Keywords: histamine, kidney, ranitidine, salt sensitivity
Abstract
Histamine is involved in the regulation of immune response, vasodilation, neurotransmission, and gastric acid secretion. Although elevated histamine levels and increased expression of histamine metabolizing enzymes have been reported in renal disease, there is a gap in knowledge regarding the mechanisms of histamine-related pathways in the kidney. We report here that all four histamine receptors as well as enzymes responsible for the metabolism of histamine are expressed in human and rat kidney tissues. In this study, we hypothesized that the histaminergic system plays a role in salt-induced kidney damage in the Dahl salt-sensitive (DSS) rat, a model characterized with inflammation-driven renal lesions. To induce renal damage related to salt sensitivity, DSS rats were challenged with 21 days of a high-salt diet (4% NaCl); normal-salt diet (0.4% NaCl)-fed rats were used as a control. We observed lower histamine decarboxylase and higher histamine N-methyltransferase levels in high-salt diet-fed rats, indicative of a shift in histaminergic tone; metabolomics showed higher histamine and histidine levels in the kidneys of high-salt diet-fed rats, whereas plasma levels for both compounds were lower. Acute systemic inhibition of histamine receptor 2 in the DSS rat revealed that it lowered vasopressin receptor 2 in the kidney. In summary, we established here the existence of the local histaminergic system, revealed a shift in the renal histamine balance during salt-induced kidney damage, and provided evidence that blockage of histamine receptor 2 in the DSS rat affects water balance and urine concentrating mechanisms.
NEW & NOTEWORTHY Histamine is a nitrogenous compound crucial for the inflammatory response. The knowledge regarding the renal effects of histamine is very limited. We showed that renal epithelia exhibit expression of the components of the histaminergic system. Furthermore, we revealed that there was a shift in the histaminergic tone in salt-sensitive rats when they were challenged with a high-salt diet. These data support the notion that histamine plays a role in renal epithelial physiological and pathophysiological functions.
INTRODUCTION
The deleterious effects of hypertension account for 26% of the reported cases of end-stage renal disease in the United States (1, 2). Salt-sensitive hypertension, which affects 30 − 50% of patients with hypertension (3, 4), increases the risk of developing comorbidities such as chronic kidney disease (CKD). In CKD, dysfunction of the immune system is well established (5); increased inflammatory markers such as cytokines, adhesion molecules, and cells of the innate immune system contribute to the development and/or perpetuation of renal injury (6, 7). Accumulation of immune cells in the tubulointerstitium of the kidney is an important factor in salt sensitivity (8).
A high-salt (HS) diet triggers damage to the renal tissue and leads to an inflammatory response that causes immune cell infiltration into the kidney and induces the production of inflammatory cytokines (9). Dahl salt-sensitive (DSS) rats fed a HS diet display a renal phenotype similar to salt-sensitive patients: albuminuria, renal fibrosis, and glomerulosclerosis. Furthermore, they exhibit increased macrophage and T cell infiltration (10). Injured glomeruli in DSS rats on a HS diet have macrophages, monocytes, and T lymphocytes infiltrating the area (11). Interestingly, when T cell infiltration was prevented in DSS rats using immunosuppressive agents or genetic approaches, this was accompanied by attenuation of hypertension (10). When T cells were deleted in DSS rats, this blunted salt-sensitive hypertension in a study by Rudemiller et al. (12); both T and B cell deletion had essentially the same effect (13). Additionally, DSS rats fed a HS diet exhibit significantly increased circulating levels of inflammatory molecules and cytokines compared with normotensive controls (11). Given the integral role of the immune/inflammatory response in renal disease progression during salt-induced renal damage, the components of this system have been identified as emerging targets of recent research.
Histamine is a biogenic amine that is involved in the inflammatory response. Histamine along with its four receptors [histamine receptor (HR)1, HR2, HR3, and HR4] is part of a flexible and responsive histaminergic system (HiS). It is created by histidine decarboxylase (HDC) and is then further metabolized by diamine oxidase (DAO) or histamine-N-methyltransferase (HNMT) (9, 14). Histamine is found in the circulation or can be released from resident immune cells such as basophils and mast cells (9). Classic functions of histamine were characterized in mucosal epithelial layers of airways and the gastrointestinal tract. Histamine release by mast cells induces signs of asthma and allergy (15). The paracrine effects of gastric mucosa involve HR2-dependent mucus production (16, 17). In the lower gastrointestinal system, histamine HR2 antagonists cause IgE production and food allergies (18). Within the kidney, histamine has been proposed to come from local tissue pools since kidney histamine levels are significantly higher than those of the circulation (19). Furthermore, HDC and HNMT have been reported to be expressed in the kidney, with HDC exhibiting effects on renal immune cell recruitment (20). All four HRs have been linked in some part to renal function (21, 22). Some studies have suggested a role of histamine in regulating glomerular filtration rate (GFR) through effects on renal blood flow autoregulation via HR1 (23). HR3 has a suspected link with urine flow and norepinephrine overflow rate given its possible localization on renal noradrenergic nerve endings (24, 25). Pini et al. (21, 26) found that an HR4 antagonist prevented the loss of megalin in a model of diabetic nephropathy. HR2 has been connected with histamine-induced renin release and potentially water excretion, renal blood flow, and renal vascular resistance (21, 23). Veglia et al. (27) reported that activation of HR1 undermines the morphological and functional integrity of the slit diaphragm. Interestingly, Kjær et al. (28) found that blockade of HR1 and 2 postdehydration blunted aldosterone reduction; it was hypothesized that histamine, acting on HR2, potentially regulates dehydration-induced vasopressin secretion. Although there have been some studies into the influence of histamine on the kidney, the details of which receptors are eliciting which functions remain to be clarified. We hypothesized in this study that the local renal HiS plays a significant role in salt-induced renal damage. Our study provided the first insight into the physiological impact of histamine-related pathways in renal disease during salt sensitivity.
METHODS
Experimental Animal Protocol
Male DSS rats were obtained from Charles River Laboratories (strain code 320) at 7 wk of age and maintained on a normal-salt (NS; 0.4% NaCl) AIN-76A-based diet upon arrival (no. 113755, Dyets, Bethlehem, PA). At 8 wk of age, rats were randomly assigned into groups that were administered either vehicle (saline) or ranitidine (selective HR2 blocker, 25 mg/kg) once daily intraperitoneally on 3 consecutive days. Water consumption and biometric parameters were recorded, and urine was collected in metabolic cages following the injections. After a 5-day recovery period, rats were switched to a HS diet (4% NaCl, AIN-76A-based chow, no. 113756) to induce renal damage, and the same animals were given vehicle (saline) or ranitidine intraperitoneally once a day on consecutive days 21–24 of the HS challenge. GFR was assessed at the end point (day 24 of the HS diet). Rats were housed at the Medical University of South Carolina Animal Services facilities under a 12:12-h light-dark cycle. All samples were collected between 10 AM and 2 PM to account for circadian variations. Access to food and water and environmental enrichment was provided ad libitum. All animal experiments were conducted in accordance with the National Institutes of Health Guide for the Care and Use of Laboratory Animals following protocol review and approval by the Medical University of South Carolina Institutional Animal Care and Use Committee. Animals were randomly assigned to specific research groups, and the sample size was estimated before the experiment using power analysis based on historical observations or pilot studies.
Kidney Flush Surgery, Blood Collection, and Tissue Isolation
For end point euthanasia and tissue harvest, rats were anesthetized with 2.5% isoflurane, and the abdominal aorta was catheterized for blood collection. The kidneys were then flushed with PBS (3 mL/min/kidney until blanched) as previously described (29). Excised kidney tissues were weighed and either immediately used for ex vivo experimentation, snap frozen for future molecular biology experiments, or fixed in 10% formalin for further staining.
GFR Measurements
GFR was measured in unrestrained conscious rats using a high-throughput method testing clearance of fluorescent FITC-labeled inulin (TdB Consultancy, Uppsala, Sweden). The method was adapted for rats from a previously described protocol (30). Predialyzed 20 mg/mL FITC-inulin solution in saline (2 µL/g body wt) was administered by a bolus tail vein injection. Then, 10 µL of blood were collected from conscious rats 3, 5, 8, 16, 25, 40, 60, 80, 100, and 120 min after the injection by tail bleed. Next, plasma was separated, and inulin clearance was quantified by FITC intensity using a NanoDrop 3300 Fluorospectrometer (Thermo Fisher Scientific, Wilmington, DE). GFR was then calculated from the observed decrease in FITC fluorescence; the GFR curves were approximated with a biexponential decay function using OriginPro 9.0 (OriginLab, Northhampton, MA), and GFR values (in mL/min) were obtained from the fitting parameters as previously described (30).
Plasma Analysis and Urinalysis
Urine and plasma electrolytes were evaluated with a Carelyte analyzer from Diamond Diagnostics (to separate plasma, blood samples obtained from the abdominal aorta before kidney flush were centrifuged immediately after collection at 1,500 g for 5 min, snap frozen in LN2, and then stored at −80°C). Plasma and urinary pH were measured using a Thermo Scientific Orion Start A111 pH meter; urine osmolality was tested with a Wescor Vapro osmometer.
Human Tissues
Human kidneys were obtained from LifeLink Foundation, which provides human donor organs and tissues (nontransplantable) for research purposes. LifeLink operates three Centers for Medicare and Medicaid Services-approved Organ Procurement Organizations in Georgia, Puerto Rico, and the west coast of Florida and a Federal Drug Administration- and American Association of Tissue Banks-certified Tissue Bank whose primary mission is to recover donor organs and tissues for life-saving and life-enhancing transplantations. Upon authorization for research, human kidneys deemed unsuitable for transplant together with deidentified clinical and medical history of the donor were made available to Augusta University (AU), a LifeLink-approved research entity that has agreed to receive such donor organs and tissues for use in studies under AU Institutional Review Board 1732545.
Human Donor Information
An unconscious female African American/Black 52-yr-old patient with an unknown medical history was admitted to the Emergency Department. During the diagnostic evaluation, a pontine hemorrhage was revealed. Given the poor prognosis and unlikelihood of recovery, the legal next of kin has chosen to change code status to do not resuscitate and proceed with withdrawal of life support and authorized organ donation after cardiac death. Since the donor did not meet the expanded criteria, the kidney was donated to Augusta University. Frozen kidney sections showed minimal interstitial fibrosis and inflammatory changes, minimal tubular atrophy, and slight thickening of arterial vessels.
Histology, Immunohistochemistry, and Tissue Damage Assessment
Immediately after harvest, tissues were fixed in 10% neutral buffered formalin, paraffin embedded, sectioned, mounted on slides, and stained with picrosirius red for protein cast and fibrosis assessment according to routinely used histological protocols. Tissues were randomized and coded before being submitted for blocking, sectioning, and staining. For the assessment of fibrosis, kidney sections were scanned with a Perkin-Elmer Vectra Polaris Automated Quantitative Pathology Imaging System Slide scanner. Fiji (National Institutes of Health) software was used to assess the area of fibrosis using the Color Deconvolution plugin and Threshold tool, and the percentage of the fibrotic area versus the total kidney slice area was calculated. Protein casts were assessed from picrosirius red-stained slides and then scored separately and blindly by two people on a scale of 0−4 (where 0 = healthy, no protein casts and 4 = a kidney with >20% protein casts) as previously described (31).
