Abstract
Objectives
Targeted testing policy for HIV/syphilis at Dutch sexual health centres (SHCs) was evaluated for its efficiency in younger heterosexuals but not for heterosexuals ≥25 years. Currently, all older heterosexuals are tested for HIV/syphilis at SHCs. To explore possibilities for increased efficiency of testing in heterosexuals aged >25 years, this study aimed to identify determinants of HIV and syphilis diagnoses that could be used in targeted testing strategies.
Design
An observational study using surveillance data from all Dutch SHC.
Participants
Women and heterosexual men aged >25 years visiting SHC between 2015 and 2021.
Primary and secondary outcome measures
The primary outcome was HIV/syphilis diagnosis, determinants of a diagnosis were analysed. Based on these determinants and their applicability in SHC practice, different targeted testing scenarios were evaluated. For each scenario, the percentage of consultations involving HIV and syphilis testing and the total amount of missed HIV and syphilis diagnoses were calculated.
Results
109 122 consultations were included among 75 718 individuals. The strongest determinants of HIV/syphilis diagnosis were HIV/syphilis-specific symptoms (adjusted OR (aOR) 34.9 (24.1–50.2)) and receiving partner notification (aOR 18.3 (13.2–25.2)), followed by low/middle education level (aOR 2.8 (2.0–4.0)), male sex (aOR 2.2 (1.6–3.0)) and age ≥30 years (aOR 1.8 (1.3–2.5)). When applying feasible determinants to targeted testing scenarios, HIV/syphilis testing would have been conducted in 54.5% of all consultations, missing 2 HIV and 3 syphilis diagnoses annually (13.4% and 11.4% of all diagnoses, respectively). In the scenario with the lowest number of missed HIV/syphilis diagnoses (0.3 HIV and 2 syphilis diagnoses annually), HIV/syphilis testing would have been conducted in 74.2% of all consultations.
Conclusions
In any targeted testing scenario studied, HIV and/or syphilis diagnoses would have been missed. This raises the question whether it is acceptable to put any of these scenarios into practice. This study contributes to a discussion about the impact of targeted testing policy.
Keywords: health policy, epidemiology, sexually transmitted disease, HIV & AIDS, syphilis, public health
Strengths and limitations of this study.
This is the first study in the Netherlands describing determinants of HIV/syphilis diagnosis among women and heterosexual men aged >25 years.
The study used nationwide surveillance data from sexual health centres.
This study was limited by the sexual behavioural variables available in the surveillance data.
Additional cost-effectiveness analyses are needed to facilitate informed decisions regarding HIV/syphilis testing policy.
Introduction
In many countries, sexually transmitted infections (STI) testing guidelines for women and heterosexual men aged >25 years are different from testing guidelines for those aged <25 years old. This is mainly the case for Chlamydia trachomatis (chlamydia) and Neisseria gonorrhoeae (gonorrhoea), where testing in women and heterosexual men aged ≥25 years is often recommended at certain indications only.1–3 However, for syphilis and HIV differentiation in testing guidelines based on age is often not described. According to Centers for Disease Control and Prevention (CDC), HIV screening should be offered to all individuals who seek care at sexual health centres (SHCs) and syphilis screening to individuals at increased risk.1 According to International Union Against Sexually Transmitted Infections (IUSTI) guidelines (contributed by European Centre for Disease Prevention and Control (ECDC) and the European Office of the WHO), both HIV and syphilis tests should be offered to all SHC attendees.4 5
In the Netherlands, all women and heterosexual men aged <25 years are eligible for testing at SHCs. Women and heterosexual men aged ≥25 years are eligible for testing at SHCs if they meet at least one of the following triage criteria: notified by a sexual contact, STI symptoms, having had an STI in the past year, female partner of men who have sex with men (MSM), commercial sex workers (CSW), originating from or having a partner from an STI-endemic area or being a victim of sexual violence.6 This older heterosexual group is routinely tested for four STI (chlamydia, gonorrhoea, HIV and syphilis) while women and heterosexual men aged <25 years are only tested for HIV and syphilis on indication.6 7 This restrictive testing among young heterosexuals was introduced to decrease costs, as government funding for SHCs changed. Evaluation of this testing policy was conducted,8 and targeted testing of HIV and syphilis on indication was found to be cost-effective; approximately three HIV and seven syphilis diagnoses were missed annually. Nevertheless, evaluation data of STI testing for older heterosexuals remains limited.
For older women and heterosexual men, more insight is needed in the characteristics of SHC visitors with HIV and syphilis diagnoses, in order to explore the possibilities for targeted testing in this group as well. Therefore, the objective of this study was to identify determinants of HIV and syphilis diagnoses among all STI clinical consultations of women and heterosexual men aged above 25 years that could possibly be used in targeted testing strategies.
