We identified next steps to reduce disparities in endometrial cancer stage by applying a person-centered framework to evidence on disparities in timeliness of care.
Abstract
OBJECTIVE:
We use the person-centered Pathway to Treatment framework to assess the scope of evidence on disparities in endometrial cancer stage at diagnosis. This report is intended to facilitate interventions, research, and advocacy that reduce disparities.
DATA SOURCES:
We completed a structured search of electronic databases: PubMed, EMBASE, Scopus, ClinicalTrials.gov, and Cochrane Central Register of Controlled Trials databases. Included studies were published between January 2000 and 2023 and addressed marginalized population(s) in the United States with the ability to develop endometrial cancer and addressed variable(s) outlined in the Pathway to Treatment.
METHODS OF STUDY SELECTION:
Our database search strategy was designed for sensitivity to identify studies on disparate prolongation of the Pathway to Treatment for endometrial cancer, tallying 2,171. Inclusion criteria were broad, yet only 24 studies addressed this issue. All articles were independently screened by two reviewers.
TABULATION, INTEGRATION, AND RESULTS:
Twenty-four studies were included: 10 on symptom appraisal, five on help seeking, five on diagnosis, and 10 on pretreatment intervals. Quality rankings were heterogeneous, between 3 and 9 (median 7.2) per the Newcastle–Ottawa Scale. We identified three qualitative, two participatory, and two intervention studies. Studies on help seeking predominantly investigate patient-driven delays. When disease factors were controlled for, delays of the pretreatment interval were independently associated with racism toward Black and Hispanic people, less education, lower socioeconomic status, and nonprivate insurance.
CONCLUSIONS:
Evidence gaps on disparities in timeliness of endometrial cancer care reveal emphasis of patient-driven help-seeking delays, reliance on health care–derived databases, underutilization of participatory methods, and a paucity of intervention studies.
SYSTEMATIC REVIEW REGISTRATION:
Given that PROSPERO was not accepting systematic scoping review protocols at the time this study began, this study protocol was shared a priori through Open Science Framework on January 13, 2021 (doi: 10.17605/OSF.IO/V2ZXY), and through peer review publication on April 13, 2021 (doi: https://doi.org/10.1186/s13643-021-01649-x).
Endometrial cancer is the most common gynecologic cancer in the United States, with incidence increasing1,2 as endometrial cancer risk factors, including obesity and older age,3,4 become more prevalent.5 Endometrial cancer survival is strongly associated with stage, grade, and histology.1,4,6–9 Of these, stage is the only modifiable factor, with 5-year mortality for early stage 50% lower than for late-stage disease.1 Advanced endometrial cancer stage is associated with lower median family income,10 lower neighborhood socioeconomics,11 and less insurance coverage.12–15 Disparities in endometrial cancer stage attributable to racism are particularly egregious and well demonstrated10,16–18 and, if resolved, could reduce the 5-year mortality for Black people by 33%.8 Aside from Medicaid expansion, reducing endometrial cancer stage in the United States has seen little progress.13–15
When an issue requires swift action, literature reviews serve crucial roles. They aim to assist researchers in building on (rather than recapitulating) existent evidence, to reduce time to translation and implementation of evidence-based practices, and to reveal the paradigm of a scientific community such that it can be checked against lived experiences. This systematic scoping review synthesizes the available scientific evidence to facilitate research, evidence translation to interventions, and multisectoral advocacy to reduce disparities in stage of endometrial cancer diagnosis. With the foundation of previous literature reviews of disparities in endometrial cancer diagnosis and outcomes,19–21 this is the first review to focus specifically on factors associated with advanced stage and to use a systematic scoping methodology to define evidence gaps.
We conceptualize reducing endometrial cancer disease stage in terms of timeliness of diagnosis and treatment after disease onset. The Walter et al22 Pathway to Treatment model, which elaborates on the Andersen et al23 Model of Total Patient Delay, provides a framework to identify sources of delay in cancer care. The Pathway to Treatment Model is divided into four intervals:
Appraisal interval: time from detection of bodily change(s) to perceiving reason to discuss symptoms with health care professional
Help-seeking interval: time from symptom onset to the first consultation with a care professional
Diagnostic interval: time from the first consultation to diagnosis
Pretreatment interval: time from diagnosis to start of treatment
We hypothesized that we would find studies addressing all four intervals and a paucity of patient-centered research, participatory methods, and interventions. In keeping with the admonition of a critical race theory critique of medical scholarship on endometrial cancer disparities,19 this review’s population–concept–context framework24 was designed to capture factors on the person, health care, and societal levels that mediate inequitable outcomes rather than reifying race as a biological reality. We assumed intersectionality of marginalized identities and experiences in creating interdependent systems of discrimination,25 so we centered racism while also seeking evidence on several other dimensions of discrimination. Given the social, political, and chronologic contexts that shape health outcomes, the review is limited to the United States and to studies published in 2000 or later.
Systematic scoping reviews adhere to guidelines adapted from traditional systematic reviews for a reproducible methodology of identifying evidence gaps in a field of research. We developed the protocol a priori using the PRISMA Extension for Scoping Reviews published in 2018 (Preferred Reporting Items for Scoping Reviews).26 Details on methods can be found in the study protocol, published and registered separately.27 The objective of this study was to identify evidence gaps on disparities in timeliness of the Pathway to Treatment of endometrial cancer to facilitate multisectoral interventions, research, and advocacy that reduce disparities in stage of endometrial cancer diagnosis.
SOURCES
The primary source of literature was a structured search of electronic databases: PubMed, EMBASE, Scopus, ClinicalTrials.gov, and Cochrane Central Register of Controlled Trials databases (Appendix 1: Search Strategy, available online at http://links.lww.com/AOG/D333). A search of Google Scholar constituted a secondary source of material. We did not consider gray literature or literature reviews without meta-analysis.
