ABSTRACT
Objective: Respiratory infections or colonization of Acinetobacter baumannii (Ab) are common in clinical practice but are treated differently. Early identification of Ab infection and colonization reduces the risk of antibiotic mismatch but objective laboratory indicators to distinguish between bacterial infections and colonization are lacking. To distinguish infection and colonization of Ab, we tested the role of two biomarkers, triggering receptor expressed on myeloid cells-1 (TREM-1) and hemolysin coregulated protein. Methods: A total of 96 inpatients with Ab were divided into infection and colonization groups. Blood samples were collected on days 1, 2, 3, 5, 8, and 10 and daily maximum body temperature was recorded. Polymerase Chain Reaction and Reverse Transcription Polymerase Chain Reaction were used to detect the presence and expression levels of the hcp gene in Ab clinical isolates. Results: sTREM-1 and procalcitonin (PCT) levels on days 1 to 10 and neutrophil classification (N%) on days 1 to 3 were different (P < 0.05) in the infection group and colonization group. Receiver operating characteristic (ROC) curves showed significant differences in N% and sTREM-1 on days 2 and 3 (P < 0.01). sTREM-1 had the highest AUCROC on days 1, 2, and 3 of all the markers. On day 1, the ROC curve of “WBC&N%&PCT&sTREM-1” was statistically different from individual indices (white blood cell count, N%, and PCT; P < 0.05) and was equal to the ROC curve of sTREM-1 (P > 0.05). Thirty five of 96 patients were classified as infection group and 61 as colonization group with hcp gene detection rates of 71.43% (25/35) and 31.15% (19/61), respectively. No differences in hcp gene presence and transcript levels were found between two groups (P > 0.05). Conclusions: Dynamic monitoring of sTREM-1 and PCT is valuable in identifying Ab infection and colonization. sTREM-1 can be improved by combination with multiple biomarkers in the early stage for identification of infection and colonization. The hcp gene was more likely to be present in the infection cohort.
KEYWORDS: Acinetobacter baumannii (Ab), soluble triggering receptor expressed on myeloid cells-1 (sTREM-1), procalcitonin (PCT), infection, colonization, hemolysin coregulated protein (Hcp)
INTRODUCTION
Acinetobacter baumannii (Ab) is a gram-negative bacterium responsible for bacteremia, pneumonia, meningitis, and urinary tract infections in patients with impaired immune function (1). Acinetobacter baumannii is carried by up to 25% of healthy people with normal immune systems and accounts for approximately 20% of intensive care unit (ICU) infections worldwide (2). Lung Ab colonization, which occurs frequently insusceptible populations, is a prerequisite for infection (3). Early distinction of Ab infection and colonization reduces the risk of antibiotic misprescription, development of drug-resistant bacteria, and prolonged hospital stays. Mistaking infection for colonization may delay treatment, worsening a patient’s condition with potentially fatal results. Therefore, early identification of A. baumannii infection or colonization is of great significance.
However, clinical experimental data and diagnosis and treatment guidelines for distinction of bacterial infection and colonization are lacking. Clinicians identify pulmonary Ab infection or colonization based on the results of bacterial culture, white blood cell count (WBC) and neutrophil classification (N%), C-reactive protein (CRP), procalcitonin (PCT), and other plasma biomarker detection results combined with time consuming and subjective imaging and symptoms analysis (4). This method not only wastes time but also depends on the subjective judgment of doctors. The accuracy rate of identification of lung Ab infection or colonization remains nonideal.
Triggering receptor expressed on myeloid cells-1 (TREM-1) is a transmembrane glycoprotein selectively expressed by intrinsic immune cells such as neutrophils, mature monocytes, macrophages, and platelets. It is involved in the pathophysiology of acute and chronic inflammatory diseases with different etiologies in which it triggers inflammation, regulates production of inflammatory factors, and amplifies inflammatory signals (5). The expression of TREM-1 is upregulated during infection and the soluble form of TREM-1, sTREM-1, reflects inflammatory status (6). The level of sTREM-1 correlated with infection severity, including hospital acquired pneumonia (HAP) caused by Ab and was barely detectable in patients with nonmicrobial inflammation (7). Feng et al. (8) demonstrated a positive correlation between serum sTREM-1 and sputum bacterial load. Hence, sTREM-1 in serum may have significance in identifying the infection or colonization of A. baumannii (2).
Currently, WBC, CRP, and PCT are used for clinical distinction between infection and colonization (4). White blood cell count increases in response to infection and inflammation but is also affected by physiological factors, resulting in poor specificity. C-reactive protein is a liver-derived positive acute phase protein, which increases in serum in response to infection, tumors, or autoimmune diseases, giving it high sensitivity but low specificity (9). Procalcitonin is a specific inflammatory infection marker related to the severity of bacterial infection but affected by immune status, microorganism type, and noninfectious diseases, such as cancer (10). Therefore, WBC, CRP, and PCT are insufficient for clinical differentiation of infection or colonization.
The Ab type VI protein secretion system (T6SS) has been recently identified as increasing bacteria-host competition, enhancing bacterial invasiveness, adhesion, and viability with in macrophages (11,12). Hemolysin coregulated protein (Hcp) is a structural and effector component of T6SS and a marker of functional T6SS (11,13,14). Hemolysin coregulated protein secretion into culture medium has been shown to reflect Ab and human respiratory epithelial cell interactions. The hcp gene (hcp +) has been detected in approximately 31.5% of clinical Ab isolates with variable expression rates (11,14,15). Thus, colonizing and infecting strains of Ab in clinical isolates may show differences in hcp gene presence and/or expression levels.
In summary, the sensitivity or specificity of a single biomarker index is insufficient for the differential diagnosis and prognosis judgment of lung infection/colonization. This study investigated the application value of single and combined application of various biomarkers in identifying pulmonary A. baumannii infection or colonization by jointly detecting the level of WBC, N%, CRP, PCT, and sTREM-1 and other infection markers in blood, as well as the level and expression of hcp gene of clinical Ab isolates. The aims were to aid clinical diagnosis and inform treatment decisions.
METHOD
Subjects
A total of 96 patients admitted to Hangzhou First People’s Hospital between November 2019 and November 2021 and from whom Ab strains were isolated from lung secretion specimens were enrolled. Fifty-three patients were excluded because of incomplete blood specimens and the remaining 43 patients were divided into the Ab infection (n = 25) and Ab colonization (n = 18) groups by two clinicians independent of the study. Twenty inpatients without infection were selected as a control group.
Grouping criteria and ethics
Patients were retrospectively grouped based on Clinical Pulmonary Infection Scores and the criteria of US Centers for Disease Control and Prevention (16). Acinetobacter baumannii colonization in lung was defined as isolation of Ab from lung specimens of patients who did not meet the above HAP criteria. Duplicate strains from the same patient were excluded. Exclusion criteria were as follows: (1) age <18 years; (2) presence of coinfections; (3) severe impairment of liver function; (4) weakened immune system and long-term treatment with hormones or immunosuppressive drugs; (5) presence of hematological disease or malignant tumor, especially lung cancer; and (6) treatment by hemodialysis, blood transfusion or radiotherapy/chemotherapy during hospitalization.
