Abstract
Objective
To estimate the ‘cost of illness’ arising from chronic wounds in Singapore.
Design
Incidence-based cost of illness study using evidence from a range of sources.
Setting
Singapore health services.
Participants
We consider 3.49 million Singapore citizens and permanent residents. There are 16 752 new individuals with a chronic wound in 2017, with 598 venous ulcers, 2206 arterial insufficiency ulcers, 6680 diabetic ulcers and 7268 pressure injuries.
Primary outcome measures expressed in monetary terms are the value of all hospital bed days lost for the population; monetary value of quality-adjusted life years (QALYs) lost in the population; costs of all outpatient visits; and costs of all poly clinic, use of Community Health Assist Scheme (CHAS) and emergency departments (EDs) visits. Intermediate outcomes that inform the primary outcomes are also estimated.
Results
Total annual cost of illness was $350 million (range $72–$1779 million). With 168 503 acute bed days taken up annually (range 141 966–196 032) that incurred costs of $139 million (range 117–161 million). Total costs to health services were $184 million (range $120–$1179 million). Total annual costs of lost health outcomes were 2077 QALYs (range −2657 to 29 029) valued at $166 million (range −212 to 2399 million).
Conclusions
The costs of chronic wounds are large to Singapore. Costs can be reduced by making positive investments for comprehensive wound prevention and treatment programmes.
Keywords: general medicine (see internal medicine), health economics, health services administration & management, wound management
STRENGTHS AND LIMITATIONS OF THIS STUDY
Reliable and relevant data sources were used to update the results.
First study to quantify the national cost of chronic wounds in a multiethnic Asian population.
Some important costs were excluded as no information was available.
The sample size for the preference-based utility weights for quality-adjusted life years were small.
Some outcomes were not adjusted for comorbidities and so might overstate the true costs.
Introduction
Chronic wounds are those that fail to heal in a time sufficient for ‘normal’ healing. They tend to present as a comorbid rather than primary condition among older individuals. Other risk factors are diabetes, poor nutrition, incontinence and reduced mobility.1 They have been described as causing a ‘silent epidemic’ that affects a large proportion of the world’s population.2 Chronic wounds are prevalent among vulnerable individuals living at home and residents of long-term care facilities. They are commonly associated with extended hospital stays but patient safety programmes have reduced healthcare-associated events.3
The burden of cost is particularly large4 with 3% of the total UK National Health Service budget5 and 4% of healthcare expenditure in Scandinavian countries used to manage the consequences of chronic wounds.6 The goal of reducing the prevalence of chronic wounds has failed to attract sustained investment from those who pay for health services.7 This contrasts with other major diseases, where payers are prepared to invest in ‘cancer moonshots’ for example,8 that will hopefully lead to better outcomes in the future. This inequity is puzzling as the technology for reducing chronic wounds is available now, saves more than it costs to implement,9 and will cause large and certain gains to health outcomes.
Ulcers of the skin are the most common type of chronic wounds and include venous ulcers, arterial ulcers, diabetic foot ulcers and pressure ulcers or injuries. These are associated with a wide range of economic costs.2 Affected individuals require frequent evidence-based treatments and if the condition becomes overwhelming, an admission to hospital is inevitable. Many patients will be admitted for other reasons, and the wound may independently prolong hospital stay.10 Debridement, minor amputations and major amputations are common among higher risk groups.11 12 Chronic wounds are prevalent among residents of aged care facilities and will incur additional costs for staff time and consumables. Home nursing services as well as charities and volunteer groups that support the frail and elderly in their homes also manage patients.13 Out-of-pocket expenditures will arise for patients and family members who travel to access services and purchase consumable items.14 Productivity losses will arise as the patients are unable to perform their normal activities, be they paid or unpaid, and family members will have to take time from waged and unwaged productive activity. Health-related quality of life, which has monetary value,15 will be reduced. All these costs can be structured by a ‘cost of illness’ method.16
The aim of this study is to estimate the ‘cost of illness’ arising from chronic wounds in Singapore. Our results could be used to stimulate decision-makers to invest in known prevention and management programmes. The findings will also aid researchers who wish to model the cost savings or the cost-effectiveness of specific interventions.
Method
Scope of the analyses
We include all resident Singapore citizens and permanent residents (n=3.49 million) and exclude resident foreign nationals and long-term employment pass holders (n=526 000) in 2017.17 Singapore has a multiethnic Asian population comprising of residents who are 76% Chinese, 15% Malay and 7.5% Indian descent.18 The perspective for this analysis includes the costs incurred by health services and the losses to health benefits, expressed as quality-adjusted life years (QALYs) foregone. We do not represent the ‘societal’ perspective as there are no data on private out of pocket costs, but we do review this omission in the Discussion section. We estimate the expected annual costs arising from incident cases of venous ulcers, arterial insufficiency ulcers, diabetic ulcers and pressure injury.
Scope of the modelling
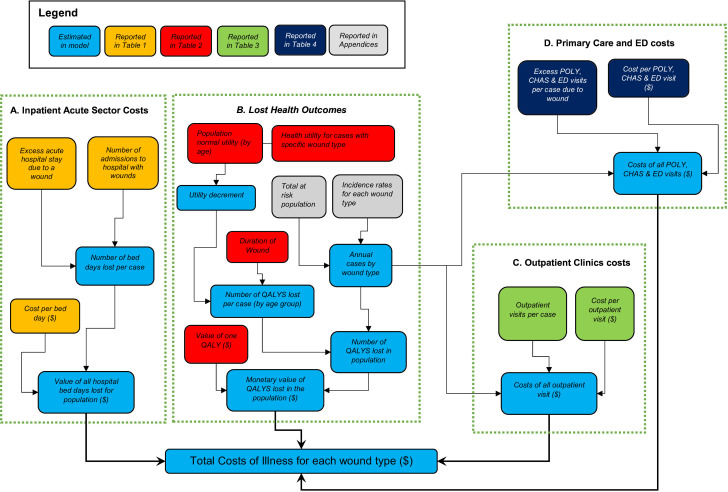
We use statistical models to estimate four primary outcomes (A to D) for the year 2017, see figure 1.