For immunohistochemical staining of rat tissues, kidney sections were cut at a 4-μm thickness, dried, paraffinized, mounted onto glass slides, and treated according to the following protocol. For deparaffinization and hydration, sample slides were treated with xylene for 5 min for three times (I, II, and III), 100% ethanol for 1 min for three times (I, II, and III), 95% ethanol for 1 min, 70% ethanol for 1 min, and deionized (DI) water for 5 min. Slides were rinsed with Tris-buffered saline with 0.1% Tween (TBST) for 5 min for three times. Antigen retrieval was done with Citrate Buffer DAKO Target Retrieval solution. Next, sample slides were treated with Target Retrieval solution (1:10 solution in DI water, 85°C) for 30 min, cooled down for 30 min at room temperature, and rinsed with DI water. For staining, samples were blocked with peroxidase block (Dako) for 25 min and rinsed with TBST for 5 min for two times. Avidin (Dako) was applied for 10 min, samples were rinsed in TBST for 5 min for three times, Biotin (Dako) was applied for 10 min, and another rinse with TBST was performed for 5 min for three times. Next, Protein Block (Dako) was applied for 30 min at room temperature. Samples were then stained with primary antibodies HRH1 (AHR-001, Alomone), HRH2 (AHR-002, Alomone), HRH3 (EPR-5631, Novus Bio), and HRH4 (AB5663P, Merck) (all 1:100 dilution) overnight at +4°C. The negative control was samples treated diluent only (no primary antibody). After incubation, samples were rinsed in TBST for 5 min for three times, stained with biotinylated secondary goat anti-rabbit IgG antibody (1:200, BA-1000-1.5, Vector Laboratories) for 30 min at room temperature, and rinsed in TBST for 5 min for three times. Streptavidin-horseradish peroxidase (Vector Laboratories) was applied for 10 min and rinsed in TBST for 5 min for three times. 3,3′-Diaminobenzidine (DAB) solution (1 drop or 20 µL of the DAB chromogen mixed well with 1 mL of Substrate Buffer, both from Dako) was applied to the samples for 5 min and rinsed gently with DI water. In the end, samples were counterstained with hematoxylin (Dako) for 1 min and rinsed with DI water, and 0.08% ammonia hydroxide in water was applied until the stain was visibly blue (30 s). Samples were rinsed with DI water for 5 min and treated with reagent alcohol (95%) for 1–2 min for two times, absolute ethanol for 1–2 min for two times, and xylene for 1–2 min for two times. Slides were mounted with mounting media (Vectashield, Vector Laboratories) and a coverglass (Corning).
For immunohistochemistry on human tissues, donor kidney samples were fixed in 10% buffered formaldehyde or 4% paraformaldehyde, processed on a Sakura Tissue TEK VIP processor [70% ethanol for 1 h, 95% ethanol for 1 h for two times, 100% ethanol for 1 h for three times, xylene for 1 h for three times, and Polyfin Embedding media (Triangle Biomedical Sciences) for 1 h for four times, 60°C] and cut into 4-μm slices. Antigen unmasking was performed using Antigen Unmasking Solution (1:100, 3 mL/300 mL DI water) in a microwave, and endogenous peroxidase was inactivated with 0.3% hydrogen peroxide for 20 min. Slides were preincubated in blocking buffer (BSA, goat serum, and Triton X-100 mixture) for 30 min. Tissue sections were incubated with anti-HDC antibodies (1:100 dilution, PA5-102775, Invitrogen), anti-HNMT antibodies (1:500 dilution, PA5-57289, Invitrogen), anti-DAO antibodies (1:250 dilution, no. 13273-1-AP, Proteintech), anti-HR1 antibodies (1:20 dilution, no. 13413-1-AP, Proteintech), anti-HR2 antibodies (1:100 dilution, AHR-002, Alomone Labs), anti-HR3 antibodies (1:100 dilution, EPR5631, Abcam), and anti-HR4 antibodies (1:100 dilution, bs-10993R, Bioss) for 2 h at room temperature or overnight at 4°C. The negative slide was incubated in the blocking buffer only. Secondary detection was performed with goat anti-rabbit biotinylated IgG (Biocare) followed by streptavidin horseradish peroxidase (Biocare) and visualized with ImmPACT DAB staining. All slides were counterstained with Harris hematoxylin, dehydrated, mounted with permanent mounting media, and then visualized with a Nikon Ti2 (inverted) microscope, Plan Fluor ×20 DIC N2 optics, and a color camera (Nikon DS-Fi3).
Western Blot Analysis
Kidney tissues were cut into 1- to 2-mm slices, and the pieces were homogenized using a bead mill homogenizer at 5 m/s for 30 s to 1 min in RIPA buffer containing a protease inhibitor cocktail (Roche) on ice and then spin cleared at 10,000 g for 10 min. The resulting supernatant was subjected to SDS-PAGE and transferred onto a nitrocellulose membrane (Bio-Rad, Hercules, CA) for probing with primary and secondary antibodies, and the bands were subsequently visualized by enhanced chemiluminescence (Thermo Scientific, Waltham, MA) using a Chemidoc Imager (Bio-Rad). The following antibodies were used: HDC (PA5-79354, Invitrogen, 1:1,000), HNMT (PA5-57289, Invitrogen, 1:500), DAO (no. 13273-1-AP, Protein Tech, 1:400), HR1 (no. 13413-1-AP, Protein Tech, 1:500), HR2 (AHR-002, Alomone, 1:500), HR3 (ab124732, Abcam, 1:1,000), HR4 (bs-10993R, Bioss, 1:1,000), tryptase (ab110413, Abcam, 1:1,000), arginine vasopressin receptor 2 (AVPR2; PA5-68479, Invitrogen, 1:600), aquaporin-2 (AQP2; PA5-78808, Invitrogen, 1:250), and Fc fragment of IgE, high affinity I, receptor for α-polypeptide (FCER1A; A1751, ABClonal, 1:750).
Measurements of Tissue Levels of Histamine and Histidine
Immediately following kidney harvest, the cortex and medulla were dissected on ice and snap frozen for metabolomic analysis. Water-soluble metabolites were extracted using an acidic acetonitrile extraction procedure (32). Metabolic profiles of renal cortices were generated using ultra high-performance liquid chromatography high-resolution mass spectrometry (UHPLC-HRMS) with a Dionex UltiMate 3000 UHPLC system (Thermo Fisher Scientific) coupled to an Exactive Plus Orbitrap MS (Thermo Fisher Scientific). Metabolites were identified by retention time exact mass [±5 ppmusing Metabolomics Analysis and Visualization Engine (MAVEN) at the Biological and Small Molecule Mass Spectrometry Core of the University of Tennessee (Knoxville, TN; RRID: SCR_021368].
Statistical Analysis
One-way ANOVA with Holm-Sidak post hoc test, Student’s t test, or one-way repeated-measures ANOVA with a Holm-Sidak post hoc test was used when applicable (see figures). Data are expressed as box plots with the whiskers indicating SDs, boxes indicating SEs, and the line showing the median. Values of P < 0.05 were considered statistically significant. Origin 2019 b was used for all statistical analysis.
RESULTS
Expression of HiS Components in Rat and Human Kidneys
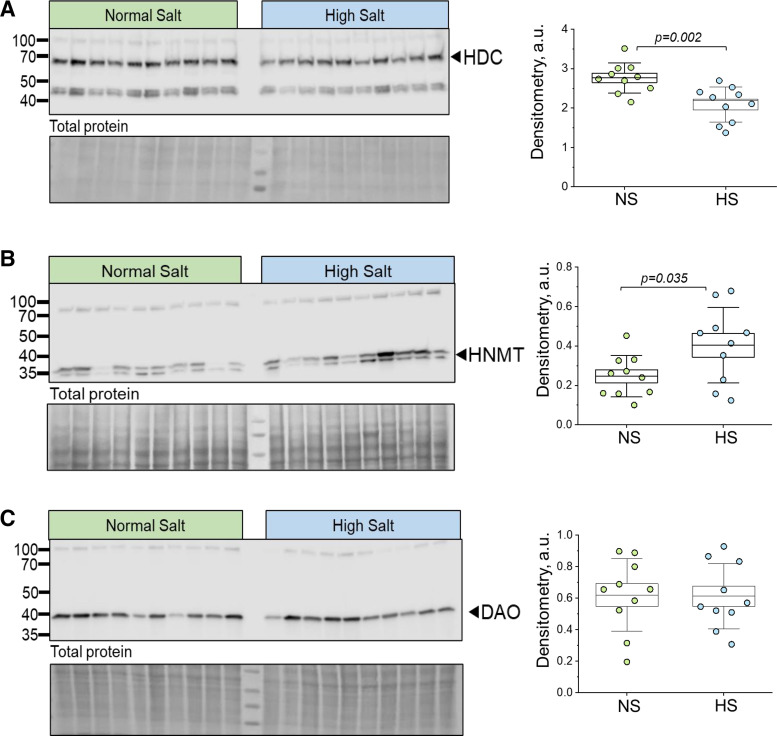
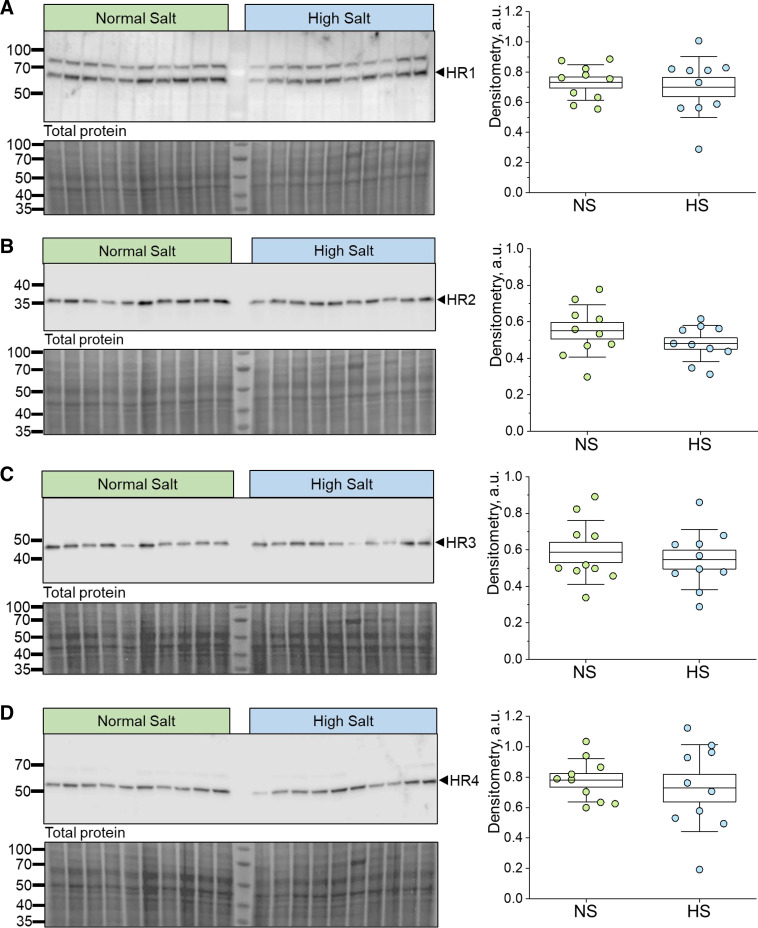
In this study, we first tested for the abundance and localization of HiS components in human and DSS rat kidneys (Figs. 1–4). Immunohistochemistry confirmed that all four HRs were present in the DSS rat and human renal cortex and medulla, with segment-specific expression (Figs. 1 and 2). In the cortical region, all four receptors showed clear localization within the glomerulus (podocytes and Bowman capsule cells) as well as the proximal tubule, where HR2 and HR4 exhibited more pronounced apical/brush border abundance. HR1, HR2, and HR3 demonstrated clear expression in the distal nephron [likely the cortical collecting duct (CD) and distal convoluted tubule], whereas HR4 expression was not detected in these cells. In the medullary region, HR2 had the most abundant expression, with the other receptors displaying clear but less profound staining in medullar tubular cells. The human renal cortex and medulla displayed expression patterns of HRs and HiS enzymes similar to those of the rat cortex (Fig. 2). Western blot analysis further confirmed expression and demonstrated similar protein levels for all four receptors in the cortex of DSS rats fed NS or HS diets (Fig. 3). Furthermore, immunohistochemistry demonstrated that all three HiS enzymes (HDC, HNMT, and DAO) were present along the nephron in DSS rat (Fig. 1, left) and human (Fig. 2) tissue. Interestingly, Western blot analysis revealed that the cortical region of HS diet-fed DSS rat kidneys exhibited substantial differences in the abundance of HDC and HNMT (Fig. 4, A and B): HS diet-fed DSS rats showed a lower level of HDC (histamine synthesis) accompanied with a higher level of HNMT (histamine metabolism). The abundance of DAO, which is less specific toward histamine, was similar between the groups (Fig. 4C). These findings suggest that there is a potential shift in the histaminergic tone in the kidney cortex toward less histamine synthesis and higher intracellular histamine metabolism during salt-induced renal damage.
