Abstract
Background:
Observational studies suggest an association of stroke with cardiac traits beyond atrial fibrillation, the leading source of cardioembolism. However, controversy remains regarding a causal role of these traits in stroke pathogenesis. Here, we leveraged genetic data to systematically assess associations between cardiac traits and stroke risk using a Mendelian Randomization framework.
Methods:
We studied 66 cardiac traits including cardiovascular diseases, magnetic resonance imaging–derived cardiac imaging, echocardiographic imaging, and electrocardiographic measures, as well as blood biomarkers in a 2-sample Mendelian Randomization approach. Genetic predisposition to each trait was explored for associations with risk of stroke and stroke subtypes in data from the MEGASTROKE consortium (40 585 cases/406 111 controls). Using multivariable Mendelian Randomization, we adjusted for potential pleiotropic or mediating effects relating to atrial fibrillation, coronary artery disease, and systolic blood pressure.
Results:
As expected, we observed strong independent associations between genetic predisposition to atrial fibrillation and cardioembolic stroke and between genetic predisposition to coronary artery disease as a proxy for atherosclerosis and large-artery stroke. Our data-driven analyses further indicated associations of genetic predisposition to both heart failure and lower resting heart rate with stroke. However, these associations were explained by atrial fibrillation, coronary artery disease, and systolic blood pressure in multivariable analyses. Genetically predicted P-wave terminal force in V1, an electrocardiographic marker for atrial cardiopathy, was inversely associated with large-artery stroke.
Conclusions:
Available genetic data do not support substantial effects of cardiac traits on the risk of stroke beyond known clinical risk factors. Our findings highlight the need to carefully control for confounding and other potential biases in studies examining candidate cardiac risk factors for stroke.
Keywords: atrial fibrillation, biomarker, cardiovascular disease, coronary artery disease, echocardiography
Cardiac conditions are among the leading risk factors for stroke with both atrial fibrillation (AF) and coronary artery disease (CAD), a proxy for systemic atherosclerosis, having an undisputed role.1 Recent observational studies suggest an involvement of alternative cardiac traits, such as atrial cardiopathy, in stroke.2,3 However, controversy remains regarding causal inference from observational findings with concerns pertaining to possible confounding, reverse causality and other biases.4
Mendelian Randomization (MR) can clarify potential causal associations based on genetic data.5 Compared with observational studies, MR, in particular, multivariable MR, is less susceptible to confounding, as genetic variants are randomly allocated at conception. Recent genome-wide association studies (GWASs) for a wide range of cardiac traits offer an opportunity to comprehensively assesses causal relationships with stroke. We, therefore, performed a large-scale, systematic MR study of cardiac traits and their effect on stroke and stroke subtypes.
Methods
The present study adheres to the Transparency and Openness Promotion guidelines. Data supporting the findings of this study are publicly available from the respective GWAS and the Supplemental Material. All included studies were approved by institutional review boards, and all participants provided written, informed consent.