Figure 1.
A diagram to show how the various parameters update the outcomes.CHAS, Community Health Assist Scheme; ED, emergency department; POLY, Polyclinics; QALYs, quality-adjusted life years.
First, are the monetary ‘value of all hospital bed days lost for the population’. The information used is summarised in Part A of figure 1 and is labelled ‘Inpatient Acute Sector Costs’. Second, are the ‘monetary value of QALYs lost in the population’. The information used is summarised in Part B of figure 1 and is labelled ‘Lost Heath Outcomes’. Third, are the ‘costs of all outpatient visits’. The information used is summarised in Part C of figure 1 and is labelled ‘Outpatient Clinics Costs’. Fourth, are the ‘costs of all poly clinic, use of Community Health Assist Scheme (CHAS) and emergency departments (ED) visits’. The information used is summarised in Part D of figure 1 and is labelled ‘Primary Care and ED costs’. CHAS is a mechanism for funding all Singapore citizens to attend medical and/or dental care at participating general practitioner and dental clinics. CHAS is particularly designed to support the management of chronic diseases.
Data, parameters and assumptions
Inpatient acute sector costs
Two outcomes, shown by blue boxes in figure 1, are estimated: ‘Number of bed days lost per case’ and ‘Value of all hospital bed days lost for population ($)’. To estimate the ‘Number of bed days lost per case’ we combined information on the ‘Number of admissions to hospital with wounds’ with information on ‘Excess acute hospital stay due to a wound’. To estimate the ‘Number of admissions to hospital with wounds’ we retrieved a population cohort of all inpatient admissions to acute hospitals in Singapore between 2012 and 2019 from the Singapore Ministry of Health, and applied the cases from 2017. From the entire dataset, we identified all inpatient episodes with any occurrence of venous ulcers, arterial insufficiency ulcers, diabetic ulcers and pressure injury or any combination of these wounds as a primary or secondary diagnosis based on the ICD-9 codes in online supplemental appendix 1.
bmjopen-2022-065692supp001.pdf (254.2KB, pdf)
To estimate ‘Excess acute hospital stay due to wound’ we extracted the length of hospital stay and relevant covariate information that could be used to explain variation in the length of stay, see online supplemental appendix 2. A parsimonious multivariable generalised linear model (GLM) with a gamma link function was used to accommodate the skew typical of lengths of stay data.19 The outcome of interest was the length of stay associated with chronic wound management in the inpatient setting. Other covariates included were age, gender, race and comorbid chronic diseases. The statistical model generated a coefficient for ‘wound type’ expressed as a rate ratio, that showed the amount of increase in length of stay associated with the presence of wound, given that other factors that predicted length of stay had been accounted for. This rate ratio was used to moderate the mean length of stay for the entire sample and an excess length of stay associated with the wound was estimated. In online supplemental appendix 3, we show a summary of the results of the multivariable GLM and in online supplemental appendix 4, we show the full model results.
To estimate the ‘Value of all hospital bed days lost for population ($)’ we combined the ‘Number of bed days lost per case’ with the ‘Cost per bed day ($)’.
All the data inputs used for this part of the model are shown in table 1.
Table 1.
Data inputs used to estimate the outcomes for the ‘Inpatient Acute Sector Costs’
| Parameter | Estimate | Distribution used for uncertainty | |
| Excess acute hospital stay due to wound (days) | |||
| Arterial | 2.37 | Gamma (757, 0.0031) | Estimated by multivariable generalised linear model |
| Venous | 0.79 | Gamma (84, 0.0094) | |
| Diabetic | 2.26 | Gamma (742, 0.003) | |
| Pressure | 1.61 | Gamma (724, 0.0022) | |
| Number of admissions to hospital with wounds | |||
| Arterial | 14 536 | Fixed | * |
| Venous | 19 210 | Fixed | |
| Diabetic | 16 999 | Fixed | |
| Pressure | 49 879 | Fixed | |
| Cost per bed day ($) | 823 | Normal (823, 2.78) | * |
*Ministry of Health, Administrative database, Ministry of Health (MOH). Accessed in 2021.
Lost health outcomes
Five outcomes, shown by blue boxes in figure 1, are estimated: Annual cases by wound type; Number of QALYs lost per case (by age group); Utility decrement; Number of QALYS lost in population; and Monetary value of QALYS lost in the population ($). The first four are intermediate outcomes that contribute information to the primary outcome of ‘Monetary value of QALYs lost in the population ($)’.
To estimate ‘Annual cases by wound type’, we apply the incidence rates for each wound type to the ‘Total at risk population’. We used published incidence rates for 2017 from a population-based study of wounds among those admitted to Singapore acute care hospitals from 2000 to 2017.20 For this work the authors identified relevant ICD-10 codes for for occurrences of venous ulcers, arterial insufficiency ulcers, diabetic ulcers and pressure ulcers or injuries, see online supplemental appendix 1, and applied them to the Singapore Ministry of Health central claims database, which includes records of all admissions to public and private acute care hospitals. The incidence rates by age band are reported alongside the at-risk population enabling the number of incident cases to be estimated, see online supplemental appendix 5 for more detail.
