Abstract
Objective
To estimate the incidence of infections among patients with psoriatic arthritis (PsA) or axial spondyloarthritis (axSpA), two distinct phenotypes included in the large group of spondyloarthritis (SpA), treated with tumour necrosis-factor-inhibitors, interleukin-17-inhibitors, Janus kinase-inhibitors, IL-23 or IL-12/23-inhibitors (IL-12/23i), phosphodiesterase 4-inhibitors or cytotoxic T-lymphocyte associated protein 4-Ig.
Methods
A meta-analysis of randomised controlled trials (RCTs), open-label extension and observational studies was conducted. Serious infections were defined as infections that were life-threatening, required intravenous antibiotics and/or hospitalisation. Non-serious infections did not meet these severity criteria. The incidence rates (IR) were reported for each diagnosis by treatment class and study type using random-effect model to create a 95% CI.
Results
Among 23 333 PsA patients and 11 457 axSpA patients, there were 1.09 serious infections per 100 patient-years (PY) (95% CI 0.85 to 1.35) with similar IR in PsA (0.96 per 100 PY 95% CI 0.69 to 1.28) and axSpA (1.09 per 100 PY 95% CI 0.76 to 1.46). The IR was lower in RCTs (0.77 per 100 PY 95% CI 0.41 to 1.20) compared with observational studies (1.68 per 100 PY 95% CI 1.03 to 2.47). In PsA patients, the lowest IR value was observed with IL-12/23i (0.29 per 100 PY 95% CI 0.00 to 1.03). There were 53.0 non-serious infections per 100 PY (95% CI 43.47 to 63.55) in 7257 PsA patients and 5638 axSpA patients. The IR was higher in RCTs (69.95 per 100 PY 95% CI 61.59 to 78.84) compared with observational studies (15.37 per 100 PY 95% CI 5.11 to 30.97).
Conclusion
Serious infections were rare events in RCTs and real-life studies. Non-serious infections were common adverse events, mainly in RCTs.
PROSPERO registration number
CRD42020196711
Keywords: Infections; Arthritis, Psoriatic; Spondylitis, Ankylosing; Biological Therapy
WHAT IS ALREADY KNOWN ON THIS TOPIC
While the risk of infections in patients with rheumatoid arthritis or psoriasis treated with biological or targeted disease-modifying agents (b/tsDMARDs) has been extensively studied, there is limited research on the incidence of serious and non-serious infections in patients with spondyloarthritis, including psoriatic arthritis (PsA) and axial spondyloarthritis (axSpA).
WHAT THIS STUDY ADDS
This meta-analysis provides an assessment of the incidence rates of serious and non-serious infections, both overall and stratified by diagnosis (PsA or axSpA), for each class of treatment in randomised trials (RCTs), open-label extensions and observational studies.
Serious infections were rare events in patients with PsA or axSpA. In observational studies, the incidence tended to be higher compared with RCTs. The lowest incidence rate was observed with the use of IL-12/23 inhibitors (IL-12/23i) and phosphodiesterase 4 inhibitors, which were specifically used in patients with PsA.
Non-serious infections showed a trend of higher incidence in RCTs and were likely under-reported in observational studies.
HOW THIS STUDY MIGHT AFFECT RESEARCH, PRACTICE OR POLICY
This meta-analysis reassures clinicians about prescription of b/tsDMARD in patients with PsA or axSpA regarding the risk of serious infections. However, it is important to note that non-serious infections were common adverse events that could have an impact on patient management.
Introduction
Spondyloarthritis (SpA) is a group of inflammatory rheumatic diseases including distinct disorders with axial inflammation and/or peripheral arthritis, enthesitis or dactylitis. Axial SpA (axSpA) refer either to ankylosing spondylitis (AS) or non-radiographic axSpA (nr-axSpA) based on radiographic structural damage according to the modified New-York criteria. Psoriatic arthritis (PsA) is another subtype of SpA with skin inflammation associated to joint involvement.1
Six biologics or targeted (b/tsDMARD) modifying disease agents are currently prescribed in PsA or axSpA with large clinical efficacy in active disease, refractory to non-steroid anti-inflammatory drugs (NSAID). These treatments act either by inhibiting an inflammatory cytokine or its receptor (tumour necrosis factor (TNF), interleukin (IL)-17, IL-23, IL-12/23), molecules involved in cell activation (cytotoxic T-lymphocyte associated protein 4 (CTLA4)) with CTLA4-Ig, or intracellular signal pathways (Janus kinase (JAK) and phosphodiesterase 4 (PDE4)).2 3 TNF-inhibitors (TNFi), IL-17-inhibitors (IL-17i) and JAK-inhibitors (JAKi) are used in PsA and axSpA, whereas IL-23-inhibitors (IL-23i), IL-12/23-inhibitors (IL-12/23i), PDE4-inhibitors (PDE4i) and CTLA4-Ig are only used in PsA due to their lack of efficacy on the axial symptoms.
Apart from the efficacy, safety and tolerance are two important parameters for therapeutic choice. Infections are a known side effect of these molecules, consistent with their mechanisms of action on the pathways of inflammation. A distinction is made between two types of infections. Serious infections are usually defined as life threatening, requiring intravenous antibiotics or hospitalisation. In the absence of consensual definition, we chose to define non-serious infections (NSI) as any infection which do not meet the criteria of serious infections.4 NSI may include upper respiratory tract infections, influenza syndromes, nasopharyngitis, urinary tract infections, gastrointestinal infections, herpes infections, opportunistic infections, candida infections and skin infections. Although NSI are not life-threatening, their occurrence could be responsible for poor treatment compliance.5
Most of the data on the risk of infection in patients treated with b/tsDMARD are known from their use in other inflammatory diseases such as rheumatoid arthritis or psoriasis. However, characteristics of patients differ from one inflammatory disease to another, particularly in terms of age, comorbidities, pathophysiology of rheumatic diseases and associated treatments. Thus, it is difficult to extrapolate safety data from different populations.
Here, we present a meta-analysis to explore the incidence rate (IR) of serious and NSI in patients with SpA including PsA and axSpA stratified by diagnosis and according to class of treatment and study design (randomised controlled trials (RCTs), open-label extension (OLE) and observational studies).
Material and methods
Literature research and study selection
The databases PubMed and Cochrane Central Register of Controlled Trial (CENTRAL) were searched from their date of inception to 30 March 2021, using a predefined search equation (online supplemental table 1). When necessary, additional studies were identified through hand searching.
rmdopen-2023-003064supp001.pdf (518.1KB, pdf)
The study was performed according to the Preferred Reporting the Items for Systematic Review and Meta-Analysis (PRISMA) statement for RCTs and OLE and in agreement with current recommendations of meta-analysis of observational studies in epidemiology group (MOOSE group) for observational studies (online supplemental table 2).
Three authors (MA, MS and SL) independently assessed titles and abstracts to identify relevant studies for inclusion. Discrepancies were discussed and when necessary, a fourth reviewer (FC-L) resolved any disagreements. The full text of the studies was obtained when necessary to confirm inclusion.
Inclusion criteria
The studies were included if they: (1) investigated the number of serious infections defined as life-threatening, requiring intravenous antibiotics or hospitalisations or NSI defined as any event coded in the MedDRA SOC Infection and Infestation (MedDRA or Medical Dictionary for Regulatory Activities https://www.meddra.org) in RCTs and OLE and defined as any infection which do not meet the criteria of serious infections in observational studies; (2) involved patients with AS fulfilling modified New-York criteria, nr-AxSpA fulfilling Assessment of SpondyloArthritis International Society classification criteria for PsA and axSpA fulfilling Classification Criteria for Psoriatic Arthritis and (3) included patients treated with bDMARD or tsDMARD (TNF inhibitors(TNFi): etanercept, infliximab, golimumab, adalimumab and certolizumab pegol; IL-17i: secukinumab, ixekinumab, bimekizumab and brodalumab; IL-23i: ustekinumab, guselkumab and risankizumab; CTLA4-Ig: abatacept; JAKi: tofacitinib, upadacitinib and filgotinib; PDE4i: apremilast).
Studies with other design than RCTs, OLE or observational studies, such as mixed data of several pathologies other than AS, nr-AxSpA or PsA and studies in other languages than English were excluded. In addition, OLE should comprise RCTs data to be included.
