Abstract

We show that in silico design of DNA secondary structures is improved by extending the base pairing alphabet beyond A–T and G–C to include the pair between 2-amino-8-(1′-β-d-2′-deoxyribofuranosyl)-imidazo-[1,2-a]-1,3,5-triazin-(8H)-4-one and 6-amino-3-(1′-β-d-2′-deoxyribofuranosyl)-5-nitro-(1H)-pyridin-2-one, abbreviated as P and Z. To obtain the thermodynamic parameters needed to include P–Z pairs in the designs, we performed 47 optical melting experiments and combined the results with previous work to fit free energy and enthalpy nearest neighbor folding parameters for P–Z pairs and G–Z wobble pairs. We find G–Z pairs have stability comparable to that of A–T pairs and should therefore be included as base pairs in structure prediction and design algorithms. Additionally, we extrapolated the set of loop, terminal mismatch, and dangling end parameters to include the P and Z nucleotides. These parameters were incorporated into the RNAstructure software package for secondary structure prediction and analysis. Using the RNAstructure Design program, we solved 99 of the 100 design problems posed by Eterna using the ACGT alphabet or supplementing it with P–Z pairs. Extending the alphabet reduced the propensity of sequences to fold into off-target structures, as evaluated by the normalized ensemble defect (NED). The NED values were improved relative to those from the Eterna example solutions in 91 of 99 cases in which Eterna-player solutions were provided. P–Z-containing designs had average NED values of 0.040, significantly below the 0.074 of standard-DNA-only designs, and inclusion of the P–Z pairs decreased the time needed to converge on a design. This work provides a sample pipeline for inclusion of any expanded alphabet nucleotides into prediction and design workflows.
Keywords: DNA secondary structure design, synthetic biology, DNA folding thermodynamics, expanded DNA alphabet
Introduction
Natural and designed nucleic acids serve a number of roles in vitro and in cells. In nature, DNA is largely an information carrier, while RNA is an information carrier, a catalyst,1 an agent for recognition of complementary sequences, and an aptamer for metabolite binding.2−4 New binding and catalytic roles can be evolved in vitro for RNA and DNA using systematic evolution of ligands by exponential enrichment (SELEX).5 RNA and DNA are also convenient scaffolds for molecular arrays6,7 and molecular machines.8 Almost all of these roles for nucleic acids involve folding into secondary and tertiary structures beyond simple duplexes.
Nucleic acids can be designed in silico to fold into specified secondary structures, and a reliable method for solving this so-called inverse folding problem would reduce the need for selection or trial and error experiments.9 Traditionally, the design process requires a search algorithm and an objective function to evaluate the candidate designs. An efficient search algorithm is necessary because an exponentially increasing number of sequences can fold to any desired target structure as a function of sequence length.10 Generally, sequences are optimized locally and subsequently assembled into larger structures. The objective function assesses the design quality by comparison to the target structure.
One approach is to guarantee that the sequence folds with minimum (most negative) free energy change from the random coil to give the target structure.11 This ensures that the most populated structure at equilibrium will be the target structure, although it does not guarantee that the probability of the desired structure will be high in the Boltzmann ensemble.
Another approach is to minimize the normalized ensemble defect (NED), the average probability that a nucleotide will be in the wrong conformation in the ensemble.12 This improves upon assessing solely the minimum free energy structure in that optimizing the NED also ensures that the helix and loop components of the desired structure will also occur with high probability.13 It can conveniently be calculated from a partition function expression14 for base pairing probabilities:
where N is the sequence length and Pi is the probability that nucleotide i is in the expected structure. The probability Pi is computed over the Boltzmann ensemble of all possible secondary structures.14 For paired nucleotides, Pi is the probability that nucleotide i is paired with its exact intended pairing partner. For unpaired nucleotides, it is the probability the nucleotide is unpaired.
The normalized ensemble defect quantifies the extent to which the desired components of the structure dominate the Boltzmann ensemble. It can be difficult to achieve a low ensemble defect, because many competing structures are often possible for a sequence. The desired structure and its close analogs must have a substantially lower folding free energy change than all of the competing structures in order to fold with high probability.
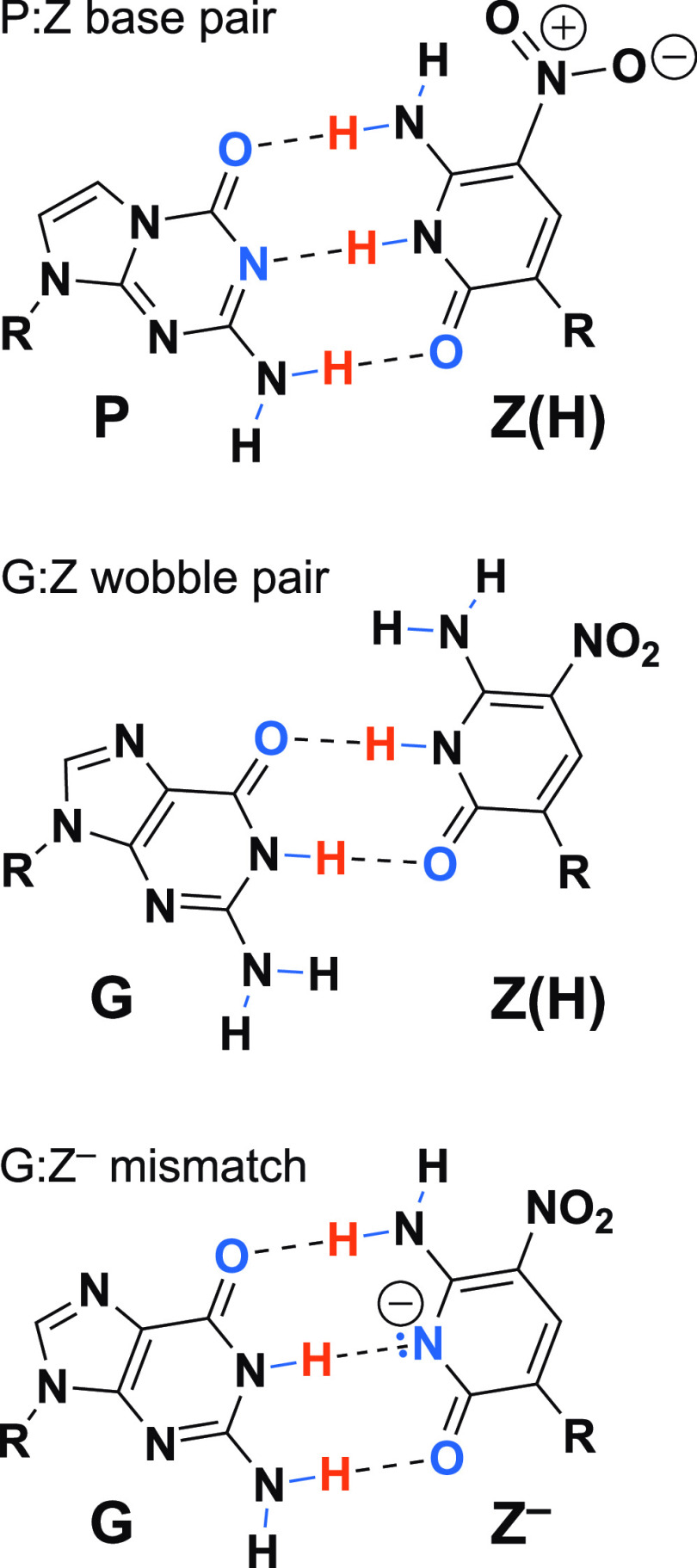
In this work, we design structures with an extended DNA alphabet, including imidazo-[1,2-a]-1,3,5-triazin-(8H)-4-one (P) and 6-amino-5-nitro-(1H)-pyridin-2-one (Z) bases of the second-generation AEGIS (Artificially Expanded Genetic Information System). P and Z form a three hydrogen-bonded base pair (Figure 1)15,16 that is largely orthogonal to the standard Watson–Crick–Franklin base pairs. Hence, P and Z can be used to add base pairs that are less likely to find alternative pairing partners than natural DNA nucleotides.
Figure 1.
(Top) The P–Z base pair has three hydrogen bonds. (Middle) The proposed G–Z “wobble” base pair with two hydrogen bonds. (Bottom) The deprotonated G–Z– pair is dominant at elevated pH.17 All results in this paper refer to pH 7.
To use P and Z in inverse folding designs, the thermodynamics of folding from the random coil need to be known in order to evaluate the design quality. Prior work established that P and Z prefer pairing with each other above all single mismatches, except that G–Z pairs are roughly as stable as A–T pairs (Figure 1).16,17 A subsequent study fit the helical nearest neighbor thermodynamic parameters for P–Z as part of the eight base “Hachimoji” system.15 In this work, we determined nearest neighbor parameters for a full P–Z extended alphabet, including fitting stacks with G–Z wobbles, better defining the P–Z stack parameter values, and extrapolating loop parameters to include P and Z bases. The RNAstructure software package was previously extended to accommodate folding alphabets of any size, and therefore it is able to handle designs using the six base alphabet.18
We demonstrate that in silico designs are improved by using the additional P–Z base pair. We chose to use the Eterna 100 benchmark structures19 and the design program from RNAstructure,20 which implements a version of the NUPACK algorithm for NED minimization.12 94 of 100 benchmark structures could be designed by RNAstructure using both standard DNA and the extended P–Z base pairs. The sequences with P–Z outperformed the sequences with standard DNA, as quantitated by showing a significantly lower NED. Both types of designs had significantly lower NED than the standard solutions provided by Eterna.
Results
For optimal designs, it is essential to have as complete a set of nearest neighbor thermodynamic parameters as possible to estimate folding stability from the random coil. Previous work showed that G–Z pairs stabilize helix formation16 and secondary structure designs have loop regions that connect helices. Therefore, we extended the existing data on P and Z thermodynamics to loops and also fit additional nearest neighbor parameters.
Stacking Nearest Neighbor Parameters for P–Z and G–Z Base Pairs
To fit nearest neighbor stacking parameters for P–Z pairs, we used the existing data set of optical melting data for duplexes with P–Z pairs15,16 and an additional 13 optical melting experiments from this work.21−24 We refined the data analysis procedure for optical melting experiments to simultaneously fit absorbance vs temperature curves at multiple concentrations in order to minimize the error with respect to all available experimental data (see Supporting Information Figure S1 and Methods section for details). Table S1 provides the optical melting stabilities determined for the 13 all-helix duplexes studied in this work.
An important consideration is whether terminal P–Z pairs need an end correction term like A–T pairs or RNA A–U pairs.25,26 The end correction term was originally motivated by hydrogen bond counting. Subsequent studies, however, found that G–U and m6A–U pairs at helix ends did not need corrections,18,27 which indicates that hydrogen bond counting does not determine the need for an end correction. It was therefore an open question of whether an end correction would be necessary for P–Z pairs at helix ends. To address this, we used linear regression to fit the P–Z stacking parameters with and without an end correction. Table S2 shows the results of these regressions, which demonstrated that the P–Z end correction was small in magnitude compared to the standard error of the regression (0.33 ± 0.21 kcal/mol).