To estimate the ‘Number of QALYs lost per case (by age group)’, we use EQ-5D-5L data. This instrument includes preference-based valuations of health states expressed as ‘health utilities’ on a scale between 0, the worst possible health state, and 1, the best possible health state.21 We used EQ-5D-5L data from 799 individuals with relevant wounds from the Singapore Wound Care Registry to inform the ‘Health utility for cases with specific wound type’. Responses were recorded at entry into the registry, when the wound was first assessed in the hospital setting and then at 1, 3 and 6 months, see online supplemental appendix 6. We used a Singapore EQ-5D-3L value set22 that was then mapped onto the EQ-5D-5L version using the SAS code in online supplemental appendix 7. The utility outcomes for the wound patients are compared with population norms for the EQ-5D index informed by Singapore preference weights for appropriate age bands,23 this informs the ‘Population normal Utility (by age)’. Using the information described above, we are able to estimate the ‘Utility Decrement’ from having a wound. The ‘Duration of Wound’ was informed by the mean durations of wounds in days for the specific wound types from the Singapore Wound Registry, see online supplemental appendix 8. The ‘Number of QALYs lost in population’ is the product of the ‘Number of QALYS lost per case’ and the ‘Annual cases by wound type’. The ‘Monetary value of QALYs lost in the population’ is the product of the ‘Number of QALYS lost in population’ and the ‘Value of one QALY’, which is set at the mean gross domestic product per capita for Singapore of SGD $80 000.24 This approach assumes the value of 1 year of perfect quality of life does not exceed the per capita gross domestic product.25
All the data inputs used for this part of the model are shown in table 2.
Table 2.
Data inputs used to estimate the outcomes for the ‘Lost Health Outcomes’
| Parameter | Estimate | Distribution used for uncertainty | |
| Health utility for cases with specific wound type | |||
| Arterial: baseline | 0.44 | Beta (0.24, 0.30) | Singapore Wound Registry |
| Venous: baseline | 0.57 | Beta (0.60, 0.46) | |
| Diabetic: baseline | 0.64 | Beta (0.21, 0.12) | |
| Pressure: baseline | 0.18 | Normal (−0.18, 0.50) | |
| Arterial: month 1 | 0.52 | Beta (0.23, 0.21) | |
| Venous: month 1 | 0.68 | Beta (0.89, 0.41) | |
| Diabetic: month 1 | 0.71 | Beta (0.04, 0.02) | |
| Pressure: month 1 | 0.00 | Normal (0.00, 0.56) | |
| Arterial: month 3 | 0.54 | Beta (0.13, 0.11) | |
| Venous: month 3 | 0.74 | Beta (0.81, 0.28) | |
| Diabetic: month 3 | 0.72 | Beta (0.28, 0.11) | |
| Pressure: month 3 | 0.18 | Normal (0.18, 0.54) | |
| Arterial: month 6 | 0.58 | Beta (0.16, 0.12) | |
| Venous: month 6 | 0.74 | Beta (0.83, 0.28) | |
| Diabetic: month 6 | 0.74 | Beta (0.05, 0.02) | |
| Pressure: month 6 | 0.11 | Normal (0.11, 0.59) | |
| Population normal utility (by age group) | |||
| <40 | 0.980 | Beta (350, 7) | 23 |
| 40–49 | 0.950 | Beta (636, 33) | |
| 50–59 | 0.940 | Beta (535, 34) | |
| 60–69 | 0.960 | Beta (193, 8) | |
| 70–79 | 0.890 | Beta (189, 23) | |
| ≥80 | 0.890 | Beta (189, 23) | |
| Value of one QALY ($) | 80 000 | Fixed | 24 |
| Durations of arterial wounds in days (by age group) | |||
| <40 | 133 | Normal (133, 92) | Singapore Wound Registry |
| 40–49 | 129 | Normal (129, 109) | |
| 50–59 | 331 | Normal (331, 394) | |
| 60–69 | 223 | Normal (223, 269) | |
| 70–79 | 307 | Normal (307, 438) | |
| ≥80 | 205 | Normal (205, 153) | |
| Durations of venous wounds in days (by age group) | |||
| <40 | 133 | Normal (133, 92) | |
| 40–49 | 129 | Normal (129, 109) | |
| 50–59 | 331 | Normal (331, 394) | |
| 60–69 | 223 | Normal (223, 269) | |
| 70–79 | 307 | Normal (307, 438) | |
| ≥80 | 205 | Normal (205, 153) | |
| Durations of diabetic wounds in days (by age group) | |||
| <40 | 177 | Normal (177, 88) | |
| 40–49 | 325 | Normal (325, 550) | |
| 50–59 | 224 | Normal (224, 216) | |
| 60–69 | 314 | Normal (314, 430) | |
| 70–79 | 256 | Normal (256, 305) | |
| ≥80 | 160 | Normal (160, 138) | |
| Durations of pressure injury in days (by age group) | |||
| <40 | 55 | Normal (55, 11) | |
| 40–49 | 86 | Normal (86, 24) | |
| 50–59 | 115 | Normal (115, 1) | |
| 60–69 | 103 | Normal (103, 59) | |
| 70–79 | 105 | Normal (105, 115) | |
| ≥80 | 62 | Normal (62, 52) | |
QALY, quality-adjusted life year.
Outpatient clinics costs
Only one outcome, ‘Costs of all outpatient visit ($)’, was estimated. Information was used for the ‘Annual cases by wound type’, ‘Cost per outpatient visit ($)’ and ‘Outpatient visits per case’.
To estimate ‘Cost per outpatient visit ($)’, we interrogated the Singapore Wound Care Registry to identify the annual number of visits for those with chronic wounds and the reported costs per visits, this information was available by wound type. There were 573 individuals for whom these data were reported. Visits were for specialist consultations specifically for their wound, and for podiatry visit or medical tests, see online supplemental appendix 9. All the data inputs used for this part of the model are shown in table 3.