Data extraction and quality assessment
The following data were independently extracted by two authors (MA and MS) for each study: NCT (National Clinical Trial) number, year of publication, study design, country, pathology, population description, type and dose of b/tsDMARD, follow-up time, characteristics of population (sex, age, disease duration, disease activity, C-reactive protein, body mass index, concomitant treatment, previous biological therapy and smoking status), number of serious infections and number of NSI. The included studies used a variety of methods to report incidence. We used patient year (PY) when it was available in studies, or we calculated it if necessary.
If data were missing in the article, the corresponding authors were contacted by e-mail. Disagreements about data extraction were resolved by discussion with another independent author (FC-L). Revised Cochrane risk-of-bias tool for randomised trials (RoB V.2, www.riskofbias.info) was used to assess the quality of RCTs and the Newcastle-Ottawa Scale (NOS) was used to assess the quality of OLE and observational studies (online supplemental table 3).
Statistical analysis
The IR of infections was separated into two major groups, according to whether these events were serious infections or NSI. Then, we performed subgroup analyses by dividing all studies according to study type and treatment class. Those analysis were stratified according to the disease (PsA, axSpA and SpA).
Forest plots were constructed from the extracted data to create a 95% CI using R V.3.4 with package ‘meta’ (V.4.9–2) and ‘metafor’ (V.2.0–0). The IR of events (serious or NSI) and their 95% CI were estimated using inverse variance method and Freeman-Tukey double arcsine transformation. IR was expressed in events per 100 PY. Heterogeneity was assessed using inconsistency index (I2). If substantial heterogeneity was observed (I2>50%), a random-effects model was used based on Hartung-Knapp adjustment. The planned subgroup analyses were the following: study design, class of treatment and disease.
Results
Search results
The initial search strategy identified 379 citations on PubMed and 348 citations on CENTRAL of which 96 were included (60 RCTs, 20 OLEs and 16 observational studies). A total of 89 studies (56 RCTs, 19 OLEs and 14 observational studies) about serious infections (n=33 892 patients with 33 168 PY of follow-up; 849 events) and 55 studies (37 RCTs, 8 OLEs and 10 observational studies) about NSI (n=13 008 patients with 12 451 PY of follow-up; 5339 events) were included (figure 1, table 1). The characteristics of patients are available in online supplemental table 4.
Figure 1.
Flowchart. axSpA, axial spondyloarthritis; PsA, psoriatic arthritis; RCT, randomised controlled trial; OLE, open-label extension.
Table 1.
Characteristics of the studies included in the meta-analysis of severe and global infections.
| Author (study name) | Year | Study design registry number | Molecule | Dose | Diagnosis | Patient in treatment group (n) | Study duration (weeks) | Outcome | |
| SI | NSI | ||||||||
| TNF-inhibitors studies | |||||||||
| Antoni (IMPACT) | 2005 | RCT phase 3 | Infliximab | 5 mg / kg / 6 w | PsA | 52 | 16 | X | |
| Van der Heijde (ASSERT) | 2005 | RCT phase 3 | Infliximab | 5 mg / kg / 6 w | AS | 201 | 24 | X | X |
| Sieper (INFAST) | 2014 | RCT phase 3 NCT00844805 | Infliximab | 5 mg / kg / 6 w | AS | 105 | 28 | X | X |
| Park (PLANETAS) | 2016 | OLE | Infliximab | 5 mg / kg / 6 w | AS | 174 | 102 | X | X |
| Davis | 2003 | RCT phase 3 | Etanercept | 25 mg / 2 w | AS | 138 | 24 | X | |
| Mease | 004 | RCT phase 3 | Etanercept | 25 mg / 2 w | PsA | 101 | 24 | X | |
| Braun | 2011 | RCT phase 3 NCT00247962 | Etanercept | 50 mg / 1 w | AS | 379 | 16 | X | |
| Dougados | 2014 | RCT phase 3 | Etanercept | 50 mg / 1 w | Nr-AxSpA | 106 | 12 | X | X |
| Mease | 2019 | RCT phase 3 NCT02376790 | Etanercept | 50 mg / 1 w | PsA | 567 | 48 | X | |
| Davis | 2007 | OLE | Etanercept | 25 mg / 2 w and 50 mg / 1 w | AS | 257 | 192 | X | X |
| Martin-Mola | 2010 | OLE | Etanercept | 25 mg / 2 w and 50 mg / 1 w | AS | 56 | 156 | X | X |
| Inman (GO RAISE) | 2008 | RCT phase 3 NCT00265083 | Golimumab | 50 or 100 mg / 4 w | AS | 278 | 24 | X | X |
| Kavanaugh (GOREVEAL) | 2009 | RCT phase 3 NCT00265096 | Golimumab | 50 or 100 mg / 4 w | PsA | 292 | 24 | X | X |
| Kavanaugh (GOVIBRANT) | 2017 | RCT phase 3 NCT02181673 | Golimumab | 2 mg / kg / 8 w IV | PsA | 240 | 24 | X | X |
| Deodhar (GO ALIVE) | 2018 | RCT phase 3 NCT02186873 | Golimumab | 2 mg / kg / 8 w IV | AS | 105 | 16 | X | X |
| Kavanaugh (GO REVEAL) | 2013 | OLE | Golimumab | 50 or 100 mg / 4 w SC | PsA | 394 | 268 | X | |
| Deodhar (GO RAISE) | 2014 | OLE | Golimumab | 50 or 100 mg / 4 w SC | AS | 353 | 268 | X | |
| Mease (ADEPT) | 2005 | RCT phase 3 NCT00195689 | Adalimumab | 40 mg / 2 w SC | PsA | 151 | 24 | X | X |
| Van der Heijde (ATLAS) | 2006 | RCT phase 3 | Adalimumab | 40 mg / 2 w SC | AS | 208 | 24 | X | X |
| Genovese | 2007 | RCT phase 3 | Adalimumab | 40 mg / 2 w SC | PsA | 51 | 12 | X | X |
| Huang | 2012 | RCT phase 3 NCT01114880 | Adalimumab | 40 mg / 2 w SC | AS | 229 | 12 | X | X |
| Sieper (ABILITY 1) | 2013 | RCT phase 3 NCT00939003 | Adalimumab | 40 mg / 2 w SC | Nr-AxSpA | 91 | 12 | X | X |
| Mease (ADEPT) | 2008 | OLE | Adalimumab | 40 mg / 2 w SC | PsA | 298 | 144 | X | X |
| Van der Heijde (ATLAS) | 2009 | OLE | Adalimumab | 40 mg / 2 w SC | AS | 311 | 104 | X | X |
| Van der Heijde (ABILITY1) | 2018 | OLE | Adalimumab | 40 mg / 2 w SC | Nr-AxSpA | 190 | 156 | X | |
| Mease (RAPID-PsA) | 2014 | RCT phase3 NCT01087762 | Certolizumabpegol | 200 mg / 2 w or 400 mg / 4 w SC | PsA | 273 | 24 | X | X |
| Landewe (RAPID-AxSpA) | 2014 | RCT phase 3 NCT01087762 | Certolizumabpegol | 200 mg / 2 w or 400 mg / 4 w SC | AS | 218 | 24 | X | X |
| Kristensen | 2007 | Observational | Several TNF-inhibitors | NA | PsA | 261 | 105.1 | X | |
| Saad | 2010 | Observational | Several TNF-inhibitors | NA | PsA | 596 | 156 | X | |
| Modesti | 2012 | Observational | Several TNF-inhibitors | NA | AS/PsA | 225 | 132.6 | X | X |
| Moura | 2019 | Observational | Several TNF-inhibitors | NA | SA | 369 | 103 | X | |
| Wallis | 2014 | Observational | Several TNF-inhibitors | NA | Nr-AxSpA | 264 | 134.7 | X | X |
| Ruberson Da Silva | 2018 | Observational | Several TNF-inhibitors | NA | PsA | 122 | 24 | X | |
| Rusman | 2018 | Observational | Several TNF-inhibitors | NA | AS | 122 | 221 | X | X |
| Wroński | 2019 | Observational | Several TNF-inhibitors | NA | AS | 103 | 72 | X | X |
| IL-17 inhibitors | |||||||||
| Baeten | 2013 | RCT phase 2 NCT00809159 | Secukinumab | 10 mg / kg / 3 w IV | AS | 24 | 28 | X | X |
| McInnes | 2014 | RCT phase 2 NCT00809614 | Secukinumab | 10 mg / kg / 3 w IV | PsA | 28 | 24 | X | |
| Baeten (MEASURE 2) | 2015 | RCT phase 3 NCT01649375 | Secukinumab | 150 or 75 mg / 4 w SC | AS | 145 | 16 | X | |