STUDY SELECTION
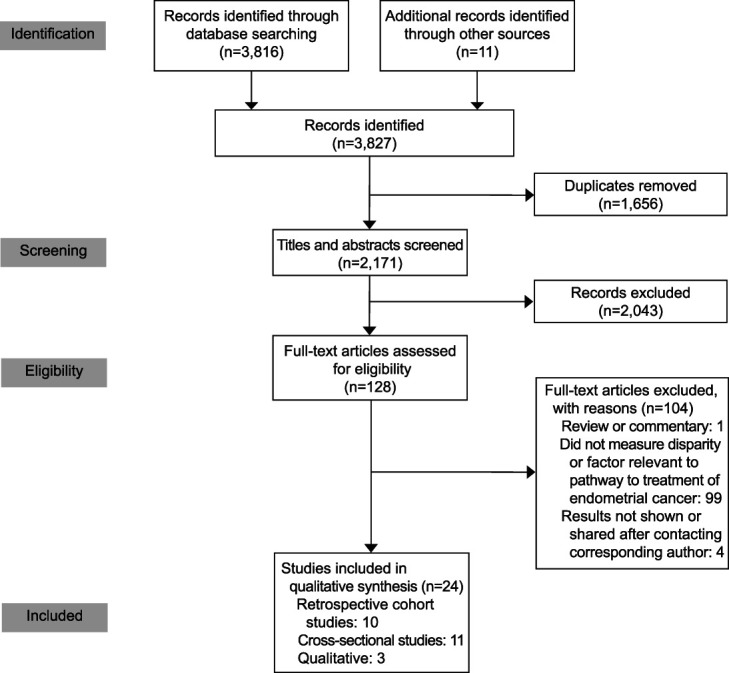
After duplicates were removed, our initial search returned 2,171 records. Two reviewers independently screened all titles and abstracts for eligibility and then the full text of all eligible articles for inclusion. Our selection protocol was designed to include all studies that assessed 1) marginalized population(s) in the United States with the ability to develop endometrial cancer and 2) variables outlined in the Walter et al22 Pathway to Treatment Model as they pertain to endometrial cancer. Studies that measured factors relevant to the endometrial cancer Pathway to Treatment in a population belonging predominantly to a marginalized group were not excluded because of the lack of a comparator group.28–30 One study of all-White participants31 was included because it was designed to serve as a comparator group for another study of all-Black participants.32 Despite our permissive inclusion criteria, 2,043 of 2,171 records were not relevant, reflecting the intended sensitivity of the database search strategy and breadth of variables outlined in the Pathway to Treatment.22 Four authors were contacted for additional data, of whom one responded but with data that did not pertain to the Pathway to Treatment; thus, all four were excluded. The search and screening processes were updated before publication. The PRISMA flow diagram details the study selection process.33 (Fig. 1).
Fig. 1. Flow diagram of references selected for study inclusion based on the PRISMA (Preferred Reporting Items for Systematic Reviews and Meta-analyses) method.
Najor. Timeliness Disparities in Endometrial Cancer Care. Obstet Gynecol 2023.
RESULTS
All studies were observational: 10 were retrospective cohorts, 11 were cross-sectional, and three were qualitative in design. Ten studies pertained to the appraisal interval, five to the help-seeking interval, five to the diagnostic interval, and 10 to the pretreatment interval (Appendix 2, available online at http://links.lww.com/AOG/D333). Results were not conducive to sensitivity testing or meta-analysis because of heterogeneity of outcomes.
Newcastle–Ottawa Scale bias and quality scores ranged from 3 to 9 (9 as maximum score) with a mean of 7.2. Critical Appraisal Skills Program for qualitative studies ranged from 9 to 10 (10 as maximum score). Four studies used overlapping National Cancer Database years.13,34–36 The literature scope reveals bias toward greater representation of the diagnostic and help-seeking intervals and representing hospitals in urban centers. We did not identify funding biases or conflicts of interest.
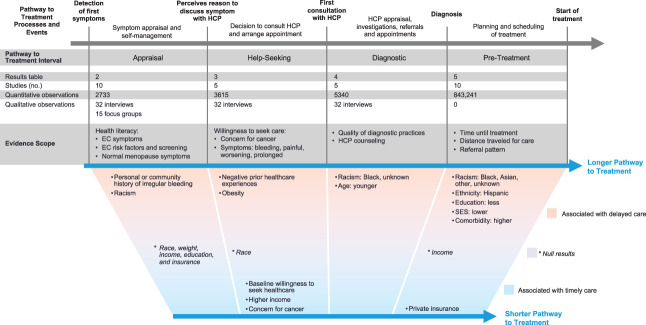
Data produced through blinded duplicate extraction processes were analyzed independently by all authors to decrease the likelihood of ignoring explanations for the observed results. Figure 2 provides a summary of key findings across the Pathway to Treatment. Appendix 2 (http://links.lww.com/AOG/D333) summarizes key findings, with additional data for the appraisal, help-seeking, diagnostic, and help-seeking intervals in Appendices 3–6 (available online at http://links.lww.com/AOG/D333). Heterogeneity of results, rather than study quality, limited qualitative synthesis.
Fig. 2. Evidence scope across the pathway to treatment. HCP, health care professional; EC, endometrial cancer; SES, socioeconomic status.
Najor. Timeliness Disparities in Endometrial Cancer Care. Obstet Gynecol 2023.
Factors relevant to the endometrial cancer appraisal interval, symptom appraisal, and self-management were described by 10 studies that included 2,733 quantitative observations, 15 qualitative observations from interviews, and 32 qualitative observations from focus groups (Appendix 3, http://links.lww.com/AOG/D333). The evidence scope of the appraisal interval focused on the effect of health literacy and past experiences on appraising endometrial cancer symptoms. Sociodemographics compared in these studies included race, ethnicity, body mass index (BMI, calculated as weight in kilograms divided by height in meters squared), education, income, and insurance. Shared themes across these studies included the perception of gynecologic cancer as an important health concern and the desire to access reliable information, including from health care professionals.28,32,37 Four studies included participants with endometrial cancer.31,37
Study populations both with and without endometrial cancer were highly aware that endometrial cancer symptoms include abnormal or postmenopausal bleeding (72.7–95.4%)29,32,38—health literacy that could facilitate perceiving the need to discuss symptoms with a health care professional. The Doll et al31 qualitative studies of the retrospective prediagnostic experiences of women with endometrial cancer found that difficulty defining abnormal menopause symptoms (partly because of past personal and familial gynecologic health experiences) presented barriers to symptom appraisal for Black women,37 whereas White women reported having more information about menopause but also experienced longer time to become concerned after personal history of irregular bleeding.
Awareness of obesity as a risk factor for endometrial cancer ranged from 38% to 44.4% in studies of endometrial cancer survivors30,39 and from 20.7% to 47.9% in studies without endometrial cancer survivors.28,40,41 Multiple studies used univariate analysis and observed that participant BMI, education, income, insurance, race, ethnicity, comorbidity, and age were not significantly associated with awareness of obesity as a risk factor for endometrial cancer.28,39–41 Awareness that age and menopause are risk factors for endometrial cancer was low, ranging from 7.4% (2.9/39)32 to 53.7% (268/499).38 Multiple study populations struggled to differentiate risk factors for endometrial cancer compared with cervical cancer.29,38,39 Access to care among those willing to seek care was not described.