We examined the residuals for the fit when the P–Z end penalty was included and observed a large residual of 2.22 kcal/mol for duplex (ZGCATGCP)2. This single experimental value drove much of the magnitude of the P–Z penalty, and the residual for (ZGCATGCP)2 was the largest across all duplexes used in the fit. Therefore, we removed this sequence from the fit as an outlier and repeated the regression. In this fit, absent the outlier, the terminal P–Z parameter is smaller in magnitude than the standard error of the regression (0.03 ± 0.19). Given this result, we do not include a terminal P–Z penalty in the fit of the stacking nearest neighbor parameters. The additional experimental data available in this study informed this decision, which differs from previous work that did include the terminal P–Z penalty.15
The P–Z stack nearest neighbor parameters for the free energy change at 37 °C are provided in Table 1, where they are compared to G–C nearest neighbor parameters. On average, a P–Z substitution for a G–C pair is stabilizing by −0.2 ± 0.4 kcal/mol. This varies across the stacks; there are two cases where P–Z substitutions of G–C pairs were unfavorable. Therefore, the full nearest neighbor treatment of P–Z pairs is required to fit the set of oligonucleotide thermodynamic data; a simple increment for P–Z substitution of G–C pairs would not suffice.
Table 1. P–Z Stack Nearest Neighbor Parameters, as Compared to Analogous G–C Stacks (Replacing P–Z with G–C)a.
| parameter | ΔG°37 (kcal/mol) | parameter | ΔG°37 (kcal/mol) | ΔΔG°37 (kcal/mol) per P–Z substitution |
|---|---|---|---|---|
| AZ | –1.44 ± 0.17 | AC | –1.4 | 0.0 |
| TP | TG | |||
| TP | –1.45 ± 0.15 | TG | –1.5 | 0.1 |
| AZ | AC | |||
| TZ | –1.49 ± 0.15 | TC | –1.3 | –0.2 |
| AP | AG | |||
| PZ | –1.57 ± 0.33 | GC | –2.2 | +0.3 |
| ZP | CG | |||
| GZ | –1.74 ± 0.16 | GC | –2.2 | +0.5 |
| CP | CG | |||
| AP | –1.85 ± 0.16 | AG | –1.3 | –0.6 |
| TZ | TC | |||
| CZ | –2.07 ± 0.13 | CC | –1.8 | –0.3 |
| GP | GG | |||
| GP | –2.28 ± 0.16 | GG | –1.8 | –0.5 |
| CZ | CC | |||
| PP | –2.35 ± 0.13 | GG | –1.8 | –0.3 |
| ZZ | CC | |||
| CP | –2.77 ± 0.16 | CG | –2.2 | –0.6 |
| GZ | GC | |||
| ZP | –3.35 ± 0.32 | CG | –2.2 | –0.6 |
| PZ | GC |
On average, the P–Z stacks are more stable by −0.2 ± 0.4 kcal/mol per P–Z pair. The free energy increment for terminal P–Z pairs is set to 0 kcal/mol, as discussed in the text.
Figure 2 is a plot of predicted ΔG°37 versus experimental ΔG°37 for all of the melting experiments, and the full table of residuals is provided in Table S3. The sequence (GTPPZZAC)2 has the largest residual with a predicted overstabilization by 1.41 kcal/mol. This sequence has four P–Z pairs in a row, and the residual might indicate a non-nearest-neighbor effect. (GAZZPPTC)2, however, also has four P–Z pairs in a row, but it has more accurately predicted stability; i.e., the residual is 0.77 kcal/mol understabilized by the prediction. Additional experiments on related sequences could be used in the future to better understand why (GTPPZZAC)2 has the largest residual and whether this reflects possible non-nearest-neighbor effects of multiple adjacent P–Z pairs.
Figure 2.
Predicted ΔG°37 as a function of experimental ΔG°37 for the P–Z stacks. The P–Z stack component is the measured folding free energy change minus the contributions of Watson–Crick–Franklin stacks, intermolecular initiation, and the symmetry correction (applied to self-complementary duplexes only). The y = x line is shown for reference. The duplex (ZGCATGCP)2 was removed from the fit as an outlier. The duplex (GTPPZZAC)2 has the largest residual for a value used in the fit, at 1.41 kcal/mol. The data were derived from Hoshika et al.,15 Wang et al.,16 and additional duplexes reported here (Table S1).
Our prior work studying the stability of P–Z pairs and mismatches with P or Z demonstrated that G–Z pairs are more stabilizing that A–T pairs, suggesting that they should be included as a wobble pair and included in secondary structure prediction, like G–U wobbles in RNA.27 Here we call these interactions G–Z pairs. In many applications designed to use P–Z pairs, G–Z pairs would be mismatches (mispairs) that occur as off-target structures. Other applications might choose to use these pairs as part of a design strategy, and in these uses, the G–Z pairs would be desired pairs. Overall, given the relative stability of G–Z pairs relative to A–T pairs, we choose to call them pairs.
Figure 1 shows two possible G–Z pairs. The first is a two hydrogen-bond wobble. The G–Z pair could also be a deprotonated three hydrogen-bond pair, as observed at higher pH; the thermodynamics alone at pH 7 cannot distinguish structures.17,28 To fit the 15 nearest neighbor parameters for G–Z stacking on A–T, G–C, P–Z, or G–Z pairs, we performed an additional 11 melts of duplexes containing G–Z pairs and combined this with 4 prior experiments.16Table S4 provides the results of these optical melting experiments.
Table 2 provides the G–Z stack nearest neighbor parameters for the free energy change at 37 °C, which were fit by linear regression (see Methods section) using fixed values of stacks with P–Z pairs (Table 1). Table 2 also compares the G–Z pair stacks to the A–T pair stacks. The number of sequences studied by optical melting is equal to the number of G–Z stack parameters, and therefore the error estimates, which are the standard errors of the regression, are underestimates. Table S5 provides the residuals from the fit of the parameters.
Table 2. G–Z Stack Nearest Neighbor Parameters, as Compared to Analogous A–T Stacks (Replacing G–Z with A–T)a.
| parameter | ΔG°37 (kcal/mol) | parameter | ΔG°37 (kcal/mol) | ΔΔG°37 (kcal/mol) per G–Z substitution |
|---|---|---|---|---|
| GZ | 1.70 ± 0.48 | AT | –0.9 | 1.3 |
| ZG | TA | |||
| GA | 0.33 ± 0.27 | AA | –1.0 | 1.3 |
| ZT | TT | |||
| GG | 0.26 ± 0.14 | AA | –1.0 | 0.6 |
| ZZ | TT | |||
| CZ | –0.17 ± 0.26 | CT | –1.3 | 1.1 |
| GG | GA | |||
| GP | –0.50 ± 0.28 | AP | –1.9 | 1.4 |
| ZZ | TZ | |||
| AG | –0.58 ± 0.14 | AA | –1.0 | 0.4 |
| TZ | TT | |||
| GG | –0.75 ± 0.20 | GA | –1.3 | 0.6 |
| CZ | CT | |||
| AZ | –1.16 ± 0.10 | AT | –0.9 | –0.3 |
| TG | TA | |||
| TG | –1.40 ± 0.24 | TA | –0.6 | -0.8 |
| AZ | AT | |||
| ZG | –1.67 ± 0.41 | TA | –0.6 | -0.5 |
| GZ | AT | |||
| CG | –1.79 ± 0.26 | CA | –1.5 | -0.3 |
| GZ | GT | |||
| GZ | –1.81 ± 0.28 | AZ | –1.5 | –0.3 |
| ZP | TP | |||
| ZP | –1.85 ± 0.20 | TP | –1.5 | –0.4 |
| GZ | AZ | |||
| GZ | –2.07 ± 0.17 | GT | –1.4 | –0.7 |
| CG | CA | |||
| PG | –2.47 ± 0.24 | PA | –1.6 | –0.9 |
| ZZ | ZT |
The error estimates are the standard errors of the regression, but these are underestimates.
On average, a G–Z pair is less stable than an A–T pair by 0.27 kcal/mol, but the average masks a wide variation across stacks (standard deviation of ΔΔG° = 1.1 kcal/mol). Adjacent to A–T or other G–Z pairs, a G–Z pair substitution for an A–T pair is often (but not always) destabilizing. For example, a G–Z pair followed by an A–T costs 1.33 kcal/mol of stability relative to an A–T followed by an A–T. In some contexts, the G–Z substitution for an A–T pair is stabilizing, such as a G–Z following a P–Z, where the substitution is more stable than the A–T following a P–Z by −0.9 kcal/mol.
Nearest Neighbor Stacking Enthalpy Changes
In addition to fitting free energy change stacking parameters, we also fit enthalpy and entropy change parameters using the same stacking model terms. These parameters enable the estimation of melting temperatures (Tms) for biotechnology applications and extrapolation of folding free energies to temperatures other than 37 °C.29 Like the free energy change parameters, the enthalpy and entropy change parameters were fit with a linear regression for P–Z pairs (Methods section).
Table S6 shows the stacking enthalpy and entropy changes for stacks with P–Z pairs. The error estimates are larger for the enthalpy parameters than for the free energy change parameters, as quantified by the fraction of the parameter value. This is expected; in fits to experimental data, the enthalpy and entropy changes are highly correlated and therefore free energy changes are determined with more precision.26
Table S7A provides the duplexes, the experimentally determined enthalpy changes, the fit enthalpy changes attributed to the P–Z, the fit values for comparison to the experiment, and the residuals. Table S7B provides the equivalent information for the fit of the entropy changes. Given the larger average uncertainty in enthalpy and entropy changes from optical melting experiments and the lack of redundancy in experiments for G–Z pair stacks, we chose to not fit enthalpy or entropy change nearest neighbor parameters for G–Z pair stacks.
Loop Stabilities
In addition to the helical stack parameters, the full set of nearest neighbor parameters required for secondary structure prediction includes parameters for estimating loop stabilities. To extrapolate a set of loop stabilities, we performed optical melting experiments on 32 duplexes that include dangling ends (Table S8), terminal mismatches (Table S9), single mismatches (from Wang et al., 2017; Table S10),16 and tandem mismatches (Table S10). Twenty-three new optical experiments for loop systems were performed for this study; the other 9 had been reported previously.16 These four types of loops were chosen because sensitivity analyses for RNA secondary structure prediction identified these parameters as important for the precision of secondary structure prediction.30,31
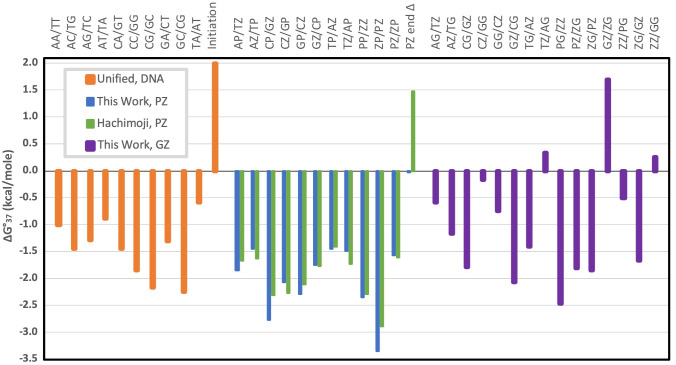
Dangling ends (single, unpaired nucleotides at the end of a helix) stabilize helix formation because the dangling nucleobase stacks on the terminal base pair.32,33 For B-form DNA helices, the 5′ dangling ends stabilize more than the 3′ dangling ends. First, we studied the stability of 5′ dangling nucleotides on a P–Z pair (Table S11A and Figure 3A). On average, for dangling A, C, G, and T, the stability of the dangling end is 0.58 ± 0.07 kcal/mol less stable on a P–Z terminal pair than on a C–G terminal pair.