Ethical approval was granted by the ethics committee of Hangzhou First People’s Hospital ([2019] KYYLS No. (020)-01) and the need for informed consent was waived.
Patient information, including sex, age, Charlson comorbidity index (CCI) score, number of days in the ICU before Ab detection, number of days of serial detection of Ab, and presence or absence of tracheotomy/intubation, was collated.
Acinetobacter baumannii isolates were preserved on the day of isolation (day 1) and venous blood collected on days 1, 2, 3, 5, 8, and 10. All specimens were stored at −80°C. White blood cell count and CRP were tested immediately. Maximum daily body temperature (T, °C) was recorded on days 1, 2, 3, 5, 8, and 10.
Detection of biomarkers
White blood cell count and neutrophil count
The patient’s venous blood (EDTA-K2 anticoagulant) was collected, WBC and neutrophil count were measured twice for each sample by the SYSMEX automated modular hematology analyzer with associated reagents (SYSMEX 800i; Sysmex Corporation, Kobe, Japan), and a mean was generated. The biological reference range was 3.5 to 9.5 × 109/L for WBC and 1.8 to 6.3 × 109/L for neutrophil count.
C-reactive protein
The patient’s venous blood (EDTA-K2 anticoagulant) was collected and CRP was measured twice for each sample by rate nephelometry using the Mindray automated hematology analyzer with associated reagents (Shenzhen Mindray Corporation, Shenzhen, China) and a mean was generated. The reference range for CRP was 0.2 to 320 mg/L.
Procalcitonin
The venous plasma of patients (heparin lithium anticoagulation) was collected and PCT was measured twice for each sample by luminescence immunoassay using Xiamen Wantai Caris-200 luminescence analyzer with associated reagents (Xiamen Innodx Biotechnology Co, Ltd, Xiamen, China) and a mean was generated. The PCT reference range was 0.02 to 50 ng/mL.
Soluble triggering receptor expressed on myeloid cells-1
The venous plasma of patients (heparin lithium anticoagulation) was collected and sTREM-1 was measured twice for each sample by an enzyme-linked immunosorbent assay using a TREM-1 ELISA Kit (R&D Systems, Minneapolis, MN) according to the operation procedure of the kit. The sTREM-1 reference range was 31.25 to 2,000 pg/mL.
Detection of Ab hcp gene
The preserved Ab isolates were seeded onto Columbia blood agar medium at 37°C for 18 h and transferred to LB broth and shaken at 37°C for 18 h. Bacterial DNA was extracted for quantitative Reverse Transcription Polymerase Chain Reaction and genomic DNA was removed for hcp gene expression detection by one-step Transcription Polymerase Chain Reaction. Relative hcp transcript levels were calculated using the 2−△△ct method and transcript levels of Ab ATCC 17978 used as the control strain.
Statistical analysis
All data were tested for normality and normally distributed data are expressed as mean ± standard deviation with differences between two independent samples tested by t test (2). Nonnormally distributed data are expressed as median (quartiles) (M [QL–QU]) and were tested using Mann-Whitney U test (17). The χ2 test was applied to the count data and ranked data and the corrected χ2 test was performed when the theoretical number of cells was 1 < T < 5. receiver operating characteristic (ROC) curves (17) and logistic regression were applied to calculate the AUCROC (area under the ROC curve), cutoff value, sensitivity/specificity, 95% confidence interval (95% CI), positive likelihood ratio/negative likelihood ratio, and positive predictive value/negative predictive value for evaluation of single and combined indices for identification of Ab colonization and infection. Z test was applied for comparison between ROC curves (6). P < 0.05 indicated that the difference was statistically significant (2) and SPSS (IBM SPSS Statistics 22.0) was used for data analyses (18).
RESULTS
Study population characteristics
Forty-three patients with complete data were divided into infection (n = 25) and colonization (n = 18) groups and 20 patients without infectious disease enrolled as controls (Table 1). The presence or absence of tracheotomy/intubation and the number of days of consecutive Ab detection in lung specimens were statistically different between infection and colonization groups (P < 0.01).
Table 1.
Comparison of basic patient information
| Basic information | Colonization group (n = 18) | Infection group (n = 25) | Control group (n = 20) | Value | P value |
|---|---|---|---|---|---|
| Sex, male/female | 12/6 | 15/10 | 12/8 | χ2 = 0.292 | 0.896 |
| Age, y | 74.0 (66.5–79.5) | 73.0 (66.0–80.5) | 66.5 (60.5–73.8) | H = 3.428 | 0.180 |
| CCI | 5.55 (5.0–6.75) | 6.0 (4.0–7.0) | 5.0 (4.3–7.0) | H = 0.260 | 0.878 |
| Days in ICU before Ab culture positive | 8 (4.25–14.75) | 10 (5.00–17.00) | — | z = −0.851 | 0.395 |
| Days of continuous Ab culture positive | 3.5 (2.0–5.0) | 10 (8.0–12.0) | — | z = −5.46 | 0.001 |
| Stay in ICU or not, yes/no | 11/7 | 22/3 | 14/6 | H = 4.253 | 0.119 |
| Use of antibiotics before admission, with/without | 5/13 | 8/17 | 7/13 | H = 0.226 | 0.893 |
| Whether tracheotomy/intubation was performed, with/without | 8/10 | 17/8 | 5/15 | H = 8.206 | 0.017 |
| Semiquantitative culture results of Ab, small/medium/large amount | 3/6/9 | 3/11/11 | — | z = −0.162 | 0.872 |
Serum levels of different biomarkers in infection and colonization groups
Dynamic changes of each index in infection and colonization groups are shown in Table 2 and Figure 1. Statistical differences were found in WBC on day 1 (z = −2.225, P < 0.05); N% on day 1(z = −2.610, P = 0.009), day 2 (z = −2.142, P = 0.032), and day 3 (z = −2.388, P = 0.014); CRP on day 5 and in both sTREM-1 and PCT on days 1 to 10 (P < 0.05). Body temperature was not different between the two groups on any day (P > 0.05).
Table 2.
