Abstract
Growth faltering in children (low length for age or low weight for length) during the first 1,000 days of life (from conception to 2 years of age) influences short-term and long-term health and survival1,2. Interventions such as nutritional supplementation during pregnancy and the postnatal period could help prevent growth faltering, but programmatic action has been insufficient to eliminate the high burden of stunting and wasting in low- and middle-income countries. Identification of age windows and population subgroups on which to focus will benefit future preventive efforts. Here we use a population intervention effects analysis of 33 longitudinal cohorts (83,671 children, 662,763 measurements) and 30 separate exposures to show that improving maternal anthropometry and child condition at birth accounted for population increases in length-for-age z-scores of up to 0.40 and weight-for-length z-scores of up to 0.15 by 24 months of age. Boys had consistently higher risk of all forms of growth faltering than girls. Early postnatal growth faltering predisposed children to subsequent and persistent growth faltering. Children with multiple growth deficits exhibited higher mortality rates from birth to 2 years of age than children without growth deficits (hazard ratios 1.9 to 8.7). The importance of prenatal causes and severe consequences for children who experienced early growth faltering support a focus on pre-conception and pregnancy as a key opportunity for new preventive interventions.
Subject terms: Malnutrition, Epidemiology, Risk factors, Developing world
Analysis of data from 33 longitudinal cohorts from low- and middle-income countries indicates that conditions during pre-conception, pregnancy and the first few months of life are crucial in determining the risk of growth faltering in young children.
Main
Growth faltering in children in the form of stunting, a marker of chronic malnutrition, and wasting, a marker of acute malnutrition, is common among young children in low-resource settings, and may contribute to child mortality and adult morbidity1,2. Worldwide, 22% of children under 5 years of age exhibit stunting and 7% exhibit wasting, with most of the burden occurring in low- and middle-income counties3 (LMICs). Current estimates attribute more than 250,000 deaths annually to stunting and more than 1 million deaths annually to wasting2. People who exhibit stunting or wasting in childhood also experience worse cognitive development4–6 and worse economic outcomes as adults7.
Despite widespread recognition of the importance of growth faltering to global public health, preventive interventions in LMICs have had limited success8. A range of nutritional interventions targeting various life stages during the fetal and childhood periods, including nutrition education, food and micronutrient supplementation during pregnancy, promotion of exclusive breastfeeding for 6 months and continued breastfeeding for 2 years, and food and micronutrient supplementation during complementary feeding, have shown beneficial effects on child growth9–11. However, postnatal breastfeeding interventions and nutritional interventions delivered to children who have begun complementary feeding have had only small effects on population-level stunting and wasting burdens, and implementation remains a substantial challenge9,12,13. Additionally, water, sanitation and hygiene interventions, which aim to reduce childhood infections that may increase the risk of wasting and stunting, have had no effect on child growth in several large randomized control trials14–16.
Modest effects of interventions to prevent stunting and wasting may reflect an incomplete understanding of the optimal manner and timing of interventions17. In recent decades, this knowledge gap has spurred renewed interest in combining rich data sources with advances in statistical methodology18 to more deeply understand the key causes of growth faltering19. Understanding the relationship between the causes and timing of growth faltering is also crucial because children who falter early could be at higher risk of more severe growth faltering subsequently. In the accompanying Articles, we present data showing that the highest rates of incident stunting and wasting occur by 3 months of age20,21.
Pooled longitudinal analyses
Here we report a pooled analysis of 33 longitudinal cohorts in 15 LMICs in south Asia, sub-Saharan Africa, Latin America and eastern Europe, in which data collection was initiated between 1987 and 2014. Our objective was to estimate relationships between child, parental and household characteristics and measures of child anthropometry, including length-for-age z-score (LAZ), weight-for-length z-score (WLZ), weight-for-age z-score (WAZ), stunting, wasting, underweight and length and weight velocities from birth to 24 months of age. The estimation of growth faltering outcomes is detailed in the accompanying Articles20,21. We also estimated associations between early growth faltering and more severe growth faltering or mortality by 24 months of age.
Cohorts were assembled as part of the Bill & Melinda Gates Foundation’s Knowledge Integration (ki) initiative, which included studies of growth and development during the first 1,000 days of life, beginning at conception. We selected longitudinal cohorts from the database that met 5 inclusion criteria: (1) they were conducted in LMICs; (2) they enroled children between birth and 24 months of age and measured their length and weight repeatedly over time; (3) they did not restrict enrolment to acutely ill children; (4) they enroled children with a median birth year after 1990; and (5) they collected anthropometric status measurements at least quarterly. These inclusion criteria ensured that we could rigorously evaluate the timing and onset of growth faltering among children who were broadly representative of populations in LMICs. Thirty-three cohorts from 15 countries met the inclusion criteria, and 83,671 children and 592,030 total measurements were included in the analysis (Fig. 1). Child mortality was rare and was not reported in many of the ki datasets, so we relaxed inclusion criteria for studies used in the mortality analysis to include studies that measured children at least twice a year. Four additional cohorts met these inclusion criterion, and 14,317 children and 70,733 additional measurements were included in mortality analyses (97,988 total children, 662,763 total observations; Extended Data Table 1). The cohorts were distributed throughout south Asia, Africa and Latin America, with a single European cohort from Belarus.
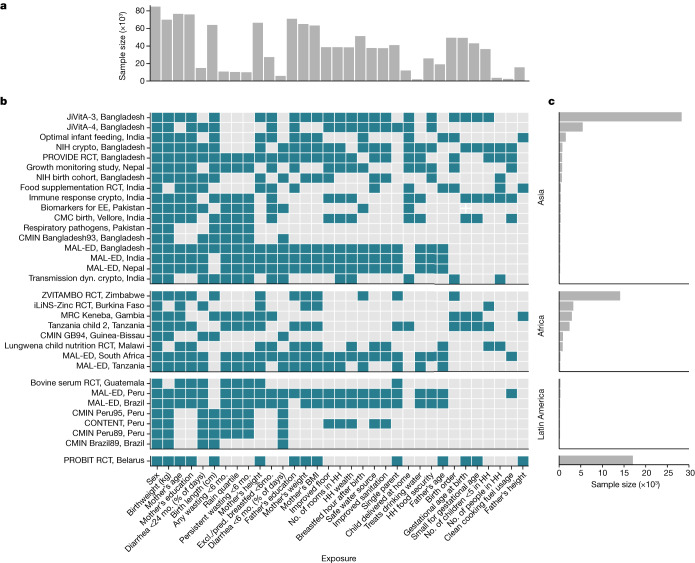
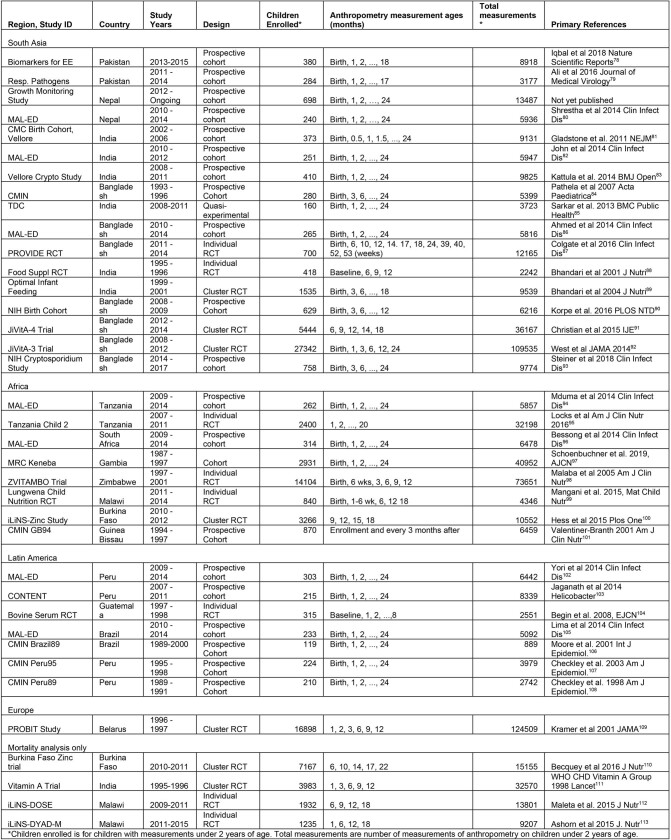
Fig. 1. Cohort sample sizes and measured exposures.
a, The total number of children with each measured exposure, sorted from left to right by the number of cohorts measuring the exposure. b, The presence of 30 exposure variables in the ki data by within each included cohort. Cohorts are sorted by geographic region and sample size. Details of the cohorts are provided in Extended Data Table 1. CMC, Christian Medical College; Crypto, Cryptosporidium; dyn., dynamics; EE, Environmental Enteropathy; Excl., exclusively; HH, household; NIH, National Institute of Health; mo., months; pred., predominantly; RCT, randomized controlled trial. c, The number of child anthropometry observations contributed by each cohort.
Extended Data Table 1.
Summary of ki cohorts
Population intervention effects
In a series of analyses, we estimated population intervention effects (PIEs) on growth faltering, the estimated change in population mean z-score if all individuals in the population had their exposure shifted from observed levels to the lowest-risk reference level22. The PIE is a policy-relevant parameter; it estimates the improvement in outcome that could be achievable through intervention for modifiable exposures, as it is a function of the degree of difference between the unexposed and the exposed in children’s anthropometry z-scores, as well as the observed distribution of exposure within the population. We selected exposures that were measured in multiple cohorts, could be harmonized across cohorts for pooled analyses, and had been identified as important predictors of stunting or wasting in prior literature (Fig. 1 and Extended Data Tables 2 and 3). Exposure measurement varied by cohort, but all estimates were adjusted for all other measured exposures that we assumed were not on the causal pathway between the exposure of interest and the outcome. For example, the association between maternal height and stunting was not adjusted for child birth weight, because low maternal height could increase stunting risk through lower child birth weight5. Parameters were estimated using targeted maximum-likelihood estimation, a doubly robust, semi-parametric method that enables valid inference while adjusting for potential confounders using ensemble machine learning18,23 (Methods). We estimated cohort-specific parameters, adjusting for measured covariates within each cohort, and then pooled estimates across cohorts using random-effects models24 (Extended Data Fig. 1). As the reference exposure for PIEs, we used the lowest risk level across cohorts. We also estimated the effects of optimal dynamic interventions, where each child’s individual low-risk level of exposure was estimated from potential confounders (Methods). The timing of exposures varied from parental and household characteristics present before birth, to fetal, at-birth or postnatal exposures. We estimated associations with growth faltering that occurred after exposure measurements to ensure temporal ordering of exposures and outcomes.
Extended Data Table 2.
Exposure variable summaries and prior published evidence – part 1
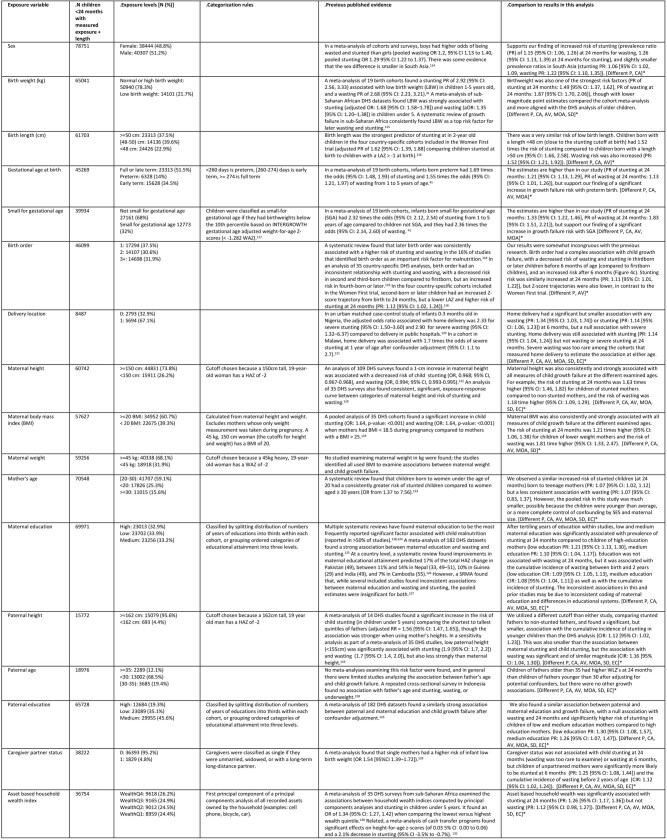
All exposures included in the analysis, as well as the categories the exposures were classified into across all cohorts, categorization rules, the total number of children, the percentage of children in each category, select evidence from prior literature, and comparisons to our results. We selected the exposures of interest based on variables present in multiple cohorts that met our inclusion criteria, were found to be important determinants of stunting and wasting in prior literature, and could be harmonized across cohorts for pooled analyses. Where possible, we cite findings from recent randomized controlled trials and systematic reviews. All results from this manuscript referenced in this table are available in Supplimentary Note 7. *Bracketed codes at the end of each cell in the “Comparison to results in this analysis” indicate limitations to comparisons with previous evidence due to differences in: P = population, CA = child age, AV = adjustment variables used in the analysis, MOA = measure of association, SD = study design, EC = exposure classification. Data are from refs. 40,114–131.
Extended Data Table 3.
Exposure variable summaries and prior published evidence – part 2
All exposures included in the analysis, as well as the categories the exposures were classified into across all cohorts, categorization rules, the total number of children, the percentage of children in each category, select evidence from prior literature, and comparisons to our results. We selected the exposures of interest based on variables present in multiple cohorts that met our inclusion criteria, were found to be important determinants of stunting and wasting in prior literature, and could be harmonized across cohorts for pooled analyses. Where possible, we cite findings from recent randomized controlled trials and systematic reviews. All results from this manuscript referenced in this table are available in Supplimentary Note 7. *Bracketed codes at the end of each cell in the “Comparison to results in this analysis” indicate limitations to comparisons with previous evidence due to differences in: P = population, CA = child age, AV = adjustment variables used in the analysis, MOA = measure of association, SD = study design, EC = exposure classification. Data are from refs. 11,14,15,101,132–150.
