Abstract
The disseminated and progressive fungal disease Penicillium marneffei penicilliosis is one of the most common infectious diseases in AIDS patients in Southeast Asia. To diagnose systemic penicilliosis, we developed an enzyme-linked immunosorbent assay (ELISA)-based antibody test with Mp1p, a purified recombinant antigenic mannoprotein of P. marneffei. Evaluation of the test with guinea pig sera against P. marneffei and other pathogenic fungi indicated that this assay was specific for P. marneffei. Clinical evaluation revealed that high levels of specific antibody were detected in two immunocompetent penicilliosis patients. Furthermore, approximately 80% (14 of 17) of the documented penicilliosis patients with human immunodeficiency virus tested positive for the specific antibody. No false-positive results were found for serum samples from 90 healthy blood donors, 20 patients with typhoid fever, and 55 patients with tuberculosis, indicating a high specificity of the test. Thus, this ELISA-based test for the detection of anti-Mp1p antibody can be of significant value as a diagnostic for penicilliosis.
Penicillium marneffei is a dimorphic pathogenic fungus endemic in Southeast Asia and southern parts of China (5, 6, 10, 11). It is the causative agent of a systemic disease, penicilliosis marneffei, in both immunocompetent and immunocompromised patients. However, penicilliosis is particularly common in people infected with human immunodeficiency virus (HIV). In certain parts of Southeast Asia, disseminated infection by P. marneffei is the third most common opportunistic infection in HIV-positive patients, after extrapulmonary tuberculosis (TB) and cryptococcal meningitis (10). In addition, infections by P. marneffei have been reported for visitors travelling to the region of endemicity (6).
Patients suffering from penicilliosis often present with nonspecific symptoms, such as low-grade fever, anemia, and weight loss. Diagnosis is often made by identifying fungal cells in bone marrow, spleen, lymph node, and sometimes, skin biopsy samples (9–11, 18). Because of the invasive nature of the procedures to obtain such specimens, diagnosis is often delayed. The clinical features of penicilliosis, however, are very similar to those of TB, particularly extrapulmonary TB, which is also difficult to diagnose. Therefore, in areas of penicilliosis endemicity, many cases of disseminated penicilliosis are frequently misdiagnosed as TB. Unfortunately, anti-TB agents are not effective against P. marneffei (10). Sometimes, penicilliosis marneffei in HIV-infected patients can also be misdiagnosed as other fungal infections, such as histoplasmosis and cryptococcosis (5, 10). Without early diagnosis and treatment, the disease is associated with a high mortality rate regardless of whether HIV infection is involved (9–11, 18).
It was shown previously that patients with penicilliosis develop elevated titers of antibodies against P. marneffei cells in immunofluorescence and immunodiffusion tests (7, 14, 18). MP1, a P. marneffei gene that encodes a highly antigenic cell wall mannoprotein, Mp1p, was previously cloned (2). By using an immunoprecipitation assay, the presence of anti-Mp1p antibodies in both P. marneffei-inoculated guinea pigs and in patients with penicilliosis was demonstrated (2). With a purified recombinant Mp1p protein, a specific and sensitive enzyme-linked immunosorbent assay (ELISA)-based antibody test was developed for the serodiagnosis of penicilliosis in both immunocompetent and immunocompromised AIDS patients.
MATERIALS AND METHODS
Strains and growth conditions.
P. marneffei PM4 is a clinical isolate from a patient with systemic penicilliosis at Queen Mary Hospital, Hong Kong. Candida albicans NGY10 was kindly provided by N. A. R. Gow, University of Aberdeen, Aberdeen, United Kingdom. Aspergillius fumigatus UPN147 is an isolate from a patient with invasive pulmonary aspergillosis on day 100 after bone marrow transplantation at Queen Mary Hospital. Histoplasma capsulatum (ATCC 26032) and Blastomyces dermatitidis (ATCC 26199) were obtained from the American Type Culture Collection (Manassas, Va.). Fungal cells were grown first on YPD agar plates (1% yeast extract, 2% Bacto Peptone, 4% glucose, 1% agar) at 37°C for 2 to 3 days to get single colonies. Fungal cultures were obtained by inoculating fungal cells from plates into the synthetic medium RPMI (Gibco-BRL, Gaithersburg, Md.) and further shaking at 37°C for 1 to 5 days to achieve a cell density of greater than 105/ml of culture. For P. marneffei, yeast cells were obtained by growth in RPMI medium at 37°C. Yeast cells from H. capsulatum, B. dermatitidis, and C. albicans were obtained similarly. The fungal cells were pelleted by centrifugation at 3,000 rpm. After careful removal of the cell culture supernatant by aspiration, approximately 50 to 100% of the fungal cells could be retained.
