Abstract
Background
Vitamin D deficiency has recently been suggested as an independent risk factor for thrombosis. Notably, vitamin D deficiency is common in pregnant populations, whom already have an increased thrombotic risk. However, pregnant women are commonly excluded from studies investigating the hemostatic system, and knowledge on the impact of vitamin D on hemostasis in pregnancy is therefore limited.
Methods
A cross-sectional study comparing the hemostatic profile of pregnant women (gestational week 12.9 ± 0.7) with vitamin D deficiency (≤50 nmol/L) (n = 70) and high adequate vitamin D status (≥100 nmol/L) (n = 59).
Results
Vitamin D deficient women displayed increased plasminogen activator inhibitor 1 levels and an increased plasminogen activator inhibitor 1/plasminogen activator inhibitor 2 ratio, even after adjusting for factors with potential influence on hemostasis (body mass index, smoking and use of fish oil supplements).
Conclusions
Vitamin D deficiency is associated with increased plasminogen activator inhibitor 1/plasminogen activator inhibitor 2 ratio in pregnant women. As an increased plasminogen activator inhibitor 1/plasminogen activator inhibitor 2 ratio with high plasminogen activator inhibitor 1 levels may increase thrombotic risk and is associated with the development of pregnancy complications, further research is needed to determine the optimal vitamin D supplementation in pregnancy.
Keywords: vitamin d, hemostasis, blood coagulation, plasminogen activator inhibitor 1, plasminogen activator inhibitor 2, pregnancy
Introduction
Deep vein thrombosis and pulmonary embolism are potentially severe complications of pregnancy. Overall, a 5 to 6 times increased thromboembolic risk is seen from early pregnancy and remains constant in all trimesters.1,2 The pregnancy-related risk of venous thromboembolism is affected by increased estrogen levels but in addition to high estrogen levels, pregnant women also display increased levels of coagulation factors, reduced protein C and S, and reduced fibrinolytic activity.3–5 Furthermore, the placenta produces factors relevant to hemostasis, specifically plasminogen activator inhibitor 2 (PAI-2), 6 which controls the fibrinolytic activity in trophoblasts, and further complicates the understanding of hemostasis and thrombotic risk during pregnancy.
Interestingly, recent studies have suggested that vitamin D deficiency may be an independent risk factor associated with an increased risk of thrombosis in both arterial and venous systems.7–13 The mechanisms linking thrombosis and vitamin D deficiency are not well-defined, but it is speculated that vitamin D deficiency may disturb the hemostatic balance leading towards a prothrombotic profile.14–23 Vitamin D deficiency is defined as a serum level of vitamin D < 50 nmol/L by the Endocrine Society Task Force on Vitamin D in the United States. 24 However, serum 25(OH)D concentrations < 25 or < 30 nmol/L are also sometimes used to define vitamin D deficiency, as concentrations below these levels are generally considered to indicate an increased risk of vitamin D related nutritional rickets and osteomalacia.25–27 Today, the optimal vitamin D level is still under debate and has been suggested to be as high as 100 nmol/L,28,29 though a level of 75 nmol/L is also considered sufficient by some.30–32 Numerous studies report a high prevalence of vitamin D deficiency in pregnant populations including Danish pregnant women.33,34 The impact of vitamin D deficiency on pregnancy health and especially the effect on thrombosis risk is however far from fully understood. This is further challenged by the fact that pregnant women are commonly excluded from studies investigating the hemostatic system, and knowledge about this population is therefore sparse.
The aim of this cross-sectional study was to investigate the effect of vitamin D deficiency on the prothrombotic profile in pregnant women by comparing hemostatic parameters in 2 groups: Pregnant women with vitamin D deficiency (vitamin D levels ≤50 nmol/L) and pregnant women with high adequate vitamin D levels (vitamin D levels ≥100 nmol/L).
Methods
Study Population
Pregnant women were recruited as a part of the large GRAVITD trial 35 at the Department of Gynecology and Obstetrics, Randers Regional Hospital, Denmark, when attending a nuchal translucency scan at 11 to 16 weeks of gestation (mean ± SD, 12.9 ± 0.7) from November 2020 to September 2021. Patients included in this cross-sectional study were included prior to randomization according to the GRAVITD trial. An initial vitamin D screening was performed on 497 pregnant women agreeing to participate, and blood samples from a total of 129 women were available for the study based on the inclusion and exclusion criteria. Inclusion criteria were a serum vitamin D level fulfilling the criteria of vitamin D deficiency (≤ 50 nmol/L) or a vitamin D level in the high adequate range (≥100 nmol/L). Exclusion criteria were known cancer, liver-, kidney-, coagulation-, or chronic inflammatory disease, as well as women reporting medical treatment affecting the coagulation system (estrogens or antithrombotic treatment).
Prepregnancy body mass index (BMI) and gestational age at inclusion were obtained from medical records. Other data eg, smoking status, vitamin use, fish oil consumption, education, and household income were self-reported by participants in an interviewer-administered questionnaire, which was filled out at the time of inclusion. The study was performed with informed consent from all participants and approved by The Regional Scientific Ethical Committee for Central Denmark (#1-10-72-54-19).
Vitamin D Analyses
Maternal vitamin D levels were determined as the serum 25-hydroxy vitamin D (25(OH)D) level, measured using high-performance liquid chromatography coupled with tandem mass spectrometry as previously described. 36 This method is considered the golden standard for determining vitamin D levels. 37
Coagulation Analyses
Nonfasting venous blood-samples were collected in 3 Vacutainer citrate tubes. Blood sampling was performed according to the guidelines recommended by the Danish Society for Clinical Biochemistry 38 with an application of minimal venous stasis. Vacutainers were immediately turned several times to secure a mixture of citrate and venous blood. Plasma samples were prepared within 2 hours and stored at −80 °C. All sample analyses were completed within 12 months of storage. Samples were investigated for levels of von Willebrand factor (vWF), thrombin generation, factor VIII, plasminogen activator inhibitor 1 (PAI-1), PAI-2, tissue plasminogen activator (tPA), fibrinogen, D-dimer and c-reactive protein (CRP) after thawing of plasma samples at 37 °C in a water bath.