| Mease (FUTURE 1) | 2015 | RCT phase 3 NCT01392326 | Secukinumab | 150 or 75 mg / 4 w, after IV loading infusion 10 mg / kg | PsA | 406 | 24 | X | |
| McInnes (FUTURE 2) | 2016 | RCT phase 3 NCT01752634 | Secukinumab | 75 or 150 or 300 mg SC | PsA | 299 | 16 | X | |
| Kivitz (MEASURE 4) | 2018 | RCT phase 3 NCT02159053 | Secukinumab | 150 mg / 4 w SC | AS | 233 | 16 | X | |
| Mease (FUTURE 5) | 2018 | RCT phase 3 NCT02404350 | Secukinumab | 150 or 300 mg / 4 w SC | PsA | 664 | 24 | X | |
| Huang (MEASURE 5) | 2020 | RCT phase 3 NCT02896127 | Secukinumab | 150 mg / 4 w SC | AS | 305 | 16 | X | |
| Deodhar (PREVENT) | 2021 | RCT phase 3 NCT02696031 | Secukinumab | 150 mg / 4 w SC | Nr-AxSpA | 369 | 20 | X | |
| McInnes (FUTURE 2) | 2017 | OLE | Secukinumab | 75 or 150 or 300 mg SC | PsA | 387 | 104 | X | X |
| Kivitz (MEASURE 4) | 2018 | OLE | Secukinumab | 150 mg / 4 w SC | AS | 346 | 104 | X | |
| Nash (FUTURE 3) | 2018 | OLE | Secukinumab | 150 or 300 mg / 4 w SC | PsA | 406 | 52 | X | |
| Baraliakos (MEASURE 1) | 2019 | OLE | Secukinumab | 150 or 75 mg / 4 w SC, after IV loading | AS | 360 | 260 | X | |
| Kivitz (FUTURE 4) | 2019 | OLE | Secukinumab | 150 mg / 4 w SC | PsA | 334 | 104 | X | |
| Huang (MEASURE 5) | 2020 | OLE | Secukinumab | 150 mg / 4 w SC | AS | 453 | 52 | X | |
| Van der Heijde (FUTURE 5) | 2020 | OLE | Secukinumab | 150 or 300 mg / 4 w SC | PsA | 255 | 52 | X | |
| Deodhar | 2021 | OLE | Secukinumab | 150 mg / 4 w SC | Nr-AxSpA | 369 | 104 | X | |
| Kiltz | 2020 | Observational | Secukinumab | MD | AS/PsA | 1001 | NA | X | |
| Klavdianou | 2020 | Observational | Secukinumab | 300 mg / 4 w SC | PsA | 75 | 52 | X | X |
| Ramonda | 2020 | Observational | Secukinumab | 150 or 300 mg / 4 w SC | PsA | 608 | 104 | X | X |
| Mease (SPIRIT P1) | 2017 | RCT phase 3 NCT01695239 | Ixekizumab | 80 mg / 2 w / 4 w SC | PsA | 210 | 24 | X | X |
| Nash (SPIRIT P2) | 2017 | RCT phase 3 NCT02349295 | Ixekizumab | 80 mg / 2 w / 4 w SC | PsA | 245 | 24 | X | X |
| Van der Heijde (COASTV) | 2018 | RCT phase 3 NCT02696785 | Ixekizumab | 80 mg / 2 w4 w SC | AS | 164 | 16 | X | X |
| Deodhar (COAST X) | 2019 | RCT phase 3 NCT02757352 | Ixekizumab | 80 mg / 2 w / 4 w SC | Nr-AxSpA | 198 | 52 | X | X |
| Deodhar (COAST W) | 2019 | RCT phase 3 NCT02696798 | Ixekizumab | 80 mg / 2 w / 4 w SC | AS | 212 | 16 | X | X |
| Dougados (COAST V/W) | 2020 | OLE | Ixekizumab | 80 mg / 2w / 4 w SC | AS | 641 | 52 | X | X |
| Orbai (SPIRIT P2) | 2021 | OLE | Ixekizumab | 80 mg / 2w / 4 w SC | PsA | 337 | 156 | X | X |
| Ritchlin (BE ACTIVE) | 2020 | RCT phase 2 NCT02969525 | Bimekizumab | 16, 160 or 320 mg / 4 w SC | PsA | 164 | 12 | X | |
| Van der Heijde (BEAGILE) | 2020 | RCT phase 2 NCT02963506 | Bimekizumab | 16, 64, 160 or 320 mg / 4 w SC | AS | 243 | 12 | X | |
| Coates (BE ACTIVE) | 2021 | OLE | Bimekizumab | 16, 64, 160 or 320 mg / 4 w SC | PsA | 204 | 152 | X | |
| Mease | 2014 | RCT phase 2 NCT01516957 | Brodalumab | 140 or 280 mg / 2 w SC | PsA | 113 | 12 | X | |
| Mease (AMVISION 1–2) | 2021 | RCT phase 3 NCT02029495NCT02024646 | Brodalumab | 140 or 210 mg / 2 w SC | PsA | 640 | 24 | X | X |
| IL-23 inhibitors | |||||||||
| Gottlieb | 2009 | RCT phase 2 NCT00267956 | Ustekinumab | 90 or 63 mg / 1 w SC | PsA | 76 | 12 | X | X |
| McInnes (PSUMITT 1) | 2013 | RCT phase 3 NCT01009086 | Ustekinumab | 45 or 90 mg SC | PsA | 409 | 16 | X | X |
| Ritchlin | 2014 | RCT phase 2 NCT01077362 | Ustekinumab | 45 or 90 mg w0–w4, then every 12 w SC | PsA | 207 | 24 | X | X |
| Chimenti | 2017 | Observational | Ustekinumab | MD | PsA | 65 | 104 | X | X |
| Deodhar | 2018 | RCT phase 2 NCT02319759 | Guselkumab | 100 mg / 8 w SC | PsA | 100 | 24 | X | X |
| Deodhar (DISCOVER 1) | 2020 | RCT phase 3 NCT03162796 | Guselkumab | 100 mg / 4 w / 8 w SC | PsA | 255 | 24 | X | X |
| Mease (DISCOVER 2) | 2020 | RCT phase 3 NCT03158285 | Guselkumab | 100 mg / 4 w / w SC | PsA | 493 | 24 | X | X |
| McInnes | 2021 | OLE | Guselkumab | 100 mg / 4 w / 8 w SC | PsA | 731 | 52 | X | |
| Baeten | 2018 | RCT phase 2 NCT02047110 | Risankizumab | 18, 90, 180 mg / 8 w SC | AS | 119 | 16 | X | X |
| CTLA4-Ig | |||||||||
| Mease | 2011 | RCT phase 2 NCT00534313 | Abatacept | 3 or 10 mg / kg (±2 initial doses of 30 mg / kg) IV / 28 days | PsA | 128 | 24 | X | |
| Mease (ASTRAEA) | 2017 | RCT phase 3 NCT01860976 | Abatacept | 125 mg / 1 w SC | PsA | 213 | 24 | X | X |
| JAK-inhibitors | |||||||||
| Gladman (OPAL Beyond) | 2017 | RCT phase 3 NCT01882439 | Tofacitinib | 5 mg or 10 mg 2x / day | PsA | 263 | 12 | X | |
| Mease (OPAL Broaden) | 2017 | RCT phase 3 NCT01877668 | Tofacitinib | 5 mg or 10 mg 2x / day | PsA | 211 | 12 | X | |
| Van der Heijde | 2017 | RCT phase 2NCT01786668 | Tofacitinib | 2, 5 or 10 mg 2x / day | AS | 156 | 16 | X | X |
| Mease (EQUATOR) | 2018 | RCT phase 2 NCT03101670 | Filgotinib | 200 mg / day | PsA | 65 | 16 | X | X |
| Van der Heijde (TORTUGA) | 2018 | RCT phase 2 NCT03117270 | Filgotinib | 200 mg / day | AS | 58 | 12 | X | X |
| Van der Heijde (SELECT-AXIS 1) | 2019 | RCT phase 2 NCT03178487 | Upadacitinib | 15 mg / day | AS | 93 | 14 | X | X |
| PDE4 inhibitors | |||||||||
| Schett | 2012 | RCT phase 2 NCT00456092 | Apremilast | 20 mg 2x / day or 40 mg / day | PsA | 136 | 12 | X | |
| Kavanaugh (PALACE 1) | 2014 | RCT phase 3 NCT01172938 | Apremilast | 20 or 30 mg 2x / day | PsA | 336 | 24 | X | |
| Cutolo (PALACE 2) | 2016 | RCT phase 3 NCT01212757 | Apremilast | 20 or 30 mg 2x / day | PsA | 325 | 16 | X | |
| Edwards (PALACE 3) | 2016 | RCT phase 3 NCT01212770 | Apremilast | 20 or 30 mg 2x / day | PsA | 336 | 24 | X | |
| Nash (ACTIVE) | 2018 | RCT phase 3 NCT01925768 | Apremilast | 30 mg 2x / day | PsA | 110 | 24 | X | |
| Wells (PALACE 4) | 2018 | RCT phase 3 NCT01307423 | Apremilast | 20 or 30 mg 2x / day | PsA | 350 | 24 | X | |
| Several treatments | |||||||||
| Ritchlin | 2019 | Observational | Ustekinumab, adalimumab, etanercept, infliximab | NA | PsA | 2041 | 256.4 | X | |
| Li | 2020 | Observational | Ixekizumab, secukinumab, ustekinumab, adalimumab, certolizumab pegol, etanercept, golimumab, infliximab | NA | PsA | 5520 | 31.2 | X | |
| Rahman | 2020 | Observational | Golimumab, infliximab, ustekinumab | NA | PsA | 462 | 416 | X | X |
| Krabbe | 2021 | Observational | Abatacept, adalimumab, certolizumab pegol, etanercept, golimumab, infliximab, secukinumab, ustekinumab | NA | AS/PsA | 5387 | 52 | X | |
AS, ankylosing spondylitis; CTLA4, cytotoxic T-lymphocyte associated protein 4-Ig; IV, intravenous; JAK, Janus kinase; MD, missing data; NA, not applicable; nr-axSpA, non-radiographic axial spondyloarthritis; NSI, non-serious infections; OLE, open-label extension; PDE4, phosphodiesterase 4; PsA, psoriatic arthritis; RCT, randomised controlled trials; SC, subcutaneous; SI, serious infections; TNF, tumour necrosis factor; w, week.