Figure 1.
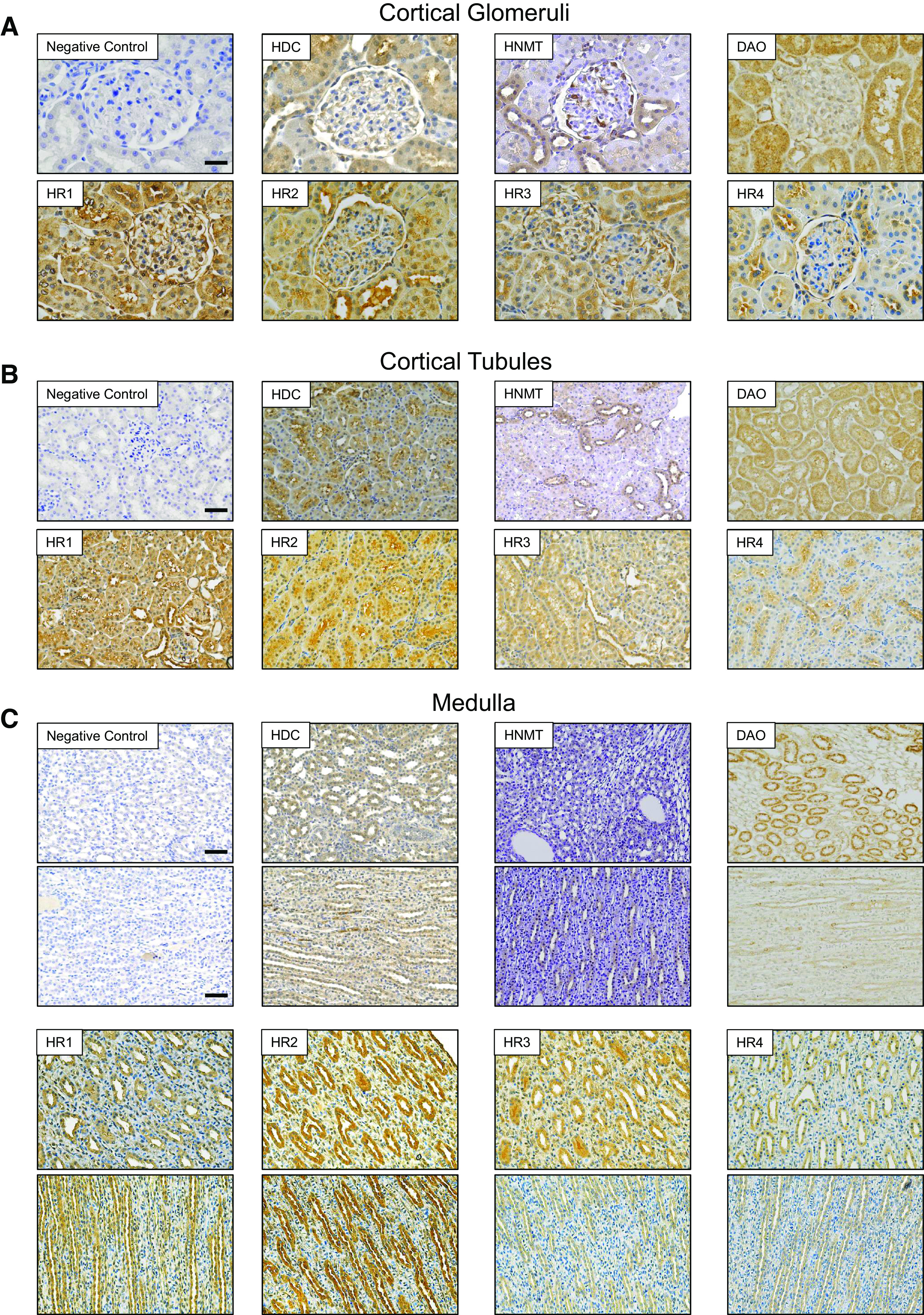
Histamine receptor (HR)1, HR2, HR3, and HR4 as well as histaminergic system enzymes are expressed within the renal cortex and medulla of male Dahl salt-sensitive (DSS) rats. A and B: representative images of immunohistochemical staining for histamine decarboxylase (HDC), histamine N-methyltransferase (HNMT), diamine oxidase (DAO), HR1, HR2, HR3, and HR4, respectively, within renal cortical glomeruli (magnification: ×40) and tubules (magnification: ×20) of male DSS rats fed a normal-salt diet. Scale bars = 25 µm for glomeruli and 50 µm for tubules. C: representative images of inner and outer medullary expression of HDC, HNMT, DAO, HR1, HR2, HR3, and HR4 in normal-salt diet-fed DSS rats. Scale bar = 50 µm. The brown coloration represents expression. Scale bars shown in the negative control image apply to all images within the same group.
Figure 4.
Enzymes related to the metabolism of histamine are expressed in the renal cortex of normal-salt (NS; 0.4% NaCl) or high-salt (HS; 4% NaCl) diet-fed male Dahl salt-sensitive rats. A–C, left: histamine decarboxylase (HDC; A), histamine N-methyltransferase (HNMT; B), and diamine oxidase (DAO; C) expression was confirmed with Western blot analysis using renal cortex samples of male Dahl salt-sensitive rats fed NS vs. HS diets. Each lane represents a sample from an individual rat. Pierce-stained membrane used for total protein analysis is shown below the membrane probed with the antibodies. A–C, right: densitometry analysis of the Western blots shown on the left; n = 10 individual animals per diet group. Boxes indicate means ± SE; whiskers indicate SDs. One-way ANOVA with a Holm-Sidak post hoc test was used for statistical analysis; a.u., arbitrary units.
Figure 2.
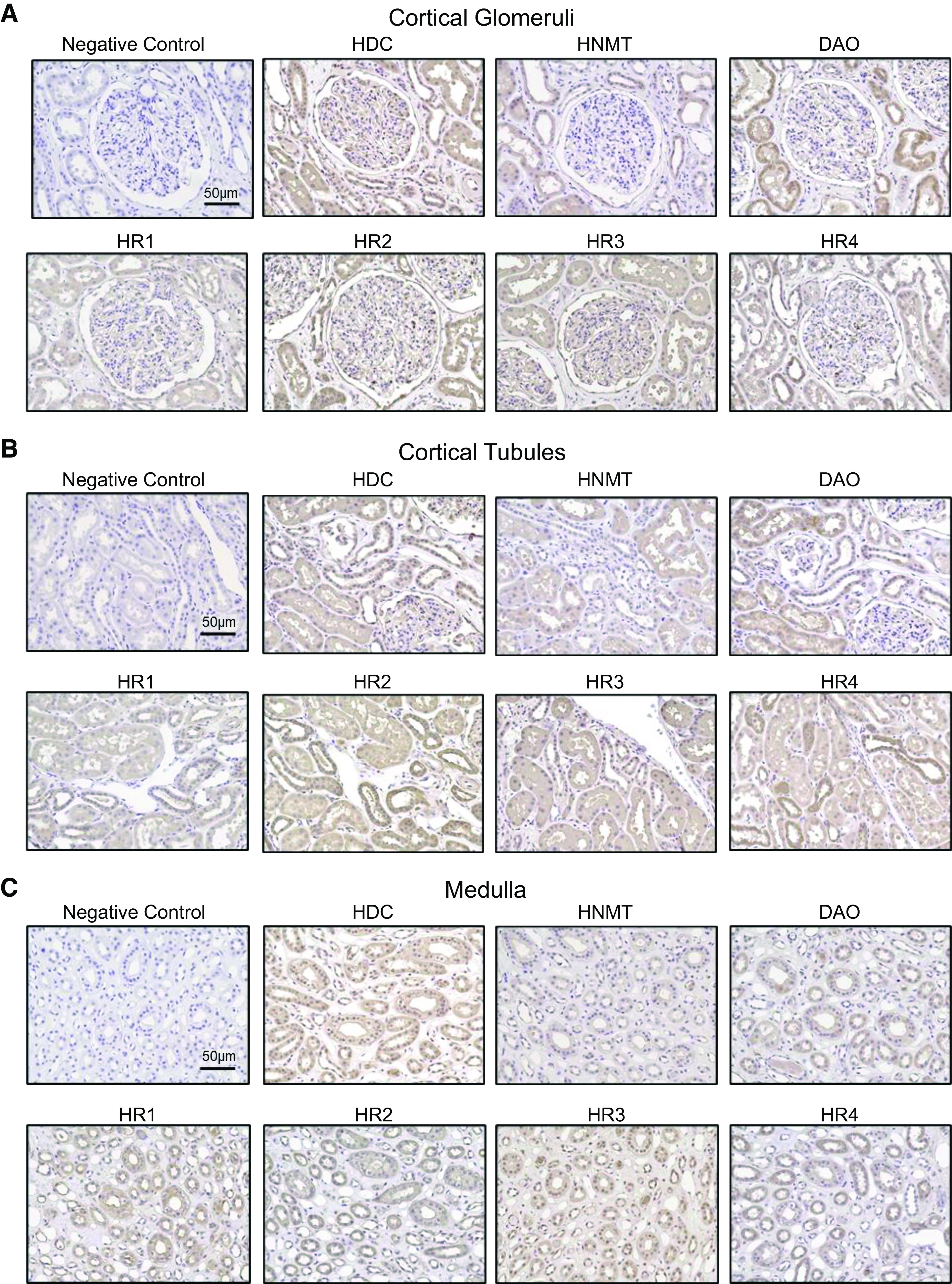
All components of the histaminergic system are abundantly expressed in the human kidney. Images represent immunohistochemistry of cortical glomeruli (A) and cortical tubular (B) and medullary (C) regions of human kidney samples. Top: representative images showing histamine decarboxylase (HDC), histamine N-methyltransferase (HNMT), and diamine oxidase (DAO) expression. Bottom: representative images showing histamine receptor (HR)1, HR2, HR3, and HR4 expression. Magnification: ×20. Scale bars = 50 µm. Scale bars shown in the negative control image apply to all images within the same group. The brown coloration represents expression.