Thrombin generation was analyzed by the calibrated automated thrombin generation assay (Thrombinoscope BV, Maastricht, The Netherlands) using a 5 pM tissue factor trigger employing the Fluoroskan Ascent microplate fluorometer (Thermo Fisher Scientific, Hvidovre, Denmark). Parameters of thrombin generation: The lag time of the thrombin formation process (min., CV 8.6%), the peak thrombin concentration (nmol/L, CV 7.5%), the time to peak (ttpeak) assessing when thrombin peak concentration was reached (min., CV 4.1%), and the endogenous thrombin potential (ETP) (nmol/L × min., CV 8.1%) recording the total amount of thrombin formed. In addition, the vWF:Ag and FVIII:C activities were analysed using an ACL TOP 350CTS coagulation analyzer (ILS Scandinavia, CV of 11.5% and 10%, respectively). CRP (CardioPhase® hsCRP) was analyzed using the nephelometer on a BNII (Siemens Healthcare Diagnostics Products, CV 3.1%). Fibrinogen and D-dimer were determined using specific assay kits (INNOVANCE D-Dimer and Dade Thrombin-Reagent, respectively) on a Siemens coagulation analyzer (Siemens Healthcare Diagnostics, CV 5% and 9%, respectively). PAI-1:Ag, PAI-2:Ag, and tPA:Ag were determined by in-house sandwich enzyme-linked immunosorbent assay using specific antibodies as previously described39,40 (CV 8.2%, 6.7%, and 5.1%, respectively).
Statistical Analyses
The background characteristics of the vitamin D deficient group and the group of women with high adequate vitamin D status were compared using chi-squared test for categorical variables, while continuous variables were tested for Gaussian distribution by inspection of QQ-plots and log transformed if appropriate. Normally distributed parameters were then compared using student's t test, while Mann–Whitney test was used for parameters that did not follow a Gaussian distribution before or after log transformation.
With respect to hemostatic parameters the 2 groups were initially compared using student's t test or Mann–Whitney test, according to the above-mentioned. As both smoking and BMI are known to potentially affect coagulation41–44 and fish oil has long been considered a natural anticoagulant,45,46 we additionally investigated the difference in hemostatic parameter levels between the 2 groups using a multiple regression model with vitamin D group, pre-pregnancy BMI, smoking status and use of fish oil supplements as independent variables. The multiple regression models were tested for multicollinearity by assessing the variance inflation factor, while potential homoscedasticity was assessed by visual inspection of residual plots. Normality of residuals was considered by visual inspection of residual QQ plots. Statistical analyses were performed using GraphPad Prism, version 9.2. A p-value < .05 was considered statistically significant.
Results
The background characteristics of the participants are presented in Table 1. When comparing women with vitamin D deficiency and women with high adequate vitamin D levels there was no statistically significant difference in maternal age, ethnicity, use of prescriptive medicine, gestational age at the time of blood sampling, education (data not shown), or household income (data not shown). The average BMI was slightly higher in pregnant women with vitamin D deficiency and notably this group of women had a higher prevalence of women with obesity, ie BMI > 30. Women in the vitamin D deficient group were also more likely to have been smoking during pregnancy and less likely to take fish oil and vitamin supplements in general. As expected, the group of pregnant women having high adequate vitamin D levels were more frequently included during the summer, while the women in the vitamin D deficient group often were included during the winter or spring.
Table 1.
Background Characteristics of the Participants.
| Characteristics | 25(OH)D ≤ 50 nmol/L n = 70 | 25(OH)D ≥ 100 nmol/L n = 59 | P-value |
|---|---|---|---|
| 25(OH)D [nmol/L] | 37.4 ± 9.9 | 114.4 ± 13.5 | < .001 |
| Age [years] | 28.9 ± 4.4 | 29.5 ± 4.1 | .355 |
| Season | < .001 | ||
| Winter | 31 (44.3) | 16 (27.1) | |
| Spring | 28 (40.0) | 10 (16.9) | |
| Summer | 4 (5.7) | 28 (47.5) | |
| Fall | 7 (10.0) | 5 (8.5) | |
| Gestational age [weeks] | 13.0 ± 0.75 | 12.8 ± 0.60 | .194 |
| BMI [kg/m2] | 27.2 ± 7.6 | 24.7 ± 4.5 | .034 |
| BMI group [kg/m2] | .015 | ||
| BMI <25 | 35 (50.0) | 32 (54.2) | |
| BMI 25-30 | 16 (22.9) | 22 (37.3) | |
| BMI >30 | 19 (27.1) | 5 (8.5) | |
| Smokers | 13 (18.6) | 4 (6.8) | .049 |
| Non-Scandinavian origin | 8 (11.4) | 7 (11.8) | .939 |
| Prescription medicine | 20 (28.6) | 15 (25.4) | .689 |
| Dietary supplements | |||
| Vitamin D | 51 (72.9) | 56 (94.9) | <.001 |
| Vitamin D, 10 µg | 47 | 43 | |
| Vitamin D, > 10 µg | 3 | 11 | |
| Vitamin D, unknown dose | 1 | 2 | |
| Calcium | 9 (12.9) | 15 (25.4) | |
| .068 | |||
| Fish oil | 13 (18.6) | 22 (37.3) | .017 |
Information on age, season of inclusion, gestational age, BMI, smoking, origin, medicine, and dietary supplements was reported by all 129 participants. Data are presented as mean ± SD or as n (%). P-values <0.05 are highlighted in bold. The season in which the women were included is divided into winter (December, January, February), spring (March, April, May), summer (June, July, August), and fall (September, October, November). Smokers cover the women answering yes to smoking at some point during pregnancy and include both women who were still smoking at time of inclusion and women who had quit prior to inclusion. Non-Scandinavian origin covers women with either one or both parents being born outside Scandinavia (Denmark, Norway, Sweden, or the Faroe Islands). Dietary supplements refer to supplements ingested by the women prior to inclusion in the study and cover the daily intake of a vitamin D containing supplement during pregnancy, as well as the intake of an additional calcium or fish oil supplement. The majority of the included women reported a daily intake of 10 μg vitamin D prior to inclusion, which is the dose recommended for pregnant women by the Danish Health Authorities. Abbreviations: 25(OH)D, 25-hydroxy vitamin D; BMI, body mass index.
The hemostatic profiles of the 2 groups were compared based on the level of vWF, thrombin generation (expressed by lag time, ETP, peak height, ttpeak and start tail), factor VIII, PAI-1, PAI-2, tPA, fibrinogen, D-dimer and CRP. In addition, the PAI-1/PAI-2 ratio, which have been considered to reflect placental function, was assessed.