Serious infections
The IR of serious infections was estimated at 1.09 events per 100 PY (95% CI 0.85 to 1.35, I2= 67%) in patients with SpA (PsA and axSpA). The IRs were 1.68 per 100 PY (95% CI 1.03 to 2.47, I2 = 91%) for observational studies, 1.48 per 100 PY (95% CI 1.26 to 1.72, I2 = 0%) for OLE and 0.77 per 100 PY (95% CI 0.41 to 1.20, I2 = 24%) for RCT (figure 2).
Figure 2.
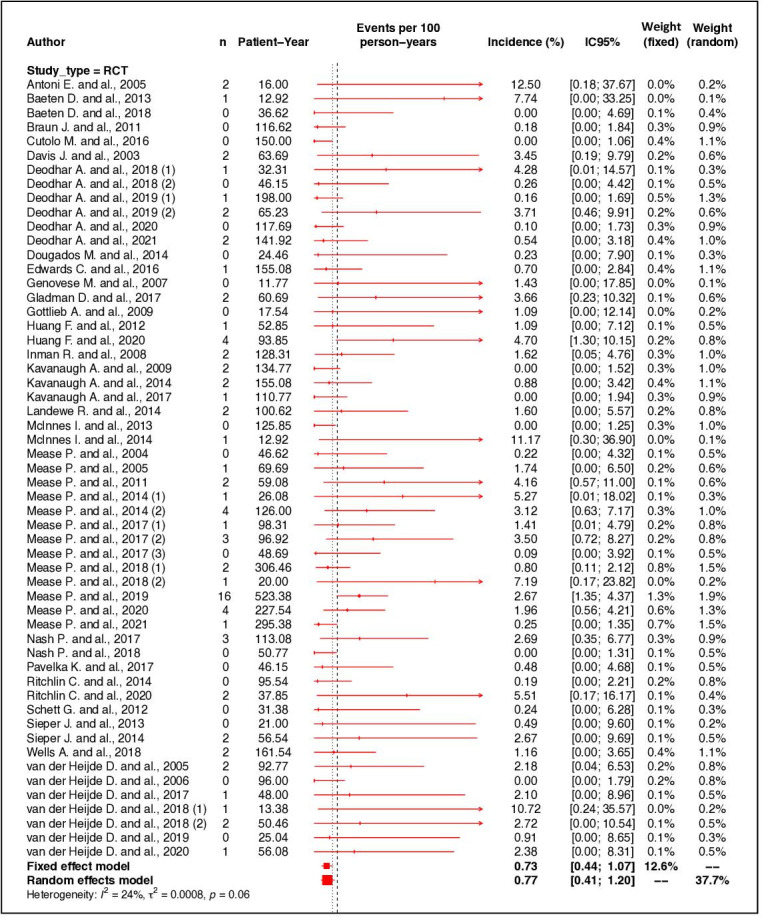
Forest plot of incidence rate of serious infections according to type of study. RCT, randomised controlled trial.
For patients with PsA, the IR of was estimated at 0.96 per 100 PY (95% CI 0.69 to 1.28, I2=52%). For patients with axSpA, the IR of was estimated at 1.09 per 100 PY (95% CI 0.76 to 1.46, I2=28%) (figure 3).
Figure 3.
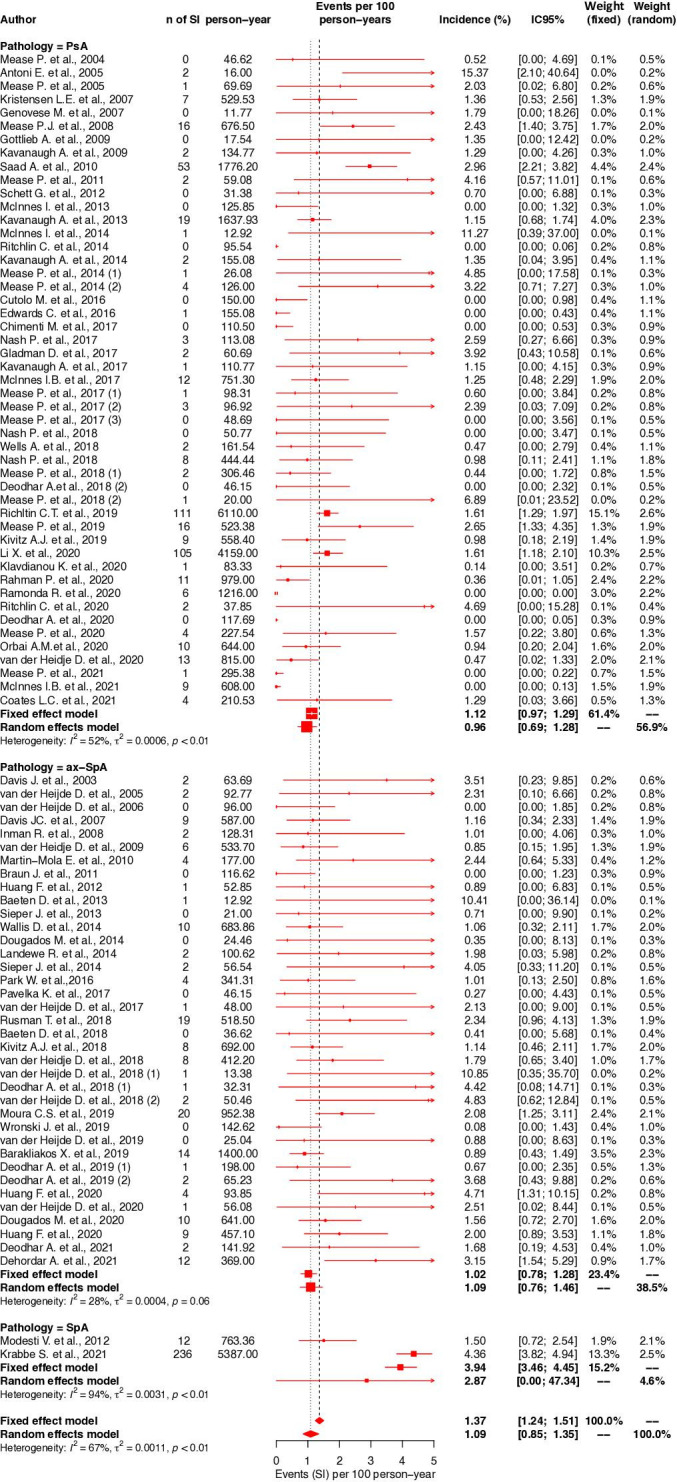
Forest plot of incidence rate of serious infections according to diagnosis. axSpA, axial spondyloarthritis; PsA, psoriatic arthritis; SpA, spondyloarthritis.