Table 3.
Data inputs used to estimate the outcomes for the ‘Outpatient Clinic Costs’
| Estimate | Distribution used for uncertainty | Source | |
| Outpatient visits per case (with consult) | Singapore Wound Registry | ||
| Arterial | 8.2 | Gamma (1.82:4.52) | |
| Venous | 5.9 | Gamma (0.98:5.99) | |
| Diabetic | 8.1 | Gamma (1.70:4.76) | |
| Pressure | 6.5 | Gamma (1.93:3.37) | |
| Outpatient visits per case (without consult) | |||
| Arterial | 8.5 | Gamma (1.77:4.79) | |
| Venous | 23.6 | Gamma (1.55:15.25) | |
| Diabetic | 14.2 | Gamma (0.08:185.15) | |
| Pressure | 9.7 | Gamma (0.81:12.00) | |
| Cost per outpatient visit (with consult) ($) | |||
| Arterial | 110 | Gamma (5.60:19.74) | |
| Venous | 133 | Gamma (2.70:49.30) | |
| Diabetic | 112 | Gamma (2.86:39.20) | |
| Pressure | 111 | Gamma (4.05:27.43) | |
| Cost per outpatient visit (without consult) ($) | |||
| Arterial | 117 | Gamma (5.60:19.74) | |
| Venous | 106 | Gamma (2.70:49.30) | |
| Diabetic | 114 | Gamma (2.86:39.20) | |
| Pressure | 124 | Gamma (4.05:27.43) | |
Primary care and ED costs
Only one outcome ‘Costs of all POLY, CHAS & ED visits ($)’ was estimated. Information was used for the ‘Annual cases by wound type’, ‘Cost per POLY, CHAS & ED visit ($)’ and ‘Excess POLY, CHAS & ED visits per case due to wound’.
For the ‘Cost per POLY, CHAS & ED visit ($), we use estimates reported by the Singapore Ministry of Health. To estimate Excess POLY, CHAS & ED visits per case due to wound’ the same population cohort for 2012 to 2019 who were admitted as inpatients were interrogated. We identify the use of the EDs of acute hospitals and all visits to community-based Polyclinics (POLY) and use of CHAS.
We sought to estimate the excess use of these services associated with any chronic wound. For the analysis each patient is counted only once, and those with wounds are only counted when they first appear with any wound, and the number of 12-month visits from the incidence date is the outcome variable. For those without wounds, their 12-month use starts from the first visit during the study period. A parsimonious GLM with a log link Poisson function was used for all regressions. The Poisson distribution was chosen over the negative binomial distribution based on fitting the model then doing model checks with diagnostic plots and relevant statistics. The outcome of interest was a count of the use of the services. Other covariates included were age, gender, race, Charlson Comorbidity Index and presence of comorbid conditions, see online supplemental appendix 10. The ensuing statistical models generated a coefficient for ‘any wound’ expressed as a rate ratio, that showed the change in the number of visits associated with the presence of wound, given that other factors that predicted variation in these outcomes. As before, the rate ratio was used to moderate the mean counts for the entire sample and an excess number of visits was estimated for all wounds, see online supplemental appendix 11. All the data inputs used for this part of the model are shown in table 4.
Table 4.
Data inputs used to estimate the outcomes for the ‘Primary care and ED Costs’
| Parameter | Estimate | Distribution used for uncertainty | Source |
| Excess visits for polyclinics (all wounds) | 0.91 | Gamma (18.33, 0.049) | Estimated by multivariable generalised linear model |
| Excess visits for CHAS (all wounds) | 3.54 | Gamma (38.51, 0.091) | |
| Excess visits for ED (all wounds) | 0.63 | Gamma (4.18, 0.15) | |
| Cost per poly clinic visit | $147 | Normal (147,2.5) | Ministry of Health, Administrative database, Ministry of Health (MOH). Accessed in 2021 |
| Cost per CHAS use | $56 | Normal (56,1.14) | |
| Cost per ED visit | $352 | Normal (352,.14) |
CHAS, Community Health Assist Scheme; ED, emergency department.
Uncertainty for all the outcomes shown in figure 1 was assessed by probabilistic sensitivity analysis. We take 5000 Monte Carlo resamples from all the parameters described in tables 1–4. We report the number of ‘acute care bed days lost’ and the number of ‘QALYs lost to chronic wounds’. We then report the findings from the resamples for the primary model outcomes: Value of all bed days lost for population; Monetary value of QALYS lost in the population; Costs of all outpatient visit; and, Costs of all POLY, CHAS & ED visits. We sum these four primary outcomes to report the ‘Total Costs of Illness for each wound type’. These processes are shown in figure 1.
Patient and public involvement
The data came from the Singapore Wound Registry and from the Ministry of Health. It was routinely reported data collected for the purpose of managing and planning health services. It was not possible to develop the research question or outcome measures based on the priorities, experience and preferences of the patients. Patients were not involved in the design, recruitment and conduct of the study. Patients who are interested will be able to read the paper.
Results
There were 16 752 ‘new’ or ‘incident’ cases for 2017, with 598 venous ulcers, 2206 arterial insufficiency ulcers, 6680 diabetic ulcers and 7268 pressure injuries. The values obtained from the model for ‘acute care bed days lost’ and the number of ‘QALYs lost to chronic wounds’ are shown in table 5.
Table 5.