Figure 3.
Dangling ends measured with P and Z nucleotides. (A) The stability of 5′ dangling nucleotides on Z–P pairs (blue), compared to C–G pairs (orange). The dangling end is less stabilizing on Z–P than on C–G pairs. (B) The stability of P and Z dangling ends, as compared to canonical purines and pyrimidines, respectively. The closing pair and orientation are indicated along the top. The dangling end nucleotide identity is directly above each blue or orange bar.
We also studied the stability provided by P and Z dangling ends (Table S11B), which we found to be highly sequence dependent. Figure 3B shows the stability of the P and Z dangling ends adjacent to Watson–Crick–Franklin pairs. A 5′ dangling Z adjacent to an A–T pair (−0.8 kcal/mol) is substantially more stable than either a C or T in the same context (−0.2 or −0.3 kcal/mol, respectively). In other cases, such as the 5′ dangling P on a C-G pair (−0.37 kcal/mol), the stability is less than analogous A or G dangling bases (−0.9 or −0.7 kcal/mol, respectively). 3′ dangling P destabilized helix formation in the two contexts studied.
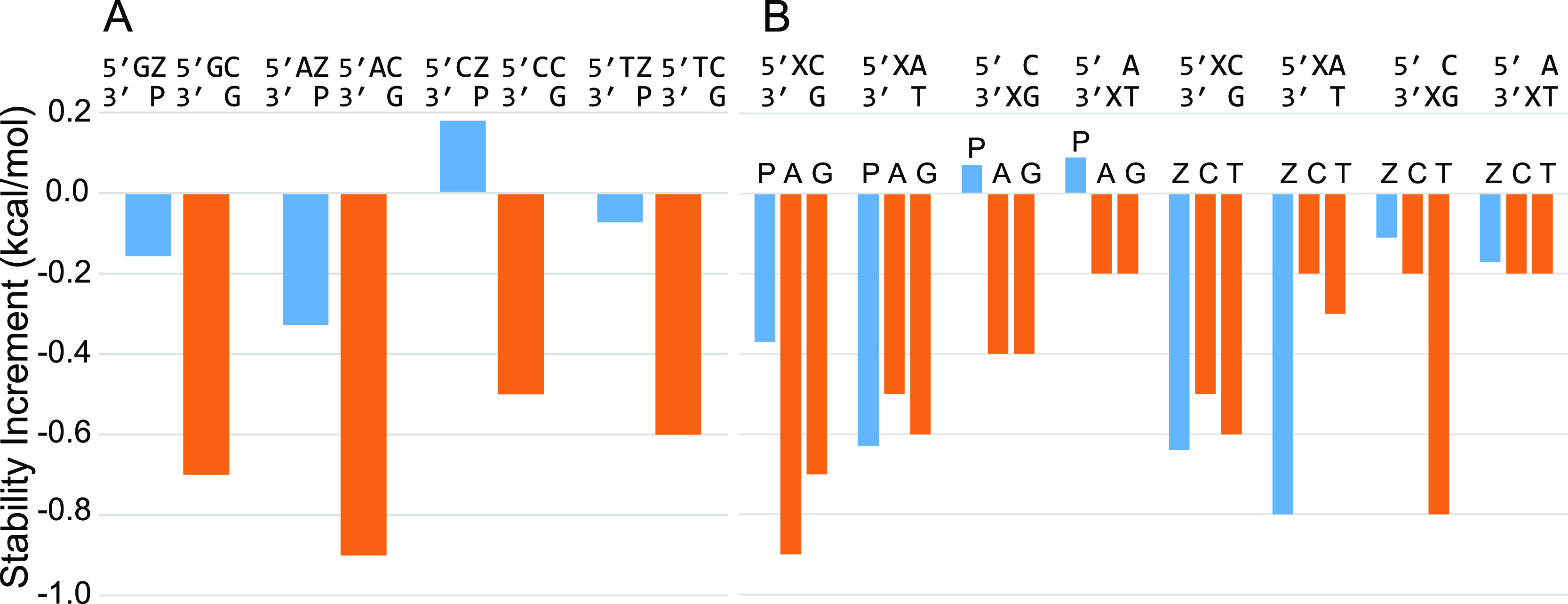
Terminal mismatches are single noncanonical pairs at the end of helices. These also stabilize helix formation because of stacking and hydrogen bonding. We studied the stability of P–P and Z–Z terminal mismatches adjacent to G–C and T–A terminal base pairs (Figure 4 and Table S12). The P–P mismatch next to a G–C pair is less stabilizing than the analogous purine mismatches G–G or A–A by 0.56 kcal/mol. The Z–Z mismatch next to a G–C pair, however, is similar in stability (at −0.76 kcal/mol) to the analogous pyrimidine mismatches C–C and T–T (with mean stability increment of −0.75 kcal/mol). The P–P mismatch next to a T–A pair is also similar in stability (−0.51 kcal/mol) to the analogous G–G and A–A mismatches (with mean stability increment of −0.5 kcal/mol). This contrasts to the Z–Z mismatch next to a T–A pair, which is more stabilizing than the analogous pyrimidine mismatches. The Z–Z mismatch next to T–A is −0.66 kcal/mol more stable (at −0.91 kcal/mol) than the mean of C–C and T–T next to T–A (at −0.25 kcal/mol). The general increased overall stability of dangling Z bases and terminal Z–Z mismatches is consistent with improved stacking of Z due to the nitro group.
Figure 4.
Stability of PP and ZZ terminal mismatches (blue), compared to G–G, A–A, C–C, and T–T terminal mismatches (orange). The left series have a G–C terminal pair and the right series have a T–A terminal pair.
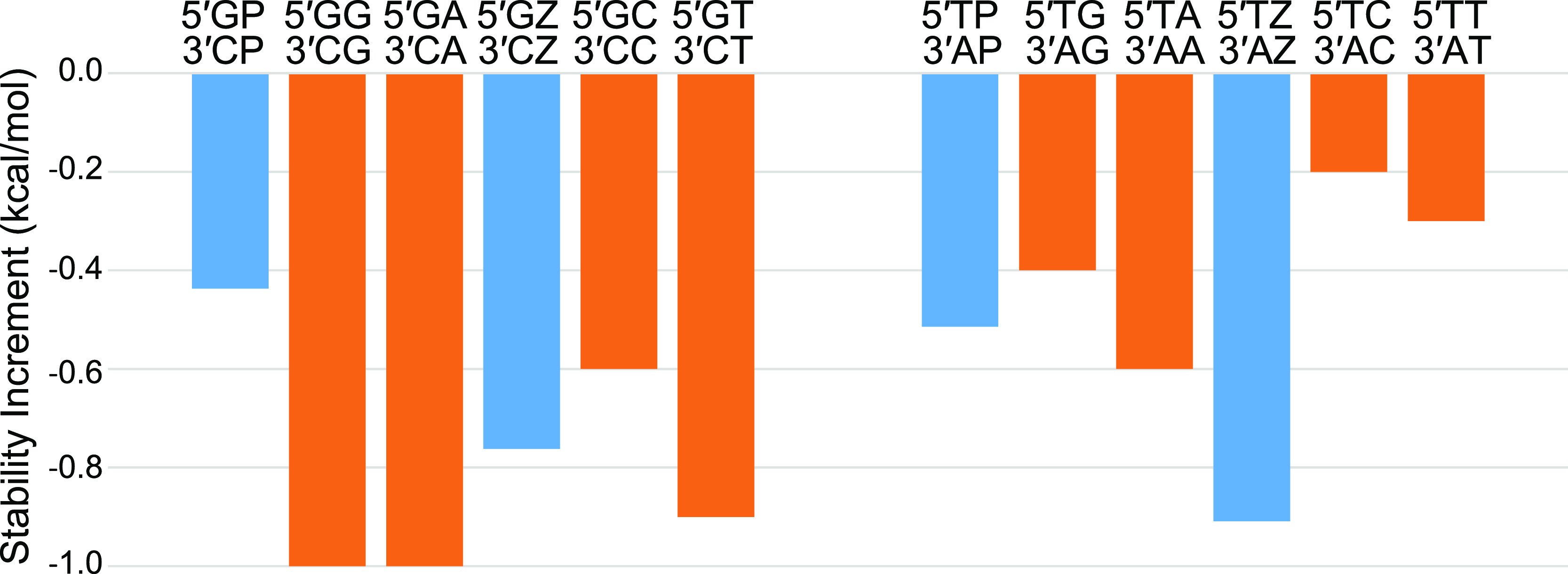
We also measured terminal mismatches on terminal P–Z base pairs (Figure 5 and Table S13). Each of these terminal mismatches was less stable than the analogous mismatch on a terminal G–C pair. On average, this stability difference is 0.7 kcal/mol. We do not discern obvious patterns that explain the different results for these terminal mismatch stabilities. Given the conformational flexibility of ssDNA, there might be a variety of accessible structures for noncanonical pairing and stacking.
Figure 5.
Stability increments of terminal mismatches on terminal P–Z pairs (blue). The stabilities of analogous terminal mismatches on G–C pairs are shown (orange).
We measured the stability of single mismatches (also called 1 × 1 internal loops): P–C, P–T, and Z–A, respectively, by optical melting. In RNA structures, many noncanonical pairs that are capable of forming pairs with two hydrogen bonds have been well characterized.34 For the alphabet of nucleotides with P and Z, possible two-hydrogen bond noncanonical pairs of P–C, P–T, and Z–A were previously proposed.16 There is a pronounced correlation of the stability with position in the helix, where single mismatches close to helix ends are stabilizing, and single mismatches that are four base pairs separated from the helix end are destabilizing to duplex formation (Figure 6 and Table S14).
Figure 6.
Stability of single mismatches (1 × 1 internal loops). Stability increments for the mismatch motif are shown, where the stabilities of the closing helices are subtracted from the duplex stability. The internal loops show a marked dependence on the distance from the helix end, where mismatches farther from helix ends are destabilizing and mismatches closer to helix ends are less destabilizing or stabilizing for helix formation.
We also observed this for control experiments with G–T and C–A mismatches. The existing database of optical melting experiments of model systems with natural DNA base mismatches focuses on mismatches with three canonical base pairs exterior to the duplex.35−39 Therefore, we developed single mismatch parameters for mismatches with P or Z by using the two total experiments with P–C and P–T mismatches in the center of the duplex.