Figure 3.
Western blot analysis verified expression of all four histamine receptors (HRs) within the renal cortex of male Dahl salt-sensitive rats on a normal-salt (NS) vs. high-salt (HS) diet. A–D, left: Western blot analysis probing for HR1 (A), HR2 (B), HR3 (C), and HR4 (D) within the renal cortex of male Dahl salt-sensitive rats fed either a NS or HS diet. Each lane represents an individual animal. Pierce-stained membranes are shown below probed membranes. A–D, right: densitometry analysis of the Western blots shown on the left comparing HR1 (A), HR2 (B), HR3 (C), and HR4 expression (D) in the renal cortex of NS or HS diet-fed rats; n = 10 per diet group; each lane is a sample from an independent animal. Boxes indicate means ± SE; whiskers indicate SDs. One-way ANOVA with a Holm-Sidak post hoc test was used for statistical analysis; a.u. arbitrary units.
Intrarenal Sources of Histamine
To investigate the potential of intrarenal production of histamine in the kidney cortex of the DSS rat, we measured levels of histidine (histamine precursor) and histamine using UHPLC-HRMS. DSS rats challenged with a HS diet exhibited a significant increase in both histidine and histamine levels in cortical regions of the kidneys (vs. the NS diet; Fig. 5, A and B). Interestingly, plasma levels of both histamine and histidine were significantly decreased following HS challenge (Fig. 5, C and D). To assess renal levels of mast cells (major contributors to local histamine release), we performed Western blot analysis to probe for markers of mast cells [tryptase (33) and FCER1A]. As shown in Fig. 5, E and F, we observed no significant changes in tryptase in response to the HS challenge in the DSS rat; FCER1A exhibited a decrease. We can surmise from these data that HS diet feeding results in elevated levels of histamine in the kidney of the DSS rat and a shift in renal histaminergic tone, as evidenced by changes in HiS enzyme expression.
Figure 5.
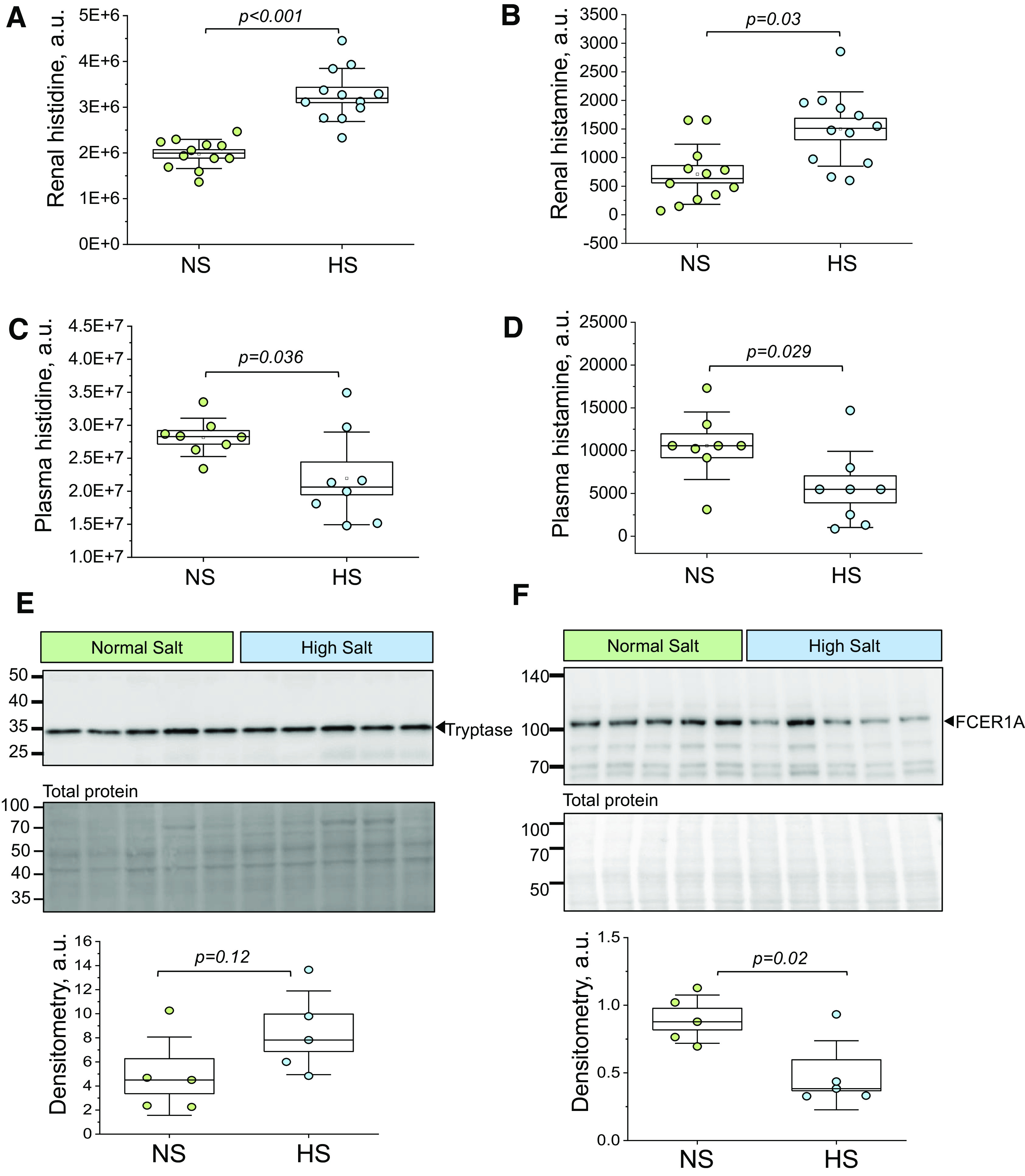
Renal and plasma levels of histamine, histidine, and expression of mast cell markers in Dahl salt-sensitive (DSS) rats. A and B: levels of histidine (A) and histamine (B) obtained with mass spectrometry in renal tissues from normal-salt (NS) and high-salt (HS) diet-fed DSS rats; n = 12 per diet group. C and D: levels of histidine (C) and histamine (D) obtained with mass spectrometry in plasma from NS and HS diet-fed DSS rats; n = 8 per diet group. E and F, top: representative Western blot images probing for mast cell markers [tryptase (E) and Fc fragment of the high-affinity IgE receptor α-polypeptide (FCER1A; F)] Ponceau-stained membranes (total protein) are shown below the probed membranes. E and F, bottom: densitometric analysis for tryptase (E) and FCER1A (F) membranes; n = 5 per diet group. Boxes indicate means ± SE; whiskers are SDs. One-way ANOVA with a Holm-Sidak post hoc test was used for statistical analysis; a.u. arbitrary units.
Effects of HR2 Blockade on Renal Tissue Damage of the DSS Rat
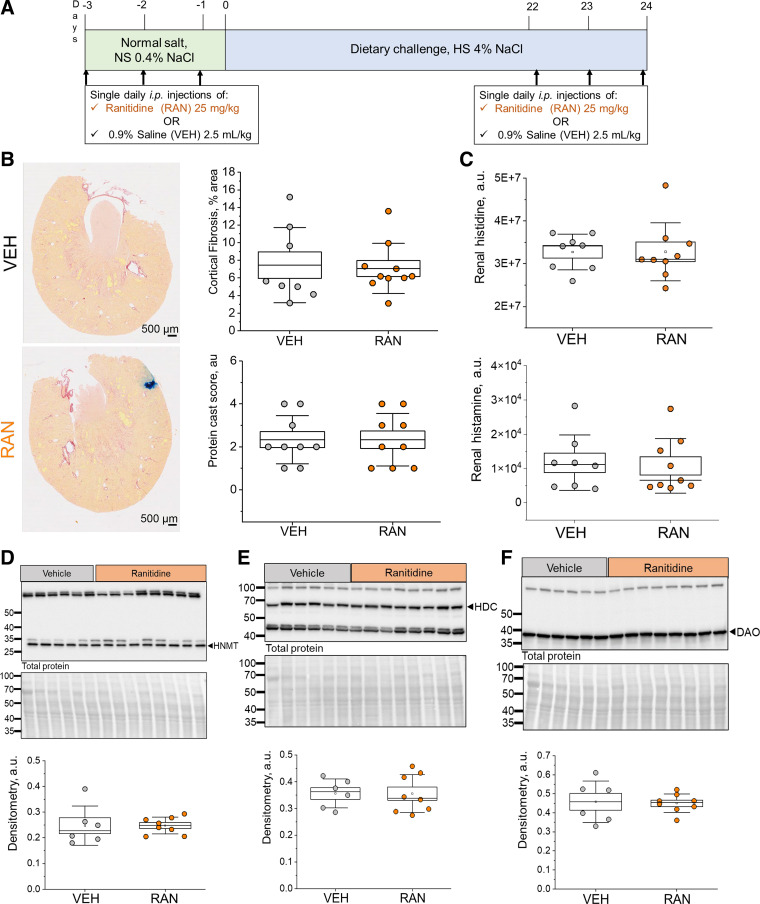
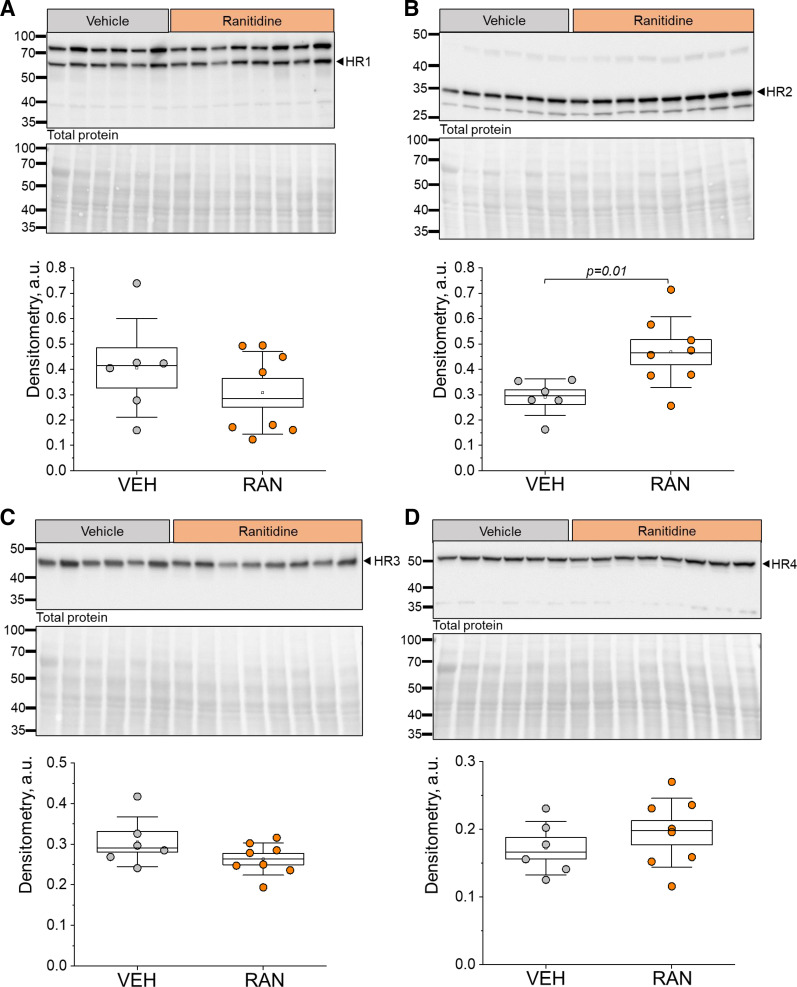
Previously, we have reported that HiS components are expressed in cultured mouse CD cells and that histamine can elicit changes in Ca2+ handling, cAMP levels, and Na+ reabsorption in these cells (22). Interestingly, HR2 exhibited the highest abundance specific to the apical membrane of these cells; we observed similar patterns in CDs of the DSS rat (Fig. 1). These findings lead us to hypothesize that HR2 is important for the regulation of renal Na+ and water reabsorption in this nephron segment. To address this hypothesis, we performed acute in vivo experiments using an HR2 blocker, ranitidine. A schematic of the experimental protocol is shown in Fig. 6A. Briefly, 8-wk-old DSS rats fed a NS diet were given a single intraperitoneal injection of 0.9% saline (vehicle; 2.5 mL/kg) or ranitidine (25 mg/kg) per day 3 days before they were switched to a HS diet (Fig. 6A). After 3 wk on HS, animals received three more daily injections of 0.9% saline or ranitidine. We report similar biometric parameters (total body weight, kidney-to-body weight ratios, heart-to-body weight ratios, plasma Na+, plasma K+, plasma Cl−, and GFR) between HS-fed vehicle- or ranitidine-treated groups at the end point (Table 1). Furthermore, histological analysis of renal tissue damage revealed that following ranitidine treatment, there were no differences in fibrosis for the cortex (7.5 ± 1.5% and 7.1 ± 0.9% for vehicle vs. ranitidine, P > 0.05) or medulla (9.6 ± 1.6% and 10.7 ± 1.3% for vehicle vs. ranitidine, P > 0.05; data not shown) of the kidneys (Fig. 6B). Protein cast analysis showed similar cast levels as well (scores of 2.3 ± 0.4 and 2.3 ± 0.4 for vehicle vs. ranitidine, P > 0.05; Fig. 6B). Figure 6C shows that ranitidine treatment did not affect histamine or histidine levels in the kidneys of the animals. Western blot analysis was used to determine the effects of acute ranitidine treatment on the abundance of HiS components in the cortex and medulla of DSS rats. HNMT, HDC, and DAO (Fig. 6, D–F), as well as HR1, HR3, and HR4 (Fig. 7, A, C, and D), exhibited similar levels in the renal cortex for vehicle- versus ranitidine-treated groups at the end of the HS diet challenge. We did, however, observe a significant increase in HR2 abundance within the renal cortex after ranitidine treatment (Fig. 7B). In the medulla, the abundance of all HiS components was similar between the treatments (see Supplemental Figs. S1 and S2). These findings suggest that acute treatment with ranitidine does not introduce an imbalance to the renal HiS; however, the compensatory increase in HR2 level following its blockade confirms the delivery of the drug to cortical renal tissue.