Methods
Study population
National surveillance data of SHCs in the Netherlands (Seksueel Overdraagbare Aandoeningen Peilstation(SOAP)) of women and heterosexual men aged above 25 years were used for this study. Consultations were selected from 2015 to 2021, as in 2015 government funding for SHC testing policy changed and consequently the characteristics of people visiting the SHCs.9 All women were included and heterosexual men were defined as men with self-reported sexual contact with women only in the past 6 months. Men who had sex with both men and women and men with unknown sexual behaviour were excluded. Age was calculated by subtracting birth year (date was not available) from consultation year. To prevent misclassification of 25 year-olds in the study population (who have different testing guidelines), people aged 26 years and older were selected. Consultations were excluded for (1) individuals with specific testing policies (eg, sex workers, transgender persons, pre-exposure prophylaxis (PrEP)), (2) consultations which did not include routine practice (not tested for chlamydia, gonorrhoea, syphilis and HIV),6 (3) consultations of people living with HIV and (4) consultations of individuals aged ≥60 years due to small numbers.
Definitions
The outcome of this study was a diagnosis of HIV and/or syphilis (infectious syphilis, being primary/secondary syphilis or syphilis latens recens). Both STI were combined in one dichotomous variable in the main analysis, as HIV and syphilis testing both require taking a blood sample. Available self-reported demographic and sexual behavioural variables were included in the model as possible determinants of an HIV/syphilis diagnosis. Age was dichotomised into categories 26–29 and ≥30 to create equally distributed groups. Education level was dichotomised to two categories: low/middle education level (no education, primary education only or vocational education) or high level education (all other education levels). Other variables included were; notified for STI (specifically for HIV/syphilis or another/unspecified STI), STI symptoms (overall and if so, HIV/syphilis specific (eg, weight loss, fever, ulcers, swollen lymph nodes), originating from an STI-endemic area (based on country of birth of both the individual and parents10), partner from risk group (STI-endemic area or MSM), STI (gonorrhoea, chlamydia, syphilis) diagnosis in the past year (persons who were not tested, were tested negative or test results were unknown were categorised as no STI history), number of partners in the past 6 months, being a client of CSW, having a chlamydia and/or gonorrhoea diagnosis at the same consultation and condom use. Before 2018 condom use was reported at last sexual contact, after 2018 this was reported in the past 6 months at vaginal and/or anal sex; both were combined in one dichotomous variable (always with a condom in the past 6 months/last sex with a condom or not always/never with a condom in the past 6 months/last sex without condom).
Statistical analyses
Determinants of an HIV/syphilis diagnosis were analysed using logistic regressions. If missing values within one variable were more than 5% they were included in analyses as a separate category, missing values less than 5% were excluded. We first checked whether we had to take into account that one person could be included in the data set with multiple consultations. The additional value of adding a random intercept on person level to the model was checked by comparing Akaike information criterion (AIC) values between the intercept-only model with and without a random intercept. Then, univariate logistic regression analyses were performed for all determinants separately as independent variable and HIV/syphilis diagnosis as dependent variable. Last, all variables were included in a multivariable model constructed based on backward elimination using AIC. For all significant determinants that remained in the final model, effect modification was examined by adding interaction terms to all univariate regressions separately. For any significant effect modifiers stratified analyses were performed.
Three sensitivity analyses were performed. First, as determinants of an HIV and syphilis diagnosis might be different, separate analyses were performed per STI. Second, the variable anal sex in the past 6 months was only collected from 2016 onwards, therefore another model was conducted over the years 2016–2021 with anal sex added as a possible determinant.11–14 Finally, a model was conducted over the years 2015–2019 to restrict the analysis to pre-COVID-19 years. During the COVID-19 pandemic, STI care in the Netherlands was downscaled, resulting in less and more targeted SHC consultations in 2020 and 2021.9 All analyses were performed in R (V.4.2.0, packages tidyverse, gtsummary, broom, janitor, lme4).
Targeted testing
In order to assess possibilities for targeted testing, different scenarios were built up. The scenarios were based on determinants in the final regression model that were also applicable for use in practice. This was also supplemented with determinants of HIV and syphilis from the separate models. For each scenario the percentage of consultations involving HIV/syphilis testing and the total and average per year of missed HIV and syphilis diagnoses between 2015 and 2021 were calculated.
Patient and public involvement
Patients and/or the public were not involved in the design, or conduct, or reporting, or dissemination plans of this research. Only data from the national surveillance system were used.