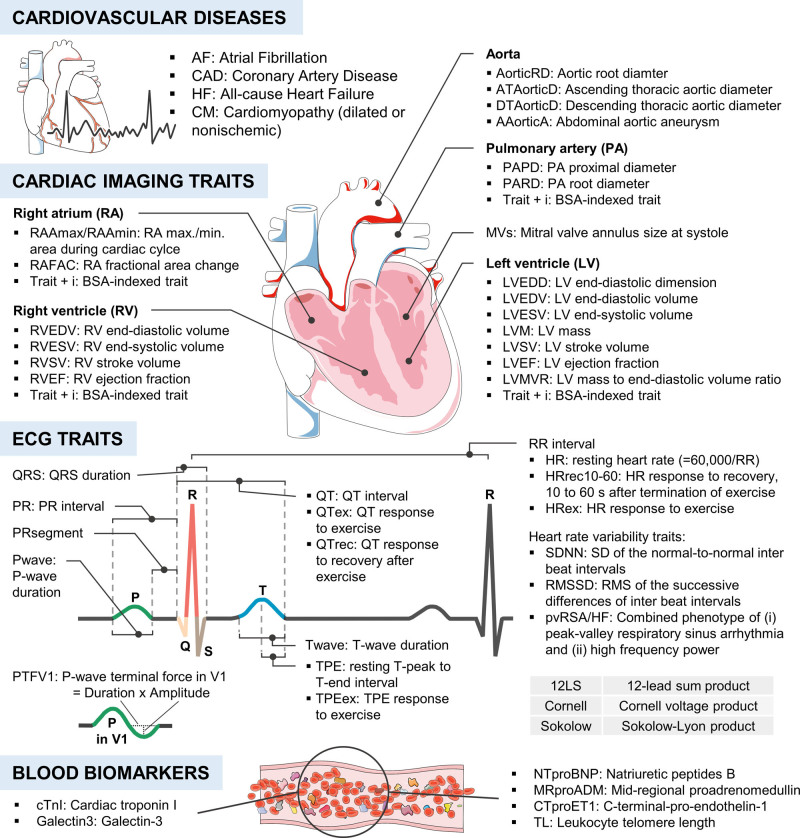
We conducted a 2-sample MR study between cardiac traits and stroke, including stroke subtypes. We first sought to confirm known associations with both AF and CAD, before taking on a data-driven approach to examine the effects of 64 additional cardiac traits (Figure 1; for sample sizes, see Table S1). Relevant associations (false discovery rate-adjusted P of inverse-variance weighted estimate <0.05) were further investigated in sensitivity and multivariable analyses. Genetic associations with any stroke (AS; 40 585 cases), any ischemic stroke (AIS; 34 217 cases), and stroke subtypes (large-artery stroke [LAS]: 4373 cases; cardioembolic stroke [CES]: 7193 cases; small-vessel stroke: 5386 cases) were derived from MEGASTROKE6 data (European subset, 406 111 controls). Stroke subtypes were classified according to TOAST (Trial of ORG 10172 in Acute Stroke Treatment). For multivariable MR,5 summary-level genetic data were acquired for AF (65 446 cases/522 744 controls),7 CAD (122 733 cases/424 528 controls),8 and systolic blood pressure (SBP; n=1 006 863).9 All analyses were repeated using the Causative Classification System (CCS),10 an alternative stroke classification in the NINDS-SiGN11 dataset (European subset, 14 300 AIS cases/26 690 controls), which further allows assessing associations with stroke of undetermined causes (Table S2).
Figure 1.
Sixty-six cardiac traits were tested for their association with stroke. BSA indicates body surface area; and RMS, root mean square. Images were adapted from Servier Medical Art (CC-BY-3.0).
