Abstract
Targeted protein degradation has emerged as a powerful approach for the selective elimination of disease-causing proteins. Cyclin-dependent kinases 4 and 6 (CDK4/6) are of significant interest in cancer research due to their crucial role in cell cycle regulation. However, resistance to CDK4/6 inhibitors is a considerable challenge. This Patent Highlight showcases the recent advances in strategies to degrade CDK4/6 to overcome drug resistance, explicitly focusing on proteolysis-targeting chimeras (PROTACs) and molecular glue degraders.
Important Compound Classes
Titles
INK4 Tumor Suppressor Proteins Mediate Resistance to CDK4/6 Kinase Inhibitors; and Small Molecule Cyclin Dependent Kinase 4/6 (CDK4/6) and IKZF2 (Helios) Degraders and Methods of Use Thereof
Patent Publication Numbers
WO 2023/039081 A2 (URL: https://patents.google.com/patent/WO2023039081A2/en?oq=WO+2023%2f039081+A2)
WO 2023/288305 A1 (URL: https://patents.google.com/patent/WO2023288305A1/en?oq=WO+2023%2f288305+A1)
Publication Dates
March 16, 2023, and January 19, 2023
Priority Applications
US 63/241,787 and US 63/222,646
Priority Dates
September 8, 2021, and July 16, 2021
Inventors
Chandarlapaty, S.; Li, Q.; Gray, N.; Jiang, B.; Sharma, A.; Mini, A. (WO 2023/039081 A2); and Verano, A.; Wang, E.; You, I.; Gray, N. (WO 2023/288305 A1)
Assignee Companies
Memorial Sloan-Kettering Cancer Center [US/US]; Memorial Hospital for Cancer and Allied Diseases [US/US]; Sloan-Kettering Institute for Cancer Research; 1275 York Avenue, New York, New York 10065, United States; Dana-Farber Cancer Institute [US/US]; 450 Brookline Ave, Boston, Massachusetts 02215, United States; and Stevens Institute of Technology [US/US]; 1 Castle Point Terrance, Hoboken, New Jersey 07030, United States (WO 2023/039081 A2); Dana-Farber Cancer Institute, Inc. [US/US]; 450 Brookline Avenue, Boston, Massachusetts 02215, United States (WO 2023/288305 A1)
Disease Area
Cancer
Biological Target
CDK4/6
Summary
Cyclin-dependent kinases 4 and 6 (CDK4/6) are essential cell cycle regulators, making them promising targets for cancer therapy. However, resistance to CDK4/6 inhibitors is a significant challenge in clinical oncology. Recent advances in targeted protein degradation offer a new paradigm to overcome this resistance.
The resistance mechanisms for CDK4/6 inhibitors are complex and multifaceted. One possible mechanism is an INK4-CDK6 complex that is insensitive to CDK4/6 inhibitors. Genetic alterations such as loss-of-function mutations in the FAT1 gene or tumor suppressor genes PTEN and ARID1A can also lead to CDK6 overexpression, promoting CDK4/6 inhibitor resistance.
INK4 proteins (including p15, p16, p18, and p19) bind to and inhibit the cyclin-dependent kinases CDK4 and CDK6, preventing them from phosphorylating the retinoblastoma protein (Rb), thus blocking the cell cycle progression. However, when it comes to the efficacy of CDK4/6 inhibitors, INK4-CDK6 complexes may play a role in resistance. When cells overexpress INK4 proteins under specific conditions, they may decrease their susceptibility to inhibition by CDK4/6 inhibitors. This resistance could result from structural changes in the CDK4/6 enzyme binding pocket that hinder the inhibitor from attaching effectively.
Researchers develop CDK4/6 inhibitors (e.g., palbociclib, ribociclib, and abemaciclib) to bind to CDK4/6 and block their interaction with cyclin D1, effectively inhibiting cell cycle progression. However, the binding of INK4 proteins to CDK6 may affect the conformation of the kinase, potentially altering the binding pocket and making it inaccessible to the inhibitors. This change could reduce the efficacy of the CDK4/6 inhibitors and result in drug resistance.
Genetic alterations that increase CDK6 expression or activity can lead to CDK6-mediated resistance to inhibitors. For example, loss-of-function mutations in the FAT1 gene, which generally suppresses CDK6 expression, can lead to CDK6 overexpression and subsequent resistance to CDK4/6 inhibitors. Additionally, loss-of-function mutations in the tumor suppressor genes PTEN or ARID1A can increase CDK6 expression and resistance to CDK4/6 inhibitors.
Targeted protein degradation, including PROTACs and molecular glue degraders, provides a mechanism to eliminate specific proteins from cells, thus offering a potential solution to inhibitor resistance. These technologies recruit an E3 ubiquitin ligase to the target protein, resulting in the ubiquitination and subsequent degradation of the target.
PROTACs targeting CDK4/6 have been developed and show promise for overcoming drug resistance. By inducing the degradation of CDK4/6, these PROTACs could overcome the resistance mechanisms associated with CDK4/6 inhibitors.
