DOSE ANTIPSYCHOTICS, Outcome 5 Behaviour: Leaving the study early (short‐term).
3.4 Adverse effects
3.4.1 TESS endpoint score
traditional ‐ Wang 2006 reported TESS scores at short‐term, but data were skewed and problematic to present (Analysis 3.6).
3.6. Analysis.
Comparison 3 ACUPUNCTURE versus STANDARD DOSE ANTIPSYCHOTICS, Outcome 6 Adverse effects: 1. General ‐ average scores (TESS, endpoint, short‐term) ‐ Skewed data.
| Adverse effects: 1. General ‐ average scores (TESS, endpoint, short‐term) ‐ Skewed data | |||||
|---|---|---|---|---|---|
| Study | Intervention | Mean | SD | N | Note |
| traditional ‐ Wang 2006 | Traditional acupuncture | 2.43 | 3.17 | 16 | P < 0.01 |
| traditional ‐ Wang 2006 | Antipsychotics | 6.93 | 5.28 | 16 | |
3.4.2 Extrapytamidal symptoms
Extrapyramidal symptoms (short‐term) were lower in the laser acupuncture group with no participants experiencing this problem but eight out of 10 people reported this in the control group (n = 21, 1 RCT, RR 0.05 CI 0.00 to 0.83, Analysis 3.7).
3.7. Analysis.
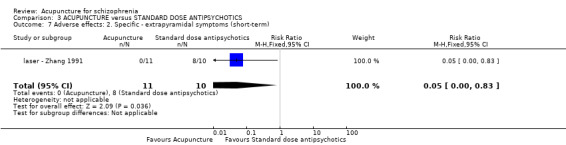
Comparison 3 ACUPUNCTURE versus STANDARD DOSE ANTIPSYCHOTICS, Outcome 7 Adverse effects: 2. Specific ‐ extrapyramidal symptoms (short‐term).
3.4.3 Numbness over the ear, upper extremity and chest on the treated side
We found no difference in numbness over the ear, upper extremity and chest on the treated side between participants given laser acupuncture and those given standard dose antipsychotics alone (n = 40, 1 RCT, RR 3.00 CI 0.13 to 69.52, Analysis 3.8).
3.8. Analysis.
Comparison 3 ACUPUNCTURE versus STANDARD DOSE ANTIPSYCHOTICS, Outcome 8 Adverse effects: 3. Specific ‐ numbness over the ear, upper extremity and chest on the treated side (short‐term).
4. COMPARISON 4: ACUPUNCTURE added to TCM DRUG versus TCM DRUG
Three studies (laser ‐ Liu 1986; traditional ‐ Zhao 2005a; traditional ‐ Zhao 2005b) compared acupuncture added to TCM drug with TCM drug.
4.1 Global state
4.1.1 Not improved
We found a significant difference for short‐term 'clinically important change' in global state between traditional acupuncture added to the TCM drug group and TCM drug group alone by studies with the same acupuncture criteria (n = 360, 2 RCTs, RR 0.11 CI 0.02 to 0.59), but no difference between electroacupuncture added to TCM drug group and TCM drug group alone by electro ‐ Zhang 1987 (n = 94, 1 RCT, RR 0.77 CI 0.57 to 1.06, Analysis 4.1).
4.1. Analysis.
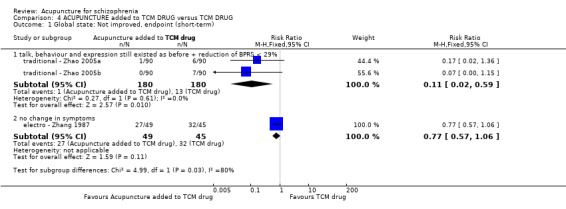
Comparison 4 ACUPUNCTURE added to TCM DRUG versus TCM DRUG, Outcome 1 Global state: Not improved, endpoint (short‐term).
4.2 Behaviour
4.2.1 Leaving the study early
We found no participants left the study early in either group (Analysis 4.2).
4.2. Analysis.
Comparison 4 ACUPUNCTURE added to TCM DRUG versus TCM DRUG, Outcome 2 Behaviour: Leaving the study early (short‐term).
5. COMPARISON 5: ACUPUNCTURE versus TCM DRUG
Three studies (electro ‐ Zhang 1987; traditional ‐ Zhao 2005a; traditional ‐ Zhao 2005b) compared acupuncture with TCM drug.
5.1 Global state
5.1.1 Not improved
Three studies (electro ‐ Zhang 1987; traditional ‐ Zhao 2005a; traditional ‐ Zhao 2005b) used two different criteria of 'not improved'. Two studies (traditional ‐ Zhao 2005a; traditional ‐ Zhao 2005b) with the same criteria indicated that there was no significant difference between traditional acupuncture group and TCM drug group in the short‐term (n = 360, 2 RCTs, RR 1.46 CI 0.74 to 2.87). However, we found that participants given electroacupuncture were significantly less likely to experience a worsening in global state (n = 88, 1 RCT, RR 0.52 CI 0.34 to 0.80, Analysis 5.1).
5.1. Analysis.
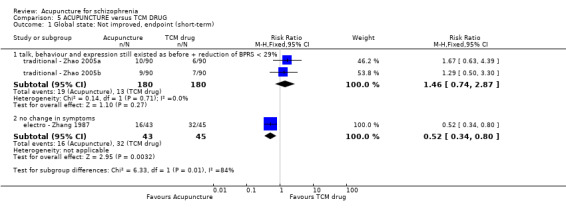
Comparison 5 ACUPUNCTURE versus TCM DRUG, Outcome 1 Global state: Not improved, endpoint (short‐term).
5.2 Behaviour
5.2.1 Leaving the study early
electro ‐ Zhang 1987, traditional ‐ Zhao 2005a and traditional ‐ Zhao 2005b reported data and we found no participant left the studies early in either acupuncture group or TCM drug group at short‐term (Analysis 5.2).
5.2. Analysis.
Comparison 5 ACUPUNCTURE versus TCM DRUG, Outcome 2 Behaviour: Leaving the study early (short‐term).
6. COMPARISION 6: ELECTRIC ACUPUNCTURE CONVULSIVE THERAPY versus ELECTRIC CONVULSIVE THERAPY
Only one study (EACT ‐ Xue 1987) compared electric acupuncture convulsive therapy with electroconvulsive therapy at short‐term.
6.1 Behaviour
6.1.1 Leaving the study early
No participants left the study early (Analysis 6.1).
6.1. Analysis.
Comparison 6 ELECTRIC ACUPUNCTURE CONVULSIVE THERAPY versus ELECTROCONVULSIVE THERAPY, Outcome 1 Behaviour: Leaving the study early (short‐term).
6.2 Adverse effects
6.2.1 Back pain
EACT ‐ Xue 1987 indicated there was no difference in the rates of back pain between electric acupuncture convulsive therapy group and standard electroconvulsive therapy group (n = 68, 1 RCT, RR 0.67 CI 0.12 to 3.74, Analysis 6.2)
6.2. Analysis.
Comparison 6 ELECTRIC ACUPUNCTURE CONVULSIVE THERAPY versus ELECTROCONVULSIVE THERAPY, Outcome 2 Adverse effects: 1. Specific ‐ back pain (short‐term).
6.2.2 Spinal fracture
Unlike back pain, the same study indicated that electric acupuncture convulsive therapy causes lower incidences of spinal fracture than electroconvulsive therapy (n = 68, 1 RCT, RR 0.33 CI 0.14 to 0.81, Analysis 6.3).
6.3. Analysis.
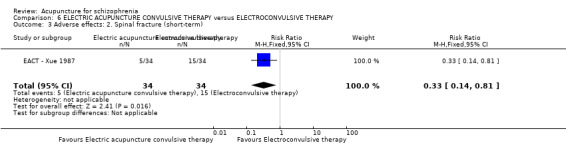
Comparison 6 ELECTRIC ACUPUNCTURE CONVULSIVE THERAPY versus ELECTROCONVULSIVE THERAPY, Outcome 3 Adverse effects: 2. Spinal fracture (short‐term).
Discussion
Summary of main results
The main results are presented in 'Summary of findings' tables. Thirty studies were included and all findings are, at the very least, at moderate risk of bias. Ten trials describe the randomisation method but only one allocation concealment (by envelopes). Eight studies used a blinding method. Most studies were short‐term and only two followed up participants to beyond six months. All but one study was undertaken in China.
1. Diagnosis and assessment criteria
Operational criteria (CCMD, ICD‐10, DSM) were used in most studies although three trials did not report criteria. The recent version of the CCMD is CCMD‐3. Whilst this refers to ICD‐10 and the DSM‐IV, it contains locally salient features that are absent in the international systems (Chen 2002). Many Chinese psychiatrists consider that this system has advantages with its inclusion of culture‐distinctive categories and the exclusion of irrelevant Western diagnostic categories (Lee 2001). The differences between local and global psychiatric classifications causes some questions about diagnosis and assessment (Lee 2009). We are reasonably confident, however, that those entering these trials would be widely recognised as suffering from schizophrenia and even if 10% of diagnoses were inaccurate, this may reflect the real world more closely than if all diagnoses were entirely accurate.
We found that different criteria for outcomes of dichotomous data from global state and mental state made it difficult to merge data and analyse. We do think there is a strong argument for rationalising outcome measures (COMET).
2. Different treatments and controls
2.1 Acupuncture methods
With the development of acupuncture and modern medical technology, there are many categories of acupuncture. In this update review we found six acupuncture categories ‐ traditional acupuncture (nine studies), electroacupuncture (12 studies), acupoint injection (three studies), laser acupuncture (three studies), acupoint catgut treatment (two studies) and another special category electric acupuncture convulsive therapy (one study). The mechanisms of action of any of these techniques still could not be explained. Even with the same category of acupuncture there are various choices for acupoints, frequencies and durations (Table 9). It remains impossible to pre‐judge clinical or physiological heterogeneity ‐ or homogeneity ‐ of different categories of acupuncture.
3. Summary of acupuncture methods.
| Study | Kind of acupuncture | Acupoints choice | Fequency and duration | Sham acupuncture (yes/no) |
| traditional ‐ Bouhlel 2011 | Traditional acupuncture | Local points, distal points diagnosed by TCM | Once for 20 minutes and 3 times a week; total 10 times | Yes |
| traditional ‐ Liu 2010 | Main acupoints and adjunct acupoints according to the TCM syndrome differentiation | Manipulated needles every 10 minutes; for 30 minutes; 4 to 5 times a week and no less than 4 times; 1 month as 1 treatment course, 3 treatment course | No | |
| traditional ‐ Luo 2006 | Two acupoint groups in turn | For 30 minutes; once a day; 20 days as a treatment course and began another treatment course after 10 non‐treatment days; 3 months treatment courses | No | |
| traditional ‐ Ma 2008 | Juque, Tanzhong, Taichong, Jianshi, Fenglong, Daling, Yingtang | For 30 minutes; once a day; 5 days treatment with 2 days non‐treatment interval; 6 weeks | No | |
| traditional ‐ Tang 2005 | According to the type of TCM | Manipulated needles every 10 minutes; for 30 minutes; 3 to 4 times a week and not less than 3 times; 3 weeks as a treatment course and 1 week interval; total 3 treatment courses | No | |
| traditional ‐ Wang 2006 | Taichong (reducing method), Hegu (reducing method), Neiguan (straight inserted), Daling (straight inserted), Renzhong (reducing method), Dazhui (straight inserted) + other acupoints choice according to type of TCM | Continued to manipulate needles 3 to 5 minutes every ten minutes; for 45 minutes; once a day; 15 times as a treatment course; 2 treatment courses | No | |
| traditional ‐ Xu 2004 | Main acupoints and adjunct acupoints (according to the TCM type of schizophrenia) and special acupoints (according to symptoms) | Manipulated needles about 3 minutes and once ten minutes; total for about 30 minutes; at first once a day then reduced to once every two days when patient was in stable condition and once a week after psychiatric symptoms disappeared; 80 days | No | |
| traditional ‐ Zhao 2005a | Shuigou, Shaoshang, Yingbai, Fengfu, Daling, Quchi, Fenglong | Once a day; for 30 minutes; 60 days | No | |
| traditional ‐ Zhao 2005b | Xinshu, Ganshu, Pishu, Shenmen, Fenglong | Once a day; for 30 minutes; 60 days | No | |
| electro ‐ Chen 2006 | Electroacupuncture | Baihui and Yingtang | For 50 minutes, once a day, 6 weeks | No |
| electro ‐ Chen 2008 | Two acupoints groups in turn ‐ Baihui and Neiguan (double) group or Shuigou and Sanyingjiao (double) group | For 45 minutes; once a day; five days a week (except Saterday and Sunday); 12 weeks as a treatment course | No | |
| electro ‐ Cheng 2009 | Tinggong, Tinghui, Yifeng, Daling, Neiguan and Sanyijiao | For 20 minutes; 5 times a week; total 30 times (6 weeks) | Yes; needles were inserted less than 5 mm superficially and about 20 mm away from each corresponding acupoint and electroacupuncture connected was with no electrical current for 20 minutes | |
| electro ‐ Cui 2000 | According to different symptoms | For 30 minutes; once two days; 20 times as a treatment course | No | |
| electro ‐ Ding 2005 | Tanzhong, Zhongwan, Shenmen, Fenglong (double), Taichong (double), Neiguan (double) | For 5 minutes; once two days | No | |
| electro ‐ Wang 2005 | Baihui, Shangxing, Yingtang, Sanyingjiao (double), Neiguan (double); added Zhongwan and Zusanli when with gastrointestinal uncomfortable symptoms and added Fenglong when with symptoms of the type of stagnation of phlegm and Qi | For 45 minutes; 5 times a week; 20 times as a treatment course; 8 weeks | No | |
| electro ‐ Xiong 2010 | Baihui and Taiyang (double) | Firstly continued to stimulate strongly 3 to 4 seconds then reduced the strength and frequency quickly, after patient's breath and face returned to normal and continued 30 to 60 seconds stimulated again; continued to stimulate 8 to 10 times; 3 times a week, total 8 weeks | No | |
| electro ‐ Yao 2006 | Baihui, Fenglong, Houxi, Ganshu | For 30 minutes; once a day; 20 to 30 times as a treatment course; total 2 treatment courses | No | |
| electro ‐ Zhang 1987 | Yifeng, Tinggong, Tounjie, Chengling, Linqi, Baihui, Dingshen | Twice a day; adjustment of treatment depended upon the condition of the patient; 20 days | No | |
| electro ‐ Zhang 1993 | Group one [Yingtang and Baihui] and group two [Shenting and Yamen] | For 45 minutes; once a day; 8 weeks as a treatment course | No | |
| electro ‐ Zhang 2001 | Baihui and Yingtang | For 45 minutes; once a day; 5 days a week except Saturday and Sunday; 6 weeks as a treatment course | No | |
| electro ‐ Zhou 1997 | According to TCM types; main acupoints (Yintang tou xinqu, Daling, Neiguan, Taiyang) and supplemental acupoints (Zusanli [Yang deficiency] | Once a day except Sunday; 36 times as a treatment course (6 weeks) | No | |
| acupoint inj ‐ Pan 2002 | Acupoint injection | According to the type of TCM (with Salviae Miltiorrhizae) | Two side acupoints in turn; once a day; 10 days as a treatment course; 7 days interval between two courses; 3 treatment courses | No |
| acupoint inj ‐ Wang 2000 | Tinggong (double) (with Clonazepam) | Once two days; 7 times | No | |
| acupoint inj ‐ Yang 2000 | Tinggong (double) (Sulpiride) | Once a day; 5 times as a treatment course; 2 intermittent treatment courses each month; total half a year | No | |
| laser ‐ Liu 1986 | Laser acupuncture | Ermen (double) (He‐Ne laser irradiation) | For 15 minutes; once a day except Sunday; 30 times as a treatment course | Yes; sham laser irradiation; the tube of the laser emitter pointed in the direction of the ear without real irradiation |
| laser ‐ Ma 1999 | Tinggong and Tinghui (He‐Ne laser irradiation) | For 30 minutes; once a day except Sunday | No | |
| laser ‐ Zhang 1991 | Two acupoint groups were used every other day alternately ‐ group 1 (Dazhui and Shenting); group 2 (Taiyang [double]) | For 15 minutes; once a day (except Sunday); 5 weeks as a treatment course | Yes; needles fixed with tape on acupoints | |
| acupoint cat ‐ Sun 2005 | Acupoint catgut treatment | Tinggong | Once 7‐10 days; 6 weeks as a treatment course | No |
| acupoint cat ‐ Wang 1997 | Tinggong (double) | 10 days as a treatment course; total 3 treatment courses | No | |
| EACT ‐ Xue 1987 | Electric acupuncture convulsive therapy | Renzhong and Baihui | Once every two days; 12 times as a treatment course | No |
2.2 Placebo methods
In this update we found traditional ‐ Bouhlel 2011 used sham traditional acupuncture, electro ‐ Cheng 2009 used sham electroacupuncture, laser ‐ Liu 1986 used sham laser irradiation and laser ‐ Zhang 1991 sham laser acupuncture. Placebo methods may reduce participants' subjectivity but it is difficult to use, especially in China where many have previous experience of acupuncture and may well know the difference between real and pseudo‐acupuncture (Li 2009; Yang 2009).
3. COMPARISON 1: ACUPUNCTURE added to STANDARD DOSE ANTIPSYCHOTICS versus STANDARD DOSE ANTIPSYCHOTICS
Please see Table 1.
3.1 Global state
Although different criteria were used to rate 'not improved' all three to 12 month studies favoured the group into which acupuncture was added. The quality of data is not good but if, by this simple addition of acupuncture, even a proportion of the effect seen in these trials was real, that would be clinically important. This finding alone merits further testing.
3.2 Mental state
These trials presented 12 different ways of recording mental state. There were some exceptions and some heterogeneous results, however, essentially, the data favoured the acupuncture group. Most of the measures indicated a modest improvement derived from trials at moderate risk of bias, but findings that were statistically if not clinically significant. These data are certainly consistent with the 'global state' outcomes above and also merit further work.
3.3 Behaviour
Attrition was remarkably low. Such high rates of retention are unusual for trials involving people with schizophrenia and probably reflect the differences in approaches to care and the care culture, rather than the treatment interventions, however, the approach and care culture of China, in which these trials were conducted applies to at least 20% of the global population. Acupuncture trials of this sort are clearly are possible in China. More, larger and clearer studies are needed.
3.4 Service outcome
Only one of the 30 included studies reported this important outcome. Acupuncture added to standard dose antipsychotics did reduce average hospitalisation days compared with standard dose antipsychotics alone. Again, this is consistent with the above results and because it was a study with 120 participants of low quality attempts should be made to replicate this finding.
3.5 Adverse effects
The wide‐ranging Treatment Emergent Symptom Scale (TESS) scores were lower for those given acupuncture added to standard dose antipsychotic treatment. These results are based on a study size of only 90 participants (electro ‐ Yao 2006), but, nevertheless, the general impression remains that acupuncture is not associated with noticeable adverse effects and also that it does little for any existing adverse effects of the medications.
4. COMPARISON 2: ACUPUNCTURE added to LOW DOSAGE ANTIPSYCHOTICS versus STANDARD DOSE ANTIPSYCHOTICS
Please see Table 2. The low dose antipsychotic was chlorpromazine at less than 300 mg/day, risperidone 1 to 2 mg/d, clozapine 100 to 150 mg/d or, a reduction of about 60% of previously daily levels.
4.1 Global state
Relapse, the primary outcome of this review, was only reported by acupoint inj ‐ Pan 2002. Acupuncture added to low dose antipsychotics did reduce the number of participants experiencing relapse compared with standard dose antipsychotics (n = 170, 1 RCT, RR 0.57 CI 0.37 to 0.89). The study followed up relapse for two years but the result is questionable because of high risk of bias and incomplete outcome data. The outcome of 'no clinically important change' in global state did not show a benefit for acupuncture group and a similar result was found for the short‐term CGI‐SI. It is, therefore, difficult to be conclusive.
4.2 Mental state
Rather weak and sometimes heterogeneous data suggest that the additional acupuncture did not really make much difference to participant's mental state. This impression remains even when we removed traditional ‐ Wang 2006. the study that caused high heterogeneity.
4.3 Behaviour
Again, very, very few people left these studies (˜2%) indicating the different circumstances in which these trials were undertaken compared to the studies of Western medicine. Just because this indicates that the studies were different to that seen in the West does not mean the findings are inapplicable. Conversely, it may reflect that people did not feel empowered to leave, and that, perhaps with that dis‐empowerment may come results of limited veracity.
4.4 Adverse effects
We found TESS endpoint score and RESES endpoint score were lower for people given acupuncture ‐ but the RESES endpoint score was calculated according to the assumption of The Cochrane Handbook or Systematic Reviews of Interventions and could incorporate even more error than other data. Data for all other adverse effects are limited and not entirely convincing. It is not clear that the reduction of antipsychotic paired with acupuncture is helpful for a wide range of adverse effects.
5. COMPARISON 3: ACUPUNCTURE versus STANDARD DOSE ANTIPSYCHOTICS
Please see Table 3.
5.1 Global state
The criteria for clinically important change of 'not improved' in global state was not standardised and findings were not consistent. The two trials using the same criteria found in favour of the acupuncture but the other three, employing different measures, were equivocal. This is surprising to those of us who are used to using standard dose antipsychotic drugs. Trials involving nearly 400 people indicate that acupuncture is as potent as antipsychotics ‐ or more so. All studies are limited by their power and quality and high‐grade replication is important.
5.2 Mental state
BPRS score at short‐term based on two small studies, traditional ‐ Wang 2006 and laser ‐ Zhang 1991. Heterogeneity exists between traditional acupuncture and laser acupuncture subgroup and data from each study drew different conclusions. Findings tend to favour acupuncture for positive symptoms and neither group for negative. Again, if a real finding, these results are similar to those that are found for trials of new antipsychotic drugs. They do tend to be biased in favour of the experimental treatment, in this case acupuncture, but that does not mean that there is a real effect.
5.3 Behaviour
Again, very high retention rates highlight the difference between these trials and what is seen in most of the rest of the world.
5.4 Adverse effects
For this comparison adverse effect data were limited. As with the other comparisons there is no clear indication of adverse effects of acupuncture, although one study reported the laser acupuncture group experienced numbness over the ear, upper extremity and chest on the treated side.
6. COMPARISON 4: ACUPUNCTURE added to TCM DRUG versus TCM DRUG
Please see Table 4.
6.1 Global state
When comparing traditional acupuncture plus capsule with capsule alone there was significant difference in global state (n = 360, 2 RCTs, RR 0.11 CI 0.02 to 0.59) but the opposite result if comparing electroacupuncture plus decoction with decoction alone (n = 94, 1 RCT, RR 0.77 CI 0.57 to 1.06). We have not really had the power to investigate the different types of acupuncture but this finding indicates that the traditional (Figure 1) may well have advantages over the electroacupuncture technique, also that the TCM drug may have little to offer, in itself, for the treatment of people with schizophrenia (Rathbone 2005b).
6.2 Behaviour
Similar to the above comparisons ‐ no participants left early. This is unusually high compliance compared to trials completed in the Western world.
7. COMPARISON 5: ACUPUNCTURE versus TCM DRUG
Please see Table 5.
7.1 Global state
Overall, there was no difference in the rates of not improved in global state, however, different approaches were used as well as different definitions of outcome. Two studies with the same criteria indicated that there was no significant difference between traditional acupuncture group and TCM drug group for one definition of 'not improved' (n = 360, 2 RCTs, RR 1.46 CI 0.74 to 2.87) but when another definition was employed a different result was found (worsening in global state, n = 88, 1 RCT, RR 0.52 CI 0.34 to 0.80, Analysis 5.1). The answer is clearly not stable and should be further investigated.
7.2 Behaviour
No participants dropped out from either the acupuncture or TCM drug group. Low attrition probably refects, at least in part, that both acupuncture and Chinese herb medicine originate from China, have a long and trusted history, and these studies were undertaken in China.
8. COMPARISON 6: ELECTRIC ACUPUNCTURE CONVULSIVE THERAPY versus ELECTROCONVULSIVE THERAPY
Please see Table 6.
8.1 Behaviour
The only relevant study (n = 68) (EACT ‐ Xue 1987), undertaken more than 20 years ago, reported no participants leaving within the 24 days of treatment. The short treatment period and a special approach to care may part of the reason for this.
8.2 Adverse effects
Electric acupuncture convulsive therapy reduced spinal fracture rate but did not reduce back pain compared with electroconvulsive therapy. Both convulsive therapies were given without anaesthetic. We still can not draw firm conclusion from weak, and hopefully, outdated evidence.
9. Missing outcomes
Even for Chinese medicine, which includes acupuncture, and has been used to treat schizophrenia‐like illnesses for over 2000 years, we found no data relating to death, engagement with services, satisfaction with treatment, quality of life or economic outcomes (details in Table 10).
4. Missing outcomes of each comparison.
| Comparison | Missing outcomes |
| ACUPUNCTURE added to STANDARD DOSE ANTIPSYCHOTICS versus STANDARD DOSE ANTIPSYCHOTICS | Death, engagement with services, satisfaction with treatment, quality of life, economic outcomes |
| ACUPUNCTURE added to LOW DOSE ANTIPSYCHOTICS versus STANDARD DOSE ANTIPSYCHOTICS | Death, service outcomes, engagement with services, satisfaction with treatment, quality of life, economic outcomes |
| ACUPUNCTURE versus STANDARD ANTIPSYCHOTICS | Death, service outcomes, engagement with services, satisfaction with treatment, quality of life, economic outcomes |
| ACUPUNCTURE added to TCM DRUG versus TCM DRUG | Death, mental state, service outcomes, adverse effects, engagement with services, satisfaction with treatment, quality of life, economic outcomes |
| ACUPUNCURE versus TCM DRUG | Death, mental state, service outcomes, adverse effects, engagement with services, satisfaction with treatment, quality of life, economic outcomes |
| ELECTRIC ACUPUCNTURE CONVULSIVE THERAPY versus ELECTRIC ACUPUNCTURE CONVULSIVE THERAPY | Death, global state, mental state, service outcomes, engagement with services, satisfaction with treatment, quality of life, economic outcomes |
Overall completeness and applicability of evidence
1. Completeness
Thirty studies were included in this update review with more than 2500 participants. We compared acupuncture added to antipsychotic drugs (regular dosage or low dosage) with standard dose antipsychotics; acupuncture plus TCM drug with TCM drug alone; acupuncture with antipsychotic drugs or TCM drug; and also electric acupuncture convulsive therapy with electroconvulsive therapy. We found many outcomes were missing such as death, engagement with services, satisfaction with treatment, quality of life or economic outcomes. Most missing outcomes are participant‐oriented data.