We independently replicated all our analyses in the UK Biobank (UKB) dataset12 (4985 AS cases/3628 AIS cases/369 419 controls). In secondary analyses, we investigated 204 single-lead ECG traits for their associations with stroke (Figure S1). Figure S2 summarizes the study design. Further details are provided in the Supplemental Methods. All MR analyses were conducted following published guidelines.13
Results
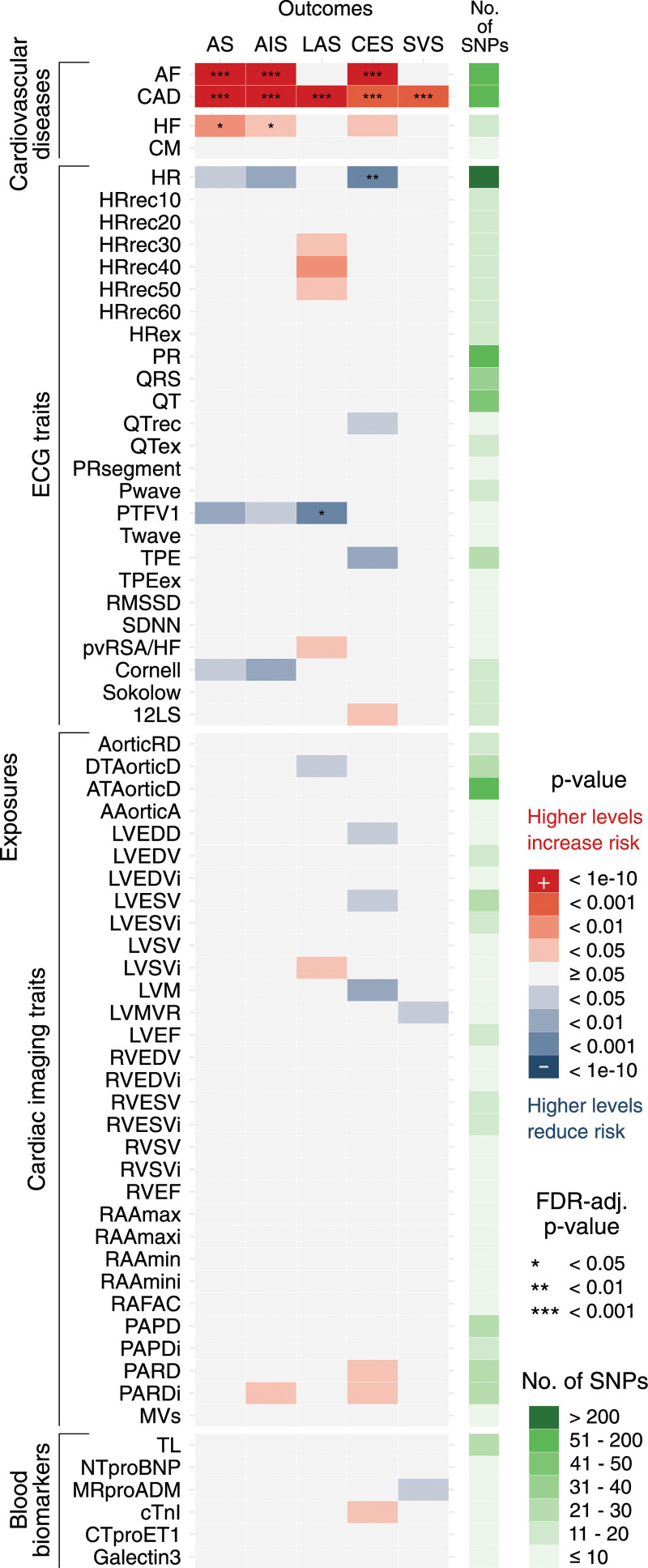
Genetic predisposition to AF was associated with AS, AIS, and CES, whereas genetic predisposition to CAD was associated with all types of stroke (Figure 2). Beyond AF and CAD, genetic predisposition to heart failure (HF) was associated with AS and AIS. Moreover, genetically predicted heart rate (HR) and genetically predicted P-wave terminal force in V1 (PTFV1) were inversely associated with CES and LAS, respectively. Twenty-five associations attained a nominal P below 0.05.
Figure 2.
Genetic associations of 66 cardiac traits and stroke. Based on inverse-variance weighted Mendelian Randomization (IVW-MR) estimates. Figure 1 provides phenotype specifications/abbreviations. AIS indicates any ischemic stroke; AS, any stroke; CES, cardioembolic stroke; LAS, large-artery stroke; and SVS, small-vessel stroke.
Sensitivity analyses (weighted-median, weighted-mode, simple-mode, MR-Egger) showed consistent results (Figure S4). Heterogeneity estimates are presented in Figure S5. In primary analyses, we found evidence for directional pleiotropy in 15 of 330 associations (Table S3) and several outliers were detected by MR-PRESSO14 (Tables S4 and S5).