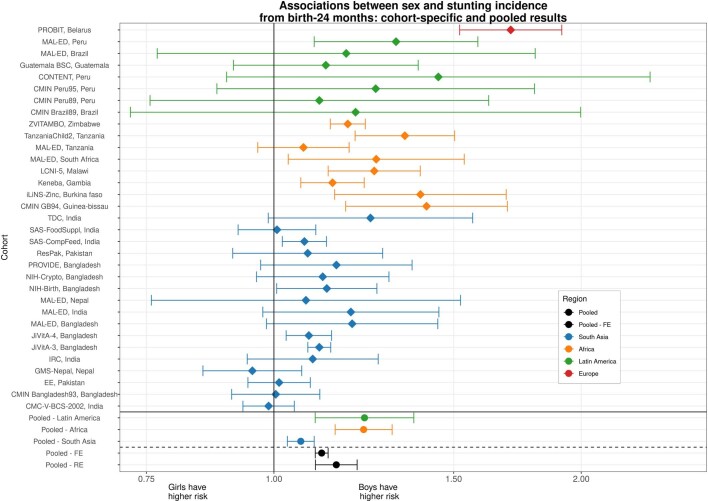
Extended Data Fig. 1. Example forest plot of cohort-specific and pooled parameter estimates.
Cohort-specific estimates of the cumulative incidence ratio of stunting are plotted on each row, comparing the risk of any stunting from birth to 24 months among boys compared to a reference level of girls. Below the solid horizontal line are region-specific pooled measures of association, pooled using random-effects models. Below the dashed line are overall pooled measures of association, comparing pooling using random or fixed effects models. The primary results reported throughout the manuscript are overall (not region stratified) estimates pooled using random effects models.
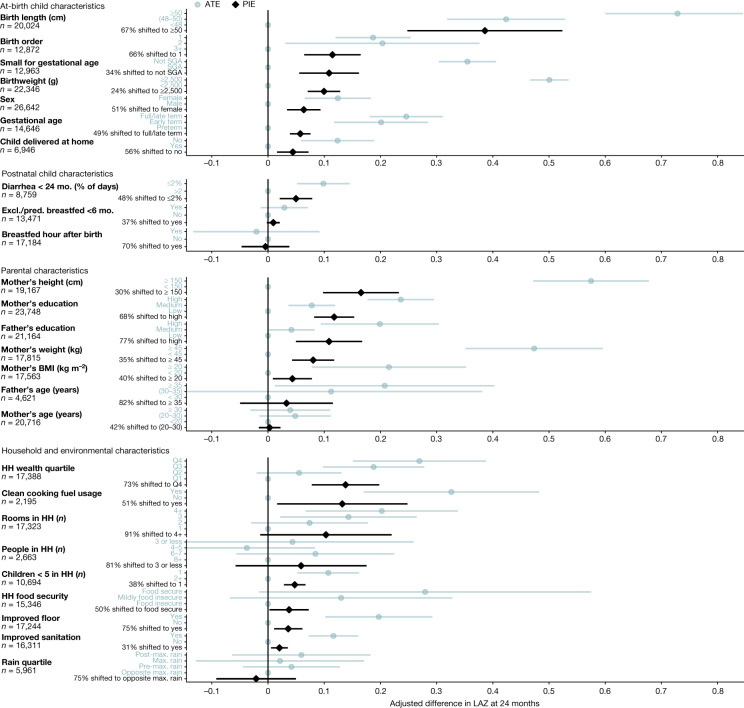
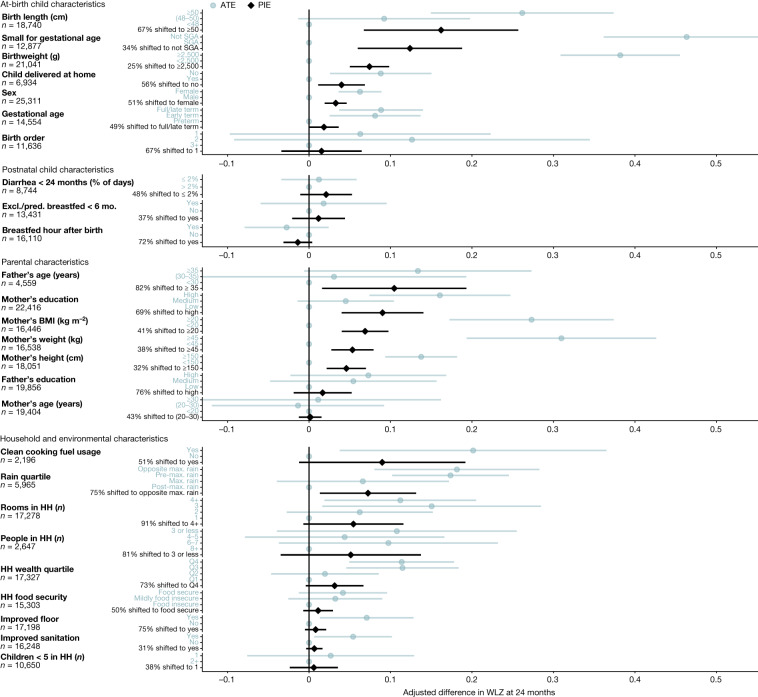
Population-level improvements in maternal height and child birth size would be expected to improve child LAZ and WLZ at 24 months of age substantially, owing to the high prevalence of suboptimal anthropometry in the populations and their strong association with attained growth at 24 months of age (Figs. 2 and 3). Beyond anthropometry, key predictors of higher z-scores included markers of better household socioeconomic status (for example, the number of rooms in the home, parental education, clean cooking fuel use and household wealth index). The pooled, cross-validated R2 for models that included the top-10 determinants for each z-score plus child sex was 0.25 for LAZ (n = 20 cohorts, 25,647 children) and 0.07 for WLZ (n = 18 cohorts, 17,853 children). The population-level effect of season on WLZ was large, with higher WLZ in drier periods (Fig. 3), consistent with seasonal differences21. Exclusive or predominant breastfeeding before 6 months of age was associated with higher WLZ but not LAZ at 6 months of age and was not a major predictor of z-scores at 24 months of age25 (Extended Data Figs. 2–4). Girls had consistently higher LAZ and WLZ than boys, potentially resulting from sex-specific differences in immunology, nutritional demands, care practices and intrauterine growth26.
Fig. 2. Population intervention effects and mean differences for child, parental, and household exposures on LAZ at 24 months of age.
Adjusted mean differences in average treatment effects (ATEs) (blue) between the labelled higher-risk level of exposures and the reference level (grey dot on the vertical line), and population intervention effects (PIEs) (black), the estimated difference in LAZ after shifting exposure levels for all children to the reference level. The number of children that contributed to each analysis is listed for each exposure. Labels on the y axis indicate the level of exposure used to estimate the ATE (blue) or the percentage of the population shifted to the lowest-risk level to estimate the PIE (black). Cohort-specific estimates were adjusted for all measured confounders using ensemble machine learning and targeted maximum-likelihood estimation (TMLE) and then pooled using random effects (Methods). Estimates are shown only for exposures measured in at least four cohorts. Max. maximum; Q, quartile; SGA, small for gestational age.
Fig. 3. PIEs and mean differences for child, parental and household exposures on WLZ at 24 months of age.
Adjusted mean differences in ATEs (blue) between the labelled higher-risk level of exposures and the reference level (grey dot on vertical line), and PIEs (black), the estimated difference in WLZ after shifting exposure levels for all children to the reference level. The number of children that contributed to each analysis is listed for each exposure. Labels on the y axis indicate the level of exposure used to estimate the ATE (blue) or the percentage of the population shifted to the lowest-risk level to estimate the PIE (black). Cohort-specific estimates were adjusted for all measured confounders using ensemble machine learning and targeted maximum-likelihood estimation (TMLE) and then pooled using random effects (Methods). Estimates are shown only for exposures measured in at least four cohorts.
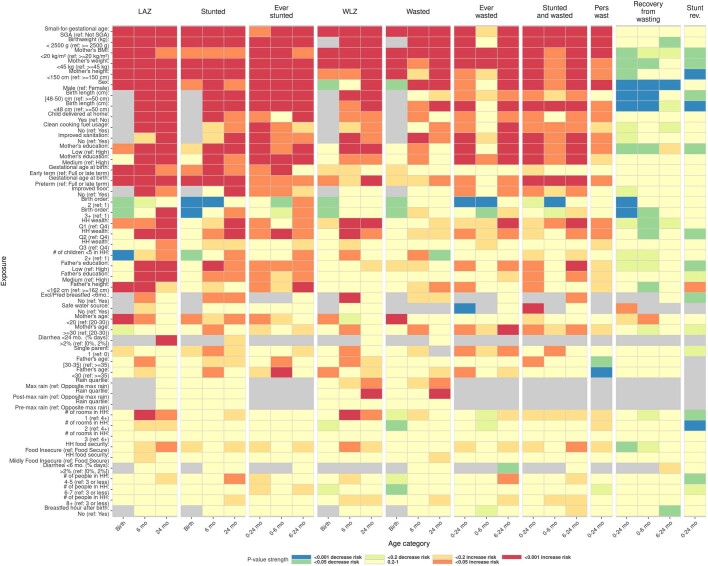
Extended Data Fig. 2. Heatmap of significance and direction across exposure-outcome combinations.
The heatmap shows the significance and direction of estimates through the cell colors, separated across primary outcomes by child age. Red and orange cells are exposures where the outcome is estimated have an increased probability of occurring compared to the reference level (harmful exposures except for recovery outcomes), while blue and green cells are exposures associated with a decreased probability of the outcome (protective exposures except for recovery outcomes). The outcomes are labeled at the top of the columns, with each set of three columns the set of three ages analyzed for that outcome. Each row is a level of an exposure variable, with reference levels excluded. Rows are sorted top to bottom by increasing average p-value. Grey cells denote comparisons that were not estimated or could not be estimated because of data sparsity in the exposure-outcome combination. All point estimates and confidence intervals for exposure-outcome pairs with P-values plotted in this figure are viewable online in Supplimentary Note 7.
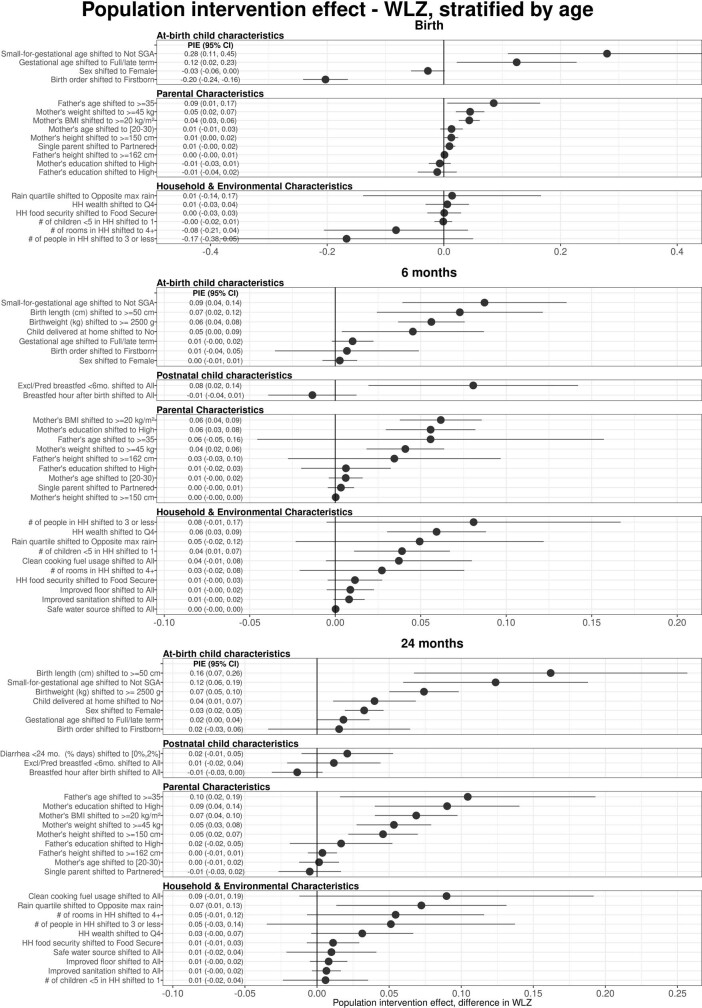
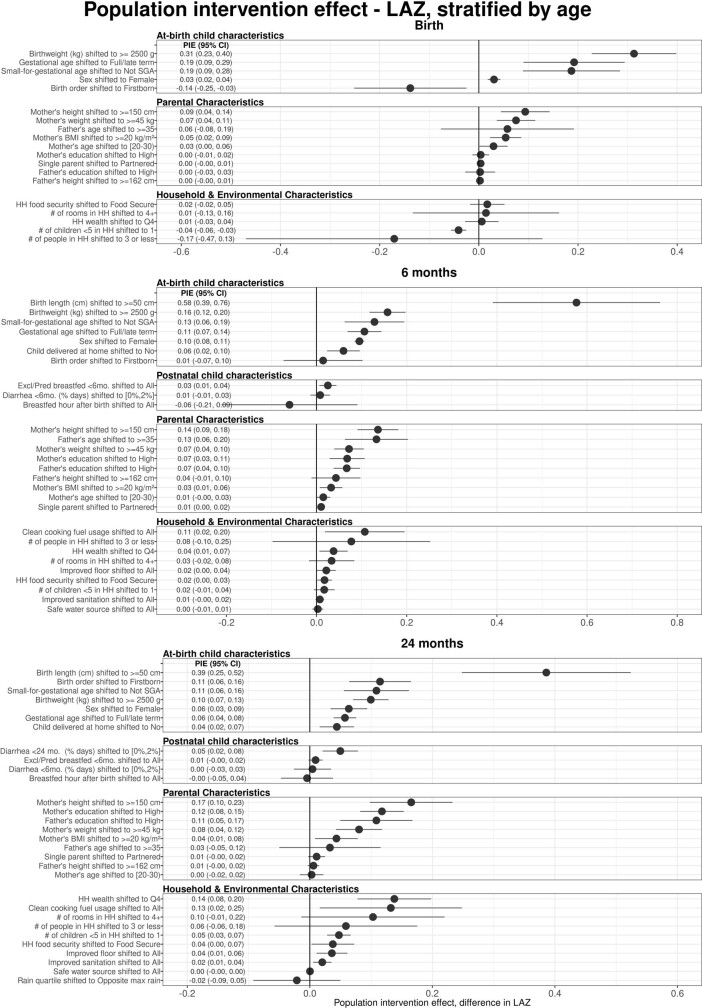
Extended Data Fig. 4. Age-stratified population intervention effects in weight-for-length Z-scores.
