Abstract
We aimed to investigate the role of the laboratory frailty index (LFI) in diabetic complications and incident disability in admitted older patients with type 2 diabetes mellitus (T2DM). We retrospectively collected the clinical data of older patients with T2DM from December 2018 to May 2020. Frailty was quantified using the LFI, which considers the accumulation of 27 items of abnormal laboratory outcomes. Univariate and multivariate analyses were performed to evaluate the relationship between LFI and diabetes-related adverse outcomes. In total, 293 consecutive older patients with T2DM were recruited for this study. According to the predefined LFI criteria, 110 (37.5%) participants were non-frail, 131 (44.7%) were prefrail, and 52 (17.8%) were frail. Univariate and multivariate analysis revealed that LFI was associated with the diabetic microangiopathy complications (odds ratio for prefrail [ORprefrail] 1.760, 95% confidence interval for prefrail [CIprefrail] 1.019-3.041, P = .043; ORfrail 4.667, 95% CIfrail 2.012-10.826, P < .001) and activities of daily living (ADL) disability (ORprefrail 2.323, 95% CIprefrail 1.209-4.463, P = .011; ORfrail 9.367, 95% CIfrail 4.030-21.775, P < .001), but not with the diabetic macroangiopathy complications and diabetic peripheral neuropathy. Frailty, as determined by the LFI, was proven to be an effective tool for the prediction of diabetic microangiopathy complications and ADL disability.
Keywords: type 2 diabetes mellitus, laboratory frailty index, microangiopathy complications, macroangiopathy complications, inflammation
What do we already know about this topic?
Previous studies have shown that older adults with diabetes tend to be frailer than those without diabetes. However, evidence for association between laboratory frailty index (LFI) and adverse health outcomes in older adults with type 2 diabetes mellitus (T2DM) is lacking.
How does your research contribute to the field?
This is the first study to investigate the role of LFI in diabetic complications and incident disability in older patients with T2DM. Frailty, defined by LFI, was proven to be an independent risk factor for diabetic microangiopathy complications and activities of daily living (ADL) disability, suggesting that the potential use of LFI in the clinical setting to determine whether the participants were prefrail or frail.
What are your research’s implications toward theory, practice, or policy?
It may be simpler to construct a frailty index using routine laboratory data commonly collected in clinical settings than based on clinical assessment data alone. Frail patients defined by the LFI require preventive multidimensional interventions to mitigate frailty development and progression of frailty.
Introduction
With an aging population and low level of physical activity, the prevalence of diabetes in China has increased by 9.73% from1980 to 2013. 1 There is an increase in diabetes prevalence with age, with diabetes affecting over 25% of individuals aged 65 years and older. 2 The public health burden of diabetes mellitus (DM) is exacerbated in the aging population and is strongly associated with higher mortality among affected individuals. 3 Diabetes-related costs are estimated at 35–40% due to vascular complications, including macroangiopathy and microangiopathy, which pose a threat to independence, self-care capacity, and quality of life. 2 Comprehensive evaluation of older patients with type 2 DM (T2DM) is essential to improve their quality of life, preserve their functionality, and avoid complications. 4
The clinical condition of frailty accounts for the most problematic manifestation of population aging. 5 Frailty is a state of diminished resilience to homeostasis after stress, resulting in a higher risk of adverse outcomes, such as falls, fractures, dementia, and even disability.5 -9 Frailty is an age-related clinical syndrome. It is prevalent among many older people. 10 Additionally, frail patients with diabetes have a higher mortality rate than robust individuals with diabetes. 11 In recent years, frailty screening and assessment have been increasingly perceived as necessary by societies. 12 Several methods have been used to measure frailty due to a lack of consensus. 13 One common approach for estimating frailty is the laboratory frailty index (LFI), which considers the accumulation of abnormal laboratory outcomes. 14 In recent studies, incipient frailty states have been identified using the LFI to assess the risk of adverse outcomes associated with clinically detectable frailty. 15 Frailty is the main determinant of poor outcomes in older individuals, 16 and is associated with a high burden of inflammation. 17 It is associated with poor control of chronic diseases, such as T2DM. 18 Moreover, sarcopenia, a major determinant of frailty, is strongly correlated with low vitamin D levels. 19 Thus, studying laboratory parameters based on frailty index in diabetic subjects is reasonable.
Several studies have shown that patients with diabetes tend to be frailer than older adults without diabetes.20 -22 There is a lack of evidence pertaining to the association between LFI and adverse health outcomes in older adults with T2DM. In this study, we investigated the role of LFI in diabetes-related complications and incident disability in admitted older adults with T2DM.