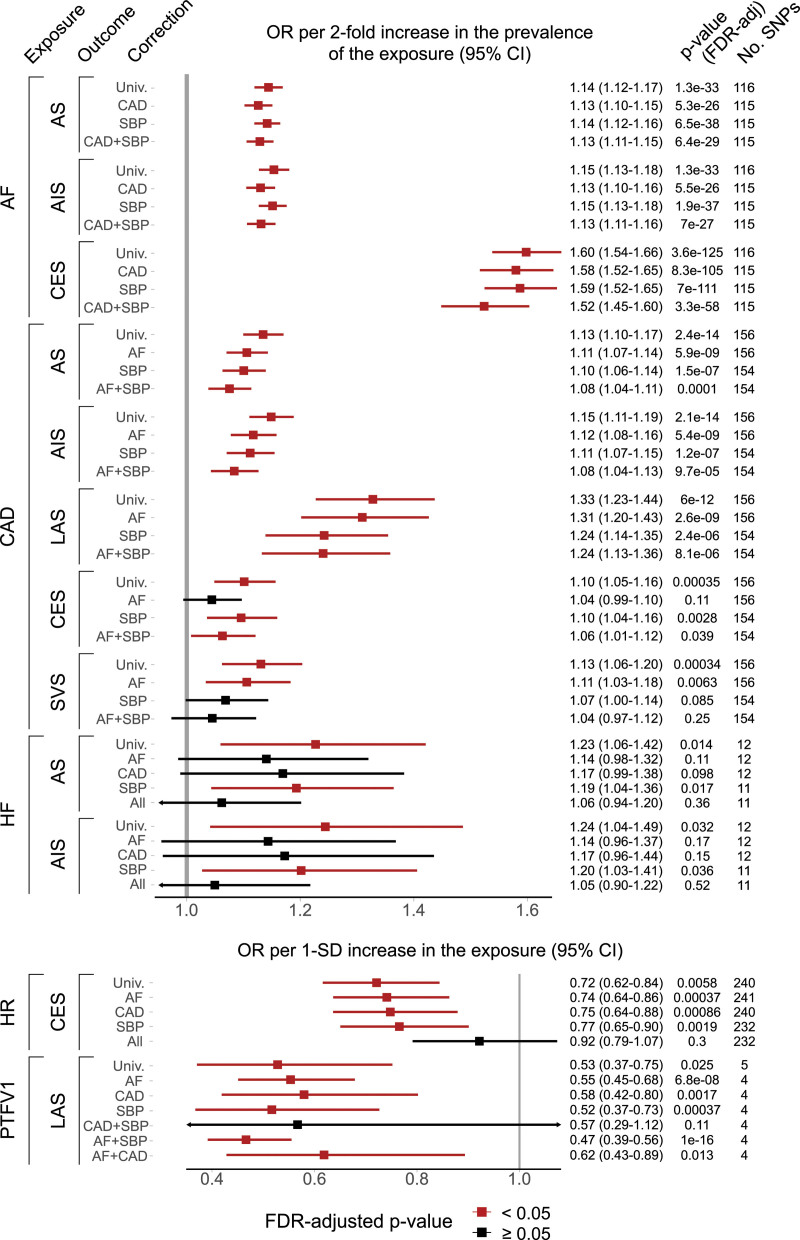
Adjusting our main findings for AF, CAD, and SBP in a multivariable MR (Figure 3), we found that the associations between HF and both AS and AIS, and between HR and CES were attenuated toward the null. The inverse association of PTFV1 with LAS was independent of AF but nonsignificant after a joint adjustment for CAD and SBP.
Figure 3.
Multivariate associations between selected cardiac traits and stroke. Estimated after adjustment for atrial fibrillation (AF), coronary artery disease (CAD), and systolic blood pressure (SBP). AIS indicates any ischemic stroke; All, joint adjustment for AF/CAD/SBP; AS, any stroke; CES, cardioembolic stroke; HF, heart failure; HR, resting heart rate; LAS, large-artery stroke; OR, odds ratio; PTFV1, P-wave terminal force in V1; SNP, single-nucleotide polymorphism; SVS, small-vessel stroke; and Univ, univariable MR.