Only one study reported our primary outcome, relapse, and even that was incomplete. One study reported a service outcome, time in hospital, which was not the pre‐stated outcome of our review. This situation is unlikely to change until there is more wide agreement about the need for specific and clinically important outcomes.
2. Applicability
The 30 included studies were published between 1986 and 2011 and almost exclusively completed in China where trial attrition is unusually low compared to the Western world. Also, although the great majority of people patients with schizophrenia are in the community most of included studies were undertaken in hospital settings from which attrition may be more difficult. Any findings of this review may be more applicable to people in hospital in China.
Quality of the evidence
Overall, the quality was not strong (Figure 3). Most studies did not adhere to the CONSORT statement; two‐thirds of the included studies did not describe how they undertook the randomisation procedure and one sixth reported incomplete outcome data. Schizophrenia is a chronic illness but most studies reported short‐term data. We have tried to be generous in our judgements regarding quality and there is a risk that we, in doing this, have downplayed a real risk of bias in this group of studies and therefore in this review.
Potential biases in the review process
Please see above (Quality of the evidence). Otherwise, we used thorough search strategies and strictly followed the review protocol in the process of study selection, data extraction and analysis. We did employ only published reports and could not entirely avoid the potential for publishing bias for negative results and small studies.
Problems continue to exist for some of the included studies (acupoint inj ‐ Pan 2002; electro ‐ Zhang 1993). We found that data from Wu 2004 were extremely similar to those of acupoint inj ‐ Pan 2002, with the exception of numbers in each group. The acupoint method and the BPRS's factor data were very similar and we were unable to gain further details about Wu 2004 and had to exclude that study in its entirety as a result.
Agreements and disagreements with other studies or reviews
1. Previous version of this Cochrane review
This review updates and improves the previous version (Rathbone 2005a). We have changed the style of the Study ID tag to be more informative, using acupuncture's category plus author's surname plus publication date. The current version now includes 30 studies, whereas the previous version included only five.
The previous Cochrane review had considered two papers reporting two different studies; now electro ‐ Zhang 1993 is the same as study 'Zhang 1994' from the previous version because we found an additional relevant reference (Zhang 1993). We changed 'Gang 1997' of the previous version to 'electro ‐ Zhou 1997'.
2. Other reviews
We identified two other acupuncture for schizophrenia reviews (Lee 2009; Nicholas 1997). The latter concluded that the results provided limited evidence overall and that the low quality studies did not allow any firm conclusions to be drawn and that international studies are needed to test whether there is any real effect. We agree with this view.
Authors' conclusions
Implications for practice.
1. For people with schizophrenia
Acupuncture has been used for treating schizophrenia‐like illnesses for over 2000 years. Though acupuncture as a complementary treatment has become more popular in the West (Zhang 2012), 29 of the 30 included studies were undertaken in China and the findings are more appropriate to a Chinese population. For this update, new studies were found to compare acupuncture added to a Traditional Chinese Medicine (TCM) drug with TCM drug, acupuncture with TCM drug, electric acupuncture convulsive therapy with electroconvulsive therapy and we separately analysed acupuncture added to standard dose antipsychotics versus standard dose antipsychotics or acupuncture adde to low dose antipsychotics versus standard dose antipsychotics. The limited evidence suggests that combining acupuncture with standard dose antipsychotics might benefit the individual's mental state especially in the short‐term. Acupuncture appears to have some benefit related to relapse when added to low dose antipsychotics compared to standard dose antipsychotics along with a reduction of drug‐related adverse effects.
2. For clinicians
Adopting acupuncture to treat schizophrenia needs co‐operation between psychiatrists and acupuncturists. Few data were available making the comparisons between acupuncture and antipsychotics, between acupuncture added to TCM drug and TCM, between acupuncture and TCM drug, and between electric acupuncture convulsive therapy and electroconvulsive therapy limited. Acupuncture treatment effects consists of three effects ‐ special effects, non‐special effects and psychological effects (Yang 2012). Without enough evidence, we could not draw conclusions if the effect of acupuncture when combining with antipsychotics is a placebo response. Without enough evidence we remain confused about the interaction between acupuncture and low dosage antipsychotic medication. Placebo and recognised effective drug‐control in three‐arm trials need to be conducted to provide strong evidence.
It is surprising that most of the studies used the Treatment Emergent Symptom Scale (TESS) or Rating Scale for Extrapyramidal Side Effects (RESES) to assess adverse effects, but few focused on acupuncture's relative adverse effects such as skin allergies. Though acupuncture has a long history, this intervention is still without a special acupuncture‐relative adverse effects scale. Adverse effects of acupuncture for schizophrenia are inadequate.
3. For managers of policy makers
Acupuncture as a valuable complementary medicine is gradually affecting the global medical treatment system. Only one study reported service outcomes and indicated that using acupuncture added to standard dose antipsychotics shorten hospitalisation days than using standard dose antipsychotics alone. This traditional treatment originating from ancient Chinese culture needs more real‐word trials to evaluate the treatment method objectively and completely, not limited by assessing treatment effects and adverse effects but also focusing on social and economic conditions.
Implications for research.
1. General
All studies should now comply with the Consolidated Standards of Reporting Trials (CONSORT, Moher 2001). More transparency in the reporting of randomised controlled trials, would enable readers to understand the design, conduct, analysis and interpretation, and to assess the validity of results. Although binary data is easier to interpret, where continuous data are used, some measure of variance should be provided. Data presented in graphs should be accompanied by exact numbers and standard deviations in the text.
2. Specific
1. Reviews
There are further refinements of this review that could take place with better inclusion of additional data (Table 7, Table 8) and possibility of additional comparisons (Table 11). This review is already large and, in the future, it may be best to split this work into separate reviews per comparison with an 'overview' review.
5. Other comparisons of relevance to this systematic review.
| Comparison | Trial |
| Different acupoints | Ma 2002; electro ‐ Zhang 1987 |
| Acupoints vs non‐acupoints | laser ‐ Liu 1986 |
2. Trials
In 2010, the STRICTA Group, CONSORT Group and the Chinese Cochrane Centre collaborated to develop STRICA (MacPherson 2010) to ensure that acupuncture trials are more accurately interpreted and more easily replicated. Further research is essential to inform both clinicians and patients about the effects of acupuncture in the treatment of schizophrenia. Trial methodology should follow both CONSORT (Moher 2001) and STRICTA guidelines.
Acupuncture is an important intervention that is likely to be widely used, at least, in China. Considering the limited data in this review, we do think that further large simple trials are indicated. This update highlights the need for standardised clinical trials, which evaluate acupuncture for people with schizophrenia, which thoroughly investigate effects of different categories of acupuncture, which investigate interaction in combination regimens, with trialists employing appropriate real‐world methodology. We suggest such a design in Table 12.
6. Suggested design for a trial.
| Methods | Allocation: randomised, clearly described, concealed.
Blindness: double, described and tested.
Duration: 4 week washout period + 24 weeks treatment period. Follow‐up: 2 years. |
| Participants | Diagnosis: schizophrenia (DSM V) with one TCM type according to TCM diagnosis standard. N = 300*. Age: any. Sex: both. History: duration of schizophrenia over 1 years; never receive acupuncture previously** (at least). |
| Interventions | 1. Electroacupuncture: N = 150.
2. Sham electroacupuncture: N = 150. The acupoints and relative parameters clearly described. |
| Outcomes | 1. Death 2. Global state 3. Mental state 4. Behaviour 5. Service outcomes 6. Adverse effects 7. Engagement with service 8. Satisfaction with treatment 9. Quality of life 10. Economic outcome |
| Notes | * Powered to be able to identify a difference of ˜20% between groups for primary outcome with adequate degree of certainty. ** This is to maximise blinding. |
There are, however, specific issues to consider.
2.1 Standardisation of acupuncture
We included 30 studies and five categories of acupuncture according to different stimulation methods and one according to special acupuncture theory application method. The majority used different acupoints and practice procedures. Although acupuncture is an important part of TCM and has the typical characteristics of this approach ‐ individualised treatment ‐ to objectively evaluate the effects of acupuncture standardisation of technique would be useful.
2.2 Blinding method and sham acupuncture
Human behaviour is influenced by what we know or believe and it is a particular risk of human healthcare research. The use of blinding methods makes it more difficult to bias results intentionally or unintentionally and helps ensure credibility of study conclusions (Simon 2000). Eight studies adapted a blinding method but only four used sham acupuncture. Though appropriate sham acupuncture is a key factor for acupuncture trials, it can also be a barrier for the trialist (Li 2009). More than 20 studies in this review probably have some degree of performance bias and only seven used blinded assessors. As acupuncture is an externally applied treatment to which special theory is associated, patient‐blinding (sham acupuncture) may be difficult, but assessor‐blinding is not so problematic. At the very least, to minimise subjectivity, assessor‐blinding does need to be practiced in further clinical trials.
2.3 Control group
A placebo‐controlled (appropriate sham acupuncture) trial is beneficial to confirm absolute effects of acupuncture. We did not find any study of this comparison. In this version of review we reported results of:
acupuncture added to standard dose antipsychotics versus standard dose antipsychotics;
acupuncture added to low dose antipsychotics versus standard dose antipsychotics;
acupuncture versus standard dose antipsychotics;
acupuncture added to TCM drug versus TCM drug;
acupuncture versus TCM drug; and a special comparison
electric acupuncture convulsive therapy versus electroconvulsive therapy.
Some of the control group treatments are definitely effective (such as antipsychotics; Rattehalli 2010; Adams 2007), but others may be more like a placebo group (e.g. TCM drug ‐ Rathbone 2005b). To evaluate the effects of acupuncture choosing recognised valid treatment as the control group would be beneficial to obtain high‐quality globally applicable evidence.
What's new
| Date | Event | Description |
|---|---|---|
| 19 August 2014 | New citation required but conclusions have not changed | Results from new studies have not significantly changed overall conclusions from previous versions of this review. |
| 17 January 2013 | New search has been performed | New search carried out Feburary 2012, 30 studies now included in the review. |
History
Protocol first published: Issue 4, 2005 Review first published: Issue 4, 2005
| Date | Event | Description |
|---|---|---|
| 7 December 2011 | Amended | Contact details updated. |
| 10 November 2010 | Amended | Contact details updated. |
| 13 November 2008 | Amended | CSG Data Extraction Tag added |
| 23 April 2008 | Amended | Converted to new review format. |
| 18 May 2006 | Amended | Minor update |
| 5 July 2005 | New citation required and conclusions have changed | Substantive amendment |
| 28 June 2005 | New citation required and conclusions have changed | Conclusions changed in light of update. |
| 20 May 2005 | New search has been performed | New studies found and included or excluded. Ammended in light of peer review comments. |
Acknowledgements
This review was initiated by John Rathbone who wrote the protocol, selected studies, data extracted, assimilated data and wrote the final first report (Rathbone 2005a). Jun Xia helped select studies, data extract, translate text and contact for that first version.
Many thanks to Samantha Roberts for the 2012 trials search. We are most grateful for the ongoing support of Claire Irving at the Cochrane Schizophrenia Group's editorial base.
The Cochrane Schizophrenia group provide a standard template for their methods section and other parts of the protocol. We have used this template and adapted it accordingly.
Authors Yuyin Pan, Cheng Luo, Guimei Cui, Jing Cheng and Professor Xueguang Bai were kind enough to respond to our telephone or e‐mail inquiries, for which we are very grateful.
Appendices
Appendix 1. Previous type of interventions (2005)
1. Acupuncture/electro‐acupuncture with or without moxibustion (a Traditional Chinese Medicine technique that involves the burning of mugwort to facilitate healing) or laser treatment, administered solely or in conjunction with antipsychotic drugs. 2. Placebo (sham acupuncture) or no treatment. 3. Antipsychotic drugs produced by pharmaceutical companies: any compound, dose, pattern or means of administration.
Appendix 2. Previous search strategy (2005)
1. Electronic searches We searched the Cochrane Schizophrenia Group's Trials Register (April 2005) using the phrase: [(*acup* OR *moxibustion*) in REFERENCE and (*acupuncture* OR *moxibustion*) in STUDY]
This register is compiled by systematic searches of major databases (Biological Abstracts CINAHL, The Cochrane Library, EMBASE, MEDLINE, RUSSMED, LILACS, PSYNDEX and PsycLIT) hand searches and conference proceedings (see Group Module).
2. We also inspected the references of all identified studies (included and excluded) for further relevant trials.
Appendix 3. Previous data collection and analysis methods (2005)
1. Selection of studies We (JR, JX) inspected all reports of studies identified from the search. A randomly selected sample of 10% of all the reports were re‐inspected in order to ensure that the selection was reliable. Where disagreement occurred, we resolved this by discussion and if there was still doubt we acquired the full article for further inspection. Once the full articles were obtained, we independently decided whether or not they met the review criteria. Again, where disagreement occurred, we resolved this by discussion and when this was not possible we sought further information from first authors and added these trials to the list of those awaiting assessment.
2. Assessment of methodological quality We allocated trials to three quality categories, as described in the Cochrane Collaboration Handbook (Alderson 2004). When disputes arose as to which category a trial was allocated, again, we attempted resolution by discussion. When this was not possible and further information was necessary to clarify into which category to allocate the trial, we did not enter data but allocated the trial to the list of those awaiting assessment. We only included trials in Category A or B in the review.
A. Low risk of bias (adequate allocation concealment) B. Moderate risk of bias (some doubt about the results) C. High risk of bias (inadequate allocation concealment). For the purpose of the analysis in this review, trials were included if they met the Cochrane Handbook criteria A or B.
3. Data extraction 3.1 Reliable extraction We independently extracted data from selected trials. When disputes arose, we attempted resolution by discussion. When this was not possible and further information was necessary to resolve the dilemma, we did not enter data but added this outcome of the trial to the list of those awaiting assessment.
3.2 Intention to treat analysis We excluded data from studies where more than 50% of participants in any group were lost to follow‐up, except for the outcome of 'leaving the study early'. In studies with less than 50% dropout rate, everyone allocated to the intervention was counted whether or not they completed follow‐up. We considered those leaving early to have had the negative outcome, except for the event of death and adverse effects.
Where attrition rates were high (25% to 50%), we analysed the impact of including this type of data in a sensitivity analysis. If inclusion of high attrition data resulted in a substantive change in the estimate of effect, then we did not pool this data, but presented the data separately.
4. Data analysis 4.1 Dichotomous/binary data In the review we used relative risk (RR), (fixed‐effect) and 95% confidence interval (CI). Where possible, we made efforts to convert relevant categorical or continuous outcome measures to dichotomous data by identifying cut off points on rating scales and dividing them into groups accordingly i.e.. 'moderate or severe impairment' and 'no better or worse'.
4.1.1 Summary statistic: For binary outcomes we calculated a standard estimate of the relative risk (RR) and its 95% confidence intervals (CI) (fixed‐effect). Where possible, we estimated the number needed to treat (NNT) using an on‐line calculator (http://www.nntonline.net/). If heterogeneity was found (see section 5) we used a random‐effects model.
4.2 Continuous data 4.2.1 Skewed data: continuous data on clinical and social outcomes are often not normally distributed. To avoid the pitfall of applying parametric tests to non‐parametric data, the following standards are applied to all data before inclusion: (a) standard deviations and means were reported in the paper or were obtainable from the authors; (b) when a scale started from the finite number zero, the standard deviation, when multiplied by two, was less than the mean (as otherwise the mean was unlikely to be an appropriate measure of the centre of the distribution, (Altman 1996); (c) if a scale started from a positive value (such as PANSS which can have values from 30‐210) the calculation described above in (b) was modified to take the scale starting point into account. In these cases skewness is present if 2SD>(S‐Smin), where S is the mean score and Smin is the minimum score. Endpoint scores on scales often have a finite start and end point and these rules can be applied to them.
4.2.2 Summary statistic: For continuous outcomes we calculated weighted mean differences (WMD) and respective 95% CI (fixed‐effect). If heterogeneity was found (see section 5) we used a random‐effects model.
4.2.3 Valid scales: A wide range of instruments are available to measure mental health outcomes. These instruments vary in quality and it has been shown that the use of rating scales which have not been described in a peer‐reviewed journal (Marshall 2000) are associated with bias, or may not be valid, or even ad hoc. Therefore, some minimum standards were set: (a) the psychometric properties of the instrument should have been described in a peer‐reviewed journal; (b) the instrument should either be a self‐report, or completed by an independent rater or relative (not the therapist); and (c) the instrument should be a global assessment of an area of functioning.
4.2.4 Endpoint versus change data: where possible, we presented endpoint data and if both endpoint and change data were available for the same outcomes then we only reported the former in this review.
4.2.5 Cluster trials Studies increasingly employ 'cluster randomisation' (such as randomisation by clinician or practice) but analysis and pooling of clustered data poses problems. Firstly, authors often fail to account for intra class correlation in clustered studies, leading to a 'unit of analysis' error (Divine 1992) whereby P values are spuriously low, confidence intervals unduly narrow and statistical significance overestimated. This causes type I errors (Bland 1997, Gulliford 1999).
Where clustering was not accounted for in primary studies, we presented the data in a table, with a (*) symbol to indicate the presence of a probable unit of analysis error. In subsequent versions of this review we will seek to contact first authors of studies to obtain intra‐class correlation co‐efficients of their clustered data and to adjust for this using accepted methods (Gulliford 1999). Where clustering has been incorporated into the analysis of primary studies, we will also present these data as if from a non‐cluster randomised study, but adjusted for the clustering effect.
We have sought statistical advice and have been advised that the binary data as presented in a report should be divided by a 'design effect'. This is calculated using the mean number of participants per cluster (m) and the intraclass correlation co‐efficient (ICC) [Design effect = 1+(m‐1)*ICC] (Donner 2002). If the ICC was not reported it was assumed to be 0.1 (Ukoumunne 1999).
5. Test for heterogeneity Firstly, we considered all of the included studies within any comparison to judge clinical heterogeneity. Then we visually inspected graphs used to investigate the possibility of statistical heterogeneity and supplemented this by using, primarily, the I‐squared statistic. This provides an estimate of the percentage of variability due to heterogeneity rather than chance alone. Where the I‐squared estimate was greater than or equal to 75%, we interpreted this as indicating the presence of high levels of heterogeneity (Higgins 2003). If inconsistency was high, we did not summate the data, but presented it separately and reasons for heterogeneity were investigated.
6. Addressing publication bias We entered all data from the included studies into a funnel graph (trial effect against trial size) in an attempt to investigate the likelihood of overt publication bias (Egger 1997).
Appendix 4. Previous description of studies
1. Excluded studies We excluded three studies. Xue 1985 and Zhuge 1993 were not randomised. Zhong 1995 compared electro‐acupuncture to computerised electrode.
2. Awaiting assessment No studies are awaiting assessment.
3. Ongoing studies We are not aware of any ongoing studies.
4. Included studies We included five studies all of which were randomised. Only electro ‐ Zhou 1997 reported on blinding, in which the raters were blind to treatment allocation.
4.1 Length of trials All included studies were short‐term (less than three months). The shortest study electro ‐ Zhang 1987 lasted for 20 days. laser ‐ Zhang 1991 was for five weeks, electro ‐ Zhou 1997 and electro ‐ Zhang 2001 were six week studies and Zhang 1994 was the longest study lasting eight weeks.
4.2 Participants All participants were diagnosed with schizophrenia. electro ‐ Zhou 1997, laser ‐ Zhang 1991 and electro ‐ Zhang 2001 used operationalised criteria (DSM/ICD/CCMD). electro ‐ Zhang 1987, Zhang 1994 did not report using predefined diagnostic criteria. electro ‐ Zhou 1997, electro ‐ Zhang 1987, laser ‐ Zhang 1991 and electro ‐ Zhang 2001 used male and female participants, whilst Zhang 1994 did not report on gender.
4.3 Setting All five trials were undertaken in a hospital setting.
4.4 Study size The numbers of participants randomised ranged between 31 laser ‐ Zhang 1991 to 88 electro ‐ Zhang 1987. Forty people were randomised by electro ‐ Zhou 1997, 69 by Zhang 1994 and 42 by electro ‐ Zhang 2001.
4.5 Interventions The treatment groups received acupuncture exclusively or in combination with antipsychotics. All the comparator groups received antipsychotics. electro ‐ Zhou 1997 used electro‐acupuncture in combination with reduced dose antipsychotics versus chlorpromazine equivalents (˜560mg/day). The treatment group in electro ‐ Zhang 1987 received electro‐acupuncture and the comparator group were given chlorpromazine (300‐600 mg/day). laser ‐ Zhang 1991 randomised people into three comparator groups, laser‐acupuncture with moxibustion, laser acupuncture with moxibustion and reduced dose chlorpromazine (150‐300 mg/day), or chlorpromazine (350‐600 mg/day). Zhang 1994 compared electro‐acupuncture to chlorpromazine equivalents (˜458 mg/day). electro ‐ Zhang 2001 compared electro‐acupuncture combined with antipsychotics to antipsychotics alone but did not report on the acupoints used or dosages. Details of acupuncture points, needles and electro‐acupuncture frequencies used (when reported) are listed in the included studies table.
4.6 Outcomes All data outcomes were reported as short‐term (less than three months). Data from some studies were unusable because raw scores were not presented. Instead outcomes were reported as P values without means and standard deviations being provided, or the scale used was not identified. electro ‐ Zhang 2001 predefined no clinical improvement as HAMD scores reduced by </ = 25%. electro ‐ Zhou 1997 predefined no clinical improvement as BPRS scores reduced by </ = 20%.
4.6.1 Outcome scales: details of scales that provided usable data are shown below. Reasons for exclusion of data from instruments are given under 'outcomes' in the included studies table.
4.6.1.1 Global state scales 4.6.1.1.1 Clinical Global Impression Scale ‐ CGI (Guy 1970) The CGI is a three‐item scale commonly used in studies on schizophrenia that enables clinicians to quantify severity of illness and overall clinical improvement. The items are: severity of illness, global improvement and efficacy index. A seven‐point scoring system is usually used with low scores indicating decreased severity and/or greater recovery. electro ‐ Zhou 1997 and laser ‐ Zhang 1991 reported CGI data.
4.6.1.2 Mental state 4.6.1.2.1 Brief Psychiatric Rating Scale ‐ BPRS (Overall 1962) The BPRS is an 18‐item scale measuring positive symptoms, general psychopathology and affective symptoms. The original scale has sixteen items, but a revised eighteen‐item scale is commonly used. Scores can range from 0 ‐126. Each item is rated on a seven‐point scale, with high scores indicating more severe symptoms. electro ‐ Zhou 1997, laser ‐ Zhang 1991and Zhang 1994 all reported BPRS data.
4.6.1.2.2 Scale for the Assessment of Negative Symptoms ‐ SANS (Andreasen 1982) This scale allows a global rating of the following negative symptoms: alogia (impoverished thinking), affective blunting, avolition‐apathy, anhedonia‐asociality and attention impairment. Assessments are made on a six‐point scale (0 = not at all to 5 = severe). Higher scores indicate more symptoms. Data for this scale were reported by Zhang 1994.
4.6.1.2.3 Scale for the Assessment of Positive Symptoms ‐ SAPS (Andreasen 1982) This six‐point scale gives a global rating of positive symptoms such as delusions, hallucinations and disordered thinking. Higher scores indicate more symptoms. Zhang 1994 was the only study to report SAPS data.
4.6.1.2.4 Hamilton Rating Scale for Depression ‐ HAMD (Hamilton 1967) This instrument is designed to be used only on patients already diagnosed as suffering from affective disorder of the depressive type. It is used for quantifying the results of an interview, and its value depends entirely on the skill of the interviewer in eliciting the necessary information. The scale contains 17 variables measured on either a five‐point or a three‐point rating scale, the latter being used where quantification of the variable is either difficult or impossible. Among the variables are: depressed mood, suicide, work and loss of interest, retardation, agitation, gastro‐intestinal symptoms, general somatic symptoms, hypochondriasis, loss of insight, and loss of weight. It is useful to have two raters independently scoring a patient at the same interview. The scores of the patient are obtained by summing the scores of the two physicians. A score of 11 is generally regarded as indicative of a diagnosis of mild depression, 14‐17 mild to moderate depression and >17 moderate to severe depression. electro ‐ Zhang 2001 reported data for this scale.
4.6.1.2.5 Zung Depression Scale ‐ ZDS (Zung 1965) The Zung Self‐Rating Depression Scale is a 20‐item self‐rated scale that is widely used as a screening tool, covering affective, psychological and somatic symptoms associated with depression. The questionnaire takes approximately ten minutes to complete and items are framed in terms of positive and negative statements. It can be effectively used in a variety of settings, including primary care, psychiatric clinics, drug trials and various research situations. Each item is scored on a Likert scale ranging from one to four. Most people with depression score between 50 and 69, while a score of 70 and above indicates severe depression. electro ‐ Zhang 2001 reported data from this scale.
4.6.1.3 Adverse events 4.6.1.3.1 Treatment Emergent Symptom Scale/Form ‐ TESS/F (Guy 1976) This checklist assesses a variety of characteristics for each adverse event, including severity, relationship to the drug, temporal characteristics (timing after a dose, duration and pattern during the day), contributing factors, course and action taken to counteract the effect. Symptoms can be listed a priori or can be recorded as observed by the investigator. Zhang 1994 reported data for this scale.
Appendix 5. Previous effects of interventions
1. The search The electronic search identified 18 reports. We were able to include five trials and we added three trials to the excluded studies table.
2. COMPARISON 1. ACUPUNCTURE versus ANTIPSYCHOTICS
2.1. Global state Two studies electro ‐ Zhang 1987 and laser ‐ Zhang 1991 reported on global state (not improved) with equivocal results.
2.2. Leaving the study early There were no losses to follow‐up in either group from two trials electro ‐ Zhang 1987 and laser ‐ Zhang 1991 by five weeks.
2.3. Adverse effects Extrapyramidal symptoms reported by laser ‐ Zhang 1991 were lower in the acupuncture group with no participants experiencing this adverse effect. The control group had significantly higher numbers with eight out of ten people reported as having extrapyramidal symptoms (n = 21, RR 0.05 CI 0.0 to 0.8, NNT 2 CI 2 to 8).
3. COMPARISON 2. ACUPUNCTURE + ANTIPSYCHOTICS versus ANTIPSYCHOTICS
3.1. Global state One study laser ‐ Zhang 1991 reported global state (not improved at five weeks) with equivocal results. Clinical Global Impression (severity of illness) scores were reported by electro ‐ Zhou 1997 with equivocal scores. electro ‐ Zhou 1997 also reported on Clinical Global Impression (global improvement) but data were skewed.
3.2. Leaving the study early Four trials (laser ‐ Zhang 1991, Zhang 1994, electro ‐ Zhou 1997, electro ‐ Zhang 2001) reported as short‐term outcomes that no participants left the study early (up to eight weeks).