Figure 6.
Effects of an acute treatment with ranitidine (RAN; histamine receptor 2 blocker) on the histaminergic system in high-salt (HS) diet-fed Dahl salt-sensitive (DSS) rats. A: outline of the experimental protocol. Rats received a single daily intraperitoneal injection of either RAN (25 mg/kg) or saline [vehicle (VEH)] for 3 consecutive days on the normal salt (NS) diet and at the end of the HS challenge. B, left: representative images of picrosirius red-stained kidneys of HS diet-fed DSS rats treated with saline (top) or RAN (bottom). B, right: graphical analysis of the cortical fibrosis level (top) in relation to the percentage of total kidney slice area and protein cast score (bottom). C: levels of histidine (top) and histamine (bottom) obtained with mass spectrometry in kidney tissues isolated from VEH- and RAN-treated DSS rats; n = 8 per diet group. D–F, top: representative Western blots probed for histamine decarboxylase (HDC), histamine N-methyltransferase (HNMT), and diamine oxidase (DAO) abundance in the renal cortex of VEH- and RAN-treated rats fed a HS diet with the Ponceau-stained membrane (total protein) shown below the antibody-probed membrane. D–F, bottom: densitometric analysis of the Western blots; n = 6–8 for VEH treatment and 8–10 for RAN treatment. Boxes indicate means ± SE; whiskers are SDs. One-way ANOVA with a Holm-Sidak post hoc test was used for statistical analysis; a.u. arbitrary units.
Table 1.
Basic biometric parameters for vehicle- vs. ranitidine-treated groups of high-salt diet-fed Dahl salt-sensitive rats
| Vehicle |
Ranitidine |
|||
|---|---|---|---|---|
| Mean | SE | Mean | SE | |
| Total body weight, g | 355 | 7.3 | 364 | 8.1 |
| Two kidneys-to-body weight ratio, mg/g | 9.8 | 0.35 | 9.7 | 0.27 |
| Heart weight-to-body weight ratio, mg/g | 3.8 | 0.12 | 3.8 | 0.09 |
| Plasma Na+, mM | 145 | 0.51 | 146 | 0.36 |
| Plasma K+, mM | 3.2 | 0.08 | 3.4 | 0.08 |
| Plasma Cl−, mM | 106 | 0.59 | 107 | 0.57 |
| Glomerular filtration rate, mL/min/100 g) | 0.8 | 0.07 | 0.8 | 0.07 |
n = 7–8 for vehicle treatment and 10 for ranitidine treatment. The following parameters were assessed: total body weight, two kidneys-to-body weight ratio, heart weight-to-body weight ratio, plasma Na+, plasma K+, and plasma Cl−. As a measure of renal function for high-salt diet-fed Dahl salt-sensitive rats for vehicle vs. ranitidine treatment, glomerular filtration rate was assessed as the filtration rate of FITC-inulin.
Figure 7.
Renal histamine receptor (HR) abundance following acute treatment with ranitidine (RAN). A–D, top: representative Western blots probed for HR1 (A), HR2 (B), HR3 (C), and HR4 (D) in renal cortex samples of vehicle (VEH)- and RAN-treated Dahl salt-sensitive rats fed a high-salt diet with the Ponceau-stained membrane representing total protein shown below the antibody probed-membrane. A–D, bottom: densitometric analysis of the Western blots; n = 6 for VEH treatment and 8 for RAN treatment. Boxes indicate means ± SE; whiskers are SDs. One-way ANOVA with a Holm-Sidak post hoc test was used for statistical analysis; a.u. arbitrary units.
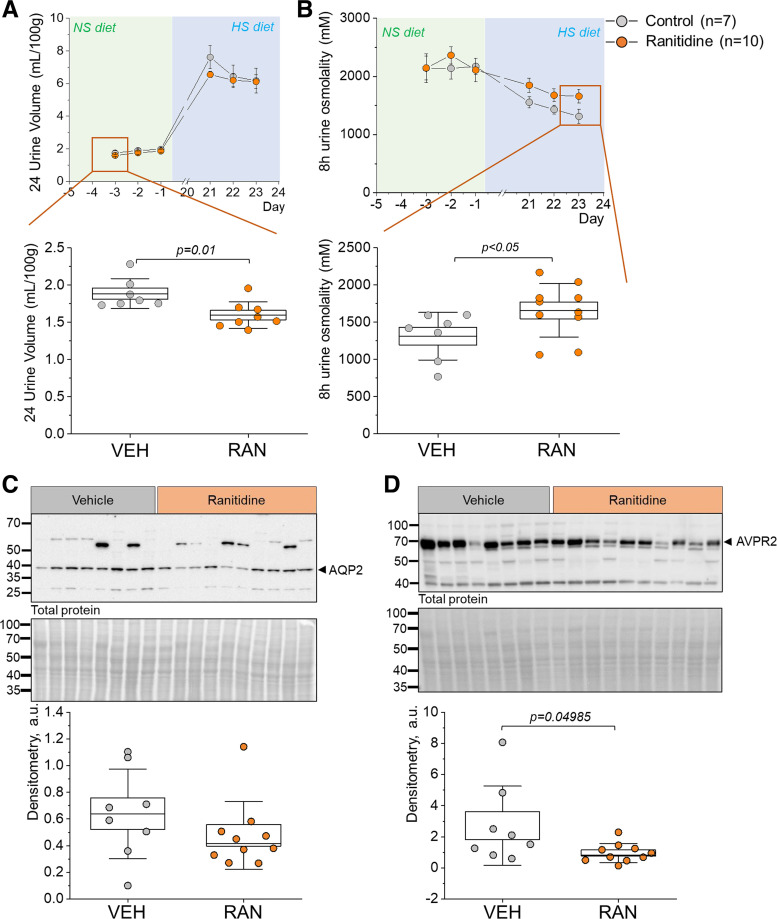
Ranitidine Alters Water Balance in the DSS Rat
To address the potential effects of HR2 inhibition on water-electrolyte balance, we measured 24-h water consumption, diuresis, urine osmolality, and electrolyte excretion for each day of the treatment window. On the NS diet, there were no considerable differences in 24-h urine Na+, K+, or 8-h osmol excretion as well as pH of the urine 8 h following treatment between the vehicle- and ranitidine-treated groups for either of the 3 days (Table 2). There was, however, a significant decrease in 24-h water consumption and an increase in 24-h urine Cl− excretion on the first day of ranitidine treatment on the NS diet (Table 2). NS diet-fed DSS rats also exhibited a significant decrease in urine volume on the first days of ranitidine treatment, with no significant differences between groups at the end of the HS diet challenge (Fig. 8A and Table 3). We determined an increase in 8-h urine osmolality following the HS challenge on the third day of ranitidine administration (Fig. 8B). The changes in diuresis and urine concentrating ability of ranitidine-treated animals as well as the reported interaction between HiS and vasopressin signaling in the brain (34) prompted further investigation. Vasopressin and AQP2 are integral players in water reabsorption and urine concentration in the CD of the kidney (35). Western blot analysis of the renal cortex for AQP2 and AVPR2 revealed that after ranitidine treatment there were no significant changes in AQP2 expression (Fig. 8C). However, we observed a drastic decrease in AVPR2 levels in the renal cortex of HS diet-fed DSS rats after ranitidine treatment (Fig. 8D).
Table 2.