Results
Study population
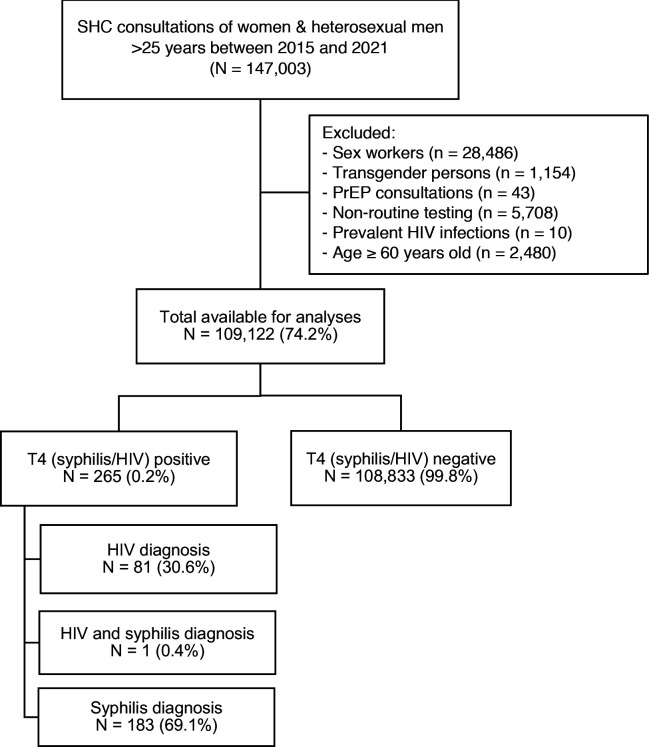
Between January 2015 and December 2021, 147 003 STI consultations among women and heterosexual men aged >25 years were registered (figure 1). In total, 37 881 (25.8%) consultations were excluded due to various reasons including sex work (n=28 486), transgender persons (n=1154), PrEP consultations (n=43), non-routine testing (n=5708), prevalent HIV infections (n=10) or age ≥60 years (n=2480), leaving 109 122 consultations for analysis among 75 718 individuals. In these consultations, 184 new syphilis diagnoses (0.2%) were reported and 82 HIV diagnoses (0.1%). In one consultation both syphilis and HIV were diagnosed.
Figure 1.
Flowchart of the included consultations in the study population. PrEP, pre-exposure prophylaxis; SHC, sexual health centre; T4, tested for chlamydia, gonorrhoea, syphilis and HIV.
In the study population, sex was equally distributed (table 1). Most people had a higher education level and originated from a non-STI/HIV-endemic area. Chlamydia was the most diagnosed STI (12.4% of all consultations). The number of consultations per year decreased over time.
Table 1.
Number and percentage of consultations by different characteristics of women and heterosexual men aged >25 years visiting Dutch sexual health centres between 2015 and 2021
| Consultations | % | |
| n | ||
| Total number of consultations | 109 122 | 100 |
| Number of individuals | 75 718 | 69.4 |
| Consultation number per individual | ||
| 1 | 75 718 | 69.4 |
| >1 | 33 404 | 30.6 |
| Sex | ||
| Men | 54 531 | 50.0 |
| Women | 54 591 | 50.0 |
| Age | ||
| 26–29 years | 50 287 | 46.1 |
| 30+ years | 58 835 | 53.9 |
| Education level* | ||
| High | 59 453 | 54.5 |
| Low/middle | 41 716 | 38.2 |
| Unknown/other | 7953 | 7.3 |
| Originating from STI/HIV-endemic area† | ||
| No | 64 782 | 59.4 |
| Yes | 44 234 | 40.5 |
| Unknown | 106 | 0.1 |
| STI diagnoses‡ | ||
| Chlamydia | 13 539 | 12.4 |
| Gonorrhoea | 2403 | 2.2 |
| Syphilis, infectious§ | 184 | 0.2 |
| HIV | 82 | 0.1 |
| Year consult | ||
| 2015 | 22 322 | 20.5 |
| 2016 | 21 306 | 19.5 |
| 2017 | 19 855 | 18.2 |
| 2018 | 15 951 | 14.6 |
| 2019 | 11 395 | 10.4 |
| 2020 | 8330 | 7.6 |
| 2021 | 9963 | 9.1 |
*Low/middle level of education: no education, elementary school, lbo, mavo, vmbo, mbo-1, havo, vwo, gymnasium. High level education: all other education levels.
†STI/HIV-endemic areas include Asia, Africa, the Dutch Caribbean islands, middle and South America.
‡Consultations could be counted double when multiple STI were found at the same consultation.
§Infectious syphilis includes primary syphilis, secondary syphilis and syphilis latens recens.
STI, sexually transmitted infections.
Determinants of HIV and/or syphilis
The strongest determinants of HIV/syphilis diagnosis in univariate analyses were HIV/syphilis specific symptoms and partner notification for HIV/syphilis (table 2). In multivariate analyses these two remained the strongest determinants (adjusted OR (aOR) 34.9; 95% CI 24.1 to 50.2 and aOR 18.3; 95% CI 13.2 to 25.2, respectively). Other significant determinants were male sex, being aged ≥30 years and low/middle education level. Persons who used condoms or had two or more sex partners in the past 6 months were less likely to have an HIV/syphilis diagnosis. Correcting for multiple consultations within one person was not necessary as the AIC values of the intercept-only model with and without a random intercept were approximately equal.
Table 2.