Exposures, rank ordered by population intervention effects on child WLZ, stratified by the age of the child at the time of anthropometry measurement. The population intervention effect is the expected difference in population mean Z-score if all children had the reference level of the exposure rather than the observed distribution. For all plots, reference levels are printed next to the name of the exposure. Cohort-specific estimates were adjusted for all measured confounders using ensemble machine learning and TMLE, and then pooled using random effects (Methods). Estimates are shown only for exposures measured in at least 4 cohorts.
These findings underscore the importance of prenatal exposures for child growth outcomes, and it may remain difficult to reduce the incidence growth faltering at the population level without broad improvements in living standards7,27. Maternal anthropometric status can influence child z-scores by affecting fetal growth and birth weight28,29. Maternal height and body mass index (BMI) could directly affect postnatal growth through breastmilk quality or could reflect family poverty, genetics, undernutrition, food insecurity or family lifestyle and diet30,31. In a secondary analysis, we estimated the associations between parental anthropometry and child z-scores, controlling for birth characteristics, and found that the associations were only partially mediated by birth size, order, hospital delivery and gestational age at birth, with adjusted z-score differences attenuated by a median of 30% (Extended Data Fig. 5).
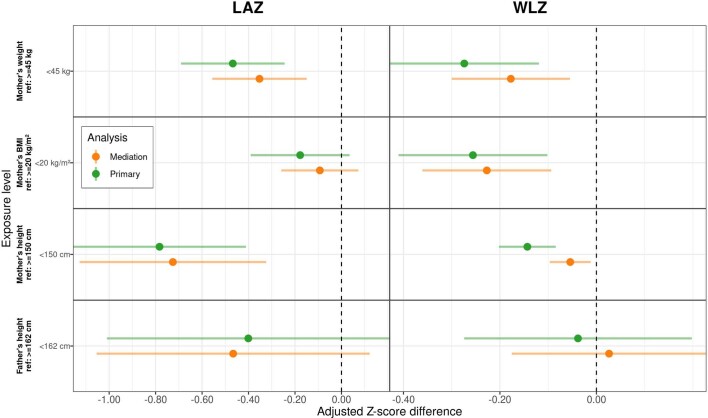
Extended Data Fig. 5. Mediation of parental anthropometry effects by birth size on child Z-scores at 24 months.
Mediating effect of adjusting for birth anthropometry and at-birth characteristics on the estimated Z-score differences between levels of parental anthropometry. Primary estimates were adjusted for all other measured exposures not on the causal pathway, while the mediation analysis estimates were additionally adjusted for birthweight, birth length, gestational age at birth, birth order, small-for-gestational age status, and home vs. hospital delivery. Only estimates from cohorts measuring at least 3 of the 6 at-birth characteristics were used to estimate the pooled Z-score differences (n = 6 cohorts, 17,124 observations). Mediation estimates were slightly attenuated toward the null, and only in the case of maternal height and child WLZ were they statistically different from the primary analysis. These results imply that the causal pathway between parental anthropometry and growth faltering operates through its effect on birth size, but most of the effect is through other pathways.
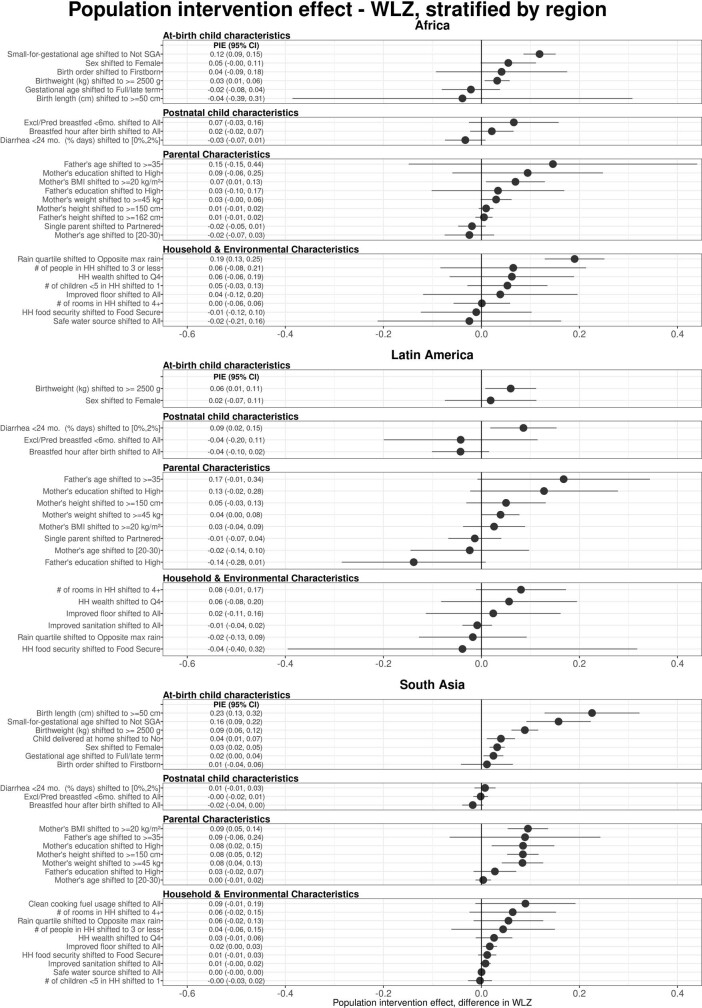
The strongest predictors of stunting and wasting estimated through population-attributable fractions closely matched those identified for child LAZ and WLZ at 24 months of age (Extended Data Figs. 6 and 7), suggesting that information embedded in continuous and binary measures of child growth provide similar inferences with respect to identifying causes relevant to public health. Potential improvements through population interventions were relatively modest. For example, if all children were born to mothers with higher BMI (20 or more) compared with the observed distribution of maternal BMI—one of the largest predictors of wasting—we estimate that the incidence of wasting by 24 months of age would be reduced by 8.2% (95% confidence interval: 4.4, 12.0; Extended Data Fig. 7). Patterns in associations across growth outcomes were broadly consistent except for preterm birth, which had a stronger association with stunting outcomes than wasting outcomes, and rainy season, which showed a strong association with wasting but not with stunting (Extended Data Fig. 2). The direction of associations did not vary across regions; however, we observed variation in the magnitude of associations across regions—notably, male sex showed a weaker association with low LAZ in south Asia (Extended Data Figs. 8 and 9).
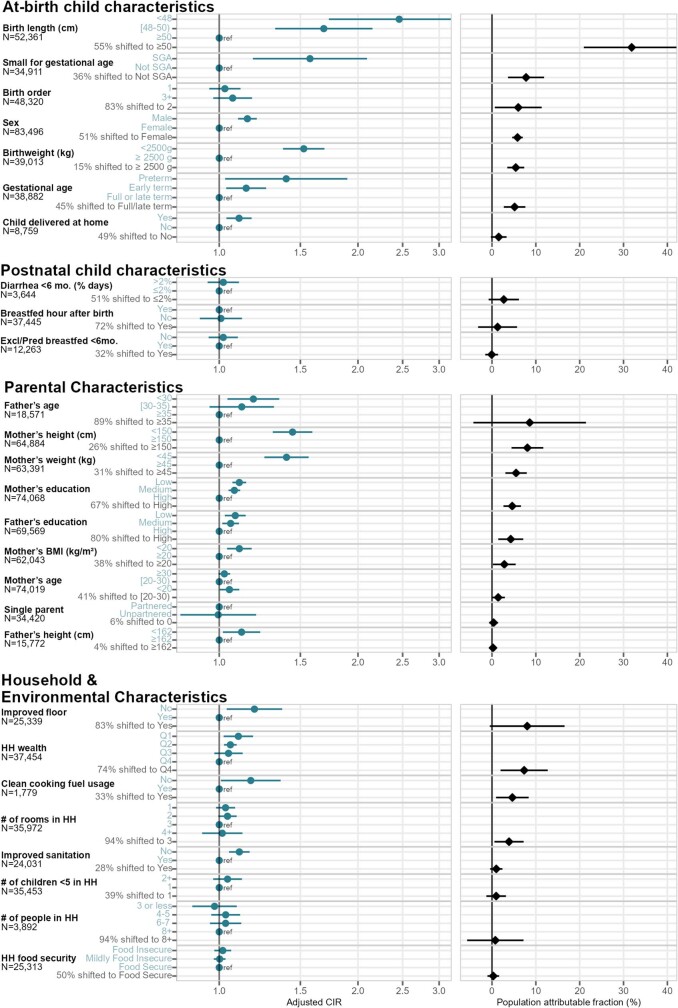
Extended Data Fig. 6. Rank-ordered associations between child, parental, and household characteristics and adjusted relative risks or population attributable fractions of stunting by age 24 months.
Blue points in the left panel show adjusted cumulative incidence ratios (CIRs) between higher-risk exposure levels and reference levels, and black points in the right panel show population attributable fractions (PAFs), the estimated proportion of the risk in the whole population that would be removed if the exposure were set to its indicated reference level. The number of children that contributed to each analysis is listed by exposure. The colored Y-axis label is either the level of exposure contrasted against the reference level to estimate the CIR, or the percent of the population shifted to the lowest-risk level to estimate the PAF. For at-birth exposures, at-birth stunting and wasting were excluded to focus on incidence of new (postnatal) cases, and for postnatal exposures (breastfeeding practice and diarrheal disease), the cumulative incidence of stunting from 6–24 months was used. Cohort-specific estimates were adjusted for all measured confounders using ensemble machine learning and TMLE, and then pooled using random effects (Methods). Estimates are shown only for exposures measured in at least 4 studies.
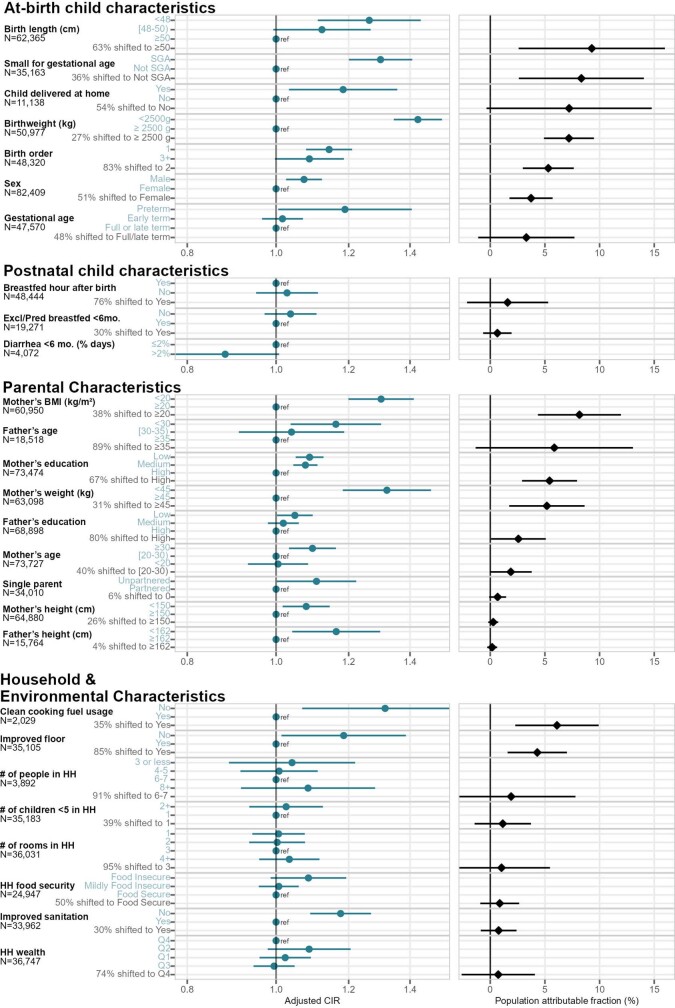
Extended Data Fig. 7. Rank-ordered associations between child, parental, and household characteristics and adjusted relative risks or population attributable fractions of wasting by age 24 months.
Blue points in the left panel show adjusted cumulative incidence ratios (CIRs) between higher-risk exposure levels and reference levels, and black points in the right panel show population attributable fractions (PAFs), the estimated proportion of the risk in the whole population that would be removed if the exposure were set to its indicated reference level. The number of children that contributed to each analysis is listed by exposure. The colored Y-axis label is either the level of exposure contrasted against the reference level to estimate the CIR, or the percent of the population shifted to the lowest-risk level to estimate the PAF. For at-birth exposures, at-birth stunting and wasting were excluded, and for postnatal exposures (breastfeeding practice and diarrheal disease), the cumulative incidence of wasting from 6-24 months was used. Cohort-specific estimates were adjusted for all measured confounders using ensemble machine learning and TMLE, and then pooled using random effects (Methods). Estimates are shown only for exposures measured in at least 4 studies. The PAF for diarrhea under 6 months was not calculable or plotted due to the unexpected CIR <1 for estimated higher diarrheal disease burden.