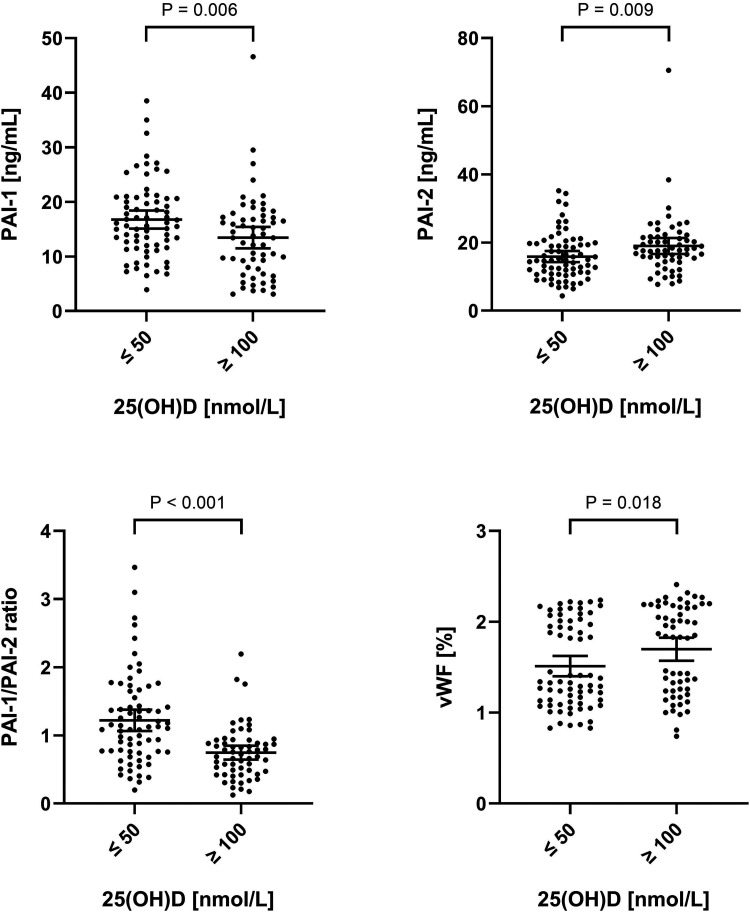
Initial comparison using Mann-Whitney test and student's t-test showed significantly increased PAI-1 levels, significantly decreased PAI-2 levels, and a higher PAI-1/PAI-2 ratio in the group of vitamin D deficient women when compared to the group of women having high adequate vitamin D status. In addition, the group of vitamin D deficient women were found to have lower levels of vWF (Figure 1).
Figure 1.
Comparison of PAI-1, PAI-2, PAI-1/PAI-2 ratio, and vWF in relation to vitamin D status. PAI-1, PAI-2, PAI-1/PAI-2 ratio, and vWF in the group of vitamin D deficient pregnant women (serum 25(OH)D levels ≤50 nmol/L) compared to the group of pregnant women with high adequate vitamin D status (serum 25(OH)D levels ≥100 nmol/L). Error bars depict mean and 95% confidence interval. P-values were calculated using Mann–Whitney tests for PAI-1 and vWF, while t-tests were used for PAI-2 and PAI-1/PAI-2 ratio after logarithmic transformation. Notably, the difference in PAI-2 levels were still significant when excluding the single measurement above 70 ng/mL (P = .018). Abbreviations: 25(OH)D, 25-hydroxy vitamin D; PAI-1, plasminogen activator inhibitor 1; PAI-2, plasminogen activator inhibitor 2; vWF, von Willebrand factor.
To further evaluate the data, the levels of each hemostatic parameter were additionally compared between the 2 groups using a multiple regression model adjusting for pre-pregnancy BMI, smoking and use of fish oil supplements, since these factors could potentially influence on hemostasis (depicted in full in supplemental table 1). In the multiple regression analyses, the statistically significantly higher levels of PAI-1 and the significantly increased PAI-1/PAI-2 ratio were maintained in the group of pregnant women with vitamin D deficiency when compared to the women with high adequate vitamin D levels. In the multiple regression analysis, vWF and PAI-2 levels were no longer found to be significantly different in the 2 groups, as smoking seemed to be determining for vWF, whereas pre-pregnancy BMI had an impact on PAI-2. Furthermore, a significant difference in thrombin generation parameters, not initially shown, were found, as the women with high adequate vitamin D status had a significantly increased lag time, ETP and peak height. No additional statistically significant differences in hemostatic parameters were observed between the 2 groups (Table 2).
Table 2.
Levels of Hemostatic Parameters.
| 25(OH)D ≤ 50 nmol/L | 25(OH)D ≥ 100 nmol/L | P-value1 | Adjusted P-value2 | |
|---|---|---|---|---|
| vWF [%] | 151 ± 47 | 170 ± 48 | .018 | .148 |
| Thrombin generation | ||||
| Lag time [min] | 2.69 ± 0.41 | 2.81 ± 0.39 | .079 | .022 |
| ETP [nmol/L x min] | 2303 ± 353 | 2395 ± 464 | .292 | .011 |
| Peak [nmol/L] | 409 ± 54.5 | 423 ± 66.4 | .248 | .017 |
| ttpeak [min] | 5.28 ± 0.60 | 5.44 ± 0.58 | .109 | .058 |
| Start tail [min] | 24.0 ± 1.83 | 23.9 ± 1.97 | .814 | .349 |
| Factor VIII [%] | 114 ± 35 | 121 ± 32 | .115 | .291 |
| PAI-1 [ng/mL] | 16.8 ± 7.0 | 13.5 ± 7.6 | .006 | .007 |
| PAI-2 [ng/mL] | 15.9 ± 6.8 | 19.0 ± 8.9 | .009 | .222 |
| PAI-1/PAI-2 ratio | 1.22 ± 0.66 | 0.75 ± 0.39 | <.001 | <.001 |
| tPA [mg/mL] | 3.96 ± 1.62 | 4.10 ± 1.65 | .629 | .421 |
| Fibrinogen [µmol/L] | 12.3 ± 2.08 | 12.4 ± 1.98 | .674 | .124 |
| D-dimer [mg/L FEU] | 1.30 ± 3.78 | 0.85 ± 2.44 | .273 | .375 |
| CRP [mg/l] | 5.08 ± 4.51 | 5.21 ± 5.88 | .509 | .294 |
Levels of the hemostatic parameters in the group of vitamin D deficient pregnant women (serum vitamin D levels ≤50 nmol/L) compared to the group of pregnant women with high adequate vitamin D status (serum vitamin D levels ≥100 nmol/L). Data are presented as mean ± SD. P-values are indicated for primary comparison1 (student's t test or Mann–Whitney test) and for multiple regression2 separately. P-values <0.05 are highlighted in bold. Multiple regression models were adjusted for pre-pregnancy, BMI, smoking status, and use of fish oil supplements. Abbreviations: 25(OH)D, 25-hydroxy vitamin D; BMI, body mass index; CRP, c-reactive protein; FEU, fibrinogen equivalent units; PAI-1, plasminogen activator inhibitor 1; PAI-2, plasminogen activator inhibitor 2; tPA, tissue plasminogen activator; vWF, von Willebrand factor; ttpeak, time to peak; ETP, endogenous thrombin potential.