Determinants of an HIV and/or syphilis diagnosis among women and heterosexual men aged >25 years visiting Dutch sexual health centres between 2015 and 2021
| HIV and/or syphilis negative | HIV and/or syphilis positive | Crude OR (95% CI) | Adjusted OR (95% CI) | |
| n (%) | n (%) | |||
| Total number of consultations* | 103 580 (99.8) | 242 (0.2) | ||
| Sex | ||||
| Women | 51 905 (99.8) | 84 (0.2) | 1 | 1 |
| Men | 51 675 (99.7) | 158 (0.3) | 1.9 (1.5 to 2.5) | 2.2 (1.6 to 3.0) |
| Age | ||||
| 26–29 years | 48 175 (99.9) | 58 (0.1) | 1 | 1 |
| 30+ years | 55 405 (99.7) | 184 (0.3) | 2.8 (2.1 to 3.7) | 1.8 (1.3 to 2.5) |
| CT and/or GO positivity at same consultation | ||||
| No | 89 023 (99.8) | 211 (0.2) | 1 | – |
| Yes | 14 557 (99.8) | 31 (0.2) | 0.90 (0.6 to 1.3) | – |
| Self-reported GO/CT/SYPH in past year | ||||
| No | 94 871 (99.8) | 215 (0.2) | 1 | – |
| Yes | 8709 (99.7) | 27 (0.3) | 1.4 (0.9 to 2.0) | – |
| Education level† | ||||
| High | 57 104 (99.9) | 54 (0.1) | 1 | 1 |
| Low/middle | 39 494 (99.6) | 142 (0.4) | 3.8 (2.8 to 5.2) | 2.8 (2.0 to 4.0) |
| Unknown/other | 6982 (99.3) | 46 (0.7) | 7.0 (4.7 to 10.3) | 4.2 (2.7 to 6.4) |
| Number of sex partners in past 6 months | ||||
| 0–1 | 23 673 (99.6) | 107 (0.4) | 1 | 1 |
| 2–3 | 42 110 (99.8) | 88 (0.2) | 0.5 (0.3 to 0.6) | 0.7 (0.5 to 0.9) |
| 4+ | 37 797 (99.9) | 47 (0.1) | 0.3 (0.2 to 0.4) | 0.4 (0.3 to 0.6) |
| Condom use‡ | ||||
| No | 86 413 (99.8) | 214 (0.2) | 1 | 1 |
| Yes | 17 167 (99.8) | 28 (0.2) | 0.7 (0.4 to 1.0) | 0.6 (0.4 to 0.9) |
| Originating from STI/HIV-endemic area§ | ||||
| No | 61 599 (99.8) | 134 (0.2) | 1 | – |
| Yes | 41 981 (99.7) | 108 (0.3) | 1.2 (0.9 to 1.5) | – |
| Received partner notification | ||||
| No | 67 817 (99.8) | 129 (0.2) | 1 | 1 |
| Yes | 34 347 (33.2) | 23 (9.5) | 0.4 (0.2 to 0.5) | 0.5 (0.3 to 0.8) |
| Yes, notified for HIV/syphilis | 1416 (1.4) | 90 (37.2) | 33.4 (25.3 to 43.9) | 18.3 (13.2 to 25.2) |
| Reported STI symptoms | ||||
| No | 58 360 (99.8) | 94 (0.2) | 1 | 1 |
| Yes, overall STI symptoms | 44 727 (99.8) | 78 (0.2) | 1.1 (0.8 to 1.5) | 1.3 (1.0 to 1.8) |
| Yes, HIV/syphilis symptoms | 493 (87.6) | 70 (12.4) | 88.2 (63.7 to 121.4) | 34.9 (24.1 to 50.2) |
| Partner in risk group¶ | ||||
| No | 55 690 (99.8) | 134 (0.2) | 1 | – |
| Yes | 47 890 (99.8) | 108 (0.2) | 0.9 (0.7 to 1.2) | – |
| Client of commercial sex worker | ||||
| No | 89 374 (99.8) | 209 (0.2) | 1 | – |
| Yes, in past 6 months | 6356 (99.6) | 23 (0.4) | 1.5 (1.0 to 2.3) | – |
| Unknown | 7850 (99.9) | 10 (0.1) | 0.5 (0.3 to 1.0) | – |
Bold ≤0.05.
*Consultations with missing values <5% on at least one of the determinants were excluded from the analyses.
†Low/middle level of education: no education, elementary school, lbo, mavo, vmbo, mbo-1, havo, vwo, gymnasium. High level education: all other education levels.
‡Before 2018, condom use was asked regarding last sexual contact. In 2018 this changed to the past 6 months and during vaginal and/or anal sex.
§STI/HIV-endemic areas include Asia, Africa, the Dutch Caribbean islands, middle and South America.
¶For heterosexual men: partner originating from a high STI/HIV endemic region. For women: partner originating from a high STI/HIV endemic region or a male partner who had sex with men.
CT, chlamydia; GO, gonorrhoea; STI, sexually transmitted infections; SYPH, syphilis.
STI symptoms and partner notification were found to be significant effect modifiers. In stratified analyses for STI symptoms (online supplemental table S1) the same determinants were found and the direction of the effects did not change. Additionally, self-reported STI in the past year became an extra determinant for persons with HIV/syphilis-specific symptoms. In stratified analyses for partner notification (online supplemental table S2) the direction of the effects also did not change. However, sex and age were no determinants anymore and chlamydia/gonorrhoeae diagnosis in the same consultation became an additional determinant for persons with HIV/syphilis-specific partner notification.