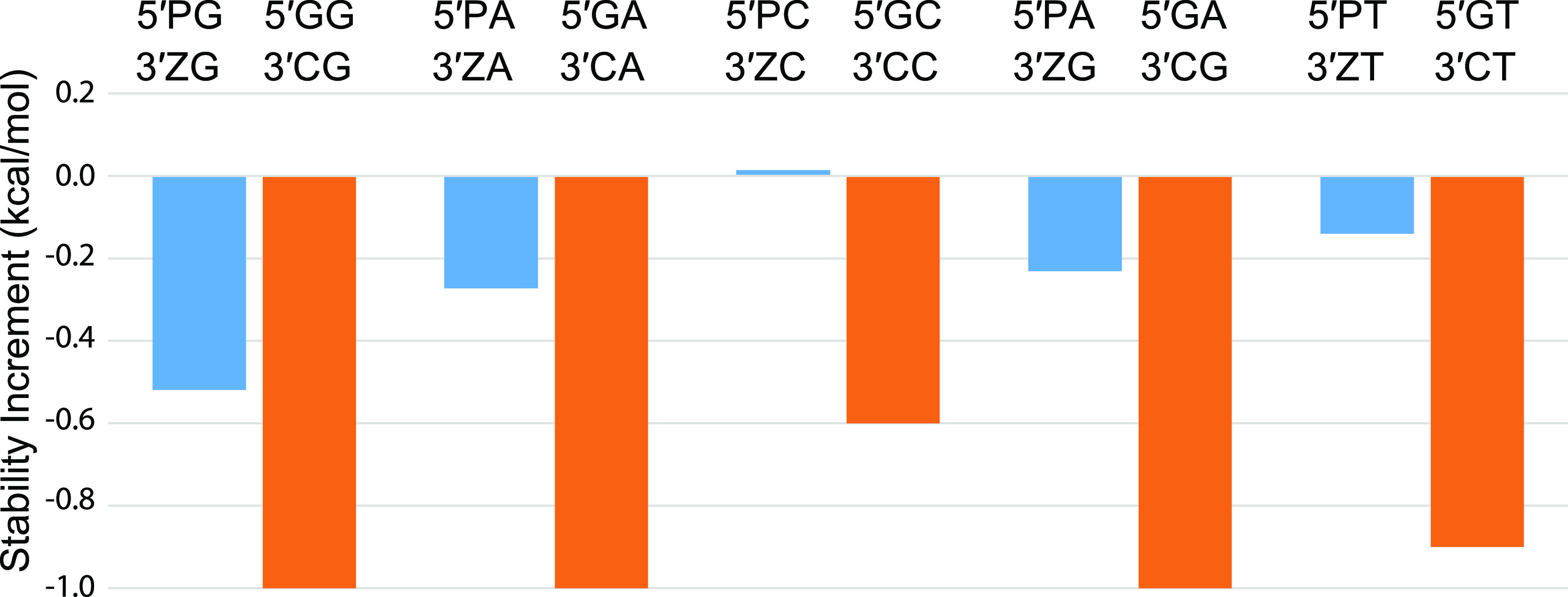
We additionally measured the stability of two tandem mismatches (also called 2 × 2 internal loops), including a tandem Z–Z/Z–Z mismatch and a tandem P–P/P–P mismatch. Tandem mismatches have been studied extensively using canonical DNA nucleotides,35,40,41 and in one study were found to have a range of stabilities spanning 5.0 kcal/mol from stabilizing to destabilizing for duplex formation.41 We found that the Z–Z/Z–Z and P–P/P–P tandem mismatches are substantially more stable than analogous tandem pyrimidine or purine mismatches (Figure 7 and Table S15). On average, the tandem Z–Z/Z–Z and tandem P–P/P–P mismatch are −0.64 kcal/mol more stable than analogous tandem mismatches of canonical nucleotides. The stability of tandem Z–Z pairs might be explained by the Z–Z base pairing motif we characterized.42
Figure 7.
Stability increments for tandem mismatches (2 × 2 internal loops). The tandem Z–Z and tandem P–P mismatches (blue) are compared to tandem pyrimidine and tandem purine mismatches (orange), respectively.
Complete Nearest Neighbor Parameters
Using the optical melting stabilities, we extrapolated the nearest neighbor terms for loops that contain P and Z or are closed by P–Z pairs. The details of the extrapolations are in the Methods section. RNAstructure was previously extended to accommodate extended alphabets beyond the canonical nucleotides.18 In summary, we developed a comprehensive set of thermodynamic parameters for A, C, G, and T, P, and Z, allowing C–G, A–T, P–Z, and G–Z base pairs, and we have integrated the expanded alphabet into the RNAstructure such that any analysis that can be done for natural DNA can be done for the expanded alphabet.
Tests of Sequence Designs that Include P and Z Nucleotides
We tested the hypothesis that extension of the canonical base pairs with P–Z pairs would improve in silico designs of sequences that fold into user-specified secondary structures. We used two types of structures for these tests: the Eterna 100 benchmark of structures,19 which is considered a challenge for automated sequence design, and a DNAzyme that had been discovered by in vitro evolution.43 The Eterna 100 data set provides a variety of design challenges ranging in length from 12 to 400 nucleotides and with mean length of 127 nucleotides. The DNAzyme consists of 104 nucleotides.
To find sequences that fold to a specified secondary structure, we used the Design program in RNAstructure.20 The default parameters were used with one exception: we allowed isolated base pairs, i.e., base pairs that do not stack on adjacent base pairs in a helix, because the Eterna 100 structures contain isolated base pairs. Each structure was attempted five times (using the computer clock to seed the random number generator), and a maximum of 5 days for each of wall time was allowed using a single processor core. RNAstructure is capable of multithreading across CPU cores using OpenMP, but we used single threading to simplify the interpretation of the benchmarks. We considered success for a given target to be finding at least one sequence design that completed in the allowed 5 days.
Design was able to design sequences for 94 of the Eterna 100 structures using canonical DNA bases only. When P–Z pairs were added to the design, 99 structures were successfully designed. When P and Z are used, we select sequences that include P–Z pairs in the target structure, but we do not choose P or Z to occur in loops. We used P and Z only for pairs to take advantage of their intrinsic pairing stability, although designs could have been chosen to use P and Z elsewhere. P–Z pairs were selected at a rate of 20%, G–C pairs are selected at a rate of 50%, and A–T pairs are selected with the remaining 30% (see Methods section).
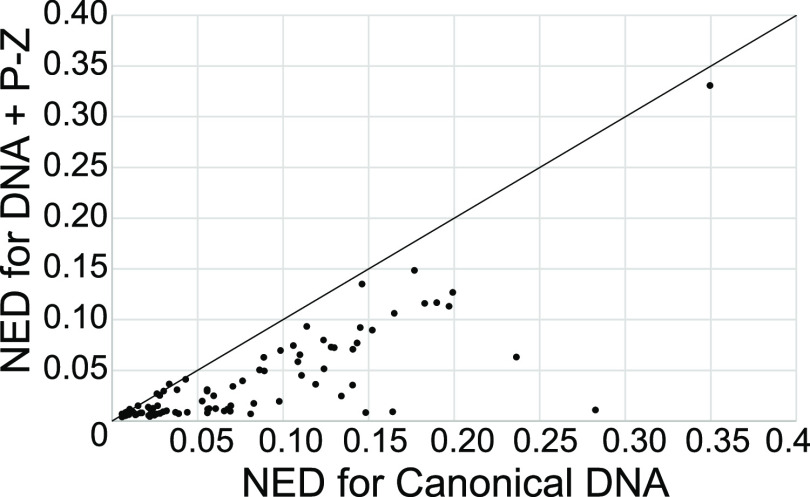
We then analyzed the results for the lowest NED for each alphabet of nucleotides. We focused on the lowest NED across calculations because Design uses stochastic refinement (by which random changes and those that improve the NED are kept and others are rejected), and the NED of the final sequence therefore varies across calculations for the same structure.20Figure 8 shows the lowest NED designed including P–Z pairs versus the lowest NED designed using only canonical nucleotides for the 94 test structures for which Design was able to solve both problems. The NED of sequences using P–Z pairs are lower in 89 cases, leaving only 5 cases where using P–Z is worse than canonical DNA nucleotides alone, and all of these 5 cases are in the lower left corner, where the NED using both alphabets is excellent (NED < 0.05).
Figure 8.
NED is significantly improved with the incorporation of P–Z pairs in designs (P = 2.7 × 10–13). This is a plot of NED for Eterna 100 designs using DNA and P–Z pairs as a function of designs using canonical DNA only. The NED of the best of five calculations is shown. Each point is a single example problem from the Eterna 100 set. Points below the diagonal line (plotted as a visual guide) are cases where incorporation of P–Z pairs improved the designs.
A one-sided paired t test indicated that the improvement in NED when incorporating P–Z pairs is significant (P = 2.7 × 10–13). The average NED for the designs including P–Z is 0.036, and the average for NED for designs with canonical DNA is 0.073 for these 94 test cases solved with both alphabets. Figure S2 shows the analogous plot of the mean NED (rather than the minimum) using P–Z versus the mean NED using only canonical pairs. The same trend for mean is observed as with lowest NED, and the improvement in mean performance is again significant (P = 1.0 × 10–13). We provide all the designed sequences in Supporting Information Data set 1, including the NED, the time used, and the random number seeds (to enable reproducibility).
We observed the most substantial improvement by incorporating P–Z pairs for the “Iron Cross” test (number 35), which improved the best NED from 0.283 to 0.011. Figure 9 shows the target structure with the best DNA sequence and the best sequence for DNA with P–Z. The structures are color-annotated by the probabilities that the nucleotides are in the desired structure. Like many of the other Eterna 100 test structures, this structure has prominent symmetry with four branches from a central multibranch loop, where each branch has a three-way multibranch loop with each helix of three base pairs. The canonical DNA design uses mostly G–C base pairs, but these can be promiscuous across the intended helices. The design that incorporates P–Z pairs has 23 P–Z pairs with the remaining 13 pairs as G–C. The target structure is stabilized by the incorporation of P–Z pairs, and alternative structures are preventable because there are additional possible combinations of P–Z and G–C pairs that form helices of three base pairs. Interestingly, P–Z pairs, although randomly chosen at a rate of 20% of pairs, became enriched above 20% during stochastic refinement against G–C and A–U pairs. The predominance of P–Z pairs after refinement in this case shows that P–Z are preferable in designed sequences that minimize NED.
Figure 9.