An independent replication in the UKB confirmed associations of a genetic predisposition to AF and CAD with both AS and AIS (Figure S6). Our analyses in CCS-defined stroke subtypes essentially confirmed our findings based on the TOAST classification, while further revealing associations of genetic predisposition to AF and CAD with stroke of undetermined causes (Figure S7).
Discussion
We confirmed strong independent associations between genetic predisposition to AF and CES, as well as between genetic predisposition to CAD, a proxy for systemic atherosclerosis, and LAS. Our data-driven analysis of 64 additional genetically predicted cardiac traits indicated associations between both HF and lower HR with stroke risk. However, these associations were attenuated in multivariable MR, suggesting pleiotropic or mediating effects involving AF, CAD, and SBP. Furthermore, we identified an inverse association of genetically predicted PTFV1 and LAS, independent of AF but not of the joint effect of CAD and SBP.
Our results provide no support for a direct causal effect of HF on stroke risk. Instead, we found pleiotropic or mediating effects involving AF and CAD. This notion is further supported by our univariable analysis of cardiac imaging traits including left ventricular ejection fraction, which indicated no association with stroke. In accordance with our genetic data, a prior observational study found stroke etiology in patients with HF to be determined by the presence of AF and by the underlying cause of HF such as CAD or hypertension.15 However, observational data regarding an independent effect of HF on stroke remain somewhat conflicting.1,3,15
A previous univariable MR study identified an inverse association between genetically predicted HR and CES.16 In multivariable analyses, we found this association to be strongly attenuated by the effects of AF, CAD, and SBP. Likewise, we found no independent association of genetically predicted HR and other stroke subtypes, consistent with observational studies in Europeans.17
Our genetic results add to the complex literature on atrial cardiopathy/abnormal atrial substrate and its interaction with AF as a potential cause of stroke.2 In some contrast to previous observational studies,2,18 our MR results provide no support for a causal association of either PTFV1 or NTproBNP, 2 commonly used markers for atrial cardiopathy,18 with AIS. Whereas the number of variants reaching genome-wide significance in prior GWAS for PTFV1 or NTproBNP remains limited (n≤5), there was no indication for weak instrument bias in our analyses (F-statistics all >30; Table S6). Future MR studies with inclusion of measures of left atrial size2 and larger datasets may provide further insights.
Strengths of our study include the comprehensiveness of the approach, providing an unprecedented overview of genetically predicted cardiac risk factors for stroke. Also, our analysis incorporated the latest and largest GWAS on cardiac traits. We further conducted sensitivity analyses to validate our inverse-variance weighted estimates and consistently corrected for multiple comparisons. As a limitation, GWAS datasets for blood biomarkers remain relatively small (most with n<20 000) and thus might cause weak instrument bias. The independent replication in the UKB suffered from restricted stroke subtyping and a limited number of stroke cases. In addition, exposure and outcome samples overlapped in several analyses, and our MR estimates are limited to linear cumulative effects of lifetime exposure to risk factors. Finally, results from this study are based on European participants limiting generalization to other ancestries.
In conclusion, from the current genetic perspective, there is little evidence for independent associations of cardiac traits and stroke beyond AF, atherosclerosis, and hypertension. Instead, our findings highlight the requirement to carefully control for confounding and other potential biases in studies examining candidate cardiac risk factors for stroke.
Article Information
Sources of Funding
European Union’s Horizon 2020 research and innovation programme No 666881, SVDs@target (to Dr Dichgans) and 667375, CoSTREAM (to Dr Dichgans); Deutsche Forschungsgemeinschaft as part of the Munich Cluster for Systems Neurology (EXC 2145 SyNergy – ID 390857198), the CRC 1123 (B3; to Dr Dichgans), DI 722/16-1 (ID: 428668490 Immunostroke), and DI 722/13-1; Vascular Dementia Research Foundation. Drs Kittner and Mitchell were supported by NIH grants: R01NS105150 and R01NS100178 and Dr Kittner was also supported by Department of Veterans Affairs grant BX004672-01A1.