Changes in dynamic indexes of A. baumannii infection group and colonization group in the lung
| Indicators | Day 1 | Day 2 | Day 3 | Day 5 | Day 8 | Day 10 |
|---|---|---|---|---|---|---|
| WBC, ×109/L | ||||||
| Infection group | 13.0 (8.1–19.1) | 13.5 (7.4–20.1) | 11.4 (7.5–18.4) | 11.4 (6.4–17.4) | 10.0 (8.0–16.9) | 10.8 (7.3–18.5) |
| Colonization group | 8.1 (5.4–12.3) | 7.8 (6.7–11.4) | 8.4 (6.4–12.2) | 9.8 (6.7–11.4) | 8.7 (7.0–10.3) | 8.6 (6.9–11.0) |
| N(%) | ||||||
| Infection group | 89.0 (82.7–93.1) | 88.9 (80.5–93.2) | 89.3 (80.2–93.3) | 85.5 (79.4–93.4) | 86.0 (81.8–95.2) | 85.4 (76.8–93.3) |
| Colonizing group | 83.0 (73.9–86.3) | 82.7 (73.6–87.9) | 82.2 (74.6–87.8) | 85.5 (79.4–93.4) | 79.9 (70.3–88.0) | 81.6 (69.4–87.8) |
| CRP, mg/L | ||||||
| Infection group | 76.0 (24.5–133.6) | 77.8 (46.0–154.1) | 62.5 (36.9–170.3) | 82.0 (28.4–150.9) | 76.0 (27.1–126.6) | 60.0 (22.1–119.0) |
| Colonizing group | 51.2 (14.2–91.4) | 52.0 (13.1–96.5) | 47.4 (14.2–81.6) | 42.3 (16.8–79.1) | 37.9 (15.5–88.8) | 49.1 (16.5–111.4) |
| PCT, ng/mL | ||||||
| Infection group | 0.94 (0.20–2.51) | 0.87 (0.32–3.23) | 1.09 (0.39–2.76) | 0.99 (0.42–2.13) | 0.67 (0.34–1.19) | 0.47 (0.27–0.90) |
| Colonizing group | 0.14 (0.08–0.50) | 0.15 (0.09–0.42) | 0.13 (0.07–0.25) | 0.11 (0.07–0.23) | 0.09 (0.02–0.30) | 0.47 (0.27–0.90) |
| sTREM-1, pg/mL | ||||||
| Infection group | 1,158.6 (779.5–1,582.3) | 1,109.5 (722.2–1,726.4) | 1,011.1 (802.5–1,817.5) | 1,052.2 (691.0–1,729.8) | 883.5 (685.7–1,268.2) | 443.4 (291.7–602.3) |
| Colonizing group | 532.8 (411.7–747.1) | 480.8 (342.2–619.6) | 450.4 (361.5–579.5) | 500.8 (358.2–608.5) | 443.4 (291.7–602.3) | 469.2 (320.1–650.3) |
| T, °C | ||||||
| Infection group | 37.6 (37.2–38.0) | 37.4 (37.1–38.0) | 37.1 (37.0–37.8) | 37.4 (37.1–37.7) | 37.0 (36.8–37.5) | 37.2 (36.8–37.6) |
| Colonizing group | 37.5 (37.1–38.3) | 37.6 (37.2–38.1) | 37.8 (37.2–38.3) | 37.2 (36.8–38.3) | 37. (36.7–37.2) | 36.9 (36.8–37.0) |
Nonnormally distributed data are represented as “median (1/4 quartile–3/4 quartile).” There was a significant difference in WBC count between the infection group and the colonization group on the first day (P < 0.05), but no difference was found on the other days (P > 0.05).
Fig. 1.
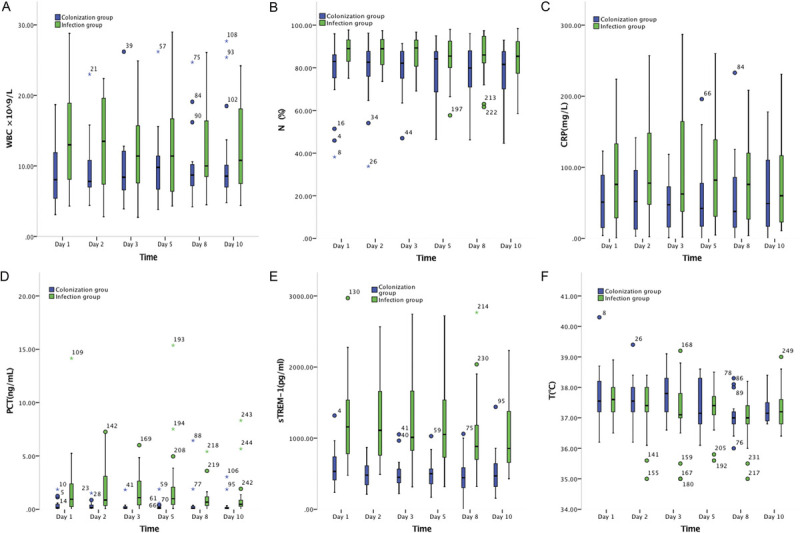
Dynamic monitoring of biomarkers in infection group and colonization group. (A) Dynamic monitoring of WBC count in infection group and colonization group. (B) Dynamic monitoring of neutrophil ratio (N%) in infection group and colonization group. (C) Dynamic monitoring of CRP in infection group and colonization group. (D) Dynamic monitoring of PCT in infection group and colonization group. (E) Dynamic monitoring of sTREM-1 in infection group and colonization group. (F) Dynamic monitoring of body T in infection group and colonization group. CRP, C-reactive protein; PCT, procalcitonin; sTREM-1, soluble triggering receptor expressed on myeloid cells-1; T, temperature; WBC, white blood cell.
Receiver operating characteristic curve analysis of single and combined biomarkers for identification of infection and colonization
Significantly different biomarkers were further assessed by construction of ROC curves for sTREM-1, PCT, and N% on days 1, 2, and 3. Single and combined ROCs are presented in Table 3 and Figures 2 to 6. The combination of these three biomarkers improved diagnostic specificity and sensitivity.
Table 3.