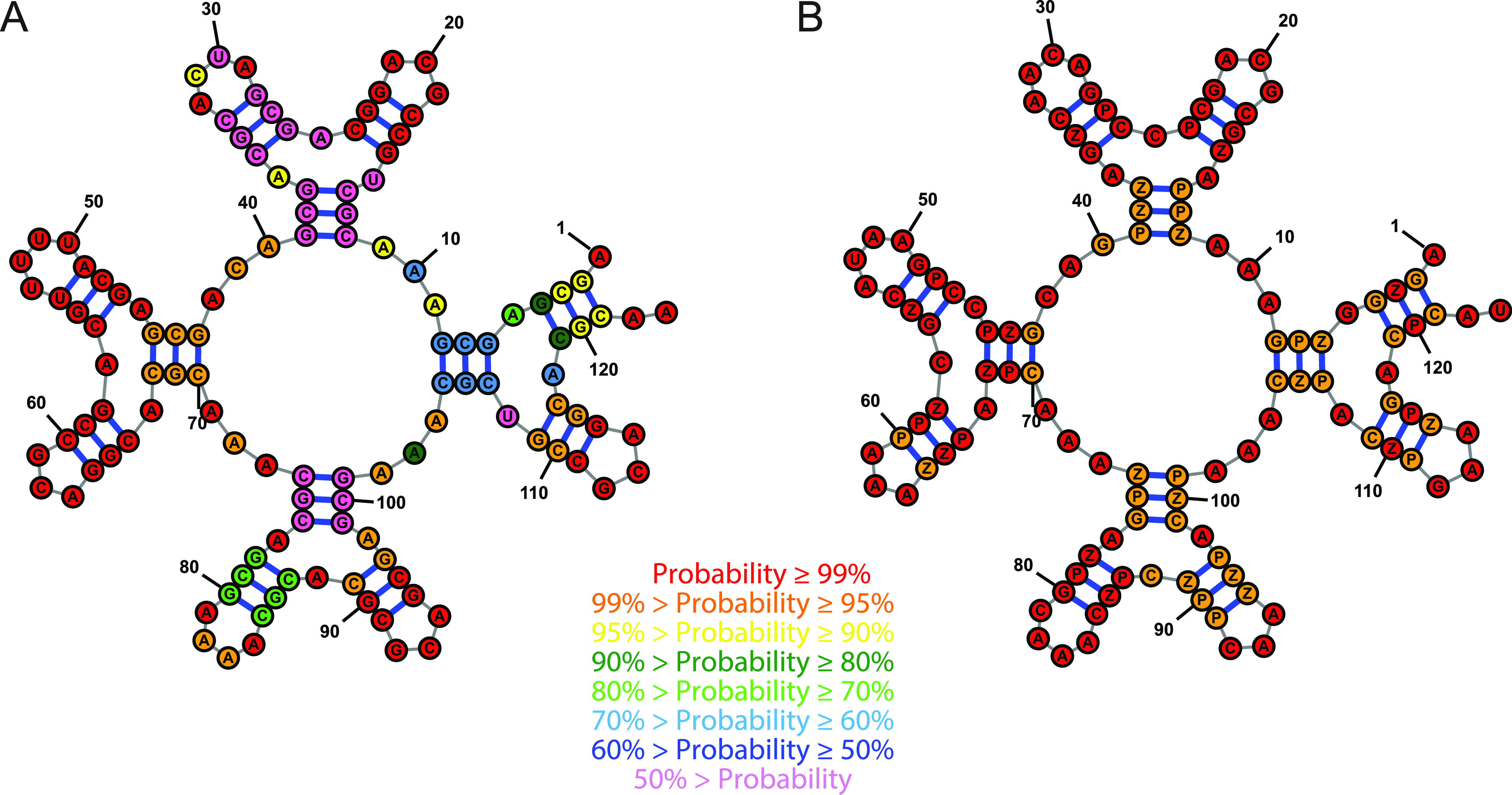
Incorporation of P–Z pairs improves the design of “Iron Cross”, problem 35 from the Eterna 100 set. Panel A shows the best design using canonical DNA only. Panel B shows the design incorporating P–Z into DNA. Bases are color-annotated with their probability of forming the correct structure, either the probability of folding into the specified target base pair or the probability of being unpaired in the target structure. The nucleotides in the P–Z containing sequence all form the desired structure with ≥95% probability. The structure composed of canonical DNA has a substantial number of nucleotides that are estimated to fold with <50% probability to the target structure.
We previously noted that there is a trade-off between time cost and NED quality when using stochastic refinement.20 In these calculations with Design, we used the default parameters, which limit the number of sequence refinements. In addition to observing an improvement in the quality of the designs (lower NED) when using P–Z pairs in the refinement, we also observe a decreased cost in the average computational time. Figure S3 shows the average time across the five calculations that incorporate P–Z pairs as a function of the average time across the five calculations that use only canonical DNA nucleotides for each Eterna 100 structure. In the calculation of average time, we use 5 days (the maximum allowed time) for calculations that failed to return a sequence. The use of P–Z nucleotides significantly improves the time cost (P = 1.7 × 10–3).
In an attempt to solve additional Eterna problems, we extended the computational time allowed to 10 days with Design. The one problem that was unsolvable (Gladius, structure 90) using P–Z remained unsolved. Three additional problems, however, were solved by extending the canonical DNA designs to 10 days (Supplementary Data set).
99 of the Eterna 100 benchmark structures have Eterna player solutions provided, with two RNA sequences being provided for each. We compared the NED of our designs, including P–Z pairs, to the Eterna solutions. Eterna designs were originally evaluated computationally as to whether the sequence is predicted to fold with the lowest free energy to the target structure. This can be achieved whether or not the NED is relatively low.13,44,45 Therefore, we expected that many of the designs would not provide low NED, despite performing well by minimum free energy folding. We compared the lowest NED for our solutions using P–Z to the lowest NED using Eterna player RNA sequences (Figure S4). Our designs, including P–Z, have significantly lower NED than the Eterna RNA designs (P = 4.28 × 10–19).
We additionally tested including P–Z base pairs in designing DNA with the same secondary structure as a DNAzyme:substrate complex, a unimolecular version of the CT10.3.29.M5 sequence bound to its target.43 The catalytic core of the DNAzyme, which includes several conserved or essential nucleotides, was specified as unpaired; the current implementation of the Design algorithm does not allow constraints on sequence. We designed 10 sequences, including P–Z pairs and 10 sequences using only canonical DNA nucleotides. Table S16 reports the results in terms of the NED and time. The NEDs for the DNAzyme designs were excellent (i.e., low) for either alphabet. Using P–Z pairs the lowest NED solution was 0.031, and using canonical DNA the best NED solution was 0.041. The average time performance using P–Z pairs was better and more consistent than when only using canonical nucleotides. The mean time with P–Z pairs was 1594 ± 134 s and the mean time for canonical nucleotides was 3232 ± 1770 s. The improvements when using P–Z pairs are significant for NED (P = 1.90 × 10–3) and time (P = 8.46 × 10–3). Within each set of designs, there was no correlation between the time required and the quality of the design.
Discussion
In this work, we tested the hypothesis that synthetic base pairs can improve the quality of DNA nanostructure designs. We used optical melting to study the folding stability of DNA containing P and Z Hachimoji nucleotides. These data informed a new nearest neighbor model for folding, including P–Z pairs, G–Z wobble pairs, and loops containing P or Z. Using the Design program in RNAstructure,20,46 which was recently enhanced to allow prediction with nucleotide alphabets beyond the canonical nucleotides,18 we demonstrated significant improvements for in silico sequence design quality.
Prior work studying RNA nearest neighbor parameters demonstrated that a subset of parameters needs to be determined with high precision to precisely estimate base pairing probabilities. That work focused our efforts here on measuring folding stability for model systems that could inform those important parameters. This approach was recently validated for the derivation of folding free energy parameters for RNA including m6A.18 A set of 98 duplexes with or without N6-methylation, including helices, bulge loops, internal loops, dangling ends, and terminal mismatches, was studied and this demonstrated that the parameters extrapolated for m6A were approximately as accurate for estimating folding stability as those for canonical bases only.47
There are limitations to the accuracy of our parameters. For example, single mismatch parameters for canonical DNA do not account for the positional dependence of the mismatch stability.33 This is because the current experimental database of DNA mismatches is limited to mostly mismatches distant from helix ends and because current structure prediction packages are not capable of accounting for this dependence. For single mismatches containing either P or Z, our parameters are based on the most destabilizing values that are consistent with being away from helix ends. This is currently a limitation in parametrization for both DNA and DNA+PZ folding alphabets. Both DNA parameters and DNA+PZ parameters would benefit from additional experiments to guide the determination of helix-position-dependent rules.
Comparison of the refined nearest neighbor thermodynamic parameters obtained here with previous DNA and PZ parameters (Figure 10) confirms that P–Z pairs are generally more stable than natural sequence DNA.15,25 Our revised parameters for PZ-containing dinucleotides generally show more negative ΔG°37 for base pair formation than the Hachimoji set, but the differences are small. As described above, one significant difference is that we do not include a free energy penalty for a terminal P–Z pair. The free energies for the G–Z pairs are more variable. The most stable G–Z containing stack, PG/ZZ, is slightly more stable than PP/ZZ, where stacks are annotated as top strand (5′ to 3′)/bottom strand (3′ to 5′) so that PG/ZZ indicates a P–Z pair followed by a G–Z pair. The least stable GZ/ZG stack, however, is substantially destabilizing (ΔG°37 > 0). This is not due solely to having two mismatches in the stack as ZG/GZ is quite stable.
Figure 10.
Nearest neighbor free energy parameters for stacks containing natural DNA, P–Z pairs, and the stable G–Z pair.15,25
There is a notable effect of the position of the Z base. For the P–Z data sets, stacks with Z at the 5′ side (e.g., XP/YZ, including ZP/PZ and PP/ZZ) have an average ΔG°37 = −2.34 kcal/mol whereas stacks with Z at the 3′ side (XZ/YP, including PP/ZZ and PZ/ZP) have ΔG° = −1.78 kcal/mol. For G–Z pairs, the average ΔG°37 for 5′ Z is −1.19 kcal/mol, and for 3′ Z it is −0.65 kcal/mol: the absolute difference is very similar to P–Z but the relative difference is much larger. Examination of X-ray crystal structures (PDB accessions: B-form 4XO0, 6MIG, and 6MIH and A-form 4XNO) does not suggest an obvious reason for this dramatic preference.48 One might guess is that the potential stacking of the Z-NO2 group could be particularly stabilizing, but the Z base appears to stack much better on its 5′ neighbor than on its 3′ neighbor. The 5′ Z is solvent exposed, and it is surrounded by ordered water in 4XNO.
Our results highlight a case where the use of P–Z pairs is crucial for high quality (i.e., low NED) designs. Structures with extensive symmetry (such as the “Iron Cross” example in Figure 9) benefit from the additional space of sequence options that are available when P–Z pairs are included. In the absence of P–Z pairs, the three base pair helices, which need the stabilization of G–C pairs, exhaust the possible sequences, and then alternative structures form when the helix sequences pair incorrectly. We hypothesize that this important diversification of sequence effect plays a smaller, but beneficial, role in other sequence designs that are not as symmetrical as “Iron Cross”.