Disclosures
None.
Supplemental Material
Supplemental Methods
Tables
Figures
Checklist for Mendelian Randomization Analyses
References
Supplementary Material
Nonstandard Abbreviations and Acronyms
- AF
- atrial fibrillation
- AIS
- any ischemic stroke
- AS
- any stroke
- CAD
- coronary artery disease
- CES
- cardioembolic stroke
- GWAS
- genome-wide association study
- HF
- heart failure
- HR
- heart rate
- LAS
- large-artery stroke
- MR
- Mendelian Randomization
- PTFV1
- P-wave terminal force in lead V1
- SBP
- systolic blood pressure
- SVS
- small-vessel stroke
This manuscript was sent to Scott E. Kasner, Guest Editor, for review by expert referees, editorial decision, and final disposition.
Supplemental Material is available at https://www.ahajournals.org/doi/suppl/10.1161/STROKEAHA.121.036306.
For Sources of Funding and Disclosures, see page e134.
Contributor Information
Simon Frerich, Email: simon.frerich@med.uni-muenchen.de.
Rainer Malik, Email: Rainer.Malik@med.uni-muenchen.de.
Marios K. Georgakis, Email: marios.georgakis@med.uni-muenchen.de.
Moritz F. Sinner, Email: moritz.sinner@med.uni-muenchen.de.
Steven J. Kittner, Email: skittner@som.umaryland.edu.
Braxton D. Mitchell, Email: bmitchel@som.umaryland.edu.
References
- 1.Wolf PA, Abbott RD, Kannel WB. Atrial fibrillation as an independent risk factor for stroke: the Framingham Study. Stroke. 1991; 22:983–988. doi: 10.1161/01.str.22.8.983 [DOI] [PubMed] [Google Scholar]
- 2.Kamel H, Okin PM, Elkind MS, Iadecola C. Atrial fibrillation and mechanisms of stroke: time for a new model. Stroke. 2016; 47:895–900. doi: 10.1161/STROKEAHA.115.012004 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Adelborg K, Szépligeti S, Sundbøll J, Horváth-Puhó E, Henderson VW, Ording A, Pedersen L, Sørensen HT. Risk of stroke in patients with heart failure: a population-based 30-year cohort study. Stroke. 2017; 48:1161–1168. doi: 10.1161/STROKEAHA.116.016022 [DOI] [PubMed] [Google Scholar]
- 4.Fewell Z, Davey Smith G, Sterne JA. The impact of residual and unmeasured confounding in epidemiologic studies: a simulation study. Am J Epidemiol. 2007; 166:646–655. doi: 10.1093/aje/kwm165 [DOI] [PubMed] [Google Scholar]
- 5.Burgess S, Thompson SG. Mendelian Randomization: Methods for Causal Inference Using Genetic Variants. 2021, Chapman and Hall/CRC [Google Scholar]
- 6.Malik R, Chauhan G, Traylor M, Sargurupremraj M, Okada Y, Mishra A, Rutten-Jacobs L, Giese AK, Van Der Laan SW, Gretarsdottir S, et al. Multiancestry GWAS of 520,000 subjects identifies 32 loci associated with stroke and stroke subtypes. Nat. Genet. 2018; 50:524–537 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Roselli C, Chaffin MD, Weng LC, Aeschbacher S, Ahlberg G, Albert CM, Almgren P, Alonso A, Anderson CD, Aragam KG, et al. Multi-ethnic genome-wide association study for atrial fibrillation. Nat Genet. 2018; 50:1225–1233. doi: 10.1038/s41588-018-0133-9 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.van der Harst P, Verweij N. Identification of 64 novel genetic loci provides an expanded view on the genetic architecture of coronary artery disease. Circ Res. 2018; 122:433–443. doi: 10.1161/CIRCRESAHA.117.312086 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Evangelou E, Warren HR, Mosen-Ansorena D, Mifsud B, Pazoki R, Gao H, Ntritsos G, Dimou N, Cabrera CP, Karaman I, et al. ; Million Veteran Program. Genetic analysis of over 1 million people identifies 535 new loci associated with blood pressure traits. Nat Genet. 