Knowledge of screening options for individuals with genetic predispositions to endometrial cancer was low in multiple studies, ranging from 29.2% in a population recruited from an organization that serves Hispanic people (n=39)32 to 48.2% in a population recruited through multimodal community outreach (n=499).38 Neither study population included people with endometrial cancer. Awareness that cervical cancer screening does not assess risk for endometrial cancer was higher, 74.6% (of 499) in one study38 and “most” participants of a qualitative study of 17 focus groups.29
Two interventions were studied to improve health literacy regarding gynecologic cancer symptoms, risk factors, and screening.32,38 Both reported significant improvements in knowledge scores from before to after the education session, although neither was tested for real-life application.
No studies estimated the length of the appraisal interval, obfuscating the magnitude of effect that could be achieved by focusing interventions on this interval, and where, how, and for whom delays are the worst. No quantitative studies were adequately powered for multivariate analysis. No studies assessed appraisal of nonendometrioid endometrial cancer symptoms.
Factors relevant to the endometrial cancer help-seeking interval, seeking care for symptoms, were described by five studies that included 3,615 quantitative observations and 32 qualitative observations from interviews (Appendix 4, http://links.lww.com/AOG/D333). The evidence scope included willingness to seek care for endometrial cancer symptoms in populations with31,37 and without endometrial cancer,32,42 with a focus on disparities by race and income. Only two studies included populations with endometrial cancer.
Willingness to seek care for symptoms associated with gynecologic cancer in study populations without endometrial cancer or endometrial cancer symptoms ranged from 5.3%32 to more than 50%.42 A larger study (n=2,991) found that income, but not (self-identified) race, significantly predicted willingness to seek care, after adjustment for concern about cancer, age, gynecologic cancer, and willingness to seek care for other conditions.42 In multivariate analysis, race was not associated with willingness to seek care for vaginal bleeding in menopausal respondents, and Black respondents reported more intention to seek care than White respondents for several gynecologic cancer symptoms.42 This study also reported that higher income was associated with willingness to seek care, but income differences by race were not described.
The studies that included endometrial cancer survivors reported that the decision to seek medical care often occurred after a personally set threshold for symptom duration or severity37 and may be prolonged for overweight women who have experienced body shaming from health care professionals.31 Similarly, a survey of self-identified women who had not had endometrial cancer reported symptom duration and unpleasantness (pain) as a significant motivator for seeking help.42 These studies found that concern for cancer was associated with perceiving a reason to discuss symptoms with a health care professional, connecting endometrial cancer symptom knowledge with the transition from the appraisal to help-seeking interval.
Although one study assessed the association between income and willingness to seek care for endometrial cancer symptoms42 and qualitative studies describe how past experiences of discrimination and racism at the hands of health care professionals can discourage help seeking,31,37 the help-seeking interval has not been adequately addressed by studies on health care access, such as the effect of insurance, transportation, rurality, immigration status, English proficiency, housing stability, and incarceration.
The quantitative evidence reviewed was untested for associations with endometrial cancer outcomes, weakening findings to the degree that recognizing the need to seek medical attention on a survey would translate to real-world endometrial cancer symptom appraisal and help seeking. Finally, as with the appraisal interval, no studies estimated the length of the help-seeking interval or prevalence of delays.
Factors relevant to the endometrial cancer diagnostic interval, health care professional appraisal and the diagnostic process, were described by 4 studies that included 5,340 quantitative observations and 32 qualitative observations from interviews (Appendix 5, http://links.lww.com/AOG/D333). Evidence was created largely by retrospective cohorts of endometrial cancer cases pulled from health care databases. The evidence scope included evaluation quality and health care professional communication. Sociodemographics compared in these studies included race and age.
A national retrospective cohort (n=4,891) demonstrated that Black people had a significantly lower odds of receiving a guideline-concordant evaluation for endometrial cancer than White people (adjusted odds ratio [aOR] 0.63, 95% CI 0.51–0.77), which was significantly associated with advanced stage (aOR 1.8, 95% CI 1.4–2.2 for Black people specifically).43 Overall documentation of bleeding status was 52%,43 similar to a study of 305 encounters for abnormal uterine bleeding (9% postmenopausal) across four Veterans Affairs hospitals that reported documentation of current bleeding status in 54% of encounters.44 The proportion of Black participants was similar in these two studies, but the Veterans Affairs population was not analyzed for differences by race.44 Interviews with endometrial cancer survivors revealed a theme of frustration that health care professionals offered vague and evasive explanations for recommending endometrial cancer testing37 and false reassurances based on inadequate evaluations.31
Evidence gaps surrounding the finding that Black people are less likely to have bleeding patterns documented or diagnostic procedures ordered include trends by geography, practice setting, health care professional specialty or training, imaging availability, insurance status, and endometrial cancer histologic type. Additional gaps include rates of (and reasons for) loss to follow-up, professionals not recognizing endometrial cancer risk, and professionals not offering diagnostic procedures. Investigating a connection between diagnostic or counseling practices and timelines of the diagnostic interval would further support a causal relationship between these factors and stage at diagnosis.
In instances in which postmenopausal bleeding has not prompted a reason to seek medical care, health care encounters can lead to endometrial cancer diagnosis if the health care professional notes bleeding or other incidental findings. The effect of having regular contact with a primary health care professional was not studied.