In subgroup analysis by therapeutic classes in PsA patients, the IRs were 1.36 per 100 PY (95% CI 0.72 to 2.16, I2=55%) with TNFi, 0.97 per 100 PY (95% CI 0.49 to 1.57, I2=47%) with IL-17i, 1.51 per 100 PY (95% CI 0.00 to 14.74, I2=31%) with JAKi, 0.29 per 100 PY (95% CI 0.00 to 1.03, I2=15%) with IL-12/23i and IL-23i, 0.38 per 100 PY (95% CI 0.00 to 1.19, I2=0%) with PDE4i and 1.71 per 100 PY (95% CI 0.00 to 43.38, I2=1%) with CTLA4-Ig (figure 4A).
Figure 4.
Forest plot of incidence rate of serious infections according to PsA (A) or axSpA (B), subgroup analysis by class of treatment. axSpA, axial spondyloarthritis; PDE4, phosphodiesterase 4; PsA, psoriatic arthritis; TNF, tumour necrosis factor.
In axSpA patients, the IRs were 1.24 per 100 PY (95% CI 0.78 to 1.77, I2=28%) with TNFi, 1.20 per 100 PY (95% CI 0.59 to 1.96, I2=40%) with IL-17i, 1.28 per 100 PY (95% CI 0.00 to 13.77, I2=0%) with JAKi (figure 4B).
Non-serious infections
The IR of NSI was estimated at 53.04 NSI per 100 PY (95% CI 43.47 to 63.55, I2= 99%) in patients with SpA (PsA or axSpA). The IRs were 69.95 per 100 PY (95% CI 61.59 to 78.84, I2 = 84%) for RCTs, 41.91 per 100 PY (95% CI 21.85 to 68.41, I2 = 99%) for OLE and 15.37 per 100 PY (95% CI 5.11 to 30.97, I2 = 99%) for observational studies (figure 5).
Figure 5.
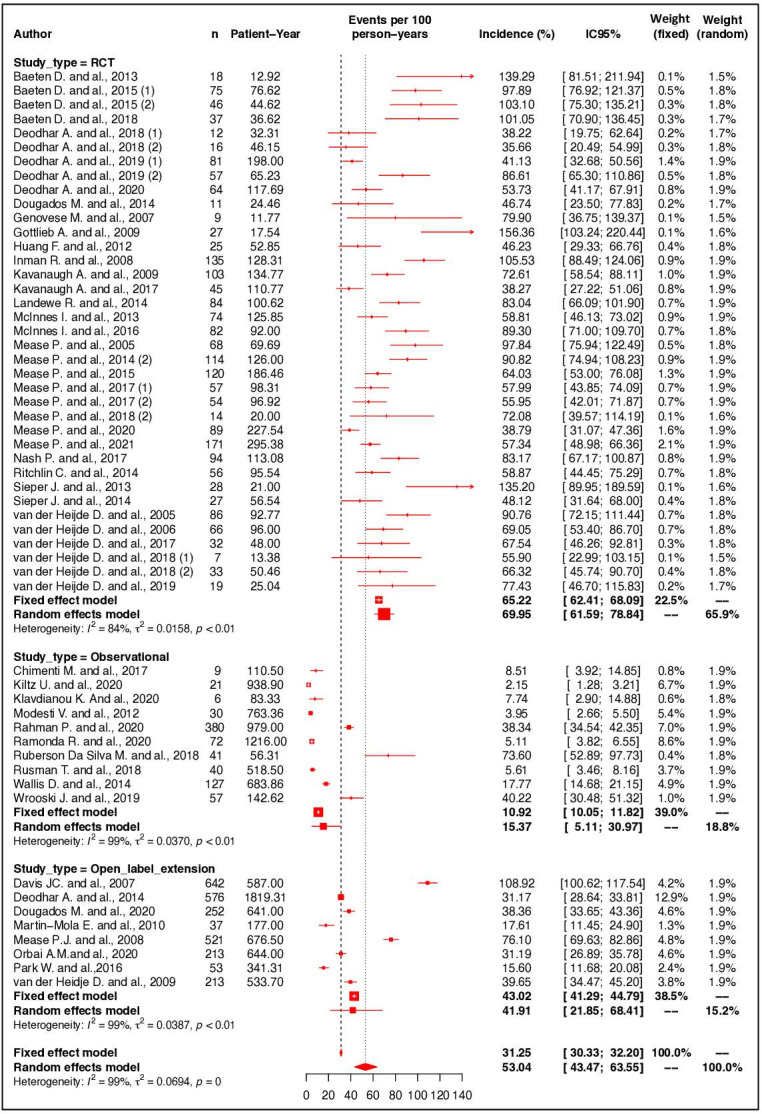
Forest plot of incidence rate of non-serious infections according to type of study. RCT, randomised controlled trial.
Similar IR was found for patients with PsA (54.08 (95% CI 40.96 to 68.99, I2=98%)) or with axSpA (58.02 per 100 PY (95% CI 44.79 to 72.94, I2=98%)) (data not shown). Subgroup analyses by class of treatment in RCTs was not performed because it resulted in too few studies in each subgroup.
Quality assessment
Concerning the quality assessment, 58% of RCTs were judged as low risk, 42% of RCTs were judged with some concerns and none of the RCTs included were judged as high risk (online supplemental table 9).
The mean NOS score in observational studies was 5.9 ranging from four to seven stars, with four stars in one study,6 five stars in five studies,7–11 six stars in four studies12–15 and seven stars in six studies.16–21
For serious infections in observational studies, meta-regression analyses did not reveal any significant association; therefore, the source of heterogeneity could not be clearly identified (see online supplemental tables 5 and 6). The visual inspection of funnel plot and Egger’s regression test (p<0.004) were in favour of small effect studies (online supplemental figure 1).
For NSI, meta-regression analyses did not reveal any significant association; therefore, the source of heterogeneity could not be clearly identified (see online supplemental tables 7 and 8). The visual inspection of funnel plot was in favour of effect studies (online supplemental figure 2).
Discussion
This large meta-analysis suggests that serious infections are rare events in the population of patients with PsA and axSpA treated with different classes of b/tsDMARD. For every 100 patients with SpA and treated with b/tsDMARD, we reported a rate of 1.09 serious infections and 53.0 NSI. In our study, the IRs of serious and NSI were similar in patients with PsA and axSpA.
