Abstract
In a slum community in northeastern Brazil 20% of a sample population was colonized with Entamoeba histolytica or Entamoeba dispar and 10.6% was colonized with E. histolytica alone. No correlation between seropositivity for anti-GalNAc lectin antibody and colonization was found. These results suggest that colonization does not necessarily produce immunity to reinfection.
Approximately 10% of the world’s population is colonized with the enteric parasites Entamoeba histolytica and Entamoeba dispar (13, 15). However, 90% of these individuals are colonized with nonpathogenic E. dispar and only 10% are colonized with pathogenic E. histolytica (5, 11). In addition, only 1 in 10 infections with E. histolytica results in symptomatic disease (12).
There is some evidence for acquired immunity to invasive amebiasis (4); however, it is controversial whether immunity to colonization develops. Some studies have suggested that individuals may be constantly reinfected throughout their lives (2, 9, 10, 12, 14), while others have demonstrated decreased colonization rates in seropositive individuals (3).
Members of our group have previously shown a high rate of seropositivity for the N-acetyl-d-galactosamine (GalNAc)-inhibitable lectin of E. histolytica in several slums in Fortaleza, Brazil (1). To examine the prevalence of E. histolytica and other parasitic infections, stool samples from 564 individuals were collected during a 1-year period, from 1996 to 1997. Informed consent was obtained from all subjects that participated in the study. The median age was 17 years (range, 1 to 80 years). A total of 43.7% (246 of 564) were males, and 56.3% (318 of 564) were females. According to questionnaires all individuals were asymptomatic.
Stool samples taken from the survey population were examined for the presence of common intestinal parasites by microscopy (Table 1). The overall parasite colonization rate was 52.4% (296 of 564), with 50.3% (149 of 296) for males and 49.6% (147 of 296) for females. A total of 44.6% (132 of 296) of parasitized individuals had mixed infections. Ascaris lumbricoides was the most prevalent parasite (33.3% [188 of 564]). E. dispar and E. histolytica were the most common protozoan parasites. Few cases of Giardia lamblia infection were found (2.4% [14 of 564]).
TABLE 1.
Prevalence of protozoan parasites in stool
| Parasite | % Prevalence (no. positive)a |
|---|---|
| Ascaris lubricoides | 33.3 (188) |
| Entamoeba colib | 16.3 (92) |
| Trichuris trichiura | 12.4 (70) |
| Entamoeba species | 8.0 (45) |
| Enterobius vermicularis | 3.0 (17) |
| Endolimax nanab | 2.5 (14) |
| Giardia lamblia | 2.5 (14) |
| Hymenolepis nana | 2.5 (14) |
| Strongyloides stercoralis | 2.3 (13) |
| Iodoamoeba buetschlii | 2.1 (12) |
| Ancylostoma | 1.8 (10) |
| Chilosmastix mesnilib | 0.17 (1) |
A total of 564 samples were tested.
Commensal organism.
E. dispar or E. histolytica was detected by microscopy in 8.0% (45 of 564) of the stools. Stools were also assayed with the Entamoeba kit, which detects either E. dispar or E. histolytica, and the E. histolytica kit, which detects only E. histolytica. Both kits were used according to the manufacturer’s instructions (Tech Lab, Blacksburg, Va.). Compared to culturing, the E. dispar and E. histolytica antigen detection test is 94% sensitive and 94% specific, and the E. histolytica antigen detection test is 86% sensitive and 98% specific (7, 8). By using the Entamoeba kit 19.6% (112 of 564) of the stools were found to be positive. The median age of individuals was 27 years (range, 1 to 72 years); 50% (56 of 112) were males and 50% (56/112) were females. By using the E. histolytica kit, 10.6% (60 of 564) of the stools were found to be positive. The median age of the individuals was 9 years (range, 3 to 64 years). A total of 51.7% (31 of 60) of the positive stools were from males and 48.3% (29 of 60) were from females. E. histolytica and E. dispar infection rates were highest in the individuals from 1 to 5 years old (8.5%) and in those above 45 years old (13.7%). E. histolytica infection rates increased slightly with age.