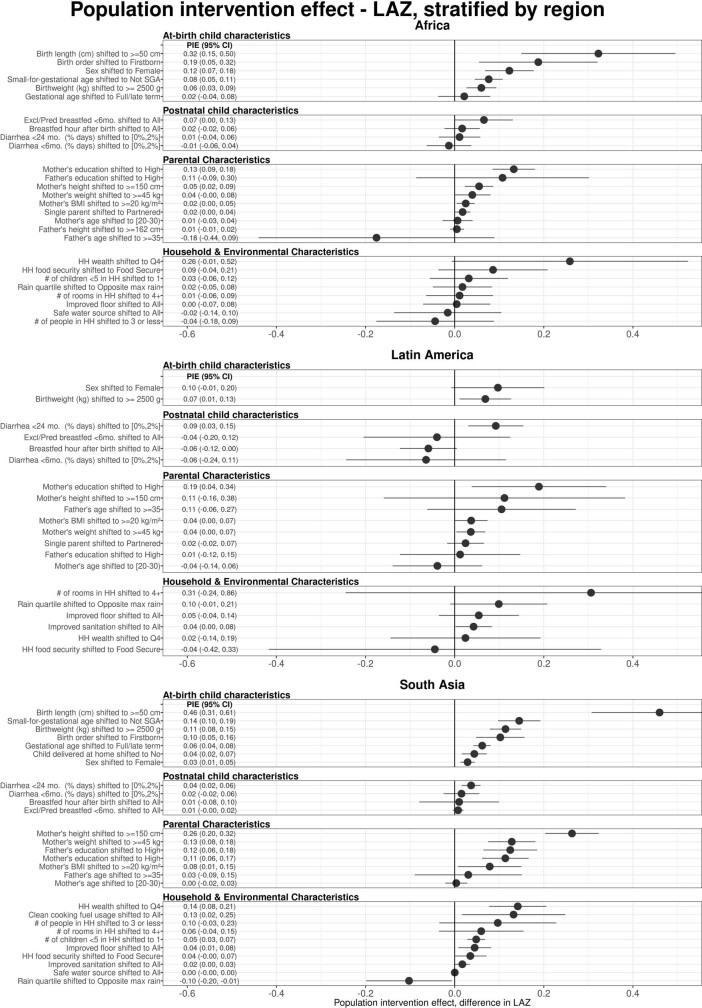
Extended Data Fig. 8. Regionally-stratified population intervention effects for length-for-age Z-scores at age 24 months.
Exposures, rank ordered by population intervention effect on child length-for-age z-score (LAZ) at age 24 months, stratified by region. The population intervention effect is the expected difference in population mean Z-score if all children had the reference level of the exposure rather than the observed distribution. For all plots, reference levels are printed next to the name of the exposure. Cohort-specific estimates were adjusted for all measured confounders using ensemble machine learning and TMLE, and then pooled using random effects (Methods). Estimates are shown only for exposures measured in at least 4 cohorts.
Extended Data Fig. 9. Regionally-stratified population intervention effects for weight-for-length Z-scores at age 24 months.
Exposures, rank ordered by population attributable difference on child weight-for-length z-score (WLZ) at age 24 months, stratified by region. The population intervention effect is the expected difference in population mean Z-score if all children had the reference level of the exposure rather than the observed distribution. For all plots, reference levels are printed next to the name of the exposure. Cohort-specific estimates were adjusted for all measured confounders using ensemble machine learning and TMLE, and then pooled using random effects (Methods). Estimates are shown only for exposures measured in at least 4 cohorts.
Age-varying effects on growth faltering
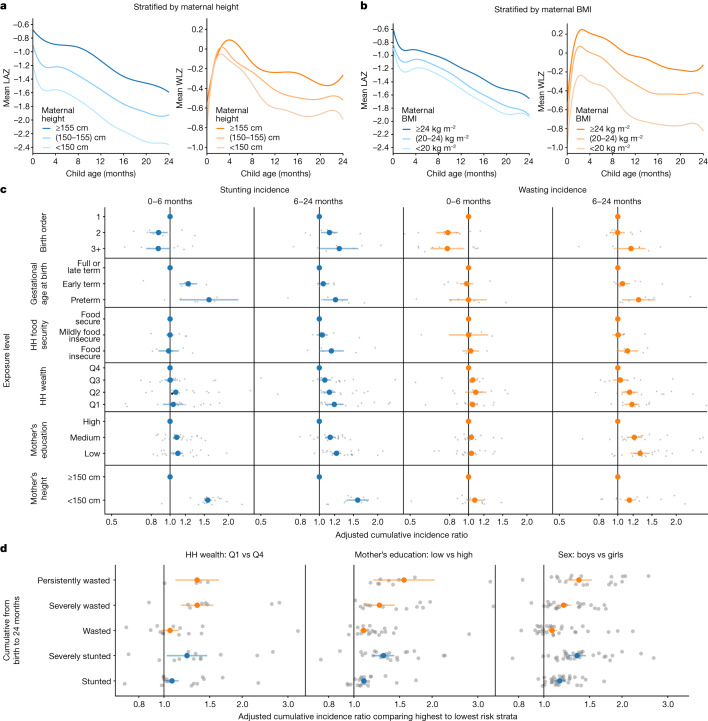
We estimated trajectories of mean LAZ and WLZ stratified by maternal height and BMI. We found that maternal height strongly influenced at-birth LAZ, and that LAZ progressed along similar trajectories up to 24 months of age regardless of maternal height (Fig. 4a), with similar but slightly less pronounced differences when stratified by maternal BMI (Fig. 4b). By contrast, children born to taller mothers had similar WLZ at birth and similar WLZ trajectories up to 3 to 4 months of age, when they diverged substantially (Fig. 4a). WLZ trajectory differences were even more pronounced when stratified by maternal BMI (Fig. 4b). These findings illustrate how maternal status strongly influences the point at which child growth trajectories begin, and how growth trajectories subsequently evolve in parallel, appearing to respond similarly to postnatal insults independently of their starting point.
Fig. 4. Effect of key exposures on the trajectories, timing and severity of child growth faltering.
a, Child LAZ and WLZ trajectories stratified by maternal height (n = 413,921 measurements, 65,061 children, 20 studies). b, Child LAZ and WLZ stratified by maternal BMI (n = 373,382 measurements, 61,933 children, 17 studies). Growth trajectories stratified by all other examined risk factors are available in Supplementary Note 5. c, Associations between key exposures and cumulative wasting incidence, stratified by age of the child during wasting incidence. Grey dots indicate cohort-specific estimates. d, Associations between key exposures and growth faltering of different severities. Cumulative incidence ratios compare the highest and lowest-risk categories of each exposure, as indicated above each graph. Grey dots indicate cohort-specific estimates.
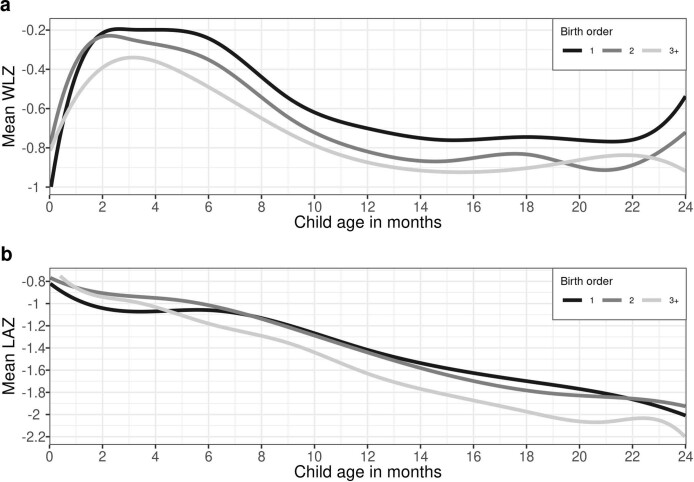
We hypothesized that causes of growth faltering could differ according to the age of growth faltering onset—for example, we expected children who were born preterm would have a higher risk of incident growth faltering immediately after birth, whereas food insecurity might increase the risk in older children, after weaning. For exposures studied in the PIE analyses, we conducted analyses stratified by age of onset and in many cases found age-varying effects (Fig. 4c). For example, most measures of socioeconomic status were associated with incident wasting or stunting only after 6 months of age, and higher birth order reduced risk for growth faltering below 6 months of age, but increased the risk thereafter. First-born babies are born with lower WLZ and catch up rapidly postnatally (Extended Data Fig. 10). This is probably because first-born babies suffer uterine constraint caused by a less developed uterine–placental–vascular supply32,33, resulting in birth weights being lower by 100–200 g in most of the studied cohorts; weight is generally more compromised than height34. The switch from a constrained uterine–placental nutrient supply line to oral nutrition permits the postnatal catch up. Stronger relationships between key socio-demographic characteristics and wasting and stunting as children age probably reflect cumulative factors that result from household conditions, particularly as complementary feeding is initiated and children begin to explore their environment and potentially face higher levels of food insecurity, especially in homes with multiple children35. When viewed across multiple definitions of growth faltering, most exposures had stronger associations with severe stunting, severe wasting or persistent wasting (more than 50% of measurements showing WLZ below –2)—rarer but more serious outcomes—than with incidence of any wasting or stunting (Fig. 4d). Additionally, the characteristics that showed strong association with lower wasting recovery by 90 days of age (birth size, small maternal stature, lower maternal education, later birth order and male sex) increased the risk of wasting prevalence and cumulative incidence (Extended Data Fig. 2).
Extended Data Fig. 10. Child growth trajectories stratified by birth order.
(a) Child weight-for-length Z-score (WLZ) trajectories, stratified by categories of child birth order. (b) Child length-for-age Z-score (LAZ) trajectories, stratified by categories of child birth order. Details on the estimation of growth trajectories are in the Methods. Child growth trajectories stratified by categories of all risk factors are available in Supplimentary Note 5.
Consequences of early growth faltering
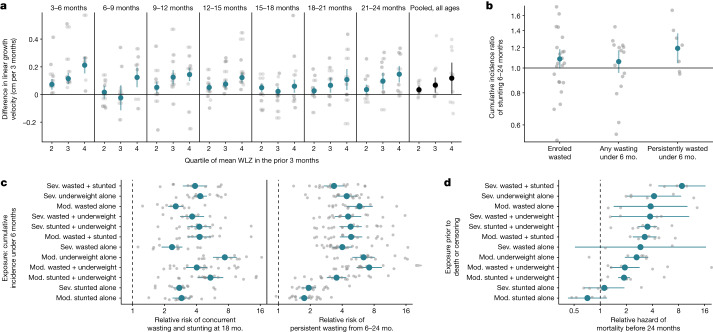
In the accompanying Articles, we document high incidence rates of wasting and stunting from birth to six months of age20,21. On the basis of previous studies, we hypothesized that early wasting could contribute to subsequent linear growth restriction, and early growth faltering could be consequential for persistent growth faltering and mortality during the first 24 months of life36–38. Among cohorts with monthly measurements, we examined age-stratified linear growth velocity by quartiles of WLZ at previous ages. We found a consistent exposure–response relationship between higher mean WLZ and faster linear growth velocity in the following 3 months (Fig. 5a). Persistent wasting from birth to 6 months of age (defined as less than 50% of measurements wasted) was the wasting exposure that showed the strongest association with incident stunting in older children (Fig. 5b).
Fig. 5. Growth faltering in early life increases risk of more severe growth faltering and mortality.
a, Adjusted differences in linear growth velocity across three-month age bands by quartile of WLZ in the preceding three months. The reference group (horizontal line) comprises children in the first quartile of WLZ in each age stratum. Far right, pooled estimates unstratified by child age. Velocity was calculated from the closest measurements within 14 days of the start and end of the age period. b, Relative risk of stunting onset between 6 and 24 months of age among children who experienced measures of early wasting before 6 months of age compared with children who did not. Grey dots indicate cohort-specific estimates. c, Association between cumulative incidence of mutually exclusive definitions of growth faltering before 6 months of age and persistent wasting from 6 to 24 months of age (33 cohorts, 6,046 cases and 68,645 children) or concurrent wasting and stunting at 18 months of age (31 cohorts, 1,447 cases, and 22,565 children). The reference group (vertical dashed line) comprises children with no measure of growth failure. Growth faltering definitions are sorted by estimates in d. d, Hazard ratios between mutually exclusive definitions of growth faltering and mortality before 24 months of age (8 cohorts, 1,689 deaths with known age of death, and 63,812 children). The reference group (vertical dashed line) comprises children with no measure of growth failure. Grey dots indicate cohort-specific estimates. Mod, moderately; sev, severely.
We next examined the relationship between measures of growth faltering during the first 6 months and serious growth-related outcomes: persistent wasting from 6–24 months and concurrent wasting and stunting at 18 months of age, both of which put children at high risk of mortality1,36. We measured concurrent wasting and stunting at 18 months because stunting prevalence peaked at this age, and because the largest number of measurements across cohorts was for children at 18 months of age20. All measures of early growth faltering were significantly associated with later, more serious growth faltering, with measures of ponderal growth faltering being among the strongest predictors (Fig. 5c).