As such, targeting the degradation of CDK4/6 could be a revolutionary approach to overcoming drug resistance in cancer therapy. Further research is needed to refine these strategies and evaluate their safety and efficacy in clinical trials.
The patent WO 2023/039081 also details a method for inducing CDK4 and/or CDK6 degradation in a patient by administering a therapeutically effective amount of a compound described herein, along with a pharmaceutically acceptable carrier. The method is used for treating, preventing, and ameliorating a CDK4- and/or CDK6-mediated disorder, disease, or condition. Another application includes treating, preventing, and mitigating breast cancer.
The patent further elaborates on scientific experiments and their results, like the formation of an INK4-CDK6 complex that promotes resistance in cells, the insensitivity of INK4-CDK6 complexes to CDK4/6 inhibitors, and the genetic alterations that lead to CDK6-mediated resistance in patients. These experiments leverage techniques such as co-immunoprecipitation, ADP-Glo kinase assays, mass spectrometry, computational modeling, and microscale thermophoresis.
The patent WO 2023/288305 Al discloses the discovery and use of a bifunctional compound that can bind with CDK4 and/or CDK6 enzymes. It also includes methods for synthesizing these compounds and their pharmaceutically acceptable salts and stereoisomers. These compounds may treat diseases or disorders characterized by abnormal CDK4 and/or CDK6 activity and Helios, a critical regulator of T cell activity. These diseases notably include various types of cancer. The bifunctional compound may reduce CDK4 and/or CDK6 levels and Helios in cells. It promotes the degradation of these proteins, which may lead to anti-tumor immunity. The compound may function by reprogramming specific ligase complexes to target these proteins for ubiquitination and degradation. The combination may further enhance the anti-tumor immune response by converting regulatory T cells into effector T cells and restoring effector T cell function in “exhausted” T cells.
The mechanism by which these compounds work is by inducing Helios’s degradation by forming a “molecular glue” with a protein called cereblon (CRBN). These molecules effectively recruit a ubiquitin ligase (CRBN) to the target protein (Helios) and act as a catalyst for targeted protein degradation. The bifunctional compound significantly affects the activity of CDK4, CDK6, and Helios, providing a new approach for treatments for diseases related to these proteins.
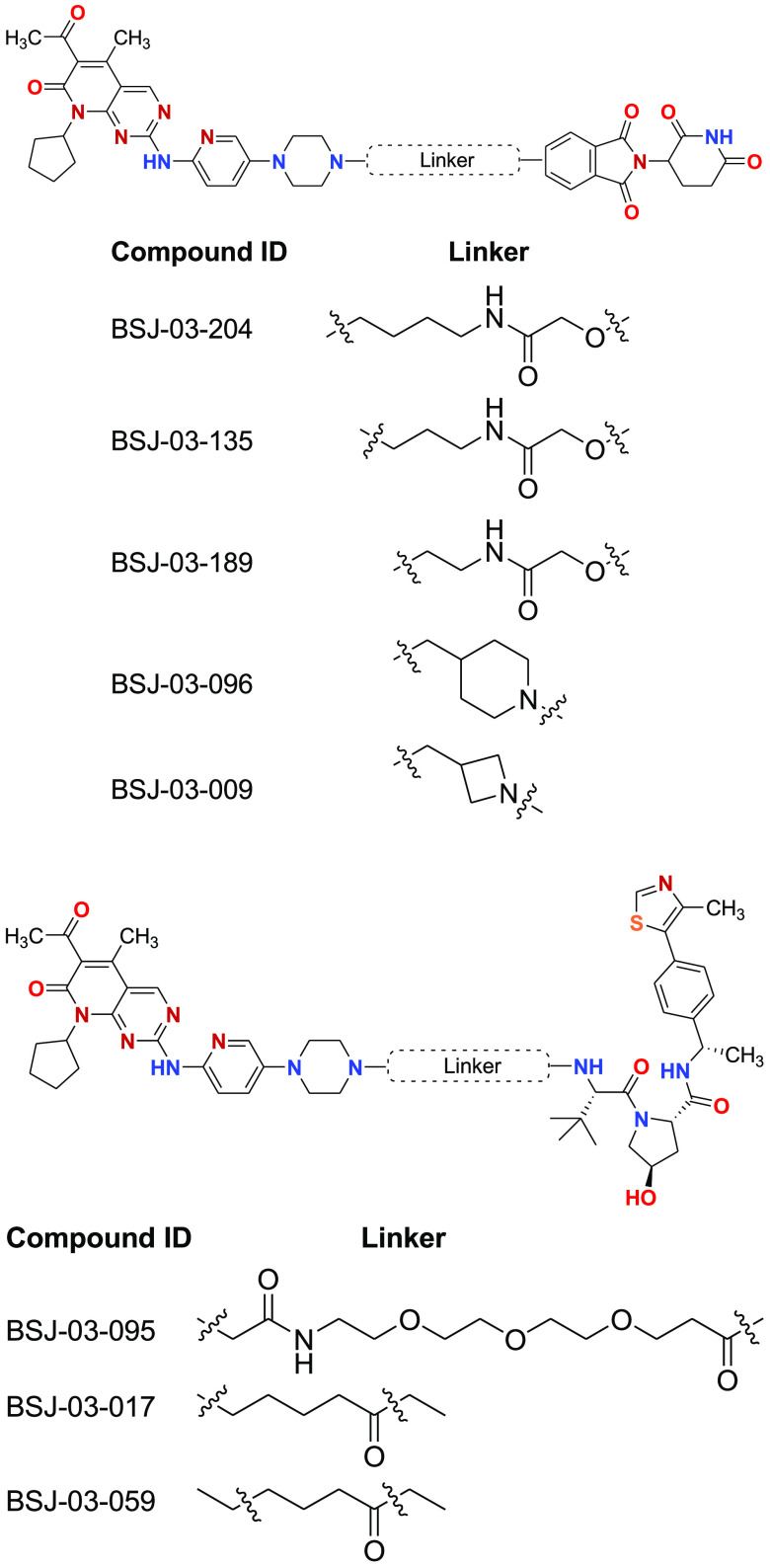
Key Structures
Biological Assay
IP-in vitro kinase assay, cell viability assay, Western blotting, and microscale thermophoresis (MST) assay. C57BL/6 male mice were dosed with BSJ-03-96 and BSJ-05-017 solution.