Materials and Methods
Patients
This study included patients who were consecutively admitted to the Endocrinology Department of our hospital between December 2018 and May 2020. Inclusion criteria included individuals aged 65 or older, diagnosed with T2DM according to the World Health Organization National Diabetic group criteria. 23 The exclusion criteria were hospital admission for acute illness or missing data. This study was conducted in accordance with the ethical principles of the Declaration of Helsinki.
The sample size required to develop predictive models for binary outcomes was estimated based on the principle of at least 10 events for each included predictor.24,25 In the present study, multivariate analyses were conducted on factors with P < .10 in the univariate analyses. Five predictors were included in the logistic regression model. In the present study, 52 individuals with frailty were identified so that more robust models could be developed.
Data Collection
Data were obtained from the inpatient hospital information system within the first 72 h of admission. Data from each patient included the following parameters: (1) clinical characteristics, such as age, sex, body mass index (BMI), systolic and diastolic blood pressure (SBP and DBP), heart rate (HR), smoking status, duration of diabetes, and hemoglobin A1c (HbA1c). (2) Adverse outcomes, including diabetic nephropathy (DN, defined as the ratio of urinary albumin to creatinine ≥30 mg/g 26 ), diabetic retinopathy (DR, was identified by dilated ophthalmoscopy and standard fundus photographs), diabetic peripheral neuropathy (DPN, was defined by either of the following criteria: (i) at least one abnormal neurological screening testing [temperature and pinprick sensation, vibration perception with 128 Hz tuning fork, touch perception with 10 g monofilament testing, and ankle reflexes] with DPN symptoms [foot sensations, such as numbness, pain, and paresthesia]; (ii) at least 2 abnormal screening examinations without DPN symptoms) 27 ; coronary artery disease (CAD, defined as coronary artery calcification detected by computed tomography and/or coronary stenosis detected by coronary angiography); cerebrovascular disease (CVD, was defined as a history of stroke or transient cerebral); peripheral arterial disease (PAD, was defined as the formation of plaque in the lower extremity artery on ultrasonography) 28 ; and activities of daily living (ADL, was assessed by the Barthel Index, which includes 10 items: feeding, bathing, grooming, dressing, controlling bowels, controlling bladder, toileting, transferring chair/bed, walking, and climbing stairs. To qualify as ADL disability, at least one of the items had to be performed with assistance). 29
Definition of Frailty
Frailty was measured using the LFI based on deficit accumulation. Supplemental Table 1 describes the scoring criteria of the LFI, which were derived from the most common laboratory tests, yielding 27 items. 15 Items were scored as normal or abnormal according to the local reference ranges. To calculate LFI, the number of abnormal results was divided by the total number of tests. For instance, if a patient had 3 abnormal test results from the 27 items, this would result in an LFI score of 0.11 (3/27). To generate a valid LFI score, at least 70%, or 19 of the 27 items, required to be measured. 15 According to the LFI score, patients were classified as non-frail (<0.15), pre-frail (0.15-0.28), or frail (≥0.28).
Statistical Analyses
The Kolmogorov-Smirnov test was used to estimate the normality of continuous variables. Analysis of variance test was used to compare continuous variables with a normal distribution, as measured by the mean ± standard deviation (SD). The median and interquartile range (IQR) were calculated for continuous variables with a non-normal distribution using the Kruskal-Wallis H test for comparison. Numbers and percentages were used to describe categorical variables by applying the Pearson’s chi square test or Fisher’s exact test for comparison. Binary logistic regression analysis was used to investigate the association between frailty and adverse outcomes. Multivariate analyses were conducted on factors with P < .10 in the univariate analyses. A logistic regression analysis was performed using odds ratios (ORs) and 95% confidence intervals (CIs). Statistical significance was determined using a 2-tailed P < .05. All statistical analyses were performed using the IBM SPSS Statistics software (version 26.0).
Results
In total, 293 consecutive patients with T2DM aged >65 years were recruited for this study. Figure 1 shows the distribution of LFI, the LFI was obviously right-skewed. According to the predefined LFI criteria, 110 (37.5%) of 293 participants were non-frail, 131 (44.7%) were prefrail, and 52 (17.8%) were frail.
Figure 1.