3.3. Mental state Dichotomised BPRS data were reported by electro ‐ Zhou 1997 (not improved </ = 20% reduction at endpoint) with equivocal results. Continuous BPRS data (Zhang 1994, electro ‐ Zhou 1997) were significant in favour of the acupuncture group (n = 109, WMD ‐4.31 CI ‐7.0 to ‐1.6). Mental state SANS and SAPS score were reported by Zhang 1994, but data were skewed and cannot be displayed graphically. Depression scores from the HAMD scale were reported by electro ‐ Zhang 2001 with scores from this smaller study being significantly lower in the acupuncture group (n = 42, WMD ‐10.41 CI ‐12.8 to ‐8.0). When the HAMD scores were dichotomised to 'not improved', again, by electro ‐ Zhang 2001 results significantly favoured the acupuncture group (n = 42, RR 0.17 CI 0.1 to 0.5, NNT 2 CI 2 to 3). electro ‐ Zhang 2001 also reported on depression using the Zung Depression Scale with results at five weeks significantly favouring the acupuncture group (n = 42, WMD ‐24.25 CI ‐28.0 to ‐20.5).
3.4. Adverse effects electro ‐ Zhou 1997 reported TESS scores at six weeks, with results significantly favouring the acupuncture group (n = 40, WMD ‐0.50 CI ‐0.9 to ‐0.1). laser ‐ Zhang 1991 found the incidences of extrapyramidal symptoms to be similar for both treatment groups.
Data and analyses
Comparison 1. ACUPUNCTURE added to STANDARD DOSE ANTIPSYCHOTICS versus STANDARD DOSE ANTIPSYCHOTICS.
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
|---|---|---|---|---|
| 1 Global state: Not improved, endpoint | 4 | Risk Ratio (M‐H, Fixed, 95% CI) | Subtotals only | |
| 1.1 medium‐term (various similar criteria) | 3 | 244 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.40 [0.28, 0.57] |
| 1.2 duration unclear | 1 | 120 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.2 [0.01, 4.08] |
| 2 Mental state: 1a. General ‐ average score (BPRS, endpoint, high score = worse) | 5 | Mean Difference (IV, Fixed, 95% CI) | Subtotals only | |
| 2.1 short‐term | 4 | 327 | Mean Difference (IV, Fixed, 95% CI) | ‐4.32 [‐5.28, ‐3.36] |
| 2.2 medium‐term | 3 | 220 | Mean Difference (IV, Fixed, 95% CI) | ‐5.51 [‐6.71, ‐4.30] |
| 3 Mental state: 1b. General ‐ average score (BPRS, endpoint, high score = worse, medium‐term) ‐ subgroup analysis | 3 | Mean Difference (IV, Random, 95% CI) | Subtotals only | |
| 3.1 traditional acupuncture | 2 | 156 | Mean Difference (IV, Random, 95% CI) | ‐7.95 [‐14.29, ‐1.61] |
| 3.2 acupoint injection | 1 | 64 | Mean Difference (IV, Random, 95% CI) | ‐0.70 [‐5.02, 3.62] |
| 4 Mental state: 2a. General ‐ average score (PANSS, endpoint, high score = worse) | 4 | Mean Difference (IV, Fixed, 95% CI) | Subtotals only | |
| 4.1 short‐term | 4 | 245 | Mean Difference (IV, Fixed, 95% CI) | ‐2.47 [‐4.31, ‐0.63] |
| 4.2 medium‐term | 2 | 135 | Mean Difference (IV, Fixed, 95% CI) | ‐3.79 [‐6.43, ‐1.15] |
| 5 Mental state: 2b. General ‐ average score (PANSS, endpoint, high score = worse, short‐term) ‐ subgroup analysis | 4 | Mean Difference (IV, Random, 95% CI) | Subtotals only | |
| 5.1 traditional acupuncture | 1 | 60 | Mean Difference (IV, Random, 95% CI) | 1.30 [‐1.56, 4.16] |
| 5.2 electroacupuncture | 3 | 185 | Mean Difference (IV, Random, 95% CI) | ‐5.64 [‐10.93, ‐0.35] |
| 6 Mental state: 2c. General ‐ average score (PANSS, endpoint, high score = worse, short‐term) ‐ electroacupuncture subgroup analysis | 2 | 135 | Mean Difference (IV, Fixed, 95% CI) | ‐3.79 [‐6.43, ‐1.15] |
| 7 Mental state: 2d. General ‐ average score (PANSS, endpoint, high score = worse) ‐ Skewed data | Other data | No numeric data | ||
| 7.1 short‐term | Other data | No numeric data | ||
| 7.2 medium‐term | Other data | No numeric data | ||
| 8 Mental state: 2e. General ‐ not improved (PANSS), endpoint | 4 | Risk Ratio (M‐H, Fixed, 95% CI) | Subtotals only | |
| 8.1 reduced rate < 25%, short‐term | 3 | 197 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.65 [0.45, 0.94] |
| 8.2 reduced rate < 30%, short‐term | 1 | 90 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.91 [0.43, 1.92] |
| 8.3 reduced rate < 30%, follow‐up, medium‐term | 1 | 90 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.69 [0.36, 1.31] |
| 9 Mental state: 3. Specific ‐ average score ‐ positive symptoms (SAPS, endpoint, high score = worse, short‐term) ‐ Skewed data | Other data | No numeric data | ||
| 10 Mental state: 4a.Specific ‐ average score ‐ negative symptoms (SANS, endpoint, high score = worse) | 1 | Mean Difference (IV, Fixed, 95% CI) | Subtotals only | |
| 10.1 short‐term | 1 | 60 | Mean Difference (IV, Fixed, 95% CI) | ‐7.66 [‐13.05, ‐2.27] |
| 10.2 medium‐term | 1 | 60 | Mean Difference (IV, Fixed, 95% CI) | ‐12.35 [‐17.54, ‐7.16] |
| 11 Mental state: 4b. Specific ‐ average score ‐ negative symptoms (SANS, endpoint, high score = worse, short‐term) ‐ Skewed data | Other data | No numeric data | ||
| 11.1 traditional acupuncture | Other data | No numeric data | ||
| 11.2 electroacupuncture | Other data | No numeric data | ||
| 12 Mental state: 5a. Specific ‐ average score ‐ depression (HAMD, endpoint, high score = worse, short‐term) | 2 | 109 | Mean Difference (IV, Random, 95% CI) | ‐8.66 [‐12.10, ‐5.22] |
| 13 Mental state: 5b. Specific ‐ not improved ‐ depression (HAMD, reduced rate < 25%, short‐term) | 2 | 109 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.17 [0.08, 0.34] |
| 14 Mental state: 7b. Specific ‐ not improved ‐ auditory hallucinations (PSYRAS‐AH, reduction < 20%, short‐term) | 1 | 60 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.27 [0.14, 0.52] |
| 15 Mental state: 7a. Specific ‐ average score ‐ auditory hallucinations (PSYRAS‐AH,endpoint, high score = worse, short‐term) | 1 | 60 | Mean Difference (IV, Fixed, 95% CI) | ‐2.17 [‐4.16, ‐0.18] |
| 16 Mental state: 6. Specific ‐ average score ‐ depression (SDS, endpoint, high score = worse, short‐term) | 1 | 42 | Mean Difference (IV, Fixed, 95% CI) | ‐24.25 [‐28.01, ‐20.49] |
| 17 Mental state: 8. Specific ‐ not improved (auditory hallucinations, endpoint) | 3 | Risk Ratio (M‐H, Fixed, 95% CI) | Subtotals only | |
| 17.1 still existed after 30 days, short‐term | 1 | 216 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.24 [0.13, 0.44] |
| 17.2 still existed or appeared frequently after disappeared, medium‐term | 1 | 64 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.32 [0.17, 0.61] |
| 17.3 no change of auditory hallucinations, duration unclear | 1 | 120 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.25 [0.03, 2.17] |
| 18 Mental state: 9. Specific ‐ average score ‐ auditory hallucinations (SAHS, endpoint, high score = worse) ‐ Skewed data | Other data | No numeric data | ||
| 18.1 short‐term | Other data | No numeric data | ||
| 18.2 medium‐term | Other data | No numeric data | ||
| 19 Mental state: 10. Specific ‐ average score ‐ hallucinations (BPRS [12th item], endpoint, high score = worse, short‐term) | 1 | 90 | Mean Difference (IV, Fixed, 95% CI) | ‐0.73 [‐1.28, ‐0.18] |
| 20 Behaviour: Leaving the study early | 15 | Risk Ratio (M‐H, Fixed, 95% CI) | Subtotals only | |
| 20.1 short‐term | 10 | 870 | Risk Ratio (M‐H, Fixed, 95% CI) | 1.33 [0.33, 5.45] |
| 20.2 medium‐term | 5 | 370 | Risk Ratio (M‐H, Fixed, 95% CI) | 3.58 [0.60, 21.27] |
| 21 Service outcomes: Time in hospital (days) | 1 | 120 | Mean Difference (IV, Fixed, 95% CI) | ‐16.0 [‐19.54, ‐12.46] |
| 22 Adverse effects: 1. General ‐ average score (TESS, endpoint, high score = worse, short‐term) | 1 | 90 | Mean Difference (IV, Fixed, 95% CI) | ‐2.80 [‐3.09, ‐2.51] |
| 23 Adverse effects: 2a. Specific ‐ extrapyramidal symptoms | 5 | Risk Ratio (M‐H, Fixed, 95% CI) | Subtotals only | |
| 23.1 short‐term ‐ overall | 3 | 202 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.66 [0.40, 1.09] |
| 23.2 short‐term ‐ specific ‐ akathisia | 1 | 75 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.58 [0.23, 1.48] |
| 23.3 short‐term ‐ specific ‐ tremor | 1 | 75 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.73 [0.24, 2.18] |
| 23.4 medium‐term ‐ overall | 2 | 156 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.62 [0.32, 1.23] |
| 23.5 medium‐term ‐ specific ‐ myotonia | 1 | 60 | Risk Ratio (M‐H, Fixed, 95% CI) | 1.0 [0.07, 15.26] |
| 23.6 medium‐term ‐ specific ‐ tremor | 1 | 60 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.67 [0.12, 3.71] |
| 23.7 medium‐term ‐ specific ‐ akathisia | 1 | 60 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.6 [0.16, 2.29] |
| 24 Adverse effects: 2b. Specific ‐ extrapyramidal symptoms ‐overall (short‐term) ‐ subgroup analysis | 3 | 202 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.66 [0.40, 1.09] |
| 24.1 traditional acupuncture | 1 | 60 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.17 [0.02, 1.30] |
| 24.2 electroacupuncture | 2 | 142 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.79 [0.47, 1.34] |
| 25 Adverse effects: 3. Specific ‐ Central Nervous System | 5 | Risk Ratio (M‐H, Fixed, 95% CI) | Subtotals only | |
| 25.1 medium‐term ‐ anxiety | 1 | 96 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.42 [0.09, 2.05] |
| 25.2 short‐term ‐ insomnia | 3 | 202 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.30 [0.11, 0.83] |
| 25.3 medium‐term ‐ insomnia | 2 | 156 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.34 [0.12, 1.02] |
| 25.4 medium‐term ‐ headache | 1 | 60 | Risk Ratio (M‐H, Fixed, 95% CI) | 3.0 [0.33, 27.23] |
| 26 Adverse effects: 4. Specific ‐ anticholinergic symptoms | 5 | Risk Ratio (M‐H, Fixed, 95% CI) | Subtotals only | |
| 26.1 short‐term ‐ dry mouth | 3 | 202 | Risk Ratio (M‐H, Fixed, 95% CI) | 1.18 [0.47, 3.00] |
| 26.2 short‐term ‐ blurred vision | 3 | 202 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.88 [0.24, 3.24] |
| 26.3 medium‐term ‐ blurred vision | 2 | 156 | Risk Ratio (M‐H, Fixed, 95% CI) | 1.0 [0.15, 6.64] |
| 26.4 medium‐term ‐ sweating | 2 | 156 | Risk Ratio (M‐H, Fixed, 95% CI) | 3.0 [0.13, 70.83] |
| 26.5 short‐term ‐ constipation | 3 | 202 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.88 [0.19, 4.06] |
| 26.6 short‐term ‐ nausea & vomiting | 3 | 202 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.41 [0.12, 1.32] |
| 27 Adverse effects: 5. Specific ‐ gastrointestinal system | 4 | Risk Ratio (M‐H, Fixed, 95% CI) | Subtotals only | |
| 27.1 medium‐term ‐ unspecified gastrointestinal symptoms | 1 | 96 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.52 [0.10, 2.71] |
| 27.2 short‐term ‐ constipation | 3 | 202 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.88 [0.19, 4.06] |
| 27.3 short‐term ‐ nausea & vomiting | 3 | 202 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.41 [0.12, 1.32] |
| 28 Adverse effects: 6. Specific ‐ cardiovascular symptoms (or headache) | 6 | Risk Ratio (M‐H, Fixed, 95% CI) | Subtotals only | |
| 28.1 short‐term ‐ dizziness | 3 | 202 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.94 [0.32, 2.75] |
| 28.2 medium‐term ‐ dizziness or headache | 1 | 96 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.35 [0.04, 3.22] |
| 28.3 short‐term ‐ tachycardia | 3 | 217 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.87 [0.42, 1.79] |
| 28.4 medium‐term ‐ tachycardia | 2 | 156 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.34 [0.04, 3.20] |
| 29 Adverse effects: 7a. Specific ‐ metabolic system | 4 | Risk Ratio (M‐H, Random, 95% CI) | Subtotals only | |
| 29.1 short‐term ‐ weight gain | 3 | 202 | Risk Ratio (M‐H, Random, 95% CI) | 0.94 [0.02, 37.33] |
| 29.2 medium‐term ‐ weight gain | 1 | 96 | Risk Ratio (M‐H, Random, 95% CI) | 0.21 [0.01, 4.23] |
| 30 Adverse effects: 7b. Specific ‐ metabolic system ‐ weight gain (short‐term) ‐ subgroup analysis | 3 | Risk Ratio (M‐H, Random, 95% CI) | Subtotals only | |
| 30.1 traditional acupuncture | 1 | 60 | Risk Ratio (M‐H, Random, 95% CI) | 0.14 [0.01, 2.65] |
| 30.2 electroacupuncture | 2 | 142 | Risk Ratio (M‐H, Random, 95% CI) | 6.15 [0.33, 115.01] |
| 31 Adverse effects: 8. Specific ‐ endocrine system | 2 | Risk Ratio (M‐H, Fixed, 95% CI) | Subtotals only | |
| 31.1 short‐term ‐ irregular menstruation | 2 | 127 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.33 [0.01, 7.87] |
| 32 Adverse effects: 9. Specific ‐ lab test | 5 | Risk Ratio (M‐H, Fixed, 95% CI) | Subtotals only | |
| 32.1 short‐term ‐ liver function abnormal | 4 | 292 | Risk Ratio (M‐H, Fixed, 95% CI) | 1.01 [0.26, 3.90] |
| 32.2 medium‐term ‐ liver function abnormal | 1 | 96 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.70 [0.12, 3.98] |
| 32.3 short‐term ‐ ECG abnormal (myocardial ischaemia) | 4 | 292 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.5 [0.05, 5.32] |
| 32.4 short‐term ‐ blood routine test abnormal (leukocyte change) | 4 | 292 | Risk Ratio (M‐H, Fixed, 95% CI) | 1.33 [0.32, 5.62] |
Comparison 2. ACUPUNCTURE added to LOW DOSE ANTIPSYCHOTICS versus STANDARD DOSE ANTIPSYCHOTICS.
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
|---|---|---|---|---|
| 1 Global state: 1. Relapse (follow‐up, long‐term) | 1 | 170 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.57 [0.37, 0.89] |
| 2 Global state: 2. Not improved (various similar criteria), endpoint (short‐term) | 4 | 272 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.83 [0.40, 1.72] |
| 2.1 no symptoms improved obviously and insight did not recover | 1 | 32 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.2 [0.01, 3.86] |
| 2.2 symptoms and orientation showed no change | 1 | 40 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.9 [0.30, 2.68] |
| 2.3 according to traditional criteria | 2 | 200 | Risk Ratio (M‐H, Fixed, 95% CI) | 1.03 [0.35, 3.02] |
| 3 Global state: 3a. CGI ‐ average score ‐ CGI‐SI (endpoint, high score = worse, short‐term) | 1 | 40 | Mean Difference (IV, Fixed, 95% CI) | ‐0.40 [‐1.08, 0.28] |
| 4 Global state: 3b. CGI ‐ average score ‐ CGI‐GI (endpoint, high score = worse, short‐term) ‐ Skewed data | Other data | No numeric data | ||
| 5 Global state: 3c. CGI ‐ average score ‐ CGI‐EI (endpoint, high score = worse, short‐term) ‐ Skewed data | Other data | No numeric data | ||
| 6 Mental state: 1a. General ‐ average score (BPRS, endpoint, high score = worse) | 5 | Mean Difference (IV, Random, 95% CI) | Subtotals only | |
| 6.1 short‐term | 5 | 332 | Mean Difference (IV, Random, 95% CI) | ‐5.55 [‐14.40, 3.29] |
| 6.2 long‐term | 1 | 137 | Mean Difference (IV, Random, 95% CI) | ‐4.87 [‐8.21, ‐1.53] |
| 7 Mental state: 1b. General ‐ average score (BPRS, endpoint, high score = worse, short‐term) ‐ subgroup analysis | 4 | 300 | Mean Difference (IV, Fixed, 95% CI) | ‐1.36 [‐3.13, 0.41] |
| 7.1 traditional acupuncture | 1 | 80 | Mean Difference (IV, Fixed, 95% CI) | 3.03 [‐1.22, 7.28] |
| 7.2 electroacupuncture | 1 | 40 | Mean Difference (IV, Fixed, 95% CI) | ‐4.5 [‐9.35, 0.35] |
| 7.3 acupoint injection | 1 | 160 | Mean Difference (IV, Fixed, 95% CI) | ‐1.87 [‐4.04, 0.30] |
| 7.4 laser acupuncture | 1 | 20 | Mean Difference (IV, Fixed, 95% CI) | ‐1.5 [‐12.40, 9.40] |
| 8 Mental state: 1c. General ‐ not improved (BPRS, short‐term) | 3 | Risk Ratio (M‐H, Fixed, 95% CI) | Subtotals only | |
| 8.1 1.1 reduced rate < 30% | 1 | 60 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.75 [0.18, 3.07] |
| 8.2 1.2 reduced rate < 25% | 1 | 170 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.62 [0.31, 1.25] |
| 8.3 1.3 reduced rate ≤ 20% | 1 | 40 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.9 [0.30, 2.68] |
| 9 Mental state: 1d. General ‐ average change scores (BPRS, low score = worse, short‐term) | 1 | 60 | Mean Difference (IV, Fixed, 95% CI) | 0.81 [‐3.17, 4.79] |
| 10 Mental state: 2a. General ‐ Average score (PANSS, endpoint, high score = worse, short‐term) ‐ Skew data | Other data | No numeric data | ||
| 11 Mental state: 2b. General ‐ not improved (PANSS, reduced rate < 30%, short‐term) | 1 | 80 | Risk Ratio (M‐H, Fixed, 95% CI) | 1.22 [0.57, 2.62] |
| 12 Mental state: 3a. Specific ‐ average score ‐ positive symptoms (SAPS, endpoint, high score = worse, short‐term) | 2 | Mean Difference (IV, Random, 95% CI) | Subtotals only | |
| 12.1 traditional acupuncture | 1 | 32 | Mean Difference (IV, Random, 95% CI) | ‐13.34 [‐16.94, ‐9.74] |
| 12.2 acupoint catgut treatment | 1 | 180 | Mean Difference (IV, Random, 95% CI) | 1.21 [0.96, 1.46] |
| 13 Mental state: 3b. Specific ‐ average score ‐ positive symptoms (SAPS, endpoint, high score = worse, short‐term) ‐ Skewed data | Other data | No numeric data | ||
| 14 Mental state: 4a. Specific ‐ average score ‐ negative symptoms (SANS, endpoint, high score = worse, short‐term) | 1 | 80 | Mean Difference (IV, Fixed, 95% CI) | 0.61 [‐3.30, 4.52] |
| 15 Mental state: 4b. Specific ‐ average score ‐ negative symptoms (SANS, endpoint, high score = worse, short‐term) ‐ Skewed data | Other data | No numeric data | ||
| 16 Behaviour: Leaving the study early (short‐term) | 8 | 662 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.81 [0.29, 2.29] |
| 17 Adverse effects: 1a. General ‐ average score (TESS, endpoint, high score = worse, short‐term) | 2 | 200 | Mean Difference (IV, Fixed, 95% CI) | ‐0.56 [‐0.86, ‐0.26] |
| 18 Adverse effects: 1b. General ‐ average score (TESS, endpoint, high score = worse, short‐term) ‐ Skewed data | Other data | No numeric data | ||
| 19 Adverse effects: 2. Specific ‐ average score (RESES, endpoint, high score = worse, short‐term) | 1 | 20 | Mean Difference (IV, Fixed, 95% CI) | ‐0.6 [‐0.73, ‐0.47] |
| 20 Adverse effects: 3. Specific ‐ extrapyramidal symptoms (short‐term) | 3 | Risk Ratio (M‐H, Fixed, 95% CI) | Subtotals only | |
| 20.1 overall | 3 | 260 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.27 [0.16, 0.46] |
| 20.2 specific ‐ myotonia | 1 | 180 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.21 [0.01, 4.29] |
| 20.3 specific ‐ tremor | 1 | 180 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.09 [0.01, 1.69] |
| 20.4 specific ‐ akathisia | 1 | 180 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.03 [0.00, 0.49] |
| 21 Adverse effects: 4. Specific ‐ Central Nervous System (short‐term) | 2 | Risk Ratio (M‐H, Fixed, 95% CI) | Subtotals only | |
| 21.1 activity increasing | 1 | 180 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.15 [0.01, 2.85] |
| 21.2 insomnia | 1 | 180 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.07 [0.00, 1.20] |
| 21.3 sleepiness | 2 | 240 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.33 [0.04, 3.03] |
| 22 Adverse effects: 5. Specific ‐ anticholinergic symptoms (short‐term) | 2 | Risk Ratio (M‐H, Fixed, 95% CI) | Subtotals only | |
| 22.1 dry mouth | 2 | 240 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.22 [0.09, 0.58] |
| 22.2 nasal congestion | 1 | 180 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.09 [0.01, 1.69] |
| 22.3 blurred vision | 2 | 240 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.22 [0.06, 0.83] |
| 22.4 constipation | 1 | 180 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.07 [0.00, 1.20] |
| 23 Adverse effects: 6. Specific ‐ cardiovascular symptoms (short‐term) | 2 | Risk Ratio (M‐H, Fixed, 95% CI) | Subtotals only | |
| 23.1 dizziness | 1 | 180 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.70 [0.12, 4.07] |
| 23.2 tachycardia | 2 | 240 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.25 [0.11, 0.53] |
| 24 Adverse effects: 7. Specific ‐ skin infection (short‐term) | 1 | 180 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.0 [0.0, 0.0] |
Comparison 3. ACUPUNCTURE versus STANDARD DOSE ANTIPSYCHOTICS.
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
|---|---|---|---|---|
| 1 Global state: Not improved, endpoint (short‐term) | 5 | Risk Ratio (M‐H, Fixed, 95% CI) | Subtotals only | |
| 1.1 various similar criteria | 3 | 141 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.88 [0.53, 1.48] |
| 1.2 talk, behaviour and expression still existed as before + reduction of BPRS < 29% | 2 | 240 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.33 [0.19, 0.59] |
| 2 Mental state: 1. General ‐ average score (BPRS, endpoint, high score = worse, short‐term) | 2 | Mean Difference (IV, Random, 95% CI) | Subtotals only | |
| 2.1 traditional acupuncture | 1 | 32 | Mean Difference (IV, Random, 95% CI) | ‐11.56 [‐16.36, ‐6.76] |
| 2.2 laser acupuncture | 1 | 21 | Mean Difference (IV, Random, 95% CI) | ‐1.07 [‐11.19, 9.05] |
| 3 Mental state: 2. Specific ‐ average score ‐ positive symptoms (SAPS, endpoint, high score = worse, short‐term) | 1 | 32 | Mean Difference (IV, Fixed, 95% CI) | ‐5.24 [‐9.06, ‐1.42] |
| 4 Mental state: 3. Specific ‐ average score ‐ negative symptoms (SANS, endpoint, high score = worse, short‐term) | 1 | 32 | Mean Difference (IV, Fixed, 95% CI) | ‐7.92 [‐17.01, 1.17] |
| 5 Behaviour: Leaving the study early (short‐term) | 6 | 421 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.0 [0.0, 0.0] |
| 6 Adverse effects: 1. General ‐ average scores (TESS, endpoint, short‐term) ‐ Skewed data | Other data | No numeric data | ||
| 7 Adverse effects: 2. Specific ‐ extrapyramidal symptoms (short‐term) | 1 | 21 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.05 [0.00, 0.83] |
| 8 Adverse effects: 3. Specific ‐ numbness over the ear, upper extremity and chest on the treated side (short‐term) | 1 | 40 | Risk Ratio (M‐H, Fixed, 95% CI) | 3.0 [0.13, 69.52] |
Comparison 4. ACUPUNCTURE added to TCM DRUG versus TCM DRUG.
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
|---|---|---|---|---|
| 1 Global state: Not improved, endpoint (short‐term) | 3 | Risk Ratio (M‐H, Fixed, 95% CI) | Subtotals only | |
| 1.1 talk, behaviour and expression still existed as before + reduction of BPRS < 29% | 2 | 360 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.11 [0.02, 0.59] |
| 1.2 no change in symptoms | 1 | 94 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.77 [0.57, 1.06] |
| 2 Behaviour: Leaving the study early (short‐term) | 3 | 454 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.0 [0.0, 0.0] |
Comparison 5. ACUPUNCTURE versus TCM DRUG.
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
|---|---|---|---|---|
| 1 Global state: Not improved, endpoint (short‐term) | 3 | Risk Ratio (M‐H, Fixed, 95% CI) | Subtotals only | |
| 1.1 talk, behaviour and expression still existed as before + reduction of BPRS < 29% | 2 | 360 | Risk Ratio (M‐H, Fixed, 95% CI) | 1.46 [0.74, 2.87] |
| 1.2 no change in symptoms | 1 | 88 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.52 [0.34, 0.80] |
| 2 Behaviour: Leaving the study early (short‐term) | 3 | 328 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.0 [0.0, 0.0] |
Comparison 6. ELECTRIC ACUPUNCTURE CONVULSIVE THERAPY versus ELECTROCONVULSIVE THERAPY.