Factors relevant to the endometrial cancer pretreatment interval, the time between diagnosis and treatment, were described by 10 studies of the pretreatment interval that included a total of 843,241 quantitative observations (Appendix 6, Tables 6.1–6.3, http://links.lww.com/AOG/D333). These were not 843,241 unique observations; four studies sampled overlapping National Cancer Database years.13,34–36 The evidence scope focused on time until treatment, distance traveled for care, and referral pattern. Sociodemographics compared in these studies included race, ethnicity, insurance status, income, and socioeconomic status. Evidence was created largely by retrospective cohorts of endometrial cancer cases pulled from health care databases. Each study defined delays differently, inhibiting meta-analysis and results comparisons.13,34,35,45,46
Insurance status was independently associated with endometrial cancer pretreatment interval delays after adjustment for endometrial cancer histology, race, education, and income. The risk of longer median time from diagnosis to surgery was 10–20% higher if the individual had no insurance, followed by about 10–15% higher for those with government insurance, compared with individuals with private insurance (which had a median 25-day wait).34 Delays were defined differently in other studies, with the likelihood of waiting more than 6 weeks for surgery 40% higher for individuals with Medicaid insurance or no insurance than for those with other insurances,35 and the likelihood of waiting more than 30 days for surgery was nonsignificantly higher in states without Medicaid expansion.13 These trends persisted for time to nonsurgical treatment.13,45,46 Longer pretreatment interval was also associated with greater medical comorbidity,34 lower educational attainment,34,35 and living in a neighborhood with low socioeconomic status (aOR 1.93, 95% CI 1.61–2.31).46
Race was significantly associated with pretreatment delays: Median wait time for surgery was 3–5 days longer for Black people (adjusted incident rate ratio 1.13 for type 1 endometrial cancer, 1.11 for type 2 endometrial cancer) compared with White people, controlling for insurance and other factors.34 This study reported that those with race other than Black or White also had a longer pretreatment interval than White people.34 The likelihood of waiting more than 6 weeks for surgery was 30–40% greater for non-Hispanic Black, 20–40% greater for Hispanic, and marginally greater for Asian people (compared with White people, all comparisons statistically significant).35 The likelihood of waiting more than 4 compared with 2–4 weeks for treatment was 10–50% greater for Hispanic and 15–70% greater for non-Hispanic Black people (compared with White, all comparisons statistically significant) in another study.46 Those with unknown or other race were similar to the White group in one study35 but experienced the longest delays in other studies.34,45
The evidence reviewed did not support the hypothesis that differences in treatment hospitals mediated the effects of racism on pretreatment interval timeliness. A large National Cancer Database cohort study (n=271,186) also found that Black people had a greater likelihood of treatment at medium-high– and high-volume centers (P<.001) and that race remained an independent predictor of mortality even when adjusted for hospital case volume.36 Similarly, a national SEER (Surveillance, Epidemiology, and End Results) cohort study (n=12,307) found that Black race was associated with treatment at hospitals with high endometrial cancer surgical volume, which was associated with having a gynecologic oncologist as the treating surgeon.47 However, this study did not reproduce the association between hospital characteristics and survival.47 A National Cancer Database cohort reported that median time until surgery was marginally shorter at community hospitals and Integrated Network Cancer Program or other centers than academic centers.34 Important regional differences may be hidden in national cohorts, as suggested by a North Carolina cohort (n=2,053) that found no significant association between referral pattern and race or ethnicity in multivariate analysis but did find that aggressive histology, later diagnostic year, and having any private insurance (compared with Medicaid insurance) predicted referral to high-volume centers.48
A national retrospective cohort (n=284,499) found that the likelihood of waiting longer for surgery was marginally higher for those who traveled more than 25 miles and about 10% higher for those who traveled between 51 and 100 miles compared with those who traveled less than 25 miles (average 26 days).34 Regional studies found differing associations between distance traveled and barriers to care. A cross-sectional study of 1,532 surgeries for endometrial cancer in Arizona found that traveling more than 50 miles for care was much more likely for individuals with government insurance (comparator: private; Arizona Health Care Cost Containment System or Medicaid: aOR 3.41, 95% CI 1.89–6.15; Medicare: aOR 2.07, 95% CI 1.38–3.13) and, after controlling for facility volume, much more likely for Hispanic (aOR 2.73, 95% CI 1.70–4.32) and Native American (aOR 8.6, 95% CI 3.44–21.52) compared with White people.49 A New York cohort saw the greatest distance traveled for those with private insurance (P<.001); that distance increased most over the study period for those with private insurance (31.8%, 95% CI 26.2–37.4) and for White people (4.2 miles, 95% CI 3.4–5.0, P<.001), with nonsignificant changes for Hispanic and Black people.50 Likewise, a Maryland cohort study reported that, after rurality was incorporated, White people were up to two times more likely to travel more than 50 miles for endometrial cancer surgery, and the effect of rurality increased when accounting for race.51
The association of distance traveled for care with race and insurance status varied between regional studies. Although the geography of these trends could reflect disinvesting in communities of color50,51 and barriers to health care access for rural communities of color,49 this has not been directly studied, and no relevant interventions were identified.
Black, Asian, and other or unknown race; less insurance coverage; and lower socioeconomic status were associated with longer time from diagnosis until treatment13,34,35,45,46 and more advanced stage at diagnosis.10–18 However, we identified no studies that address how these factors create community-level barriers to accessing timely treatment. Studies are needed to guide intervention design.
DISCUSSION
Mapping evidence gaps elucidated future studies with the potential to address disparities in stage of diagnosis of endometrial cancer. We designed our protocol to capture intervention studies yet only found two,32,38 both of which addressed knowledge about endometrial cancer. Defining clinically significant delays and the degree to which the Pathway to Treatment intervals contribute to total delay is an important next step to determine needs assessments and intervention goals.
The scope of literature on the appraisal and help-seeking intervals disproportionately investigates patient-driven delays, outlining the neoliberal perspective that individuals are ultimately responsible for their health outcomes.52 Stage of endometrial cancer is associated with access to primary care53 and insurance,12,14,15 pointing toward expanded health care access as a means to shorten, or even bypass, the appraisal and help-seeking intervals. The role of infrastructure, politics, racism, and other “isms” in creating conditions for poor endometrial cancer outcomes warrants further study. For example, no studies on transportation infrastructure were identified, even within studies on distance traveled for endometrial cancer treatment.
Evidence regarding the diagnostic interval showed that inferior diagnostic evaluations were provided to Black people nationally, which was associated with later endometrial cancer stage. Underutilization of endometrial sampling significantly contributes to guideline discordance.43,44 Possible explanations that warrant investigation include discrimination, higher rates of loss to follow-up from barriers to accessing health care, geographic differences in rates of guideline concordance, and higher prevalence of leiomyomas, making ultrasonograms less sensitive.54
Another factor possibly contributing to diagnostic delays and guideline discordance is whether health care professionals disclose concern for cancer.37 The (knowledge to have) concern for cancer was significant across the appraisal, help-seeking, and diagnostic intervals and was one of the only factors identified that could plausibly reduce time to diagnosis,29,32,37,38,42 and participants of multiple studies stated their desire to know more about gynecologic cancer, particularly from health care professionals.28,32,37 Not disclosing when tests are for cancer attenuates personal agency to advocate for care and may encourage some to decline care.55
Future studies could help focus interventions by further characterizing populations at risk of Pathway to Treatment delays with an intersectional lens.25 For example, sexual orientation may also contribute to disparate delays characterized by interconnected social identities25; self-identified lesbians have an increased prevalence of chronic disease and risk factors for endometrial cancer, including nulliparity and obesity,56–59 as well as less contact with the health care system, particularly with respect to gynecologic health screening.60 The greater likelihood that the gynecologic concerns and pain of people of color are not addressed by their health care professionals61–63 significantly intersects with other identities25 and warrants further study as a mechanism of delay.