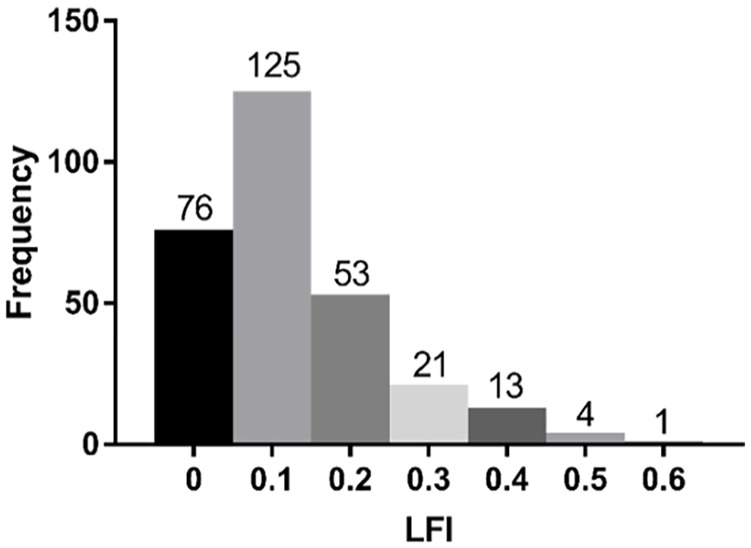
The distribution of the LFI.
Table 1 summarized the baseline characteristics of the population according to the LFI categories. On enrollment, they had a median age of 72 years, a median height of 1.60 m, a median weight of 62.5 kg, a median BMI of 24.3 kg/m2, a median SBP of 142 mmHg, a median DBP of 74 mmHg, a median HR of 78 bpm, a median duration of diabetes of 11 years, and a median HbA1c of 8.50%. Among the recruited patients, 150 (51.2%) were males and 143 (48.8%) were females. Among the LFI categories, there were significant differences in DBP, HR, and the use of metformin and insulin but no significant differences were observed in age, sex, height, weight, BMI, SBP, duration of diabetes, smoking status, drinking status, or HbA1c levels.
Table 1.
Patient Demographic and Clinical Characteristics.
| Characteristics | Total (n = 293) | Non-Frail (n = 110) | Pre-frail (n = 131) | Frail (n = 52) | P |
|---|---|---|---|---|---|
| Age, median (IQR), years | 72 (10) | 71 (8) | 72 (11) | 74 (11) | .451 |
| Sex, n (%) | .108 | ||||
| Male | 150 (51.2) | 62 (56.4) | 56 (42.7) | 25 (48.1) | |
| Female | 143 (48.8) | 48 (43.6) | 75 (57.3) | 27 (51.9) | |
| Height, median (IQR), m | 1.60 (0.12) | 1.60 (0.13) | 1.60 (0.13) | 1.60 (0.12) | .913 |
| Weight, median (IQR), kg | 62.5 (16.0) | 63.0 (18.0) | 63 (15.0) | 60.5 (14.0) | .645 |
| BMI, median (IQR), kg/m2 | 24.3 (4.4) | 24.5 (4.8) | 24.3 (4.2) | 23.5 (4.3) | .585 |
| BMI group, n (%) | .572 | ||||
| Underweight (<18.5) | 6 (2.0) | 2 (1.8) | 2 (1.5) | 2 (3.8) | |
| Normal (18.5-23.9) | 130 (44.4) | 44 (40.0) | 60 (45.8) | 26 (50.0) | |
| Overweight (≥24) | 157 (53.6) | 64 (58.2) | 69 (52.7) | 24 (46.2) | |
| SBP, median (IQR), mmHg | 142 (28) | 144 (26) | 142 (26) | 136 (36) | .236 |
| DBP, median (IQR), mmHg | 74 (15) | 75 (12) | 74 (14) | 72 (12) | .029 * |
| HR, median (IQR), b.m.p | 78 (14) | 76 (12) | 77 (15) | 80 (14) | .007 * |
| Duration of diabetes, years | 11 (13) | 11 (13) | 11 (13) | 14 (14) | .494 |
| Smoking status, n (%) | .211 | ||||
| Never | 211 (72.0) | 85 (77.3) | 85 (65.6) | 40 (76.9) | |
| Previous | 43 (14.7) | 13 (11.8) | 22 (16.8) | 8 (15.4) | |
| Current | 39 (13.3) | 12 (10.9) | 23 (17.6) | 4 (7.7) | |
| Drinking status, n (%) | .540 | ||||
| Never | 224 (76.4) | 88 (80.0) | 95 (72.5) | 41 (76.5) | |
| Previous | 31 (10.6) | 8 (7.3) | 19 (14.5) | 4 (10.6) | |
| Current | 38 (13.0) | 14 (12.7) | 17 (14.0) | 7 (12.9) | |
| Drugs | |||||
| Metformin, n (%) | 174 (59.4) | 80 (72.7) | 81 (61.8) | 13 (25.0) | <.001 * |
| Insulin, n (%) | 160 (54.6) | 50 (45.5) | 72 (55.0) | 38 (73.1) | .004 * |
| HbA1c, median (IQR), % | 8.50 (3.10) | 8.65 (2.83) | 8.40 (3.20) | 8.50 (4.05) | .938 |
IQR = interquartile range; BMI = body mass index; SBP = systolic blood pressure; DBP = diastolic blood pressure; HR = heart rate; HbA1c = hemoglobin A1c.