Comparison of indicators for differentiation of pulmonary A. baumannii infection and colonization
| Index | AUCROC | Cutoff | Sensitivity | Specificity | 95% CI | PPV, % | NPV, % | LR(+) | LR(−) |
|---|---|---|---|---|---|---|---|---|---|
| WBC | |||||||||
| Day 1 | 0.701 | 16.1 | 40.0 | 94.4 | 0.546–0.856 | 91 | 53 | 7.14 | 0.64 |
| N | |||||||||
| Day 1 | 0.736 | 87.1 | 60.0 | 83.0 | 0.583–0.889 | 83.0 | 60.0 | 3.60 | 0.48 |
| Day 2 | 0.693 | 88.7 | 52.0 | 89.0 | 0.534–0.853 | 87.0 | 57.0 | 4.68 | 0.54 |
| Day 3 | 0.716 | 91.5 | 48.0 | 100.0 | 0.563–0.868 | 100.0 | 58.0 | — | 52.0 |
| PCT | |||||||||
| Day 1 | 0.732 | 0.59 | 60.0 | 83.0 | 0.601–0.892 | 83.0 | 60.0 | 3.60 | 0.48 |
| Day 2 | 0.808 | 0.75 | 64.0 | 89.0 | 0.674–0.937 | 89.0 | 64.0 | 5.76 | 0.41 |
| Day 3 | 0.864 | 0.40 | 76.0 | 94.0 | 0.757–0.981 | 95.0 | 74.0 | 13.68 | 0.25 |
| Day 5 | 0.900 | 0.20 | 96.0 | 78.0 | 0.800–1.000 | 86.0 | 93.0 | 4.32 | 0.05 |
| Day 8 | 0.794 | 0.31 | 84.0 | 78.0 | 0.641–0.948 | 84.0 | 78.0 | 3.78 | 0.21 |
| Day 10 | 0.831 | 0.20 | 92.0 | 83.0 | 0.686–0.977 | 88.0 | 88.0 | 5.52 | 0.10 |
| sTREM-1 | |||||||||
| Day 1 | 0.867 | 774.5 | 80.0 | 83.0 | 0.760–0.974 | 87.0 | 75.0 | 4.80 | 0.24 |
| Day 2 | 0.929 | 648.9 | 88.0 | 83.0 | 0.857–1.000 | 88.0 | 83.0 | 5.28 | 0.14 |
| Day 3 | 0.902 | 662.3 | 92.0 | 89.0 | 0.805–0.999 | 92.0 | 89.0 | 8.28 | 0.09 |
| Day 5 | 0.883 | 583.4 | 88.0 | 78.0 | 0.783–0.983 | 85.0 | 82.0 | 3.96 | 0.15 |
| Day 8 | 0.858 | 592.2 | 88.0 | 78.0 | 0.741–0.974 | 85.0 | 82.0 | 3.96 | 0.15 |
| Day 10 | 0.847 | 647.1 | 84.0 | 78.0 | 0.724–0.969 | 84.0 | 78.0 | 3.78 | 0.21 |
| WBC &N&PCT &sTREM-1 | |||||||||
| Day 1 | 0.931 | — | 84.0 | 94.0 | 0.856–1.000 | 95.0 | 81.0 | 15.12 | 0.17 |
| N&PCT &sTREM-1 | |||||||||
| Day 2 | 0.951 | — | 88.0 | 94.0 | 0.889–1.000 | 96.0 | 85.0 | 15.84 | 0.13 |
| Day 3 | 0.938 | — | 92.0 | 94.0 | 0.859–1.000 | 96.0 | 89.0 | 16.56 | 0.08 |
| PCT &sTREM-1 | |||||||||
| Day 1 | 0.902 | — | 84.0 | 89.0 | 0.809–0.995 | 91.0 | 80.0 | 7.56 | 0.18 |
| Day 2 | 0.951 | — | 88.0 | 94.0 | 0.890–1.000 | 96.0 | 85.0 | 15.84 | 0.13 |
| Day 3 | 0.929 | — | 96.0 | 89.0 | 0.844–1.000 | 92.0 | 94.0 | 8.64 | 0.05 |
| Day 5 | 0.911 | — | 84.0 | 94.0 | 0.818–1.000 | 95.0 | 81.0 | 15.12 | 0.17 |
| Day 8 | 0.864 | — | 88.0 | 78.0 | 0.751–0.978 | 85.0 | 82.0 | 3.96 | 0.15 |
| Day 10 | 0.860 | — | 80.0 | 83.0 | 0.743–0.977 | 87.0 | 75.0 | 4.80 | 0.24 |
LR(+), positive likelihood ratio; LR(−), negative likelihood ratio; NPV, negative predictive value; PPV, positive predictive value.
Fig. 2.
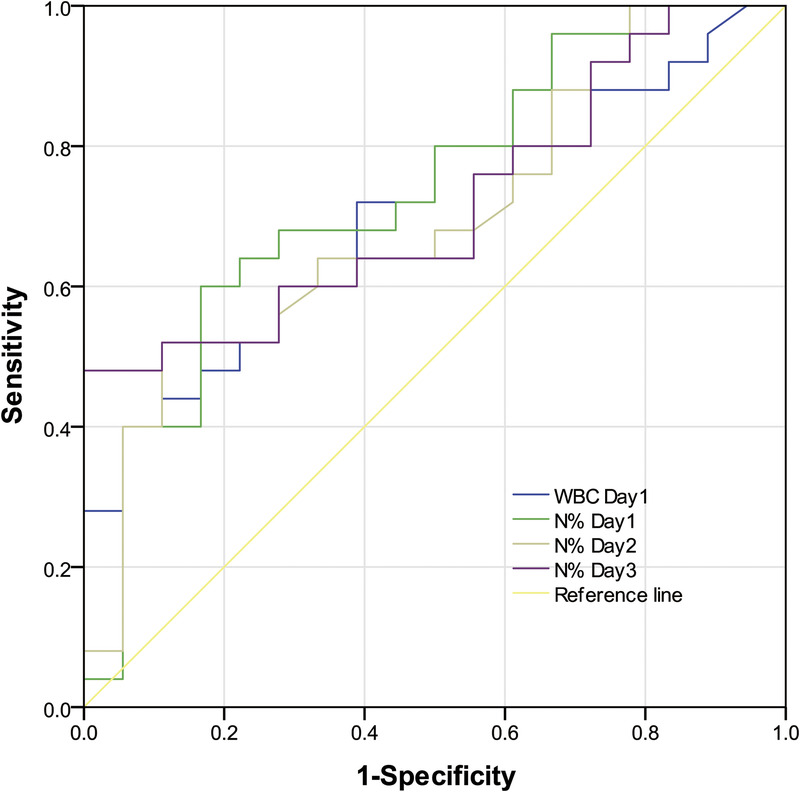
The ROC curves of WBC count on day 1 and N% on days 1–3 were used to differentiate infection group and colonization group. ROC, receiver operating characteristic; WBC, white blood cell.
Fig. 6.
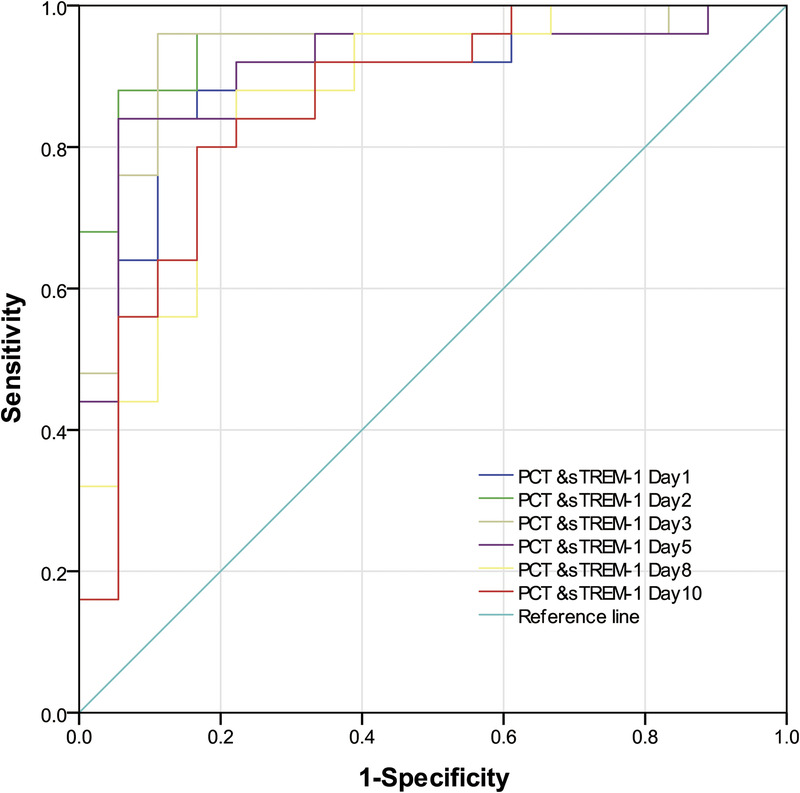
The ROC curves of the combination of sTREM-1 and PCT on days 1–10 for identifying infection and colonization. PCT, procalcitonin; ROC, receiver operating characteristic; sTREM-1, soluble triggering receptor expressed on myeloid cells-1.