Finally, we estimated hazard ratios of all-cause mortality by 2 years of age associated with measures of growth faltering in 8 cohorts that reported ages of death, which included 1,689 child deaths by 24 months of age (2.4% of children in the 8 cohorts). The included cohorts were highly monitored, and in most cohorts mortality rates were lower than in the general population (Extended Data Table 4). Additionally, the data included only deaths that occurred after anthropometry measurements, so many neonatal deaths may have been excluded, and lacked data on cause-specific mortality, so some deaths may have occurred from causes unrelated to growth faltering. Despite these caveats, growth faltering increased the hazard of death before 24 months for all measures except stunting alone, with the strongest associations observed for severe wasting and stunting (hazard ratio = 8.7, 95% confidence interval: 4.7 to 16.4) and severe underweight alone (hazard ratio = 4.2, 95% confidence interval: 2.0 to 8.6) (Fig. 5d).
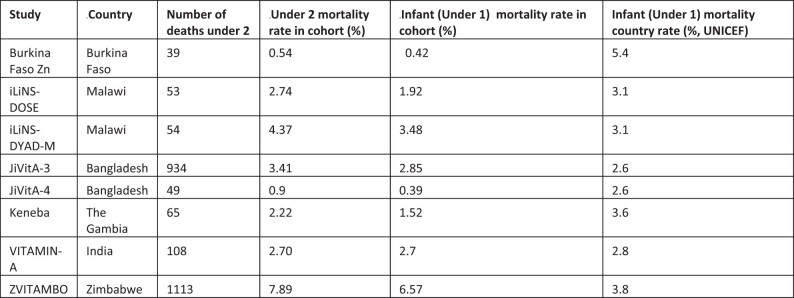
Extended Data Table 4.
ki cohort and country-level mortality rates
Under 1-year country-specific mortality rate is from UNICEF (https://data.unicef.org/country), and is higher than the cohort-specific under 2-year mortality rate for all cohorts used in the mortality analysis.
Discussion
This synthesis of cohorts during the first 1,000 days of life from LMICs has provided new insights into the principal causes and near-term consequences of growth faltering. Our use of a semi-parametric method to adjust for potential confounding provided a harmonized approach to estimate PIEs that spanned child-, parent- and household-level exposures with unprecedented breadth (30 exposures) and scale (662,763 anthropometric measurements from 33 cohorts). Our focus on the effects of shifting population-level exposures on continuous measures of growth faltering reflects a growing appreciation that growth faltering is a continuous process39. The results show that children in LMICs stand to benefit from interventions to support optimal growth during the first 1,000 days of life. Combining information from high-resolution, longitudinal cohorts enabled us to study critically important outcomes—such as persistent wasting and mortality—that it would not be not possible to study in smaller studies or in cross-sectional data, as well as to examine risk factors by age.
Maternal, prenatal and at-birth characteristics were the strongest predictors of growth faltering across regions in LMICs. Our results underscore prenatal exposures as key determinants of child growth faltering40. The limited effect of exclusive or predominant breastfeeding during the first 6 months of life (+0.01 LAZ) aligns with a meta-analysis of breastfeeding promotion25, but our finding of a limited effect of reducing diarrhea during the first 24 months (+0.05 LAZ) contrasts with some observational studies41,42. Many predictors such as child sex, birth order and season are not modifiable but could guide interventions that mitigate their effects, such as seasonally targeted supplementation or enhanced monitoring among boys. Strong associations between maternal anthropometry and early growth faltering highlight the role of intergenerational transfer of growth deficits between mothers and their children30. Shifting several key population exposures (maternal height or BMI, education and birth length) to their observed low-risk level would improve LAZ by up to 0.40z and WLZ by up to 0.15z in target populations and could be expected to prevent 8% to 32% of incident stunting and wasting (Figs. 2 and 3 and Extended Data Figs. 6 and 7). Maternal anthropometric status was highly influential on child birth size, but the parallel drop in postnatal z-scores among children born to different maternal phenotypes was much larger than differences at birth, indicating that growth trajectories were not fully ‘programmed’ at birth (Fig. 4a,b). This is in accordance with the transition from a placental to oral nutrient supply at birth.
There are caveats to these analyses. The PIEs were based on exposure distributions in the 33 cohorts, which were not necessarily representative of the general population in each setting. The use of external exposure distributions from population-based surveys would be difficult because many key exposures that we considered, such as at-birth characteristics or longitudinal diarrhea prevalence, are not measured in such surveys. In some cases, detailed exposure measurements such as longitudinal breastfeeding or diarrhea history were coarsened to simpler measures to harmonize definitions across cohorts, potentially attenuating their association with growth faltering. Other key exposures such as dietary diversity, nutrient consumption, micronutrient status, maternal and child morbidity indicators, pathogen-specific infections and sub-clinical inflammation and intestinal dysfunction were measured in only a few cohorts, and were therefore not included43,44. The absence of these exposures in the analysis, some of which have been found to be important within individual contributed cohorts44,45, means that our results emphasize exposures that were more commonly collected, but probably exclude some additional causes of growth faltering. A final caveat is that we studied consequences up to 24 months of age—the primary age range of contributed ki cohort studies—and thus did not consider effects on longer-term outcomes. Several studies have suggested that puberty could be another potential window for intervention to enhance catch-up growth46. Improving girls’ stature at any point up to the end of puberty could help to reduce intergenerational transfer of growth faltering by increasing maternal height47, which could in turn improve outcomes among their children (Figs. 2, 3 and 4a,b).
The countries that have shown the greatest reductions in stunting have undergone improvements in maternal education, nutrition and maternal and newborn healthcare and reductions in the number of pregnancies48, reinforcing the importance of interventions from conception to 1 year of age, when fetal and infant growth velocity is high and energy expenditure for growth and development is about 50% above adult values49 (adjusted for fat-free mass). A stronger focus on prenatal interventions should not distract from renewed efforts on postnatal prevention. The prenatal and postnatal growth faltering that we observed reinforce the need for sustained support of mothers and children throughout the first 1,000 days of life. Efficacy trials that deliver prenatal nutrition supplements to pregnant women50–53, therapies to reduce infection and inflammation in pregnant women54–58 and nutritional supplements to children aged 6–24 months11,12 have reduced child growth faltering but have fallen short of completely preventing it. Our results suggest that the next generation of preventive interventions should focus on the early period of a child’s first 1,000 days—throughout the period from pre-conception to 24 months of age—because maternal status and at-birth characteristics are key determinants of growth faltering during the first 24 months of life. Halting the cycle of growth faltering early should reduce the risk of severe consequences, including mortality, during this formative window of child development. Long-term investments and patience may be required, as it will take decades to eliminate the intergenerational factors that limit maternal height.
Methods
Study designs and inclusion criteria
We included all longitudinal observational studies and randomized trials available through the ki project on 1 April 2018 that met 5 inclusion criteria: (1) they were conducted in LMICs; (2) they enroled children between birth and 24 months of age and measured their length and weight repeatedly over time; (3) they did not restrict enrolment to acutely ill children; (4) they enroled children with a median birth year after 1990; and (5) they collected anthropometric status measurements at least quarterly. We included all children under 24 months of age, assuming months were 30.4167 days, and we considered a child’s first measure recorded by 7 days after birth as their anthropometry at birth. Four additional studies with high-quality mortality information that measured children at least every 6 months were included in the mortality analyses (The Burkina Faso Zinc trial, The Vitamin-A trial in India, and the iLiNS-DOSE and iLiNS-DYAD-M trials in Malawi).
Statistical analysis
Analyses were conducted in R version 4.0.5.
Outcome definitions
We calculated LAZ, WAZ and WLZ using World Health Organization (WHO) 2006 growth standards59. We used the medians of triplicate measurements of heights and weights of children from pre-2006 cohorts to re-calculate z-scores to the 2006 standard. We dropped 1,190 (0.2%) unrealistic measurements of LAZ (>+6 or <–6z), 1,330 (0.2%) measurements of WAZ (>5 or <–6z), and 1,670 (0.3%) measurements of WLZ (>+5 or <–5z), consistent with WHO recommendations60. Further details on cohort inclusion and assessment of anthropometry measurement quality are provided in the accompanying Article20. We also calculated the difference in linear and ponderal growth velocities over three-month periods. We calculated the change in LAZ, WAZ, length in cm and weight in kg within three-month age intervals, including measurements within a two-week window around each age in months to account for variation in the age at each length measurement.
We defined stunting as LAZ <–2, severe stunting as LAZ <–3, underweight as WAZ <–2, severe underweight as WAZ <–3, wasting as WLZ <–2, severe wasting as WLZ <–3, and concurrent stunting and wasting as LAZ <–2 and WLZ <–2. Children with ≥50% of WLZ measurements <–2 and at least 4 measurements over a defined age range were classified as persistently wasted (for example, birth to 24 months, median interval between measurements: 80 days, interquartile range: 62–93). Children were assumed to never recover from stunting episodes, but children were classified as recovered from wasting episodes (and at risk for a new episode of wasting) if their measured WLZ was at or above –2 for at least 60 days (details in the accompanying Article21). Stunting reversal was defined as children stunted under 3 months whose final 2 measurements before 24 months were non-stunted. Child mortality was all-cause and was restricted to children who died after birth and before age 24 months. For child morbidity outcomes (Fig. 4c), concurrent wasting and stunting prevalences at 18 months of age were estimated using the anthropometry measurement taken closest to 18 months of age, and within 17–19 months of age, while persistent wasting was estimated from child measurements between 6 and 24 months of age. We chose 18 months to calculate concurrent wasting and stunting because it maximized the number of child observations at later ages when concurrent wasting and stunting was most prevalent, and used ages of 6–24 months to define persistent wasting to maximize the number of anthropometry measurements taken after the early growth faltering exposure measurements21.
Estimating relationships between child, parental and household exposures and measures of growth faltering
Exposure definitions
We selected the exposures of interest based on variables present in multiple cohorts that met our inclusion criteria, were found to be important predictors of stunting and wasting in prior literature and could be harmonized across cohorts for pooled analyses. Extended Data Tables 2 and 3 list all exposures included in the analysis, as well as exposure categories used across cohorts, and the total number of children in each category. For parental education and asset-based household wealth, we categorized to levels relative to the distribution within each cohort. Continuous biological characteristics (gestational age, birth weight, birth height, parental weight, parental height and parental age) were classified based on a common distribution, pooling data across cohorts. Our rationale was that the meaning of socioeconomic variables is culturally context-dependent, whereas biological variables should have a more universal meaning.
Risk set definition
For exposures that occur or exist before birth, we considered the child at risk of incident outcomes at birth. Therefore, we classified children who were born stunted (or wasted) as incident episodes of stunting (or wasting) when estimating the relationship between household characteristics, paternal characteristics, and child characteristics such as gestational age, sex, birth order and birth location.
For postnatal exposures (for example, breastfeeding practices, water, sanitation and hygiene characteristics and birth weight), we excluded episodes of stunting or wasting that occurred at birth. Children who were born wasted could enter the risk set for postnatal exposures if they recovered from wasting during the study period21. This restriction ensured that for postnatal exposures, the analysis only included postnatal, incident episodes. Children born or enroled wasted were included in the risk set for the outcome of recovery from wasting within 90 days for all exposures (prenatal and postnatal).
Estimating differences in outcomes across categories of exposures
We estimated measures of association between exposures and growth faltering outcomes by comparing outcomes across categories of exposures in four ways:
Mean difference of the comparison levels of the exposure on LAZ, WLZ at birth, 6 months, and 24 months. The z-scores used were the measures taken closest to the age of interest and within 1 month of the age of interest, except for z-scores at birth which only included a child’s first measure recorded by 7 days after birth. We also calculated mean differences in LAZ, WAZ, weight and length velocities.
Prevalence ratios between comparison levels of the exposure, compared to the reference level at birth, 6 months, and 24 months. Prevalence was estimated using anthropometry measurements closest to the age of interest and within one month of the age of interest, except for prevalence at birth which only included measures taken on the day of birth.
Cumulative incidence ratios (CIRs) between comparison levels of the exposure, compared to the reference level, for the incident onset of outcomes between birth and 24 months, 6 and 24 months, and birth and 6 months.
Mean z-scores by continuous age, stratified by levels of exposures from birth to 24 months were fit within individual cohorts using cubic splines with the bandwidth chosen to minimize the median Akaike information criterion across cohorts61. We estimated splines separately for each exposure category. We pooled spline curves across cohorts into a single prediction, offset by mean z-scores at one year, using random-effects models62.
Estimating population-attributable parameters
We estimated three measures of the population-level effect of exposures on growth faltering outcomes:
Population intervention effect (PIE), a generalization of population-attributable risk, was defined as the change in population mean z-score if the entire population’s exposure was set to an ideal reference level. For each exposure, we chose reference levels based on prior literature or as the category with the highest mean LAZ or WLZ across cohorts.
Population-attributable fraction (PAF) was defined as the proportional reduction in cumulative incidence if the entire population’s exposure was set to an ideal low-risk reference level. We estimated the PAF for the prevalence of stunting and wasting at birth, 6, and 24 months and cumulative incidence of stunting and wasting from birth to 24 months, 6 to 24 months, and from birth to 6 months. For each exposure, we chose the reference level as the category with the lowest risk of stunting or wasting.
Optimal individualized intervention impact. We used a variable importance measure methodology to estimate the impact of an optimal individualized intervention on an exposure63. The optimal intervention on an exposure was determined through estimating individualized treatment regimes, which give an individual-specific rule for the lowest-risk level of exposure based on individuals’ measured covariates. The covariates used to estimate the low-risk level are the same as those used for the adjustment documented in section 6 below. The impact of the optimal individualized intervention is derived from the variable importance measure, which is the predicted change in the population mean outcome from the observed outcome if every child’s exposure was shifted to the optimal level. This differs from the PIE and PAF parameters in that we did not specify the reference level; moreover, the reference level could vary across participants.