Discussion
Despite the markedly increased risk of thromboembolic events during pregnancy, this study is, to our knowledge, the first investigation of vitamin D and the hemostatic system focusing on pregnant women. Moreover, the study assesses hemostatic parameters covering both primary hemostasis, secondary hemostasis, and fibrinolysis as well as the placental specific factor PAI-2 which has never been investigated in relation to vitamin D status. Among the hemostatic parameters investigated, we found significantly higher levels of PAI-1 and an increased PAI-1/PAI-2 ratio in pregnant women with vitamin D deficiency (≤50 nmol/L) when compared to pregnant women with high adequate vitamin D levels (≥100 nmol/L). A finding that remained significant, when considering other potentially relevant factors, namely pre-pregnancy BMI, smoking status, and use of fish oil supplements.
PAI-1 level is a well-known marker of fibrinolytic activity and increased levels of PAI-1 impair fibrin clearance. A reduced fibrinolytic capacity is associated with a prothrombotic profile, and high plasma levels of PAI-1 have been associated with increased risk of cardiovascular disease. 47 Likewise, transgenic mice over-expressing PAI-1 suffer severe venous thrombosis, 48 while PAI-1 knock-out mice display reduced venous thrombosis and atherosclerosis 49 supporting a possible clinical benefit of reduced PAI-1 levels in pregnant women with high adequate vitamin D levels as presented in our study. However, prospective data is needed to validate this assumption.
The inverse relationship between PAI-1 and vitamin D found in this study, has also previously been reported in other studies performed in non-pregnant populations, including a large multi-ethnic cross-sectional study with 6443 participants by Blondon et al 21 and a study by Moin et al 50 in 99 women with PCOS. Furthermore, a randomized controlled trial by Witham et al, 18 also showed a significant reduction in PAI-1 levels after vitamin D supplementation compared to placebo in a non-pregnant population. However, no significant associations were found in 5 other randomized controlled trials and intervention studies.15,51–54 In vitro vitamin D has been shown to suppress PAI-1 production in a number of cell types including osteoblast-like cells and human coronary artery and aortic smooth muscle cells.55,56 While these observations appear to be important, the exact mechanism underlying vitamin D suppression of PAI-1 remains to be defined.
The exact role of PAI-1 in gynecological and obstetrical diseases has not been established 57 but some studies suggest a role of PAI-1 in the occurrence and development of pregnancy complications such as preeclampsia 58 and fetal growth restriction (FGR). 59 Increased PAI-1 may result in the excessive deposition of fibrin and reduced blood flow at the maternal-fetal interface, which affects the growth and development of the fetus. Interestingly, a range of pregnancy complications, including preeclampsia and FGR, have also been associated with low maternal vitamin D levels.60–62
PAI-2 is an inhibitor of fibrinolysis and is produced in the placenta, hence being unique for pregnancy. Reduced levels of PAI-2 in vitamin D deficient pregnant women were found in the initial comparison, however, in the multiple regression analysis, we found PAI-2 levels to be affected by maternal pre-pregnancy BMI, which could relate to BMI-related effects on placental function or on vitamin D levels. 63 Notably, PAI-2 has been suggested to play a role in pregnancy complications, especially preeclampsia, where PAI-2 levels, opposed to PAI-1 levels, have been found to be decreased,39,64 suggesting a complex role of PAI-2 in pregnancy complications.
In line with this, the ratio between PAI-1 and PAI-2 levels has been suggested as an indicator of placental function. With a low PAI-1/PAI-2 ratio indicating good placental function and a high PAI-1/PAI-2 ratio indicating poor placental function and an increased risk of preeclampsia.64,65 In the present study, we found a higher PAI-1/PAI-2 ratio in pregnant women with vitamin D deficiency, which could be associated with the increased prothrombotic profile and pregnancy complications.
Unexpectedly, this study showed an association between high adequate vitamin D status and increased levels of vWF. However, after adjusting for smoking this association was no longer statistically significant. It is well known that smoking affects levels of vWF in healthy adolescent females, independently of other risk factors. 66 In accordance with our findings, 3 cross-sectional studies19,21,50 and 2 interventional studies67,68 previously investigating the association between vWF and vitamin D in non-pregnant populations did not find any association between high vitamin D levels and increased levels of vWF.
Analyses of thrombin generation is often considered a global measure of hemostasis.69–71 Multiple regression models in the present study show a significantly decreased lag time, ETP and peak height in vitamin D deficient women compared to women with high adequate vitamin D status. This indicates both antithrombotic (ETP and peak height) and prothrombotic (lag time) properties of low vitamin D levels. These results are somewhat puzzling, as we expected that subjects with low vitamin D status would have a higher thrombotic potential. Although ETP may not completely reflect fibrinolytic activity, the clinical significance of these divergent results on thrombin generation is unclear. Accordingly, previous studies investigating the association between vitamin D and thrombin generation parameters in nonpregnant populations have also shown similar divergent results.15,51,67,72
No significant differences were shown between the 2 groups of pregnant women in the present study when comparing the additional hemostatic parameters. According to our recent systematic review, 73 findings on the effect of vitamin D on hemostatic parameters in nonpregnant populations have generally been inconclusive. However, of particular note, Blondon et al 15 suggested that the influence of vitamin D status on hemostasis may be restricted to individuals with a severe vitamin D deficiency (<25 nmol/L). However, in the present study, very few of the participants had vitamin D levels <25 nmol/L limiting our ability to test the effect of severe vitamin D deficiency in pregnancy.