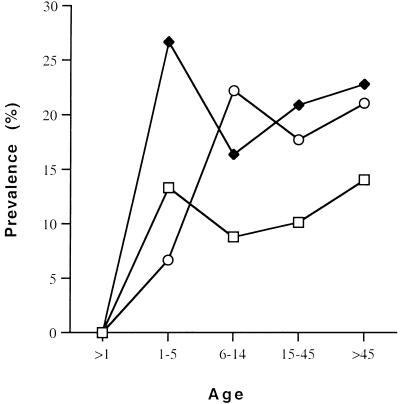
To determine if there was any correlation between intestinal colonization and seropositivity, serum samples were obtained from 401 of the 564 individuals from whom stools were collected. Seropositivity for the GalNAc lectin was determined by the enzyme-linked immunosorbent assay (ELISA) method described by Ravdin et al. (11). Samples whose anti-GalNAc concentrations were 3 standard deviations above the value for the negative control were considered positive. The overall rate of seropositivity for anti-GalNAc immunoglobulin G (IgG) antibodies was 19.7% (79 of 401). Figure 1 displays the age distribution of seropositivity for anti-GalNAc IgG antibodies and stool colonization in these 401 individuals. Antilectin seropositivity rates reached a maximum for those 6 to 14 years of age (22.2%), but the rates remained nearly the same throughout the older individuals. Stool colonization rates followed a similar pattern, although the colonization rates peaked earlier in the individuals aged 1 to 5 years.
FIG. 1.
Age-related rates of anti-GalNAc lectin seropositivity and stool colonization with E. histolytica and E. dispar. Open squares and solid diamonds indicate individuals colonized with E. histolytica and with E. dispar and/or E. histolytica, respectively; open circles indicate individuals that were seropositive for anti-GalNAc lectin antibody.
For these individuals 19.4% (78 of 401) of the stool samples were positive for E. dispar and/or E. histolytica and 10.2% (41 of 401) were positive for E. histolytica. Ten stools were negative in the E. dispar-E. histolytica ELISA but positive in the E. histolytica ELISA. Therefore, we had a total of 88 individuals colonized by E. dispar and/or E. histolytica. Anti-GalNAc lectin antibodies were detected in 22 of the 88 individuals whose stools were positive (25%). Of the 41 individuals with E. histolytica-positive stools only 8 were seropositive (19.5%). The seropositivity rate of individuals not colonized with E. dispar or E. histolytica was 18.2% (57 of 313). The seropositivity rates were not statistically significantly different between individuals colonized or not colonized by E. dispar and/or E. histolytica.
In this sample from a slum-dwelling population in northeastern Brazil approximately 20% was colonized with E. histolytica or E. dispar and 10% was colonized with E. histolytica based on results obtained with ELISA antigen detection kits (7, 8). The prevalence of E. histolytica colonization increased with age from 8.5% in 1- to 5-year-olds to 13.7% in individuals above 45 years old. Increased incidences of amebiasis in individuals more than 45 years old have been reported by others (2, 10, 14).
We found no correlation between seropositivity and stool colonization for E. dispar and/or E. histolytica. The overall rate of seropositivity among all groups for anti-GalNAc lectin IgG antibodies was 19.7%. In our study, only 19.5% of the individuals that were colonized with E. histolytica were seropositive, 25% of those colonized with E. dispar or E. histolytica were seropositive, and 18.2% of individuals whose stools were Entamoeba negative were seropositive. This suggests that individuals may be constantly reinfected throughout their lives and that there may be no immunity, or that there is at least incomplete immunity, to colonization with E. histolytica.
Ours results contrast with the results of previous studies. One, in South Africa, found that 99% of liver abscess patients and 100% of asymptomatic patients had serum antilectin antibodies (11). However, only four asymptomatic individuals were included in that study. In another epidemiological survey in Durban, South Africa, 17 of 17 asymptomatic subjects colonized with E. histolytica pathogenic zymodemes were seropositive for crude antigen (6). Our data also differ from those of a study in India that found that 12.8% of seropositive individuals were colonized whereas 20.3% of seronegative individuals were colonized. Results similar to ours have been reported from Colombia, where asymptomatic cyst passers and noninfected subjects were found to have similar rates of seropositivity (12).
One possible explanation for these conflicting studies is that there may be restricted invasiveness of some strains of E. histolytica and that these strains may consequently fail to elicit a circulating-antibody response. This may be reflected in the low incidence of invasive amebiasis found in the communities mentioned above. Also, since our study was a one-time sampling we do not know how long our subjects were colonized. A controlled prospective study of colonization, the development of seropositivity, and the incidence of reinfection will be useful in defining the nature of immunity in amebiasis.
Acknowledgments
This work was supported by NIH grant AI-32615 and NIH Fogarty Research Collaboration Award TW00509 to B.J.M.