Figure 8 demonstrates cases when the NED of the best sequence designed including P–Z pairs is worse than that of the best sequence designed with DNA only. Given enough search time or enough search attempts, this would not happen because the DNA designs including P–Z pairs could find the better DNA-only solution. We observe these cases because Design stochastically refines the sequences and returns solutions when it has achieved a specified threshold NED or when it has exhausted a specified number of trials. For this work, we chose to not refine the default parameters for the search, although it might be possible to improve the best NED by changing these settings.
As alphabets expand, the number of parameters needed to describe even the simplest nearest-neighbor models expands combinatorially. Comprehensive description of mismatch and internal loop thermodynamics is out of reach for low-throughput measurements such as those described here. Systematic evaluation of which parameters are most critical,30,31 high throughput measurements of enthalpy and entropy as well as free energy changes,49 position-dependent rules, and eventually integration of 3-D motif stability50,51 will be necessary to tackle more demanding design problems like structures that change their folds upon ligand binding or as conditions change.
Prior work demonstrated an advantage to combining DNA with synthetic polymers in nanostructure design.52 In that work, peptide nucleic acid (PNA) with three positively charged terminal lysines (called PNA3K) was used in a fluorescence detection assay and a component of DNA origami. The PNA3K optimized the nanostructure yield by stabilizing the nanostructures in the buffers that were studied. Future work could use PNA,53 additional synthetic bases,54,55 naturally occurring modified bases,56 or a combination of these components to construct specific structures. Here we provide a roadmap for determination of folding thermodynamics for expanded nucleotide alphabets, their incorporation in secondary structure prediction, and their use in automated design algorithms.
Methods
Oligonucleotide Synthesis, DNA Melting, and Analysis of Melting Curves
P/Z containing DNA oligonucleotides were synthesized as described,16 and unmodified oligonucleotides were purchased from IDT (Coralville, Iowa). The purity of all oligonucleotides was evaluated by denaturing polyacrylamide gel electrophoresis (PAGE), staining with SYBR Gold, and imaging at 312 nm. We note that Z-containing oligonucleotides quench SYBR fluorescence in the gel; Z has a broad absorption peak centered at about 380 nm.
Absorbance melting curves were performed as described for the P–Z pairs and loop energy oligos.16,57 For G–Z melts, we improved the standard procedure in several ways. We acquired absorbance vs temperature curves for each of the single strands individually, both to determine their diluted stock concentrations directly and to use actual data instead of a linearly extrapolated ssDNA trendline. We carried out simultaneous dsDNA melts at two concentrations, nominally 1 μM and 5 μM total strand concentration, CT, which enabled rapid identification of pipetting errors and set constraints on the concentrations of the two individual strands in the dsDNA melt cuvettes. We used equations for predicting total absorbance that explicitly consider unequal concentrations of the single strands and the absorbance of the remaining excess single strand.
We carried out global fits to both of the absorbance vs temperature curves with a set of six fit parameters: two ssDNA concentrations for each melt (the other two being determined by the total absorbance), a common % hypochromicity for the duplex, and a common slope for the dsDNA absorbance vs T, ΔH°, and ΔS°. The fit was repeated four times to consider each pair of possibilities in each melt for which the strand is in excess. The fit with the lowest residual that also had minimal errors in concentrations relative to nominal values was selected. This procedure allows explicit correction for pipetting errors, which are typically in the range of 5%, and the global fitting to dsDNA absorbance decreases the total number of parameters by two for each melt. We observe that typical estimated errors in ΔH° and ΔS° are reduced from 8 to 10% to about 5% by following this procedure. It could readily be extended to melts at additional CT, as is done with the alternative ln(CT) vs 1/Tm procedure for determining ΔH° and ΔS°.24 An example of the output from our Matlab routines is provided as Figure S1 and the code is available at GitHub at https://github.com/jasondkahn. We note that other recent work is available for improved fitting of optical melting data.58
P–Z and G–Z Stacking Free Energy Change Nearest Neighbor Fits
A custom Python program was written to fit the P–Z nearest neighbor parameters. We used all available data across prior papers (with the exception of (ZGCATGCP)2 as explained above) to fit these terms.15,16 We expect that optical melting experiments are well reproduced (within approximately 3% for ΔG°37) across laboratories and the residuals in Table S3 support this.59 First, the sum of the Watson–Crick–Franklin stacks, the intermolecular initiation, and the symmetry correction (if needed for self-complementary duplexes) was subtracted for each duplex. For compatibility with RNAstructure, we used the parameters that are provided with the RNAstructure package.46 Then, the nearest neighbor stacks were fit by linear regression to the remaining stabilities with the statsmodels ordinary least-squares class (OLS).60 This fixes the stability of the Watson–Crick–Franklin stacks with their existing values. Similarly, the stacks with G–Z pairs were fit with a separate custom Python program, fixing the values of the Watson–Crick–Franklin and P–Z stacks. Error estimates for parameters are the standard error of the regression.
P–Z and G–Z Stacking Enthalpy and Entropy Change Nearest Neighbor Fits
To fit the enthalpy change nearest neighbor parameters, a custom Python program was written. The Watson–Crick–Franklin stack enthalpy changes and intermolecular initiation enthalpy changes were subtracted from the experimentally determined enthalpies for each duplex. The Watson–Crick–Franklin enthalpy change parameters were those reported by SantaLucia and Hicks.33 Then the enthalpy change nearest neighbor parameters were fit by linear regression using the statsmodels ordinary least-squares class (OLS).60 A second custom Python program was used to fit the G–Z enthalpy change stacking parameters.
The enthalpy changes of the Watson–Crick–Franklin and P–Z stacking enthalpy changes and initiation were subtracted from the total experimentally determined enthalpy changes. The G–Z enthalpy change terms were then fit to this remaining stability by linear regression. Error estimates for the enthalpy change parameters are the standard errors of the regression. Following the same methods used to fit the enthalpy change nearest neighbor parameters, two additional Python programs were written to fit the entropy change nearest neighbor parameters for stacks with P–Z pairs and for stacks with G–Z pairs.
Determination of Loop Motif Stability Increments
Loop stability increments are determined by subtracting the helix stabilities from the optical melting stability determined for model systems. In this work, nonself-complementary duplexes were used to determine dangling end, terminal mismatch, and internal loops. For the dangling ends and terminal mismatches, the stability increment of the motif is determined by subtracting a reference helix stability from the stability of the duplex with the motif:
The stability of the reference duplex is estimated with stacking nearest neighbor parameters.
For internal loops, the stability is the total stability of the duplex minus the helical stacks (estimated with nearest neighbor parameters):
Extrapolation of the Loop Nearest Neighbor Parameters
For the basis of our free energy change nearest neighbor parameters, we used the DNA parameters incorporated in RNAstructure. A description of the parameter tables and the exact data that informs sequence-dependent extrapolations for P and Z nucleotides is provided in the Supporting Information. Below we summarize the extrapolations used.
For the DNA+PZ alphabet parameters, we removed GT pairs, which were traditionally included as part of the DNA secondary structures. GT pairs are less stable than AT pairs and GZ pairs,35 and they are treated as mismatches in the DNA+PZ alphabet.
For 5′ dangling ends on terminal Z–P pairs, we used the experimental values (Table S11A) for A, C, G, and T. The dangling end parameters for DNA are not very sequence dependent. The largest differences are for 3′ dangling ends on terminal CG pairs, which range from −0.4 to −1.1 kcal/mol in stability. For other terminal pairs, the dangling end stabilities (3′ or 5′) are in the range of 0.4 kcal/mol.
Therefore, we approximate a 5′ dangling A, C, G, or T on a terminal P–Z pair as the mean of the measured values on the Z–P pair (−0.1 ± 0.2 kcal/mol). For 3′ dangling ends, we are conservative and use the same value of −0.1 kcal/mol. For dangling ends on P–Z or Z–P terminal pairs, we use the experimental values when available (Table S11B). For 3′ P dangling ends that were not measured, we use the mean value of those measured (Table S11B; 0.1 kcal/mol). For 5′ P dangling ends that were not measured, we use the mean value of those measured (Table S11B; −0.5 kcal/mol). Similarly, for 3′ or 5′ Z dangling ends that were not measured, we use the mean of the measured values, which are −0.1 or −0.7 kcal/mol, respectively (Table S11B).
For terminal mismatches, we used our measured values when they were available. For P–P terminal mismatches that were not measured, we used the mean from other P–P mismatches (Table S12; −0.5 kcal/mol). Likewise, for Z–Z terminal mismatches not measured, we used the mean from other Z–Z mismatches (Table S12; −0.8 kcal/mol). For terminal mismatches on P–Z, Z–G, or G–Z pairs, including mismatches with P or Z (but not PP or ZZ), we use the mean value of the terminal mismatches on Z–P pairs (Table S13; −0.2 ± 0.2 kcal/mol). Terminal mismatch parameters are used in secondary structure prediction for mismatches in exterior loops, hairpin loops, internal loops (other than 1 × 1, 1 × 2, 2 × 2, or 1 × n, where n > 2), and multibranch loops. The terminal stack parameters also provide one of the terms needed to estimate the stability of mismatch-mediated coaxial stacking.61−63
For single mismatches, also known as 1 × 1 internal loops, parameters were informed by experiments reported in Table S14. We use mismatches in the center of adjacent canonical helices to be consistent with prior studies of DNA single mismatches. The single mismatch parameter table in RNAstructure contains all sequences (including the mismatch and two closing base pairs). When an experimental value has been measured, we use that value. The following extrapolations were used to determine parameters involving P or Z: We map P–Z closure to entries for G–C closure and we map G–Z closure to entries for A–T. In the loops, we map Z to C and P to G. If the mapping results in a Watson–Crick–Franklin pair, we remap to an A–C mismatch, preserving the purine-pyrimidine orientation. We then lookup the entry as mapped to canonical nucleotides and stabilize mismatches with P or Z by −0.6 kcal/mol, which is the mean additional stabilization observed for the measured mismatches (Table S14) compared to analogous AC mismatches.
For tandem mismatches, we use a similar approach to single mismatches. RNAstructure uses a table with all possible sequence entries. We use the same remapping used for single mismatches to look up an entry composed of canonical nucleotides. Then, for each mismatch with a P or Z, we stabilize by an additional −0.6 kcal/mol, which is the mean stabilization per mismatch observed for tandem mismatches as compared to analogous loops with canonical nucleotides (Table S15).
For 2 × 1 internal loops, RNAstructure also uses a table with all sequence entries. Similar to single mismatches, we map entries with P or Z back to entries with canonical nucleotides only. For loops that contain either P or Z, we stabilize the loop by −0.6 kcal/mol, the average stabilization observed per mismatch with P or Z in single mismatches (Table S14).
Following the nearest neighbor parameters for DNA, the PZ alphabet tables for first mismatches in hairpin loops, mismatches at helix ends in internal loops (larger than 2 × 2 internal loops), and for mismatches at helix ends in coaxial stacks are set equal to the terminal mismatch values.