In the present study, 71% of the pregnant women with vitamin D deficiency (<50 nmol/L) received a minimum of 10 µg of vitamin D supplement as recommended by the Danish Health authorities. This underlines, that vitamin D levels as determined by 25(OH)D is highly dependent on both dietary intake and the endogenous synthesis in the skin in response to sun exposure,74–76 with a higher need for supplementation during winter months. Currently, there are much debate about the optimal vitamin D level during pregnancy. To this end, daily doses of up to 100 µg (4000 IU) have been tested and found safe in pregnancy. 77 The Endocrine Society 32 and the Danish Society for Obstetrics and Gynecology 30 currently suggests that sufficient vitamin D levels should be defined as a minimum level of 75 nmol/L in pregnancy. However, so far, no studies have considered whether emphasis should be put on the role of vitamin D in hemostatic regulation when determining the optimal vitamin D level in pregnancy. As this study is limited by the exclusion of women having vitamin D levels in the 50 to 100 nmol/L range, further studies are needed to clarify if 75 nmol/L also moderates the prothrombotic profile of pregnancy. This study is further limited by the sample size, as small differences in the prevalence of life-style habits and body composition among the 2 groups might have a more prominent impact on the results with the current sample size. Furthermore, as we did not register the specific time for each blood sampling for this study, it can not be excluded that the circadian rhythm displayed by some of the fibrinolytic parameters might also have an impact on the results.
Conclusion
Compared to women with high adequate vitamin D status, PAI-1 levels, and PAI-1/PAI-2 ratio were statistically significantly increased in pregnant women with vitamin D deficiency. This could indicate fibrinolytic differences in vitamin D deficiency and possible thrombo-protective properties of vitamin D in pregnancy. Noting that increased PAI-1 levels and PAI-1/PAI-2 ratio are also associated with increased risk of pregnancy complications, a deeper understanding on how vitamin D affects the hemostatic system and how hemostatic factors may contribute to pregnancy-related diseases are needed, especially in the light of the high prevalence of vitamin D deficiency in many pregnant populations.
Supplemental Material
Supplemental material, sj-docx-1-cat-10.1177_10760296231201855 for Vitamin D Deficiency is Associated With Increased Plasminogen Activator Inhibitor 1/Plasminogen Activator Inhibitor 2 Ratio in Pregnancy by Matilde Kanstrup Andersen, Isabella Hangaard Rüdiger, Anna Louise Vestergaard, Yaseelan Palarasah, Pinar Bor, Agnete Larsen, and Mustafa Vakur Bor, in Clinical and Applied Thrombosis/Hemostasis
Acknowledgement
The authors are deeply grateful to all those who contributed to this research project. We would like to thank the staff at the Department of Gynecology and Obstetrics and the Clinical Research Unit, Randers Regional Hospital, for making the project possible. We would also like to thank medical laboratory scientists Anette Larsen and Kathrine Overgaard at the Department of Clinical Biochemistry, University Hospital of Southern Denmark, for excellent technical assistance in the analyses of blood samples. Furthermore, we would like to express our sincere gratitude to the funds which provided financial support for this research. Without their generous contributions, this project would not have been possible.
List of abbreviations
- 25(OH)D
25-hydroxy vitamin D
- BMI
Body mass index
- CRP
c-reactive protein
- ELISA
Enzyme-linked immunosorbent assay
- ETP
endogenous thrombin potential
- FEU
fibrinogen equivalent units
- FGR
fetal growth restriction
- tPA
tissue plasminogen activator
- ttpeak
time to peak
- vWF
von Willebrand factor
- PAI-1
plasminogen activator inhibitor 1
- PAI-2
plasminogen activator inhibitor 2
Footnotes
Authors’ Contributions: The GRAVITD trial was planned by ALV, PB, and AL, and the idea for the present study, as a part of the GRAVITD trial, was conceived by PB and MVB. The planning of the present study was performed by IHR, ALV, PB, AL, and MVB and the study was carried out by MKA, IHR, ALV, PB, AL, and MVB. The inclusion of study participants and collection of blood samples were performed by MKA, IHR, and ALV. MVB supervised the analysis of all hemostatic parameters except PAI-2, while YP supervised the analysis of the PAI-2 content. Data analyses and interpretation of the results were carried out by MKA and IHR under the supervision of ALV, PB, AL, and MVB. First draft of the manuscript was prepared by MKA and IHR. All authors have contributed and agreed on the final version of the manuscript.
Availability of Data and Materials: The full dataset is not publicly available, since we do not have consent from the participants to publish the full dataset. However, a de-identified dataset will be available from the corresponding author upon reasonable request.
The authors declared the following potential conflicts of interest with respect to the research, authorship, and/or publication of this article: ALV is the primary investigator of a larger double-blinded randomized controlled trial (the GRAVITD trial) investigating the effect of increased vitamin supplementation during pregnancy, for which vitamin D and placebo tablets have been donated by Orkla Care Denmark. Orkla Care Denmark had no role in any part of either the GRAVITD trial or the present study.
Ethics Approval: The study was performed in accordance with the Declaration of Helsinki and approved by The Regional Scientific Ethical Committee for Central Denmark (Reference number #1-10-72-54-19).
Funding: The authors disclosed receipt of the following financial support for the research, authorship, and/or publication of this article: This work was supported by the Frimodt-Heineke Fonden, Murermester Lauritz Peter Christensen og hustru Kirsten Sigrid Christensens Fond, Regionshopitalet Randers’ spiremidler 2020, Snedkermester Sophus Jacobsen og Hustru Astrid Jacobsens Fond (grant number 3051415 jhj/mj/is). The funding sources played no role in neither study design, data collection, data analysis, interpretation of data, nor in the writing of the manuscript.
Informed Consent: Written informed consent was obtained from the patients for their anonymized information to be published in this article.
ORCID iD: Matilde Kanstrup Andersen https://orcid.org/0009-0007-2950-8288
Supplemental Material: Supplemental material for this article is available online.