REFERENCES
- 1.Braga L L, Lima A M, Sears C L, Newman R D, Wuhib T, Paiva C A, Guerrant R L, Mann B J. Seroepidemiology of Entamoeba histolytica in a slum in Northeastern Brazil. Am J Trop Med Hyg. 1996;55:693–697. doi: 10.4269/ajtmh.1996.55.693. [DOI] [PubMed] [Google Scholar]
- 2.Bray R S, Harris W G. The epidemiology of infection with Entamoeba histolytica in the Gambia, West Africa. Trans R Soc Trop Med Hyg. 1977;71:401–407. doi: 10.1016/0035-9203(77)90038-4. [DOI] [PubMed] [Google Scholar]
- 3.Choudhuri G, Prakash V, Kumar A, Shahi S K, Sharma M. Protective immunity to Entamoeba histolytica infection in subjects with antiamoebic antibodies residing in a hyperendemic zone. Scand J Infect Dis. 1991;23:771–776. doi: 10.3109/00365549109024306. [DOI] [PubMed] [Google Scholar]
- 4.DeLeon, A. 1970. Prognostico tardio en el absceso hepatica amebiano. Arch. Investig. Med. (Mexico) 1(Suppl.):S205. [PubMed]
- 5.Gathiram V, Jackson T F H G. Frequency distribution of Entamoeba histolytica zymodemes in a rural South African population. Lancet. 1985;i:719–721. doi: 10.1016/s0140-6736(85)91263-2. [DOI] [PubMed] [Google Scholar]
- 6.Gathiram V, Jackson T F H G. A longitudinal study of asymptomatic carriers of pathogenic zymodemes of Entamoeba histolytica. S Afr J Med Sci. 1987;72:669–672. [PubMed] [Google Scholar]
- 7.Haque R, Ali I K M, Akther S, Petri W A., Jr Comparison of PCR, isoenzyme analysis, and antigen detection for diagnosis of Entamoeba histolytica infection. J Clin Microbiol. 1998;36:449–452. doi: 10.1128/jcm.36.2.449-452.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Haque R, Neville L M, Hahn P, Petri W A., Jr Rapid diagnosis of Entamoeba infection by using Entamoeba and Entamoeba histolytica stool antigen detection kits. J Clin Microbiol. 1995;33:2558–2561. doi: 10.1128/jcm.33.10.2558-2561.1995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Irusen E M, Jackson T F, Simjee A E. Asymptomatic intestinal colonization by pathogenic Entamoeba histolytica in amebic liver abscess: prevalence, response to therapy, and pathogenic potential. Clin Infect Dis. 1992;14:889–893. doi: 10.1093/clinids/14.4.889. [DOI] [PubMed] [Google Scholar]
- 10.Oyerinde J P, Alonge A A, Adegbite-Hollist A F, Ogunbi O. The epidemiology of Entamoeba histolytica in a Nigerian urban population. Int J Epidemiol. 1979;8:55–59. doi: 10.1093/ije/8.1.55. [DOI] [PubMed] [Google Scholar]
- 11.Ravdin J I, Jackson T F H G, Petri W A, Jr, Murphy C F, Ungar B L P, Gathiram V, Skilogiannis J, Simjee A E. Association of serum antibodies to adherence lectin with invasive amebiasis and asymptomatic infection with pathogenic Entamoeba histolytica. J Infect Dis. 1990;162:768–772. doi: 10.1093/infdis/162.3.768. [DOI] [PubMed] [Google Scholar]
- 12.Stamm W P, Ashley M J, Bell K. The value of amoebic serology in an area of low endemicity. Trans R Soc Trop Med Hyg. 1976;70:49–53. doi: 10.1016/0035-9203(76)90006-7. [DOI] [PubMed] [Google Scholar]
- 13.Walsh J A. Problems in recognition and diagnosis of amebiasis: estimation of the global magnitude of morbidity and mortality. Rev Infect Dis. 1986;8:228–238. doi: 10.1093/clinids/8.2.228. [DOI] [PubMed] [Google Scholar]
- 14.Wanke C, Butler T, Islam M. Epidemiological and clinical features of invasive amebiasis in Bangladesh: a case-control comparison with other diarrheal diseases and postmortem findings. Am J Trop Med Hyg. 1988;38:335–341. doi: 10.4269/ajtmh.1988.38.335. [DOI] [PubMed] [Google Scholar]
- 15.World Health Organization. The world health report 1995: bridging the gaps. Report of the director-general. Geneva, Switzerland: World Health Organization; 1995. [Google Scholar]