Annual outcomes for bed days lost and QALYs lost for incident cases (n=16 752)
| Mean (min:max) | |
| Number of QALYs lost | |
| Arterial | 544 (−340:5436) |
| Venous | 75 (−127:0971) |
| Diabetic | 856 (−1670:16 962) |
| Pressure | 602 (−520:6631) |
| Number of bed days lost | |
| Arterial | 34 389 (29 269:38 674) |
| Venous | 15 161 (10 044:22 166) |
| Diabetic | 38 423 (33 445:43 573) |
| Pressure | 80 530 (69 208:91 619) |
QALYs, quality-adjusted life years.
The values obtained from the model for the four primary outcomes are shown in table 6.
Table 6.
Annual cost outcomes for the primary outcomes (n=16 752)
| Value of all bed days lost for population | Mean (min:max) |
| Arterial | $28 302 139 ($24 148 856:$31 912 933) |
| Venous | $12 477 776 ($8 243 954:$18 212 629) |
| Diabetic | $31 622 397 ($27 431 880:$35 693 113) |
| Pressure | $66 276 250 ($57 237 092:$75 718 807) |
| Monetary value of QALYs lost in the population | |
| Arterial | $43 491 588 (−$27 170 499:$434 890 646) |
| Venous | $5 975 553 (−$10 153 391:$77 645 170) |
| Diabetic | $68 498 429 (−$133 624 494:$1 356 989 736) |
| Pressure | $48 199 291 (−$41 617 890:$530 453 557) |
| Costs of all outpatient visit | |
| Arterial | $4 233 236 ($84 899:$35 360 192) |
| Venous | $2 017 189 ($8957:$27 539 296) |
| Diabetic | $16 337 217 ($3058:$785 613 392) |
| Pressure | $13 859 689 ($42 824:$142 991 574) |
| Primary care and ED costs | |
| Costs polyclinic visits—all wounds | $2 245 437 ($877 197:$5 715 449) |
| Costs CHAS episodes of care—all wounds | $3 296 040 ($1 849 062:$5 930 304) |
| Costs ED visits—all wounds | $3 730 682 ($235 741:$14 456 940) |
CHAS, Community Health Assist Scheme; ED, emergency department; QALYs, quality-adjusted life years.
The aggregate of these cost outcomes are the ‘Total Costs of Illness for each wound type’ and are shown in table 7 for the entire population of Singapore, and for the average individual.
Table 7.
Annual total costs of illness for each wound type (n=16 752)
| Total costs of illness for each wound type | Mean (min:max) | |
| Population | Individual | |
| Arterial | $77 247 993 ($6 047 354:$469 696 045) | $35 364 ($383:$257 207) |
| Venous | $20 801 577 ($5388 353:$91 673 830) | $35 023 ($9064:$162 054) |
| Diabetic | $120 155 304 (−$97 359 748:$1 395 843 120) | $18 095 (−$17 094:$231 815) |
| Pressure | $132 358 039 ($42 035 656:$624 864 715) | $18 161 ($3829:$73 175) |
| Total cost of illness | $350 562 913 ($72 814 108:$1 779 366 924) | $21 002 ($2664:$100 366) |
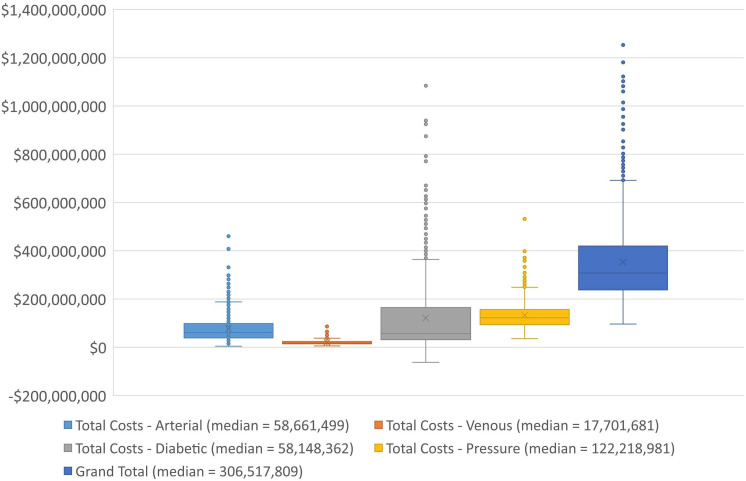
Based on our estimates pressure injuries account for 48% of the 168 503 bed days lost to chronic wounds and 38% of the $28.4 million outpatients costs. For the non-hospital sector, the costs of CHAS services and ED visits account for most of the burden. The QALY burdens are large for diabetic foot ulcers, arterial and pressure injury with venous ulcers having lesser impact. Box plots for the ‘Total Costs’ outcomes are shown in figure 2.
Figure 2.
Total cost outcomes for the cost of illness by wound type.
There is a 100% probability that the costs of chronic wounds are positive with the most likely value suggesting an annual cost to Singapore of $350 million. The findings are uncertain with the range of total costs between $79 million and $1.78 billion. More than half of the total costs arise from use of health services ($185 million, 53%) with outpatients accounting for $37 million (10%) and the use of acute bed days accounting for $139 million (40%). The value of the lost health by QALYs is substantial at $165 million, 47% of the total burden.