Human and animal sera.
Human sera were obtained from patients with documented penicilliosis proven by either biopsy (bone marrow or lymph node) and/or blood culture results. Serum specimens were obtained from two penicilliosis patients (n = 2) without HIV infection or other conditions of immunodeficiency (Queen Mary Hospital). Additional serum specimens were obtained from HIV-positive penicilliosis patients (n = 17; Queen Elizabeth Hospital, Hong Kong). Control serum specimens were obtained from healthy blood donors (n = 90), patients with documented TB (n = 55), and patients with typhoid fever (n = 20) (Queen Mary Hospital). Guinea pig antisera against the fungal pathogens P. marneffei, C. albicans, A. fumigatus, H. capsulatum, and B. dermatitidis were produced as follows. After growth in RPMI medium for 1 to 5 days, the fungal yeast cells (with the exception of A. fumigatus mycelial cells) were harvested by centrifugation at 3,000 rpm. The cells were then resuspended in phosphate-buffered saline (13.7 mM sodium chloride, 0.27 mM potassium chloride, 1 mM phosphate buffer [pH 7.4] with 0.05% phenol) at a McFarland turbidity standard of 3. An equal volume of complete Freund’s adjuvant was mixed with 500 μl of yeast suspension, and 500 μl of the final suspension was injected intramuscularly into the thigh of the guinea pigs. Incomplete Freund’s adjuvant was used in subsequent immunizations in a procedure identical to the first immunization in which complete Freund’s adjuvant was used. A total of four inoculations were completed in 2 months, with one injection done every 2-week period.
Western blot analysis.
A glutathione S-transferase (GST) gene fusion system (Pharmacia, Uppsala, Sweden) was used for the expression and purification of P. marneffei antigenic protein Mp1p (2). For Western blot analysis, 100 ng of purified GST-Mp1p protein was loaded onto a sodium dodecyl sulfate (SDS)–10% polyacrylamide gel and subsequently electroblotted onto a nitrocellulose membrane (Bio-Rad, Hercules, Calif.). The blot was incubated with a 1:2,000 dilution of a guinea pig anti-P. marneffei antibody and detected with an ECL fluorescence system (Amersham Life Science, Buckinghamshire, England).
Serological test.
For the penicilliosis antibody test, each well of a Nunc (Roskilde, Denmark) immunoplate was coated with 0.5 ng of purified GST-Mp1p protein for 12 h and then blocked in phosphate-buffered saline with 2% bovine serum albumin. Testing was performed as described previously (4). Specifically, 100 μl of serially diluted animal serum (as indicated) or 1 μl of human serum (10 μl of 1:10-diluted serum) was added to the wells of the GST-Mp1p-coated plates in a total volume of 100 μl and incubated at 37°C for 2 h. After the plates were washed, 1:4,000-diluted alkaline phosphatase-conjugated goat anti-guinea pig or goat anti-human antibody was added (Cappel ICN Pharmaceuticals, Aurora, Ill.). The detection was carried out with p-nitrophenyl phosphate substrate (Sigma Immuno Chemicals, St. Louis, Mo.).
RESULTS
ELISA-based antibody test for penicilliosis marneffei.
To produce recombinant Mp1p protein, the GST-Mp1p fusion protein was expressed in Escherichia coli and subsequently purified (2). The purified fusion protein was separated on SDS-polyacrylamide gels followed by Coomassie blue staining or Western blot analysis with serum from a guinea pig inoculated with P. marneffei cells. A prominent immunoreactive protein band of 75 kDa was visible on the Western blot; this size was consistent with the expected size of 73 kDa for the full-length GST-Mp1p fusion protein (Fig. 1). This result showed the presence of specific anti-Mp1p antibody in guinea pig serum after P. marneffei cell inoculation and the immunoreactivity of the purified GST-Mp1p recombinant protein.
FIG. 1.

Western blot analysis of the purified recombinant Mp1p protein antigen with serum from a P. marneffei-inoculated guinea pig. Molecular size markers (in kilodaltons) are shown on the left.