We choose to focus our study on the IR of infections to describe the absolute risk of infection in the population of patients with PsA or axSpA treated with b/tsDMARD. Indeed, while physicians and patients have a better understanding of the differences in risk when results are presented as absolute risk rather than relative risk, few studies in the literature have focused on the assessment of this absolute risk in population of patients with PsA and axSpA.22 23
Compared with rheumatoid arthritis, there is a trend toward a lower IR of serious infections in patients with PsA and axSpA. In fact, a meta-analysis including 66 RCTs and 22 OLEs, estimated the IR of serious infections at 4.90 per 100 PY in patients with rheumatoid arthritis treated with TNFi and 3.04 per 100 PY with CTLA4-Ig.24 In observational registries, IR of serious infections varies between 3.1 and 6.4 infections per 100 PY.25–33 Compared with psoriasis, the IR of serious infections in patients with PsA and axSpA appears to be similar. In a meta-analysis of RCTs, the IR of serious infections was estimated at 1.29 per 100 PY with IL-17i.34 In trials PHOENIX 1, 2 and ACCEPT, the IR of serious infections in patients treated with ustekinumab was estimated at 1.4 per 100 PY.35 In a nationwide cohort study from France involved data from the National Health Data System, IR of serious infections in was 25.0 (95% CI (23.8 to 26.2)) per 1000 PY among patients newly treated with biological treatment.36There is very limited data in the literature about IR of serious infections in patients without inflammatory disease or immunosuppressive treatment. In a large population-based study, Smollen et al found that patients with rheumatoid arthritis treated with any type of therapy (biological or not) have an IR of serious infections at 3.86 per 100 PY and estimated IR of SI of healthy individuals of general population at 1.25 per 100 PY.37
To estimate the IR of infections, we have included many studies with various designs. We found a trend towards a higher IR of serious infections in observational studies than in RCTs. This could be explained by differences in population characteristics. In fact, despite RCTs are the gold standard as only randomisation and double blinding can allow for proper group comparison, these studies are of limited duration and often apply very strict inclusion criteria. Because of the longer time of follow-up and the inclusion of less selected patients sometimes with comorbidities, observational studies offer a valuable source of information for rare serious adverse events such as serious infections and might be more representative of the population encountered in daily practice.38 39 In our study, mean treatment exposure was 6 months in RCTs, 2.4 years in OLE and 2.5 years in observational studies, which could imply an increase in the risk of infection with increasing duration of exposure to treatment. However, it is known from studies in rheumatoid arthritis, that risk of serious infections is increased in the first 6 months of treatment and higher IR of serious infections were reported for shorter study duration in rheumatoid arthritis.24 27 28 On this issue, our results are mixed. On the one hand, there is a trend of a higher incidence of infection in OLE than in RCTs. This hypothesis is supported by an observational study in which mean serious infections onset time was 25.5 months in patients with AS.6 However, we excluded many OLE during data extraction because of missing data or because they reported number of patients with serious infections instead of number of serious infections.40 In this context, the population of OLE included in our study is not strictly comparable with that of RCTs and other confounding factors besides the duration of follow-up could explain differences in IR of serious infections. On the other hand, when we look at the IR of serious infections in RCTs and in their corresponding OLE, we also observe mixed results with some studies finding a higher IR of serious infections in RCTs41–46 and other studies where the IR of serious infections seems higher in OLE.47–49 More studies designed to evaluate the IR of serious infections over time in SpA are therefore needed. Also, in our meta-analysis, patients of observational studies have longer disease duration and were treated with more concomitant drugs than in RCTs (concomitant corticoid was used by 28% of patients in observational studies and 14% of patients in RCTs). A prospective study assessing the IR of serious infections in a cohort of rheumatoid arthritis patients before the era of b/tsDMARD showed an increased risk in the presence of corticosteroid therapy (RR 2.2; 95% CI 1.5 to 3.4).50 Another study showed a dose-dependent effect of corticosteroid therapy on the risk of severe pneumonia in patients with rheumatoid arthritis.51 Another explanation is the more regular follow-up of patients in RCTs which benefit from closer monitoring and therefore more antibiotic prescriptions.
In the case of NSI, we found a trend towards a lower IR of NSI in observational studies compared with RCTs. Indeed, because these infections are not serious, patients are more likely to under-report them in studies with an observational design. It is not possible to compare the rates obtained in our study with existing data in the literature as the methods of collecting this adverse event vary from one study to another. In rheumatoid arthritis, a prospective observational cohort study BSBR-RA was estimated IR of NSI at 27 NSI per 100 PY from clinician questionnaires and patients diaries every 6 months for 3 years.52 In a meta-analysis of 33 RCTs including patients with any inflammatory diseases treated with TFNi, IR of NSI range from 30 NSI per 100 PY with infliximab to eight NSI per 100 PY with etanercept.53 RCTs therefore appear to be the best source for estimating the occurrence of NSI, as those are systematically recorded. It may be interesting to study whether NSI represent a risk factor for serious infections and could be used as a surrogate endpoint in RCTs.
Regarding therapeutic choice, the lowest IR of serious infections were found for IL-23i and PDE4i prescribed exclusively in PsA. This result is in agreement with a recent cohort study in patients with PsA or psoriasis showing that patients treated with ustekinumab had 1.3–3.0 times lower risk of hospitalised serious infections than patients initiating other biologics as TNFi.54 Li et al reported in a cohort of patients with psoriasis (8010 PY) or PsA (4159 PY of follow-up) that patients treated with an IL-23i were less likely to display a hospitalised serious infections than patients treated with a TNFi.12 In a meta-analysis of patient with psoriasis, the rate of overall adverse event including serious infections was significantly higher in biologics targeting the IL-17 compared with biologics targeting IL-23.55 The IR of serious infections with TNFi in axSpA was estimated at 1.24 events per 100 PY. To the best of our knowledge, only one other meta-analysis of nine RCTs estimated the IR of serious infections with TNFi at 1.9 per 100 PY.56 The lower rate of serious infections in this study could be explained by the inclusion of more recent publications in which the population benefited of the current European Alliance of Associations for Rheumatology recommendations of vaccination against influenza and pneumococcal disease and systematic pretherapeutic assessment of infection. In SpA, IR of serious infections were similar with TNFi (1.29 events per 100 PY), IL-17i (1.05 events per 100 PY) and JAKi (1.30 events per 100 PY). Those results are in line with observational studies included in our meta-analysis which evaluated risk of infection in patients with PsA or psoriasis treated with IL-17i or TNFi and found no significant difference regarding serious infections.12 54 However, a recent observational study from the Nordic Countries suggest a higher risk of infection with secukinumab than TNFi in patients with SpA. The authors moderate this result as the crude excess risk seemed largely explained by more frequent secukinumab use in difficult-to-treat patients especially in patients older, with longer disease duration and who were not naïve from biologic (53% bionaive in TNF group vs 11% bionaive in secukinumab group).57
The main limitation of this meta-analysis was the substantial heterogeneity in observational studies, without source identification. Higher methodological and clinical heterogeneity is often expected in MOOSE compared with RCTs. Subgroup analyses did not improve the heterogeneity and meta-regression analyses for year of publication, duration of follow-up and number of patients with concomitant corticoid, NSAID or conventional synthetic DMARD were not significant in univariate analysis. This high heterogeneity could explain a part of the funnel plot asymmetry. Moreover, we could not exclude publication bias given the results of Egger’s regression test and funnel plot, although the asymmetry of funnel plot may be related to the smaller cohorts being at increased risk of infection or, in observational studies, related to the easier publication of positive studies with significant results.
We attempted to estimate the IR as precisely as possible using data from various study designs. However, we found a lack of large observational studies evaluating the IR of serious infections in this population, in particular in national cohorts, unlike in rheumatoid arthritis. Because of the absence of a control group in observational studies, it was not possible to evaluate the relative risk of serious and NSI in those studies. We did not include studies concerning biosimilars because our search equation was based on the international non-proprietary nomenclature of drugs, whereas biosimilars have names specific to each laboratory that develops them. Only one study with a biosimilar was included (PLANETAS OLE) as the biosimilar was compared with the reference originator infliximab arm and only this last one was included in our meta-analysis.
Conclusion
Our meta-analysis highlights that serious infections are rare events in the specific population of patients with PsA or axSpA treated with b/tsDMARD despite a trend of a higher incidence in observational studies compared with RCTs.
NSI were frequent in RCTs although their impact is currently poorly studied in the literature. The impact of the frequent finding of NSI in patients treated with b/tsDMARD, mainly in RCTs studies, needs to be explored.
Acknowledgments
We wish to thank all patients who participated in studies included in our paper.
Footnotes
Twitter: @ncabroj
Contributors: MA and MS equally contributed to this article. MA, MS and SL contributed to data collection. NC and J-CL contributed to the statistical analysis. MA and FC-L wrote the manuscript. All the authors participated in the interpretation of the data. All authors critically appraised the manuscript for important intellectual content and approved the final manuscript. FC-L is the guarantor for this manuscript.
Funding: The authors have not declared a specific grant for this research from any funding agency in the public, commercial or not-for-profit sectors.
Competing interests: None declared.
Provenance and peer review: Not commissioned; externally peer reviewed.
Supplemental material: This content has been supplied by the author(s). It has not been vetted by BMJ Publishing Group Limited (BMJ) and may not have been peer-reviewed. Any opinions or recommendations discussed are solely those of the author(s) and are not endorsed by BMJ. BMJ disclaims all liability and responsibility arising from any reliance placed on the content. Where the content includes any translated material, BMJ does not warrant the accuracy and reliability of the translations (including but not limited to local regulations, clinical guidelines, terminology, drug names and drug dosages), and is not responsible for any error and/or omissions arising from translation and adaptation or otherwise.
Data availability statement
All data relevant to the study are included in the article or uploaded as supplementary information.
Ethics statements
Patient consent for publication
Not required.
Ethics approval
Not applicable.