References
- 1.Kujovich JL. Hormones and pregnancy: Thromboembolic risks for women. Br J Haematol . 2004;126(4):443-454. [DOI] [PubMed] [Google Scholar]
- 2.Jacobsen AF, Skjeldestad FE, Sandset PM. Incidence and risk patterns of venous thromboembolism in pregnancy and puerperium—a register-based case-control study. Am J Obstet Gynecol . 2008;198(2):233.e231-237. [DOI] [PubMed] [Google Scholar]
- 3.Abou-Ismail MY, Citla Sridhar D, Nayak L. Estrogen and thrombosis: A bench to bedside review. Thromb Res . 2020;192:40-51. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Bremme KA. Haemostatic changes in pregnancy. Best Pract Res Clin Haematol . 2003;16(2):153-168. [DOI] [PubMed] [Google Scholar]
- 5.Brenner B. Haemostatic changes in pregnancy. Thromb Res . 2004;114(5-6):409-414. [DOI] [PubMed] [Google Scholar]
- 6.Astedt B, Lindoff C, Lecander I. Significance of the plasminogen activator inhibitor of placental type (PAI-2) in pregnancy. Semin Thromb Hemost . 1998;24(5):431-435. [DOI] [PubMed] [Google Scholar]
- 7.Korkmaz UTK, Ersoy S, Yuksel A, et al. Association between vitamin D levels and lower-extremity deep vein thrombosis: A case-control study. Sao Paulo Med J . 2021;139(3):279-284. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Epstein D, Kvanta A, Lindqvist PG. Seasonality and incidence of central retinal vein occlusion in Sweden: A 6-year study. Ophthalmic Epidemiol . 2015;22(2):94-97. [DOI] [PubMed] [Google Scholar]
- 9.Huang J, Wang Z, Hu Z, Jiang W, Li B. Association between blood vitamin D and myocardial infarction: A meta-analysis including observational studies. Clin Chim Acta . 2017;471:270-275. [DOI] [PubMed] [Google Scholar]
- 10.Lindqvist PG, Epstein E, Olsson H. Does an active sun exposure habit lower the risk of venous thrombotic events? A D-lightful hypothesis. J Thromb Haemost . 2009;7(4):605-610. [DOI] [PubMed] [Google Scholar]
- 11.Shi H, Chen H, Zhang Y, et al. 25-Hydroxyvitamin D level, vitamin D intake, and risk of stroke: A dose-response meta-analysis. Clin Nutr . 2020;39(7):2025-2034. [DOI] [PubMed] [Google Scholar]
- 12.Su C, Jin B, Xia H, Zhao K. Association between vitamin D and risk of stroke: A PRISMA-compliant systematic review and meta-analysis. Eur Neurol . 2021;84(6):399-408. [DOI] [PubMed] [Google Scholar]
- 13.Wan J, Yuan J, Li X, et al. Association between serum vitamin D levels and venous thromboembolism (VTE): A systematic review and meta-analysis of observational studies. Complement Ther Med . 2020;54:102579. [DOI] [PubMed] [Google Scholar]
- 14.Aleva FE, Tunjungputri RN, van der Vorm LN, et al. Platelet integrin αIIbβ3 activation is associated with 25-hydroxyvitamin D concentrations in healthy adults. Thromb Haemost . 2020;120(5):768-775. [DOI] [PubMed] [Google Scholar]
- 15.Blondon M, Biver E, Braillard O, Righini M, Fontana P, Casini A. Thrombin generation and fibrin clot structure after vitamin D supplementation. Endocr Connect . 2019;8(11):1447-1454. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Da Porto A, Cavarape A, Catena C, Colussi G, Casarsa V, Sechi LA. Interactions between vitamin D levels, cardiovascular risk factors, and atherothrombosis markers in patients with symptomatic peripheral artery disease. Vasc Med . 2021;26(3):315-316. [DOI] [PubMed] [Google Scholar]
- 17.Topaloglu O, Arslan MS, Karakose M, et al. Is there any association between thrombosis and tissue factor pathway inhibitor levels in patients with vitamin D deficiency? Clin Appl Thromb Hemost . 2015;21(5):428-433. [DOI] [PubMed] [Google Scholar]
- 18.Witham MD, Adams F, Kabir G, Kennedy G, Belch JJ, Khan F. Effect of short-term vitamin D supplementation on markers of vascular health in south Asian women living in the UK—a randomised controlled trial. Atherosclerosis . 2013;230(2):293-299. [DOI] [PubMed] [Google Scholar]
- 19.Hypponen E, Berry D, Cortina-Borja M, Power C. 25-Hydroxyvitamin D and pre-clinical alterations in inflammatory and hemostatic markers: A cross sectional analysis in the 1958 British birth cohort. PLoS One . 2010;5(5):e10801. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Jorde R, Haug E, Figenschau Y, Hansen JB. Serum levels of vitamin D and haemostatic factors in healthy subjects: The tromsø study. Acta Haematol . 2007;117(2):91-97. [DOI] [PubMed] [Google Scholar]
- 21.Blondon M, Cushman M, Jenny N, et al. Associations of Serum 25-hydroxyvitamin D with hemostatic and inflammatory biomarkers in the multi-ethnic study of atherosclerosis. J Clin Endocrinol Metab . 2016;101(6):2348-2357. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Kendrick J, Targher G, Smits G, Chonchol M. 25-Hydroxyvitamin D deficiency is independently associated with cardiovascular disease in the third national health and nutrition examination survey. Atherosclerosis . 2009;205(1):255-260. [DOI] [PubMed] [Google Scholar]
- 23.Targher G, Pichiri I, Lippi G. Vitamin D, thrombosis, and hemostasis: More than skin deep. Semin Thromb Hemost . 2012;38(1):114-124. [DOI] [PubMed] [Google Scholar]
- 24.Holick MF, Binkley NC, Bischoff-Ferrari HA, et al. Evaluation, treatment, and prevention of vitamin D deficiency: An Endocrine Society clinical practice guideline. J Clin Endocrinol Metab . 2011;96(7):1911-1930. [DOI] [PubMed] [Google Scholar]
- 25.Institute of Medicine Committee to Review Dietary Reference Intakes for Vitamin D, Calcium . The National Academies Collection: Reports funded by National Institutes of Health. In: Ross AC, Taylor CL, Yaktine AL, Del Valle HB, eds. Dietary Reference Intakes for Calcium and Vitamin D . Washington (DC) : National Academies Press (US).Copyright © 2011, National Academy of Sciences.; 2011. [PubMed] [Google Scholar]
- 26.EFSA Panel on Dietetic Products N, Allergies. Dietary reference values for vitamin D. EFSA Journal . 2016;14(10):e04547. [Google Scholar]
- 27.Munns CF, Shaw N, Kiely M, et al. Global consensus recommendations on prevention and management of nutritional rickets. J Clin Endocrinol Metab . 2016;101(2):394-415. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Cannell JJ, Hollis BW, Zasloff M, Heaney RP. Diagnosis and treatment of vitamin D deficiency. Expert Opin Pharmacother . 2008;9(1):107-118. [DOI] [PubMed] [Google Scholar]
- 29.Bischoff-Ferrari HA, Giovannucci E, Willett WC, Dietrich T, Dawson-Hughes B. Estimation of optimal serum concentrations of 25-hydroxyvitamin D for multiple health outcomes. Am J Clin Nutr . 2006;84(1):18-28. [DOI] [PubMed] [Google Scholar]
- 30.DSOG. D-vitamin mangel. http://gynobsguideline.dk/sandbjerg/D-vitaminmangelGuideline2013.pdf. Accessed2013.