Future studies should use appropriate methods for measuring and analyzing racism. Not only is race a rough proxy for racism, but the classification of race was imprecise in that most studies did not specify how race was assigned, some studies excluded all participants who were neither White nor Black,36,39,42,43,47 and others grouped all non-White participants together.13,48 This imprecision obfuscates the experiences of at-risk populations; this review found that those with other or unknown race had significantly different outcomes compared with White comparator groups.34,36,50 Analytical design can perpetuate gaps in data representation. Asian people were explicitly represented in 14 studies, five of which performed multivariate analysis, but only two included Asian people in multivariate analyses. Federal policies also create data representation gaps, as seen with Arab Americans, a large population subjected to widespread discrimination and significant cancer epidemiologic differences from non-Hispanic White people,64,65 whose health outcomes are invisible because of a lack of federal recognition.66–68 The causal relationship between data representation and health outcomes is not ascertainable from endometrial cancer literature but is often bidirectional.69
Health care–generated data are inadequate to disentangle the effects of racism and other institutions on the endometrial cancer Pathway to Treatment. Diagnostic and billing codes only roughly represent care provided,68 and chart reviews offer information from the perspective of health care professionals or systems with limited information on social determinants of health and on the appraisal and help-seeking intervals. Few studies included,29 let alone centered,37 the priorities and voices of diverse people with endometrial cancer. It is paramount that communities of diverse endometrial cancer survivors should drive efforts to understand the Pathway to Treatment of endometrial cancer.
This review has several strengths. Adherence to best practice standards for systematic scoping reviews supports the reliability and reproducibility of our findings.26,70 The Pathway to Treatment theory-driven methodology improves useability and person-centeredness of the evidence. The qualitative and geographically regional quantitative studies add important context to quantitative evidence.
The Pathway to Treatment model is limited by the assumed linear progression from the detection of first body symptoms to the start of treatment. In addition, the evidence evaluated for this review could not be exhaustively represented here, so themes discussed were selected on the basis of our assessment of clinical significance. Finally, we acknowledge the power imbalance (and, therefore, unmeasured content bias) in academic knowledge creation.
In conclusion, qualitative studies of endometrial cancer survivors suggest that personal and community health and health care experiences may affect symptom appraisal and the process of deciding to seek care. No studies directly assessed the effect of health care access on the help-seeking or diagnostic intervals. Delays of the pretreatment interval were associated with later endometrial cancer stage and with several dimensions of discrimination, including racism (measured in proxy by race). However, no studies assessed the prevalence of delays in the appraisal, help-seeking, and diagnostic intervals. Few studies used person-centering or participatory methods. Two intervention studies were identified, both of which targeted health care literacy.
Footnotes
This review did not benefit from dedicated funding apart from the institutional support for each authors' time. Funding sources for the studies included in this review include the CDC, Robert Wood Johnson Foundation, National Comprehensive Center Network Foundation, Veterans Affairs Services Research & Development, National Comprehensive Cancer Network Foundation, and National Cancer Institute.
Financial Disclosure Jamie N. Bakkum-Gamez disclosed that money was paid to their institution from the following: NIH/NCI—P30CA15083 (PI: Willman); V Foundation grant—T2016-001-03 (PI: Bakkum-Gamez); and NIH/NCI—CA136393 (PI: Kaufmann). They also disclosed the following: Exact Sciences–contracted research agreement between Mayo Clinic and Exact 5. They are listed as an inventor on IP that is licensed by both Exact and Mayo Clinic and received no royalties to date. They are a member of the Foundation for Women's Cancer Board of Directors. Andrew M. Kaunitz received payment from Myovant. He authored chapters in the e-textbook for UpToDate on Contraception, Abnormal Uterine Bleeding, Dysmenorrhea, and Menstrual Suppression. Avonne E. Connor has been a grant reviewer for the American Cancer Society. Christopher C. Destephano received payment from Abiomed, purchased by Johnson and Johnson, stock therefore became cash. Transferred as gift from parent. Family is an important influence on my work but the gift is unrelated to the manuscript submitted. Mark Sherman receives support from Exact Sciences for cancer detection research. The other authors did not report any potential conflicts of interest.
Presented at the American College of Obstetricians and Gynecologists’ 70th Annual Clinical and Scientific Meeting, May 6–8, 2022, San Diego, California.
The authors thank the Sioux Nation because much of the study occurred on their occupied land; Leslie Hassett, MLS, for assistance creating the literature search strategy; Dyda Dao, MD, MSc, for assistance with study selection; Kemi M. Doll, MD, MCSR, for consultation during the conceptualization phase; and Mayo Clinic media services, for assistance with infographic production.
The authors' Positionality Statement is available online at http://links.lww.com/AOG/D332.
Each author has confirmed compliance with the journal's requirements for authorship.
Peer reviews and author correspondence are available at http://links.lww.com/AOG/D334.
Figure.