Fig. 3.
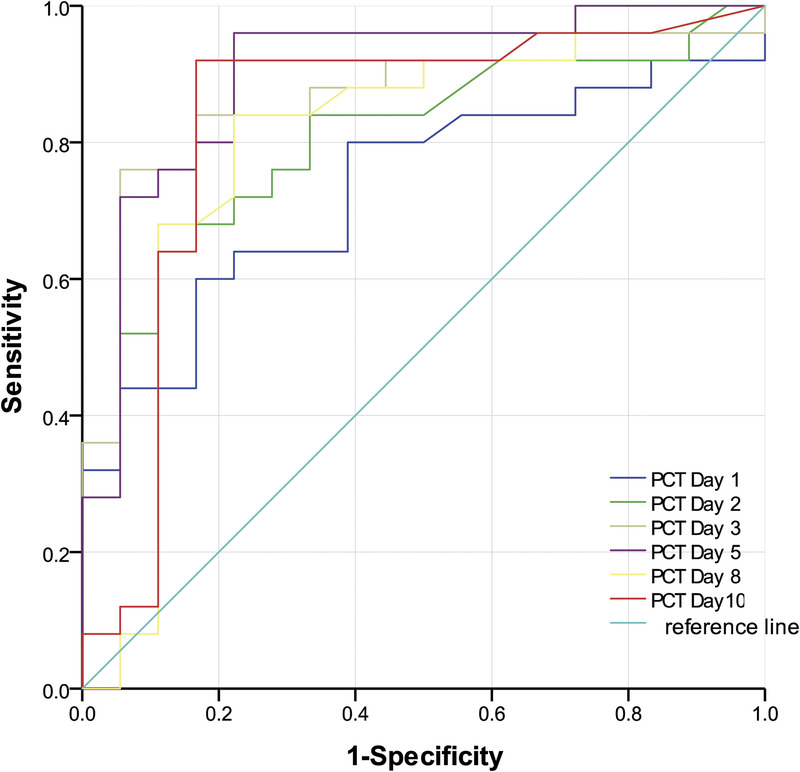
The ROC curves of PCT on days 1–10 were used to differentiate infection group and colonization group. PCT, procalcitonin; ROC, receiver operating characteristic.
Fig. 4.
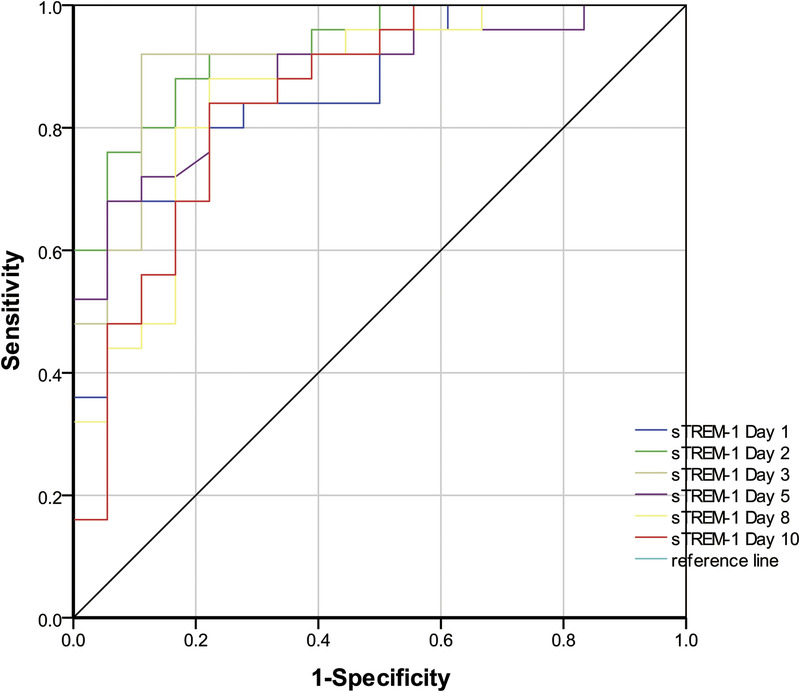
The ROC curves of sTREM-1 on days 1–10 were used to differentiate infection group and colonization group. ROC, receiver operating characteristic; sTREM-1, soluble triggering receptor expressed on myeloid cells-1.
Fig. 5.
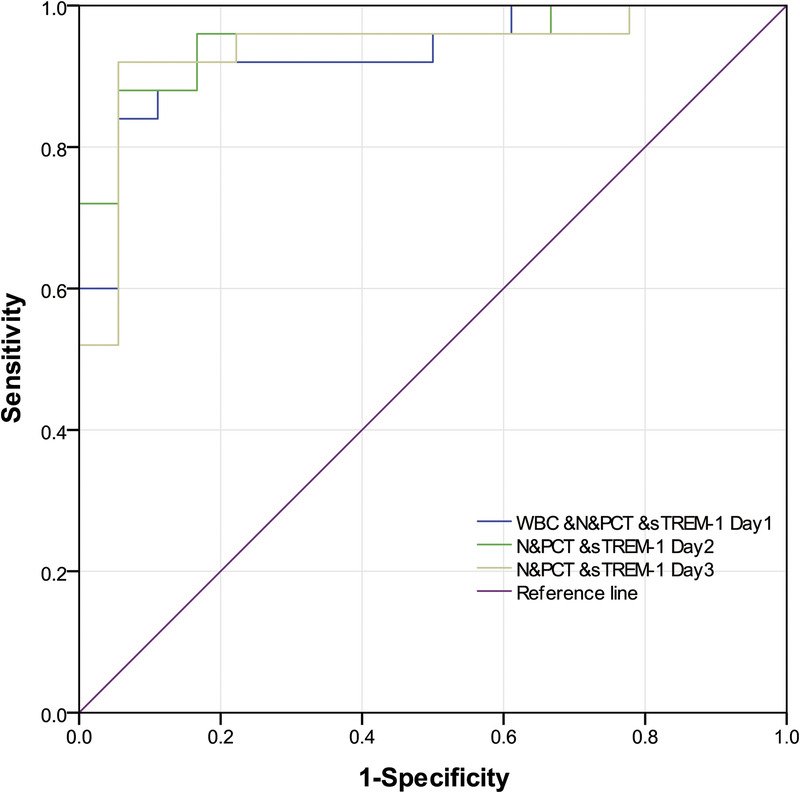
The ROC curves of multiple indicators on days 1–3 combined to identify infection and colonization. ROC, receiver operating characteristic.