PIE and PAF parameters assume a causal relationship between exposure and outcome. For some exposures, we considered attributable effects to have a pragmatic interpretation — they represent a summary estimate of relative importance that combines the exposure’s strength of association and its prevalence in the population64. Comparisons between optimal intervention estimates and PIE estimates are shown in Extended Data Fig. 11.
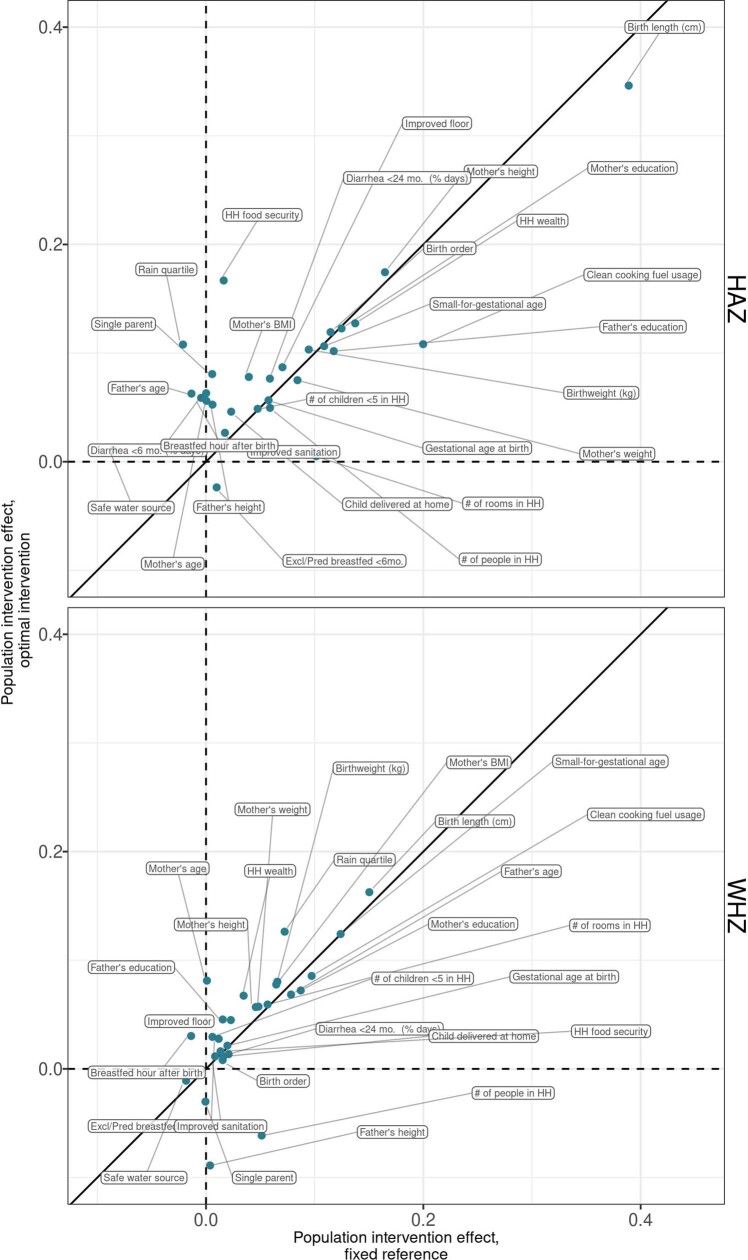
Extended Data Fig. 11. Comparing fixed-reference and optimal intervention estimates of the population intervention effect.
Pooled population intervention effects on child LAZ and WHZ at 24 months, with the X-axis showing attributable differences using a fixed, and the Y-axis showing the optimal intervention attributable difference, where the level the exposure is shifted to can vary by child. Points are labeled with the specific risk factor. Estimates farther from the diagonal line have larger differences between the static and optimal intervention estimates. The optimal intervention attributable differences, which are not estimated with an a-priori specified low-risk reference level, were generally close to the static attributable differences, indicating that the chosen reference levels were the lowest-risk strata in most or all children.
Estimation approach
Estimation of cohort-specific effects
For each exposure, we used the directed acyclic graph framework to identify potential confounders from the broader set of exposures used in the analysis65. We did not adjust for characteristics that were assumed to be intermediate on the causal path between any exposure and the outcome, because while controlling for mediators may help adjust for unmeasured confounders in some conditions, it can also lead to collider bias66,67. Detailed lists of adjustment covariates used for each analysis are available in Supplementary Note 1. Confounders were not measured in every cohort, so there could be residual confounding in cohort-specific estimates.
Analyses used a complete-case approach that only included children with non-missing exposure and outcome measurements. For additional covariates in adjusted analyses, we used the following approach to impute missing covariate values68. Within each cohort, if there was <50% missingness in a covariate, we imputed missing measurements as the median (continuous variables) or mode (categorical variables) among all children, and analyses included an indicator variable for missingness in the adjustment set. Covariates with >50% missingness were excluded from the potential adjustment set. When calculating the median for imputation, we used children as independent units rather than measurements so that children with more frequent measurements were not over-represented.
Unadjusted prevalence ratios and CIRs between the reference level of each exposure and comparison levels were estimated using logistic regressions69. Unadjusted mean differences for continuous outcomes were estimated using linear regressions.
To flexibly adjust for potential confounders and reduce the risk of model misspecification, we estimated adjusted prevalence ratios, CIRs, and mean differences using TMLE, a two-stage estimation strategy that incorporates state-of-the-art machine learning algorithms (super learner) while still providing valid statistical inference23,70. The effects of covariate adjustment on estimates compared to unadjusted estimates is shown in Extended Data Fig. 12, and E-values, summary measures of the strength of unmeasured confounding needed to explain away observed significant associations71, are plotted in Extended Data Fig. 13. The super learner is an ensemble machine learning method that uses cross-validation to select a weighted combination of predictions from a library of algorithms72. We included in the library simple means, generalized linear models, LASSO penalized regressions73, generalized additive models74, and gradient boosting machines75. The super learner was fit to maximize the tenfold cross-validated area under the receiver operator curve (AUC) for binomial outcomes, and minimize the tenfold cross-validated mean-squared error (MSE) for continuous outcomes. That is, the super learner was fit using nine-tenths of the data, while the AUC/MSE was calculated on the remaining one-tenth of the data. Each fold of the data was held out in turn and the cross-validated performance measure was calculated as the average of the performance measures across the ten folds. This approach is practically appealing and robust in finite samples, since this cross-validation procedure uses unseen sample data to measure the estimator’s performance. Also, the super learner is asymptotically optimal in the sense that it is guaranteed to outperform the best possible algorithm included in the library as sample size grows. The initial estimator obtained via super learner is subsequently updated to yield an efficient double-robust semi-parametric substitution estimator of the parameter of interest23. To estimate the R2 of models including multiple exposures, we fit super learner models, without the targeted learning step, and within each cohort measuring the exposures. We then pooled cohort-specific R2 estimates using fixed-effects models.
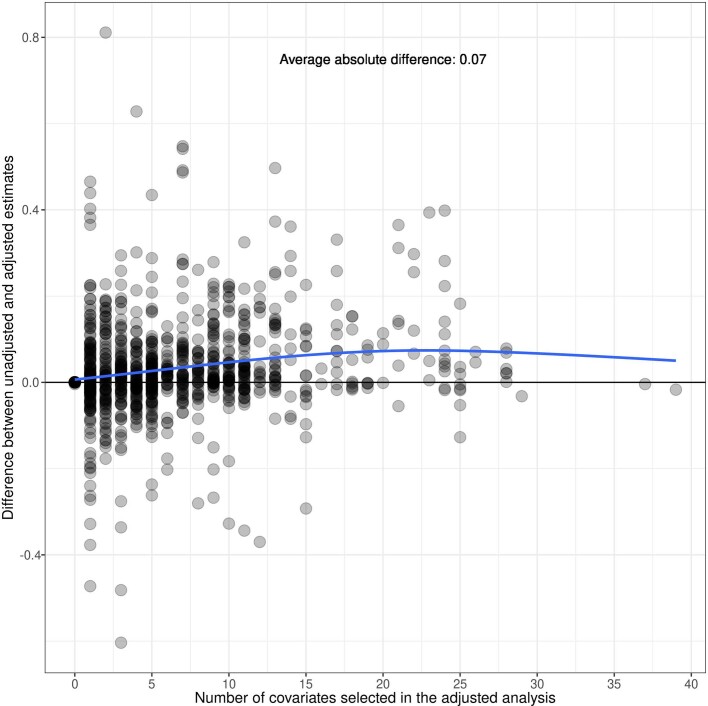
Extended Data Fig. 12. Difference between adjusted and unadjusted Z-score effects by number of selected adjustment variables.
Points mark the difference in estimates unadjusted and adjusted estimates of the difference in average Z-scores between exposed and unexposed children across 33 cohorts, 30 exposures and length-for-age and weight-for-length Z-score outcomes included in the analysis. Different cohorts measured different sets of exposures, and a different number of adjustment covariates were chosen for each cohort-specific estimate based on outcome sparsity, so cohort-specific estimates adjust for different covariates and numbers of covariates. The plot shows no systematic bias between unadjusted and adjusted estimates based on number of covariates chosen. The blue line shows the average difference between adjusted estimates from unadjusted estimates, fitted using a cubic spline.
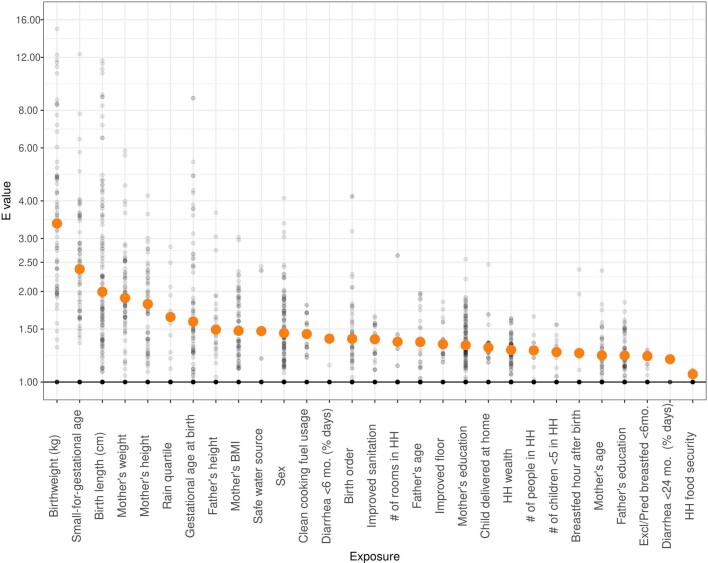
Extended Data Fig. 13. Assessing sensitivity of estimates to unmeasured confounding using E-values.
An E-value is the minimum strength of association in terms of relative risk that an unmeasured confounder would need to have with both the exposure and the outcome to explain away an estimated exposure–outcome association71. Orange points mark the E-values for the pooled estimates of relative risk for each exposure. Grey points are cohort-specific E-values for each exposure-outcome relationship. Non-significant pooled estimates have points plotted at 1.0. Orange points are median E-values among statistically significant estimates for each exposure. As an example, an unmeasured confounder would on average need to almost double the risk of both the exposure and the outcome to explain away observed significant associations for the birth length exposure.
We estimated influence curve-based, clustered standard errors to account for repeated measures in the analyses of recovery from wasting or progression to severe wasting. We assumed that the children were the independent units of analysis unless the original study had a clustered design, in which case the unit of independence in the original study were used as the unit of clustering. We used clusters as the unit of independence for the iLiNS-Zinc, Jivita-3, Jivita-4, Probit, and SAS Complementary Feeding trials. We estimated 95% confidence intervals for incidence using the normal approximation.
Mortality analyses estimated hazard ratios using Cox proportional hazards models with a child’s age in days as the timescale, adjusting for potential confounders, with the growth faltering exposure status updated at each follow-up that preceded death or censoring by 24 months of age. Growth faltering exposures included moderate (between –2z and –3z) wasting, stunting, and underweight, severe (below –3z) wasting, stunting, and underweight, and combinations of concurrent wasting, stunting, and underweight. Growth faltering categories were mutually exclusive within moderate or severe classifications, so children were classified as only wasted, only stunted, or only underweight, or some combination of these categories. We estimated the hazard ratio associated with different anthropometric measures of child growth failure in separate analyses, considering each as an exposure in turn with the reference group defined as children without the deficit. For children who did not die, we defined their censoring date as the administrative end of follow-up in their cohort, or age 24 months (730 days), whichever occurred first. Because mortality was a rare outcome, estimates are adjusted only for child sex and trial treatment arm. To avoid reverse causality, we did not include child growth measures occurring within 7 days of death. Extended Data Table 4 lists the cohorts used in the mortality analysis, the number of deaths in each cohort, and a comparison to country-level infant mortality rates.
Data sparsity
We did not estimate relative risks between a higher level of exposure and the reference group if there were 5 or fewer cases in either stratum. In such cases, we still estimated relative risks between other exposure strata and the reference strata if those strata were not sparse. For rare outcomes, we only included one covariate for every 10 observations in the sparsest combination of the exposure and outcome, choosing covariates based on ranked deviance ratios.
Pooling parameters
We pooled adjusted estimates from individual cohorts using random-effects models, fit using restricted maximum-likelihood estimation. The pooling methods are detailed in the accompanying Article20. All parameters were pooled directly using the cohort-specific estimates of the same parameter, except for population-attributable fractions. Pooled PAFs were calculated from random-effects pooled population intervention effects (PIEs), and pooled outcome prevalence in the population using the following formulas76:
| 1 |
| 2 |
For PAFs of exposures on the cumulative incidence of wasting and stunting, the pooled cumulative incidence was substituted for the outcome prevalence in the above equations. We used this method instead of direct pooling of PAFs because unlike PAFs, PIEs are unbounded with symmetrical confidence intervals.