An ELISA-based P. marneffei serology test was developed with this recombinant Mp1p protein for the detection of antibodies specific for this protein. Box titration was carried out with different dilutions of Mp1p coating antigen and a guinea pig anti-GST-Mp1p specific antibody. The results identified 0.5 ng of purified GST-Mp1p protein per ELISA well as the ideal amount for plate coating. Subsequently, this antibody test was evaluated for its sensitivity and specificity in an animal model and in clinical specimens.
Fungal specificity of the assay in a guinea pig model.
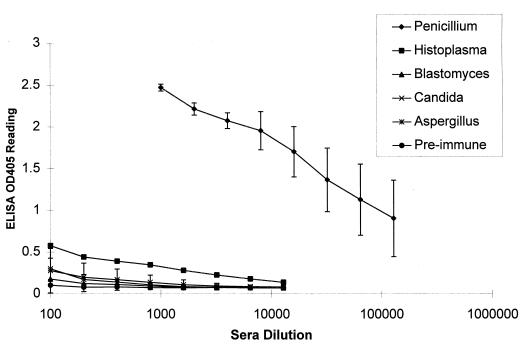
Since previous studies showed some cross-reactivity of P. marneffei with serological tests developed for other fungal pathogens (1, 12, 17), an animal model system was developed to examine this penicilliosis antibody test for potential cross-reactivity with antibodies to other fungal pathogens. Sera were collected from guinea pigs inoculated with the fungal pathogens P. marneffei, C. albicans, A. fumigatus, H. capsulatum, and B. dermatitidis. Serial dilutions of these antisera were made and subjected to the penicilliosis serology study, and the results of this study are shown in Fig. 2. The standard deviations of optical density values at 405 nm (OD405 values) at each dilution were obtained with the Microsoft Excel program, with the exception of H. capsulatum, for which only one immunized guinea pig was analyzed. The results indicated that the P. marneffei-inoculated guinea pigs developed high levels of specific antibody in this assay. In fact, the OD405 values for anti-P. marneffei sera at a 1:128,000 dilution were higher than those for all other antifungal sera at a 1:100 dilution. Thus, the serum antibody titers for P. marneffei-inoculated guinea pigs were at least 1,000-fold higher than those inoculated with other fungal pathogens.
FIG. 2.
ELISA-based antibody test detects high levels of specific antibodies only in P. marneffei-inoculated guinea pigs. Serial dilutions of the guinea pig sera were made, and an ELISA was performed to determine the antibody levels in the animal sera. OD405 values were obtained and plotted. Standard deviations for each serum group were obtained, with the exception of H. capsulatum, for which only one serum specimen was tested. The numbers of guinea pigs used for each group were as follows: preimmune, 3; P. marneffei inoculated, 3; C. albicans inoculated, 3; A. fumigatus inoculated, 3; H. capsulatum inoculated, 1; and B. dermatitidis inoculated, 2.
High antibody titer in two immunocompetent penicilliosis patients.
Previous results indicated that immunocompetent penicilliosis patients had high levels of specific antibodies against Mp1p protein (2). To determine their titers in this new ELISA antibody test, serum samples from two acute penicilliosis patients without HIV infection or other conditions of immunodeficiency were used. The results indicated that one of these patients had a serum titer (largest dilution factor showing positive result) of 20,000 and the other one had a serum titer of 80,000. Ten negative control serum samples from healthy blood donors in the same study showed no detectable signal at a 1:100 dilution (titer, <100). Since none of the healthy controls tested gave a positive signal at a 1:100 dilution, all subsequent evaluations with human sera were done at a 1:100 dilution.
Clinical evaluation of the antibody test for penicilliosis patients with AIDS.