Statistically significant (P < .05).
Table 2 presented descriptive statistics of the cross-sectional outcome measures of the LFI categories. The most common diabetic complications were DN (n = 138, 47.1%), DR (n = 111, 37.9%), CAD (n = 165, 56.3%), CVD (n = 165, 56.3%), PAD (n = 40,13.7%) and DPN (n = 185, 63.1%). We found that the LFI categories were associated with diabetic microangiopathy complications (P = .002*) and ADL disability (P < .001*) but not with diabetic macroangiopathy complications (P = .099). Both DN (P < .001*) and DR (P = .002*) occurred more frequently in the frail group.
Table 2.
Adverse Outcomes.
| Outcomes | Total (n = 293) | Non-Frail (n = 110) | Pre-Frail (n = 131) | Frail (n = 52) | P |
|---|---|---|---|---|---|
| Diabetic microangiopathy | 183 (62.5) | 57 (51.8) | 84 (64.1) | 42 (80.8) | .002 * |
| DN, n (%) | 138 (47.1) | 36 (32.7) | 64 (48.9) | 38 (73.1) | <.001 * |
| DR, n (%) | 111 (37.9) | 30 (27.3) | 52 (39.7) | 29 (55.8) | .002 * |
| Diabetic macroangiopathy | 213 (72.7) | 76 (69.1) | 93 (71.0) | 44 (84.6) | .099 |
| CAD, n (%) | 165 (56.3) | 63 (57.3) | 67 (51.1) | 35 (67.3) | .134 |
| CVD, n (%) | 109 (37.2) | 33 (30.0) | 53 (40.5) | 23 (44.2) | .126 |
| PAD, n (%) | 40 (13.7) | 12 (10.9) | 18 (13.7) | 10 (19.2) | .354 |
| DPN, n (%) | 185 (63.1) | 67 (60.9) | 88 (67.2) | 30 (57.7) | .404 |
| ADL disability, n (%) | 105 (35.8) | 23 (20.9) | 48 (36.6) | 34 (65.4) | <.001 * |
DN = diabetic nephropathy; DR = diabetic retinopathy; CAD = coronary artery disease; CVD = cerebrovascular disease; PAD = peripheral arterial disease; DPN = diabetic peripheral neuropathy; ADL = activities of daily living.
Statistically significant (P < .05).
Table 3 illustrated the results of the association between the LFI categories and outcomes using binary logistic regression analysis. We found LFI categories were associated with the diabetic microangiopathy complications (ORprefrail 1.760, 95% CIprefrail 1.019-3.041, P = .043*; ORfrail 4.667, 95% CIfrail 2.012-10.826, P < .001*), DN (ORprefrail 1.987, 95% CIprefrail 1.141-3.461, P = .015*; ORfrail 5.798, 95% CIfrail 2.671-12.588, P < .001*), DR (ORprefrail 1.770, 95% CIprefrail 1.009-3.103, P = .046*; ORfrail 3.291, 95% CIfrail 1.598-6.778, P < .001*) and ADL disability (ORprefrail 2.323, 95% CIprefrail 1.209-4.463, P = .011*; ORfrail 9.367, 95% CIfrail 4.030-21.775, P < .001*), which were consistent with the findings from the univariate analysis.
Table 3.
Binary Logistic Regression Frailty and Outcomes.
| Outcomes | OR | 95% CI | P |
|---|---|---|---|
| Diabetic microangiopathy | .001 * | ||
| Non-Frail | Ref. | ||
| Pre-Frail | 1.760 | 1.019-3.041 | .043 * |
| Frail | 4.667 | 2.012-10.826 | <.001 * |
| DN | <.001 * | ||
| Non-Frail | Ref. | ||
| Pre-Frail | 1.987 | 1.141-3.461 | .015 * |
| Frail | 5.798 | 2.671-12.588 | <.001 * |
| DR | .005 * | ||
| Non-Frail | Ref. | ||
| Pre-Frail | 1.770 | 1.009-3.103 | .046 * |
| Frail | 3.291 | 1.598-6.778 | .001 * |
| Diabetic macroangiopathy | .164 | ||
| Non-Frail | Ref. | ||
| Pre-Frail | 1.040 | 0.545-1.984 | .906 |
| Frail | 2.653 | 0.929-7.580 | .069 |
| CAD | .352 | ||
| Non-Frail | Ref. | ||
| Pre-Frail | 0.754 | 0.430-1.323 | .325 |
| Frail | 1.269 | 0.563-2.859 | .565 |
| CVD | .212 | ||
| Non-Frail | Ref. | ||
| Pre-Frail | 1.442 | 0.817-2.546 | .207 |
| Frail | 1.946 | 0.885-4.280 | .098 |
| PAD | .708 | ||
| Non-Frail | Ref. | ||
| Pre-Frail | 1.121 | 0.466-2.697 | .799 |
| Frail | 1.615 | 0.510-5.114 | .415 |
| DPN | .461 | ||
| Non-Frail | Ref. | ||
| Pre-Frail | 1.191 | 0.670-2.116 | .551 |
| Frail | 0.737 | 0.334-1.627 | .451 |
| ADL disability | <.001 * | ||
| Non-Frail | Ref. | ||
| Pre-Frail | 2.323 | 1.209-4.463 | .011 * |
| Frail | 9.367 | 4.030-21.775 | <.001 * |
Ref. = reference; DN = diabetic nephropathy; DR = diabetic retinopathy; CAD = coronary artery disease; CVD = cerebrovascular disease; PAD = peripheral arterial disease; DPN = diabetic peripheral neuropathy; ADL = activities of daily living.