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
|---|---|---|---|---|
| 1 Behaviour: Leaving the study early (short‐term) | 1 | 68 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.0 [0.0, 0.0] |
| 2 Adverse effects: 1. Specific ‐ back pain (short‐term) | 1 | 68 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.67 [0.12, 3.74] |
| 3 Adverse effects: 2. Spinal fracture (short‐term) | 1 | 68 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.33 [0.14, 0.81] |
Characteristics of studies
Characteristics of included studies [ordered by study ID]
acupoint cat ‐ Sun 2005.
| Methods | Allocation: randomised. Blindness: not reported. Duration: 6 weeks. | |
| Participants | Diagnosis: schizophrenia with auditory hallucinations (CCMD‐3).
N = 180.
Age: mean age (28.10 ± 6.90) years (catgut treatment + low dose risperidone group); (30.40 ± 10.72) years (risperidone group).
Sex: 86 women and 94 men. History: average duration of illness (5.85 ± 4.80) months (acupoint catgut treatment + low dose risperidone group); (4.92 ± 3.69) years (risperidone group). Setting: hospitalised patients. Country: China. Inclusion criteria: had a CCMD‐3 diagnosis of schizophrenia; the duration of illness more than 3 months and auditory hallucinations continued longer than 1 month; never received any antipsychotics or washout period was longer than 1 week; age between 18 and 60 years. Exclusion criteria: elderly and frail; pregnant women; severe somatic diseases; cerebral organic diseases. |
|
| Interventions | 1. Acupoint catgut treatment + low dose risperidone: acupoint catgut treatment (Tinggong; opened mouth; once 7‐10 days; 6 weeks as a treatment course) + low dose risperidone (1 to 2 mg/d; average dose (1.38 ± 0.52) mg/d). N = 88. 2. Risperidone: initial dose 1 mg, adjusted the dose according to treatment courses and side effects and achieved to 4 to 10 mg/d within 2 weeks; average dose (5.95 ± 1.76) mg/d. N = 92. Combination therapy: could add artane, propranolol and benzodiazepine drugs and could not received any other antipsychotics. |
|
| Outcomes | Global state: no clinically important change in global state1. Mental state2: PANSS; SAPS. Behaviour: leaving the study early. Adverse effects: TESS; skin infection. Unable to use: Adverse effects: lab test (kidney function; blood sodium; blood chloride; blood glucose; stool routine test) (reported no impact in both groups but no data). Lab test: EEG (not clinical outcome). |
|
| Notes | 1. Assessment criteria (according to traditional criteria): recovery; marked improvement; improvement; no effect. 2. The ratings were assessed before treatment and, 1 week after treatment, 2 weeks after treatment, 4 weeks after treatment and 6 weeks after treatment. |
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Report stated ‐ "using random sampling". |
| Allocation concealment (selection bias) | Unclear risk | Not stated. |
| Blinding of participants and personnel (performance bias) All outcomes | High risk | Not used sham acupoint catgut treatment. |
| Blinding of outcome assessment (detection bias) All outcomes | Unclear risk | Not stated. |
| Incomplete outcome data (attrition bias) Outcomes | Low risk | No participants left the study early. |
| Selective reporting (reporting bias) | Unclear risk | We were unable to locate the protocol, unsure if selective reporting occurred. |
| Other bias | Unclear risk | This study was supported by the Health Department of Shanxi Province. |
acupoint cat ‐ Wang 1997.
| Methods | Allocation: randomised. Blindness: not reported. Duration: 3 treatment courses. | |
| Participants | Diagnosis: schizophrenia with auditory hallucinations (CCMD‐2‐R).
N = 216. Age: between 15 and 60 years (mean age (36.4 ± 2.1) years) (acupoint catgut treatment + antipsychotics group); between 20 and 58 years (mean age (35.7 ± 1.2) years) (antipsychotics group). Sex: 109 women and 107 men. History: duration of illness between 1 and 8 years (average duration 2.3 years) (acupoint catgut treatment + antipsychotics group); between 0.6 and 9.0 years (average duration 2.1 years) (antipsychotics group). Setting: hospitalised patients. Country: China. Inclusion criteria: had a CCMD‐2‐R diagnosis of schizophrenia; auditory hallucinations still existed after 8‐weeks treatment with equivalent chlorpromazine dose 400 to 600 mg/d. |
|
| Interventions | 1. Acupoint catgut treatment + antipsychotics: acupoint catgut treatment (Tinggong [double]; opened mouth; 10 days as a treatment course; total 3 treatment courses) + antipsychotics (no further details). N = 108. 2. Antipsychotics: no further details. N = 108. |
|
| Outcomes | Mental state: no clinically important change in specific symptoms (auditory hallucinations)1. Behaviour: leaving the study early. Unable to use: Adverse effects: local pain when eating (existed in acupoint catgut treatment + antipsychotics group but no data). |
|
| Notes | 1. Assessment criteria: marked improvement (auditory hallucinations disappeared within 10 days); improvement (auditory hallucinations disappeared within 20 days); no effect (auditory hallucinations still existed after 30 days). 2. The equivalent chlorpromazine dose of two compared groups was no difference. |
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | Report stated ‐ "randomly divided into treatment group and control group according to admission order". No further details. |
| Allocation concealment (selection bias) | Unclear risk | Not stated. |
| Blinding of participants and personnel (performance bias) All outcomes | Unclear risk | Not used; sham acupoint catgut treatment. |
| Blinding of outcome assessment (detection bias) All outcomes | Unclear risk | Not stated. |
| Incomplete outcome data (attrition bias) Outcomes | Low risk | No participants left the study early. |
| Selective reporting (reporting bias) | Unclear risk | We were unable to locate the protocol, unsure if selective reporting occurred. |
| Other bias | Low risk | Not obvious. |
acupoint inj ‐ Pan 2002.
| Methods | Allocation: randomised.
Blindness: assessor blind.
Duration: 1 week washout period and 3 treatment courses. Follow‐up: 2 years. |
|
| Participants | Diagnosis: schizophrenia (CCMD‐2‐R).
N = 170.
Age1: between 16 and 67 years (mean age (32.24 ± 10.40) years) (acupoint injection + chlorpromazine group); between 17 and 60 years (mean age (32.07 ± 10.17) years) (chlorpromazine group). History1: duration of illness between 3 and 240 months (average duration (54.72 ± 58.75) months) (acupoint injection + chlorpromazine group); between 3 and 304 months (average duration (65.81 ± 67.98) months) (chlorpromazine group). Sex: women and men. Setting: hospitalised patients. Country: China. Inclusion criteria: had a CCMD‐2‐R diagnosis of schizophrenia; BPRS > 36. Exclusion criteria: severe somatic and haemorrhagic diseases; acupuncture syncope and had aggressive behaviour. Follow‐up: participants with effective treatment. N = 144/170. Age: between 16 and 60 years (mean age (31.59 ± 10.28) years(acupoint injection + chlorpromazine group); between 17 and 59 years (mean age (30.79 ± 9.61) years) (chlorpromazine group). Sex: 43 women and 101 men. History: duration of illness between 3 and 232 months (average duration (55.87 ± 59.76) months (acupoint injection + chlorpromazine group); between 3 and 301 months (average duration (53.02 ± 58.32) months) (chlorpromazine group). |
|
| Interventions | 1. Acupoint injection + low dose chlorpromazine: acupoint injection* (Salviae Miltiorrhizae; acupoints choice according to the type of TCM**) + low dose chlorpromazine (Wintermin; less than 300 mg/d; average dose (253.92 ± 42.25) mg/d1). N = 105. 2. Chlorpromazine: Wintermin; over 400 mg/d; average dose was (465.29 ± 72.92) mg/d1. N = 65. Combination therapy: dose could be adjusted within the provision range or added anticholinergic drugs when adverse reactions took place; could add benzodiazepine drugs when needed. Follow‐up: 1. Acupoint injection + chlorpromazine: Wintermin; maintained the same antipsychotics; average dose (149 ± 55) mg/d. N = 92/105. 2. Chlorpromazine: Wintermin; maintained the same antipsychotics; average dose (160 ± 58) mg/d. N = 52/65. Combination therapy: the same as treatment period. * Acupoint injection: two side acupoints in turn; once a day; 10 days as a treatment course; 7‐day interval between two courses. ** Acupoints choice according to type of TCM: Type of phlegm‐fire attacking upwards: Shangqiu, Fenglong, Yangjiao, Ququan, Baihui. Type of internal retention of phlegm and dampness; Sanjingjiao, Shangqiu, Fenglong, Yanglingquan, Baihui. Type of Qi stagnation and Blood stasis: Ligou, Benshen, Yangjiao, Ququan, Baihui. Type of Yin deficiency and fire excess: Shaohai, Zhizheng, Zhubing, Feiyang, Baihui. Type of Yang deficiency: Sanyingjiao, Zusanli, Dazhong, Feiyang, Baihui. |
|
| Outcomes | Mental state: BPRS2,3. Behaviour: leaving the study early. Adverse effects: TESS4. Follow‐up: Global state: relapse. Mental state:BPRS5 (BPRS score was assessed continuously during follow‐up). |
|
| Notes | 1. Data reported from only 160 participants. 2. The rating was assessed before treatment and after each treatment course. 3. Another assessment standard (according to reduced rate): recovery (≥ 75%); marked improvement (≥ 50%); improvement (≥ 25%); no effect (< 25%). 4. The rating was assessed after each treatment course. 5. The rating was assessed before follow‐up, one year and two years; we used data of two years. 6. Contact made with author as there was little difference between two references. Author Yufeng Pan had retired and author Yuying Pan clarified that the two references referred to the same study. For those outcomes containing different data from the two papers we did not extract the data. |
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | Report stated ‐ "randomly divided into the two groups". No further details. |
| Allocation concealment (selection bias) | Unclear risk | Not stated. |
| Blinding of participants and personnel (performance bias) All outcomes | High risk | Sham acupoint injection not used. |
| Blinding of outcome assessment (detection bias) All outcomes | Low risk | Report stated ‐ "the assessors were blind to the drug allocation". |
| Incomplete outcome data (attrition bias) Outcomes | High risk | Ten participants left the study early during the study and the follow‐up period. |
| Selective reporting (reporting bias) | Unclear risk | We were unable to locate the protocol, unsure if selective reporting occurred. |
| Other bias | High risk | There were inconsistencies in the data between the 2 reports of the same study. |
acupoint inj ‐ Wang 2000.
| Methods | Allocation: randomised. Blindness: assessor blind. Duration: 2 weeks. | |
| Participants | Diagnosis: schizophrenia with auditory hallucinations (CCMD‐2‐R).
N = 90. Age: between 18 and 59 years (mean age (39.54 ± 10.93) years) (acupoint injection + antipsychotics group); between 18 and 58 years (mean age (38.27 ± 9.78) years) (antipsychotics group). Sex: 31 women and 59 men. History: duration of illness (12.70 ± 8.31) years (acupoint injection + antipsychotics group); (11.5 ± 8.31) years (antipsychotics group). Setting: hospitalised patients. Country: China. Inclusion criteria: had a CCMD‐2‐R diagnosis of schizophrenia; existed all kinds of auditory hallucinations and the scores of the twelfth item of BPRS ≥ 3; auditory hallucinations did not disappear after 2 months systemic treatment with one antipsychotics. Exclusion criteria: organic diseases and other hallucinations. |
|
| Interventions | 1. Acupoint injection + antipsychotics: acupoint injection (clonazepam 1.0 mg; Tinggong [double], opened mouth, once two days, 7 times) + antipsychotics (remained previous antipsychotics treatment; equivalent chlorpromazine dose (685 ± 240) mg/d). N = 45. 2. Antipsychotics: remained previous antipsychotics treatment; equivalent chlorpromazine dose (633 ± 247) mg/d. N = 45. |
|
| Outcomes | Mental state: BPRS (the scores of the twelfth item). Behaviour: leaving the study early. |
|
| Notes | ||
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Report stated ‐ "randomly divided into experimental group and control group using draw lots method". |
| Allocation concealment (selection bias) | Unclear risk | Not stated. |
| Blinding of participants and personnel (performance bias) All outcomes | High risk | Sham acupoint injection not used. |
| Blinding of outcome assessment (detection bias) All outcomes | Low risk | Report stated ‐ " two doctors with extensive clinical experience and not attending the trial assessed outcomes before treatment and 2 weeks after treatment". |
| Incomplete outcome data (attrition bias) Outcomes | Low risk | No participants left the study early. |
| Selective reporting (reporting bias) | Unclear risk | We were unable to locate the protocol, unsure if selective reporting occurred. |
| Other bias | High risk | One author worked for drug industry. |
acupoint inj ‐ Yang 2000.
| Methods | Allocation: randomised. Blindness: not reported. Duration: half a year. | |
| Participants | Diagnosis: schizophrenia with auditory hallucinations (CCMD‐2).
N = 64. Age: mean age 30.7 years (acupoint injection + antipsychotics group); 28.8 years (antipsychotics group). Sex: 10 women and 54 men. History: average duration of illness 5.2 years (acupoint injection + antipsychotics group) and 5.8 years (antipsychotics group). Country: China. Inclusion criteria: had CCMD‐2‐R diagnosis of schizophrenia; > 2 hospitalisations; systemic treatments of multiple antipsychotics but persistent auditory hallucinations; after other treatments (acupuncture and Chinese herbs) persistent auditory hallucinations or reappeared; current auditory hallucinations > 8 weeks; BPRS < 30. |
|
| Interventions | 1. Acupoint injection + antipsychotics: acupoint injection (sulpiride 50 mg; Tinggong [double], once a day, 5 times as a treatment course, 2 intermittent treatment courses each month, total half a year) + antipsychotics (remained antipsychotics treatment; equivalent chlorpromazine dose (668.4 ± 221.6) mg/d). N = 34. 2. Antipsychotics: remained antipsychotics treatment (except some cases reduced dose or added artane for extrapyramidal reaction); equivalent chlorpromazine dose (651 ± 20.84) mg/d. N = 30. |
|
| Outcomes | Mental state: BPRS1; no clinically important change in specific symptoms (auditory hallucinations)2. Behaviour: leaving the study early. |
|
| Notes | 1. The rating was assessed 1 week before treatment and, 3 months and half a year after treatment. 2. Criteria: recovery (auditory hallucinations disappeared completely; did not reappear about half a year); improvement (times of auditory hallucinations reduced; had insight of auditory hallucinations; did not effect daily life; or auditory hallucinations appeared occasionally after disappeared); no effect (auditory hallucination still existed or appeared frequently after disappeared). |
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | Report stated ‐ "randomly divided into two groups". No further details. |
| Allocation concealment (selection bias) | Unclear risk | Not stated. |
| Blinding of participants and personnel (performance bias) All outcomes | High risk | Sham acupoint injection not used. |
| Blinding of outcome assessment (detection bias) All outcomes | Unclear risk | Not stated. |
| Incomplete outcome data (attrition bias) Outcomes | Low risk | No participants left the study early. |
| Selective reporting (reporting bias) | Unclear risk | We were unable to locate the protocol, unsure if selective reporting occurred. |
| Other bias | Low risk | Not obvious. |
EACT ‐ Xue 1987.
| Methods | Allocation: randomised. Blindness: not reported. Duration: a treatment course. | |
| Participants | Diagnosis: schizophrenia.
N = 68. Age: between 19 and 37 years (electric acupuncture convulsive therapy group) and between 17 and 48 years (electroconvulsive therapy group); most of them were middle‐aged patients. Sex: 23 women and 45 men. Country: China. |
|
| Interventions | 1. Electric acupuncture convulsive therapy: Renzhong and Baihui; average electricity consumption 1.27 Joule/331 times; once every two days; 12 times as a treatment course. N = 34. 2. Electric convulsive therapy: electrode position of two temporal; average electricity consumption 34.97 Joule/286 times; once every two days; 12 times as a treatment course. N = 34. |
|
| Outcomes | Behaviour: leaving the study early. Adverse effects: back pain; spinal fracture1. Unable to use: Physical exam: tendon reflexes; patellar clonus (not clinical outcomes). |
|
| Notes | 1. Twelve patients suffered a spinal fracture during the treatment period and their data could not be used. 2. Authors compared three groups (electric acupuncture convulsive therapy group, electroconvulsive therapy group and grand mal epilepsy group) but only patients with schizophrenia randomly divided into electric acupuncture convulsive therapy group and electroconvulsive therapy group. |
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | Report stated ‐ "randomly divided into two groups". No further details. |
| Allocation concealment (selection bias) | Unclear risk | Not stated. |
| Blinding of participants and personnel (performance bias) All outcomes | High risk | Author compared two groups and used electrodes with different positions. |
| Blinding of outcome assessment (detection bias) All outcomes | Unclear risk | Not stated. |
| Incomplete outcome data (attrition bias) Outcomes | Low risk | No participants left the study early. |
| Selective reporting (reporting bias) | Unclear risk | We were unable to locate the protocol, unsure if selective reporting occurred. |
| Other bias | Low risk | Not obvious. |
electro ‐ Chen 2006.
| Methods | Allocation: randomised. Blindness: not reported. Duration: 6 weeks. | |
| Participants | Diagnosis: post‐schizophrenic depression1 (CCMD‐3). N = 67. Age: mean age (30.2 ± 11.0) years (electroacupuncture + antipsychotics group) and (2.9 ± 10.7) years2 (antipsychotics group). Sex: 27 women and 40 men. History: average duration of illness (5.6 ± 2.9) years (electroacupuncture + antipsychotics group) and (4.9 ± 3.8) years (antipsychotics group). Setting: both inpatients and outpatients. Country: China. |
|
| Interventions | 1. Electroacupuncture + antipsychotics: electroacupuncture* (Baihui and Yingtang) + antipsychotics (remained previous medication). N = 33. 2. Antipsychotics: remained previous medication. N = 34. * Electroacupuncture: continuous wave; 2‐4 Hz, 50 minutes, once a day. |
|
| Outcomes | Mental state: HAMD3,4. Behaviour: leaving the study early. Adverse effects: TESS3. |
|
| Notes | 1. Diagnosis of post‐schizophrenia depression: diagnosed with schizophrenia in the last 1 year; symptoms of depression appeared when the condition improved but not cured; the depression continued at least two weeks; HAMD > 20. 2. Although the author reported that there was no significant age difference between the two groups the table appears to contain a typographical error in the antipsychotics' group. 3. The rating was assessed before treatment and at 2 weeks, 4 weeks and 6 weeks after treatment. 4. Another assessment standard (according to reduced rate): recovery (≥ 75%); marked improvement (≥ 50%); improvement (≥ 50%); no effect (< 25%, reduced rate = [total scores before treatment‐total scores after treatment]/total scores before treatment*100%). |
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | Report stated ‐ "randomly". No further details. |
| Allocation concealment (selection bias) | Unclear risk | Not stated. |
| Blinding of participants and personnel (performance bias) All outcomes | High risk | Sham electroacupuncture not used. |
| Blinding of outcome assessment (detection bias) All outcomes | Unclear risk | Not stated. |
| Incomplete outcome data (attrition bias) Outcomes | Low risk | No participants left the study early. |
| Selective reporting (reporting bias) | Unclear risk | We were unable to locate the protocol, unsure if selective reporting occurred. |
| Other bias | Low risk | Not obvious. |
electro ‐ Chen 2008.
| Methods | Allocation: randomised. Blindness: not report. Duration: 12 weeks. | |
| Participants | Diagnosis: schizophrenia with negative symptoms (type II schizophrenia) (CCMD‐3; Andreasen's diagnosis standard of type II schizophrenia) . N = 62. Age: between 19 and 54 years (mean age (27 ± 13) years) 1 (electroacupuncture + aripiprazole group); between 17 and 60 years (mean age (28 ± 15) years) (aripiprazole group). Sex: 22 women and 38 men1. History: duration of illness between 3 and 24 years1 (electroacupuncture + aripiprazole group); between 2 and 19 years (aripiprazole group). Setting: hospitalised patients. Country: China. Inclusion criteria: had a CCMD‐3 and a type II diagnosis of schizophrenia; with mainly negative symptoms and PANSS > 60, the scores of negative factors > 30; age between 16 and 60 years and duration of illness more than 1 year; liver and kidney function test, lipids test, blood glucose test, ECG, blood routine test, urine routine test, and stool routine test normal; patients or family members gave their consent. Exclusion criteria: combined with other diseases; had serious physical illnesses or brain organic diseases or epilepsy and alcohol or drug abuse; pregnant or lactating women; with similar drug allergic history and unable to adapt electroacupuncture history. |
|
| Interventions | 1. Electroacupuncture + aripiprazole: electroacupuncture* (Baihui and Neiguan [double] group or Shuigou and Sanyingjiao [double] group) + aripiprazole (Bosiqing; initial dosage 5 mg/d; gradually added to the therapeutic dosage 10 to 30 mg/d within two weeks; average dosage (18.4 ± 6.2) mg/d). N = 32. 2. Aripiprazole: Bosiqing; initial dosage 5 mg/d; gradually added to the therapeutic dosage 10 to 30 mg/d within two weeks; average dosage (20.1 ± 4.3) mg/d. N = 30. Washout period: those treated with other antipsychotics or mood stablisers needed 2‐weeks' washout period. Combination therapy: could not receive any other antipsychotics during the observation period; symptomatic treatment drugs could be used according to patient's condition when with adverse drug reactions and could receive benzodiazepines when with poor sleep. * Electroacupuncture: used two acupoints groups in turn; once for 45 minutes; once a day; five days a week (except Saturday and Sunday); 12 weeks as a treatment course. |
|
| Outcomes | Mental state: PANSS2,3. Behaviour: leaving the study early. Adverse effects: TESS2 (mainly reported insomnia; myotonia; tremor; akathisia; blurred vision; sweating; headache; tachycardia). Unable to use: Adverse effects: lab test (kidney function test, lipids test, blood glucose test, stool routine test) (reported no obvious abnormal before or after treatment in both groups and no data). |
|
| Notes | 1. Two patients in the electroacupuncture + aripiprazole group left the study early and author reported the age, sex and duration of illness of this group excluding those two patients. 2. The ratings were assessed before treatment and at 2 weeks, 4 weeks, 8 weeks and 12 weeks after treatment. 3. Another assessment standard (according to reduced rate): recovery (≥ 75%); marked improvement (51% to 75%); improvement (25% to 50%); no effect (< 25%, reduced rate = [scores before treatment‐scores after treatment]/scores before treatment*100%). |
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Report stated ‐ "random systematic sampling according to admission order". |
| Allocation concealment (selection bias) | Unclear risk | Not stated. |
| Blinding of participants and personnel (performance bias) All outcomes | High risk | Sham electroacupuncture not used. |
| Blinding of outcome assessment (detection bias) All outcomes | Unclear risk | Not stated. |
| Incomplete outcome data (attrition bias) Outcomes | High risk | Two participants (electroacupuncture + aripiprazole group) left the study early. |
| Selective reporting (reporting bias) | Unclear risk | We were unable to locate the protocol, unsure if selective reporting occurred. |
| Other bias | Low risk | Not obvious. |
electro ‐ Cheng 2009.
| Methods | Allocation: randomised.
Blindness: patient and assessor blind. Duration: 4‐week baseline evaluation and risperidone run‐in phase + 6 weeks treatment period. |
|
| Participants | Diagnosis: schizophrenia with auditory hallucinations (DSM IV) . N = 60. Age: mean age (31.20 ± 5.79) years (electroacupuncture + risperidone group) and (28.97 ± 5.49) years (sham electroacupuncture + risperidone group). Sex: 32 women and 28 men. History: average duration of auditory hallucinations (10.13 ± 2.89) years (electroacupuncture + risperidone group) and (9.03 ± 2.94) years (sham electroacupuncture + risperidone group). Setting: hospitalised patients. Country: China. Inclusion criteria: were 18‐60 years of age; had a DSM IV diagnosis of schizophrenia using Structured Clinical Interview for DSM IV; had previously been treated with risperidone following documented treatment failure after two antipsychotics were administered for an adequate duration with sufficient dose; demonstrated a documented failure to show a satisfactory clinical response to an adequate trial of risperidone (three or more months of at least 4 mg/day of oral risperidone); had persistent and distressing auditory hallucinations, evidenced by a score of 11 or more on the total of PSYRATS‐AH; gave informed consent. Exclusion criteria: mental retardation; seizure disorder; substance abuse/dependence; pregnant; severe and unstable physical illnesses; danger of attack or extreme agitation; or previously received acupuncture (to maximise blinding). |
|
| Interventions | 1. Electroacupuncture + risperidone: electroacupuncture* (Tinggong, Tinghui, Yifeng, Daling, Neiguan and Sanyijiao) + risperidone (before initiation of the baseline observation period all patients had been on a stable dose of risperidone for at least 4 weeks and baseline doses of risperidone remained stable throughout the study; the average initial risperidone dosage (5.15 ± 0.46) mg/d). N = 30. 2. Sham electroacupuncture + risperidone: sham electroacupuncture (20 mm away from each corresponding acupoint) + risperidone (before initiation of the baseline observation period all patients had been on a stable dose of risperidone for at least 4 weeks and baseline doses of risperidone remained stable throughout the study; the average initial risperidone dosage (5.22 ± 0.47) mg/d). N = 30. Combination therapy: lorazepam (< 4 mg) was allowed to counteract sleep problems; antidepressants, mood stabilisers and antipsychotic drugs other than risperidone were not allowed during the study. * Electroacupuncture: needled bilaterally; depth 15to 30 mm; once the 'De‐Qi' sensation was elicited, the handles of the needles inserted into Tinggong, Tinghui and Yifeng were connected to an electrical input via an electroacupuncture machine (sparse dense wave; frequency 2 to 10 Hz; intensity 2 to 3 mA); for 20 minutes; 5 times a week; total 30 times (6 weeks). ** Sham electroacupuncture: depth less than 5 mm; the handles of the needles inserted into points near Tinggong, Tinghui and Yifeng were connected to the electroacupuncture machine with no electrical current; for 20 minutes; 5 times a week; total 30 times (6 weeks) (simulated a real electroacupuncture procedure and the acupuncturist did not manipulate the needles and no 'De‐Qi' sensation was elicited). |
|
| Outcomes | Mental state: PSYRATS‐AH1,2; PANSS1. Behaviour: leaving the study early. |
|
| Notes | 1.The ratings were assessed at baseline, 2 weeks, 4 weeks and 6 weeks. 2. Another assessment standard: (according to reduction scores from baseline): a treatment response (≥ 20%). 3. This study is a part of Bai's study (see the reference) and they only reported this study (obtained the information from author Jing Chen). 4. Author analysed data using intention‐to‐treat method. |
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Report stated ‐ "randomly assigned to either the real electroacupuncture group or the sham electroacupuncture group by the SAS program". |
| Allocation concealment (selection bias) | Low risk | Report stated ‐ "maintained using opaque sealed envelopes". |
| Blinding of participants and personnel (performance bias) All outcomes | Low risk | Report stated ‐ "blinded to the treatment allocation"; the acupuncturist was "instructed not to communicate with the patients and the clinical investigators"; the participants were "acupuncture‐naive patients" and "there was also limited contact between the study participants and restricted conversation between acupuncturist and participants during treatment". |
| Blinding of outcome assessment (detection bias) All outcomes | Low risk | Report stated ‐ "blinded to the treatment allocation" and the acupuncturist was "instructed not to communicate with the patients and the clinical investigators". |
| Incomplete outcome data (attrition bias) Outcomes | Low risk | Though seven participants left the study early the author analysed data using intention‐to‐treat method. |
| Selective reporting (reporting bias) | Unclear risk | We were unable to locate the protocol, unsure if selective reporting occurred. |
| Other bias | Unclear risk | This study was supported by the Stanley Medical Research Institue (SMRI), Chevy Chase, Maryland. |
electro ‐ Cui 2000.
| Methods | Allocation: randomised.