No available caption
REFERENCES
- 1.Cote ML, Ruterbusch JJ, Olson SH, Lu K, Ali-Fehmi R. The growing burden of endometrial cancer: a major racial disparity affecting Black women. Cancer Epidemiol Biomarkers Prev 2015;24:1407–15. doi: 10.1158/1055-9965.Epi-15-0316 [DOI] [PubMed] [Google Scholar]
- 2.Rahib L, Smith BD, Aizenberg R, Rosenzweig AB, Fleshman JM, Matrisian LM. Projecting cancer incidence and deaths to 2030: the unexpected burden of thyroid, liver, and pancreas cancers in the United States. Cancer Res 2014;74:2913–21. doi: 10.1158/0008-5472.Can-14-0155 [DOI] [PubMed] [Google Scholar]
- 3.Reeves KW, Carter GC, Rodabough RJ, Lane D, McNeeley SG, Stefanick ML, et al. Obesity in relation to endometrial cancer risk and disease characteristics in the Women's Health Initiative. Gynecol Oncol 2011;121:376–82. doi: 10.1016/j.ygyno.2011.01.027 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Amant F, Moerman P, Neven P, Timmerman D, Van Limbergen E, Vergote I. Endometrial cancer. Lancet 2005;366:491–505. doi: 10.1016/s0140-6736(05)67063-8 [DOI] [PubMed] [Google Scholar]
- 5.Wang Y, Beydoun MA. The obesity epidemic in the United States–gender, age, socioeconomic, racial/ethnic, and geographic characteristics: a systematic review and meta-regression analysis. Epidemiol Rev 2007;29:6–28. doi: 10.1093/epirev/mxm007 [DOI] [PubMed] [Google Scholar]
- 6.Gal D, Recio FO, Zamurovic D. The new International Federation of Gynecology and Obstetrics surgical staging and survival rates in early endometrial carcinoma. Cancer 1992;69:200–2. doi: [DOI] [PubMed] [Google Scholar]
- 7.Collins Y, Holcomb K, Chapman-Davis E, Khabele D, Farley JH. Gynecologic cancer disparities: a report from the Health Disparities Taskforce of the Society of Gynecologic Oncology. Gynecol Oncol 2014;133:353–61. doi: 10.1016/j.ygyno.2013.12.039 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Doll KM, Winn AN, Goff BA. Untangling the Black-White mortality gap in endometrial cancer: a cohort simulation. Am J Obstet Gynecol 2017;216:324–5. doi: 10.1016/j.ajog.2016.12.023 [DOI] [PubMed] [Google Scholar]
- 9.Smotkin D, Nevadunsky NS, Harris K, Einstein MH, Yu Y, Goldberg GL. Histopathologic differences account for racial disparity in uterine cancer survival. Gynecol Oncol 2012;127:616–9. doi: 10.1016/j.ygyno.2012.08.025 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Madison T, Schottenfeld D, James SA, Schwartz AG, Gruber SB. Endometrial cancer: socioeconomic status and racial/ethnic differences in stage at diagnosis, treatment, and survival. Am J Public Health 2004;94:2104–11. doi: 10.2105/ajph.94.12.2104 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Von Behren J, Abrahao R, Goldberg D, Gomez SL, Setiawan VW, Cheng I. The influence of neighborhood socioeconomic status and ethnic enclave on endometrial cancer mortality among Hispanics and Asian Americans/Pacific Islanders in California. Cancer Causes Control 2018;29:875–81. doi: 10.1007/s10552-018-1063-7 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Doll KM, Meng K, Basch EM, Gehrig PA, Brewster WR, Meyer AM. Gynecologic cancer outcomes in the elderly poor: a population-based study. Cancer 2015;121:3591–9. doi: 10.1002/cncr.29541 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Albright BB, Nasioudis D, Craig S, Moss HA, Latif NA, Ko EM, et al. Impact of Medicaid expansion on women with gynecologic cancer: a difference-in-difference analysis. Am J Obstet Gynecol 2021;224:195.e1–17. doi: 10.1016/j.ajog.2020.08.007 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Barrington DA, Sinnott JA, Calo C, Cohn DE, Cosgrove CM, Felix AS. Where you live matters: a National Cancer Database study of Medicaid expansion and endometrial cancer outcomes. Gynecol Oncol 2020;158:407–14. doi: 10.1016/j.ygyno.2020.05.018 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Smith AJB, Fader AN. Effects of the Affordable Care Act on young women with gynecologic cancers. Obstet Gynecol 2018;131:966–76. doi: 10.1097/aog.0000000000002592 [DOI] [PubMed] [Google Scholar]
- 16.Bregar AJ, Alejandro Rauh-Hain J, Spencer R, Clemmer JT, Schorge JO, Rice LW, et al. Disparities in receipt of care for high-grade endometrial cancer: a National Cancer Data Base analysis. Gynecol Oncol 2017;145:114–21. doi: 10.1016/j.ygyno.2017.01.024 [DOI] [PubMed] [Google Scholar]
- 17.Wright JD, Fiorelli J, Schiff PB, Burke WM, Kansler AL, Cohen CJ, et al. Racial disparities for uterine corpus tumors: changes in clinical characteristics and treatment over time. Cancer 2009;115:1276–85. doi: 10.1002/cncr.24160 [DOI] [PubMed] [Google Scholar]
- 18.Sud S, Holmes J, Eblan M, Chen R, Jones E. Clinical characteristics associated with racial disparities in endometrial cancer outcomes: a Surveillance, Epidemiology and End Results analysis. Gynecol Oncol 2018;148:349–56. doi: 10.1016/j.ygyno.2017.12.021 [DOI] [PubMed] [Google Scholar]
- 19.Doll KM, Snyder CR, Ford CL. Endometrial cancer disparities: a race-conscious critique of the literature. Am J Obstet Gynecol 2018;218:474–82.e2. doi: 10.1016/j.ajog.2017.09.016 [DOI] [PubMed] [Google Scholar]
- 20.Fader AN, Habermann EB, Hanson KT, Lin JF, Grendys EC, Dowdy SC. Disparities in treatment and survival for women with endometrial cancer: a contemporary national cancer database registry analysis. Gynecol Oncol 2016;143:98–104. doi: 10.1016/j.ygyno.2016.07.107 [DOI] [PubMed] [Google Scholar]
- 21.Pergialiotis V, Haidopoulos D, Tzortzis AS, Antonopoulos I, Thomakos N, Rodolakis A. The impact of waiting intervals on survival outcomes of patients with endometrial cancer: a systematic review of the literature. Eur J Obstet Gynecol Reprod Biol 2020;246:1–6. doi: 10.1016/j.ejogrb.2020.01.004 [DOI] [PubMed] [Google Scholar]
- 22.Walter F, Webster A, Scott S, Emery J. The Andersen model of total patient delay: a systematic review of its application in cancer diagnosis. J Health Serv Res Pol 2012;17:110–8. doi: 10.1258/jhsrp.2011.010113 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Andersen BL, Cacioppo JT, Roberts DC. Delay in seeking a cancer diagnosis: delay stages and psychophysiological comparison processes. Br J Soc Psychol 1995;34:33–52. doi: 10.1111/j.2044-8309.1995.tb01047.x [DOI] [PubMed] [Google Scholar]