Statistically significant (P < .05).
Discussion
Previous studies typically employed Fried’s frailty phenotype and the FRAIL scale, defined by a comprehensive geriatric assessment.20,30,31 However, the frailty phenotype and FRAIL scale may not capture all the factors that affect an individual’s life expectancy. Previous LFI studies demonstrated that combining self-reports with clinical measures increased the accuracy of mortality prediction.14,22,32 It may be simpler to construct a frailty index using routine laboratory data commonly collected in clinical settings than based on clinical assessment data alone. Howlett et al. assessed a 21-item LFI based on 21 laboratory data points and DBP and SBP measures in community-dwelling older adults and institutionalized individuals. 14 Similarly, Blodgett et al . evaluated a 23-item LFI based on routine blood tests and standard physical measurements in community-dwelling older adults. 33 Adding more deficit factors to the LFI may enhance its predictive capability. Therefore, in the current study, we operationalized the LFI using 27 of the most common laboratory parameters based on blood samples. Notably, the LFI has the advantage that it can be analyzed using a single laboratory report from a general laboratory without having to consider additional variables, such as standard physical examinations or more specialized blood tests.
To the best of our knowledge, the association between the LFI and adverse outcomes in older patients with T2DM has not been evaluated in previous studies. This is the first study to evaluate the relationship between LFI and adverse outcomes in older patients with T2DM. In the present study, we demonstrated that the prevalence of frailty measured using the LFI was 17.8% in older patients with T2DM. The proportion of frailty was lower than that reported by Ellis et al . 15 because the average patient age in their study was 84.8 years, which is much higher than the median age of patients in this study (72 years). Several studies have shown that the LFI can predict mortality.34,35 In contrast, Blodgett et al . found that the LFI and mortality did not show a significant association in 20 to 39 years old patients. 32 Therefore, the LFI may have a different role in predicting death among varying age groups.
The present study showed that the LFI was a useful screening method to predict adverse outcomes, including microangiopathy complications and ADL disability in patients with T2DM. It is unclear how laboratory tests fit into major and minor deficits. Deficits in clinical health result from unrepaired and unremoved insults that accumulate over time and cause widespread time-dependent damage at the cellular, tissue, and organ levels. According to this concept, deficits at the cellular or molecular levels eventually lead to dysfunction at the macroscopic organ level.36,37 Consequently, frailty can be viewed as a dynamic process that originates at the subcellular level and affects tissues, organs, and ultimately the overall organism function.35,37 It was recently proposed that, in general, this process reflects the accumulation of damage, both unrepaired and unremoved, and could be modeled at a systemic level. 38 A higher level of frailty increases the risk of adverse frailty outcomes such as hospitalization and mortality. 39 Our group was motivated to pursue further investigations based on these ideas.
There is evidence that treatment can slow or reverse frailty progression and help minimize potential health consequences.40,41 Management strategies for diabetes in older adults with a long life expectancy are similar to those in younger individuals. However, individuals who are frail are unlikely to benefit from such strategies. 5 First, the target should be less stringent control (HbA1c ≤ 8.0%-8.5%) for frail older adults with increased susceptibility to hypoglycemia or at a high risk of hypoglycemia.42,43 Second, multicomponent exercise interventions, including aerobic activity and resistance training, have proven to be the best strategies for reducing the risk of frailty and seem to be the most effective strategies for improving balance, gait, and strength, as well as preventing older individuals from falling and helping them maintain their functional abilities. 44 Exercise should be encouraged for all older adults who can safely engage in such activities. 43 Third, malnutrition is a major problem in older people, and restrictive diets have not been proven to be beneficial for this population.45,46 Therefore, older adults should consume optimal nutrition and protein. 43 We suggest that older individuals with diabetes and frailty should consume diets rich in protein and energy to prevent malnutrition and weight loss. 47 These proposed measures may prevent adverse outcomes in older patients with T2DM and reduce the burden on both individual expenditures and national health systems to a great degree, in view of the high prevalence of frailty among Chinese adults over the age of 65.
