Abstract
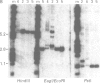
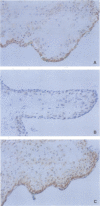
The fragile X syndrome, an X linked mental retardation syndrome, is caused by an expanded CGG repeat in the first exon of the FMR1 gene. In patients with an expanded repeat the FMR1 promoter is methylated and, consequently, the gene is silenced and no FMR1 protein (FMRP) is produced, thus leading to the clinical phenotype. Here we describe a prenatal diagnosis performed in a female from a fragile X family carrying a large premutation. In chorionic villus DNA of the male fetus the normal maternal CGG allele and a normal pattern on Southern blot analysis were found in combination with the FRAXAC2 and DXS297 allele of the maternal at risk haplotype. A second chorionic villus sampling was performed giving identical results on DNA analysis and, in addition, expression of FMRP was shown by immunohistochemistry. We concluded that the male fetus was not affected with the fragile X syndrome. Subsequent detailed haplotype analysis showed a complex recombination pattern resembling either gene conversion or a double crossover within a 20 kb genomic region.
Full text
PDF


Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Antiñolo G., Borrego S., Cabeza J. C., Sánchez R., Sánchez J., Sánchez B. Reverse mutation in fragile X syndrome. Am J Hum Genet. 1996 Jan;58(1):237–239. [PMC free article] [PubMed] [Google Scholar]
- Brown W. T., Houck G. E., Jr, Ding X., Zhong N., Nolin S., Glicksman A., Dobkin C., Jenkins E. C. Reverse mutations in the fragile X syndrome. Am J Med Genet. 1996 Aug 9;64(2):287–292. doi: 10.1002/(SICI)1096-8628(19960809)64:2<287::AID-AJMG11>3.0.CO;2-B. [DOI] [PubMed] [Google Scholar]
- Fu Y. H., Kuhl D. P., Pizzuti A., Pieretti M., Sutcliffe J. S., Richards S., Verkerk A. J., Holden J. J., Fenwick R. G., Jr, Warren S. T. Variation of the CGG repeat at the fragile X site results in genetic instability: resolution of the Sherman paradox. Cell. 1991 Dec 20;67(6):1047–1058. doi: 10.1016/0092-8674(91)90283-5. [DOI] [PubMed] [Google Scholar]
- Kremer E. J., Pritchard M., Lynch M., Yu S., Holman K., Baker E., Warren S. T., Schlessinger D., Sutherland G. R., Richards R. I. Mapping of DNA instability at the fragile X to a trinucleotide repeat sequence p(CCG)n. Science. 1991 Jun 21;252(5013):1711–1714. doi: 10.1126/science.1675488. [DOI] [PubMed] [Google Scholar]
- O'Hoy K. L., Tsilfidis C., Mahadevan M. S., Neville C. E., Barceló J., Hunter A. G., Korneluk R. G. Reduction in size of the myotonic dystrophy trinucleotide repeat mutation during transmission. Science. 1993 Feb 5;259(5096):809–812. doi: 10.1126/science.8094260. [DOI] [PubMed] [Google Scholar]
- Oostra B. A., Jacky P. B., Brown W. T., Rousseau F. Guidelines for the diagnosis of fragile X syndrome. National Fragile X Foundation. J Med Genet. 1993 May;30(5):410–413. doi: 10.1136/jmg.30.5.410. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Smeets H. J., Smits A. P., Verheij C. E., Theelen J. P., Willemsen R., van de Burgt I., Hoogeveen A. T., Oosterwijk J. C., Oostra B. A. Normal phenotype in two brothers with a full FMR1 mutation. Hum Mol Genet. 1995 Nov;4(11):2103–2108. doi: 10.1093/hmg/4.11.2103. [DOI] [PubMed] [Google Scholar]
- Turner G. Finding genes on the X chromosome by which homo may have become sapiens. Am J Hum Genet. 1996 Jun;58(6):1109–1110. [PMC free article] [PubMed] [Google Scholar]
- Verkerk A. J., Pieretti M., Sutcliffe J. S., Fu Y. H., Kuhl D. P., Pizzuti A., Reiner O., Richards S., Victoria M. F., Zhang F. P. Identification of a gene (FMR-1) containing a CGG repeat coincident with a breakpoint cluster region exhibiting length variation in fragile X syndrome. Cell. 1991 May 31;65(5):905–914. doi: 10.1016/0092-8674(91)90397-h. [DOI] [PubMed] [Google Scholar]
- Vits L., De Boulle K., Reyniers E., Handig I., Darby J. K., Oostra B., Willems P. J. Apparent regression of the CGG repeat in FMR1 to an allele of normal size. Hum Genet. 1994 Nov;94(5):523–526. doi: 10.1007/BF00211019. [DOI] [PubMed] [Google Scholar]
- Väisänen M. L., Haataja R., Leisti J. Decrease in the CGGn trinucleotide repeat mutation of the fragile X syndrome to normal size range during paternal transmission. Am J Hum Genet. 1996 Sep;59(3):540–546. [PMC free article] [PubMed] [Google Scholar]
- Willemsen R., Oosterwijk J. C., Los F. J., Galjaard H., Oostra B. A. Prenatal diagnosis of fragile X syndrome. Lancet. 1996 Oct 5;348(9032):967–968. doi: 10.1016/s0140-6736(05)65388-3. [DOI] [PubMed] [Google Scholar]
- Zhong N., Dobkin C., Brown W. T. A complex mutable polymorphism located within the fragile X gene. Nat Genet. 1993 Nov;5(3):248–253. doi: 10.1038/ng1193-248. [DOI] [PubMed] [Google Scholar]
- van den Ouweland A. M., Deelen W. H., Kunst C. B., Uzielli M. L., Nelson D. L., Warren S. T., Oostra B. A., Halley D. J. Loss of mutation at the FMR1 locus through multiple exchanges between maternal X chromosomes. Hum Mol Genet. 1994 Oct;3(10):1823–1827. doi: 10.1093/hmg/3.10.1823. [DOI] [PubMed] [Google Scholar]