For Fig. 4a,b, mean trajectories estimated using cubic splines in individual studies and then curves were pooled using random effects62. Curves estimated from all anthropometry measurements of children taken from birth to 24 months of age within studies that measured the measure of maternal anthropometry.
Sensitivity analyses
We examined covariate missingness by study and assessed the effect of covariate missingness by comparing results with median/mode missingness imputation to a complete-case analysis (Supplementary Note 2). We compared estimates pooled using random-effects models, which are more conservative in the presence of heterogeneity across studies, with estimates pooled using fixed effects (Supplementary Note 3), and we compared adjusted estimates with estimates unadjusted for potential confounders (Supplementary Note 4). We also plotted splines of child growth trajectories, stratified by exposure levels, for all exposures in Supplementary Note 5. We re-estimated the attributable differences of exposures on WLZ and LAZ at 24 months, dropping the PROBIT trial, the only European study (Supplementary Note 6). Point estimates and confidence intervals from all age, exposure and growth outcome combinations (as presented in Extended Data Fig. 2) are plotted in Supplementary Note 7.
Inclusion and ethics
This study analysed data that was collected in 15 LMICs that were assembled by the Bill & Melinda Gates Foundation Ki initiative. The datasets are owned by the original investigators that collected the data. Members of the Ki Child Growth Consortium were nominated by each study’s leadership team to be representative of the country and study teams that originally collected the data. Consortium members reviewed their cohort’s data within the i database to ensure external and internal consistency of cohort-level estimates. Consortium members provided significant input on the statistical analysis plan, interpretation of results and manuscript writing. Per the request of consortium members, the manuscript includes cohort-level and regional results to maximize the utility of the study findings for local investigators and public health agencies. Analysis code has been published with the manuscript to promote transparency and extensions of our research by local and global investigators.
Reporting summary
Further information on research design is available in the Nature Portfolio Reporting Summary linked to this article.
Online content
Any methods, additional references, Nature Portfolio reporting summaries, source data, extended data, supplementary information, acknowledgements, peer review information; details of author contributions and competing interests; and statements of data and code availability are available at 10.1038/s41586-023-06501-x.
Supplementary information
This file contains Supplementary Notes 1–7
Acknowledgements
This research was financially supported by a global development grant (OPP1165144) from the Bill & Melinda Gates Foundation to the University of California, Berkeley, CA, USA. J.B.-C. acknowledges funding from the National Institute of Allergy and Infectious Diseases under Award K01AI141616. J.B.-C. is a Chan Zuckerberg Biohub investigator. The authors thank the following collaborators on the included cohorts and trials for their contributions to study planning, data collection, and analysis: M. Sharif, S. Kerio, Urosa, Alveen, S. Hussain, V. Paudel, A. Costello, N. Rouamba, J.-B. Ouédraogo, L. Prince, S. A. Vosti, B. Torun, L. M. Locks, C. M. McDonald, R. Kupka, R. J. Bosch, R. Kisenge, S. Aboud, M. Wang, Azaduzzaman, A. A. Shamim, R. Haque, R. Klemm, S. Mehra, M. Mitra, K. Schulze, S. Taneja, B. Nayyar, V. Suri, P. Khokhar, B. Nayyar, P. Khokhar, J. E. Rohde, T. Kumar, J. Martines, M. K. Bhan and all other members of the study staff and field teams; all study participants and their families for their important contributions; the LCNI5 and iLiNS research teams, participants and people of Lungwena, Namwera, Mangochi and Malindi; and our research assistants for their positive attitude, support and help in all stages of the studies.
Extended data figures and tables
Extended Data Fig. 3. Age-stratified population intervention effects in length-for-age Z-scores.
Exposures, rank ordered by population intervention effect on child LAZ, stratified by the age of the child at the time of anthropometry measurement. The population intervention effect is the expected difference in mean Z-score if all children had the reference level of the exposure rather than the observed exposure distribution. Reference levels are printed in the exposure label. Cohort-specific estimates were adjusted for all measured confounders using ensemble machine learning and TMLE, and then pooled using random effects (Methods). Estimates are shown only for exposures measured in at least 4 cohorts.
Author contributions
Conceptualization: A.M., J.B.-C., J.M.C., K.H.B., P.C. and B.F.A. Funding acquisition: J.M.C., A.E.H., M.J.v.d.L. and B.F.A. Data curation: A.M., J.B.-C., J.C., O.S., W. Cai, A.N., N.N.P., W.J., E.J., E.O.C., S.R., N.H., I. Malenica, H.L., R. Hafen, V.S., J.H. and T.N. Formal analyses: A.M., J.B.-C., J.C., O.S., W. Cai, A.N., N.N.P., W.J., E.J., E.O.C., S.R. S.D., N.H., I. Malenica, H.L., V.S. and B.F.A. Methodology: A.M., J.B.-C., J.M.C., J.C., O.S., N.H., I. Malenica, A.E.H., M.J.v.d.L., K.H.B., P.C. and B.F.A. Visualization: A.M., J.B.-C., A.N., N.N.P., S.R., A.S., E.J., J.C., R. Hafen, S.D. K.H.B., P.C. and B.F.A. Writing, original draft preparation: A.M., J.B.-C. and B.F.A. Writing, review and editing: A.M., J.B.-C., J.M.C., K.H.B., P.C., B.F.A. and all other authors.
Peer review
Peer review information
Nature thanks Nicholas Kassebaum, Theis Lange, Bruno Masquelier and the other, anonymous, reviewer(s) for their contribution to the peer review of this work. Peer review reports are available.
Data availability
The data that support the findings of this analysis are a combination of data from multiple principal investigators and institutions. The data are available, upon reasonable request, to the requestor by contacting the individual principal investigators. The individuals and the contact information to help the requestor obtain access to the data are listed at https://www.synapse.org/#!Synapse:syn51570682/wiki/. The analysis dataset is at https://www.synapse.org/#!Synapse:syn51570682/datasets/. This dataset is access controlled and not available publicly for privacy reasons.
Code availability
Code used in the study has been deposited at Zenodo: https://zenodo.org/record/793781177.
Competing interests
T. Norman is an employee of the Bill & Melinda Gates Foundation. K.H.B. and P. Christian are former employees of the Bill & Melinda Gates Foundation. J.C., V.S., R. Hafen and J.H. are research contractors funded by the Bill & Melinda Gates Foundation.
Footnotes
Publisher’s note Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
A list of authors and their affiliations appears at the end of the paper
Contributor Information
Andrew Mertens, Email: amertens@berkeley.edu.
Benjamin F. Arnold, Email: ben.arnold@ucsf.edu
The Ki Child Growth Consortium:
Souheila Abbeddou, Linda S. Adair, Tahmeed Ahmed, Asad Ali, Hasmot Ali, Per Ashorn, Rajiv Bahl, Mauricio L. Barreto, Elodie Becquey, France Begín, Pascal Obong Bessong, Maharaj Kishan Bhan, Nita Bhandari, Santosh K. Bhargava, Zulfiqar A. Bhutta, Robert E. Black, Ladaporn Bodhidatta, Delia Carba, William Checkley, Parul Christian, Jean E. Crabtree, Kathryn G. Dewey, Christopher P. Duggan, Caroline H. D. Fall, Abu Syed Golam Faruque, Wafaie W. Fawzi, José Quirino da Silva Filho, Robert H. Gilman, Richard L. Guerrant, Rashidul Haque, S. M. Tafsir Hasan, Sonja Y. Hess, Eric R. Houpt, Jean H. Humphrey, Najeeha Talat Iqbal, Elizabeth Yakes Jimenez, Jacob John, Sushil Matthew John, Gagandeep Kang, Margaret Kosek, Michael S. Kramer, Alain Labrique, Nanette R. Lee, Aldo Ângelo Moreira Lima, Tjale Cloupas Mahopo, Kenneth Maleta, Dharma S. Manandhar, Karim P. Manji, Reynaldo Martorell, Sarmila Mazumder, Estomih Mduma, Venkata Raghava Mohan, Sophie E. Moore, Robert Ntozini, Mzwakhe Emanuel Nyathi, Maribel Paredes Olortegui, Césaire T. Ouédraogo, William A. Petri, Prasanna Samuel Premkumar, Andrew M. Prentice, Najeeb Rahman, Manuel Ramirez-Zea, Harshpal Singh Sachdev, Kamran Sadiq, Rajiv Sarkar, Monira Sarmin, Naomi M. Saville, Saijuddin Shaikh, Bhim P. Shrestha, Sanjaya Kumar Shrestha, Alberto Melo Soares, Bakary Sonko, Aryeh D. Stein, Erling Svensen, Sana Syed, Fayaz Umrani, Honorine D. Ward, Keith P. West, Jr, Lee Shu Fune Wu, Seungmi Yang, and Pablo Penataro Yori
Extended data
is available for this paper at 10.1038/s41586-023-06501-x.
Supplementary information
The online version contains supplementary material available at 10.1038/s41586-023-06501-x.
References
- 1.McDonald CM, et al. The effect of multiple anthropometric deficits on child mortality: meta-analysis of individual data in 10 prospective studies from developing countries. Am. J. Clin. Nutr. 2013;97:896–901. doi: 10.3945/ajcn.112.047639. [DOI] [PubMed] [Google Scholar]
- 2.Forouzanfar MH, et al. Global, regional, and national comparative risk assessment of 79 behavioural, environmental and occupational, and metabolic risks or clusters of risks, 1990–2015: a systematic analysis for the Global Burden of Disease Study 2015. Lancet. 2016;388:1659–1724. doi: 10.1016/S0140-6736(16)31679-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Joint Child Malnutrition Estimates—Levels and Trends, 2019 edn (World Health Organization, 2019); http://www.who.int/nutgrowthdb/estimates2018/en/.
- 4.Sudfeld CR, et al. Malnutrition and its determinants are associated with suboptimal cognitive, communication, and motor development in Tanzanian children. J. Nutr. 2015;145:2705–2714. doi: 10.3945/jn.115.215996. [DOI] [PubMed] [Google Scholar]
- 5.Black RE, et al. Maternal and child undernutrition and overweight in low-income and middle-income countries. Lancet. 2013;382:427–451. doi: 10.1016/S0140-6736(13)60937-X. [DOI] [PubMed] [Google Scholar]
- 6.Grantham-McGregor S, et al. Developmental potential in the first 5 years for children in developing countries. Lancet. 2007;369:60–70. doi: 10.1016/S0140-6736(07)60032-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.McGovern ME, Krishna A, Aguayo VM, Subramanian SV. A review of the evidence linking child stunting to economic outcomes. Int. J. Epidemiol. 2017;46:1171–1191. doi: 10.1093/ije/dyx017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Bhutta ZA, et al. What works? Interventions for maternal and child undernutrition and survival. Lancet. 2008;371:417–440. doi: 10.1016/S0140-6736(07)61693-6. [DOI] [PubMed] [Google Scholar]
- 9.Panjwani A, Heidkamp R. Complementary feeding interventions have a small but significant impact on linear and ponderal growth of children in low- and middle-income countries: a systematic review and meta-analysis. J. Nutr. 2017;147:2169S–2178S. doi: 10.3945/jn.116.243857. [DOI] [PubMed] [Google Scholar]
- 10.Bhutta ZA, et al. Evidence-based interventions for improvement of maternal and child nutrition: what can be done and at what cost? Lancet. 2013;382:452–477. doi: 10.1016/S0140-6736(13)60996-4. [DOI] [PubMed] [Google Scholar]
- 11.Dewey KG, et al. Preventive small-quantity lipid-based nutrient supplements reduce severe wasting and severe stunting among young children: an individual participant data meta-analysis of randomized controlled trials. Am. J. Clin. Nutr. 2022;116:1314–1333. doi: 10.1093/ajcn/nqac232. [DOI] [PubMed] [Google Scholar]
- 12.Dewey KG, et al. Characteristics that modify the effect of small-quantity lipid-based nutrient supplementation on child growth: an individual participant data meta-analysis of randomized controlled trials. Am. J. Clin. Nutr. 2021;114:15S–42S. doi: 10.1093/ajcn/nqab278. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Stewart CP, Iannotti L, Dewey KG, Michaelsen KF, Onyango AW. Contextualising complementary feeding in a broader framework for stunting prevention. Matern. Child. Nutr. 2013;9:27–45. doi: 10.1111/mcn.12088. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Null C, et al. Effects of water quality, sanitation, handwashing, and nutritional interventions on diarrhoea and child growth in rural Kenya: a cluster-randomised controlled trial. Lancet Glob. Health. 2018;6:e316–e329. doi: 10.1016/S2214-109X(18)30005-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Luby SP, et al. Effects of water quality, sanitation, handwashing, and nutritional interventions on diarrhoea and child growth in rural Bangladesh: a cluster randomised controlled trial. Lancet Glob. Health. 2018;6:e302–e315. doi: 10.1016/S2214-109X(17)30490-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Humphrey JH, et al. Independent and combined effects of improved water, sanitation, and hygiene, and improved complementary feeding, on child stunting and anaemia in rural Zimbabwe: a cluster-randomised trial. Lancet Glob. Health. 2019;7:e132–e147. doi: 10.1016/S2214-109X(18)30374-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Young, H. & Marshak, A. Persistent Global Acute Malnutrition (Boston: Feinstein International Center, Tufts University, 2017).
- 18.Coyle, J. R. et al. Targeted learning. Wiley StatsRef10.1002/9781118445112.stat08414 (2023).