bmjopen-2023-072862supp001.pdf (65.6KB, pdf)
bmjopen-2023-072862supp002.pdf (65KB, pdf)
In all sensitivity analyses (HIV/syphilis separately, including anal sex and excluding COVID-19 years) (online supplemental table S3) the same determinants and direction of effects were found as the initial model, except for reported HIV/syphilis symptoms which was not a determinant of HIV diagnosis. An additional significant determinant of HIV diagnosis was originating from an STI/HIV-endemic area, while this was protective for syphilis. For syphilis diagnosis, self-reported STI in the past year was an additional determinant. In analyses including anal sex, anal sex was an additional significant determinant of HIV/syphilis diagnosis. Finally, restricting the analyses to pre-COVID-years made no large differences to the initial model.
bmjopen-2023-072862supp003.pdf (52.6KB, pdf)
Targeted testing
If targeted testing was only applied to SHC consultations who reported HIV/syphilis symptoms (the strongest determinant), in 0.6% of all consultations between 2015 and 2021 HIV/syphilis testing would have been conducted (table 3, scenario 1). Yet 95.1% of HIV diagnoses and 58.2% of syphilis diagnoses would then be missed, which corresponds to 11 and 15 missed diagnoses per year. If notified for HIV/syphilis by a partner would be added as testing criterium (the second most strongest determinant; scenario 2), in approximately 2% of all consultations HIV/syphilis testing would have been conducted, diagnosing 36.6% of all HIV and 64.7% of all syphilis diagnoses. Other significant determinants were education level, sex and age. Only age was assessed as applicable to SHC practice and age >30 years was added to scenario 3, resulting in 54.5% of all consultations wherein HIV/syphilis testing would have been conducted resulting in missing two HIV and three syphilis diagnoses annually. Finally, when adding the separate determinants of HIV and syphilis diagnosis (self-reported STI in the past year and originating from STI/HIV-endemic area; scenario 4), in 74.2% of all consultations HIV/syphilis testing would still have been conducted, missing 0.3 HIV and 2 syphilis diagnoses on average per year.
Table 3.
Number of missed HIV and/or syphilis diagnoses in targeted test options among women and heterosexual men aged >25 years visiting Dutch sexual health centres between 2015 and 2021
| Scenario | Targeted testing | Consultations tested for HIV and/or syphilis | Diagnosed HIV and/or syphilis in total 2015–2021 | Missed HIV and/or syphilis diagnoses in total 2015–2021 | Missed HIV and/or syphilis diagnoses on average per year | |||
| HIV | Syphilis | HIV | Syphilis | HIV | Syphilis | |||
| n (%) | n (%) | n (%) | n (%) | n (%) | n | n | ||
| Based on significant determinants of HIV/syphilis | ||||||||
| 1 | Reported HIV/syphilis symptoms(1). | 611 (0.6) | 4 (4.9) | 77 (41.8) | 78 (95.1) | 107 (58.2) | 11 | 15 |
| 2 | Reported HIV/syphilis symptoms (1) and/or consultations of persons who received partner notification for HIV/syphilis (2). | 2125 (1.9) | 30 (36.6) | 119 (64.7) | 52 (63.4) | 65 (35.3) | 7 | 9 |
| 3 | Reported HIV/syphilis symptoms (1), consultations of persons who received partner notification for HIV/syphilis (2) and/or aged >30 years (3). | 59 451 (54.5) | 71 (86.6) | 163 (88.6) | 11 (13.4) | 21 (11.4) | 2 | 3 |
| Based on additional significant determinants of HIV and syphilis in separate models | ||||||||
| 4 | Reported HIV/syphilis symptoms (1), consultations of persons who received partner notification for HIV/syphilis (2), aged >30 years (3), self-reported STI in past year (4) and/or originating from an STI/HIV-endemic area (5).* | 80 964 (74.2) | 80 (97.6) | 171 (92.9) | 2 (2.4) | 13 (7.1) | 0.3 | 2 |
| Total number of consultations, 2015–2021 | 109 122 (100) | 82 (100) | 184 (100) | |||||
*STI/HIV-endemic areas include Asia, Africa, the Dutch Caribbean islands, middle and South America.
STI, sexually transmitted infections.
Discussion
The strongest determinants of an HIV/syphilis diagnosis in women and heterosexual men aged over 25 years visiting SHCs were received partner notification for HIV/syphilis and reported HIV/syphilis symptoms. Persons aged ≥30 years were also more likely to have an HIV/syphilis diagnosis. When applying these determinants to targeted testing scenarios, HIV/syphilis testing would still have been conducted in 54.5% of all consultations, missing two HIV and three syphilis diagnoses annually. The scenario that resulted in the lowest number of missed HIV/syphilis diagnoses was when determinants of HIV or syphilis separately were also included, resulting in 0.3 HIV and 2 syphilis diagnoses missed annually. However, only in 26% of all consultations an HIV/syphilis test would have been omitted between 2015 and 2021.