Biological Data
The table below shows data from a pharmacokinetics
study in mice. For human subjects, a total of 1366 metastatic tumors
from 1115 patients with HR+/HER2-metastatic breast cancer were analyzed.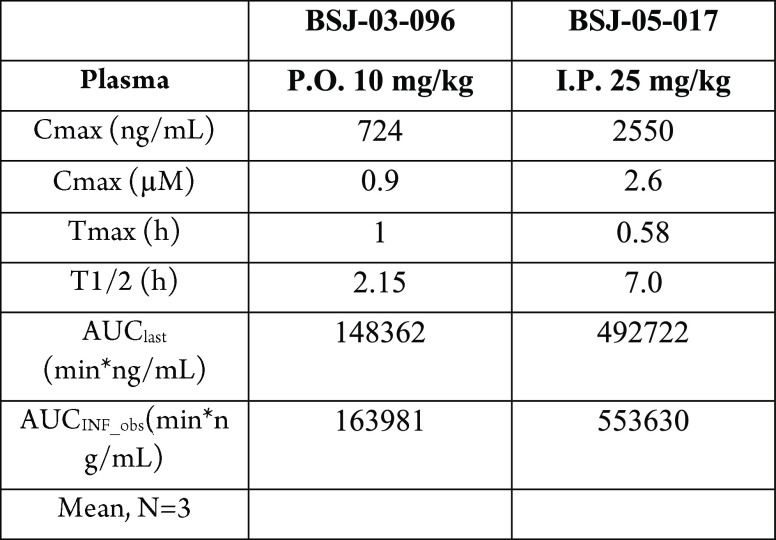
Recent Review Articles
The author declares no competing financial interest.
References
- Zhu Z.; Zhu Q. Differences in Metabolic Transport and Resistance Mechanisms of Abemaciclib, Palbociclib, and Ribociclib. Front. Pharmacol. 2023, 14, 1212986. 10.3389/fphar.2023.1212986. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shastry M.; Hamilton E. Novel Estrogen Receptor-Targeted Agents for Breast Cancer. Curr. Treat. Options Oncol. 2023, 24, 821–844. 10.1007/s11864-023-01079-y. [DOI] [PubMed] [Google Scholar]
- Corti C.; De Angelis C.; Bianchini G.; Malorni L.; Giuliano M.; Hamilton E.; Jeselsohn R.; Jhaveri K.; Curigliano G.; Criscitiello C. Novel Endocrine Therapies: What is Next in Estrogen Receptor Positive, HER2 Negative Breast Cancer?. Cancer Treat. Rev. 2023, 117, 102569. 10.1016/j.ctrv.2023.102569. [DOI] [PubMed] [Google Scholar]
- Nilmani; D’costa M.; Bothe A.; Das S.; Udhaya Kumar S.; Gnanasambandan R.; George Priya Doss C. CDK Regulators-Cell Cycle Progression or Apoptosis-Scenarios in Normal Cells and Cancerous Cells. Adv. Protein Chem. Struct. Biol. 2023, 135, 125–177. 10.1016/bs.apcsb.2022.11.008. [DOI] [PubMed] [Google Scholar]
- Rezaeian A. H.; Inuzuka H.; Wei W. Insights into the Aberrant CDK4/6 Signaling Pathway as a Therapeutic Target in Tumorigenesis. Adv. Protein Chem. Struct. Biol. 2023, 135, 179–201. 10.1016/bs.apcsb.2022.11.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Niu Z.; Jin R.; Zhang Y.; Li H. Signaling Pathways and Targeted Therapies in Lung Squamous Cell Carcinoma: Mechanisms and Clinical Trials. Signal. Transduct. Target. Ther. 2022, 7, 353. 10.1038/s41392-022-01200-x. [DOI] [PMC free article] [PubMed] [Google Scholar]