Inpatients with diabetes should undergo routine frailty assessment to adjust therapy, monitor hypoglycemia, and determine hospital outcome. Subsequent research endeavors pertaining to older inpatients with diabetes could include an examination of the effects of inpatient interventions on individuals with diabetes exhibiting diverse levels of frailty, including the utilization of distinct categories of diabetes medications and the implementation of standardized insulin orders. There is insufficient knowledge regarding the effects of different blood glucose targets in frail older adults in both inpatient and outpatient settings. Further investigations are necessary to determine appropriate and safe targets that effectively mitigate the progression of symptoms and complications, while ensuring patient safety.
This study had several limitations. First, this was a single-center study; therefore, bias within the population may be inevitable. However, we collaborated with other centers and further studies are ongoing to confirm the conclusions of this investigation. In future studies, we will examine the effect of different classes of diabetes medications and standardized insulin orders on older patients with diabetes and varying levels of frailty. Second, this study did not include long-term outcomes, which may have limited its conclusions. We are currently collecting long-term follow-up data to explore whether LFI affects the survival and quality of life in older patients with T2DM.
Conclusion
Our study assessed the prevalence of frailty among older individuals with T2DM according to the LFI. Frailty was proven to be an independent risk factor for diabetic microangiopathy complications and ADL disability, suggesting that clinicians can use the LFI to determine whether participants are prefrail or frail. In this regard, frail individuals require multidimensional preventive interventions to mitigate the development of frailty.
Supplemental Material
Supplemental material, sj-docx-1-inq-10.1177_00469580231201022 for Frailty Index was Associated With Adverse Outcomes in Admitted Elderly Patients With Type 2 Diabetes Mellitus by Yi Lin, Xiaochong Shi, Lingling Huang, Aixia Chen and Haihui Zhu in INQUIRY: The Journal of Health Care Organization, Provision, and Financing
Footnotes
Author Contributions: Study conception and design: Haihui Zhu and Yi Lin. Acquisition of data: Lingling Huang, Aixia Chen. Analysis and interpretation of data: Xiaocong Shi. Drafting of manuscript: Yi Lin. Critical revision of manuscript: Haihui Zhu.
Data Availability Statement: Data are available upon reasonable request. The datasets generated during and/or analyzed during the current study are not publicly available due to privacy or ethical restrictions but are available from the corresponding author on reasonable request.
The author(s) declared no potential conflicts of interest with respect to the research, authorship, and/or publication of this article.
Funding: The author(s) disclosed receipt of the following financial support for the research, authorship, and/or publication of this article: This work was supported by Wenzhou Science and Technology Bureau (Y20180615).
Ethics Approval: This study was approved by the Human Subjects Committee of the Third Affiliated Hospital of Shanghai University, Wenzhou People’s Hospital (KY-2022-274).
ORCID iD: Haihui Zhu  https://orcid.org/0000-0002-4396-5481
https://orcid.org/0000-0002-4396-5481
Supplemental Material: Supplemental material for this article is available online.