This is the first study in the Netherlands to describe determinants of an HIV/syphilis diagnosis among women and heterosexual men aged >25 years. By the use of national surveillance data of SHCs a large study sample was guaranteed. However, there were some limitations. First, we were limited to variables as available in SOAP data. For example, HIV/syphilis-specific symptoms is one combined variable. We do note that clinical symptoms of recent HIV infection and early syphilis infection do overlap, so a clinical distinction would not be possible. More detailed clinical data may have improved the results of the regression model and the application of possible targeted testing scenarios in clinical practice. In addition, SOAP data did not allow to include all variables in the analyses as some questions contained too many missings. Especially victim of sexual violence would have been interesting as it is an HIV/syphilis test criterium for heterosexuals <25 years but could not be included due to too many missings. However, in consultations that did contain information on sexual violence, only one HIV diagnosis was found among victims of sexual violence, so we do not expect that including this variable would have changed our results. Second, HIV and syphilis were included in one combined outcome variable, while one might argue that the main analyses should have been separated in advance. However, as we intended to explore the effectiveness of potential STI targeted testing strategies in this study, we think that combined HIV/syphilis testing would be most effective for SHC practice as both HIV and syphilis tests are conducted on a blood sample. Once blood is taken, integrated testing for HIV and syphilis is most convenient. Furthermore, since the number of diagnoses were small, combining the two also increased the power. Sensitivity analyses showed different determinants when separating the two. For example, origin from an STI-endemic area was a determinant of HIV only and reported HIV/syphilis symptoms was a strong determinant of syphilis but not for HIV. This could be explained by syphilis symptoms being more often present and more recognisable than HIV symptoms.15 Third, in this study we estimated missed HIV/syphilis diagnoses annually based on numbers of HIV/syphilis diagnoses between 2015 and 2021 and did not take into account an effect of time to diagnosis. Delayed diagnoses could lead to, for example, delayed healthcare and/or further HIV/syphilis transmission, causing different annual numbers of missed HIV/syphilis diagnoses in reality then estimated in this study. Finally, it should be noted that the results of our study might be different when evaluating future years, based on possible differences in population and/or STI testing policy. Therefore continuous evaluation remains needed.
To our knowledge, no other studies have been performed on determinants of both HIV and/or syphilis diagnoses as one outcome, apart from co-infections. For determinants of HIV and syphilis diagnoses separately the targeted populations between studies differ greatly, hampering comparison of our study results.16–23 However, determinants in our study consistent with existing data were partner notification and lower education level, found to be determinants of both HIV and syphilis6 9 16–18 and STI symptoms and male sex found to be determinants of syphilis only.6 19 Yet an unexpected result in our study was that persons with two or more partners would be at decreased risk for HIV/syphilis diagnosis, as multiple partners are usually determinants of STI.9 17 22 This difference could be explained by the strict triage criteria for heterosexuals ≥25 years at SHC, making this a higher risk group compared with, for example, heterosexuals <25 years who are all eligible for STI testing. Another explanation for these reversed effects in our study might be by unmeasured variables like reasons for testing.
Using the determinants of an HIV/syphilis diagnosis, we constructed potential strategies for targeted testing. The testing scenarios were built up based on significant determinants in the model, combined with feasibility in SHC practice. Targeted testing based on sex and education level were considered not feasible as this might lead to discrimination and/or stigmatisation. Yet these results do stress the importance of reaching out to persons with low/middle-education level and making sure that STI care at SHC is accessible for this group.24 The regression model showed that the only outstanding determinants of HIV/syphilis diagnosis were HIV/syphilis specific symptoms and partner notification. Partner notification contributed to approximately half of all HIV/syphilis diagnoses found in our study. This underlines the great potential of partner notification in STI case detection, and stresses the importance of partner notification in STI control. All other determinants in the regression model had ORs close to one, meaning that specific risk groups were hard to identify within the group of heterosexuals older than 25 years at SHC. Also, when adding all of these significant determinants to targeted testing, most participants would still have been tested (74%). This raises the question whether you would be able to call this targeted testing. This might indicate that the current triage criteria for this group to be eligible for STI testing at SHC are effective in finding the persons at higher risk for STI and might need to remain as they are for surveillance purposes.
In every targeted scenario evaluated, HIV and/or syphilis diagnoses will be missed. It should be questioned whether it is acceptable in an era of aiming at going towards zero new HIV infections to put any of these targeted testing scenarios into practice. A study on targeted HIV/syphilis testing for heterosexuals <25 years estimated that three missed HIV and seven missed syphilis diagnoses annually were considered to be limited, when €3.3 million could be saved.8 An evaluation of test cost savings for women and heterosexual men aged >25 years is needed to make informed decisions. To find the optimal strategy, HIV and syphilis treatment costs should also be included in these evaluations. Additionally, ethical aspects should be considered to decide how many diagnoses are acceptable to be missed. The Joint United Nations Programme on HIV/AIDS (UNAIDS) announced the target to reach zero HIV infections in 203025 and STI AIDS Netherlands also set the aim to reach zero new HIV infections as soon as possible.15 To reach this, any missed diagnosis would be too much and timely diagnosis of HIV is necessary. In the Netherlands, diagnosis of late-stage HIV is more common among women and heterosexual men compared with MSM26 and also in the UK it is shown that syphilis often remains undiagnosed, especially among heterosexual men.27 Untreated syphilis could lead to latent syphilis with severe neurological and cardiovascular damage.28 Finally, complications of non-detected cases could lead to increased costs, either through treatment of severe disease or additional testing in general practice or hospitals. We recommend all these considerations to be taken into account when assessing targeted testing policy.