There were significant differences in the proportion of neutrophils N% between the infection group and the colonization group on days 1 to 3 (P < 0.05). There was a significant difference in CRP between the infection group and the colonization group on the fifth day (P < 0.05), but there was no difference on the other days (P > 0.05). There were significant differences in PCT and STREM-1 between infection group and colonization group on days 1 to 10 (P < 0.05). There was no significant difference in body temperature T between infection group and colonization group on days 1 to 10 (P > 0.05).
On the first day, there were no significant differences in WBC count, N%, PCT, and sTREM-1 (P > 0.05). On day 2 and day 3, the ROC curves of N% and sTREM-1 were significantly different (P < 0.01). There was no significant difference in the ROC curves of PCT and sTREM-1 from day 1 to day 10 (P > 0.05), and there was no significant difference in the ROC curves of PCT and sTREM-1 from day 1 to day 10 (P > 0.05). On the first day, the ROC curves of combined “WBC &N&PCT &sTREM-1” were statistically different from those of single index (WBC count, N%, and PCT) (P < 0.05), but not from those of single index sTREM-1 (P > 0.05). On the second and third day, the ROC curve of combined “N&PCT &sTREM-1” was significantly different from that of single index N% (P < 0.05). The ROC curves of “PCT &sTREM-1” combined curve and single index (PCT and sTREM-1) had no statistical difference from day 1 to day 10 (P > 0.05), and the ROC curves of “PCT &sTREM-1” combined curve had no statistical difference (P > 0.05).
hcp analysis in Ab isolates
The presence of the hcp gene was detected by PCR in 44 of 96 Ab strains (45.83%) from lung specimens 25/35 (71.43%) from the infection and 19/61 (31.15%) from the colonization groups. hcp presence was significantly different between the two groups (P < 0.05) but the amount of hcp gene, calculated using the 2−△△ct method, was not different (P > 0.05; Table 4).
Table 4.
Comparison of hcp gene quantity and hcp gene expression (2−△△ct)
| Groups | Relative amount | P | Relative expression | P |
|---|---|---|---|---|
| Infection (n = 25) | 0.53 (0.48–0.57) | > 0.05 | 0.51 (0.42–0.54) | > 0.05 |
| Colonization(n = 19) | 0.61 (0.53–0.77) | 0.57 (0.47–0.76) |
Nonnormally distributed data are represented as “median (1/4 quartile–3/4 quartile).”
mRNA of hcp was measured in the 44 hcp+ Ab strains and no difference in transcript levels between infection and colonization groups was found (P > 0.05; Table 4).
DISCUSSION
Acinetobacter baumannii is a common clinical pathogen, which accounted for the top three respiratory tract isolates in 2016–2019, according to the China Antimicrobial Surveillance Network. Multidrug-resistant strains of HAP have become more frequent in clinical settings (14,19), contributing to a 52% Ab mortality rate (4). Hence, early identification of Ab colonization and infection is vital to prevent disease progression, reduce antibiotic abuse, and halt bacterial drug resistance.
The current study found Ab infection rates to be 36.46% (35/96) and colonization rates to be 63.54% (61/96). Rates among the 53 cases (53/96, 55.21%) from the ICU, were 58.49% (31/53) infection and 41.51% (22/53) colonization. Variation among infection and colonization found at different hospitals has been reported (20). Martín-Aspas et al. (21) reported a 40% (49/122) Ab infection rate and a 60% (73/122) colonization rate while Rodríguez-Baño et al. (22) found Ab colonization to be approximately 50% in some Spanish hospitals. Differences may be attributed to local socioeconomic conditions, hospital management, epidemiological differences, and patients’ basic conditions. Higher Ab infection rates in ICUs than in other wards may be due to complications experienced by ICU patients, weakened immunity, more invasive treatments, and the closed nature of ICU wards, which renders them prone to aggregated infections. Moreover, the tracheotomy/intubation rate was significantly higher among the infection compared with the colonization group of the current cohort (P < 0.05), indicating that invasive operations of the respiratory tract constitute risk factors for Ab infection (16,23–25). Furthermore, the number of days of continuous Ab strain detection was greater for the infection group (P < 0.01). This observation indicates the possibility of distinguishing Ab infection and colonization based on duration of Ab detection. However, this method is time consuming, increasing patients’ financial burdens and prolonging hospital stays. No significant differences were found between the two groups in terms of sex, age, CCI, antibiotic use within 3 months, or amounts of Ab. Thus, our preliminary conclusion is that a history of an ICU stay and invasive operations were the main risk factors for protracted lung Ab infection.
Microbial culture, WBC count and classification, CRP, PCT, and temperature monitoring are used to distinguish infection from colonization. A new inflammatory marker, sTREM-1 (8,16,26,27), for identifying bacterial infection and colonization in experimental animals has recently been reported (2). The current study found sTREM-1 and PCT to be significantly higher in the infection relative to the colonization group on days 1 to 10 (P < 0.05), demonstrating the potential utility of these two biomarkers. PCT and sTREM-1 ROC curves on days 1 to 10 were similar (P > 0.05), as were combined “PCT & sTREM-1” and single index (PCT or sTREM-1; P > 0.05). Thus, the combination of these two biomarkers possessed a similar test efficacy to individual indices in identifying Ab infection or colonization. Procalcitonin and sTREM-1 day 3 ROC curves had the highest positive likelihood ratios (13.68 and 8.28), indicating optimal diagnostic value. The sTREM-1 day 2 ROC curve showed the highest AUC value of 0.929 with optimal cutoff point 648.9 pg/L, sensitivity 88.0%, and specificity 83.0%. All data demonstrated the value of PCT and sTREM-1 for identifying lung Ab infection or colonization and early detection maximized their utility. Richard et al (28) also found an sTREM-1 AUC of 0.87 (95% CI, 0.81–0.92), superior to PCT and CRP and consistent with the current findings.