Blindness: not reported. Duration: 6 weeks. |
|
| Participants | Diagnosis: schizophrenia (CCMD‐2‐R) . N = 60. Age: between 20 and 50 years (mean age (35.1 ± 7) years). Sex: 48 women and 12 men. History: duration of illness between 5 and 10 years. Setting: hospitalised patients. Country: China. Inclusion criteria: had a CCMD‐2‐R diagnosis of schizophrenia; without severe heart, liver and kidney dysfunction or other severe somatic diseases; without chlorpromazine allergic history. |
|
| Interventions | 1. Electroacupuncture + chlorpromazine: electroacupuncture* (acupoint choice according to different symptoms) + chlorpromazine (100 to 300 mg/d). N = 30. 2. Chlorpromazine: 400 to 500 mg/d. N = 30. Combination therapy: not combined with any other antipsychotics; could received symptomatic treatment when with extrapyramidal reactions, tachycardia or elevated transaminase. * Electroacupuncture: with mainly positive symptoms used Xinshu, Ganshu, Pishu, Shenmen, Fenglong; with mainly negative symptoms used Dazhui, Fengfu, Neiguan, Fenglong; with both positive and negative symptoms added or removed acupoints according to the symptoms; refusing to eat added Hegu and Zusanli; not speaking added Lianquan and Yamen; excitement and hyperactivity added Renzhong and Quchi; intensity according to patients feeling; frequency 20 to 40 times/second ; for 30 minutes; once two days; 20 times as a treatment course. |
|
| Outcomes | Mental state: BPRS1,2. Behaviour: leaving the study early. Adverse effects: TESS3 (mainly reported extrapyramidal symptoms; tachycardia; dry mouth; blurred vision; sleepiness). Others4: adding medication. |
|
| Notes | 1.The rating was assessed before treatment and, 2 weeks, 4 weeks and 6 weeks after treatment. 2. Another assessment standard (according to reduced rate): improvement (≥ 30%); marked improvement (≥ 50%); recovery (≥ 80%); no effect (< 30%). 3. The ratings were assessed at 2 weeks, 4 weeks and 6 weeks after treatment. 4. Author reported this outcome. |
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Report state ‐ "randomly divided into two groups according to age and gender matching". Contacted the author who said she used coin‐tossing method . |
| Allocation concealment (selection bias) | Unclear risk | Not stated. |
| Blinding of participants and personnel (performance bias) All outcomes | High risk | Sham electroacupuncture not used. |
| Blinding of outcome assessment (detection bias) All outcomes | Unclear risk | Not stated. |
| Incomplete outcome data (attrition bias) Outcomes | Low risk | No participants left the study early. |
| Selective reporting (reporting bias) | Unclear risk | We were unable to locate the protocol, unsure if selective reporting occurred. |
| Other bias | Low risk | Not obvious. |
electro ‐ Ding 2005.
| Methods | Allocation: randomised.
Blindness: not reported. Duration: 2 months. |
|
| Participants | Diagnosis: chronic schizophrenia (CCMD‐3). N = 50. Age: between 47 and 60 years (mean age (57.4 ± 5.71) years). Sex: 50 men and no women. History: duration of illness between 19 and 39 years (average duration (32.25 ± 6.31) years); duration of continued hospitalisation between 11 and 22 years (average duration (18.17 ± 7.35) years); received antipsychotics more than 10 years. Setting: hospitalised patients. Country: China. |
|
| Interventions | 1. Electroacupuncture + antipsychotics: electroacupuncture* (Tanzhong, Zhongwan, Shenmen, Fenglong [double], Taichong [double], Neiguan [double]) + antipsychotics (remained previous medication). N = 25. 2. Antipsychotics: remained previous medication. N = 25. * Electroacupuncture: reinforcing‐reducing method for deficiency syndrome and reducing method for excess syndrome; connected needles to the electroacupuncture machine once the sensation was elicited; pulse current; 5 minutes; once two days. |
|
| Outcomes | Mental state: BPRS; PANSS. Behaviour: leaving the study early. |
|
| Notes | 1. Contact made with the author, Fenggang Li, and no participants left the study early. | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | Report stated ‐ "randomly divided into treatment group and control group". No further details. |
| Allocation concealment (selection bias) | Unclear risk | Not stated. |
| Blinding of participants and personnel (performance bias) All outcomes | High risk | Not used sham electroacupuncture and doctors often told patients (electroacupuncture + antipsychotics group) the importance and treatment mechanism of acupuncture. |
| Blinding of outcome assessment (detection bias) All outcomes | Unclear risk | Not stated. |
| Incomplete outcome data (attrition bias) Outcomes | Low risk | No participants left the study early. |
| Selective reporting (reporting bias) | Unclear risk | We were unable to locate the protocol, unsure if selective reporting occurred. |
| Other bias | Unclear risk | Participants were men and unsure if gender selection bias occurred and the study lasted longer than 20 years. |
electro ‐ Wang 2005.
| Methods | Allocation: randomised. Blindness: not reported. Duration: 8 weeks. | |
| Participants | Diagnosis: schizophrenia with negative symptoms (type II schizophrenia) (CCMD‐3; Andreasen's diagnosis standard of type II schizophrenia).
N = 75. Age: mean age (41.7 ± 10.6) years (electroacupuncture + risperidone group); (39.3 ± 9.2) years (risperidone group). Sex: 41 women and 34 men. History: duration of illness (11.7 ± 6.5) years (electroacupuncture + risperidone group) and (13.2 ± 7.4) years (risperidone group); average number of hospitalisations (4.2 ± 2.5) times (electroacupuncture + risperidone group) and (3.9 ± 2.2) times (risperidone group) . Setting: hospitalised patients. Country: China. Inclusion criteria: had a CCMD‐3 diagnosis of schizophrenia; had an Andreasen's diagnosis of type II schizophrenia; PANSS > 60; the scores of negative factors > 30; women and men; age between 18 and 55 years; patients receiving any other antipsychotics, mood stabilisers or antidepressants needed 2 weeks washout period and total scores of PANSS decreased < 20. Exclusion criteria: had cerebral organic diseases or severe physical illnesses; combined with other psychotic diseases; with similar drugs allergic history; drug or alcohol dependence or abuse; pregnant or lactating women. |
|
| Interventions | 1. Electroacupuncture + risperidone: electroacupuncture* (Baihui, Shangxing, Yingtang, Sanyingjiao [double], Neiguan [double]) + risperidone (Weisitong; initial dose 1 mg/d and added to therapeutic dose 3 to 6 mg/d in 10 days according to patient's conditions and adverse reactions; average dose (4.9 ± 1.4) mg/d). N = 40. 2. Risperidone: Weisitong; initial dose 1 mg/d and added to therapeutic dose 3 to 6 mg/d in 10 days according to patient's conditions and adverse reactions; average dose (5.3 ± 1.2) mg/d. N = 35. Combination therapy: could add artane, propranolol when extrapyramidal reactions appeared; could add benzodiazepine drugs to treat patients with pool sleep. * Electroacupuncture: frequency 20 to 40 times/minute; intermittent wave; for 45 minutes; 5 times a week; 20 times as a treatment course; added Zhongwan and Zusanli when with gastrointestinal uncomfortable symptoms and added Fenglong when with symptoms of the type of stagnation of phlegm and Qi. |
|
| Outcomes | Mental state: PANSS1,2. Behaviour: leaving the study early. Adverse effects: TESS1 (main: extrapyramidal symptoms; dry mouth; insomnia; blurred vision; dizziness; constipation; weight gain; nausea and vomiting); lab test (blood routine test; urine routine test; liver function and kidney function; and ECG). Others3: adding medication. |
|
| Notes | 1. The ratings were assessed at the end of 0 week, 4 weeks and 8 weeks. 2. Another assessment standard (according to reduced rate): recovery (≥ 75%); marked improvement (51% to 75%); improvement (25% to 50%); no effect (< 25%). 3. Author reported this outcome. |
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | Report stated ‐ "randomly divided into experimental group and control group". No further details. |
| Allocation concealment (selection bias) | Unclear risk | Not stated. |
| Blinding of participants and personnel (performance bias) All outcomes | High risk | Sham electroacupuncture not used. |
| Blinding of outcome assessment (detection bias) All outcomes | Unclear risk | Not stated. |
| Incomplete outcome data (attrition bias) Outcomes | Low risk | No participants left the study early. |
| Selective reporting (reporting bias) | Unclear risk | We were unable to locate the protocol, unsure if selective reporting occurred. |
| Other bias | Low risk | Not obvious. |
electro ‐ Xiong 2010.
| Methods | Allocation: randomised. Blindness: assessor blind. Duration: 8 weeks. | |
| Participants | Diagnosis: refractory schizophrenia (CCMD‐3, the defined standard of refractory schizophrenia1).
N = 80. Age: mean age (29.4 ± 11.3) years (electroacupuncture + low dose clozapine group); (28.1 ± 12.2) years (clozapine group). Sex: 34 women and 46 men. History: average duration of illness (35.2 ± 12.2) months (electroacupuncture + low dose clozapine group); (36.3 ± 13.2) months (clozapine group). Setting: hospitalised patients. Country: China. Inclusion criteria: had a CCMD‐3 diagnosis of schizophrenia; had a diagnosis of refractory schizophrenia; age between 14 and 60 years; women and men; patients already receiving antipsychotics needed 7 days' washout period; gave informed consent. Exclusion criteria: had severe heart, liver and kidney primary diseases. |
|
| Interventions | 1. Electroacupunture + low dose clozapine: electroacupuncture* (Baihui and Taiyang [double]) + low dose clozapine (initial dose 50 mg/d; added to 100 to 150 mg/d within 1 week). N = 40. 2.Clozapine: initial dose 50 to 100 mg/d, added to 200 to 500 mg/d within 1 week. N = 40. * Electroacupuncture: 10 voltage; continuous wave; frequency 60Hz; current intensity was limited when neck and facial muscles cramped and breath‐hold and hypoxia appeared; firstly continued to stimulate strongly 3 to 4 seconds then reduced the strength and frequency quickly, after patient's breath and face returned to normal and continued 30 to 60 seconds stimulated again; continued to stimulate 8 to 10 times; 3 times a week, total 8 weeks. |
|
| Outcomes | Mental state: PANSS2,3. Behaviour: leaving the study early. Adverse effects: TESS4. |
|
| Notes | 1. The defined standard of refractory schizophrenia: no effect after receiving at least 2 or more different antipsychotic chemical drugs with enough doses and enough treatment courses; no effect after receiving electric shock 7 to 12 times enough treatment courses' treatments; PANSS ≥ 60. 2. The rating was assessed before treatment and, 2 weeks, 4 weeks and 8 weeks after treatment. 3. Another assessment standard (according to reduced rate): recovery (≥80%); marked improvement (≥ 50%, < 80%); improvement (≥ 30%, < 50%); no effect (< 30%, reduced rate = [scores before treatment‐scores after treatment]/scores before treatment*100%). 4. The rating was assessed 8 weeks after treatment. |
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | Report stated ‐ "randomised into" two groups. No further details. |
| Allocation concealment (selection bias) | Unclear risk | Not stated. |
| Blinding of participants and personnel (performance bias) All outcomes | High risk | Sham electroacupuncture not used. |
| Blinding of outcome assessment (detection bias) All outcomes | Low risk | Report stated ‐ "two doctors who were blind the drug allocation assessed outcomes". |
| Incomplete outcome data (attrition bias) Outcomes | Low risk | No participants left the study early. |
| Selective reporting (reporting bias) | Unclear risk | We were unable to locate the protocol, unsure if selective reporting occurred. |
| Other bias | Low risk | Not obvious. |
electro ‐ Yao 2006.
| Methods | Allocation: randomised.
Blindness: not reported. Duration: 8 weeks. Follow‐up: 6 months (effective case). |
|
| Participants | Diagnosis: schizophrenia (CCMD‐3) . N = 90. Age: between 18 and 60 years (mean age (31 ± 12) years) (electroacupuncture + clozapine group); between 18 and 60 years (mean age (29 ± 13) years) (clozapine group). Sex: 40 women and 50 men. History: duration of this episodes between 1 and 16 months (average duration (8 ± 5) years) (electroacupuncture + clozapine group), between 1.5 and 15 months (average duration (9 ± 3) months) (clozapine group). Type: paranoid type 31cases, undifferentiated type 9 cases, hebephrenic type 5 cases and 8 cases with positive family history (electroacupuncture + clozapine group); paranoid type 29 cases, undifferentiated type 12 cases, hebephrenic 4 cases and 10 cases with positive family history (clozapine group). Setting: hospitalised patients. Country: China. Inclusion criteria: had a CCMD‐3 diagnosis of schizophrenia; not received any antipsychotics within the last two weeks. Exclusion criteria: severe heart, brain, blood vessels and other physical illnesses; drug or alcohol dependence; pregnant or lactating women. |
|
| Interventions | 1. Electroacupuncture + clozapine: electroacupuncture* (Baihui, Fenglong, Houxi, Ganshu) + clozapine (total dose 200‐300 mg/d; twice a day). N = 45. 2. Clozapine: total dose 200 to 300 mg/d; twice a day. N = 45. * Electroacupuncture: sparse dense wave; for 30 minutes; once a day; 20 to 30 times as a treatment course; total 2 treatment courses. |
|
| Outcomes | Mental state: PANSS1.2. Behaviour: leaving the study early. Adverse effects: TESS1,3. Follow‐up: Mental state: PANSS2,4. |
|
| Notes | 1. The ratings were assessed before treatment and, 2 weeks, 4 weeks, 6 weeks and 8 weeks after treatment. 2. Another assessment standard (according to reduced rate): recovery (> 95%); marked improvement (60% to 94%); improvement (30‐59%); no effect (< 30%). 3. Lab test included in TESS was tested before treatment and after treatment. 4. Effective cases included recovery cases, marked improvement cases and improvement cases. Effective cases were assessed 6 weeks of follow‐up period (converted to dichotomous data). |
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Reported state ‐ " randomly divided into two group using draw lots method". |
| Allocation concealment (selection bias) | Unclear risk | Not stated. |
| Blinding of participants and personnel (performance bias) All outcomes | High risk | Sham electroacupuncture not used. |
| Blinding of outcome assessment (detection bias) All outcomes | Unclear risk | Not stated. |
| Incomplete outcome data (attrition bias) Outcomes | High risk | No participants left the study early but only followed up effective cases and six participants left the study early at follow‐up period. |
| Selective reporting (reporting bias) | Unclear risk | We were unable to locate the protocol, unsure if selective reporting occurred. |
| Other bias | Low risk | Not obvious. |
electro ‐ Zhang 1987.
| Methods | Allocation: randomised. Blindness: not reported. Duration: 20 days. | |
| Participants | Diagnosis: schizophrenia. First stage: N = 182. Age: between 17 and 43 years (electroacupuncture + Dang Gui Cheng Qi Tang group); between 17 and 51 years (decoction of herbs group); between 17 and 48 years (electroacupuncture group); between 16 and 48 years (chlorpromazine group). Sex: 97 women and 85 men. History: mostly first admissions. Setting: hospitalised patients. Country: China. Inclusion criteria: as for readmitted patients only those who experienced marked improvement or cure at the end of the previous course of treatment were included. Exclusion criteria: those whose illness had lasted over two years; weak or pregnant; had symptoms of deterioration; physically frail or had a history of peptic ulcer, cardiac or renal disease; those who showed rapid remission of symptoms on admission. Second stage: N = 99. Age: under 20 years 16 cases; between 21 and 30 years 52 cases; between 31 and 40 years 18 cases; between 41 and 50 years 10 cases; over 50 years 3 cases. Sex: all men and no women. History: duration of illness within 2 years 66 cases and over 2 years 33 cases; first admissions 32 cases, second admissions 21 cases and more than 3 hospitalisation times 9 cases. Setting: hospitalised patients. Country: China. Inclusion criteria: as for readmitted patients only those who experienced marked improvement or cure at the end of the previous course of treatment were included. Ecliusion criteria: those whose illness had lasted over two years were included; others were the same as the first stage. |
|
| Interventions | First stage: 1. Electroacupuncture + Dang Gui Cheng Qi Tang: electroacupuncture* (Yifeng, Tinggong, Tounjie, Chengling, Linqi, Baihui, Dingshen) + Dang Gui Cheng Qi Tang**. N = 49. 2. Decoction of herbs: Dang Gui Cheng Qi Tang**. N = 45. 3. Electrocupuncture*: Yifeng, Tinggong, Tounjie, Chengling, Linqi, Baihui, Dingshen. N = 43. 4. Chlorpromazine (Thorazine): any dose when necessary; 300‐600 mg/day; not combined with other antipsychotics. N = 45. Second stage: 1. Electroacupuncture + Dang Gui Cheng Qi Tang: electroacupuncture* (Yifeng, Tinggong, Chengling and Tounie) + Dang Gui Cheng Qi Tang**. N = 26. 2. Electroacupuncture + Dang Gui Cheng Qi Tang (with additives): electroacupuncture* (Yifeng, Tinggong, Chengling and Tounie) + Dang Gui Cheng Qi Tang** (with additives***). N = 25. 3. Electroacupuncture*: Tounie, Chengling and Linqi. N = 23. 4. Electroacupuncture*: Yifeng, Tinggong, Chengling and Tounie. N = 25. * Electroacupuncture: successive waves of 120cycles (Hz)/sec and 500 μsec pulse width; the intensity of stimulation varied with the individual and could be generalised into three grades (strong stimulation, moderate stimulation and mild stimulation); twice a day; adjustment of treatment depended upon the condition of the patient. ** Dang Gui Cheng Qi Tang: Radix Angelicae Sinensis 30 g, Radix et Rhizoma Rhei 30 g, Natrii Sulfas 15 g, Poncirus Trifoliata (L) Raf 12 g and Fruetus Trichosanthis 150 mL; all herbs except the Natrii Sulfas were decocted into a 100 mL solution, then the Natrii Sulfas was added and dissolved in it; usually 50 mL two times a day but the dose maybe increased to 150 to 200 mL daily when necessary. *** Additional herbs of Dang Gui Cheng Qi Tang (with additives): Swmen Persicae, Radix Curcumae, Radix Paeoniae Rubrae, Radix Bupleuri, Radix Scutellariae, Flos Carthami, Rhizoma Ligustici Chuanxiong, Radix Srephaniae tetrandrae, Radix Ledebouriellae, Poria, Radix Polygalae, Fructus Ziziphi Jujubae, Radix Rehmanniae, Rhizoma Acori Graminei, Os Draconis, Concha Osreeae, Haematitum, Herba Leonuri, Parata, Cortex Cinnamoni, Rhizoma Coptidis; according to the different condition of patient. Combination therapy: 10% chloral hydrate 10 to 20 mL orally, hyminal 0.1 to 0.2 g orally, phenobarbital 0.03 to 0.1 g or paraldehyde 4 to 5 mL orally or intramuscularly for patient who could not fall asleep at night and could not to be used for a long time. |
|
| Outcomes | Global state: no clinically important change in global state1. Behaviour: leaving the study early. Unable to use; Adverse effects: reported electroacupuncture relative adverse effects (holding breath, facial cyanosis, arrhythmia, transient increase of blood pressure, injury of teeth, tongue and lips, epileptic attacks with strong stimulation) and TCM relative adverse effects (diarrhoea) existed but no data. |
|
| Notes | 1. Assessment criteria: recovery (disappearance of all symptoms); marked improvement (50% relief of symptoms with moderate insight); mild improvement (25% relief of symptoms with no insight); no effect (no change in symptoms). 2. Only included the first stage of the study and could not include the second stage of the study because of interventions. |
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | Report stated ‐ "divided into groups randomly". No further details. |
| Allocation concealment (selection bias) | Unclear risk | Not stated. |
| Blinding of participants and personnel (performance bias) All outcomes | High risk | Sham electroacupuncture or any other dummy treatments not used. |
| Blinding of outcome assessment (detection bias) All outcomes | Unclear risk | Not stated. |
| Incomplete outcome data (attrition bias) Outcomes | Low risk | No participants left the study early. |
| Selective reporting (reporting bias) | Unclear risk | We were unable to locate the protocol, unsure if selective reporting occurred. |
| Other bias | Low risk | Not obvious. |
electro ‐ Zhang 1993.
| Methods | Allocation: randomised. Blindness: not reported. Duration: 8 weeks . | |
| Participants | Diagnosis: refractory schizophrenia (CCMD‐2‐R).
N: unclear1. Age: both references reported mean age. History: both references reported average duration of illness. Setting: hospitalised patients. Country: China. Inclusion criteria: had a CCMD‐2‐R diagnosis of schizophrenia; after at least 2 times hospitalisations and systemic treatments of multiple antipsychotics most of the psychiatric symptoms disappeared but auditory hallucinations appeared repeatedly; after other treatments (acupuncture and Chinese herbs) auditory hallucinations still existed or reappeared after disappeared; auditory hallucinations this time continued more than 8 weeks; BPRS < 30. |
|
| Interventions | 1. Electroacupuncture + antipsychotics: electroacupuncture* (CCEA; group one [Yingtang and Baihui] and group two [Shenting and Yamen]) + antipsychotics** (remained unchanged doses and kinds of medicines). 2. Antipsychotics**: remained unchanged doses and kinds of medicines. * Electroacupuncture: both groups were alternately used; sine‐wave; frequency 12, 10, 8, 6 Hz respectively for 10, 10, 10 and 15 minutes; frequency of fundamental waves 250 Hz and 750 Hz; maxim voltage 2 to 9 V; for 45 minutes; once a day; 8 weeks as a treatment course. ** From the date of two months before dividing into two groups to the end of the study the doses and kinds of medicines taken remained unchanged. |
|
| Outcomes | Mental state2: SANS; SAPS. Unable to use: Global state: no clinically important change in global state3 (data could not to be used). Mental state: BPRS (data could not to be used). Behaviour: leaving the study early (data could not to be used). Adverse effects: TESS2(only reported no adverse effects in electroacupuncture + antipsychotics group and compared ECG no difference between two groups). Lab test: EEG; T3; T4; RUR; FT4I; TSH; rT3; TG; TM; LH; FSH; T (not clinical outcomes). |
|
| Notes | 1. Two references with different participants' numbers but some data were the same and only extracted the same data with the same participants' number which could be used. No further detail. 2. The ratings were assessed before treatment, in 2nd, 4th, 6th and 8th week after treatment. 3. The effects were assessed after treatment with four degree which were worked out in Nanjing in 1958 (recovery, remarkable improvement, improvement and inefficacy). |
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | Report stated ‐ "divided at random into two groups". No further details. |
| Allocation concealment (selection bias) | Unclear risk | Not stated. |
| Blinding of participants and personnel (performance bias) All outcomes | High risk | Sham electroacupuncture not used. |
| Blinding of outcome assessment (detection bias) All outcomes | Unclear risk | Not stated. |
| Incomplete outcome data (attrition bias) Outcomes | High risk | Some of the graph showed data of 100 participants and some showed data of 69 participants. No further details. |
| Selective reporting (reporting bias) | Unclear risk | We were unable to locate the protocol, unsure if selective reporting occurred. |
| Other bias | High risk | Some important data were the same but with different participants' number in two references. No further details. |
electro ‐ Zhang 2001.
| Methods | Allocation: randomised. Blindness: not reported. Duration: 6 weeks. | |
| Participants | Diagnosis: schizophrenia with depressive symptoms (ICD‐10; CCMD‐2‐R).
N = 42.
Age: between 17 and 54 years (mean age (32.19 ± 9.13) years).
Sex: 19 women and 23 men. History: duration of illness between 7 months and 16 years (average (6.83 ± 4.77) years). Setting: hospitalised patients. Education level: higher than high school. Country: China. Inclusion criteria: had a CCMD‐2‐R and ICD‐10 diagnosis of schizophrenia; HAMD ≥ 20 and without severe tendency of suicide; without severe somatic diseases; without extreme excited, stupor, silent and any other uncooperative state; all the patients received simple antipsychotics and without receiving any antidepressants and anxiolytics. |
|
| Interventions | 1. Electroacupuncture + antipsychotics: electroacupuncture (Intelligent electroacupuncture; Baihui and Yingtang; peak voltage 3‐10 VP; for 45 minutes; once a day; 5 days a week except Saturday and Sunday; 6 weeks as a treatment course) + antipsychotics (no further details). N = 22. 2. Antipsychotics: no further details. N = 20. |
|
| Outcomes | Mental state: HAMD1, SDS. Behaviour: leaving the study early. |
|
| Notes | 1. Another assessment standard (according to reduced rate): marked improvement (≥ 50%); improvement (≥ 25%); no effect (< 25%, reduced rate = [scores before treatment‐scores after treatment]/scores before treatment*100%). | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Report stated ‐ "randomly sampled". |
| Allocation concealment (selection bias) | Unclear risk | Not stated. |
| Blinding of participants and personnel (performance bias) All outcomes | High risk | Sham electroacupuncture not used. |
| Blinding of outcome assessment (detection bias) All outcomes | Unclear risk | Not stated. |
| Incomplete outcome data (attrition bias) Outcomes | Low risk | No participants left the study early. |
| Selective reporting (reporting bias) | Unclear risk | We were unable to locate the protocol, unsure if selective reporting occurred. |
| Other bias | Low risk | Not obvious. |
electro ‐ Zhou 1997.
| Methods | Allocation: randomised. Blindness: assessor blind. Duration: 6 weeks. | |
| Participants | Diagnosis: schizophrenia (DSM III; CCMD).
N = 40.
Age: mean age (31.8 ± 8.9) years.