- 31.Dawson-Hughes B, Heaney RP, Holick MF, Lips P, Meunier PJ, Vieth R. Estimates of optimal vitamin D status. Osteoporos Int . 2005;16(7):713-716. [DOI] [PubMed] [Google Scholar]
- 32.Holick MF, Binkley NC, Bischoff-Ferrari HA, et al. Evaluation, treatment, and prevention of vitamin D deficiency: An endocrine society clinical practice guideline. J Clin Endocrinol Metab. 2011;96(7):1911-1930. [DOI] [PubMed] [Google Scholar]
- 33.Vestergaard AL, Justesen S, Volqvartz T, et al. Vitamin D insufficiency among Danish pregnant women-prevalence and association with adverse obstetric outcomes and placental vitamin D metabolism. Acta Obstet Gynecol Scand . 2021;100(3):480-488. [DOI] [PubMed] [Google Scholar]
- 34.Andersen LB, Abrahamsen B, Dalgård C, et al. Parity and tanned white skin as novel predictors of vitamin D status in early pregnancy: A population-based cohort study. Clin Endocrinol (Oxf) . 2013;79(3):333-341. [DOI] [PubMed] [Google Scholar]
- 35.Vestergaard AL, Christensen M, Andreasen MF, Larsen A, Bor P. Vitamin D in pregnancy (GRAVITD) - a randomised controlled trial identifying associations and mechanisms linking maternal vitamin D deficiency to placental dysfunction and adverse pregnancy outcomes - study protocol. BMC Pregnancy Childbirth . 2023;23(1):177. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Højskov CS, Heickendorff L, Møller HJ. High-throughput liquid-liquid extraction and LCMSMS assay for determination of circulating 25(OH) vitamin D3 and D2 in the routine clinical laboratory. Clin Chim Acta . 2010;411(1-2):114-116. [DOI] [PubMed] [Google Scholar]
- 37.Garg U. 25-Hydroxyvitamin D testing: Immunoassays versus tandem mass spectrometry. Clin Lab Med. 2018;38(3):439-453. [DOI] [PubMed] [Google Scholar]
- 38.The Clinical & Laboratory Standards Institute (CLSI). Wayne P. Collection, transport, and processing of blood specimens for testing plasma-based coagulation assays and molecular hemostasis assays; approved guideline. 5th edn. CLSI; 2008. CLSI H21-A5. [Google Scholar]
- 39.Godtfredsen AC, Sidelmann JJ, Dolleris BB, et al. Fibrinolytic changes in women with preeclampsia. Clin Appl Thromb Hemost . 2022;28:1–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Espenhain Landgrebe L, Schlosser Mose L, Palarasah Y, Sidelmann JJ, Bladbjerg E.M. The effects of sampling from a peripheral venous catheter compared to repeated venepunctures on markers of coagulation, inflammation, and endothelial function. Scand J Clin Lab Invest . 2019;79(8):584-589. [DOI] [PubMed] [Google Scholar]
- 41.Kaye SM, Pietiläinen KH, Kotronen A, et al. Obesity-related derangements of coagulation and fibrinolysis: A study of obesity-discordant monozygotic twin pairs. Obesity (Silver Spring). 2012;20(1):88-94. [DOI] [PubMed] [Google Scholar]
- 42.Samad F, Ruf W. Inflammation, obesity, and thrombosis. Blood . 2013;122(20):3415-3422. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Leone A. Smoking, haemostatic factors, and cardiovascular risk. Curr Pharm Des . 2007;13(16):1661-1667. [DOI] [PubMed] [Google Scholar]
- 44.Buyukhatipoglu H, Tiryaki O, Usalan C. Impaired fibrinolytic and blunted nitric oxide response to phlebotomy in cigarette smoking healthy blood donors. J Int Med Res . 2009;37(3):674-679. [DOI] [PubMed] [Google Scholar]
- 45.Calder PC. Mechanisms of action of (n-3) fatty acids. J Nutr . 2012;142(3):592s-599s. [DOI] [PubMed] [Google Scholar]
- 46.Bays HE. Safety considerations with omega-3 fatty acid therapy. Am J Cardiol . 2007;99(6a):35c-43c. [DOI] [PubMed] [Google Scholar]
- 47.Nordt TK, Peter K, Ruef J, Kübler W, Bode C. Plasminogen activator inhibitor type-1 (PAI-1) and its role in cardiovascular disease. Thromb Haemost . 1999;82(Suppl 1):14-18. [PubMed] [Google Scholar]
- 48.Erickson LA, Fici GJ, Lund JE, Boyle TP, Polites HG, Marotti KR. Development of venous occlusions in mice transgenic for the plasminogen activator inhibitor-1 gene. Nature . 1990;346(6279):74-76. [DOI] [PubMed] [Google Scholar]
- 49.Carmeliet P, Stassen JM, Schoonjans L, et al. Plasminogen activator inhibitor-1 gene-deficient mice. II. Effects on hemostasis, thrombosis, and thrombolysis. J Clin Invest . 1993;92(6):2756-2760. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Moin ASM, Sathyapalan T, Butler AE, Atkin SL. Vitamin D association with coagulation factors in polycystic ovary syndrome is dependent upon body mass index. J Transl Med . 2021;19(1):239. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Jorde R, Sneve M, Torjesen P, Figenschau Y, Hansen JB. Parameters of the thrombogram are associated with serum 25-hydroxyvitamin D levels at baseline, but not affected during supplementation with vitamin D. Thromb Res . 2010;125(5):e210-e213. [DOI] [PubMed] [Google Scholar]
- 52.Stricker H, Tosi Bianda F, Guidicelli-Nicolosi S, Limoni C, Colucci G. Effect of a single, oral, high-dose vitamin D supplementation on endothelial function in patients with peripheral arterial disease: A randomised controlled pilot study. Eur J Vasc Endovasc Surg . 2012;44(3):307-312. [DOI] [PubMed] [Google Scholar]
- 53.Sinha-Hikim I, Duran P, Shen R, Lee M, Friedman TC, Davidson MB. Effect of long term vitamin D supplementation on biomarkers of inflammation in Latino and African-American subjects with pre-diabetes and hypovitaminosis D. Horm Metab Res . 2015;47(4):280-283. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Amarasekera AT, Assadi-Khansari B, Liu S, et al. Vitamin D supplementation lowers thrombospondin-1 levels and blood pressure in healthy adults. PLoS One . 2017;12(5):e0174435. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Fukumoto S, Allan EH, Martin TJ. Regulation of plasminogen activator inhibitor-1 (PAI-1) expression by 1,25-dihydroxyvitamin D-3 in normal and malignant rat osteoblasts. Biochim Biophys Acta . 1994;1201(2):223-228. [DOI] [PubMed] [Google Scholar]