The N% on days 1 to 3 was significantly higher in the infection than in the colonization group (P < 0.05). N% AUCROCs on days 1, 2, and 3 were 0.736, 0.693, and 0.716, respectively, indicating that N% alone was not an ideal biomarker in distinguishing Ab infection from colonization. The WBC&N%&PCT &sTREM-1 AUCROC of 0.931 on day 1 was statistically different from individual index AUCROCs (WBC count, N%, and PCT; P < 0.05). N%&PCT&sTREM-1 AUCROC of 0.951 (day 2) and 0.938 (day 3) were significantly different from those for N% alone (P < 0.05) but not for sTREM-1 alone (P > 0.05). Thus, combined multiple indices improve the sensitivity and specificity of identifying lung Ab infection or colonization. This may be due to the excellent early-stage test efficacy of sTREM-1 and PCT, which is improved by addition of WBC count and N%. Moreover, variations in onset time, degree of increase, and duration affect inflammatory biomarkers individually, resulting in varied efficacy of markers in combination or individually.
White blood cell counts on day 1(z = −2.228, P = 0.026) and CRP (z = −1.994, P = 0.046) on day 4 showed differences between the two groups, whereas no differences were found for temperature. These three indices have little utility for distinguishing Ab colonization from infection. Ito and Ishida (29) and Li et al. (30) have reported low specificity and sensitivity for WBC and both WBC and CRP can also increase during viral infection. Temperature fluctuates greatly in inpatients because of condition changes, coinfections, and anti-inflammatory treatment, giving it poor specificity. Elderly patients or patients with severe infections occasionally have normal temperature and WBC (31). Such studies illustrate the poor specificity of these three biomarkers, in agreement with our findings.
The T6SS is a gram-negative bacteria virulence system, which enables bacterial competition in the host, enhances invasiveness and adhesion, and improves bacterial viability within macrophages. T6SS is central to the pathogenesis of Ab infection (18–20,22,32,33). The T6SS marker, Hcp, shows different presence and expression levels in clinical isolates (11,14,15). We found a higher presence rate of the hcp gene in the infection (71.43%) than in the colonization group (31.15%) (P < 0.05), suggesting the utility of hcp presence in distinguishing Ab infection and colonization. Different studies have reported diverse hcp presence rates (11,14,15,33), perhaps related to local epidemiology, different sites of isolation, grouping criteria, and sample sizes. Presence and expression levels of hcp were similar in the two groups of the current cohort, which may reflect grouping criteria, multiple infections, and the sample size.
In summary, we propose that early-stage PCT and sTREM-1 values (within 3 days of Ab detection) may be used to identify Ab infection or colonization and combination with WBC and N% improves sensitivity and specificity. Dynamic monitoring of PCT and sTREM-1 aids the clarification of infection versus colonization. Detection of the hcp gene presence rate assists this process.
We acknowledge some limitations to the current study. First, no gold standard for distinguishing between pulmonary infection and colonization of A. baumannii exists. We can only use a widely used scoring tool reported in the literature, scored by two experienced attending physicians, which has potential bias. Second, the sample size was small and the subjects enrolled were from the same hospital, so that results may not be universally applicable. Third, ICU patients had complicated conditions influencing all infection biomarkers, which may affect the identification of Ab colonization and infection. Fourth, histological analysis is normally considered the criterion standard for diagnosis of pulmonary infections and the emphasis on clinical symptoms, imaging, and hematological examinations in the current study may have affected results. Fifth, hcp gene expression is influenced by many factors and their impact on identification of Ab infection and colonization has not been discussed. Lastly, lung Ab infection and colonization states are dynamic processes, as affected by bacterial virulence, drug resistance, human immunity, and medical level. Further investigations of the factors and mechanisms affecting host infection/colonization were required.
Therefore, it is of great significance that early identification of A. baumannii infection or colonization. As biomarkers (WBC, CRP, PCT, and sTREM-1) and body temperature are nonspecific indicators, the identification scheme proposed in this study from the aspect of body immunity has some false positives and false negatives. Therefore, this study explored the identification of lung Ab infection or colonization from the bacterial aspect (hcp gene carrier rate and its expression) and the body aspect (biomarkers and body temperature) and found that: (1) the identification of Ab infection and colonization requires consideration of high risk factors of infection (ICU hospitalization history and respiratory tract invasive operation) and the duration of infection/colonization; (2) the dynamic monitoring of blood biomarkers and body temperature has significance in distinguishing Ab infection and colonization, but there may still be the possibility of false positive and false negative; and (3) the carrier rate of hcp gene was higher in the infected group. In summary, biomarkers and the hcp gene are valuable for the identification of Ab infection or colonization in lung. Further exploration is required to verify the current findings.
ACKNOWLEDGMENTS
Authors’ contributions: D.Y. did the conceptualization; X.W., J.J., C.W., W.Y., J.C., X.D. and D.Y. did the methodology; X.W., J.J., C.W., W.Y., J.C., X.D. and H.W. did the formal analysis; X.W., J.J., C.W., W.Y., J.C., X.D., H.W. and D.Y. did the investigation; X.W., J.J., and D.Y. did the writing—original draft; H.W., and D.Y. did the writing—review and editing; D.Y. did the project administration; D.Y., H.W. and X.W did the funding acquisition.
Availability of supporting data: The data in this article have not been published elsewhere and are available from the corresponding author upon request
Footnotes
XW, JJ, CW, and WY contributed equally to this work.
This work was supported by the National Natural Science Foundation of China (grant number 81930111), the Natural Science Foundation of Zhejiang Province (grant number LZ22H190002), the Health & Medical Sci-Tech Project of Hangzhou Municipal Health Commission (Z2021005), the Science and Technology Project of Hangzhou Municipal (grant number 202204A001), and the Health & Medical Sci-Tech Project of Health Commission of Zhejiang Province (grant number 2020KY206, 2022PY016).
The authors report no conflict of interests.
Contributor Information
Xiaolei Wang, Email: zhaoliling.00@163.com.
Jiahui Jiang, Email: jjhzju@zju.edu.cn.
Chenxing Wei, Email: 22018546@zju.edu.cn.
Wenjie Yang, Email: ywj020040@163.com.
Jian Chen, Email: chenjian090@126.com.
Xueyan Dong, Email: dongxueyan82@163.com.