References
- 1.Claudepierre P, Wendling D, Breban M, et al. Ankylosing spondylitis, spondyloarthropathy, spondyloarthritis, or spondylarthritis: what's in a name? Joint Bone Spine 2012;79:534–5. 10.1016/j.jbspin.2012.06.003 [DOI] [PubMed] [Google Scholar]
- 2.Coates LC, Corp N, van der Windt DA, et al. GRAPPA treatment recommendations: an update from the 2020 GRAPPA annual meeting. J Rheumatol 2021:jrheum. 10.3899/jrheum.201681 [DOI] [PubMed] [Google Scholar]
- 3.Wendling D, Lukas C, Prati C, et al. 2018 update of French society for rheumatology (SFR) recommendations about the everyday management of patients with spondyloarthritis. Joint Bone Spine 2018;85:275–84. 10.1016/j.jbspin.2018.01.006 [DOI] [PubMed] [Google Scholar]
- 4.International conference on Harmonisation of technical requirements for registration of pharmaceuticals for human use. ICH harmonized tripartite guideline: guideline for good clinical practice. J Postgrad Med 2001;47:45–50. [PubMed] [Google Scholar]
- 5.Pan SMD, Dehler S, Ciurea A, et al. Comparison of drug retention rates and causes of drug discontinuation between anti-tumor necrosis factor agents in rheumatoid arthritis. Arthritis Rheum 2009;61:560–8. 10.1002/art.24463 Available: http://doi.wiley.com/10.1002/art.v61%3A5 [DOI] [PubMed] [Google Scholar]
- 6.Modesti V, Ramonda R, Ortolan A, et al. Infection relapse in Spondyloarthritis treated with biological drugs: a single-centre study. Scand J Rheumatol 2012;41:490–1. 10.3109/03009742.2012.698393 [DOI] [PubMed] [Google Scholar]
- 7.da Silva MRR, Dos Santos JBR, Almeida AM, et al. Effectiveness and safety of anti-TNF in psoriatic arthritis patients in Brazil: a post-incorporation analysis. J Comp Eff Res 2018;7:989–1000. 10.2217/cer-2018-0017 [DOI] [PubMed] [Google Scholar]
- 8.Saad AA, Ashcroft DM, Watson KD, et al. Efficacy and safety of anti-TNF therapies in psoriatic arthritis: an observational study from the British society for rheumatology biologics register. Rheumatology (Oxford) 2010;49:697–705. 10.1093/rheumatology/kep423 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Chimenti MS, Ortolan A, Lorenzin M, et al. Effectiveness and safety of Ustekinumab in Naïve or TNF-inhibitors failure psoriatic arthritis patients: a 24-month prospective Multicentric study. Clin Rheumatol 2018;37:397–405. 10.1007/s10067-017-3953-6 [DOI] [PubMed] [Google Scholar]
- 10.Wallis D, Thavaneswaran A, Haroon N, et al. Tumour necrosis factor inhibitor therapy and infection risk in axial spondyloarthritis: results from a longitudinal observational cohort. Rheumatology (Oxford) 2015;54:152–6. 10.1093/rheumatology/keu255 [DOI] [PubMed] [Google Scholar]
- 11.Rusman T, Ten Wolde S, Euser SM, et al. Gender differences in retention rate of tumor necrosis factor alpha inhibitor treatment in ankylosing spondylitis: a retrospective cohort study in daily practice. Int J Rheum Dis 2018;21:836–42. 10.1111/1756-185X.13271 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Li X, Andersen KM, Chang H-Y, et al. Comparative risk of serious infections among real-world users of Biologics for psoriasis or psoriatic arthritis. Ann Rheum Dis 2020;79:285–91. 10.1136/annrheumdis-2019-216102 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Wroński J, Fiedor P, Głuszko P. Adverse events in patients with ankylosing spondylitis treated with TNF inhibitors: a cross-sectional study. Int J Clin Pharm 2019;41:864–71. 10.1007/s11096-019-00859-7 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Ritchlin CT, Stahle M, Poulin Y, et al. Serious infections in patients with self-reported Psoriatic arthritis from the psoriasis longitudinal assessment and Registry (PSOLAR) treated with biologics. BMC Rheumatol 2019;3:52. 10.1186/s41927-019-0094-3 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Klavdianou K, Lazarini A, Grivas A, et al. Real life efficacy and safety of Secukinumab in biologic-experienced patients with psoriatic arthritis. Front Med 2020;7:288. 10.3389/fmed.2020.00288 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Kristensen LE, Gülfe A, Saxne T, et al. Efficacy and tolerability of anti-tumour necrosis factor therapy in psoriatic arthritis patients: results from the South Swedish arthritis treatment group register. Ann Rheum Dis 2008;67:364–9. 10.1136/ard.2007.073544 [DOI] [PubMed] [Google Scholar]
- 17.Kiltz U, Sfikakis PP, Gaffney K, et al. Secukinumab use in patients with moderate to severe psoriasis, psoriatic arthritis and ankylosing spondylitis in real-world setting in Europe: baseline data from SERENA study. Adv Ther 2020;37:2865–83. 10.1007/s12325-020-01352-8 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Ramonda R, Lorenzin M, Carriero A, et al. Effectiveness and safety of Secukinumab in 608 patients with psoriatic arthritis in real life: a 24-month prospective, multicentre study. RMD Open 2021;7:e001519. 10.1136/rmdopen-2020-001519 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Krabbe S, Grøn KL, Glintborg B, et al. Risk of serious infections in arthritis patients treated with biological drugs: a matched cohort study and development of prediction model. Rheumatology (Oxford) 2021;60:3834–44. 10.1093/rheumatology/keaa876 [DOI] [PubMed] [Google Scholar]
- 20.Moura CS, Rahme E, Maksymowych WP, et al. Use of disease-modifying anti-rheumatic or anti-tumour necrosis factor drugs and risk of hospitalized infection in ankylosing spondylitis. Scand J Rheumatol 2019;48:121–7. 10.1080/03009742.2018.1470253 [DOI] [PubMed] [Google Scholar]
- 21.Rahman P, Arendse R, Khraishi M, et al. Long-term effectiveness and safety of Infliximab, Golimumab and Ustekinumab in patients with psoriatic arthritis from a Canadian prospective observational registry. BMJ Open 2020;10:e036245. 10.1136/bmjopen-2019-036245 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Gigerenzer G, Gaissmaier W, Kurz-Milcke E, et al. Helping doctors and patients make sense of health Statistics. Psychol Sci Public Interest 2007;8:53–96. 10.1111/j.1539-6053.2008.00033.x [DOI] [PubMed] [Google Scholar]
- 23.Zipkin DA, Umscheid CA, Keating NL, et al. Evidence-based risk communication: a systematic review. Ann Intern Med 2014;161:270–80. 10.7326/M14-0295 [DOI] [PubMed] [Google Scholar]
- 24.Strand V, Ahadieh S, French J, et al. Systematic review and meta-analysis of serious infections with tofacitinib and biologic disease-modifying antirheumatic drug treatment in rheumatoid arthritis clinical trials. Arthritis Res Ther 2015;17:362. 10.1186/s13075-015-0880-2 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Cecconi M, Ranza R, Titton DC, et al. Incidence of infectious adverse events in patients with rheumatoid arthritis and spondyloarthritis on biologic drugs—data from the brazilian registry for biologics monitoring. J Clin Rheumatol 2020;26:73–8. 10.1097/RHU.0000000000000935 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Zink A, Manger B, Kaufmann J, et al. Evaluation of the RABBIT risk score for serious infections. Ann Rheum Dis 2014;73:1673–6. 10.1136/annrheumdis-2013-203341 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Dixon WG, Watson K, Lunt M, et al. Rates of serious infection, including site-specific and bacterial intracellular infection, in rheumatoid arthritis patients receiving anti–tumor necrosis factor therapy: results from the British society for rheumatology biologics register. Arthritis Rheum 2006;54:2368–76. 10.1002/art.21978 [DOI] [PubMed] [Google Scholar]
- 28.Askling J, Fored CM, Brandt L, et al. Time-dependent increase in risk of Hospitalisation with infection among Swedish RA patients treated with TNF antagonists. Ann Rheum Dis 2007;66:1339–44. 10.1136/ard.2006.062760 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Pérez-Sola MJ, Torre-Cisneros J, Pérez-Zafrilla B, et al. Infections in patients treated with tumor necrosis factor antagonists: incidence, etiology and mortality in the BIOBADASER registry. Med Clin (Barc) 2011;137:533–40. 10.1016/j.medcli.2010.11.032 [DOI] [PubMed] [Google Scholar]