- 24.Joanna Briggs Institute reviewers' manual: 2015 edition/supplement. The Joanna Briggs Institute; 2015. [Google Scholar]
- 25.Carbado DW, Crenshaw KW, Mays VM, Tomlinson B. Intersectionality: mapping the movements of a theory. Du Bois Rev 2013;10:303–12. doi: 10.1017/s1742058x13000349 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Tricco AC, Lillie E, Zarin W, O'Brien KK, Colquhoun H, Levac D, et al. PRISMA Extension for Scoping Reviews (PRISMA-ScR): checklist and explanation. Ann Intern Med 2018;169:467–73. doi: 10.7326/m18-0850 [DOI] [PubMed] [Google Scholar]
- 27.Najor AJ, Dao D, Bakkum-Gamez JN, Sherman ME, Connor AE, Destephano CC. Disparities and interventions in the timeliness of endometrial cancer diagnosis and treatment in the United States: a scoping review protocol. Syst Rev 2021;10:107. doi: 10.1186/s13643-021-01649-x [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Washington CR, Haggerty A, Ronner W, Neff PM, Ko EM. Knowledge of endometrial cancer risk factors in a general gynecologic population. Gynecol Oncol 2020;158:137–42. doi: 10.1016/j.ygyno.2020.03.032 [DOI] [PubMed] [Google Scholar]
- 29.Cooper CP, Polonec L, Gelb CA. Women's knowledge and awareness of gynecologic cancer: a multisite qualitative study in the United States. J Womens Health 2011;20:517–24. doi: 10.1089/jwh.2011.2765 [DOI] [PubMed] [Google Scholar]
- 30.Connor EV, Raker CA, Clark MA, Stuckey AR. Obesity risk awareness in women with endometrial cancer. Arch Gynecol Obstet 2017;295:965–9. doi: 10.1007/s00404-017-4301-4 [DOI] [PubMed] [Google Scholar]
- 31.Doll KM, Nguyen A, Alson JG. A conceptual model of vulnerability to care delay among women at risk for endometrial cancer. Gynecol Oncol 2022;164:318–24. doi: 10.1016/j.ygyno.2021.11.010 [DOI] [PubMed] [Google Scholar]
- 32.Puckett M, Townsend J, Patterson JR, Shaw D, Wasilewski Y, Stewart SL. Using inside knowledge campaign materials to improve gynecologic cancer knowledge in underserved women. J Womens Health 2019;28:1185–92. doi: 10.1089/jwh.2019.7968 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Moher D, Shamseer L, Clarke M, Ghersi D, Liberati A, Petticrew M, et al. Preferred Reporting Items for Systematic Review and Meta-Analysis Protocols (PRISMA-P) 2015 statement. Syst Rev 2015;4:1. doi: 10.1186/2046-4053-4-1 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.AlHilli MM, Elson P, Rybicki L, Khorana AA, Rose PG. Time to surgery and its impact on survival in patients with endometrial cancer: a national cancer database study. Gynecol Oncol 2019;153:511–6. doi: 10.1016/j.ygyno.2019.03.244 [DOI] [PubMed] [Google Scholar]
- 35.Strohl AE, Feinglass JM, Shahabi S, Simon MA. Surgical wait time: a new health indicator in women with endometrial cancer. Gynecol Oncol 2016;141:511–5. doi: 10.1016/j.ygyno.2016.04.014 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Buskwofie A, Huang Y, Tergas AI, Hou JY, Ananth CV, Neugut AI, et al. Impact of hospital volume on racial disparities and outcomes for endometrial cancer. Gynecol Oncol 2018;149:329–36. doi: 10.1016/j.ygyno.2018.02.019 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Doll KM, Hempstead B, Alson J, Sage L, Lavallee D. Assessment of prediagnostic experiences of Black women with endometrial cancer in the United States. JAMA Netw Open 2020;3:e204954. doi: 10.1001/jamanetworkopen.2020.4954 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Novinson D, Puckett M, Townsend J, Tortolero-Luna G, Patterson JR, Gelb CA, et al. Increasing awareness of uterine cancer risks and symptoms by using campaign materials from inside knowledge: get the facts about gynecologic cancer. J Cancer Educ 2019;34:1190–7. doi: 10.1007/s13187-018-1427-4 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Sekhon S, Massad LS, Hagemann AR, Dick R, Leon A, Zamorano AS, et al. Patients with endometrial cancer continue to lack understanding of their risks for cancer. Gynecol Oncol Rep 2019;29:106–10. doi: 10.1016/j.gore.2019.07.013 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Cardozo ER, Neff LM, Brocks ME, Ekpo GE, Dune TJ, Barnes RB, et al. Infertility patients' knowledge of the effects of obesity on reproductive health outcomes. Am J Obstet Gynecol 2012;207:509.e1–10. doi: 10.1016/j.ajog.2012.08.020 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Soliman PT, Bassett RL, Jr, Wilson EB, Boyd-Rogers S, Schmeler KM, Milam MR, et al. Limited public knowledge of obesity and endometrial cancer risk: what women know. Obstet Gynecol 2008;112:835–42. doi: 10.1097/AOG.0b013e318187d022 [DOI] [PubMed] [Google Scholar]
- 42.Trivers KF, Rodriguez JL, Hawkins NA, Cooper CP, Polonec L, Gelb CA. Intention to seek care for symptoms associated with gynecologic cancers, HealthStyles survey, 2008. Prev Chronic Dis 2011;8:A144. [PMC free article] [PubMed] [Google Scholar]
- 43.Doll KM, Khor S, Odem-Davis K, He H, Wolff EM, Flum DR, et al. Role of bleeding recognition and evaluation in Black-White disparities in endometrial cancer. Am J Obstet Gynecol 2018;219:593.e1–14. doi: 10.1016/j.ajog.2018.09.040 [DOI] [PubMed] [Google Scholar]
- 44.Cordasco KM, Yuan AH, Danz MJ, Farmer MM, Jackson L, Yee EF, et al. Guideline adherence of Veterans Health Administration primary care for abnormal uterine bleeding. Womens Health Issues 2019;29:144–52. doi: 10.1016/j.whi.2018.12.004 [DOI] [PubMed] [Google Scholar]