- 56.Wu-Wong JR, Nakane M, Ma J. Vitamin D analogs modulate the expression of plasminogen activator inhibitor-1, thrombospondin-1 and thrombomodulin in human aortic smooth muscle cells. J Vasc Res . 2007;44(1):11-18. [DOI] [PubMed] [Google Scholar]
- 57.Zhai J, Li Z, Zhou Y, Yang X. The role of plasminogen activator inhibitor-1 in gynecological and obstetrical diseases: An update review. J Reprod Immunol . 2022;150:103490. [DOI] [PubMed] [Google Scholar]
- 58.Pinheiro MB, Gomes KB, Dusse LM. Fibrinolytic system in preeclampsia. Clin Chim Acta . 2013;416:67-71. [DOI] [PubMed] [Google Scholar]
- 59.Seferovic MD, Gupta MB. Increased umbilical cord PAI-1 levels in placental insufficiency are associated with fetal hypoxia and angiogenesis. Dis Markers . 2016;2016:7124186. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.O'Callaghan KM, Kiely M. Systematic review of vitamin D and hypertensive disorders of pregnancy. Nutrients . 2018;10(3):294. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Wagner CL, Hollis BW, Kotsa K, Fakhoury H, Karras SN. Vitamin D administration during pregnancy as prevention for pregnancy, neonatal and postnatal complications. Rev Endocr Metab Disord . 2017;18(3):307-322. [DOI] [PubMed] [Google Scholar]
- 62.Wei SQ, Qi HP, Luo ZC, Fraser WD. Maternal vitamin D status and adverse pregnancy outcomes: A systematic review and meta-analysis. J Matern Fetal Neonatal Med . 2013;26(9):889-899. [DOI] [PubMed] [Google Scholar]
- 63.Pereira-Santos M, Costa PR, Assis AM, Santos CA, Santos DB. Obesity and vitamin D deficiency: A systematic review and meta-analysis. Obes Rev . 2015;16(4):341-349. [DOI] [PubMed] [Google Scholar]
- 64.Reith A, Booth NA, Moore NR, Cruickshank DJ, Bennett B. Plasminogen activator inhibitors (PAI-1 and PAI-2) in normal pregnancies, pre-eclampsia and hydatidiform mole. Br J Obstet Gynaecol . 1993;100(4):370-374. [DOI] [PubMed] [Google Scholar]
- 65.Stewart FM, Freeman DJ, Ramsay JE, Greer IA, Caslake M, Ferrell WR. Longitudinal assessment of maternal endothelial function and markers of inflammation and placental function throughout pregnancy in lean and obese mothers. J Clin Endocrinol Metab . 2007;92(3):969-975. [DOI] [PubMed] [Google Scholar]
- 66.Prisco D, Fedi S, Brunelli T, et al. The influence of smoking on von willebrand factor is already manifest in healthy adolescent females: The floren-teen (florence teenager) study. Int J Clin Lab Res . 1999;29(4):150-154. [DOI] [PubMed] [Google Scholar]
- 67.Elbers LPB, Wijnberge M, Meijers JCM, et al. Coagulation and fibrinolysis in hyperparathyroidism secondary to vitamin D deficiency. Endocr Connect . 2018;7(2):325-333. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Witham MD, Dove FJ, Khan F, Lang CC, Belch JJ, Struthers AD. Effects of vitamin D supplementation on markers of vascular function after myocardial infarction—a randomised controlled trial. Int J Cardiol . 2013;167(3):745-749. [DOI] [PubMed] [Google Scholar]
- 69.Kalz J, ten Cate H, Spronk HM. Thrombin generation and atherosclerosis. J Thromb Thrombolysis . 2014;37(1):45-55. [DOI] [PubMed] [Google Scholar]
- 70.van Paridon PCS, Panova-Noeva M, van Oerle R, et al. Relationships between coagulation factors and thrombin generation in a general population with arterial and venous disease background. Thromb J . 2022;20(1):32. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.van Paridon PCS, Panova-Noeva M, van Oerle R, et al. Thrombin generation in cardiovascular disease and mortality—results from the gutenberg health study. Haematologica . 2020;105(9):2327-2334. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Saliba W, Awad K, Ron G, Elias M. The effect of vitamin D supplementation on thrombin generation assessed by the calibrated automated thrombogram. Clin Appl Thromb Hemost. 2014;22(4):340-345. [DOI] [PubMed] [Google Scholar]
- 73.Rüdiger IH, Andersen MK, Vestergaard AL, Bor P, Larsen A, Bor MV. Is Vitamin D Deficiency Prothrombotic? A Systematic Review . Semin Thromb Hemost . 2023;49(5):453–470. [DOI] [PubMed] [Google Scholar]
- 74.O'Neill CM, Kazantzidis A, Ryan MJ, et al. Seasonal changes in Vitamin D-Effective UVB availability in Europe and associations with population serum 25-hydroxyvitamin D. Nutrients . 2016;8(9):533. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75.McLaughlin M, Raggatt PR, Fairney A, Brown DJ, Lester E, Wills MR. Seasonal variations in serum 25-hydroxycholecalciferol in healthy people. Lancet . 1974;1(7857):536-538. [DOI] [PubMed] [Google Scholar]
- 76.Macdonald HM, Mavroeidi A, Fraser WD, et al. Sunlight and dietary contributions to the seasonal vitamin D status of cohorts of healthy postmenopausal women living at northerly latitudes: A major cause for concern? Osteoporos Int . 2011;22(9):2461-2472. [DOI] [PubMed] [Google Scholar]
- 77.Hollis BW, Johnson D, Hulsey TC, Ebeling M, Wagner CL. Vitamin D supplementation during pregnancy: Double-blind, randomized clinical trial of safety and effectiveness. J Bone Miner Res . 2011;26(10):2341-2357. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Supplemental material, sj-docx-1-cat-10.1177_10760296231201855 for Vitamin D Deficiency is Associated With Increased Plasminogen Activator Inhibitor 1/Plasminogen Activator Inhibitor 2 Ratio in Pregnancy by Matilde Kanstrup Andersen, Isabella Hangaard Rüdiger, Anna Louise Vestergaard, Yaseelan Palarasah, Pinar Bor, Agnete Larsen, and Mustafa Vakur Bor, in Clinical and Applied Thrombosis/Hemostasis