Design Calculations
The Design program from RNAstructure was used for the calculations.20,46 We modified the program to allow isolated base pairs, i.e., base pairs that are not stacked on adjacent base pairs, and this revision will be available in RNAstructure version 6.5. The other parameters were set to defaults. The software was compiled by using GCC version 4.8.5. All calculations were run on one core on machines equipped with two Intel Xeon CPU E52695v4 (2.10 GHz) processors and 124 GB of RAM. The CPU time was extracted from a Slurm queuing report.
Design calculations were all run by using the thermodynamic parameters that include P and Z nucleotides. Design randomly selected pairs and unpaired nucleotides were designed for initial sequences and then subsequently for the refinement of the sequence. We extended Design to allow user-specified biases for the selection of pairs and unpaired nucleotides. These biases are used to initialize the sequence and also to select revised sequences during the iterative refinement stage. For calculations with only canonical nucleotides, G–C and A–T pairs were selected with equal probability. For designs that included P–Z pairs, P–Z was selected with a probability of 20%, G–C with a probability of 50%, and A–T with a probability of 30%. For loop sequences, A, C, G, and T were selected with probabilities of 60%, 10%, 10%, and 20%, respectively. This reflects a bias for A occurring in loops.64 P and Z were not selected for incorporation in loops.
Statistical Tests
For analysis of the Eterna 100 set designs, we used SciPy 1.7.3 to perform paired t tests for NED or time.65 Our null hypothesis is that the quality of designs with PZ pairs is not better (NED or time) than the designs with canonical nucleotides only. Similarly, for the DNAzyme calculations, our null hypothesis is that the quality of designs with PZ pairs is not better (NED or time) than the designs with canonical nucleotides only. We tested this null hypothesis using Welch’s t tests for the NED or time. For all tests, we used a one-sided test with the type I error rate, α, set to 0.05.
Acknowledgments
Sequence design calculations were performed using resources provided by the University of Rochester Center for Integrated Research Computing. We thank our two anonymous reviewers for providing helpful and detailed feedback.
Glossary
Abbreviations
- e.u.
entropy units (cal mol–1 K–1)
- NED
normalized ensemble defect.
Supporting Information Available
The Supporting Information is available free of charge at https://pubs.acs.org/doi/10.1021/acssynbio.3c00358.
Author Contributions
T.M.P. and M.Z. carried out folding and design calculations. H.S. and D.H.M. performed regression analysis. T.M., K.K.S., and X.W. performed absorbance melting experiments. S.H. synthesized, purified, and characterized all of the oligonucleotides containing nonstandard nucleotides. R.J.P. and S.A.B. consulted on all aspects of the work, and reviewed the manuscript. J.D.K. wrote analysis programs and supervised experimental work. D.H.M. supervised the computational work. T.M.P., J.D.K., and D.H.M. wrote the manuscript with input from all of the authors.
Author Contributions
# T.M.P., T.M., and H.S. contributed equally to this paper.
This work was supported by NIH grant R35GM145283 to D.H.M. and an SBIR award from the U.S. Army (W911NF-12-C-0060) to Celadon Laboratories (later DNA Analytics). This work was also supported by R01GM141391-01A1 to S.A.B.
The authors declare the following competing financial interest(s): DNA Analytics applied for a U.S. patent, PCT/US23/64288 (Generating Parameters to Predict Hybridization Strength of Nucleic Acid Sequences), to protect the applicable optical melt curve fit methods.
Supplementary Material
References
- Doudna J. A.; Cech T. R. The chemical repertoire of natural ribozymes. Nature 2002, 418 (6894), 222–228. 10.1038/418222a. [DOI] [PubMed] [Google Scholar]
- Serganov A.; Nudler E. A decade of riboswitches. Cell 2013, 152 (1–2), 17–24. 10.1016/j.cell.2012.12.024. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Morais P.; Adachi H.; Yu Y. T. Spliceosomal snRNA Epitranscriptomics. Front. Genet. 2021, 12, 652129. 10.3389/fgene.2021.652129. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ghildiyal M.; Zamore P. D. Small silencing RNAs: an expanding universe. Nat. Rev. Genet. 2009, 10 (2), 94–108. 10.1038/nrg2504. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gold L. SELEX: How It Happened and Where It will Go. J. Mol. Evol. 2015, 81 (5–6), 140–3. 10.1007/s00239-015-9705-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Seeman N. C. DNA in a material world. Nature 2003, 421 (6921), 427–31. 10.1038/nature01406. [DOI] [PubMed] [Google Scholar]
- Castro C. E.; Kilchherr F.; Kim D. N.; Shiao E. L.; Wauer T.; Wortmann P.; Bathe M.; Dietz H. A primer to scaffolded DNA origami. Nat. Methods 2011, 8 (3), 221–9. 10.1038/nmeth.1570. [DOI] [PubMed] [Google Scholar]
- Lund K.; Manzo A. J.; Dabby N.; Michelotti N.; Johnson-Buck A.; Nangreave J.; Taylor S.; Pei R.; Stojanovic M. N.; Walter N. G.; Winfree E.; Yan H. Molecular robots guided by prescriptive landscapes. Nature 2010, 465 (7295), 206–10. 10.1038/nature09012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hofacker I. L.; Fontana W.; Stadler P. F.; Bonhoeffer L. S.; Tacker M.; Schuster P. Fast folding and comparison of RNA secondary structures. Monatsh. Chem. 1994, 125 (2), 167–168. 10.1007/BF00818163. [DOI] [Google Scholar]
- Zuker M.; Sankoff D. RNA secondary structures and their prediction. Bull. Math. Biol. 1984, 46 (4), 591–621. 10.1016/S0092-8240(84)80062-2. [DOI] [Google Scholar]
- Garcia-Martin J. A.; Clote P.; Dotu I. RNAiFOLD: a constraint programming algorithm for RNA inverse folding and molecular design. J. Bioinform. Comput. Biol. 2013, 11 (2), 1350001. 10.1142/S0219720013500017. [DOI] [PubMed] [Google Scholar]
- Zadeh J. N.; Wolfe B. R.; Pierce N. A. Nucleic acid sequence design via efficient ensemble defect optimization. J. Comput. Chem. 2011, 32 (3), 439–452. 10.1002/jcc.21633. [DOI] [PubMed] [Google Scholar]
- Yamagami R.; Kayedkhordeh M.; Mathews D. H.; Bevilacqua P. C. Design of highly active double-pseudoknotted ribozymes: a combined computational and experimental study. Nucleic Acids Res. 2019, 47 (1), 29–42. 10.1093/nar/gky1118. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McCaskill J. S. The equilibrium partition function and base pair probabilities for RNA secondary structure. Biopolymers 1990, 29 (6–7), 1105–1119. 10.1002/bip.360290621. [DOI] [PubMed] [Google Scholar]
- Hoshika S.; Leal N. A.; Kim M. J.; Kim M. S.; Karalkar N. B.; Kim H. J.; Bates A. M.; Watkins N. E. Jr; SantaLucia H. A.; Meyer A. J.; DasGupta S.; Piccirilli J. A.; Ellington A. D.; SantaLucia J. Jr; Georgiadis M. M.; Benner S. A. Hachimoji DNA and RNA: A genetic system with eight building blocks. Science 2019, 363 (6429), 884–887. 10.1126/science.aat0971. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang X.; Hoshika S.; Peterson R. J.; Kim M. J.; Benner S. A.; Kahn J. D. Biophysics of Artificially Expanded Genetic Information Systems. Thermodynamics of DNA Duplexes Containing Matches and Mismatches Involving 2-Amino-3-nitropyridin-6-one (Z) and Imidazo[1,2-a]-1,3,5-triazin-4(8H)one (P). ACS Synth. Biol. 2017, 6 (5), 782–792. 10.1021/acssynbio.6b00224. [DOI] [PubMed] [Google Scholar]
- Reichenbach L. F.; Sobri A. A.; Zaccai N. R.; Agnew C.; Burton N.; Eperon L. P.; de Ornellas S.; Eperon I. C.; Brady R. L.; Burley G. A. Structural basis of the mispairing of an artificially expanded genetic information system. Chem 2016, 1, 946–958. 10.1016/j.chempr.2016.11.009. [DOI] [Google Scholar]
- Kierzek E.; Zhang X.; Watson R. M.; Kennedy S. D.; Szabat M.; Kierzek R.; Mathews D. H. Secondary structure prediction for RNA sequences including N(6)-methyladenosine. Nat. Commun. 2022, 13 (1), 1271. 10.1038/s41467-022-28817-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Anderson-Lee J.; Fisker E.; Kosaraju V.; Wu M.; Kong J.; Lee J.; Lee M.; Zada M.; Treuille A.; Das R. Principles for Predicting RNA Secondary Structure Design Difficulty. J. Mol. Biol. 2016, 428 (5), 748–757. 10.1016/j.jmb.2015.11.013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bellaousov S.; Kayedkhordeh M.; Peterson R. J.; Mathews D. H. Accelerated RNA Secondary Structure Design Using Pre-Selected Sequences for Helices and Loops. RNA 2018, 24 (11), 1555–1567. 10.1261/rna.066324.118. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schroeder S. J.; Turner D. H. Optical melting measurements of nucleic acid thermodynamics. Methods Enzymol. 2009, 468, 371–87. 10.1016/S0076-6879(09)68017-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Siegfried N. A.; Bevilacqua P. C. Thinking inside the box: designing, implementing, and interpreting thermodynamic cycles to dissect cooperativity in RNA and DNA folding. Methods Enzymol. 2009, 455, 365–93. 10.1016/S0076-6879(08)04213-4. [DOI] [PubMed] [Google Scholar]
- Mergny J. L.; Lacroix L. Analysis of thermal melting curves. Oligonucleotides 2003, 13 (6), 515–37. 10.1089/154545703322860825. [DOI] [PubMed] [Google Scholar]
- Andronescu M.; Condon A.; Turner D. H.; Mathews D. H. The determination of RNA folding nearest neighbor parameters. Methods Mol. Biol. 2014, 1097, 45–70. 10.1007/978-1-62703-709-9_3. [DOI] [PubMed] [Google Scholar]
- SantaLucia J. Jr., A unified view of polymer, dumbbell, and oligonucleotide DNA nearest-neighbor thermodynamics. Proc. Natl. Acad. Sci. U.S.A. 1998, 95 (4), 1460–1465. 10.1073/pnas.95.4.1460. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Xia T.; SantaLucia J. Jr; Burkard M. E.; Kierzek R.; Schroeder S. J.; Jiao X.; Cox C.; Turner D. H. Thermodynamic parameters for an expanded nearest-neighbor model for formation of RNA duplexes with Watson-Crick base pairs. Biochemistry 1998, 37 (42), 14719–35. 10.1021/bi9809425. [DOI] [PubMed] [Google Scholar]