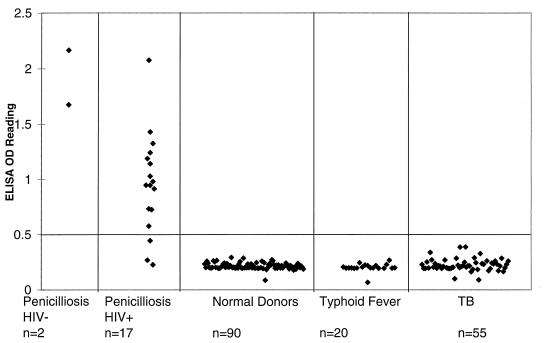
Since most systemic penicilliosis patients were also immunocompromised (HIV positive), serum samples from 17 confirmed penicilliosis patients who were HIV seropositive (n = 17) were investigated. The ELISA OD405 readings of these specimens were plotted, as shown in Fig. 3, together with those from two previous immunocompetent penicilliosis patients. To establish the baseline for the test, serum samples from 90 healthy blood donors were also tested in the penicilliosis antibody ELISA. For the 90 specimens from healthy controls, the mean ELISA OD405 value was 0.214, with a standard deviation of 0.028. An absorbance value of 0.49 was selected as the cutoff value that equals the sum of the mean value for the healthy control (0.21) and 10 times the standard deviation (0.28). The choice of a high cutoff value was to eliminate any potential false-positive results. For values under this cutoff value, the sensitivity of the test for penicilliosis-AIDS patients tested was 82% (14 of 17 patients). Although the number of immunocompetent penicilliosis patients was small (n = 2), one might expect that the assay should work at least as well as that for the AIDS patients. The specificity of the test was 100% since none of the 90 specimens from healthy controls was positive in the assay (0 of 90).
FIG. 3.
Evaluation of sensitivity and specificity of P. marneffei antibody test of penicilliosis patients. To evaluate the sensitivity and specificity of the P. marneffei antibody test, serum specimens were obtained from 17 documented penicilliosis patients with HIV infection. In addition, serum specimens from two penicilliosis patients without HIV infection or other conditions of immunodeficiency were also included in the evaluation. The control serum specimens for the test came from 90 healthy blood donors, 55 tuberculosis patients, and 20 typhoid fever patients. All serum samples were diluted 1:100 before the ELISA test was performed. The test results at OD405 were plotted. The cutoff line for positive diagnosis is drawn at a value that equals the sum of the mean value and 10 times the standard deviation of healthy blood donors.
Since P. marneffei penicilliosis is frequently misdiagnosed as TB, it was important to evaluate this antibody test by using sera from TB patients. The results for 55 TB patients indicated that the mean antibody test OD value was 0.226, with a standard deviation of 0.055 (Fig. 3). With the cutoff value set at 0.49, none of the serum specimens from TB patients scored positive at a 1:100 dilution. Similarly, none of the 20 serum specimens from patients with typhoid fever was positive. Both results further supported the high specificity of the penicilliosis antibody test. On the basis of the data from clinical evaluations and the analysis of the animal models with different fungal inoculations, the ELISA-based antibody test for penicilliosis with purified recombinant Mp1p protein antigen appears sensitive and specific.
DISCUSSION
In this study, an immunoassay was developed with a recombinant antigenic P. marneffei protein (Mp1p) for the serodiagnosis of penicilliosis of patients who are immunocompetent or immunocompromised (HIV seropositive). The results of the study indicate that immunocompetent penicilliosis patients have high serology titers in the immunoassay. More importantly, this assay is sufficiently sensitive for the diagnosis of penicilliosis in immunocompromised (HIV-positive) patients with approximately 80% (14 of 17 patients) sensitivity. Additional results show that this antibody test for penicilliosis is specific. First, of the animal sera obtained from guinea pigs inoculated with different fungal pathogens, only those inoculated with P. marneffei had high antibody titers. These titers were at least 1,000-fold higher than those from animals inoculated with any other fungal pathogen. Second, none of the 90 local healthy blood donors was positive in the assay. Finally, this assay was adequate to differentiate penicilliosis from TB since none of the serum samples from TB patients (0 of 55) tested was positive.
Thus, the results indicate that a specific serological test for antibodies against the Mp1p protein of P. marneffei may have significant value in the diagnosis of penicilliosis in both immunocompetent and immunocompromised patients. This result resembles other antibody tests for a similar fungal disease, histoplasmosis, for which serological antibody tests are often the first laboratory evidence of the disease (16). Previously, there were several reports on antibody detection for penicilliosis based on immunodiffusion (7, 8) or immunofluorescence tests (18) with either crude antigen preparation or whole fungal cells. However, there are limitations in both the sensitivity and specificity of those tests. Recently, two studies identified several specific protein antigens of P. marneffei that are immunologically reactive in approximately 50% of penicilliosis serum specimens on Western blot assays (3, 13). However, the genes for these protein antigens are not known. MP1 is the first gene cloned that codes for an antigenic protein, Mp1p, of P. marneffei. It was shown in this study that an ELISA antibody test developed with the purified recombinant Mp1p offers significantly improved sensitivity and specificity for the serodiagnosis of penicilliosis marneffei. This result is likely due to the higher sensitivity of an ELISA and the better specificity with a pure recombinant protein antigen.