References
- 1. Wang L, Gao P, Zhang M, et al. Prevalence and ethnic pattern of diabetes and prediabetes in China in 2013. JAMA. 2017;317:2515-2523. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2. Sinclair A, Dunning T, Rodriguez-Mañas L. Diabetes in older people: new insights and remaining challenges. Lancet Diabetes Endocrinol. 2015;3:275-285. [DOI] [PubMed] [Google Scholar]
- 3. de Miguel-Yanes JM, Jiménez-García R, Hernández-Barrera V, Méndez-Bailón M, de Miguel-Díez J, Lopez-de-Andrés A. Impact of type 2 diabetes mellitus on in-hospital-mortality after major cardiovascular events in Spain (2002-2014). Cardiovasc Diabetol. 2017;16:126. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Gómez-Huelgas R, Gómez Peralta F, Rodríguez Mañas L, et al. Treatment of type 2 diabetes mellitus in elderly patients. Rev Clin Esp. 2018;218:74-88. [DOI] [PubMed] [Google Scholar]
- 5. Clegg A, Young J, Iliffe S, Rikkert MO, Rockwood K. Frailty in elderly people. Lancet. 2013;381:752-762. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Shamliyan T, Talley KM, Ramakrishnan R, Kane RL. Association of frailty with survival: a systematic literature review. Ageing Res Rev. 2013;12:719-736. [DOI] [PubMed] [Google Scholar]
- 7. Yoon SJ, Kim KI. Frailty and disability in diabetes. Ann Geriatr Med Res. 2019;23:165-169. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Li G, Prior JC, Leslie WD, et al. Frailty and risk of fractures in patients with type 2 diabetes. Diabetes Care. 2019;42:507-513. [DOI] [PubMed] [Google Scholar]
- 9. Rogers NT, Steptoe A, Cadar D. Frailty is an independent predictor of incident dementia: evidence from the english longitudinal study of ageing. Sci Rep. 2017;7:15746. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Cesari M, Calvani R, Marzetti E. Frailty in older persons. Clin Geriatr Med. 2017;33:293-303. [DOI] [PubMed] [Google Scholar]
- 11. Yanase T, Yanagita I, Muta K, Nawata H. Frailty in elderly diabetes patients. Endocr J. 2018;65:1-11. [DOI] [PubMed] [Google Scholar]
- 12. Vellas B, Cestac P, Morley JE. Implementing frailty into clinical practice: we cannot wait. J Nutr Health Aging. 2012;16:599-600. [DOI] [PubMed] [Google Scholar]
- 13. de Vries NM, Staal JB, van Ravensberg CD, Hobbelen JS, Olde Rikkert MG, Nijhuis-van der Sanden MW. Outcome instruments to measure frailty: a systematic review. Ageing Res Rev. 2011;10:104-114. [DOI] [PubMed] [Google Scholar]
- 14. Howlett SE, Rockwood MR, Mitnitski A, Rockwood K. Standard laboratory tests to identify older adults at increased risk of death. BMC Med. 2014;12:171. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Ellis HL, Wan B, Yeung M, et al. Complementing chronic frailty assessment at hospital admission with an electronic frailty index (FI-laboratory) comprising routine blood test results. CMAJ. 2020;192:E3-E8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. Bilgin S, Aktas G, Kurtkulagi O, Atak BM, Duman TT. Edmonton frail score is associated with diabetic control in elderly type 2 diabetic subjects. J Diabetes Metab Disord. 2020;19:511-514. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. Bilgin S, Aktas G, Kahveci G, Atak BM, Kurtkulagi O, Duman TT. Does mean platelet volume/lymphocyte count ratio associate with frailty in type 2 diabetes mellitus? Bratisl Lek Listy. 2021;122:116-119. [DOI] [PubMed] [Google Scholar]
- 18. Akan S, Aktas G. Relationship between frailty, according to three frail scores, and clinical and laboratory parameters of the geriatric patients with type 2 diabetes mellitus. Rev Assoc Med Bras. 2022;68:1073-1077. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. Kocak MZ, Aktas G, Atak B, et al. The association between vitamin D levels and handgrip strength in elderly men. Acta Endocrinol. 2020;16:263-266. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. Cacciatore F, Testa G, Galizia G, et al. Clinical frailty and long-term mortality in elderly subjects with diabetes. Acta Diabetol. 2013;50:251-260. [DOI] [PubMed] [Google Scholar]
- 21. Kang S, Oh TJ, Cho BL, et al. Sex differences in sarcopenia and frailty among community-dwelling Korean older adults with diabetes: the Korean frailty and aging cohort study. J Diabetes Invest. 2021;12:155-164. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22. Aguayo GA, Hulman A, Vaillant MT, et al. Prospective Association among diabetes diagnosis, HbA1c, glycemia, and frailty trajectories in an elderly population. Diabetes Care. 2019;42:1903-1911. [DOI] [PubMed] [Google Scholar]
- 23. Alberti KG, Zimmet PZ. Definition, diagnosis and classification of diabetes mellitus and its complications. Part 1: diagnosis and classification of diabetes mellitus provisional report of a WHO consultation. Diabet Med. 1998;15:539-553. [DOI] [PubMed] [Google Scholar]
- 24. Concato J, Feinstein AR, Holford TR. The risk of determining risk with multivariable models. Ann Intern Med. 1993;118:201-210. [DOI] [PubMed] [Google Scholar]