Discussion
These findings suggest that the costs of chronic wounds to Singapore are large and account for approximately 0.07% of GDP. The total cost burden accounts for 3.14% of the 2019 Government Health Expenditure on services26 and 2.3% of total economy-wide expenditure on services. Our estimates roughly align with those from other countries. In Australia 2% of the total national health expenditure is used for chronic wounds and in the UK 3% of the national health expenditure is taken up.27 Two per cent of the European health budget28 is for care of chronic wounds and for Scandinavian countries the costs were found to be 2%–4% of the total healthcare expenditure.6
While the findings are lower than the annual costs of diabetes in Singapore, estimated to be US$787 million in 2010,29 the policy response to diabetes has been considerable with a ‘War on Diabetes’ declared in 2016 to mobilise a national programme to reduce the problem of diabetes.30 We found two other studies reporting costs of chronic venous leg and neuroischaemic ulcers in Singapore, but neither study were at a population level instead focusing on average costs per patient.31
This study likely underestimates the extent of the costs of chronic wounds as relevant information was not available for many costs we suspect are present. Our data came primarily from patients who were admitted to the hospital for their wounds. Thus patients with less serious wounds managed in the community are excluded. We were also unable to identify and include estimates of the private costs incurred by patients and family members. Other studies have found that such costs can be substantial. For the German setting Purwins et al32 found patients with leg ulcers in a given year spent €424 on topical treatments and drugs, €486 on out of pocket incidentals, €254 on drug prescriptions and €740 on non-drug treatments.
Although no data were available on the time away from work and other production losses, we addressed this by estimating and valuing lost QALYs. We assume that time in reduced health states has a relationship with an ability to be economically productive. Thus, the dollar valuations of the lost QALYs can be thought of as representing lost production from chronic wounds. Most governments are willing to pay money for services that increase the number of QALYs in a population, given a programme achieves a marginal QALY below a designated cost.33 Importantly we did not consider the costs of lost production for informal carers, which we expect are substantial.
There are further limitations to our study. Regarding the estimation of QALY losses, the sample sizes for EQ-5D were quite small for pressure injury with only 51 patients providing data. It is possible the estimate would change with a larger and more representative sample. It should be noted that the lowest health utilities arise from this sample. For example, the values for baseline were −0.18 indicating a health state valued worse than death, and a value of 0.00, the worst possible health state, was observed for month 1. Values remained low at 0.18 and 0.11 for the 3-month and 6-month follow-ups. We assumed that the observed decrement between the population norms for health utility and the estimates from the wound registry were wholly attributable to the presence of a wound. These QALY estimates did not adjust for the other health conditions that patients may have, and as such may overstate the QALY losses.
To attribute excess acute bed days to the presence of a chronic wound we developed generalised linear regression models with the outcome of length of stay. While we did not have an exhaustive list of control variables, we did find that factors such as race, age, gender, myocardial infarction, cancer, liver disease, peptic ulcer disease, peripheral vascular disease, renal disease, chronic obstructive pulmonary disease, dementia, diabetes, heart failure, hyperlipidaemia, lymphoproliferative disease, major depression, Parkinson’s, schizophrenia and stroke all played a role in explaining variation in the observed length of stay. We fitted the best models possible, but acknowledge there may be some covariate information missing.
In summary, this recurring and unnecessary cost burden is a deadweight loss to Singapore health services, and society in general. It could be reduced if evidence-based and relatively simple prevention and management programmes were implemented. International evidence9 reveals that using optimal prevention practices for diabetic foot ulcers34 was cost-saving in Peru,35 Australia,36 Thailand37 and China.38 For the prevention of pressure injury nursing-led interventions, a quality improvement collaborative and the standardised use of pressure injury bundles were found to be cost-saving in Denmark,39 USA and UK.40–43 And for the prevention of venous leg ulcers, compression therapy, clinical assessments and use of guidelines were found to be cost-saving in the UK44 45 and USA.46 47
Our findings provide fundamental information for researchers who wish to model the cost-effectiveness of programmes that will improve wound outcomes in the future. Understanding the baseline of costs and QALY outcomes form a useful start-point for any evaluation of interventions.
Conclusions
The costs of chronic wounds are large in Singapore, but many of them could be avoided by making positive investments in integrated and comprehensive wound prevention and treatment programmes.
Supplementary Material
Footnotes
Contributors: NG, GG, KT, OG, JH, TTC, PB, DC, ASY, YZN, ZL, YE, FABA, WZ and KH made substantial contributions to the conception and design of the paper, the acquisition, analysis and interpretation of data; drafting and reviewing versions of the manuscript; and final approval of the version submitted. All authors are accountable for all aspects of the work. NG is guarantor and accepts full responsibility for the work and/or the conduct of the study, had access to the data, and controlled the decision to publish.
Funding: We would like to acknowledge the funding supported from the Agency for Science, Technology and Research (A*STAR) under its Industry Alignment Fund-Pre-Positioning Programme (IAF-PP) grant number H1901a00Y9/ and H17/01/a0/0CC9 as part of the Wound Care Innovation for the Tropics (WCIT) Programme.
Competing interests: None declared.
Patient and public involvement: Patients and/or the public were not involved in the design, or conduct, or reporting or dissemination plans of this research.
Provenance and peer review: Not commissioned; externally peer reviewed.
Supplemental material: This content has been supplied by the author(s). It has not been vetted by BMJ Publishing Group Limited (BMJ) and may not have been peer-reviewed. Any opinions or recommendations discussed are solely those of the author(s) and are not endorsed by BMJ. BMJ disclaims all liability and responsibility arising from any reliance placed on the content. Where the content includes any translated material, BMJ does not warrant the accuracy and reliability of the translations (including but not limited to local regulations, clinical guidelines, terminology, drug names and drug dosages), and is not responsible for any error and/or omissions arising from translation and adaptation or otherwise.
Data availability statement
No data are available. No data from the wound registry or MOH administrative databases are available. This is due to the privacy considerations of the Singapore MOH and the Singapore Wound Registry.
Ethics statements
Patient consent for publication
Not applicable.