Water consumption and urinary electrolyte and osmol excretion in normal-salt diet-fed (day −3 to day −1) Dahl salt-sensitive rats (vehicle- vs. ranitidine-treated groups)
| Vehicle |
Ranitidine |
|||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|
|
Day −3
|
Day −2
|
Day −1
|
Day −3
|
Day −2
|
Day −1
|
|||||||
| Mean | SE | Mean | SE | Mean | SE | Mean | SE | Mean | SE | Mean | SE | |
| 24-h water consumption, mL | 13.4* | 1.9 | 14 | 1 | 14.3 | 1 | 11.7* | 0.5 | 13.7 | 0.6 | 13.2 | 0.6 |
| 24-h Na+ excretion, µmol | 625 | 92 | 798 | 83.3 | 852 | 54.2 | 882 | 141.9 | 668 | 69.9 | 718 | 70.5 |
| 24-h Cl− excretion, µmol | 728* | 101.6 | 1,026 | 82.3 | 1,100 | 59.3 | 1,108* | 131.7 | 930 | 57.3 | 1,131 | 73.9 |
| 24-h K+ excretion, µmol | 1,498 | 549.1 | 1,375 | 350.4 | 1,515 | 352.4 | 1,634 | 409.3 | 1,231 | 221.8 | 1,294 | 86.1 |
| 8-h osmol excretion, mmol | 2.1 | 0.4 | 3.1 | 0.3 | 3 | 0.2 | 3.2 | 0.4 | 2.7 | 0.2 | 2.7 | 0.2 |
| 8-h urine pH, mmol | 5.3 | 0.1 | 5.3 | 0.1 | 5.4 | 0.04 | 5.4 | 0.04 | 5.8 | 0.4 | 5.9 | 0.4 |
n = 7 for vehicle treatment and 10 for ranitidine treatment. The following parameters were assessed: water consumption, urinary Na+ excretion, urinary Cl− excretion, and urinary K+ excretion for the 24-h period as well as 8-h osmol excretion and urine pH levels. *Statistical significance (P < 0.05) for vehicle vs. ranitidine treatment. Repeated-measures ANOVA with a Holm-Sidak post hoc test was used for statistical analysis.
Figure 8.
Changes in urine volume and osmolality in ranitidine (RAN)-treated Dahl salt-sensitive rats. A and B, top: line and symbol graphs highlighting the 3 days of RAN treatment on normal-salt (NS; day −3 to day −1) and high-salt (HS; day 21 to day 23) diets in Dahl salt-sensitive rats. Orange circles represent the RAN-treated group; light gray circles represent the vehicle (VEH)-treated group. A and B, bottom: box plots emphasize the first day of treatment while on the NS diet and the last day of treatment while on the HS diet, respectively. C and D, top: representative Western blots probed for aquaporin 2 (AQP2; C) and the type 2 arginine vasopressin receptor (AVPR2; D) in HS diet-fed animals (top) with the Ponceau-stained membrane representing total protein shown below the antibody-probed membrane. C and D, bottom: densitometric analysis of the Western blots; n = 7–8 for VEH treatment and 8–10 for RAN treatment. Boxes indicate means ± SE; whiskers are SDs. Repeated-measures ANOVA with a Holm-Sidak post hoc test was used for statistical analysis; a.u. arbitrary units.
Table 3.
Water consumption and urinary electrolyte and osmol excretion in high-salt diet-fed (days 21−23) Dahl salt-sensitve rats (vehicle- vs. ranitidine-treated groups)
| Vehicle |
Ranitidine |
|||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|
|
Day 21
|
Day 22
|
Day 23
|
Day 21
|
Day 22
|
Day 23
|
|||||||
| Mean | SE | Mean | SE | Mean | SE | Mean | SE | Mean | SE | Mean | SE | |
| 24-h water consumption, mL | 32.4 | 2 | 33.5 | 2.8 | 35.1 | 3.1 | 32.2 | 1.4 | 30.6 | 1.3 | 30.3 | 1.2 |
| 24-h Na+ excretion, µmol | 8,655 | 758.1 | 7,774 | 842.3 | 5,996 | 982.4 | 8,330 | 553.3 | 8,372 | 629 | 6,131 | 1,087.3 |
| 24-h Cl− excretion, µmol | 9,187 | 735.8 | 8,418 | 754.9 | 6,674 | 1,037.1 | 8,776 | 688 | 9,458 | 451.8 | 7,177 | 1,224.3 |
| 24-h K+ excretion, µmol | 1,919 | 130.7 | 1,557 | 142.8 | 1,196 | 159.6 | 1,744 | 138.6 | 1,757 | 63 | 1,336 | 200.1 |
| 8-h osmol excretion, mmol | 3.9 | 0.4 | 4.5 | 0.6 | 3.8 | 0.6 | 4.1 | 0.4 | 4 | 0.8 | 3.8 | 0.4 |
| 8-h urine pH, mmol | 6 | 0.3 | 6.9 | 0.5 | 6.4 | 0.5 | 6.1 | 0.4 | 6.6 | 0.4 | 6.5 | 0.5 |
n = 7 for vehicle treatment and 10 for ranitidine treatment. The following parameters were assessed: water consumption, urinary Na+ excretion, urinary Cl− excretion, and urinary K+ excretion for the 24-h period as well as 8-h osmol excretion and urine pH levels. Repeated-measures ANOVA with a Holm-Sidak post hoc test was used for statistical analysis.
DISCUSSION
Our data demonstrate that renal epithelial cells express essential components of the HiS, indicating a potential for local intrarenal metabolism of histamine as well as the capacity for these cells to serve as functional targets that would conduct the signaling elicited by this compound. This is in line with studies that have previously alluded to local production being the main source of histamine in the kidney (9). Of note, although we have shown all four HRs and three HiS enzymes (HDC, HNMT, and DAO) in renal epithelial cells, it is important to acknowledge the similarities and differences in expression patterns that we established between species as well as different published sources. We have shown that all four HRs are expressed in the cortex and medulla of the DSS rat kidney. Other investigators reported that in rat renal tissues, HR1 and HR2 are both abundant in the glomerular capsule, mesangial cells, the proximal tubule, and the distal tubule, whereas HR3 can be found in the apical membrane of principal cells of the CD, and HR4 was shown to reside in the apical membrane of the loop of Henle and proximal tubular cells (21, 36–38). In human kidneys, we detected differential expression of all components of the HiS along the nephron. The Kidney Interactive Transcriptomics (KIT) database (39) reports that HRH1 and HRH4 have an abundance of expression within the CD, podocytes, loop of Henle, and proximal tubule. The database also reports that HRH2 showed little expression in the podocytes, distal tubule, CD, loop of Henle, and proximal tubule; however, HRH3 expression was not found (21, 39). In summary, our data are in accordance with the previously accumulated knowledge and allow us to confidently establish the expression of HRs in renal cells.
To our knowledge, there are very limited reports available regarding the abundance or localization of HNMT, DAO, and HDC in kidney tissues, and we are presenting here the first comprehensive evidence of their renal expression. Harvima et al. (40) demonstrated that HNMT can be purified from rat kidneys with a very high yield. An immunofluorescence study detected the presence of HNMT in proximal tubules of rat kidneys (41). According to the KIT database (39), in human kidneys, HNMT is abundantly expressed in all segments of the proximal tubule, loop of Henle, podocytes, and mesangial cells. Our immunochemistry results demonstrated that HiS enzymes are abundant in both rat and human kidney tissue.
The confirmed existence of the machinery comprising the local renal HiS brings to light the question of potential renal histamine sources and especially the changes in histaminergic signaling that can be elicited by pathological conditions. We stipulated here that renal histamine production and its effects are involved in the development of salt-induced renal damage. The local pool of histamine in the kidney is greater than that of the circulation, and the population of histamine-producing resident mast cells is low (9). According to our data reported here, the renal epithelium possesses the HiS apparatus capable of histamine production. Thus we hypothesized that the renal epithelium, rather than mast cells, is the major source of histamine production. Thus we showed that both histamine and its precursor, histidine, exhibit higher levels in renal tissues of DSS rats fed a HS diet while plasma levels of these compounds are lower in these animals. A previous study has provided evidence that plasma histamine levels are increased in nephrotic syndrome, end‐stage renal failure, and renal insufficiency (21). The data regarding intrarenal histamine levels are limited; it is known, however, that circulating histamine concentration is significantly lower than in kidney tissue (19, 42, 43). Abboud et al. (44) revealed that in experimental nephrosis induced with puromycin, the content of histamine was remarkably higher in the cortex of nephrotic rats versus controls. Zimmermann et al. (19) noted that kidneys have medium concentrations of histamine compared with other organs, whereas the level of N-methylhistamine (histamine metabolite formed by HNMT) is substantial in the kidney, lungs, and intestine and negligible in other tissues. Our findings indicate that the histaminergic balance is shifted toward lower production of histamine and its more active degradation (as indicated by lower renal expression of HDC and higher expression of HNMT). Although nothing is known regarding HNMT profile changes in pathophysiology, there are some reports indicating that HDC levels fluctuate in kidney tissues in altered physiological states. For example, in pregnancy, HDC is upregulated in superficial cortical nephrons in mice and humans (potentially to increase renal blood flow) (20, 45). Based on our data, we can surmise that in DSS rats on HS, the renal HiS is significantly affected, as evidenced by changes in the enzyme expression profile and elevated intrarenal histamine levels. It remains to be elucidated, however, if the observed shift is caused by the production of histamine by epithelial cells, which possess the machinery to do so, the release from mast cells residing in the tissue, or both. We can therefore speculate that histamine can be produced by the renal epithelium; however, despite the similar (tryptase) or even decreased (FCER1A) levels of mast cell markers we revealed, it cannot be excluded that potential release from mast cells is contributing to an increase in renal histamine levels observed on HS. Our findings echo the study of Noguchi et al. (46), which showed that histamine alleviates cardiorenal damage. When the authors assessed mast cell infiltration in cardiac and renal tissues, the results were inconclusive and prompted discussion regarding a different cell type that could release histamine or its paracrine effects (47). We posit here that the increase in histamine level results from local intrarenal shifts in histamine production and degradation rather than mast cell release since we detected only moderate changes in mast cell markers between the NS- and HS diet-fed groups. Interestingly, despite reduced production and increased degradation of histamine in the cortex, histidine and histamine levels are higher on the HS diet than NS diet. We can speculate that the HS diet stimulates the uptake of histidine from the circulation (its plasma concentration may reach up to 100 µM) and its accumulation in renal tissues where existing HDC converts it to histamine. Increased histamine level through a negative feedback mechanism then decreases HDC and induces HNMT expression. The search for the histidine uptake pathways at play in the renal epithelial cells during HS load can provide new targets for antihypertensive therapy.