- 30.Strangfeld A, Eveslage M, Schneider M, et al. Treatment benefit or survival of the fittest: what drives the time-dependent decrease in serious infection rates under TNF inhibition and what does this imply for the individual patient Ann Rheum Dis 2011;70:1914–20. 10.1136/ard.2011.151043 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Atzeni F, Sarzi-Puttini P, Botsios C, et al. Long-term anti-TNF therapy and the risk of serious infections in a cohort of patients with rheumatoid arthritis: comparison of adalimumab, etanercept and infliximab in the GISEA registry. Autoimmun Rev 2012;12:225–9. 10.1016/j.autrev.2012.06.008 [DOI] [PubMed] [Google Scholar]
- 32.Komano Y, Tanaka M, Nanki T, et al. Incidence and risk factors for serious infection in patients with rheumatoid arthritis treated with tumor necrosis factor inhibitors: a report from the registry of Japanese rheumatoid arthritis patients for longterm safety. J Rheumatol 2011;38:1258–64. 10.3899/jrheum.101009 [DOI] [PubMed] [Google Scholar]
- 33.Harrold LR, Griffith J, Zueger P, et al. Longterm, real-world safety of Adalimumab in rheumatoid arthritis: analysis of a prospective US-based Registry. J Rheumatol 2020;47:959–67. 10.3899/jrheum.190260 [DOI] [PubMed] [Google Scholar]
- 34.Loft ND, Vaengebjerg S, Halling A ‐S., et al. Adverse events with IL‐17 and IL‐23 inhibitors for psoriasis and Psoriatic arthritis: a systematic review and meta‐analysis of phase III studies. J Eur Acad Dermatol Venereol 2020;34:1151–60. 10.1111/jdv.16073 Available: https://onlinelibrary.wiley.com/toc/14683083/34/6 [DOI] [PubMed] [Google Scholar]
- 35.Ghosh S, Gensler LS, Yang Z, et al. Ustekinumab safety in psoriasis, psoriatic arthritis, and Crohn’s disease: an integrated analysis of phase II/III clinical development programs. Drug Saf 2019;42:809. 10.1007/s40264-019-00816-3 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Penso L, Dray-Spira R, Weill A, et al. Association between biologics use and risk of serious infection in patients with psoriasis. JAMA Dermatol 2021;157:1056–65. 10.1001/jamadermatol.2021.2599 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Smolen JS, Aletaha D, Barton A, et al. Rheumatoid arthritis. Nat Rev Dis Primers 2018;4:18001. 10.1038/nrdp.2018.1 [DOI] [PubMed] [Google Scholar]
- 38.Van Spall HGC, Toren A, Kiss A, et al. Eligibility criteria of randomized controlled trials published in high-impact general medical journals: a systematic sampling review. JAMA 2007;297:1233–40. 10.1001/jama.297.11.1233 [DOI] [PubMed] [Google Scholar]
- 39.Sedrak MS, Freedman RA, Cohen HJ, et al. Older adult participation in cancer clinical trials: a systematic review of barriers and interventions. CA Cancer J Clin 2021;71:78–92. 10.3322/caac.21638 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Sterne JAC, Sutton AJ, Ioannidis JPA, et al. Recommendations for examining and interpreting funnel plot asymmetry in meta-analyses of randomised controlled trials. BMJ 2011;343. 10.1136/bmj.d4002 [DOI] [PubMed] [Google Scholar]
- 41.Kavanaugh A, McInnes IB, Mease P, et al. Clinical efficacy, radiographic and safety findings through 5 years of subcutaneous golimumab treatment in patients with active psoriatic arthritis: results from a long-term extension of a randomised. Ann Rheum Dis 2014;73:1689–94. 10.1136/annrheumdis-2013-204902 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Huang F, Sun F, Wan W-G, et al. Secukinumab provided significant and sustained improvement in the signs and symptoms of ankylosing spondylitis: results from the 52-week, phase III China-centric study, MEASURE 5. Chin Med J (Engl) 2020;133:2521–31. 10.1097/CM9.0000000000001099 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.van der Heijde D, Mease PJ, Landewé RBM, et al. Secukinumab provides sustained low rates of radiographic progression in psoriatic arthritis: 52-week results from a phase 3 study, FUTURE 5. Rheumatology (Oxford) 2020;59:1325–34. 10.1093/rheumatology/kez420 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Orbai A-M, Gratacós J, Turkiewicz A, et al. Efficacy and safety of Ixekizumab in patients with psoriatic arthritis and inadequate response to TNF inhibitors: 3-year follow-up (SPIRIT-P2). Rheumatol Ther 2021;8:199–217. 10.1007/s40744-020-00261-0 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Coates LC, Warren RB, Ritchlin CT, et al. Bimekizumab safety and efficacy in patients with psoriatic arthritis: 3-year results from a phase 2B open-label extension study. Ann Rheum Dis 2021;80(Suppl 1):779–80. 10.1136/annrheumdis-2021-eular.124 [DOI] [Google Scholar]
- 46.Davis JC, van der Heijde DM, Braun J, et al. Efficacy and safety of up to 192 weeks of etanercept therapy in patients with ankylosing spondylitis. Ann Rheum Dis 2008;67:346–52. 10.1136/ard.2007.078139 [DOI] [PubMed] [Google Scholar]
- 47.Mease PJ, Ory P, Sharp JT, et al. Adalimumab for long-term treatment of Psoriatic arthritis: 2-year data from the adalimumab effectiveness in psoriatic arthritis trial (ADEPT). Ann Rheum Dis 2009;68:702–9. 10.1136/ard.2008.092767 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.van der Heijde D, Schiff MH, Sieper J, et al. Adalimumab effectiveness for the treatment of ankylosing spondylitis is maintained for up to 2 years: long-term results from the ATLAS trial. Ann Rheum Dis 2009;68:922–9. 10.1136/ard.2007.087270 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Sieper J, van der D, Dougados M, et al. Efficacy and safety of adalimumab in patients with non-radiographic axial Spondyloarthritis:results of a randomised placebo-controlled trial (ABILITY-1). Ann Rheum Dis 2013;72:815–22. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Franklin J, Lunt M, Bunn D, et al. Risk and predictors of infection leading to hospitalisation in a large primary-care-derived cohort of patients with inflammatory polyarthritis. Ann Rheum Dis 2007;66:308–12. 10.1136/ard.2006.057265 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Wolfe F, Caplan L, Michaud K. Treatment for rheumatoid arthritis and the risk of hospitalization for pneumonia: associations with prednisone, disease-modifying antirheumatic drugs, and anti–tumor necrosis factor therapy. Arthritis Rheum 2006;54:628–34. 10.1002/art.21568 [DOI] [PubMed] [Google Scholar]
- 52.Bechman K, Halai K, Yates M, et al. Nonserious infections in patients with rheumatoid arthritis: results from the British society for rheumatology biologics register for rheumatoid arthritis. Arthritis Rheumatol 2021;73:1800–9. 10.1002/art.41754 Available: https://onlinelibrary.wiley.com/toc/23265205/73/10 [DOI] [PubMed] [Google Scholar]
- 53.Dao KH, Herbert M, Habal N. Nonserious infections. Rheum Dis Clin North Am 2012;38:707–25. 10.1016/j.rdc.2012.08.016 [DOI] [PubMed] [Google Scholar]
- 54.Jin Y, Lee H, Lee MP, et al. Risk of hospitalization for serious infection after initiation of ustekinumab or other Biologics in patients with psoriasis or psoriatic arthritis. Arthritis Care Res (Hoboken) 2022;74:1792–805. 10.1002/acr.24630 [DOI] [PubMed] [Google Scholar]
- 55.Cui L, Chen R, Subedi S, et al. “Corrigendum to "efficacy and safety of Biologics targeting IL-17 and IL-23 in the treatment of moderate-to-severe plaque psoriasis: a systematic review and meta-analysis of randomized controlled trials" [INT. Immunopharmacol. 62 (2018) 46-58]”. Int Immunopharmacol 2018;64:432. 10.1016/j.intimp.2018.08.039 [DOI] [PubMed] [Google Scholar]
- 56.Fouque-Aubert A, Jette-Paulin L, Combescure C, et al. Serious infections in patients with ankylosing spondylitis with and without TNF blockers: a systematic review and meta-analysis of randomised placebo-controlled trials. Ann Rheum Dis 2010;69:1756–61. 10.1136/ard.2008.098822 [DOI] [PubMed] [Google Scholar]
- 57.Glintborg B, Di Giuseppe D, Wallman JK, et al. Is the risk of infection higher during treatment with secukinumab than with TNF inhibitors? an observational study from the Nordic countries. Rheumatology (Oxford) 2023;62:647–58. 10.1093/rheumatology/keac358 [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
rmdopen-2023-003064supp001.pdf (518.1KB, pdf)
Data Availability Statement
All data relevant to the study are included in the article or uploaded as supplementary information.