- Chen J. L.; Dishler A. L.; Kennedy S. D.; Yildirim I.; Liu B.; Turner D. H.; Serra M. J. Testing the nearest neighbor model for canonical RNA base pairs: revision of GU parameters. Biochemistry 2012, 51 (16), 3508–22. 10.1021/bi3002709. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hutter D.; Benner S. A. Expanding the genetic alphabet: non-epimerizing nucleoside with the pyDDA hydrogen-bonding pattern. J. Org. Chem. 2003, 68 (25), 9839–42. 10.1021/jo034900k. [DOI] [PubMed] [Google Scholar]
- Lu Z. J.; Turner D. H.; Mathews D. H. A set of nearest neighbor parameters for predicting the enthalpy change of RNA secondary structure formation. Nucleic Acids Res. 2006, 34 (17), 4912–4924. 10.1093/nar/gkl472. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zuber J.; Cabral B. J.; McFadyen I.; Mauger D. M.; Mathews D. H. Analysis of RNA Nearest Neighbor Parameters Reveals Interdependencies and Quantifies the Uncertainty in RNA Secondary Structure Prediction. RNA 2018, 24 (11), 1568–1582. 10.1261/rna.065102.117. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zuber J.; Sun H.; Zhang X.; McFadyen I.; Mathews D. H. A sensitivity analysis of RNA folding nearest neighbor parameters identifies a subset of free energy parameters with the greatest impact on RNA secondary structure prediction. Nucleic Acids Res. 2017, 45 (10), 6168–6176. 10.1093/nar/gkx170. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bommarito S.; Peyret N.; SantaLucia J. Jr. Jr., Thermodynamic parameters for DNA sequences with dangling ends. Nucleic Acids Res. 2000, 28 (9), 1929–1934. 10.1093/nar/28.9.1929. [DOI] [PMC free article] [PubMed] [Google Scholar]
- SantaLucia J.; Hicks D. The thermodynamics of DNA structural motifs. Annu. Rev. Biophys. Biomol. Struct. 2004, 33, 415–40. 10.1146/annurev.biophys.32.110601.141800. [DOI] [PubMed] [Google Scholar]
- Leontis N. B.; Stombaugh J.; Westhof E. The non-Watson-Crick base pairs and their associated isostericity matrices. Nucleic Acids Res. 2002, 30 (16), 3497–3531. 10.1093/nar/gkf481. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Allawi H. T.; SantaLucia J. Jr., Thermodynamics and NMR of internal G·T mismatches in DNA. Biochemistry 1997, 36 (34), 10581–94. 10.1021/bi962590c. [DOI] [PubMed] [Google Scholar]
- Allawi H. T.; SantaLucia J. Jr., Nearest-neighbor thermodynamics of internal A·C mismatches in DNA: sequence dependence and pH effects. Biochemistry 1998, 37 (26), 9435–44. 10.1021/bi9803729. [DOI] [PubMed] [Google Scholar]
- Allawi H. T.; SantaLucia J. Jr., Thermodynamics of internal C·T mismatches in DNA. Nucleic Acids Res. 1998, 26 (11), 2694–701. 10.1093/nar/26.11.2694. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Allawi H. T.; SantaLucia J. Jr., Nearest neighbor thermodynamic parameters for internal G·A mismatches in DNA. Biochemistry 1998, 37 (8), 2170–9. 10.1021/bi9724873. [DOI] [PubMed] [Google Scholar]
- SantaLucia J. Jr; Allawi H. T. Thermodynamics and NMR of internal G·T mismatches in DNA. Biochemistry 1997, 36, 10581–10594. 10.1021/bi962590c. [DOI] [PubMed] [Google Scholar]
- Li Y.; Agrawal S. Oligonucleotides containing G·A pairs: effect of flanking sequences on structure and stability. Biochemistry 1995, 34 (31), 10056–62. 10.1021/bi00031a030. [DOI] [PubMed] [Google Scholar]
- Bourdelat-Parks B. N.; Wartell R. M. Thermodynamic stability of DNA tandem mismatches. Biochemistry 2004, 43 (30), 9918–25. 10.1021/bi049161w. [DOI] [PubMed] [Google Scholar]
- Benner S.; Wang B.; Rocca J.; Hoshika S.; Chen C.; Yang Z.; Esmaeeli R.; Wang J.; Pan X.; Lu J.; Wang K.; Cao Y. C.; Tan W.. A New Folding Motif Formed with an Expanded Genetic Alphabet. Research Square, September 23, 2022. 10.21203/rs.3.rs-1754248/v1. [DOI] [PMC free article] [PubMed]
- Lam J. C.; Withers J. B.; Li Y. A complex RNA-cleaving DNAzyme that can efficiently cleave a pyrimidine-pyrimidine junction. J. Mol. Biol. 2010, 400 (4), 689–701. 10.1016/j.jmb.2010.05.047. [DOI] [PubMed] [Google Scholar]
- Kayedkhordeh M.; Yamagami R.; Bevilacqua P. C.; Mathews D. H. Inverse RNA Folding Workflow to Design and Test Ribozymes that Include Pseudoknots. Methods Mol. Biol. 2021, 2167, 113–143. 10.1007/978-1-0716-0716-9_8. [DOI] [PubMed] [Google Scholar]
- Ward M.; Courtney E.; Rivas E. Fitness functions for RNA structure design. Nucleic Acids Res. 2023, 51 (7), e40 10.1093/nar/gkad097. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Reuter J. S.; Mathews D. H. RNAstructure: software for RNA secondary structure prediction and analysis. BMC Bioinformatics 2010, 11, 129. 10.1186/1471-2105-11-129. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Szabat M.; Prochota M.; Kierzek R.; Kierzek E.; Mathews D. H. A Test and Refinement of Folding Free Energy Nearest Neighbor Parameters for RNA Including N(6)-Methyladenosine. J. Mol. Biol. 2022, 434, 167632. 10.1016/j.jmb.2022.167632. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Georgiadis M. M.; Singh I.; Kellett W. F.; Hoshika S.; Benner S. A.; Richards N. G. Structural basis for a six nucleotide genetic alphabet. J. Am. Chem. Soc. 2015, 137 (21), 6947–55. 10.1021/jacs.5b03482. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bae J. H.; Fang J. Z.; Zhang D. Y. High-throughput methods for measuring DNA thermodynamics. Nucleic Acids Res. 2020, 48 (15), e89 10.1093/nar/gkaa521. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Denny S. K.; Bisaria N.; Yesselman J. D.; Das R.; Herschlag D.; Greenleaf W. J. High-Throughput Investigation of Diverse Junction Elements in RNA Tertiary Folding. Cell 2018, 174 (2), 377–390. 10.1016/j.cell.2018.05.038. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yesselman J. D.; Eiler D.; Carlson E. D.; Gotrik M. R.; d’Aquino A. E.; Ooms A. N.; Kladwang W.; Carlson P. D.; Shi X.; Costantino D. A.; Herschlag D.; Lucks J. B.; Jewett M. C.; Kieft J. S.; Das R. Computational design of three-dimensional RNA structure and function. Nat. Nanotechnol. 2019, 14 (9), 866–873. 10.1038/s41565-019-0517-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pedersen R. O.; Kong J.; Achim C.; LaBean T. H. Comparative Incorporation of PNA into DNA Nanostructures. Molecules 2015, 20 (9), 17645–58. 10.3390/molecules200917645. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gupta A.; Mishra A.; Puri N. Peptide nucleic acids: Advanced tools for biomedical applications. J. Biotechnol. 2017, 259, 148–159. 10.1016/j.jbiotec.2017.07.026. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Moran S.; Ren R. X.; Rumney S.; Kool E. T. Difluorotoluene, a Nonpolar Isostere for Thymine, Codes Specifically and Efficiently for Adenine in DNA Replication. J. Am. Chem. Soc. 1997, 119 (8), 2056–2057. 10.1021/ja963718g. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hoshika S.; Singh I.; Switzer C.; Molt R. W. Jr; Leal N. A.; Kim M. J.; Kim M. S.; Kim H. J.; Georgiadis M. M.; Benner S. A. ″Skinny″ and ″Fat″ DNA: Two New Double Helices. J. Am. Chem. Soc. 2018, 140 (37), 11655–11660. 10.1021/jacs.8b05042. [DOI] [PubMed] [Google Scholar]
- McCown P. J.; Ruszkowska A.; Kunkler C. N.; Breger K.; Hulewicz J. P.; Wang M. C.; Springer N. A.; Brown J. A. Naturally occurring modified ribonucleosides. Wiley Interdiscip Rev. RNA 2020, 11 (5), e1595 10.1002/wrna.1595. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang X.Thermodynamics and Application of a Pair of Synthetic Nucleobases from Artificially Expanded Genetic Information System. Ph.D. dissertation, University of Maryland, College Park, 2016. [Google Scholar]
- Sieg J. P.; Arteaga S. J.; Znosko B. M.; Bevilacqua P. C. MeltR software provides facile determination of nucleic acid thermodynamics. Biophys. Rep. 2023, 3 (2), 100101. 10.1016/j.bpr.2023.100101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- SantaLucia J. Jr; Turner D. H. Measuring the thermodynamics of RNA secondary structure formation. Biopolymers 1997, 44 (3), 309–19. . [DOI] [PubMed] [Google Scholar]
- Seabold S.; Perktold J.. Statsmodels: Econometric and Statistical Modeling with Python. In Proceedings of the 9th PYTHON in Science Conference (SCIPY 2010), 2010; pp 92–96.
- Mathews D. H.; Disney M. D.; Childs J. L.; Schroeder S. J.; Zuker M.; Turner D. H. Incorporating chemical modification constraints into a dynamic programming algorithm for prediction of RNA secondary structure. Proc. Natl. Acad. Sci. U.S.A. 2004, 101 (19), 7287–92. 10.1073/pnas.0401799101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kim J.; Walter A. E.; Turner D. H. Thermodynamics of coaxially stacked helices with GA and CC mismatches. Biochemistry 1996, 35 (43), 13753–13761. 10.1021/bi960913z. [DOI] [PubMed] [Google Scholar]
- Walter A. E.; Turner D. H.; Kim J.; Lyttle M. H.; Müller P.; Mathews D. H.; Zuker M. Coaxial stacking of helixes enhances binding of oligoribonucleotides and improves predictions of RNA folding. Proc. Natl. Acad. Sci. U.S.A. 1994, 91 (20), 9218–9222. 10.1073/pnas.91.20.9218. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Smit S.; Yarus M.; Knight R. Natural selection is not required to explain universal compositional patterns in rRNA secondary structure categories. RNA 2006, 12 (1), 1–14. 10.1261/rna.2183806. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Virtanen P.; Gommers R.; Oliphant T. E.; Haberland M.; Reddy T.; Cournapeau D.; Burovski E.; Peterson P.; Weckesser W.; Bright J.; van der Walt S. J.; Brett M.; Wilson J.; Millman K. J.; Mayorov N.; Nelson A. R. J.; Jones E.; Kern R.; Larson E.; Carey C. J.; Polat I.; Feng Y.; Moore E. W.; VanderPlas J.; Laxalde D.; Perktold J.; Cimrman R.; Henriksen I.; Quintero E. A.; Harris C. R.; Archibald A. M.; Ribeiro A. H.; Pedregosa F.; van Mulbregt P.; et al. SciPy 1.0: fundamental algorithms for scientific computing in Python. Nat. Methods 2020, 17 (3), 261–272. 10.1038/s41592-019-0686-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.