2018; 50:1412–1425. doi: 10.1038/s41588-018-0205-x [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Ay H, Benner T, Arsava EM, Furie KL, Singhal AB, Jensen MB, Ayata C, Towfighi A, Smith EE, Chong JY, et al. A computerized algorithm for etiologic classification of ischemic stroke: the Causative Classification of Stroke System. Stroke. 2007; 38:2979–2984. doi: 10.1161/STROKEAHA.107.490896 [DOI] [PubMed] [Google Scholar]
- 11.Pulit SL, McArdle PF, Wong Q, Malik R, Worrall BB, de Bakker PI, Kittner SJ, Mitchell BD, Rosand J; NINDS Stroke Genetics Network (SiGN). Loci associated with ischaemic stroke and its subtypes (SiGN): a genome-wide association study. Lancet Neurol. 2016; 15:174–184. doi: 10.1016/S1474-4422(15)00338-5 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Bycroft C, Freeman C, Petkova D, Band G, Elliott LT, Sharp K, Motyer A, Vukcevic D, Delaneau O, O’Connell J, et al. The UK Biobank resource with deep phenotyping and genomic data. Nature. 2018; 562:203–209. doi: 10.1038/s41586-018-0579-z [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Burgess S, Davey Smith G, Davies NM, Dudbridge F, Gill D, Glymour MM, Hartwig FP, Holmes MV, Minelli C, Relton CL, et al. Guidelines for performing Mendelian randomization investigations. Wellcome Open Res. 2019; 4:186. doi: 10.12688/wellcomeopenres.15555.2 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Verbanck M, Chen CY, Neale B, Do R. Detection of widespread horizontal pleiotropy in causal relationships inferred from MR between complex traits and diseases. Nat Genet. 2018; 50:693–698. doi: 10.1038/s41588-018-0099-7 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Vemmos K, Ntaios G, Savvari P, Vemmou AM, Koroboki E, Manios E, Kounali A, Lip GY. Stroke aetiology and predictors of outcome in patients with heart failure and acute stroke: a 10-year follow-up study. Eur J Heart Fail. 2012; 14:211–218. doi: 10.1093/eurjhf/hfr172 [DOI] [PubMed] [Google Scholar]
- 16.Larsson SC, Drca N, Mason AM, Burgess S. Resting heart rate and cardiovascular disease. Circ Genom Precis Med. 2019; 12:e002459. doi: 10.1161/CIRCGEN.119.002459 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Aune D, Sen A, Ó’Hartaigh B, Janszky I, Romundstad PR, Tonstad S, Vatten LJ. Resting heart rate and the risk of cardiovascular disease, total cancer, and all-cause mortality – a systematic review and dose–response meta-analysis of prospective studies. Nutr Metab Cardiovasc Dis. 2017; 27:504–517. doi: 10.1016/j.numecd.2017.04.004 [DOI] [PubMed] [Google Scholar]
- 18.Kamel H, Bartz TM, Elkind MSV, Okin PM, Thacker EL, Patton KK, Stein PK, deFilippi CR, Gottesman RF, Heckbert SR, et al. Atrial Cardiopathy and the Risk of Ischemic Stroke in the CHS (Cardiovascular Health Study). Stroke. 2018; 49:980–986. doi: 10.1161/STROKEAHA.117.020059 [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.