In our previous studies, we identified that all HRs and enzymes of the HiS are expressed in cultured mouse CD cells, where histamine elicits a functional dose-dependent signaling response (cAMP production and Ca2+ transients) and inhibits epithelial Na+ channel-mediated currents (22). Our published data demonstrated that HR2 is abundantly expressed in the apical membrane of CD cells, and we hypothesized that the observed responses are predominantly regulated via this receptor. Here, we aimed to test this in vivo in the DSS rat that was acutely administered a selective HR2 blocker, ranitidine, intraperitoneally for 3 consecutive days before and at the end of the HS challenge. Most importantly, our experiments showed that ranitidine acutely decreased water consumption and urine volume in these animals on the NS diet because, perhaps, it took a day for the animals to adapt to the taste of the drug in drinking water. Interestingly, ranitidine administration caused a marked increase in urine osmolality at the end of the chronic HS challenge and a concurrent decrease in AVPR2 expression in the kidney. We can speculate that although the concentrating ability of the distal nephron should have been decreased by AVPR2 downregulation, the influence of ranitidine on transport in the upstream nephron segments could lead to differential regulation of substances building up osmolality (such as urea). The effects of H2 blockers or histamine in general on urea transport are poorly presented in the literature and may become a point of interest for future studies. Histamine is known to cause vasopressin release by histaminergic neurons via activation of HR1 and HR2 (48–50). In goats and dogs, histamine infusion resulted in antidiuresis (51, 52). We presume that in our acute experiments, the effects of antidiuresis evoked by ranitidine are independent of the central nervous system. Although ranitidine does not easily cross the blood-brain barrier (53), there is some potential for central nervous system penetration, and therefore we cannot fully exclude that it might have affected histaminergic neurons in the brain and thus vasopressin and related cascades. Vasopressin is an established regulator of water transport via AQP2 in CD cells (54). In our hands, although AVPR2 abundance was significantly lower after ranitidine, interestingly, we did not observe changes in total AQP2 level, which points to potential compensatory mechanisms and involvement of other regulators of the water and electrolyte balance. It is possible that although total AQP2 levels did not change, ranitidine changed surface (apical) expression of AQP2. There is very limited knowledge available regarding the effects of HR inhibition/activation on renal water transport. Interestingly, HR4 knockout mice presented a lower renal basal expression of AQP2 (55). In salivary glands, histamine (via HR1) induced a cytosolic Ca2+ increase and aquaporin-5 translocation to the plasma membrane (56), while in the nasal epithelium histamine downregulated this water channel (57). More research is needed to establish the mechanistic interplay between histamine, vasopressin, and water transport in the kidney.
Taken together, our data help establish the existence of the local HiS in renal epithelial cells, suggesting an intrarenal epithelial source of histamine, and indicate a shift in the renal histaminergic tone during the development of salt-induced renal damage. Further research is required to discern the molecular pathways downstream of specific HRs as well as the consequences of chronic administration of HR-specific pharmacology, such as the antihistamine medications widely used to counteract allergic reactions, in renal pathophysiology. Additional attention in future studies should be devoted to the separation of the systemic, renal epithelial, and vascular effects of histamine. Given our finding and the recent data that demonstrated beneficial cardiac and renal effects of HR3 activation in a mouse model of cardiac dysfunction (46), more preclinical and clinical studies should be devoted to the investigation of the mechanisms mediated by the HiS in the kidney, potential for repurposing of the existing drugs, and effects of antihistamines in individuals with kidney disease.
SUPPLEMENTAL MATERIAL
Supplemental Figs. S1 and S2: https://doi.org/10.6084/m9.figshare.22383640.v1.
DATA AVAILABILITY
Data will be made available upon reasonable request.
GRANTS
This work was supported by National Institutes of Health Grants R01HL148114 (to D.V.I.), R01DK123266 (to T.S.P.), and R01HL161231 (to D.L.M.), American Heart Association Postdoctoral Fellowship 903584 (to D.R.S.), and Department of Physiology, Augusta University, startup funds (to D.V.I.).
DISCLOSURES
No conflicts of interest, financial or otherwise, are declared by the authors.
AUTHOR CONTRIBUTIONS
M.D., T.S.P., and D.V.I. and conceived and designed research; D.R.S., R.S.S., M.D., C.C., S.P., F.A., E.B., M.I.S., S.D.W., A.S.Z., T.A., S.N.A., C.J.C., S.R.C., D.L.M., T.S.P., and D.V.I. performed experiments; D.R.S. R.S.S., M.D., C.C., S.P., F.A., S.D.W., A.S.Z., T.A., S.N.A., C.J.C., S.R.C., T.S.P., and D.V.I. analyzed data; D.R.S. R.S.S., M.D., C.C., S.P., F.A., E.B., A.S.Z., T.A., D.L.M., T.S.P., and D.V.I. interpreted results of experiments; D.R.S., R.S.S., M.D., C.C., S.P., F.A., A.S.Z., T.A., and D.V.I. prepared figures; D.R.S., R.S.S., and D.V.I. and drafted manuscript; D.R.S., R.S.S., M.D., C.C., S.P., F.A., E.B., M.I.S., S.D.W., A.S.Z., T.A., S.N.A., C.J.C., S.R.C., D.L.M., T.S.P., and D.V.I. edited and revised manuscript; D.R.S., R.S.S., M.D., C.C., S.P., F.A., E.B., M.I.S., S.D.W., A.S.Z., T.A., S.N.A., C.J.C., S.R.C., D.L.M., T.S.P., and D.V.I. approved final version of manuscript.
ACKNOWLEDGMENTS
We acknowledge Dr. Andrey V. Ilatovskiy for helpful discussions of the initial idea for the project. Dylan Brower (South Carolina Governor’s School of Science and Mathematics) is recognized for the initial help with histological imaging. We thank the organ procurement organization that provided human renal tissue for this study (LifeLink Foundation). We also thank the Histology and Electron Microscopy Core Laboratory at Augusta University and the Histology Core at the Medical University of South Carolina for assistance with tissue processing and staining.
REFERENCES
- 1.World Health Organization. Hypertension. Key Facts. https://www.who.int/news-room/fact-sheets/detail/hypertension [12 Jan 2022].
- 2.Centers for Disease Control and Prevention. Chronic Kidney Disease in the United States, 2021. Atlanta, GA: U.S. Department of Health and Human Services, 2019. [Google Scholar]
- 3. Yang P, Zhao X, Zhou L, Jin Y, Zheng X, Ouyang Y, Chen M, Zeng L, Chen S, Chen X, Tian Z. Protective effect of oral histidine on hypertension in Dahl salt-sensitive rats induced by high-salt diet. Life Sci 270: 119134, 2021. doi: 10.1016/j.lfs.2021.119134. [DOI] [PubMed] [Google Scholar]
- 4. Balafa O, Kalaitzidis RG. Salt sensitivity and hypertension. J Hum Hypertens 35: 184–192, 2021. doi: 10.1038/s41371-020-00407-1. [DOI] [PubMed] [Google Scholar]
- 5. Tecklenborg J, Clayton D, Siebert S, Coley SM. The role of the immune system in kidney disease. Clin Exp Immunol 192: 142–150, 2018. doi: 10.1111/cei.13119. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Rapa SF, Di Iorio BR, Campiglia P, Heidland A, Marzocco S. Inflammation and oxidative stress in chronic kidney disease-potential therapeutic role of minerals, vitamins and plant-derived metabolites. Int J Mol Sci 21: 263, 2019. doi: 10.3390/ijms21010263. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Yamaguchi J, Tanaka T, Nangaku M. Recent advances in understanding of chronic kidney disease. F1000Res 4: 1212, 2015. doi: 10.12688/f1000research.6970.1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Rodriguez-Iturbe B, Johnson RJ. Role of inflammatory cells in the kidney in the induction and maintenance of hypertension. Nephrol Dial Transplant 21: 260–263, 2006. doi: 10.1093/ndt/gfi319. [DOI] [PubMed] [Google Scholar]
- 9. Sudarikova AV, Fomin MV, Yankelevich IA, Ilatovskaya DV. The implications of histamine metabolism and signaling in renal function. Physiol Rep 9: e14845, 2021. doi: 10.14814/phy2.14845. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Wade B, Abais-Battad JM, Mattson DL. Role of immune cells in salt-sensitive hypertension and renal injury. Curr Opin Nephrol Hypertens 25: 22–27, 2016. doi: 10.1097/MNH.0000000000000183. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Mattson DL. Immune mechanisms of salt-sensitive hypertension and renal end-organ damage. Nat Rev Nephrol 15: 290–300, 2019. doi: 10.1038/s41581-019-0121-z. [DOI] [PubMed] [Google Scholar]
- 12. Rudemiller N, Lund H, Jacob HJ, Geurts AM, Mattson DL, PhysGen Knockout Program. CD247 modulates blood pressure by altering T-lymphocyte infiltration in the kidney. Hypertension 63: 559–564, 2014. doi: 10.1161/HYPERTENSIONAHA.113.02191. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Mattson DL, Lund H, Guo C, Rudemiller N, Geurts AM, Jacob H. Genetic mutation of recombination activating gene 1 in Dahl salt-sensitive rats attenuates hypertension and renal damage. Am J Physiol Regul Integr Comp Physiol 304: R407–R414, 2013. doi: 10.1152/ajpregu.00304.2012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Branco A, Yoshikawa FS, Pietrobon AJ, Sato MN. Role of histamine in modulating the immune response and inflammation. Mediators Inflamm 2018: 9524075, 2018. doi: 10.1155/2018/9524075. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. White MV. The role of histamine in allergic diseases. J Allergy Clin Immunol 86: 599–605, 1990. doi: 10.1016/s0091-6749(05)80223-4. [DOI] [PubMed] [Google Scholar]
- 16. Kolivas S, Shulkes A. Regulation of expression of the receptors controlling gastric acidity. Regul Pept 121: 1–9, 2004. doi: 10.1016/j.regpep.2003.09.009. [DOI] [PubMed] [Google Scholar]
- 17. Kobayashi T, Tonai S, Ishihara Y, Koga R, Okabe S, Watanabe T. Abnormal functional and morphological regulation of the gastric mucosa in histamine H2 receptor-deficient mice. J Clin Invest 105: 1741–1749, 2000. doi: 10.1172/JCI9441. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. Smolinska S, Jutel M, Crameri R, O'Mahony L. Histamine and gut mucosal immune regulation. Allergy 69: 273–281, 2014. doi: 10.1111/all.12330. [DOI] [PubMed] [Google Scholar]