References
- 1.Raeder K, Jachan DE, Müller-Werdan U, et al. Prevalence and risk factors of chronic wounds in nursing homes in Germany: a cross-sectional study. Int Wound J 2020;17:1128–34. 10.1111/iwj.13486 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Graves N, Phillips CJ, Harding K. A narrative review of the epidemiology and economics of chronic wounds. Br J Dermatol 2022;187:141–8. 10.1111/bjd.20692 [DOI] [PubMed] [Google Scholar]
- 3.Dávila Torres J. [To err is human, but to not put processes in place to avoid errors from becoming fatal is inhumane. 5th International summit of the patient safety movement (PSM), California, USA, 2017]. Cir Cir 2017;85:101–3. 10.1016/j.circir.2017.03.001 [DOI] [PubMed] [Google Scholar]
- 4.Olsson M, Järbrink K, Divakar U, et al. The humanistic and economic burden of chronic wounds: a systematic review. Wound Repair Regen 2019;27:114–25. 10.1111/wrr.12683 [DOI] [PubMed] [Google Scholar]
- 5.Posnett J, Franks PJ. The burden of chronic wounds in the UK. Nurs Times 2008;104:44–5. [PubMed] [Google Scholar]
- 6.Gottrup F, Holstein P, Jørgensen B, et al. A new concept of a multidisciplinary wound healing center and a national expert function of wound healing. Arch Surg 2001;136:765–72. 10.1001/archsurg.136.7.765 [DOI] [PubMed] [Google Scholar]
- 7.Pacella RE, Tulleners R, McCosker L, et al. Reimbursement for the cost of compression therapy for the management of venous leg ulcers in Australia. Int Wound J 2019;16:1069–72. 10.1111/iwj.13152 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Gyawali B, Sullivan R, Booth CM. Cancer groundshot: going global before going to the moon. Lancet Oncol 2018;19:288–90. 10.1016/S1470-2045(18)30076-7 [DOI] [PubMed] [Google Scholar]
- 9.Cheng Q, Graves N, Pacella RE. Economic evaluations of guideline-based care for chronic wounds: a systematic review. Appl Health Econ Health Policy 2018;16:633–51. 10.1007/s40258-018-0403-9 [DOI] [PubMed] [Google Scholar]
- 10.Lo ZJ, Lim X, Eng D, et al. Clinical and economic burden of wound care in the tropics: a 5-year institutional population health review. Int Wound J 2020;17:790–803. 10.1111/iwj.13333 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Fitridge R, Pena G, Mills JL. The patient presenting with chronic limb-threatening ischaemia. Does diabetes influence presentation, limb outcomes and survival? Diabetes Metab Res Rev 2020;36 Suppl 1:e3242. 10.1002/dmrr.3242 [DOI] [PubMed] [Google Scholar]
- 12.Sorber R, Abularrage CJ. Diabetic foot ulcers: epidemiology and the role of multidisciplinary care teams. Semin Vasc Surg 2021;34:47–53. 10.1053/j.semvascsurg.2021.02.006 [DOI] [PubMed] [Google Scholar]
- 13.Martinengo L, Olsson M, Bajpai R, et al. Prevalence of chronic wounds in the general population: systematic review and meta-analysis of observational studies. Ann Epidemiol 2019;29:8–15. 10.1016/j.annepidem.2018.10.005 [DOI] [PubMed] [Google Scholar]
- 14.Kapp S, Santamaria N. The financial and quality-of-life cost to patients living with a chronic wound in the community. Int Wound J 2017;14:1108–19. 10.1111/iwj.12767 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.McCabe C, Claxton K, Culyer AJ. The NICE cost-effectiveness threshold: what it is and what that means. Pharmacoeconomics 2008;26:733–44. 10.2165/00019053-200826090-00004 [DOI] [PubMed] [Google Scholar]
- 16.Jo C. Cost-of-illness studies: concepts, scopes, and methods. Clin Mol Hepatol 2014;20:327–37. 10.3350/cmh.2014.20.4.327 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Ministry of health population in brief . 2022. Available: https://www.population.gov.sg/images/PublicationImages/population-in-brief-2017.pdf
- 18.Department of Statistics, M.o.T.I, Republic of Singapore . Census of population 2020 statistical release 1: demographic characteristics, education, language and religion,. 2020Available: https://www.singstat.gov.sg/-/media/files/publications/cop2020/sr1/cop2020sr1.ashx
- 19.Dodd S, Bassi A, Bodger K, et al. A comparison of multivariable regression models to analyse cost data. J Eval Clin Pract 2006;12:76–86. 10.1111/j.1365-2753.2006.00610.x [DOI] [PubMed] [Google Scholar]
- 20.Goh OQ, Ganesan G, Graves N, et al. Incidence of chronic wounds in Singapore, a multiethnic Asian country, between 2000 and 2017: a retrospective cohort study using a nationwide claims database. BMJ Open 2020;10:e039411. 10.1136/bmjopen-2020-039411 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Devlin N, Parkin D, Janssen B. Methods for analysing and reporting EQ-5D Data. Springer, 2020. 10.1007/978-3-030-47622-9 [DOI] [PubMed] [Google Scholar]
- 22.Luo N, Wang P, Thumboo J, et al. Valuation of EQ-5D-3L health states in Singapore: modeling of time trade-off values for 80 empirically observed health states. Pharmacoeconomics 2014;32:495–507. 10.1007/s40273-014-0142-1 [DOI] [PubMed] [Google Scholar]
- 23.Abdin E, Subramaniam M, Vaingankar JA, et al. Population norms for the EQ-5D index scores using Singapore preference weights. Qual Life Res 2015;24:1545–53. 10.1007/s11136-014-0859-5 [DOI] [PubMed] [Google Scholar]
- 24.World Bank . GDP per capita (current US$) - Singapore. 2018. Available: https://data.worldbank.org/indicator/NY.GDP.PCAP.CD?locations=SG [Accessed Jun 2018].