Sex: 17 women and 23 men. History: average duration of illness (8.2 ± 7.0) years. Setting: hospitalised patients. Country: China. Inclusion criteria: scores higher than 35 points on BPRS1. Exclusion criteria: severe heart, liver, kidney or other somatic diseases. |
|
| Interventions | 1. Electroacupuncture + low dose antipsychotics: electroacupuncture* (acupoints choice according to TCM types) + antipsychotics (a reduction of ˜60% of their previously daily levels). N = 25. 2. Antipsychotics: continued to receive their usual antipsychotics; equivalent chlorpromazine dose (560 ± 71.2) mg/day. N = 15. * Electroacupuncture: main acupoints (Yintang tou xinqu, Daling, Neiguan, Taiyang) and supplemental acupoints (Zusanli [Yang deficiency], Sanyinjiao [Yin deficiency], Fenglong [ Persistent Phlegm]);180 cycles/second; 500 ms pulse width; up to 60 mA; once a day except Sunday; 36 times as a treatment course (6 weeks). |
|
| Outcomes | Global state: no clinically important change in global state1; CGI (CGI‐SI, CGI‐GI, EI).
Mental state: BPRS2,3. Behaviour: leaving the study early. Adverse effects: TESS. Unable to use: Lab test: cAMP; cGMP; the neuropeptide beta‐endorphin (not clinical outcome). |
|
| Notes | 1. Results were classified as: marked improvement (the majority of symptoms were eliminated and orientation was recovered); improvement (some of the symptoms were eliminated and orientation was partly recovered); no effect (symptoms and orientation showed no change). 2.This rating scale was written in Chinese and was assessed before treatment and at 2 weeks, 4 weeks and 6 weeks after treatment. 3. Another assessment criteria (according to reduced rate): marked improvement (≥ 80%); improvement (50%to 80%); slight improvement (20% to 50%); no effect (≤ 20%). |
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | Report stated ‐ "randomly divided into two study groups". No further details. |
| Allocation concealment (selection bias) | Unclear risk | Not stated. |
| Blinding of participants and personnel (performance bias) All outcomes | High risk | Author compared two groups and did not use sham electroacupuncture. |
| Blinding of outcome assessment (detection bias) All outcomes | Low risk | Report stated ‐ "The subjects were evaluated clinically before and after the study course by specialists in psychiatry who were blinded as to which group the subjects belonged". |
| Incomplete outcome data (attrition bias) Outcomes | Low risk | No participants left the study early. |
| Selective reporting (reporting bias) | Unclear risk | We were unable to locate the protocol, unsure if selective reporting occurred. |
| Other bias | Low risk | Not obvious. |
laser ‐ Liu 1986.
| Methods | Allocation: randomised. Blindness: not reported. Duration: a treatment course. | |
| Participants | Diagnosis: schizophrenia with auditory hallucinations. N = 60. Age: mean age (35.8 ± 13.0) years (He‐Ne laser irradiation group (Ermen)); (33.2 ± 6.9) years (chlorpromazine + sham laser irradiation group); (37.7 ± 10.8) years (He‐Ne laser irradiation group (non‐acupoint)). Sex: 32 women and 28 men. History: average duration of illness 5.1 years (He‐Ne laser irradiation group (Ermen)); 6.2 years (chlorpromazine + sham laser irradiation group); 7.3 years (He‐Ne laser irradiation group (non‐acupoint)). Country: China. Inclusion criteria: had auditory hallucinations definitely schizophrenic in origin for more than one month. |
|
| Interventions | 1. He‐Ne laser irradiation (Ermen): Ermen (double); irradiated with mouths partially open; the tube of the emitter held 30 cm away; power 4.7 hw; patch of red light 0.5 cm; wavelength 6328 Å; optimal current output 6 to 7.5 mA; for 15 minutes; once a day except Sunday; 30 times as a treatment course. N = 20. 2. Chlorpromazine + sham laser irradiation: chlorpromazine (average dosage 450 mg/d) + sham laser irradiation (the tube of the laser emitter pointed in the direction of the ear without real irradiation; once a day except Sunday; for 15 minutes; 30 times as a treatment course). N = 20. 3. He‐Ne laser irradiation (non‐acupoint): laser irradiation was thrown on the inner aspect of earlobe where no acupoint was located; once a day except Sunday; for 15 minutes; 30 times as a treatment course. N = 20. Combination therapy: not received any supplementary medication. |
|
| Outcomes | Behaviour: leaving the study early. Adverse effects: numbness over the ear, upper extremity and chest on the treated side (only reported the data of the He‐Ne laser irradiation group (Ermen)). Unable to use: Mental state: rating scale of auditory hallucinations (for this particular trial). Adverse effects: sensation of heat at irradiated site and a feeling of plugged auditory canal (equivocal data). |
|
| Notes | ||
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | Report stated ‐ "randomly numbered and treated as three groups". |
| Allocation concealment (selection bias) | Unclear risk | Not stated. |
| Blinding of participants and personnel (performance bias) All outcomes | High risk | Author reported that three groups all used laser irradiation (two real and one sham) and one group combined with chlorpromazine but did not reported the other two groups used dummy drug. |
| Blinding of outcome assessment (detection bias) All outcomes | Unclear risk | Not stated. |
| Incomplete outcome data (attrition bias) Outcomes | Low risk | No participants left the study early. |
| Selective reporting (reporting bias) | Unclear risk | We were unable to locate the protocol, unsure if selective reporting occurred. |
| Other bias | Low risk | Not obvious. |
laser ‐ Ma 1999.
| Methods | Allocation: randomised. Blindness: not reported. Duration: unclear. | |
| Participants | Diagnosis: schizophrenia with auditory hallucinations (CCMD‐2). N = 120. Age: between 17 and 53 years (mean age (32.02 ± 8.4) years) (He‐Ne laser irradiation + chlorpromazine group); between 16 and 55 years (mean age (31.08 ± 8.6) years) (chlorpromazine group). Sex: 26 women and 94 men. History: duration of illness ≥ 15 days (He‐Ne laser irradiation + chlorpromazine group); ≥ 10 days (chlorpromazine group). Setting: hospitalised patients. Country: China. Inclusion criteria: had a CCMD‐2 diagnosis of schizophrenia; BPRS ≥ 35. Exclusion criteria: brain organic diseases or brain organic diseases. |
|
| Interventions | 1. He‐Ne laser irradiation + chlorpromazine: He‐Ne laser irradiation (Tinggong and Tinghui; wavelength 6328 Å; optimal current output 10 ± mA; negative ion generator 10‐4 A; output total laser power 14‐28 mµ; guided beam Φ3ⅹ300; focus divergent optical lens; for 30 minutes; once a day except Sunday) + chlorpromazine (dose 300 to 550 mg/d; average dose (395 ± 55) mg/d). N = 60. 2. Chlorpromazine: dose 300 to 600 mg/d; average dose (400 ± 85 mg/d). N = 60. Patients receiving antipsychotics before the study commenced needed a 2‐week washout period. Combination therapy: not combined with any other antipsychotics. |
|
| Outcomes | Global state: no clinically important change in global state1. Mental state: BPRS (4 weeks); no clinically important change in specific symptoms (auditory hallucinations)2. Behaviour: leaving the study early (4 week). Service outcome: time to hospitalisation. Others3: Time to auditory hallucinations disappeared. |
|
| Notes | 1. Assessment criteria: according to usual used 4 evaluation clinical criteria (recovery, marked improvement, improvement, no effect). 2. Assessment criteria: auditory hallucinations disappeared completely (disappeared completely); marked improvement (frequency of auditory hallucinations reduced obviously; clarity was significantly reduced; essentially no effect on patient's thinking or behaviour); improvement (frequency of auditory hallucinations reduced slightly; clarity was slightly reduced; partly effect on patient's thinking or behaviour); no effect (no change of auditory hallucinations). 3. Author reported this outcome. |
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | Report stated ‐ "randomly divided into two groups". No further details. |
| Allocation concealment (selection bias) | Unclear risk | Not stated. |
| Blinding of participants and personnel (performance bias) All outcomes | Unclear risk | Sham laser irradiation not used. |
| Blinding of outcome assessment (detection bias) All outcomes | Unclear risk | Not stated. |
| Incomplete outcome data (attrition bias) Outcomes | Low risk | No participants left the study early. |
| Selective reporting (reporting bias) | Unclear risk | We were unable to locate the protocol, unsure if selective reporting occurred. |
| Other bias | Low risk | Not obvious. |
laser ‐ Zhang 1991.
| Methods | Allocation: randomised. Blindness: assessor blind. Duration: 1 week washout period and 5 weeks treatment period. | |
| Participants | Diagnosis: schizophrenia (paranoid type) (DSM III; CCMD‐2‐R).
N = 31.
Age: between 17 and 55 years (mean age 32.4 years).
Sex: 11 women and 20 men. History: duration of illness between 3 months and 22 years (average duration (6.2 ± 5) years). Setting: hospitalised patients. Country: China. Exclusion criteria: somatic diseases. |
|
| Interventions | 1. Laser acupuncture*: group 1 (Dazhui and Shenting); group 2 (Taiyang [double]). N = 11. 2. Laser acupuncture + low dose chlorpromazine: laser acupuncture* (group 1 [Dazhui and Shenting] and group 2 [Taiyang [double]]) + chlorpromazine (150 to 300 mg/day). N = 10. 3. Sham laser acupuncture + chlorpromazine: sham laser acupuncture (needles fixed with tape on acupoints) + chlorpromazine (350 to 600 mg/day). N = 10. *Laser acupuncture: using the two acupoints groups every other day alternately; needles inserted acupoints; for 15 minutes; once a day (except Sunday); 5 weeks as a treatment course; optical fibre output power > 2 MW, output laser distributing angle < 20°; core diameter of optical fibre 300 micron. Combination therapy (laser acupuncture group and laser acupuncture + low dose chlorpromazine group): only could use diazepam or chloral hydrate when patient with insomnia. |
|
| Outcomes | Global state: no clinically important change in global state1,2. Mental state: BPRS3. Behaviour: leaving the study early. Adverse effects: RESES3. Unable to use: Global state: CGI3 (no useful data). |
|
| Notes | 1. The rating was assessed after treatment. 2. Assessment criteria (according to traditional criteria): recovery; marked improvement; improvement; no effect. 3. The ratings were assessed before treatment and each week after treatment. |
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | Report stated ‐ "divided into laser acupuncture group, laser acupuncture and low dose chlorpromazine group and sham laser acupuncture and high dose chlorpromazine group using random allocation method". No further details. |
| Allocation concealment (selection bias) | Unclear risk | Not stated. |
| Blinding of participants and personnel (performance bias) All outcomes | High risk | Though author compared 3 groups and used sham laser acupuncture did not use dummy medications. |
| Blinding of outcome assessment (detection bias) All outcomes | Low risk | Report stated ‐ "two experience doctors assessed outcomes using blind evaluation method". |
| Incomplete outcome data (attrition bias) Outcomes | Low risk | No participants left the study early. |
| Selective reporting (reporting bias) | Unclear risk | We were unable to locate the protocol, unsure if selective reporting occurred. |
| Other bias | Low risk | Not obvious. |
traditional ‐ Bouhlel 2011.
| Methods | Allocation: randomised. Blindness: double‐blind. Duration: 24 days. | |
| Participants | Diagnosis: schizophrenia or schizoaffective disorder (DSM IV). N = 36. Age1: between 21 and 56 years (mean age (36.6 ± 10.06) years). Sex:1 12 women and 19 men. History1: duration of disorders between 1 year and 37 years (average duration (12.68 ± 9.39) years) and the number of previous hospitalisations ranged from 0 to 28 times (average number (6.19 ± 7.02) times). Setting: hospitalised patients. Country: Tunisia. Inclusion criteria: patient who met DSM IV diagnosis of schizophrenia and who gave their consent. Exclusion criteria: patients who were suffering from organic disorder and those who were included in other research protocols. |
|
| Interventions | 1. Traditional acupuncture + antipsychotics: traditional acupuncture* (local points, distal points diagnosed by TCM) + antipsychotics (no further details). N = 151. 2. Sham acupuncture + antipsychotics: no further details. N = 161. * Traditional acupuncture: a simple manipulation; once for 20 minutes and 3 times a week; total 10 times. |
|
| Outcomes | Mental state: PANSS; SAPS; SANS. Unable to use: Behaviour: leaving the study early (no useful data) (no further details). |
|
| Notes | 1. These data were from 31 participants. 2. The author reported outcomes of 31 hospitalised patients and reported that four other participants left the study before completing the 10 sessions and another patient left study after the first session, thus there were a total of 36 participants and we did not know which group the five participants who left the study early came from. |
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | Report stated ‐ "a clinical randomised trial". No further details. |
| Allocation concealment (selection bias) | Unclear risk | Not stated. |
| Blinding of participants and personnel (performance bias) All outcomes | Low risk | Reports stated ‐ "double‐blind". The author reported that both the patient and the psychiatrist did not know if it was a true traditional acupuncture treatment or a placebo (sham acupuncture). |
| Blinding of outcome assessment (detection bias) All outcomes | Low risk | Report stated ‐ "outcome assessments were performed by the same treating psychiatrist and the psychiatrist did not know patient treated by a true traditional acupuncture treatment or sham acupuncture". |
| Incomplete outcome data (attrition bias) Outcomes | High risk | Five participants left the study early. |
| Selective reporting (reporting bias) | Unclear risk | We were unable to locate the protocol, unsure if selective reporting occurred. |
| Other bias | Low risk | Not obvious. |
traditional ‐ Liu 2010.
| Methods | Allocation: randomised. Blindness: not reported. Duration: 3 months. | |
| Participants | Diagnosis: schizophrenia with refractory auditory hallucinations (CCMD‐3).
N = 100.
Age: mean age (34.05 ± 8.35) years (traditional acupuncture + risperidone group); (35.12 ± 7.57) years (risperidone group).
Sex: 43 women and 57 men. History: average duration of illness (4.27 ± 3.70) years (traditional acupuncture + risperidone group); (4.02 ± 3.91) years (risperidone group). Setting: hospitalised patients. Country: China. Inclusion criteria: had a CCMD‐3 diagnosis of schizophrenia and agreed to receive treatment; hospitalised and received systematic therapy with various antipsychotics, at least two, and the majority of psychiatric symptoms disappeared but the auditory hallucinations appeared repeatedly; had received other treatments (such as electric shock, acupuncture or Chinese herbs) but auditory hallucinations remained or reappeared; the duration of auditory hallucinations>8 weeks; BPRS < 30; expected length of hospitalisations > 3 months. Exclusion criteria: with allergic reactions or severe adverse effects; unwilling to receive treatment with the reasons of poor efficacy, side effects or others during trial process; with other severe physical complications during trial process. |
|
| Interventions | 1. Traditional acupuncture + risperidone: traditional acupuncture* (used main acupoints** and adjunct acupoints*** according to the TCM syndrome differentiation) + risperidone (Weisitong; initial dosage 1 mg [before breakfast]; gradually increased to the therapeutic dosage [2 to 6 mg/d] within 2 weeks; average dosage (2.3 ± 0.8) mg/d; daily total dosage less than 3 mg once a day before breakfast; otherwise twice a day before breakfast and dinner). N = 50. 2. Risperidone: Weisitong; initial dosage 1 mg (before breakfast); gradually increased to the therapeutic dosage (2 to 6 mg/d) within 2 weeks; daily total dosage less than 3 mg once a day before breakfast; otherwise twice a day before breakfast and dinner. N = 50. combination therapy: could not combine with any other antipsychotics, antidepressants, mood stabilisers or electric shock; could add artane, scopolamine injections and propranolol with temporary therapeutic dosage according to the condition to enhance patient's compliance and stopped to use when patient's somatic complaints alleviated. * Traditional acupuncture: manipulated needles every 10 minutes; for 30 minutes; 4 to 5 times a week and no less than 4 times; 1 month as 1 treatment course. ** Main acupoints: Shenmen (double); Daling (double); Taichong (double); Tinggong(double); Yifeng (double); Baihui. *** Adjunct acupoints: Type of stagnation of phlegm and Qi: Fenglong (double) and Tanzhong (double). Type of failure of the heart and kidney integrating: Taixi (double) and Laogong (double). Type of phlegm‐fire attacking upwards: Yongquan (double) and Houxi (double) Type of deficiency of both the heart and spleen: Zusanli (double) and Sanyinjiao (double). |
|
| Outcomes | Global state: no clinically important change in global state1. Mental state2: BPRS; SAHS. Behaviour: leaving the study early. Adverse effects: TESS3. |
|
| Notes | 1. Assessment criteria: recovery (auditory hallucinations disappeared completely; without any other psychiatric symptoms; insight recovered completely); marked improvement (the majority of auditory hallucinations disappeared; other psychiatric symptoms improved; insight partly recovered); improvement (the number of auditory hallucinations reduced; voice clarity was fuzzy; partly affected patient's daily life; insight was not complete); no effect (the number, duration and content of auditory hallucinations without marked change or became worse). 2. The ratings were assessed before treatment and at the end of 1, 2 and 3 treatment courses. 3. The rating was assessed at the end of 1, 2 and 3 treatment courses but lab test repeated twice (before and after third treatment). |
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Report stated ‐ "randomly into two groups using the random number table according to the admission order". |
| Allocation concealment (selection bias) | Unclear risk | Not stated. |
| Blinding of participants and personnel (performance bias) All outcomes | High risk | Sham electroacupuncture not used. |
| Blinding of outcome assessment (detection bias) All outcomes | Unclear risk | Not stated. |
| Incomplete outcome data (attrition bias) Outcomes | High risk | Four participants left the study early. |
| Selective reporting (reporting bias) | Unclear risk | We were unable to locate the protocol, unsure if selective reporting occurred. |
| Other bias | Unclear risk | This study was the Lanzhou's 2008 second batch technology program. |
traditional ‐ Luo 2006.
| Methods | Allocation: randomised. Blindness: single‐blind1. Duration: 1 week washout period and 3 months treatment courses. | |
| Participants | Diagnosis: type II syndrome of schizophrenia (CCMD‐3).
N = 60.
Age: between 15 and 54 years (mean age (26.81 ± 7.43) years) (traditional acupuncture + risperidone group); between 16 and 56 years (mean age (27.47 ± 6.78) years) (risperidone group).
Sex: 26 women and 34 men. History: duration of illness between 1.5 and 18 years (average duration (6.81 ± 5.42) years) (traditional acupuncture + risperidone group); between 1.5 and 20 years (average duration (7.73 ± 5.18) years) (risperidone group). Country: China. Inclusion criteria: had a CCMD‐3 diagnosis of schizophrenia; BPRS > 35; SANS ≥ 60; SAPS < 8. Exclusion criteria: severe somatic diseases; cerebral organic psychosis; ethyl alcohol and drug dependence. |
|
| Interventions | 1. Traditional acupuncture + risperidone: traditional acupuncture* (two acupoints groups** in turn) + risperidone (initial dose 1 mg/d; added to 4 to 6 mg/d within 10 days; twice a day). N = 30. 2.Risperidone: initial dose 1 mg/d; added to 4 to 6 mg/d within 10 days; twice a day. N = 30. Combination therapy: supporting symptomatic treatment, psychological and occupational and recreational rehabilitation. * Traditinal acupuncture: for 30 minutes; once a day; 20 days as a treatment course and began another treatment course after 10 non‐treatment days. ** Acupoints group: Group A: Baihui (reinforcing method); Shenting Tou Shangxing (reinforcing method); Zusanli (left), Yanglingquan (left), Neiguan (left), Shenmen (left), Yongquan (left) (reinforcing‐reducing method). Group B: Sishencong (reinforcing method); Yingtang Tou face acupuncture heart area (reinforcing method); Zusanli (right), Yanglingquan (right), Neiguan (right), Shenmen (right), Yongquan (right) (reinforcing‐reducing method). |
|
| Outcomes | Global state: no clinically important change in global state2. Mental state3: BPRS; SANS. Behaviour: leaving the study early. |
|
| Notes | 1. Contact made with author Cheng Luo, however, still unclear about single‐blind method. 2. Assessment criteria (according to criteria of Neuropsychiatric Association of Chinese Medical Association): recovery; marked improvement; effect; no effect. 3. The ratings were assessed before treatment and at 1 month, 2 moths and 3 months after treatment. |
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | Report stated ‐ "randomly divided into two groups". No further details. |
| Allocation concealment (selection bias) | Unclear risk | Not stated. |
| Blinding of participants and personnel (performance bias) All outcomes | Unclear risk | Report stated ‐ "using single‐blind method". No further details. |
| Blinding of outcome assessment (detection bias) All outcomes | Unclear risk | Report stated ‐ "using single‐blind method". No further details. |
| Incomplete outcome data (attrition bias) Outcomes | Low risk | No participants left the study early. |
| Selective reporting (reporting bias) | Unclear risk | We were unable to locate the protocol, unsure if selective reporting occurred. |
| Other bias | Low risk | Not obvious. |
traditional ‐ Ma 2008.
| Methods | Allocation: randomised. Blindness: not reported. Duration: 1 week washout period and 6 weeks treatment period. | |
| Participants | Diagnosis: schizophrenia (type of stagnation of phlegm and Qi) (CCMD‐3, diagnosis of TCM).
N = 60.
Age: between 16 and 48 years (mean age (29.2 ± 6.2) years) (traditional acupuncture + risperidone group); between 17 and 51 years (mean age (30.6 ± 3.6) years) (risperidone group).
Sex: 22 women and 38 men. History: duration of illness between 1 month and 9 years (average duration (4.6 ± 3.42) years) (traditional acupuncture + risperidone group); between 2 months and 11 years (average duration (5.3 ± 3.6) years) (risperidone group). Setting: both inpatients and outpatients. Country: China. Inclusion criteria: had a CCMD‐3 diagnosis of schizophrenia; PANSS ≥ 60; had a TCM diagnosis of type of stagnation of phlegm and Qi. Exclusion criteria: pregnant or lactating women; severe organic diseases; alcohol and drug dependence. |
|
| Interventions | 1. Traditional acupuncture + risperidone: traditional acupuncture* (Juque, Tanzhong, Taichong, Jianshi, Fenglong, Daling, Yingtang) + risperidone (Silishu; initial dose 1 mg/d; added to 2 to 4 mg/d within 2 weeks). N = 30. 2.Risperidone: Silishu; initial dose 1 mg/d; added to 2 to 6 mg/d within 2 weeks. N = 30. * Traditional acupuncture: reducing by rotating needles; for 30 minutes; once a day; 5 days treatment with 2 days non‐treatment interval. |
|
| Outcomes | Mental state: PANSS1,2. Behaviour: leaving the study early. Adverse effects: TESS1. Unable to use: Adverse effects: lab test (kidney function test ‐not reported. |
|
| Notes | 1. The ratings were assessed before treatment and at 2 weeks, 4 weeks and 6 weeks after treatment. 2. Another assessment standard (according to reduced score): recovery (≥ 75%); marked improvement (50% to 74%); improvement (25% to 49%); no effect (< 25%). |
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | Report stated ‐ "randomly divided into two groups". No further details. |
| Allocation concealment (selection bias) | Unclear risk | Not stated. |
| Blinding of participants and personnel (performance bias) All outcomes | High risk | Sham electroacupuncture not used. |
| Blinding of outcome assessment (detection bias) All outcomes | Unclear risk | Not stated. |
| Incomplete outcome data (attrition bias) Outcomes | Low risk | No participants left the study early. |
| Selective reporting (reporting bias) | Unclear risk | 1.We were unable to locate the protocol, unsure if selective reporting occurred. 2. Kidney function tests were completed but not reported and we are unsure of the reason for this omission. |
| Other bias | Low risk | Not obvious. |
traditional ‐ Tang 2005.
| Methods | Allocation: randomised. Blindness: not reported. Duration: 3 months. | |
| Participants | Diagnosis: schizophrenia with auditory hallucinations (CCMD‐3).
N = 84.
Sex: 20 women and 64 men.
Setting: hospitalised patients. Country: China. Inclusion criteria: had a CCMD‐3 diagnosis of schizophrenia; duration of hospitalisation>6 months; received antipsychotics with good compliance but auditory hallucinations did not disappear. |
|
| Interventions | 1. Traditional acupuncture + antipsychotics: traditional acupuncture* (acupoints choice according to the type of TCM**) + antipsychotics (remained previous antipsychotics treatment). N = 42. 2. Antipsychotics: remained previous antipsychotics treatment. N = 42. Combination therapy: could add artane, propranolol and benzodiazepine drugs and could not received any other antipsychotics. * Traditional acupuncture: acupoint near ear and on the head used transport point needling method and twisting and reducing method; acupoint on the limbs used quick‐slow supplementation and draining method and directional supplementation and draining method; manipulated needles every 10 minutes; for 30 minutes; 3 to 4 times a week and not less than 3 times; 3 weeks as a treatment course and 1‐week interval between two treatment courses; total 3 treatment courses. ** Acupoints choice according to type of TCM: Type of stagnation of phlegm and Qi: Taichong, Fenglong, Tinggong, Yifeng, Baihui, Daling, Tanzhong. Type of failure of the heart and kidney integrating: Tinggong, Taixi, Shenmen, Sanyingjiao, Baihui, Tongtian. Type of phlegm‐fire attacking upwards: Laogong, Yongquan, Daling, Taixi, Yifeng, Shenmen, Quchi. Type of deficiency of both the heart and spleen: Baihui, Pishu, Xinshu, Shenshu, Sanyingjiao, Zusanli. |
|
| Outcomes | Global state: no clinically important change in global state1. Behaviour: leaving the study early. |
|
| Notes | 1. Assessment criteria: marked improvement (auditory hallucinations disappeared; psychiatric symptoms cause by auditory hallucinations improved markedly); improvement (times of auditory hallucinations reduced or sound was fuzzy; other psychiatric symptoms improved); no effect (auditory hallucinations and psychiatric symptoms did not improve). | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | Report stated ‐ "randomly divided into two groups". No further details. |
| Allocation concealment (selection bias) | Unclear risk | Not stated. |
| Blinding of participants and personnel (performance bias) All outcomes | High risk | Sham electroacupuncture not used. |
| Blinding of outcome assessment (detection bias) All outcomes | Unclear risk | Not stated. |
| Incomplete outcome data (attrition bias) Outcomes | Low risk | No participants left the study early. |
| Selective reporting (reporting bias) | Unclear risk | We were unable to locate the protocol, unsure if selective reporting occurred. |
| Other bias | Low risk | Not obvious. |
traditional ‐ Wang 2006.
| Methods | Allocation: randomised. Blindness: not reported. Duration: 30 days. | |
| Participants | Diagnosis: hebephrenic schizophrenia (CCMD‐2‐R).