Altogether, this study is a first step in considering targeted testing for HIV and syphilis of women and heterosexual men aged >25 years in the Netherlands. It is indicated that no specific group can be identified for targeted testing without missing any HIV/syphilis diagnoses. A discussion with a multidisciplinary team consisting of public health professionals, policymakers, ethicists, economists, epidemiologists and all others involved about the public health impact of targeted testing policy is needed.
Supplementary Material
Acknowledgments
The authors thank Birgit van Benthem, Susan van den Hof and Jaap van Dissel for their constructive comments on the manuscript. Also, Maarten Schipper is thanked for statistical advice on the analyses.
Footnotes
Contributors: IJMW, HMG and JCMH designed the study. IJMW and MV cleaned the data. IJMW analysed the data and drafted the manuscript. All authors contributed to the interpretation of the results, commented on the manuscript and approved the final version. JCMH acts as guarantor for the overall content.
Funding: The authors have not declared a specific grant for this research from any funding agency in the public, commercial or not-for-profit sectors.
Competing interests: None declared.
Patient and public involvement: Patients and/or the public were not involved in the design, or conduct, or reporting, or dissemination plans of this research.
Provenance and peer review: Not commissioned; externally peer reviewed.
Supplemental material: This content has been supplied by the author(s). It has not been vetted by BMJ Publishing Group Limited (BMJ) and may not have been peer-reviewed. Any opinions or recommendations discussed are solely those of the author(s) and are not endorsed by BMJ. BMJ disclaims all liability and responsibility arising from any reliance placed on the content. Where the content includes any translated material, BMJ does not warrant the accuracy and reliability of the translations (including but not limited to local regulations, clinical guidelines, terminology, drug names and drug dosages), and is not responsible for any error and/or omissions arising from translation and adaptation or otherwise.
Data availability statement
Data are available upon reasonable request. This study uses data from the Dutch national registration of sexual health centre consultations (SOAP). Data can be requested for scientific use from the SOAP registration committee (contact soap@rivm.nl).
Ethics statements
Patient consent for publication
Not applicable.
Ethics approval
Ethical approval for the study was not necessary following the Dutch Medical Research (involving Human Subjects) Act, as the study uses routinely collected, anonymous surveillance data (Wet medisch-wetenschappelijk onderzoek met mensen 1998 §1 artikel 1).
References
- 1.Workowski KA, Bachmann LH, Chan PA, et al. Sexually transmitted infections treatment guidelines, 2021. MMWR Recomm Rep 2021;70:1–187. 10.15585/mmwr.rr7004a1 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Lanjouw E, Ouburg S, de Vries HJ, et al. “Background review for the '2015 European guideline on the management of Chlamydia Trachomatis infections'” Int J STD AIDS 2015:0956462415618838. 10.1177/0956462415618838 [DOI] [PubMed] [Google Scholar]
- 3.Unemo M, Ross J, Serwin AB, et al. European guideline for the diagnosis and treatment of Gonorrhoea in adults Int J STD AIDS 2021;32:108–26. 10.1177/0956462420948739 [DOI] [PubMed] [Google Scholar]
- 4.Janier M, Unemo M, Dupin N, et al. European guideline on the management of Syphilis. J Eur Acad Dermatol Venereol 2021;35:574–88. 10.1111/jdv.16946 [DOI] [PubMed] [Google Scholar]
- 5.Gökengin D, Wilson-Davies E, Nazlı Zeka A, et al. European guideline on HIV testing in Genito-urinary medicine settings. J Eur Acad Dermatol Venereol 2021;35:1043–57. 10.1111/jdv.17139 [DOI] [PubMed] [Google Scholar]
- 6.National Institute for Public Health and the Environment (RIVM) . Draaiboek voor Centra Seksuele Gezondheid in de Publieke Gezondheidszorg. 2022. Available: https://lci.rivm.nl/draaiboeken/consult-seksuele-gezondheid
- 7.Suijkerbuijk AWM, Over EAB, Koedijk FDH, et al. More efficient testing policy at STI clinics. Ned Tijdschr Geneeskd 2014;158:A6980. Available: https://pubmed.ncbi.nlm.nih.gov/24642118/ [PubMed] [Google Scholar]
- 8.Suijkerbuijk AWM, Over EAB, van Aar F, et al. Consequences of restricted STI testing for young Heterosexuals in the Netherlands on test costs and QALY losses. Health Policy 2018;122:198–203. 10.1016/j.healthpol.2017.12.001 [DOI] [PubMed] [Google Scholar]
- 9.van DA, Visser M, van F, et al. Sexually transmitted infections in the Netherlands in 2021. Bilthoven: National Institute for Public Health and the Environment (RIVM), 2022. [Google Scholar]