- 25. Riley RD, Ensor J, Snell KIE, et al. Calculating the sample size required for developing a clinical prediction model. BMJ. 2020;368:m441. [DOI] [PubMed] [Google Scholar]
- 26. Molitch ME, DeFronzo RA, Franz MJ, et al. Nephropathy in diabetes. Diabetes Care. 2004;27 Suppl 1:S79-S83. [DOI] [PubMed] [Google Scholar]
- 27. Jia W, Weng J, Zhu D, et al. Standards of medical care for type 2 diabetes in China 2019. Diabetes Metab Res Rev. 2019;35:e3158. [DOI] [PubMed] [Google Scholar]
- 28. Mohler Er. 3rd Peripheral arterial disease: identification and implications. Arch Intern Med. 2003;163:2306-2314. [DOI] [PubMed] [Google Scholar]
- 29. Li X, Zheng T, Guan Y, et al. ADL recovery trajectory after discharge and its predictors among baseline-independent older inpatients. BMC Geriatr. 2020;20:86. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30. Li Y, Zou Y, Wang S, et al. A pilot study of the FRAIL scale on predicting outcomes in Chinese elderly people with type 2 diabetes. J Am Med Dir Assoc. 2015;16:714.e7-714.e12. [DOI] [PubMed] [Google Scholar]
- 31. Chao CT, Wang J, Chien KL. Both pre-frailty and frailty increase healthcare utilization and adverse health outcomes in patients with type 2 diabetes mellitus. Cardiovasc Diabetol. 2018;17:130. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32. Blodgett JM, Theou O, Howlett SE, Rockwood K. A frailty index from common clinical and laboratory tests predicts increased risk of death across the life course. GeroScience. 2017;39:447-455. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33. Blodgett JM, Theou O, Howlett SE, Wu FC, Rockwood K. A frailty index based on laboratory deficits in community-dwelling men predicted their risk of adverse health outcomes. Age Ageing. 2016;45:463-468. [DOI] [PubMed] [Google Scholar]
- 34. Rockwood K, McMillan M, Mitnitski A, Howlett SE. A frailty index based on common laboratory tests in comparison with a clinical frailty index for older adults in long-term care facilities. J Am Med Dir Assoc. 2015;16:842-847. [DOI] [PubMed] [Google Scholar]
- 35. Hao Q, Sun X, Yang M, Dong B, Dong B, Wei Y. Prediction of mortality in Chinese very old people through the frailty index based on routine laboratory data. Sci Rep. 2019;9:221. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36. Mitnitski A, Song X, Rockwood K. Assessing biological aging: the origin of deficit accumulation. Biogerontology. 2013;14:709-717. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37. Lopez-Otin C, Blasco MA, Partridge L, et al. The hallmarks of aging. Cell. 2013;153:1194-1217. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38. Mitnitski AB, Rutenberg AD, Farrell S, Rockwood K. Aging, frailty and complex networks. Biogerontology. 2017;18:433-446. [DOI] [PubMed] [Google Scholar]
- 39. Op Het Veld LPM, Ament BHL, van Rossum E, et al. Can resources moderate the impact of levels of frailty on adverse outcomes among (pre-) frail older people? A longitudinal study. BMC Geriatr. 2017;17:185. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40. Rolland Y, Dupuy C, Abellan van Kan G, Gillette S, Vellas B. Treatment strategies for sarcopenia and frailty. Med Clin North Am. 2011;95:427-38. [DOI] [PubMed] [Google Scholar]
- 41. Dent E, Martin FC, Bergman H, Woo J, Romero-Ortuno R, Walston JD. Management of frailty: opportunities, challenges, and future directions. Lancet. 2019;394:1376-1386. [DOI] [PubMed] [Google Scholar]
- 42. American Diabetes A. 6. Glycemic targets: standards of medical care in diabetes–2019. Diabetes Care. 2019;42:S61-S70. [DOI] [PubMed] [Google Scholar]
- 43. American Diabetes A. 12. Older adults: standards of medical care in diabetes-2019. Diabetes Care. 2019;42:S139-S147. [DOI] [PubMed] [Google Scholar]
- 44. Assar ME, Laosa O, Rodríguez Mañas L. Diabetes and frailty. Curr Opin Clin Nutr Metab Care. 2019;22:52-57. [DOI] [PubMed] [Google Scholar]
- 45. Khagram L, Martin CR, Davies MJ, Speight J. Psychometric validation of the self-care inventory-Revised (SCI-R) in UK adults with type 2 diabetes using data from the AT.Lantus follow-on study. Health Qual Life Outcomes. 2013;11:24. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46. Wing RR, Reboussin D, Lewis CE. Intensive lifestyle intervention in type 2 diabetes. New Engl J Med. 2013;369:2358-2359. [DOI] [PubMed] [Google Scholar]
- 47. LeRoith D, Biessels GJ, Braithwaite SS, et al. Treatment of diabetes in older adults: an endocrine society* clinical practice guideline. J Clin Endocrinol Metab. 2019;104:1520-1574. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Supplemental material, sj-docx-1-inq-10.1177_00469580231201022 for Frailty Index was Associated With Adverse Outcomes in Admitted Elderly Patients With Type 2 Diabetes Mellitus by Yi Lin, Xiaochong Shi, Lingling Huang, Aixia Chen and Haihui Zhu in INQUIRY: The Journal of Health Care Organization, Provision, and Financing