N = 48. Age: mean age (15.87 ± 2.48) years (traditional acupuncture + low dose antipsychotics group); (16.11 ± 2.73 years (traditional acupuncture group); (15.74 ± 2.89) years (traditional antipsychotics group). Sex: 18 women and 30 men. History: average duration of illness (1.54 ± 1.29) years (traditional acupuncture + low dose antipsychotics group); (1.60 ± 1.21) years (traditional acupuncture group); (1.52 ± 1.17) years (traditional antipsychotics group). Setting: hospitalised patients. Country: China. Inclusion criteria: had a CCMD‐2‐R diagnosis of hebephrenic schizophrenia; BPRS ≥ 35. Exclusion criteria: had a CCMD‐2‐R diagnosis of hebephrenic schizophrenia but BPRS < 35; with different treatments caused by combining with other diseases; could not continue to receive treatment during the study period; changed to other treatments. |
|
| Interventions | 1. Traditional acupuncture + low dose antipsychotics: traditional acupuncture* (Taichong [reducing method], Hegu [reducing method], Neiguan [straight inserted], Daling [straight inserted], Renzhong [reducing method], Dazhui [straight inserted] + acupoints choice according to type of TCM**) + low dose antipsychotics (≤equivalent chlorpromazine dose 0.2 g/d). N = 16. 2. Traditional acupuncture*:Taichong (reducing method), Hegu (reducing method), Neiguan (straight inserted), Daling (straight inserted), Renzhong (reducing method), Dazhui (straight inserted) + acupoints choice according to type of TCM**. N = 16. 3. Antipsychotics: enough dose antipsychotics (equivalent chlorpromazine dose 0.4 to 0.6 g/d). N = 16. Combination therapy: psychological counsel every day with parents according to patient's condition. * Traditional acupuncture: continued to manipulate needles 3 to 5 minutes every ten minutes; for 45 minutes; once a day; 15 times as a treatment course; 2 treatment courses. ** Acupoints choice according to type of TCM: Type of Qi stagnation and blood stasis: Yanglingquan, Sanyingjiao. Type of Internal retention of phlegm and dampness; Fenglong. Type of internal disturbance of pyrophlegm: Fenglong, Xingjian. Type of Yin deficiency and fire excess: Fuliu. Type of Yang deficiency: Shenshu (moxibustion), Pishu, Guanyuan (moxibustion). |
|
| Outcomes | Global state: no clinically important change in global state1,2. Mental state3: BPRS, SAPS, SANS. Behaviour: leaving the study early. Adverse effects3: TESS. |
|
| Notes | 1. Assessment criteria: recovery (psychiatric symptoms disappeared completely; insight recovered); effect (marked improvement [psychiatric symptoms disappeared completely or the majority main psychiatric symptoms disappeared; insight partly recovered or not recovered]; improvement [symptoms disappeared partly; insight did not recover); no effect (no symptoms improved obviously and insight did not recover). 2. The rating was assessed 30 days after treatment. 3. The ratings were assessed before treatment and each 15 days after treatment. |
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Report stated ‐ "randomly divided into 3 groups using random allocation table according to the admission date and case number". |
| Allocation concealment (selection bias) | Unclear risk | Not stated. |
| Blinding of participants and personnel (performance bias) All outcomes | High risk | Sham electroacupuncture not used. |
| Blinding of outcome assessment (detection bias) All outcomes | Unclear risk | Not stated. |
| Incomplete outcome data (attrition bias) Outcomes | Low risk | No participants left the study early. |
| Selective reporting (reporting bias) | Unclear risk | We were unable to locate the protocol, unsure if selective reporting occurred. |
| Other bias | Low risk | Not obvious. |
traditional ‐ Xu 2004.
| Methods | Allocation: randomised. Blindness: not reported. Duration: 80 days. | |
| Participants | Diagnosis: schizophrenia (CCMD‐2‐R).
N = 80. Age: mean age (31.74 ± 10.23) years (traditional acupuncture + low dose antipsychotics group); (30.12 ± 8.06) years (antipsychotics group). Sex: 80 men and no women. History: average duration of illness (5.53 ± 6.08) years (start age (25.33 ± 8.12) years) (traditional acupuncture + low dose antipsychotics group); (6.04 ± 3.47) years (start age (26.17 ± 7.32) years) (antipsychotics group). Type: hebephrenic schizophrenia 2 cases, paranoid type 28 cases, simple schizophrenia 1 case, undifferentiated type 9 cases (traditional acupuncture + low dose antipsychotics group); hebephrenic schizophrenia 1 case, paranoid type 26 cases, simple schizophrenia 2 cases, catatonic type 1 case, undifferentiated type 10 cases (antipsychotics group) Setting: hospitalised patients. Country: China. Inclusion criteria: had a CCMD‐2‐R diagnosis of schizophrenia; BPRS ≥ 35. Exclusion criteria: had a CCMD‐2‐R diagnosis of schizophrenia but BPRS < 35 or had diseases which would affect trial results. |
|
| Interventions | 1. Traditional acupuncture + low dose antipsychotics: traditional acupuncture* (used main acupoints** and adjunct acupoints*** [according to the TCM type of schizophrenia] and special acupoints**** [according to symptoms]) + low dose antipsychotics (equivalent chlorpromazine dose ≤ 0.2 g/d). N = 40. 2. Antipsychotics: maximum daily dose equivalent chlorpromazine dose 0.4 to 0.7 g/d. N = 40. Combination therapy: added artane when patient with severe extrapyramidal side effects; added propranolol when patient with severe palpitation and heart rate > 100 times/minute. * Traditional acupuncture: used lifting‐thrusting supplementation and draining method and twirling supplementation and draining method according to patient's condition; manipulated needles about 3 minutes and once ten minutes; total for about 30 minutes; at first once a day then reduced to once every two days when patient was in stable condition and once a week after psychiatric symptoms disappeared. ** Main acupoints: Shuigou, Baihui, Neiguan, Sanyingjiao. ***Adjunct acupoints (according to the CTM type of schizophrenia): Type of internal disturbance of pyrophlegm: Zhongwan, Fenglong, Xingjian. Type of Internal Retention of phlegm and dampness: Fenglong, Yinglingquan, Zusanli. Type of Qi stagnation and Blood stasis: Xuehai, Geshu. Type of Yin deficiency and fire excess: Shenmen, Fuliu. Type of Yang deficiency: Taixi, Guanyuan (moxibustion). Other miscellaneous types such as deficiency of both heart and spleen: Anmian, Shenmen. **** Special acupoints (according to symptoms): Zhongzhu, Tinggong (auditory hallucinations); Yuyao (phosphenes); Hegu, Laogong (agitation). |
|
| Outcomes | Mental state1: BPRS; SAPS; SANS. Behaviour: leaving the study early. Adverse effects: TESS2. Unable to use: Global state: no clinically important change in global state3,4 (only reported P value, P value > 0.05). |
|
| Notes | 1. The ratings were assessed before treatment and each 20 days after treatment.
2. The rating was assessed each 20 days after treatment. 3. Assessment criteria: recovery (psychiatric symptoms disappeared completely; insight recovered); marked improvement (psychiatric symptoms disappeared completely or the majority main psychiatric symptoms disappeared; insight partly recovered or not recovered); improvement (symptoms disappeared partly; insight did not recover); no effect (no symptoms improved obviously and insight did not recover). 4. The rating was assessed after treatment. |
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Report stated ‐ "randomly divided into experimental group and control group using random allocation table according to the admission date and case number". |
| Allocation concealment (selection bias) | Unclear risk | Not stated. |
| Blinding of participants and personnel (performance bias) All outcomes | High risk | Sham electroacupuncture not used. |
| Blinding of outcome assessment (detection bias) All outcomes | Unclear risk | Not stated. |
| Incomplete outcome data (attrition bias) Outcomes | Low risk | Three participants left the study early but results graphs (BPRS, SAPS, SANS, TESS) showed that author analysed 40 cases in each group. |
| Selective reporting (reporting bias) | Unclear risk | We were unable to locate the protocol, unsure if selective reporting occurred. |
| Other bias | Unclear risk | Participants were men and unsure if gender selection bias occurred. |
traditional ‐ Zhao 2005a.
| Methods | Allocation: randomised. Blindness: not reported. Duration: 60 days. | |
| Participants | Diagnosis: schizophrenia (type I; Kuang type) (CCMD‐2‐R; Diagnosis and Effect Standard of Diseases in TCM [1994]) .
N = 300.
Sex: 162 women and 138 men. History: longer than 3 months. Setting: outpatients. Country: China. Inclusion criteria: had a CCMD‐2‐R diagnosis of schizophrenia; had a TCM diagnosis of Kuang type of phlegm‐fire disturbing mind; stopped to receive relative drug treatment more than 1 month. Exclusion criteria: cerebral organic mental disorders; mental disorders caused by somatic diseases; mental disorders caused by psychoactive substances and non‐dependent substances; had both schizophrenia and mood disorders symptoms but the duration of illness of disruptive symptoms was shorter than that of mood disorder symptoms or no longer than 2 weeks; with severe cardiovascular and cerebrovascular, liver and haematopoietic system diseases. |
|
| Interventions | 1. Fuyuankang capsule: 5 capsules (0.4 g/capsule) every time; 3 times a day; with warm water. N = 90. 2. Traditional acupuncture: Shuigou, Shaoshang, Yingbai, Fengfu, Daling, Quchi, Fenglong; once a day; for 30 minutes. N = 90. 3. Traditional acupuncture + Fuyankang capsule: traditional acupuncture (Shuigou, Shaoshang, Yingbai, Fengfu, Daling, Quchi, Fenglong; once a day; for 30 minutes) + Fuyuankang capsule (5 capsules every time; 3 times a day; with warm water). N = 90. 4. Weisitong (risperidone tablet): initial dose 1 mg/d (after dinner); added 1 mg after 3 days then added 0.1 mg every week until daily dose 4 mg. N = 30. * Author used placebo with therapeutic medications; no further details. |
|
| Outcomes | Global state: no clinically important change in global state1. Behaviour: leaving the study early. Unable to use: Global state: TCM Syndromes Scale2(no useful data). Mental state: BPRS (no data). Adverse effects: lab test (ECG; blood routine test; urine routine test; stool test; liver function; kidney function); blood pressure; adverse reaction (only reported data for traditional acupuncture + Fuyuankang group). Lab test: SOD (not clinical outcomes). |
|
| Notes | 1. Assessment criteria: recovery (talk, behaviour and expression all returned to normal state; could deal with daily affairs; the reduction of BPRS ≥ 95%; improvement (talk, behaviour and expression returned to normal state approximately or obviously improved; the reduction of BPRS 30% to 94%); no effect (talk, behaviour and expression still existed as before; the reduction of BPRS < 29%). 2. Author also reported the global state of TCM. 3. The study of 'Zhao 2005' consisted of two parts ‐ 'traditional ‐ Zhao 2005a' (type I schizophrenia) and 'traditional ‐ Zhao 2005b' (type II schizophrenia). 4. The allocation rate of groups of each type schizophrenia was 3:3:3:1. |
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | Report stated ‐ "randomly divided". No further details. |
| Allocation concealment (selection bias) | Unclear risk | Not stated. |
| Blinding of participants and personnel (performance bias) All outcomes | High risk | Used placebo but did not use sham acupuncture. |
| Blinding of outcome assessment (detection bias) All outcomes | Unclear risk | Not stated. |
| Incomplete outcome data (attrition bias) Outcomes | Low risk | No participants left the study early. |
| Selective reporting (reporting bias) | High risk | Although the protocol was not located, the author reported that BPRS was one of the outcomes, however, only reported global state. |
| Other bias | Low risk | Not obvious. |
traditional ‐ Zhao 2005b.
| Methods | Allocation: randomised. Blindness: not reported. Duration: 60 days. | |
| Participants | Diagnosis: schizophrenia (type II; Dian type) (CCMD‐2‐R; Diagnosis and Effect Standard of Diseases in TCM [1994]) .
N = 300.
Sex: 156 women and 144 men. History: longer than 3 months. Setting: outpatients. Country: China. Inclusion criteria: had a CCMD‐2‐R diagnosis of schizophrenia; had a TCM diagnosis of Dian type of phlegm‐Qi stagnation and blinding; stopped to receive relative drug treatment more than 1 month. Exclusion criteria: cerebral organic mental disorders; mental disorders caused by somatic diseases; mental disorders caused by psychoactive substances and non‐dependent substances; had both schizophrenia and mood disorders symptoms but the duration of illness of disruptive symptoms was shorter than that of mood disorder symptoms or no longer than 2 weeks; with severe cardiovascular and cerebrovascular, liver and haematopoietic system diseases. |
|
| Interventions | 1. Fuyuankang capsule: 5 capsules (0.4 g/capsule) every time; 3 times a day; with warm water. N = 90. 2. Traditional acupuncture: Xinshu, Ganshu, Pishu, Shenmen, Fenglong; once a day; for 30 minutes. N = 90. 3. Traditional acupuncture + Fuyankang capsule: traditional acupuncture (Xinshu, Ganshu, Pishu, Shenmen, Fenglong; once a day; for 30 minutes) + Fuyuankang capsule (5 capsules every time; 3 times a day; with warm water). N = 90. 4. Weisitong (risperidone tablet): initial dose 1 mg/d (after dinner); added 1 mg after 3 days then added 0.1 mg every week until daily dose 4 mg. N = 30. * Author used placebo with therapeutic medications; no further details. |
|
| Outcomes | Global state: no clinically important change in global state1. Behaviour: leaving the study early. Unable to use: Global state: TCM Syndromes Scale2(no useful data). Mental state: BPRS. Adverse effects: lab test (ECG; blood routine test; urine routine test; stool test; liver function; kidney function); blood pressure; adverse reaction (only reported data of traditional acupuncture + Fuyuankang group). Lab test: SOD (not clinical outcomes). |
|
| Notes | 1. Criteria: recovery (talk, behaviour and expression all returned to normal state; could deal with daily affairs; the reduction of BPRS ≥ 95%; improvement (talk, behaviour and expression returned to normal state approximately or obviously improved; the reduction of BPRS 30% to 94%); no effect (talk, behaviour and expression still existed as before; the reduction of BPRS < 29%). 2. Author also reported the global state of TCM. 3. The study of 'Zhao 2005' consisted of two part ‐ 'traditional ‐ Zhao 2005a' (type I schizophrenia) and 'traditional ‐ Zhao 2005b' (type II schizophrenia). 4. The allocation rate of groups of each type schizophrenia was 3:3:3:1. |
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | Report stated ‐ "randomly divided". No further details. |
| Allocation concealment (selection bias) | Unclear risk | Not stated. |
| Blinding of participants and personnel (performance bias) All outcomes | High risk | Used placebo but did not use sham acupuncture. |
| Blinding of outcome assessment (detection bias) All outcomes | Unclear risk | Not stated. |
| Incomplete outcome data (attrition bias) Outcomes | Low risk | No participants left the study early. |
| Selective reporting (reporting bias) | High risk | Although the protocol was not located, the author reported that BPRS was one of the outcomes, however, only reported global state. |
| Other bias | Low risk | Not obvious. |
Diagnostic tools and scales: DSM: Diagnostic and Statistical Manual. ICD 10: International Classification of Diseases. CCMD: Chinese Classification of Mental Disorders.
Global state: CGI: Clinical Global Impression Scale. CGI‐SI: Clinical Global Impression ‐ Severity of illness. CGI‐GI: Clinical Global Impression ‐ Improvement scale. CGI‐EI: Clinical Global Impression ‐ Efficacy Index.
Mental state: BPRS: Brief Psychiatric Rating Scale. PANSS: Positive and Negative Syndrome Scale. SANS: Scale for the Assessment of Negative Symptoms. SAPS: Scale for the Assessment of Positive Symptoms. SAHS: Specific Auditory Hallucination Scale. HAMD: Hamilton Rating Scale for Depression. SDS: Zung Self‐Rating Depression Scale. PSYRATS‐AH: Psychotic Symptom Rating Scales Auditory Hallucination Subscale.
Adverse effects: TESS: Treatment Emergent Symptom Scale. RESES: Rating Scale for Extrapyramidal Side Effects.
Test: ECG: electrocardiogram. EEG: electroencephalogram. cAMP: cyclic adenosine monophosphate. cGMP: cyclic guanosine monophosphate. T3: triiodothyronine. T4: thyroid hormone. RUR: T Resin Test. FT4I: free T index. TSH: thyroid‐stimulation hormone. rT3: trans triiodothyronine. TG: thyroglobulin antibody. TM: thyroid microsome. LH: luteinizing hormone. FSH: follicle stimulating hormone. T: testosterone. SOD: superoxide dismutase.
Others: TCM: Traditional Chinese Medicine. CCEA: Computer‐Controlled Electric Acupuncture. mg/d: mg per day.
Characteristics of excluded studies [ordered by study ID]
| Study | Reason for exclusion |
|---|---|
| Luo 2006a | Allocation: randomised. Participants: people with schizophrenia or mood disorders (affective disorders) manic episode. Interventions: acupuncture + antipsychotics vs antipsychotics. Outcomes: not reported by diagnostic group. |
| Ma 2002 | Allocation: randomised. Participants: people with chronic schizophrenia. Interventions: antipsychotics + electroacupuncture (Yingtang and Baihui) versus antipsychotics + electroacupuncture (Baihui and Spiritual and emotional movement area) (compared electroacupuncture with different acupoints), not acupuncture vs other treatments. |
| Ma 2004 | Allocation: not randomised. |
| Sun 1994 | Allocation: not randomised, case series. |
| Wu 2004 | Allocation: randomised. Participants: people with schizophrenia. Interventions: acupoint injection + low dose chlorpromazine (Wintermin) versus chlorpromazine (Wintermin). Outcomes: BPRS (mental state); TESS (adverse effects). * Some important data from this study (conducted in same institution as acupoint inj ‐ Pan 2002) were the same as acupoint inj ‐ Pan 2002 (baseline and endpoint total BPRS), but numbers allocated, the acupoint method, and BPRS' factor data were different; we could not contact author of Wu 2004 but author of acupoint inj ‐ Pan 2002 had no knowledge of this study. |
| Xiong 2009 | Allocation: not randomised, quasi‐randomised (according to the admission order; patient with odd number into electroacupuncture group and with even number into MECT group). |
| Xue 1985 | Allocation: divided randomly to three intervention groups but unclear how allocated within one key intervention group Participants: people with schizophrenia. Interventions: Electroacupuncture/acuclip convulsive therapy (EACT) (involved mixture of techniques ‐ not all of which are acupuncture ‐ several of which could be applied in a single person) versus electroconvulsive therapy (ECT) versus ECT or EACT (unclear how allocation to EACT undertaken within this group), not acupuncture versus none/other treatment. |
| Zhong 1995 | Allocation: randomised. Participants: people with common mental diseases. Interventions: electroacupuncture versus computerised electrode. |
| Zhuge 1993 | Allocation: not randomised, allocated according to age and sex. |
Mental state: BPRS: Brief Psychiatric Rating Scale.
Adverse effects: TESS: Treatment Emergent Symptom Scale. Test: ECT: electroconvulsive therapy. EACT: electric acupuncture convulsive therapy.
Other: MECT: modified electroconvulsive therapy
Characteristics of studies awaiting assessment [ordered by study ID]
NCT01167348.
| Methods | Allocation: randomised.
Blindness: single‐blind (assessor).
Duration: 2 months. Design: parallel. |
| Participants | Diagnosis: schizophrenia with obesity (ICD). N = 86 (estimated enrolment). Sex: women and men. Inclusion criteria: ICD:295, living in chronic ward for more than 2 months; body mass index (BMI) > or = 24; aged between 20 to 60; psychotic status stable and can communicate. Exclusion criteria: unstable psychotic status; participants who have endocrine disease; participants who have cardiac disease; participants who have immunological disease; participants who have liver or renal function impairment; pregnant or lactating woman; cerebrovascular accident (CVA) stroke and disability; participants who attend weight control programs in last 3 months; any conditions that treating doctors refuse to join in this study. |
| Interventions | 1. Auricular acupressure: with a 0.5 mm x 0.5 mm seed on the specific positions on the ear and press these specific positions 3 times a day. 2. Placebo: no further details. |
| Outcomes | Metabolic: Body weight scale, waste circumference, fat percentage by BIA. |
| Notes | Contacted with Han‐Yi Ching using the email address from the ClinicalTrials.gov but no feedback. No further details and unable to confirm whether the study has finished and unable to locate the full text. |
BIA: Bio‐Impedance Analysis ICD: International Classification of Diseases.
Differences between protocol and review
1. Methodology update
The protocol used for this update has been changed and updated: taken from the most recent methodology of the Cochrane Schizophrenia Group, it acknowledges changes in Cochrane methodology since this review was first published. For example, use of 'Summary of findings' tables instead of calculating NNT/NNH and changes in the 'Risk of bias' tables and 'methods' being described in more detail. We do not think the use of these new methods affects the results or integrity of the review. For previous methodology please see Appendix 3.
2. Type of interventions
The title of this review is broad ‐ 'Acupuncture for schizophrenia'. The previous version used a more narrow range of comparisons and, we felt, left out some that are important. We have added all categories of acupuncture alone or in combination regimens compared with placebo (or no treatment) or any other treatments. For previous types of interventions please see Appendix 1.
Contributions of authors
Xiaohon Shen ‐ selected studies, extracted data and updated the full review.
Jun Xia ‐ selected studies and extracted data.
Clive E Adams ‐ assisted with data extraction, undertook analysis and writing the final report.
Sources of support
Internal sources
Shanghai Shuguang Hospital ‐ affiliated to Shanghai University of Traditional Chinese Medicine, China.
University of Nottingham, UK.
External sources
No sources of support supplied
Declarations of interest
Xiaohong Shen ‐ none known.
Jun Xia ‐ none known.
Clive E Adams ‐ none known.
New search for studies and content updated (no change to conclusions)
References
References to studies included in this review
acupoint cat ‐ Sun 2005 {published data only}
- Sun L, Zhang H, Xia S, Gong F, Wang J, Guo M, et al. Control study of hallucinations of schizophrenia by the acupoint catgut treatment [穴位埋线治疗分裂症幻觉的对照研究]. Practical Clinical Journal of Integrated Traditional Chinese and Western Medicine [实用中西医结合临床] 2005;5(6):5‐6. [Google Scholar]
acupoint cat ‐ Wang 1997 {published data only}
- Wang J, Ye Y. Effective analysis of Tinggong embedding catgut method combining with antipsychotics on auditory hallucinations treatment [听宫穴埋线法合并抗精神病药治疗幻听的疗效分析]. Chinese Journal of Psychiatry 1997;30(4):202. [Google Scholar]
acupoint inj ‐ Pan 2002 {published data only}
- Pan Y, Wang J, Liu S, Wu C, Wang L, Shen L. Treatment for schizophrenia patients with acupoint injection [穴位注药治疗精神分裂症的研究]. Journal of Taishan Medical College 2002;23(2):128‐30. [Google Scholar]
- Pan Y, Wu C, Pan Y. A control study of schizophrenia patients according to types [精神分裂症分型施治的对照研究]. Journal of the Handan Medical College 2003;16(6):493‐4. [Google Scholar]
- Pan Y, Pan Y, Wu C, Li M. A follow‐up study of schizophrenia patients treated by acupuncture and drug [针药并用治疗精神分裂症的随访研究]. Health Psychology Journal 2002;10(6):456‐7. [Google Scholar]
acupoint inj ‐ Wang 2000 {published data only}
- Wang G, Ji R, Pei G. The control study of injection of clonazepami into T'ingkung for refractory auditory hallucination of schizophrenia [听宫穴注射氯硝西泮治疗精神分裂症顽固性幻听对照研究]. Journal of Clinical Psychosomatic Diseases [临床心身疾病杂志] 2000;6(3):143‐4. [Google Scholar]
acupoint inj ‐ Yang 2000 {published data only}
- Yang S, Liu G. Observation on intractable auditory hallucination treated by injecting sulpiride into acupoints [穴位注射舒必利治疗顽固性幻听疗效观察]. Journal of Practical Traditional Chinese Medicine 2000;16(7):24‐5. [Google Scholar]
EACT ‐ Xue 1987 {published data only}
- Xue C, Wang J, Tang M. Comparative study of spinal fractures in electric acupuncture convulsive therapy, electroconvulsive therapy and epilepsy [电针抽搐治疗、电抽搐治疗与癫痫所致的脊柱骨折]. Chinese Journal of Neurology and Psychiatry [Chung Hua Shen Ching Ching Shen Ko Tsa Chih] 1987;20(6):346‐9. [PubMed] [Google Scholar]
electro ‐ Chen 2006 {published data only}
- Chen Z, Song H, Wen N. [Title only available in Chinese characters] [电针治疗精神分裂症后抑郁]. Modern Journal of Integrated Traditional Chinese and Western Medicine 2006;15(13):1776‐7. [Google Scholar]
electro ‐ Chen 2008 {published data only}
- Chen K, Li D, Ye L. The effect of electric acupuncture combined with aripiprazole in treating negative symptoms of schizophrenia [电针合并阿立哌唑治疗精神分裂症阴性症状的疗效观察]. Medical Journal of Chinese People's Health [中国民康医学] 2008;20(1):7‐10. [Google Scholar]
electro ‐ Cheng 2009 {published data only}
- Bai X. An 8‐week randomized study of acupuncture in the treatment of auditory hallucinations in refractory and non‐refractory schizophrenic patients. Stanley Foundation Research Programs. China, 2002.
- Cheng J, Wang G, Xiao L, Wang H, Wang X, Li C. Electro‐acupuncture versus sham electro‐acupuncture for auditory hallucinations in patients with schizophrenia: a randomized controlled trial. Clinical Rehabilitation 2009;23(7):579‐88. [DOI] [PubMed] [Google Scholar]
electro ‐ Cui 2000 {published data only}
- Cui G, Wang P. The clinical study of combining electrostimulation and chlorpromazine in schizophrenia [电针合并氯丙嗪治疗精神分裂症的临床研究]. Modern Journal of Integrated Chinese Traditional and Western Medicine 2000;9(16):1536‐7. [Google Scholar]
electro ‐ Ding 2005 {published data only}
- Ding G, Ling F, Zhang J. [only Chinese Title available] [针灸治疗慢性精神分裂症的临床效果分析]. Modern Journal of Integrated Traditional Chinese and Western Medicine 2005;14(1):53‐4. [Google Scholar]
electro ‐ Wang 2005 {published data only}
- Wang P, Wang X, Li X, Wu X, Duan D. Controlled studies on combination of electroacupuncture and risperidone in negative symptoms of schizophrenia [电针合并利培酮治疗精神分裂症阴性症状对照研究]. Liaoning Journal of Traditional Chinese Medicine 2005;32(7):710‐1. [Google Scholar]
electro ‐ Xiong 2010 {published data only}
- Xiong D, Liu L, Yi Y, Ye F. Observation on the therapeutic effect of electroacupuncture combined with small dose of clozapine in clinical treatment of refractory schizophrenia [电针联合小剂量氯氮平治疗难治性精神分裂症的疗效观察]. Chen Tzu Yen Chiu / Acupuncture Research 2010;35(2):134‐7. [MEDLINE: ] [PubMed] [Google Scholar]
electro ‐ Yao 2006 {published data only}
- Yao F, Sun F, Zhang Z. Short‐term curative effect of electroacupuncture as an adjunctive treatment on schizophrenia [电针辅助治疗精神分裂症的近期疗效观察]. Chinese Journal of Integrated Traditional and Western Medicine 2006;26(3):253‐5. [MEDLINE: ] [PubMed] [Google Scholar]
electro ‐ Zhang 1987 {published data only}
- Wu T. Personal Communication 2008.