The specificity of the antibody test for penicilliosis with a pure recombinant protein appears to be very good. The study with animal models shows very limited cross-reactivity with antibodies to different fungal pathogens in the penicilliosis antibody test. This is probably because Mp1p protein is unique to P. marneffei or it is not highly conserved among fungal pathogens. In fact, our unpublished findings favor the second possibility since Western blot analysis of extracts from several fungal pathogens, including C. albicans, H. capsulatum, and Cryptococcus neoformans, with specific anti-Mp1p antibody revealed that the immunoreactive protein was present only in P. marneffei. Therefore, we suggest that a positive result should be a very specific indicator of penicilliosis. In contrast, tests with crude antigen preparations often have significant cross-reactivity. Previous studies of antibody responses in rabbits immunized independently with H. capsulatum, Paracoccidioides brasiliensis, and B. dermatitidis demonstrated that all rabbit sera had significant immunoreactivity with a crude antigen extracted from H. capsulatum (1). Such cross-reactions between these fungi were also demonstrated in antibody tests of sera from penicilliosis patients (15).
Clinical evaluation further strengthens the specificity of the serology test. Although P. marneffei is endemic in this area, there is little evidence for the presence of specific antibodies against this fungal protein antigen in the general population. The results obtained from healthy blood donors indicated that none of the 90 specimens was positive in the assay. TB is commonly found in the local population, especially in immunocompromised patients, with a clinical presentation very similar to that of penicilliosis. Since TB patients need to be treated differently from those with penicilliosis, it is clinically important to differentiate these two diseases. The results show that none of the serum samples from 55 TB patients was positive in the test. Therefore, a positive result in the test should be a good indicator of penicilliosis.
It should be noted that both antibody and antigen tests are needed for the serodiagnosis of penicilliosis. An antibody test could be more informative for patients with better humoral immunity, i.e., sufficient to remove fungal antigens in the blood, or, alternatively, it could be useful when the fungal load is low. However, with lower immunity and increased fungal load, an antigenemia test could be more useful. Immunodiffusion (7) and latex agglutination (8) tests which appear to be specific and reasonably sensitive for detecting P. marneffei antigenemia have recently been developed. These tests should complement the ELISA-based antibody test presented here for the clinical diagnosis of penicilliosis.
ACKNOWLEDGMENTS
We thank Wai Ting Hui for excellent technical assistance and J. S. M. Peiris and N. Chan for the critical reading of the manuscript.
This work is supported by grants from CRCG of the University of Hong Kong (to L.C.) and from the Hong Kong Industry Support Fund (AF/55/96).
REFERENCES
- 1.Azuma I, Kanetsuna F, Tanaka Y, Yamamura Y, Carbonell L M. Chemical and immunological properties of galactomannans obtained from: Histoplasma duboissii, Histoplasma capsulatum, Paracoccidioides brasiliensis, and Blastomyces dermatitidis. Mycopathol Mycol Appl. 1974;54:111–125. doi: 10.1007/BF02055979. [DOI] [PubMed] [Google Scholar]
- 2.Cao L, Chan C-M, Lee C, Wong S S-Y, Yuen K-Y. MP1 encodes an abundant and highly antigenic cell wall mannoprotein in the pathogenic fungus Penicillium marneffei. Infect Immun. 1998;66:966–973. doi: 10.1128/iai.66.3.966-973.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Chongtrakool P, Chaiyarol S C, Vithayasai V, Trawatcharegon S, Teanpaisan R, Kalnawakul S, Sirisinha S. Immunoreactivity of a 38-kilodalton Penicillium marneffei antigen with human immunodeficiency virus-positive sera. J Clin Microbiol. 1997;35:2220–2223. doi: 10.1128/jcm.35.9.2220-2223.1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Coligan J E, Kruisbeek A M, Hargulies D H, Shevach E M, Strober W, editors. Current protocols in immunology. New York, N.Y: John Wiley & Sons, Inc.; 1994. [Google Scholar]