REFERENCES
- 1.Weiner LM Webb AK Limbago B, et al. Antimicrobial-resistant pathogens associated with healthcare-associated infections: summary of data reported to the National Healthcare Safety Network at the Centers for Disease Control and Prevention, 2011–2014. Infect Control Hosp Epidemiol. 2016;37(11):1288–1301. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Hu C Jiang J Li Z, et al. Expression pattern of soluble triggering receptor expressed on myeloid cells-1 in mice with Acinetobacter baumannii colonization and infection in the lung. J Thorac Dis. 2018;10(3):1614–1621. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Mateo-Estrada V Fernández-Vázquez JL Moreno-Manjón J, et al. Accessory genomic epidemiology of cocirculating Acinetobacter baumannii clones. mSystems. 2021;6(4):e0062621. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Yu J Tycksen E Yang W, et al. Use of host response to refine the diagnosis of group A streptococcal pharyngitis. J Pediatric Infect Dis Soc. 2022;11(11):482–491. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Carrasco K Boufenzer A Jolly L, et al. TREM-1 multimerization is essential for its activation on monocytes and neutrophils. Cell Mol Immunol. 2019;16(5):460–472. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Jiang J Wang X Cheng T, et al. Dynamic monitoring of sTREM-1 and other biomarkers in acute cholangitis. Mediators Inflamm. 2020;2020:8203813. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Yu Y Zhu C Liu C, et al. Diagnostic performance of soluble triggering receptor expressed on myeloid cells-1 in ventilator-associated pneumonia of patients with ischemic stroke. Can J Infect Dis Med Microbiol. 2017;2017:9513690. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Feng JY Su WJ Pan SW, et al. Role of TREM-1 in pulmonary tuberculosis patients—analysis of serum soluble TREM-1 levels. Sci Rep. 2018;8(1):8223. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Sproston NR, Ashworth JJ. Role of C-reactive protein at sites of inflammation and infection. Front Immunol. 2018;9:754. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Gao Q Li Z Mo X, et al. Combined procalcitonin and hemogram parameters contribute to early differential diagnosis of gram-negative/gram-positive bloodstream infections. J Clin Lab Anal. 2021;35(9):e23927. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Kim J Lee JY Lee H, et al. Microbiological features and clinical impact of the type VI secretion system (T6SS) in Acinetobacter baumannii isolates causing bacteremia. Virulence. 2017;8(7):1378–1389. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Fitzsimons TC Lewis JM Wright A, et al. Identification of novel Acinetobacter baumannii type VI secretion system antibacterial effector and immunity pairs. Infect Immun. 2018;86(8):e00297–e00218. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Ruiz FM Santillana E Spínola-Amilibia M, et al. Crystal structure of Hcp from Acinetobacter baumannii: a component of the type VI secretion system. PLoS One. 2015;10(6):e0129691. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Repizo GD. Prevalence of Acinetobacter baumannii strains expressing the type 6 secretion system in patients with bacteremia. Virulence. 2017;8(7):1099–1101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Weber BS Miyata ST Iwashkiw JA, et al. Genomic and functional analysis of the type VI secretion system in Acinetobacter. PloS One. 2013;8(1):e55142. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Fan CY Lee WT Hsu TC, et al. Effect of chlorhexidine bathing on colonization or infection with Acinetobacter baumannii: a systematic review and meta-analysis. J Hosp Infect. 2019;103(3):284–292. [DOI] [PubMed] [Google Scholar]
- 17.Ebrahimi ZS Nasli-Esfahani E Nadjarzade A, et al. Effect of symbiotic supplementation on glycemic control, lipid profiles and microalbuminuria in patients with non-obese type 2 diabetes: a randomized, double-blind, clinical trial. J Diabetes Metab Disord. 2017;16:23. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Wang F, An W, Zhang X. Copeptin combined with National Early Warning Score for predicting survival in elderly critical ill patients at emergency department. Am J Emerg Med. 2021;49:153–157. [DOI] [PubMed] [Google Scholar]
- 19.Gao J Zou Y Wang Y, et al. Breath analysis for noninvasively differentiating Acinetobacter baumannii ventilator-associated pneumonia from its respiratory tract colonization of ventilated patients. J Breath Res. 2016;10(2):027102. [DOI] [PubMed] [Google Scholar]
- 20.Di Venanzio G Flores-Mireles AL Calix JJ, et al. Urinary tract colonization is enhanced by a plasmid that regulates uropathogenic Acinetobacter baumannii chromosomal genes. Nat Commun. 2019;10(1):2763. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Martín-Aspas A Guerrero-Sánchez FM García-Colchero F, et al. Differential characteristics of Acinetobacter baumannii colonization and infection: risk factors, clinical picture, and mortality. Infect Drug Resist. 2018;11:861–872. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Pan P Wang X Chen Y, et al. Effect of Hcp iron ion regulation on the interaction between Acinetobacter baumannii with human pulmonary alveolar epithelial cells and biofilm formation. Front Cell Infect Microbiol. 2022;12:761604. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Rodríguez-Baño J Cisneros JM Fernández-Cuenca F, et al. , Grupo de Estudio de Infección Hospitalaria (GEIH) . Clinical features and epidemiology of Acinetobacter baumannii colonization and infection in Spanish hospitals. Infect Control Hosp Epidemiol. 2004;25(10):819–824. [DOI] [PubMed] [Google Scholar]
- 24.Arvaniti K Lathyris D Ruimy R, et al. The importance of colonization pressure in multiresistant Acinetobacter baumannii acquisition in a Greek intensive care unit. Crit Care. 2012;16(3):R102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Henig O Weber G Hoshen MB, et al. Risk factors for and impact of carbapenem-resistant Acinetobacter baumannii colonization and infection: matched case-control study. Eur J Clin Microbiol Infect Dis. 2015;34(10):2063–2068. [DOI] [PubMed] [Google Scholar]
- 26.Gallop D Scanlon KM Ardanuy J, et al. Triggering receptor expressed on myeloid cells-1 (TREM-1) contributes to Bordetella pertussis inflammatory pathology. Infect Immun. 2021;89(10):e0012621. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Wang Y Zhang S Li L, et al. The usefulness of serum procalcitonin, C-reactive protein, soluble triggering receptor expressed on myeloid cells 1 and Clinical Pulmonary Infection Score for evaluation of severity and prognosis of community-acquired pneumonia in elderly patients. Arch Gerontol Geriatr. 2019;80:53–57. [DOI] [PubMed] [Google Scholar]
- 28.Richard-Greenblatt M Boillat-Blanco N Zhong K, et al. Prognostic accuracy of soluble triggering receptor expressed on myeloid cells (sTREM-1)-based algorithms in febrile adults presenting to Tanzanian outpatient clinics. Clin Infect Dis. 2020;70(7):1304–1312. [DOI] [PubMed] [Google Scholar]
- 29.Ito A, Ishida T. Diagnostic markers for community-acquired pneumonia. Ann Transl Med. 2020;8(9):609. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Li Y, Min L, Zhang X. Usefulness of procalcitonin (PCT), C-reactive protein (CRP), and white blood cell (WBC) levels in the differential diagnosis of acute bacterial, viral, and mycoplasmal respiratory tract infections in children. BMC Pulm Med. 2021;21(1):386. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Kushimoto S Gando S Saitoh D, et al. , JAAM Sepsis Registry Study Group . The impact of body temperature abnormalities on the disease severity and outcome in patients with severe sepsis: an analysis from a multicenter, prospective survey of severe sepsis. Crit Care. 2013;17(6):R271. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Lopez J, Ly PM, Feldman MF. The tip of the VgrG spike is essential to functional type VI secretion system assembly in Acinetobacter baumannii. mBio. 2020;11(1):e02761–e02719. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Corral J Pérez-Varela M Sánchez-Osuna M, et al. Importance of twitching and surface-associated motility in the virulence of Acinetobacter baumannii. Virulence. 2021;12(1):2201–2213. [DOI] [PMC free article] [PubMed] [Google Scholar]