- 19. Zimmermann AS, Burhenne H, Kaever V, Seifert R, Neumann D. Systematic analysis of histamine and N-methylhistamine concentrations in organs from two common laboratory mouse strains: C57Bl/6 and Balb/c. Inflamm Res 60: 1153–1159, 2011. doi: 10.1007/s00011-011-0379-5. [DOI] [PubMed] [Google Scholar]
- 20. Morgan TK, Montgomery K, Mason V, West RB, Wang L, van de Rijn M, Higgins JP. Upregulation of histidine decarboxylase expression in superficial cortical nephrons during pregnancy in mice and women. Kidney Int 70: 306–314, 2006. doi: 10.1038/sj.ki.5001553. [DOI] [PubMed] [Google Scholar]
- 21. Grange C, Gurrieri M, Verta R, Fantozzi R, Pini A, Rosa AC. Histamine in the kidneys: what is its role in renal pathophysiology? Br J Pharmacol 177: 503–515, 2020. doi: 10.1111/bph.14619. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22. Sudarikova AV, Fomin MV, Sultanova RF, Zhao Y, Perez S, Domondon M, Shamatova M, Lysikova DV, Spires DR, Ilatovskaya DV. Functional role of histamine receptors in the renal cortical collecting duct cells. Am J Physiol Cell Physiol 322: C775–C786, 2022. doi: 10.1152/ajpcell.00420.2021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23. Banks RO, Inscho EW, Jacobson ED. Histamine H1 receptor antagonists inhibit autoregulation of renal blood flow in the dog. Circ Res 54: 527–535, 1984. doi: 10.1161/01.res.54.5.527. [DOI] [PubMed] [Google Scholar]
- 24. Montesino H, Villar M, Vega E, Rudolph MI. Histamine, a neuromodulator of noradrenergic transmission in uterine horns from mice in diestrus. Biochem Pharmacol 50: 407–411, 1995. doi: 10.1016/0006-2952(95)00140-u. [DOI] [PubMed] [Google Scholar]
- 25. Tanida M, Kaneko H, Shen J, Nagai K. Involvement of the histaminergic system in renal sympathetic and cardiovascular responses to leptin and ghrelin. Neurosci Lett 413: 88–92, 2007. doi: 10.1016/j.neulet.2006.11.035. [DOI] [PubMed] [Google Scholar]
- 26. Pini A, Grange C, Veglia E, Argenziano M, Cavalli R, Guasti D, Calosi L, Ghè C, Solarino R, Thurmond RL, Camussi G, Chazot PL, Rosa AC. Histamine H4 receptor antagonism prevents the progression of diabetic nephropathy in male DBA2/J mice. Pharmacol Res 128: 18–28, 2018. doi: 10.1016/j.phrs.2018.01.002. [DOI] [PubMed] [Google Scholar]
- 27. Veglia E, Pini A, Moggio A, Grange C, Premoselli F, Miglio G, Tiligada K, Fantozzi R, Chazot PL, Rosa AC. Histamine type 1-receptor activation by low dose of histamine undermines human glomerular slit diaphragm integrity. Pharmacol Res 114: 27–38, 2016. doi: 10.1016/j.phrs.2016.10.011. [DOI] [PubMed] [Google Scholar]
- 28. Kjaer A, Knigge U, Jørgensen H, Warberg J. Dehydration-induced vasopressin secretion in humans: involvement of the histaminergic system. Am J Physiol Endocrinol Metab 279: E1305–E1310, 2000. doi: 10.1152/ajpendo.2000.279.6.E1305. [DOI] [PubMed] [Google Scholar]
- 29. Domondon M, Polina I, Nikiforova AB, Sultanova RF, Kruger C, Vasileva VY, Fomin MV, Beeson GC, Nieminen AL, Smythe N, Maldonado EN, Stadler K, Ilatovskaya DV. Renal glomerular mitochondria function in salt-sensitive hypertension. Front Physiol 10: 1588, 2019. doi: 10.3389/fphys.2019.01588. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30. Ilatovskaya DV, Levchenko V, Winsor K, Blass GR, Spires DR, Sarsenova E, Polina I, Zietara A, Paterson M, Kriegel AJ, Staruschenko A. Effects of elevation of ANP and its deficiency on cardiorenal function. JCI Insight 7: e148682, 2022. doi: 10.1172/jci.insight.148682. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31. Polina I, Spicer MJ, Domondon M, Schibalski RS, Sarsenova E, Sultanova RF, Ilatovskaya DV. Inhibition of neprilysin with sacubitril without RAS blockage aggravates renal disease in Dahl SS rats. Ren Fail 43: 315–324, 2021. doi: 10.1080/0886022X.2021.1879856. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32. Rabinowitz JD, Kimball E. Acidic acetonitrile for cellular metabolome extraction from Escherichia coli. Anal Chem 79: 6167–6173, 2007. doi: 10.1021/ac070470c. [DOI] [PubMed] [Google Scholar]
- 33. Payne V, Kam PC. Mast cell tryptase: a review of its physiology and clinical significance. Anaesthesia 59: 695–703, 2004. doi: 10.1111/j.1365-2044.2004.03757.x. [DOI] [PubMed] [Google Scholar]
- 34. Yagi K. Effects of a histamine H2-receptor antagonist, ranitidine on the vasopressin and oxytocin responses to novelty stress in the rat. Neurosci Res 19: 357–364, 1994. doi: 10.1016/0168-0102(94)90076-0. [DOI] [PubMed] [Google Scholar]
- 35. Jung HJ, Kwon TH. Molecular mechanisms regulating aquaporin-2 in kidney collecting duct. Am J Physiol Renal Physiol 311: F1318–F1328, 2016. doi: 10.1152/ajprenal.00485.2016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36. Rosa AC, Grange C, Pini A, Katebe MA, Benetti E, Collino M, Miglio G, Bani D, Camussi G, Chazot PL, Fantozzi R. Overexpression of histamine H4 receptors in the kidney of diabetic rat. Inflamm Res 62: 357–365, 2013. doi: 10.1007/s00011-012-0587-7. [DOI] [PubMed] [Google Scholar]
- 37. Veglia E, Grange C, Pini A, Moggio A, Lanzi C, Camussi G, Chazot PL, Rosa AC. Histamine receptor expression in human renal tubules: a comparative pharmacological evaluation. Inflamm Res 64: 261–270, 2015. doi: 10.1007/s00011-015-0807-z. [DOI] [PubMed] [Google Scholar]
- 38. Pini A, Chazot PL, Veglia E, Moggio A, Rosa AC. H3 receptor renal expression in normal and diabetic rats. Inflamm Res 64: 271–273, 2015. doi: 10.1007/s00011-015-0808-y. [DOI] [PubMed] [Google Scholar]
- 39. Wu H, Uchimura K, Donnelly EL, Kirita Y, Morris SA, Humphreys BD. Comparative analysis and refinement of human PSC-derived kidney organoid differentiation with single-cell transcriptomics. Cell Stem Cell 23: 869–881.e868, 2018. doi: 10.1016/j.stem.2018.10.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40. Harvima RJ, Kajander EO, Harvima IT, Fraki JE. Purification and partial characterization of rat kidney histamine-N-methyltransferase. Biochim Biophys Acta 841: 42–49, 1985. doi: 10.1016/0304-4165(85)90272-7. [DOI] [PubMed] [Google Scholar]
- 41. Fukuda H, Kiyama H, Maeyama K, Kubota H, Yamatodani A, Watanabe T, Tohyama M, Wada H. Immunohistochemical demonstration of histamine N-methyltransferase-like structures in rat kidney. Cell Tissue Res 243: 681–684, 1986. doi: 10.1007/BF00218079. [DOI] [PubMed] [Google Scholar]
- 42. Burtin C, Scheinmann P, Paupe J, Canu P, Goy J. Tissue histamine levels in male and female normal and nude mice. Agents Actions 12: 199–200, 1982. doi: 10.1007/BF01965144. [DOI] [PubMed] [Google Scholar]
- 43. Sedor JR, Abboud HE. Histamine modulates contraction and cyclic nucleotides in cultured rat mesangial cells. Differential effects mediated by histamine H1 and H2 receptors. J Clin Invest 75: 1679–1689, 1985. doi: 10.1172/JCI111876. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44. Abboud HE, Ou SL, Velosa JA, Shah SV, Dousa TP. Dynamics of renal histamine in normal rat kidney and in nephrosis induced by aminonucleoside of puromycin. J Clin Invest 69: 327–336, 1982. doi: 10.1172/jci110456. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45. Rosengren E, Steinhardt C. Elevated histidine decarboxylase activity in the kidney of the pregnantmouse. Experientia 17: 544, 1961. doi: 10.1007/BF02156410. [DOI] [PubMed] [Google Scholar]
- 46. Noguchi K, Ishida J, Kim JD, Muromachi N, Kako K, Mizukami H, Lu W, Ishimaru T, Kawasaki S, Kaneko S, Usui J, Ohtsu H, Yamagata K, Fukamizu A. Histamine receptor agonist alleviates severe cardiorenal damages by eliciting anti-inflammatory programming. Proc Natl Acad Sci USA 117: 3150–3156, 2020. doi: 10.1073/pnas.1909124117. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47. Tharaux PL. Histamine provides an original vista on cardiorenal syndrome. Proc Natl Acad Sci USA 117: 5550–5552, 2020. doi: 10.1073/pnas.2001336117. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48. Tuomisto L, Eriksson L, Fyhrquist F. Vasopressin release by histamine in the conscious goat. Eur J Pharmacol 63: 15–24, 1980. doi: 10.1016/0014-2999(80)90112-0. [DOI] [PubMed] [Google Scholar]
- 49. Tuomisto L, Eriksson L, Fyhrquist F. Plasma vasopressin levels after I.C.V. infusion of histamine agonists in the conscious goat. Agents Actions 14: 558–560, 1984. doi: 10.1007/BF01973871. [DOI] [PubMed] [Google Scholar]
- 50. Kjaer A, Knigge U, Rouleau A, Garbarg M, Warberg J. Dehydration-induced release of vasopressin involves activation of hypothalamic histaminergic neurons. Endocrinology 135: 675–681, 1994. doi: 10.1210/endo.135.2.8033816. [DOI] [PubMed] [Google Scholar]
- 51. Tuomisto L, Eriksson L. Antidiuresis induced by infusions of histamine into the brain ventricles of conscious hydrated goats. Eur J Pharmacol 54: 191–201, 1979. doi: 10.1016/0014-2999(79)90077-3. [DOI] [PubMed] [Google Scholar]
- 52. Bhargava KP, Kulshrestha VK, Santhakumari G, Srivastava YP. Mechanism of histamine-induced antidiuretic response. Br J Pharmacol 47: 700–706, 1973. doi: 10.1111/j.1476-5381.1973.tb08196.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53. Vial T, Goubier C, Bergeret A, Cabrera F, Evreux JC, Descotes J. Side effects of ranitidine. Drug Saf 6: 94–117, 1991. doi: 10.2165/00002018-199106020-00002. [DOI] [PubMed] [Google Scholar]
- 54. Chen L, Jung HJ, Datta A, Park E, Poll BG, Kikuchi H, Leo KT, Mehta Y, Lewis S, Khundmiri SJ, Khan S, Chou CL, Raghuram V, Yang CR, Knepper MA. Systems biology of the vasopressin V2 receptor: new tools for discovery of molecular actions of a GPCR. Annu Rev Pharmacol Toxicol 62: 595–616, 2022. doi: 10.1146/annurev-pharmtox-052120-011012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55. Verta R, Gurrieri M, Borga S, Benetti E, Pollicino P, Cavalli R, Thurmond RL, Chazot PL, Pini A, Rosa AC, Grange C. The interplay between histamine H4 receptor and the kidney function: the lesson from H4 receptor knockout mice. Biomolecules 11: 1517, 2021. doi: 10.3390/biom11101517. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56. Kim JH, Park SH, Moon YW, Hwang S, Kim D, Jo SH, Oh SB, Kim JS, Jahng JW, Lee JH, Lee SJ, Choi SY, Park K. Histamine H1 receptor induces cytosolic calcium increase and aquaporin translocation in human salivary gland cells. J Pharmacol Exp Ther 330: 403–412, 2009. doi: 10.1124/jpet.109.153023. [DOI] [PubMed] [Google Scholar]
- 57. Wang W, Wang X, Ma L, Zhang R. Histamine downregulates aquaporin 5 in human nasal epithelial cells. Am J Rhinol Allergy 29: 188–192, 2015. doi: 10.2500/ajra.2015.29.4181. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Supplemental Figs. S1 and S2: https://doi.org/10.6084/m9.figshare.22383640.v1.
Data Availability Statement
Data will be made available upon reasonable request.