- 5.Deng Z L, Ribas J L, Gibson D W, Connor D H. Infections caused by Penicillium marneffei in China and Southeast Asia: review of eighteen published cases and report of four more Chinese cases. Rev Infect Dis. 1988;10:640–652. doi: 10.1093/clinids/10.3.640. [DOI] [PubMed] [Google Scholar]
- 6.Duong A D. Infection due to Penicillium marneffei, an emerging pathogen: review of 155 reported cases. Clin Infect Dis. 1996;23:125–130. doi: 10.1093/clinids/23.1.125. [DOI] [PubMed] [Google Scholar]
- 7.Imwidthaya P, Sekhon A S, Mastro T D, Garg A K, Ambrosie E. Usefulness of a microimmunodiffusion test for the detection of Penicillium marneffei antigenemia, antibodies and exoantigens. Mycopathologia. 1997;138:51–55. doi: 10.1023/a:1006826907109. [DOI] [PubMed] [Google Scholar]
- 8.Kaufman L, Standard P G, Jalbert M, Kantipong P, Limpakarnjanarat K, Mastro T D. Diagnostic antigenemia tests for penicilliosis marneffei. J Clin Microbiol. 1996;34:2503–2505. doi: 10.1128/jcm.34.10.2503-2505.1996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Supparatpinyo K, Chiewchanvit S, Hirunsri P, Uthammachai C, Nelson K E, Sirisanthana T. Penicillium marneffei infection in patients infected with human immunodeficiency virus. Clin Infect Dis. 1992;14:871–874. doi: 10.1093/clinids/14.4.871. [DOI] [PubMed] [Google Scholar]
- 10.Supparatpinyo K, Khamwan C, Baosoung V, Nelson K E, Sirisanthana T. Disseminated Penicillium marneffei infection in Southeast Asia. Lancet. 1994;344:110–113. doi: 10.1016/s0140-6736(94)91287-4. [DOI] [PubMed] [Google Scholar]
- 11.Tsui W M S, Ma K F, Tsang D N C. Disseminated Penicillium marneffei infection in HIV-infected subject. Histopathology. 1991;20:287–293. doi: 10.1111/j.1365-2559.1992.tb00985.x. [DOI] [PubMed] [Google Scholar]
- 12.Van Cutsem J, Meulemans L, Van Gerven F, Stynen D. Detection of circulating galactomannan by Pastorex Aspergillus in experimental invasive aspergillosis. Mycoses. 1990;33:61–69. doi: 10.1111/myc.1990.33.2.61. [DOI] [PubMed] [Google Scholar]
- 13.Vanittanakom N, Mekaprateep M, Sittisombut N, Supparatpinyo K, Kanjanasthiti P, Nelson K E, Sirisanthana T. Western immunoblot analysis of protein antigens of Penicillium marneffei. J Med Vet Mycol. 1997;35:123–131. doi: 10.1080/02681219780001011. [DOI] [PubMed] [Google Scholar]
- 14.Viviani M A, Tortorano A M, Rizzardini G, Quirino T, Kaufman L, Padhye A A, Ajello L. Treatment and serological studies of an Italian case of penicilliosis marneffei contracted in Thailand by a drug addict infected with the human immunodeficiency virus. Eur J Epidemiol. 1993;9:79–85. doi: 10.1007/BF00463094. [DOI] [PubMed] [Google Scholar]
- 15.Wheat L J, French M L V, Kamel S, Tewari R P. Evaluation of cross-reactions in Histoplasma capsulatum serological tests. J Clin Microbiol. 1986;23:493–499. doi: 10.1128/jcm.23.3.493-499.1986. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Wheat L J. Fungal infections in the immunocompromised host. In: Rubin R H, Young L S, editors. Clinical approach to infection in the compromised host. New York, N.Y: Plenum Press; 1994. pp. 211–236. [Google Scholar]
- 17.Wheat L J, Wheat H, Connolly P, Kleiman M, Supparatpinyo K, Nelson K E, Bradsher R, Restrepo A. Cross-reactivity in Histoplasma capsulatum variety capsulatum antigen assays of urine samples from patients with endemic mycoses. Clin Infect Dis. 1997;24:1169–1171. doi: 10.1086/513647. [DOI] [PubMed] [Google Scholar]
- 18.Yuen K Y, Wong S S, Tsang D N, Chau P Y. Serodiagnosis of Penicillium marneffei infection. Lancet. 1994;344:444–445. doi: 10.1016/s0140-6736(94)91771-x. [DOI] [PubMed] [Google Scholar]