- 10.Statistics Netherlands (CBS) . Migratieachtergrond. 2022. Available: https://www.cbs.nl/nl-nl/onze-diensten/methoden/begrippen/migratieachtergrond
- 11.Elmes J, Silhol R, Hess KL, et al. Receptive Anal sex contributes substantially to Heterosexually acquired HIV infections among at-risk women in twenty US cities: results from a Modelling analysis. Am J Reprod Immunol 2020;84:e13263. 10.1111/aji.13263 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.O’Leary A, DiNenno E, Honeycutt A, et al. Contribution of Anal sex to HIV prevalence among Heterosexuals: A modeling analysis. AIDS Behav 2017;21:2895–903. 10.1007/s10461-016-1635-z [DOI] [PubMed] [Google Scholar]
- 13.Rahman N, Ghanem KG, Gilliams E, et al. Factors associated with sexually transmitted infection diagnosis in women who have sex with women, women who have sex with men and women who have sex with both. Sex Transm Infect 2021;97:423–8. 10.1136/sextrans-2020-054561 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.van Liere GAFS, Dukers-Muijrers NHTM, Levels L, et al. High proportion of Anorectal Chlamydia Trachomatis and Neisseria Gonorrhoeae after routine universal Urogenital and Anorectal screening in women visiting the sexually transmitted infection clinic. Clin Infect Dis 2017;64:1705–10. 10.1093/cid/cix243 [DOI] [PubMed] [Google Scholar]
- 15.National Institute for Public Health and the Environment (RIVM) . Richtlijnen & Draaiboeken. 2022. Available: https://lci.rivm.nl/richtlijnen
- 16.Gupta N, Niyas VKM, Nischal N, et al. Epidemiological trends in patients living with human immunodeficiency virus: a 13-year experience from a tertiary care center in India. Infez Med 2019;27:308–15. Available: https://pubmed.ncbi.nlm.nih.gov/31545775/ [PubMed] [Google Scholar]
- 17.Nishiki S, Arima Y, Yamagishi T, et al. Syphilis in Heterosexual women: case characteristics and risk factors for recent Syphilis infection in Tokyo, Japan, 2017-2018. Int J STD AIDS 2020;31:1272–81. 10.1177/0956462420945928 [DOI] [PubMed] [Google Scholar]
- 18.Hurtado I, Alastrue I, Pavlou M, et al. Increased Syphilis trend among patients in an AIDS information and prevention center. Gac Sanit 2011;25:368–71. 10.1016/j.gaceta.2010.04.007 [DOI] [PubMed] [Google Scholar]
- 19.Spiteri G, Unemo M, Mårdh O, et al. The resurgence of Syphilis in high-income countries in the 2000S: a focus on Europe. Epidemiol Infect 2019;147:e143. 10.1017/S0950268819000281 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Erdinc FS, Dokuzoguz B, Unal S, et al. Temporal trends in the epidemiology of HIV in Turkey. Curr HIV Res 2020;18:258–66. 10.2174/1570162X18666200427223823 [DOI] [PubMed] [Google Scholar]
- 21.Saxton PJW, McAllister SM, Thirkell CE, et al. Population rates of HIV, Gonorrhoea and Syphilis diagnoses by sexual orientation in New Zealand. Sex Transm Infect 2022;98:376–9. 10.1136/sextrans-2021-055186 [DOI] [PubMed] [Google Scholar]
- 22.Harrington P, Onwubiko U, Qi M, et al. Factors associated with HIV seroconversion among women attending an urban health clinic in the south: A matched case–control study. AIDS Patient Care and STDS 2020;34:124–31. 10.1089/apc.2019.0259 [DOI] [PubMed] [Google Scholar]
- 23.Chen M, Ma Y, Chen H, et al. Demographic characteristics and spatial clusters of recent HIV-1 infections among newly diagnosed HIV-1 cases in Yunnan. BMC Public Health 2019;19. 10.1186/s12889-019-7557-8 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Slurink IA, Götz HM, van Aar F, et al. Educational level and risk of sexually transmitted infections among clients of Dutch sexual health centres. Int J STD AIDS 2021;32:1004–13. 10.1177/09564624211013670 [DOI] [PubMed] [Google Scholar]
- 25.UNAIDS Joint United Nations Programme on HIV/AIDS . Fast-track: ending the AIDS epidemic by 2030. Geneva: UNAIDS, 2014. [Google Scholar]
- 26.van Sighem AI, Wit F, Boyd A, et al. Monitoring Report 2021. Human Immunodeficiency Virus (HIV) Infection in the Netherlands (stichting HIV monitoring). Amsterdam, 2021. [Google Scholar]
- 27.Harvala H, Reynolds C, Fabiana A, et al. Lessons learnt from Syphilis-infected blood donors: a timely reminder of missed opportunities. Sex Transm Infect 2022;98:293–7. 10.1136/sextrans-2021-055034 [DOI] [PubMed] [Google Scholar]
- 28.World health Organization (WHO) [Syphilis]. 2022. Available: https://www.who.int/health-topics/syphilis#tab=tab_1
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
bmjopen-2023-072862supp001.pdf (65.6KB, pdf)
bmjopen-2023-072862supp002.pdf (65KB, pdf)
bmjopen-2023-072862supp003.pdf (52.6KB, pdf)
Data Availability Statement
Data are available upon reasonable request. This study uses data from the Dutch national registration of sexual health centre consultations (SOAP). Data can be requested for scientific use from the SOAP registration committee (contact soap@rivm.nl).