- 45.Dolly D, Mihai A, Rimel BJ, Fogg L, Rotmensch J, Guirguis A, et al. A delay from diagnosis to treatment is associated with a decreased overall survival for patients with endometrial cancer. Front Oncol 2016;6:31. doi: 10.3389/fonc.2016.00031 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Baskovic M, Lichtensztajn DY, Nguyen T, Karam A, English DP. Racial disparities in outcomes for high-grade uterine cancer: a California cancer registry study. Cancer Med 2018;7:4485–95. doi: 10.1002/cam4.1742 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Armstrong K, Randall TC, Polsky D, Moye E, Silber JH. Racial differences in surgeons and hospitals for endometrial cancer treatment. Med Care 2011;49:207–14. doi: 10.1097/MLR.0b013e3182019123 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Doll KM, Meng K, Gehrig PA, Brewster WR, Meyer AM. Referral patterns between high- and low-volume centers and associations with uterine cancer treatment and survival: a population-based study of Medicare, Medicaid, and privately insured women. Am J Obstet Gynecol 2016;215:447.e1–13. doi: 10.1016/j.ajog.2016.04.020 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Benjamin I, Dalton H, Qiu Y, Cayco L, Johnson WG, Balducci J. Endometrial cancer surgery in Arizona: a statewide analysis of access to care. Gynecol Oncol 2011;121:83–6. doi: 10.1016/j.ygyno.2010.11.028 [DOI] [PubMed] [Google Scholar]
- 50.Knisely A, Huang Y, Melamed A, Tergas AI, St. Clair CM, Hou JY, et al. Effect of regionalization of endometrial cancer care on site of care and patient travel. Am J Obstet Gynecol 2020;222:58.e1–10. doi: 10.1016/j.ajog.2019.07.026 [DOI] [PubMed] [Google Scholar]
- 51.Gunderson CC, Tergas AI, Fleury AC, Diaz-Montes TP, Giuntoli RL. Primary uterine cancer in Maryland impact of distance on access to surgical care at high-volume hospitals. Int J Gynecol Cancer 2013;23:1244–51. doi: 10.1097/IGC.0b013e31829ea002 [DOI] [PubMed] [Google Scholar]
- 52.Gibson AF, Lee C, Crabb S. “Take ownership of your condition”: Australian women's health and risk talk in relation to their experiences of breast cancer. Health Risk Soc 2015;17:132–48. doi: 10.1080/13698575.2015.1032215 [DOI] [Google Scholar]
- 53.Alcalá HE, Roby DH, Grande DT, McKenna RM, Ortega AN. Insurance type and access to health care providers and appointments under the Affordable Care Act. Med Care 2018;56:186–92. doi: 10.1097/mlr.0000000000000855 [DOI] [PubMed] [Google Scholar]
- 54.Romano SS, Doll KM. The impact of fibroids and histologic subtype on the performance of US clinical guidelines for the diagnosis of endometrial cancer among Black women. Ethn Dis 2020;30:543–52. doi: 10.18865/ed.30.4.543 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Vandborg MP, Christensen RD, Kragstrup J, Edwards K, Vedsted P, Hansen DG, et al. Reasons for diagnostic delay in gynecological malignancies. Int J Gynecol Cancer 2011;21:967–74. doi: 10.1097/IGC.0b013e31821d2770 [DOI] [PubMed] [Google Scholar]
- 56.McCune KC, Imborek KL. Clinical care of lesbian and bisexual women for the obstetrician gynecologist. Clin Obstet Gynecol 2018;61:663–73. doi: 10.1097/GRF.0000000000000399 [DOI] [PubMed] [Google Scholar]
- 57.Trinh MH, Agénor M, Austin SB, Jackson CL. Health and healthcare disparities among U.S. women and men at the intersection of sexual orientation and race/ethnicity: a nationally representative cross-sectional study. BMC Public Health 2017;17:964. doi: 10.1186/s12889-017-4937-9 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Boehmer U, Bowen DJ, Bauer GR. Overweight and obesity in sexual-minority women: evidence from population-based data. Am J Public Health 2007;97:1134–40. doi: 10.2105/ajph.2006.088419 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Zaritsky E, Dibble SL. Risk factors for reproductive and breast cancers among older lesbians. J Womens Health 2010;19:125–31. doi: 10.1089/jwh.2008.1094 [DOI] [PubMed] [Google Scholar]
- 60.Solazzo AL, Agenor M, Austin SB, Chavarro JE, Charlton BM. Sexual orientation differences in cervical cancer prevention among a cohort of U.S. women. Womens Health Issues 2020;30:306–12. doi: 10.1016/j.whi.2020.02.002 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Labuski CM. A Black and White issue? Learning to see the intersectional and racialized dimensions of gynecological pain. Soc Theor Health 2017;15:160–81. doi: 10.1057/s41285-017-0027-4 [DOI] [Google Scholar]
- 62.Greenwood BN, Hardeman RR, Huang L, Sojourner A. Physician-patient racial concordance and disparities in birthing mortality for newborns. Proc Natl Acad Sci U S A 2020;117:21194–200. doi: 10.1073/pnas.1913405117 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Hoffman KM, Trawalter S, Axt JR, Oliver MN. Racial bias in pain assessment and treatment recommendations, and false beliefs about biological differences between Blacks and Whites. Proc Natl Acad Sci U S A 2016;113:4296–301. doi: 10.1073/pnas.1516047113 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Hensley Alford S, Schwartz K, Soliman A, Johnson CC, Gruber SB, Merajver SD. Breast cancer characteristics at diagnosis and survival among Arab–American women compared to European– and African–American women. Breast Cancer Res Treat 2009;114:339–46. doi: 10.1007/s10549-008-9999-z [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Bergmans R, Soliman AS, Ruterbusch J, Meza R, Hirko K, Graff J, et al. Cancer incidence among Arab Americans in California, Detroit, and New Jersey SEER registries. Am J Public Health 2014;104:e83–91. doi: 10.2105/AJPH.2014.301954 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Chebli P, Reyes K, Muramatsu N, Watson K, Fitzgibbon M, Abboud S, et al. Perspectives of multisectoral community stakeholders on Arab American cancer patients' needs and suggested interventions. Support Care Cancer 2021;29:5915–25. doi: 10.1007/s00520-021-06169-x [DOI] [PubMed] [Google Scholar]
- 67.Abboud S, Chebli P, Rabelais E. The contested Whiteness of Arab identity in the United States: implications for health disparities research. Am J Public Health 2019;109:1580–3. doi: 10.2105/ajph.2019.305285 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Agency for Healthcare Research and Quality. Improving data collection across the health care system. Accessed November 20, 2021. https://ahrq.gov/research/findings/final-reports/iomracereport/reldata5.html
- 69.Perez CC. Invisible women: data bias in a world designed for men. Abrams; 2019. [Google Scholar]
- 70.Arksey H, O'Malley L. Scoping studies: towards a methodological framework. Int J Soc Res Methodol 2005;8:19–32. doi: 10.1080/1364557032000119616 [DOI] [Google Scholar]