- 25.Williams A. What could be nicer than NICE? OHE annual lecture. O.O.H. economics London, Available: ///C:/Users/gmsgnic/Downloads/292%20-%202004_What_Could_Be_Nicer_Williams.pdf [Google Scholar]
- 26.Ministry of Health Singapore . Government health expenditure and healthcare financing. 2022. Available: https://www.moh.gov.sg/resources-statistics/singapore-health-facts/government-health-expenditure-and-healthcare-financing
- 27.Posnett J, Franks PJ. The costs of skin breakdown and ulceration in the UK, in skin breakdown: the silent epidemic. Hull: Smith & Nephew Foundation; 2007. [Google Scholar]
- 28.Böttrich JG. Challenges in chronic wound care: the need for interdisciplinary collaboration, Available: http://www.eucomed.be/blog/108/59/blog/2012/02/23/Challenges-in-chronic-wound-care-the-need-for-interdisciplinary-collaboration [Accessed 02 Mar 2013].
- 29.Png ME, Yoong J, Phan TP, et al. Current and future economic burden of diabetes among working-age adults in Asia: conservative estimates for Singapore from 2010-2050 BMC Public Health 2016;16:589. 10.1186/s12889-016-3164-0 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Ministry of Health . War on diabetes. 2022. Available: https://www.moh.gov.sg/wodcj
- 31.Nazeha N, Lee JY, Saffari SE, et al. The burden of costs on health services from patients with venous leg ulcers in Singapore. Int Wound J 2023;20:845–52. 10.1111/iwj.13931 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Purwins S, Herberger K, Debus ES, et al. Cost-of-illness of chronic leg ulcers in Germany. Int Wound J 2010;7:97–102. 10.1111/j.1742-481X.2010.00660.x [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Rawlins MD, Culyer AJ. National institute for clinical excellence and its value judgments. BMJ 2004;329:224–7. 10.1136/bmj.329.7459.224 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Foot, I.W.G.o.t.D . International consensus on the diabetic foot. Amsterdam, 1999. [Google Scholar]
- 35.Cárdenas MK, Mirelman AJ, Galvin CJ, et al. The cost of illness attributable to diabetic foot and cost-effectiveness of secondary prevention in Peru. BMC Health Serv Res 2015;15:483.:483. 10.1186/s12913-015-1141-4 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Cheng Q, Lazzarini PA, Gibb M, et al. A cost-effectiveness analysis of optimal care for diabetic foot ulcers in Australia. Int Wound J 2017;14:616–28. 10.1111/iwj.12653 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Rerkasem K, Kosachunhanun N, Tongprasert S, et al. A multidisciplinary diabetic foot protocol at Chiang Mai University Hospital: cost and quality of life. Int J Low Extrem Wounds 2009;8:153–6. 10.1177/1534734609344143 [DOI] [PubMed] [Google Scholar]
- 38.Wu B, Wan X, Ma J. Cost-effectiveness of prevention and management of diabetic foot ulcer and amputation in a health resource-limited setting. J Diabetes 2018;10:320–7. 10.1111/1753-0407.12612 [DOI] [PubMed] [Google Scholar]
- 39.Mathiesen ASM, Nørgaard K, Andersen MFB, et al. Are labour-intensive efforts to prevent pressure ulcers cost-effective? J Med Econ 2013;16:1238–45. 10.3111/13696998.2013.832256 [DOI] [PubMed] [Google Scholar]
- 40.Padula WV, Mishra MK, Makic MBF, et al. Improving the quality of pressure ulcer care with prevention: a cost-effectiveness analysis. Med Care 2011;49:385–92. 10.1097/MLR.0b013e31820292b3 [DOI] [PubMed] [Google Scholar]
- 41.Thomson JS, Brooks RG. The economics of preventing and treating pressure ulcers: a pilot study. J Wound Care 1999;8:312–6. 10.12968/jowc.1999.8.6.25879 [DOI] [PubMed] [Google Scholar]
- 42.Xakellis GC, Frantz RA. The cost-effectiveness of interventions for preventing pressure ulcers. J Am Board Fam Pract 1996;9:79–85. [PubMed] [Google Scholar]
- 43.Xakellis GC, Frantz RA, Lewis A, et al. Cost-effectiveness of an intensive pressure ulcer prevention protocol in long-term care. Adv Wound Care 1998;11:22–9. [PubMed] [Google Scholar]
- 44.Bosanquet N, Franks P, Moffatt C, et al. Community leg ulcer clinics: cost-effectiveness. Health Trends 1993;25:146–8. [PubMed] [Google Scholar]
- 45.Simon DA, Freak L, Kinsella A, et al. Community leg ulcer clinics: a comparative study in two health authorities. BMJ 1996;312:1648–51. 10.1136/bmj.312.7047.1648 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Korn P, Patel ST, Heller JA, et al. Why insurers should reimburse for compression stockings in patients with chronic venous stasis. J Vasc Surg 2002;35:950–7. 10.1067/mva.2002.121984 [DOI] [PubMed] [Google Scholar]
- 47.McGuckin M, Waterman R, Brooks J, et al. Validation of venous leg ulcer guidelines in the United States and United Kingdom. Am J Surg 2002;183:132–7. 10.1016/s0002-9610(01)00856-x [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
bmjopen-2022-065692supp001.pdf (254.2KB, pdf)
Data Availability Statement
No data are available. No data from the wound registry or MOH administrative databases are available. This is due to the privacy considerations of the Singapore MOH and the Singapore Wound Registry.