- Zhang L, Tang Y, Zhu W, Xu S. Comparative study of schizophrenia treatment with electroacupuncture, herbs and chlorpromazine. Chinese Medical Journal 1987;100:152‐7. [PubMed] [Google Scholar]
- Zhang L, Xu S, Tang Y, Zhu W. A comparative study of the treatment of schizophrenia with electric acupuncture, herbal decoction and chlorpromazine. American Journal of Acupuncture 1990;18(1):11‐4. [MEDLINE: ; PMID 10416732] 10416732 [Google Scholar]
electro ‐ Zhang 1993 {published data only}
- Zhang B, Meng S, Yu J, Quan C, Li W, Sun H, et al. A controlled study of therapeutic effects of computer‐controlled electric acupuncture treatment on refractory schizophrenia. World Journal of Acupuncture‐Moxibustion 1993;3(4):3‐9. [Google Scholar]
- Zhang B, Meng S, Yu J, Quan C, Li W, Sun H, et al. Clinical therapeutic effect of mentality electroacupuncture on schizophrenia [智能电针仪治疗精神分裂症临床疗效对照研究]. Chinese Acupuncture and Moxibustion 1994;17(1):17‐20. [MEDI9402] [Google Scholar]
electro ‐ Zhang 2001 {published data only}
- Zhang Y, Shao H, Zhao X, Liu W, He J, Chai L, et al. The clinical efficacy with intelligent electro‐acupuncture of treating schizophrenia with depressive symptoms [智能电针治疗精神分裂症伴发抑郁症状的临床疗效观察]. Chinese Journal of Behavioral Medical Science 2001;10(1):44‐5. [MEDI0104] [Google Scholar]
electro ‐ Zhou 1997 {published data only}
- Zhou G, Jin S, Zhang L. Comparative clinical study on the treatment of schizophrenia with electroacupuncture and reduced doses of antipsychotic drugs. American Journal of Acupuncture 1997;25(1):25‐31. [153rd Annual Meeting of the American Psychiatric Association [CD‐ROM]: MARATHON Multimedia, 2000 NR80] [Google Scholar]
laser ‐ Liu 1986 {published data only}
- Liu Z, Wang Y, Zhang S, He A, Chen Y, Liu X. Therapeutic effect of He‐Ne laser irradiation of point erman in schizophrenic auditory hallucination‐‐a clinical assessment. Journal of Traditional Chinese Medicine = Chung i tsa chih ying wen pan / sponsored by All‐China Association of Traditional Chinese Medicine, Academy of Traditional Chinese Medicine 1986;6(4):253‐6. [PUBMED: 3600018] [PubMed] [Google Scholar]
laser ‐ Ma 1999 {published data only}
- Ma Z, Li X, Lv Y. A control study of auditory hallucination treated with point‐stimulating therapy of helium neon [氦氖激光穴位照射治疗具有幻听症状的精神分裂症对照研究]. Sichuan Mental Health [四川精神卫生] 1999;12(2):90‐1. [Google Scholar]
laser ‐ Zhang 1991 {published data only}
- Zhang B, Quan C, Yu J, Li W, Sun H, Liu S, et al. A controlled study of clinical therapeutic effects of laser acupuncture for schizophrenia [激光针灸治疗精神分裂症临床疗效的对照研究]. Chung Hua Shen Ching Ching Shen Ko Tsa Chi (Chinese Journal of Neurology and Psychiatry) 1991;24(2):81‐3, 124. [MEDLINE: ; PMID 1860386] [PubMed] [Google Scholar]
traditional ‐ Bouhlel 2011 {published data only}
- Bouhlel S, El‐Hechmi S, Ghanmi L, Ghaouar M, Besbes C, Khaled M, et al. Effectiveness of acupuncture in treating schizophrenia: A clinical randomised trial about 31 patients. Tunisie Medicale 2011;89(10):774‐8. [PubMed] [Google Scholar]
traditional ‐ Liu 2010 {published data only}
- Liu X. Clinical observation of acupuncture treatment in 50 schizophrenia with refractory auditory hallucinations [针刺治疗精神分裂症顽固性幻听患者50例临床观察]. Shanghai Journal of Traditional Chinese Medicine [Shang Hai Zhong Yi Yao Za Zhi][中医杂志] 2010;7:621‐4. [Google Scholar]
traditional ‐ Luo 2006 {published data only}
- Luo C, Zhou W. Study of acupuncture adjunctive therapy on type II syndrome of schizophrenia [针灸辅助治疗精神分裂症II型综合征的研究]. Modern Journal of Integrated Traditional Chinese and Western Medicine 2006;15(2):148‐9. [Google Scholar]
traditional ‐ Ma 2008 {published data only}
- Ma L, Zhang E, Lu S. Control study of acupuncture combining with risperidone for schizophorenia [针刺合用利培酮治疗精神分裂症对照研究]. Medical Journal of Chinese People's Health [中国民康医学] 2008;20(17):1981. [Google Scholar]
traditional ‐ Tang 2005 {published data only}
- Tang Y, Huang Q, Guo J. Clinical analysis of acupuncture for auditory hallucinations [针刺治疗幻听临床分析]. Chinese Journal of Current Traditional and Western Medicine 2005;3(5):431‐2. [Google Scholar]
- Tang Y, Huang Q, Li H, Wen Y, Guo J. Clinical analysis of acupuncture in the treatment of phonism [针刺治疗幻听临床分析]. Chinese Journal of Health Psychology [中国健康心理学杂志] 2007;15(4):367. [Google Scholar]
traditional ‐ Wang 2006 {published data only}
- Wang L, Xie Y. Clinical study of acupuncture for hebephrenic schizophrenia [针刺治疗青春期精神分裂症的临床研究]. Journal of Clinical Acupuncture and Moxibustion 2006;22(9):12‐4. [Google Scholar]
traditional ‐ Xu 2004 {published data only}
- Xu T, Diao H, Xu P, Gan H, Sun Z, Xu L, et al. Clinical study on acupuncture combined with small dose of antipsychotics for treatment of 40 cases of schizophrenia [針刺配合小劑量抗精神病藥物治療精神分裂癥40例臨床研究]. Journal of Traditional Chinese Medicine [Chung i tsa chih ying wen pan][中醫雜志] 2004;45(1):22‐5. [Google Scholar]
traditional ‐ Zhao 2005a {published data only}
- Zhao Y. Acupuncture Is It Recover Health Capsule Trear Schizophrenia and Influence to Patient's Blood Free Radical Supersession to Share [针刺合用复元康胶囊治疗精神分裂症及其对患者血自由基代谢影响]. Doctoral Dissertation of Heilongjiang University of TCM 2005.
traditional ‐ Zhao 2005b {published data only}
- Zhao Y. Acupuncture Is It Recover Health Capsule Trear Schizophrenia and Influence to Patient's Blood Free Radical Supersession to Share [针刺合用复元康胶囊治疗精神分裂症及其对患者血自由基代谢影响]. Doctoral Dissertation of Heilongjiang University of TCM 2005.
References to studies excluded from this review
Luo 2006a {published data only}
- Luo C, Zhang Y, Xiong K, Zhou W. A study of acupunncture on prevent side ‐ effect caused by antipsychotics [针刺预防抗精神病药物副反应110例临床观察]. Journal of the Yunnan College of Traditional Chinese Medicine 2006;29(3):27‐8. [Google Scholar]
Ma 2002 {published data only}
- Ma H, Guo Y, Fan H, Li Y, Yue S. [Title only available in Chinese characters] [头部电针治疗慢性精神分裂症临床研究]. Proceedings of the 7th Psychiatry Conference of the Society of Intergreted Traditional Chinese and Western Medicine. 2002:213‐6.
- Yang S, Guo Y, Yue S. [Title only available in Chinese characters] [头部电针治疗慢性精神分裂症临床疗效及护理]. Medical Journal of Chinese People's Health [中国民康医学] 2003;15(10):630‐1. [Google Scholar]
Ma 2004 {published data only}
- Ma L. [Title only available in Chinese characters] [电针百会、神庭穴对精神分裂症患者认知功能影响的临床研究]. MSc dissertation submitted to the Beijing University of Chinese Medicine, China 2004.
- Ma L, Gu S, Zhang X. Effect of electroacupuncture at baihui and shenting on cognitive function of patients with convalescent schizophrenia. Chinese Journal of Clinical Rehabilitation 2005;9(4):99‐101. [Google Scholar]
Sun 1994 {published data only}
- Sun Z, Wu H, Sun Y, Zhao A. Observation on treatment of 350 cases of schizophrenia with electroacupuncture and acupoint‐injection. Chinese Acupuncture and Moxibustion 1994, issue S1:53‐4.
Wu 2004 {published data only}
- Wu C, Li M. A study of acupuncture point injection in the treatment of schizophrenia. Chinese General Practice [中国全科医学] 2004;7(19):1382‐4. [Google Scholar]
Xiong 2009 {published data only}
- Xiong D, Yi Y, Zhu X, Hu W. Controlled study on therapeutic effects of electroacupuncture and modified electroconvulsive therapy on catatonic schizophrenia [电针与无抽搐电休克治疗紧张型精神分裂症]. Chinese Acupuncture and Moxibustion 2009;29(10):804‐6. [MEDLINE: ] [PubMed] [Google Scholar]
Xue 1985 {published data only}
- Xue C, Xie H, Ruan Q, Cheng Y, Liu D. Electric acupuncture convulsive therapy. Convulsive Therapy 1985;1(4):242‐51. [MEDLINE: ; PMID 8251722] [PubMed] [Google Scholar]
Zhong 1995 {published data only}
- Zhong H, Feng X, Luo H, Jia Y, Zhao X. Comparative observation on treatment of psychosis with electrode and electro‐acupuncture controlled by processors. World Journal Acupuncture‐Moxibustion 1995;5(3):24‐7. [MEDI95S5] [Google Scholar]
Zhuge 1993 {published data only}
- Zhuge D, Chen J. Comparison between electro‐acupuncture with chlorpromazine and chlorpromazine alone in 60 schizophrenic patients. Chung‐Kuo Chung Hsi i Chieh Ho Tsa Chih 1993;13(7):408‐9. [MEDLINE: ; PMID 8251722] [PubMed] [Google Scholar]
References to studies awaiting assessment
NCT01167348 {published data only}
- NCT01167348. The effectiveness of auricular acupressure on bodybweight parameters on patients with schizophrenia. http://ClinicalTrials.gov/show/NCT01167348 2010.
Additional references
Adams 2007
- Adams EC, Awad G, Rathbone J, Thornley B. Chlorpromazine versus placebo for schizophrenia. Cochrane Database of Systematic Reviews 2007, Issue 2. [DOI: 10.1002/14651858.CD000284] [DOI] [PubMed] [Google Scholar]
Alderson 2004
- Alderson P, Green S, Higgins JPT. Cochrane Reviewers' Handbook 4.2.2 [updated December 2003]. The Cochrane Library. Chichester, UK: John Wiley & Sons, Ltd, 2004. [In: The Cochrane Library, Issue 1, 2004. Chichester, UK: John Wiley & Sons, Ltd.] [Google Scholar]
Altman 1996
- Altman DG, Bland JM. Detecing skewness from summary information. BMJ 1996;313:1200. [OLZ020600] [DOI] [PMC free article] [PubMed] [Google Scholar]
Andreasen 1982
- Andreasen NC. Negative symptoms in schizophrenia. Definition and reliability. Archives of General Psychiatry 1982;39:784‐8. [DOI] [PubMed] [Google Scholar]
Bland 1997
- Bland JM. Statistics notes. Trials randomised in clusters. BMJ 1997;315:600. [DOI] [PMC free article] [PubMed] [Google Scholar]
Boissel 1999
- Boissel JP, Cucherat M, Li W, Chatellier G, Gueyffier F, Buyse M, et al. The problem of therapeutic efficacy indices. 3. Comparison of the indices and their use [Apercu sur la problematique des indices d'efficacite therapeutique, 3: comparaison des indices et utilisation. Groupe d'Etude des Indices D'efficacite]. Therapie 1999;54(4):405‐11. [PUBMED: 10667106] [PubMed] [Google Scholar]
Chen 2002
- Chen Y. Chines Classification of Mental Disorders (CCMD‐3): Towards Integration in International Classification. Psychopathology 2002;35(2‐3):171‐5. [DOI] [PubMed] [Google Scholar]
Deeks 2000
- Deeks J. Issues in the selection for meta‐analyses of binary data. Proceedings of the 8th International Cochrane Colloquium; 2000 Oct 25‐28; Cape Town. Cape Town: The Cochrane Collaboration, 2000.
Divine 1992
- Divine GW, Brown JT, Frazier LM. The unit of analysis error in studies about physicians' patient care behavior. Journal of General Internal Medicine 1992;7(6):623‐9. [DOI] [PubMed] [Google Scholar]
Donner 2002
- Donner A, Klar N. Issues in the meta‐analysis of cluster randomized trials. Statistics in Medicine 2002;21:2971‐80. [DOI] [PubMed] [Google Scholar]
Egger 1997
- Egger M, Davey‐Smith G, Schneider M, Minder CSO. Bias in meta‐analysis detected by a simple, graphical test. BMJ 1997;13:629‐34. [DOI] [PMC free article] [PubMed] [Google Scholar]
Elbourne 2002
- Elbourne D, Altman DG, Higgins JPT, Curtina F, Worthingtond HV, Vaile A. Meta‐analyses involving cross‐over trials: methodological issues. International Journal of Epidemiology 2002;31(1):140‐9. [DOI] [PubMed] [Google Scholar]
Ernst 2001
- Ernst E, A White. Prospective studies of the safety of acupuncture: a systematic review. American Journal of Medicine 2001;110:481‐5. [DOI] [PubMed] [Google Scholar]
Fan 2010
- Fan F, Hong X, Song J. Research and prospect on laser acupuncture [激光针灸的研究现状与展望]. Laser Journal 2010;31(5):58‐9. [Google Scholar]
Furukawa 2006
- Furukawa TA, Barbui C, Cipriani A, Brambilla P, Watanabe N. Imputing missing standard deviations in meta‐analyses can provide accurate results. Journal of Clinical Epidemiology 2006;59(7):7‐10. [DOI] [PubMed] [Google Scholar]
Gulliford 1999
- Gulliford MC. Components of variance and intraclass correlations for the design of community‐based surveys and intervention studies: data from the Health Survey for England 1994. American Journal of Epidemiology 1999;149:876‐83. [DOI] [PubMed] [Google Scholar]
Guo 2010
- Guo Y, Shi Y, Li Y, Liu Y, Zhang Y. Research progress of Traditional Chinese Medicine on schizophrenia treatment [中药治疗精神分裂症的研究进展]. Modern Journal of Integrated Traditional Chinese and Western Medicine 2010;19(27):3543‐5. [Google Scholar]
Guy 1970
- Guy W, Bonato RR, eds. Clinical global impressions. Manual for the ECDEU Assessment Battery 2. Rev ed. National Institute of Mental Health, 1970. [Google Scholar]
Guy 1976
- Guy W. ECDEU Assessment Manual for Psychopharmacology (DOTES: Dosage Record and Treatment Emergent Symptom Scale). Rockville: National Institute of Mental Health, 1976. [Google Scholar]
Haddock 1999
- Haddock G, McCarron J, Tarrier N, Faragher EB. Scale to measure dimensions of hallucinations and delusions: the psychotic symptom rating scales (PSYRATS). Psychological Medicine 1999;29(4):879‐89. [DOI] [PubMed] [Google Scholar]
Hamilton 1967
- Hamilton M. Development of a rating scale for primary depressive illness. British Journal of Social and Clinical Psychology 1967;4(Dec, 6):278‐96. [DOI] [PubMed] [Google Scholar]
Higgins 2003
- Higgins JP, Thompson SG, Deeks JJ, Altman DG. Measuring inconsistency in meta‐analyses. BMJ 2003;327:557‐60. [DOI] [PMC free article] [PubMed] [Google Scholar]
Higgins 2011
- Higgins JPT, Green S (editors). Cochrane Handbook for Systematic Reviews of Interventions Version 5.0.2 [updated September 2011]. The Cochrane Collaboration, 2011. Available from www.cochrane‐handbook.org.
Hutton 2009
- Hutton Jane L. Number needed to treat and number needed to harm are not the best way to report and assess the results of randomised clinical trials. British Journal of Haematology 2009;146(1):27‐30. [DOI] [PubMed] [Google Scholar]
Kaptchuk 2002
- Kaptchuk TJ. Acupuncture: theory, efficacy, and practice. American College of Physicians ‐ American Society of Internal Medicine 2002;136:374‐83. [DOI] [PubMed] [Google Scholar]
Kay 1986
- Kay SR, Opler LA, Fiszbein A. Positive and Negative Syndrome Scale (PANSS) Manual. North Tonawanda, NY: Multi‐Health Systems, 1986. [Google Scholar]
Lee 2001
- Lee S. From diversity to unit. The classification of mental disorders in 21st‐century China. Psychiatric Clinics of North America 2001;24(3):421‐31. [DOI] [PubMed] [Google Scholar]
Lee 2009
- Lee MS, Shin BC, Ronan P, Ernst E. Acupuncture for schizophrenia: a systematic review and meta‐analysis. International Journal of Clinical Practice 2009;63(11):1622‐33. [PUBMED: 19832819] [DOI] [PubMed] [Google Scholar]
Leucht 2005
- Leucht S, Kane JM, Kissling W, Hamann J, Etschel E, Engel RR. What does the PANSS mean?. Schizophrenia Research 2005;79(2‐3):231‐8. [PUBMED: 15982856] [DOI] [PubMed] [Google Scholar]
Leucht 2005a
- Leucht S, Kane JM, Kissling W, Hamann J, Etschel E, Engel R. Clinical implications of brief psychiatric rating scale scores. British Journal of Psychiatry 2005;187:366‐71. [PUBMED: 16199797] [DOI] [PubMed] [Google Scholar]
Leucht 2007
- Leucht S, Engel RR, Bauml J, Davis JM. Is the superior efficacy of new generation antipsychotics an artifact of LOCF?. Schizophrenia Bulletin 2007;33(1):183‐91. [PUBMED: 16905632] [DOI] [PMC free article] [PubMed] [Google Scholar]
Li 2009
- Li J. Research progress of placebo method in acupuncture clinical trials [安慰方法在针灸临床试验中的研究进展]. Journal of Complementary and Alternative Medicine 2009;25(1):53‐4. [Google Scholar]
Lu 2006
- Lu W, Shi J, Zhou X, Chen F. Effective observation of body acupuncture combine with auricular acupuncture to treat schizophrenia refractory auditory hallucinations [体针合并耳穴压籽治疗精神分裂症顽固性幻听的疗效观察]. Sichuan Mental Health 2006;19(4):236‐7. [Google Scholar]
MacPherson 2001
- MacPherson H, Thomas K, Walters S, Fitter M. The York acupuncture safety study: prospective survey of 34 000 treatments by traditional acupuncturists. 2001 BMJ;323(Sept):486‐7. [DOI] [PMC free article] [PubMed] [Google Scholar]
MacPherson 2010
- MacPherson H, Altman DG, Hammerschlag R, Youping L, Taixiang W, White A, et al. STRICTA Revision Group. Revised STandards for Reporting Interventions in Clinical Trials of Acupuncture (STRICTA): extending the CONSORT statement. Acupuncture in Medicine 2010;28:83‐93. [DOI] [PMC free article] [PubMed] [Google Scholar]
Marshall 2000
- Marshall M, Lockwood A, Bradley C, Adams C, Joy C, Fenton M. Unpublished rating scales: a major source of bias in randomised controlled trials of treatments for schizophrenia. British Journal of Psychiatry 2000;176:249‐52. [DOI] [PubMed] [Google Scholar]
Meng 2012
- Meng S, Lv J, Wen X. Recent state on clinical application of acupoint catgut treatment [穴位埋线疗法临床应用近况]. Shanxi Journal of Traditional Chinese Medicine 2012;28(2):56‐8. [Google Scholar]
Ming 2001
- Translated by Zhu Ming. The Medical Classics of the Yellow Emperor. Beijing: Foreign Language Press, 2001. [Google Scholar]
Moher 2001
- Moher D, Schulz KF, Altman D. The CONSORT statement: revised recommendations for improving the quality of reports of parallel‐group randomized trials. JAMA 2001;285:1987‐91. [DOI] [PubMed] [Google Scholar]
Nicholas 1997
- Beecroft N, Rampes H. Review of acupuncture for schizophrenia. Acupuncture in Medicine 1997;15:91‐4. [Google Scholar]
Overall 1962
- Overall JE, Gorham DR. The Brief Psychiatric Rating Scale. Psychological Reports 1962;10:799‐812. [Google Scholar]
Rathbone 2005b
- Rathbone J, Zhang L, Zhang M, Xia J, Liu X, Yang Y. Chinese herbal medicine for schizophrenia. Cochrane Database of Systematic Reviews 2005, Issue 4. [DOI: 10.1002/14651858.CD003444] [DOI] [PMC free article] [PubMed] [Google Scholar]
Rattehalli 2010
- Rattehalli RD, Jayaram MB, Smith M. Risperidone versus placebo for schizophrenia. Cochrane Database of Systematic Reviews 2010, Issue 1. [DOI: 10.1002/14651858.CD006918.pub2] [DOI] [PubMed] [Google Scholar]
Schünemann 2008
- Schünemann HJ, Oxman AD, Vist GE, Higgins JPT, Deeks JJ, Glasziou P, et al. Chapter 12: Interpreting results and drawing conclusions. In: Higgins JPT, Green S editor(s). Cochrane Handbook for Systematic Reviews of Interventions. The Cochrane Collaboration, 2008:359‐83. [Google Scholar]
Shi 2007
- Shi X. Science of Acupuncture and Moxibustion. Beijing: Chinese Publishing House of Traditional Chinese Medicine, 2007. [Google Scholar]
Shi 2010
- Shi J, Zhou X, Gao M, Wang P. Status of acupuncture for schizophrenia [针灸治疗精神分裂症的现状]. Medical Journal of National Defending Forces in North China 2010;22(4):100‐2. [Google Scholar]
Simon 2000
- Simon JD, Douglas GA. Blinding in clinical trials and other studies. BMJ 2000;321:19‐26. [DOI] [PMC free article] [PubMed] [Google Scholar]
Simpson 1970
- Simpson GM, Angus JWS. A rating scale for extrapyramidal side effects. Acta Psychoiatrica Scandinavica 1970;212:11‐9. [DOI] [PubMed] [Google Scholar]
Tang 2010
- Tang Z, Chen H. Research progress of effects and mechanism of acupoint injection [穴位注射作用效应及机制的研究进展]. Zhejiang JITCWM 2010;20(2):119‐20. [Google Scholar]
The Psychosis Professional Committee 1988
- The Psychosis Professional Committee of Chinese Integrative Medicine Association. The standard of integrative medicine syndrome type of schizophrenia [精神分裂症的中西医结合辨证分型标准]. Chinese Journal of Integrative Medicine 1988;8(2):127. [Google Scholar]
Ukoumunne 1999
- Ukoumunne OC, Gulliford MC, Chinn S, Sterne JAC, Burney PGJ. Methods for evaluating area‐wide and organistation‐based intervention in health and health care: a systematic review. Health Technology Assessment 1999;3(5):1‐75. [PubMed] [Google Scholar]
Wang 2007
- Wang Y. Overview of Chinese Medicine and acupuncture for schizophrenia [精神分裂症的中药及针灸治疗概况]. Yunnan Journal of Traditional Chinese Medicine and Materia Medica 2007;28(10):54‐5. [Google Scholar]
Xia 2009
- Xia J, Adams CE, Bhagat N, Bhagat V, Bhoopathi P, El‐Sayeh H, et al. Loss to outcomes stakeholder survey: the LOSS study. Psychiatric Bulletin 2009;33(7):254‐7. [Google Scholar]
Xu 1991
- Xu X. The English‐Chinese Encyclopedia of Practical Traditional Chinese Medicine. Beijing: Higher Education Press, 1991. [Google Scholar]
Xu 2010
- Xu T, Su J. Clinical status and thinking of acupuncture for schizophrenia [针灸治疗精神分裂症的临床现状与思考]. CJITWM 2010;30(11):1130‐2. [Google Scholar]
Yang 2009
- Yang Z, Zheng Q, Li Y, Wu X. Overview of adoption and research of sham acupuncture in acupuncture clinical research [针灸临床研究中假针刺的运用及研究概况]. Journal of New Chinese Medicine 2009;41(3):93‐5. [Google Scholar]
Yang 2012
- Yang Y, Wei Y, Wang Y, Yin L, Xu Y, Liu Y. Current status and fundamental strategies for clinical research on acupuncture and moxibustion [针灸临床研究现状与基本策略]. Shanghai Journal of Traditional Chinese Medicine 2012;46(1):7‐10. [Google Scholar]
Zhang 1993
- Zhang B, Meng S, Yu J, Quan C, Li W, Sun H, et al. A controlled study of therapeutic effects of computer‐controlled electric acupuncture treatment on refractory schizophrenia. World Journal of Acupuncture‐Moxibustion 1993;3(4):3‐9. [Google Scholar]
Zhang 1996
- Zhang J. Current status of integrated traditional and Western medicine study on schizophrenia. Chinese Journal of Integrated Traditional and Western Medicine 1996;16(11):643‐5. [PubMed] [Google Scholar]
Zhang 2012
- Zhang Y, Lao L, Ceballos R. Acupuncture use among American adults: What acupuncture practitioners can learn from National Health Interview Survey 2007?. Evidence‐Based Complementary and Alternative Medicine 2012. [DOI: 10.1155/2012/710750] [DOI] [PMC free article] [PubMed]
Zung 1965
- Zung WW. A self‐rating depression scale. Archives of General Psychiatry 1965;12:63‐70. [DOI] [PubMed] [Google Scholar]
References to other published versions of this review
Rathbone 2005a
- Rathbone J, Xia J. Acupuncture for schizophrenia. Cochrane Database of Systematic Reviews 2005, Issue 4. [DOI: 10.1002/14651858.CD005475] [DOI] [PubMed] [Google Scholar]