- 19.Keats EC, et al. Effective interventions to address maternal and child malnutrition: an update of the evidence. Lancet Child Adolesc. Health. 2021;5:367–384. doi: 10.1016/S2352-4642(20)30274-1. [DOI] [PubMed] [Google Scholar]
- 20.Benjamin-Chung, J. et. al. Early-childhood linear growth faltering in low- and middle-income countries. Nature10.1038/s41586-023-06418-5 (2023). [DOI] [PMC free article] [PubMed]
- 21.Mertens, A. et al. Child wasting and concurrent stunting in low- and middle-income countries. Nature10.1038/s41586-023-06480-z (2023). [DOI] [PMC free article] [PubMed]
- 22.Westreich D. From patients to policy: population intervention effects in epidemiology. Epidemiology. 2017;28:525–528. doi: 10.1097/EDE.0000000000000648. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.van der Laan, M. J. & Rose, S. Targeted Learning: Causal Inference for Observational and Experimental Data (Springer, 2011).
- 24.Raudenbush, S. W. Analyzing Effect Sizes: Random-Effects Models. in The Handbook of Research Synthesis and Meta-Analysis (eds Cooper, H. et al.) 295–315 (Russell Sage Foundation, 2009).
- 25.Giugliani ERJ, Horta BL, de Mola CL, Lisboa BO, Victora CG. Effect of breastfeeding promotion interventions on child growth: a systematic review and meta-analysis. Acta Paediatr. 2015;104:20–29. doi: 10.1111/apa.13160. [DOI] [PubMed] [Google Scholar]
- 26.Thurstans S, et al. Understanding sex differences in childhood undernutrition: a narrative review. Nutrients. 2022;14:948. doi: 10.3390/nu14050948. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Haddad L, Alderman H, Appleton S, Song L, Yohannes Y. Reducing child malnutrition: how far does income growth take us? World Bank Econ. Rev. 2003;17:107–131. doi: 10.1093/wber/lhg012. [DOI] [Google Scholar]
- 28.Young MF, et al. Role of maternal preconception nutrition on offspring growth and risk of stunting across the first 1000 days in Vietnam: a prospective cohort study. PLoS ONE. 2018;13:e0203201. doi: 10.1371/journal.pone.0203201. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Addo OY, et al. Maternal height and child growth patterns. J. Pediatr. 2013;163:549–554.e1. doi: 10.1016/j.jpeds.2013.02.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Martorell R, Zongrone A. Intergenerational influences on child growth and undernutrition. Paediatr. Perinat. Epidemiol. 2012;26:302–314. doi: 10.1111/j.1365-3016.2012.01298.x. [DOI] [PubMed] [Google Scholar]
- 31.Bzikowska-Jura A, et al. Maternal nutrition and body composition during breastfeeding: association with human milk composition. Nutrients. 2018;10:1379. doi: 10.3390/nu10101379. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Gluckman PD, Hanson MA. Maternal constraint of fetal growth and its consequences. Semin. Fetal. Neonatal Med. 2004;9:419–425. doi: 10.1016/j.siny.2004.03.001. [DOI] [PubMed] [Google Scholar]
- 33.Hafner E, Schuchter K, Metzenbauer M, Philipp K. Uterine artery Doppler perfusion in the first and second pregnancies. Ultrasound Obstet. Gynecol. 2000;16:625–629. doi: 10.1046/j.1469-0705.2000.00290.x. [DOI] [PubMed] [Google Scholar]
- 34.Ong KKL, et al. Size at birth and early childhood growth in relation to maternal smoking, parity and infant breast-feeding: longitudinal birth cohort study and analysis. Pediatr. Res. 2002;52:863–867. doi: 10.1203/00006450-200212000-00009. [DOI] [PubMed] [Google Scholar]
- 35.Garg A, Morduch J. Sibling rivalry and the gender gap: evidence from child health outcomes in Ghana. J. Popul. Econ. 1998;11:471–493. doi: 10.1007/s001480050080. [DOI] [PubMed] [Google Scholar]
- 36.Myatt M, et al. Children who are both wasted and stunted are also underweight and have a high risk of death: a descriptive epidemiology of multiple anthropometric deficits using data from 51 countries. Arch. Public Health. 2018;76:28. doi: 10.1186/s13690-018-0277-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Richard SA, et al. Wasting is associated with stunting in early childhood. J. Nutr. 2012;142:1291–1296. doi: 10.3945/jn.111.154922. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Stobaugh HC, et al. Children with poor linear growth are at risk for repeated relapse to wasting after recovery from moderate acute malnutrition. J. Nutr. 2018;148:974–979. doi: 10.1093/jn/nxy033. [DOI] [PubMed] [Google Scholar]
- 39.Perumal N, Bassani DG, Roth DE. Use and misuse of stunting as a measure of child health. J. Nutr. 2018;148:311–315. doi: 10.1093/jn/nxx064. [DOI] [PubMed] [Google Scholar]
- 40.Christian P, et al. Risk of childhood undernutrition related to small-for-gestational age and preterm birth in low- and middle-income countries. Int. J. Epidemiol. 2013;42:1340–1355. doi: 10.1093/ije/dyt109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Schlaudecker EP, Steinhoff MC, Moore SR. Interactions of diarrhea, pneumonia, and malnutrition in childhood: recent evidence from developing countries. Curr. Opin. Infect. Dis. 2011;24:496–502. doi: 10.1097/QCO.0b013e328349287d. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Checkley W, et al. Multi-country analysis of the effects of diarrhoea on childhood stunting. Int. J. Epidemiol. 2008;37:816–830. doi: 10.1093/ije/dyn099. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Black RE, et al. Maternal and child undernutrition: global and regional exposures and health consequences. Lancet. 2008;371:243–260. doi: 10.1016/S0140-6736(07)61690-0. [DOI] [PubMed] [Google Scholar]
- 44.Kosek M, et al. Fecal markers of intestinal inflammation and permeability associated with the subsequent acquisition of linear growth deficits in infants. Am. J. Trop. Med. Hyg. 2013;88:390–396. doi: 10.4269/ajtmh.2012.12-0549. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Investigators M-EN. Relationship between growth and illness, enteropathogens and dietary intakes in the first 2 years of life: findings from the MAL-ED birth cohort study. BMJ Glob. Health. 2017;2:e000370. doi: 10.1136/bmjgh-2017-000370. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Prentice AM, et al. Critical windows for nutritional interventions against stunting123. Am. J. Clin. Nutr. 2013;97:911–918. doi: 10.3945/ajcn.112.052332. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Georgiadis A, et al. Growth recovery and faltering through early adolescence in low- and middle-income countries: determinants and implications for cognitive development. Soc. Sci. Med. 2017;179:81–90. doi: 10.1016/j.socscimed.2017.02.031. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Bhutta ZA, et al. How countries can reduce child stunting at scale: lessons from exemplar countries. Am. J. Clin. Nutr. 2020;112:894S–904S. doi: 10.1093/ajcn/nqaa153. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Pontzer H, et al. Daily energy expenditure through the human life course. Science. 2021;373:808–812. doi: 10.1126/science.abe5017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Lassi ZS, et al. Effects of nutritional interventions during pregnancy on birth, child health and development outcomes: a systematic review of evidence from low- and middle-income countries. Campbell Syst. Rev. 2021;17:e1150. doi: 10.1002/cl2.1150. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Hambidge KM, et al. A multicountry randomized controlled trial of comprehensive maternal nutrition supplementation initiated before conception: the Women First trial. Am. J. Clin. Nutr. 2019;109:457–469. doi: 10.1093/ajcn/nqy228. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Callaghan-Gillespie M, et al. Trial of ready-to-use supplemental food and corn-soy blend in pregnant Malawian women with moderate malnutrition: a randomized controlled clinical trial. Am. J. Clin. Nutr. 2017;106:1062–1069. doi: 10.3945/ajcn.117.157198. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Argaw A, et al. Fortified balanced energy–protein supplementation during pregnancy and lactation and infant growth in rural Burkina Faso: a 2 × 2 factorial individually randomized controlled trial. PLoS Med. 2023;20:e1004186. doi: 10.1371/journal.pmed.1004186. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.WHO Recommendations on Antenatal Care for a Positive Pregnancy Experience (World Health Organization, 2016). [PubMed]
- 55.ter Kuile FO, van Eijk AM, Filler SJ. Effect of sulfadoxine-pyrimethamine resistance on the efficacy of intermittent preventive therapy for malaria control during pregnancy: a systematic review. JAMA. 2007;297:2603–2616. doi: 10.1001/jama.297.23.2603. [DOI] [PubMed] [Google Scholar]
- 56.Kayentao K, et al. Intermittent preventive therapy for malaria during pregnancy using 2 vs 3 or more doses of sulfadoxine-pyrimethamine and risk of low birth weight in Africa: systematic review and meta-analysis. JAMA. 2013;309:594–604. doi: 10.1001/jama.2012.216231. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Hendrixson DT, et al. A novel intervention combining supplementary food and infection control measures to improve birth outcomes in undernourished pregnant women in Sierra Leone: a randomized, controlled clinical effectiveness trial. PLoS Med. 2021;18:e1003618. doi: 10.1371/journal.pmed.1003618. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Hallamaa L, et al. Child health outcomes after presumptive infection treatment in pregnant women: a randomized trial. Pediatrics. 2018;141:e20172459. doi: 10.1542/peds.2017-2459. [DOI] [PubMed] [Google Scholar]
- 59.WHO Multicentre Growth Reference Study Group. WHO Child Growth Standards based on length/height, weight and age. Acta Paediatr.95, 76–85 (2006). [DOI] [PubMed]
- 60.World Health Organization & UNICEF. Recommendations for Data Collection, Analysis and Reporting on Anthropometric Indicators in Children Under 5 Years Old (World Health Organization, 2019).
- 61.Wood SN, Pya N, Säfken B. Smoothing parameter and model selection for general smooth models. J. Am. Stat. Assoc. 2016;111:1548–1563. doi: 10.1080/01621459.2016.1180986. [DOI] [Google Scholar]
- 62.Gasparrini A, Armstrong B, Kenward MG. Multivariate meta-analysis for non-linear and other multi-parameter associations. Stat. Med. 2012;31:3821–3839. doi: 10.1002/sim.5471. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Luedtke AR, van der Laan MJ. Super-learning of an optimal dynamic treatment rule. Int. J. Biostat. 2016;12:305–332. doi: 10.1515/ijb-2015-0052. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Jewell, N. P. Statistics for Epidemiology (Chapman & Hall/CRC, 2004).
- 65.Greenland S, Robins JM, Pearl J. Confounding and collapsibility in causal inference. Stat. Sci. 1999;14:29–46. doi: 10.1214/ss/1009211805. [DOI] [Google Scholar]
- 66.VanderWeele TJ, Shpitser I. A new criterion for confounder selection. Biometrics. 2011;67:1406–1413. doi: 10.1111/j.1541-0420.2011.01619.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Groenwold RHH, Palmer TM, Tilling K. To adjust or not to adjust? When a “confounder” is only measured after exposure. Epidemiology. 2021;32:194–201. doi: 10.1097/EDE.0000000000001312. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Stuart, E. A. Matching methods for causal inference: a review and a look forward. Stat. Sci. (2010). [DOI] [PMC free article] [PubMed]
- 69.McNutt L-A, Wu C, Xue X, Hafner JP. Estimating the relative risk in cohort studies and clinical trials of common outcomes. Am. J. Epidemiol. 2003;157:940–943. doi: 10.1093/aje/kwg074. [DOI] [PubMed] [Google Scholar]
- 70.Gruber, S. & van der Laan, M. Targeted Maximum Likelihood Estimation: A GentleIntroduction. Working Paper Series (Univ. California, Berkeley, Division of Biostatistics,2009).
- 71.VanderWeele TJ, Ding P. Sensitivity analysis in observational research: introducing the E-value. Ann. Intern. Med. 2017;167:268. doi: 10.7326/M16-2607. [DOI] [PubMed] [Google Scholar]
- 72.van der Laan, M., Polley, E. & Hubbard, A. Super Learner, Working Paper Series (Univ. California, Berkeley, Division of Biostatistics, 2007).
- 73.Tibshirani R. Regression shrinkage and selection via the lasso. J. R. Stat. Soc. B. 1996;58:267–288. [Google Scholar]
- 74.Hastie T, Tibshirani R. Generalized additive models. Stat. Sci. 1986;1:297–310. doi: 10.1177/096228029500400302. [DOI] [PubMed] [Google Scholar]
- 75.Chen, T. & Guestrin, C. XGBoost: a scalable tree boosting system. In Proc. 22nd ACM SIGKDD International Conference on Knowledge Discovery and Data Mining 785–794 (Association for Computing Machinery, 2016).
- 76.Kirch, W. (ed.) in Encyclopedia of Public Health 1117–1118 (Springer, 2008); 10.1007/978-1-4020-5614-7_2685.
- 77.Mertens, A. child-growth/ki-longitudinal-growth: Manuscript acceptance replication code. Zenodohttps://zenodo.org/badge/DOI/10.5281/zenodo.7937811.svg (2023).
- 78.Iqbal NT, et al. Promising biomarkers of environmental enteric dysfunction: a prospective cohort study in Pakistani children. Sci. Rep. 2018;8:2966. doi: 10.1038/s41598-018-21319-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79.Ali A, et al. Respiratory viruses associated with severe pneumonia in children under 2 years old in a rural community in Pakistan. J. Med. Virol. 2016;88:1882–1890. doi: 10.1002/jmv.24557. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 80.Shrestha PS, et al. Bhaktapur, Nepal: the MAL-ED Birth Cohort Study in Nepal. Clin. Infect. Dis. 2014;59:S300–S303. doi: 10.1093/cid/ciu459. [DOI] [PubMed] [Google Scholar]
- 81.Gladstone BP, et al. Protective effect of natural rotavirus infection in an Indian birth cohort. N. Engl. J. Med. 2011;365:337–346. doi: 10.1056/NEJMoa1006261. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 82.John SM, et al. Establishment of the MAL-ED Birth Cohort Study site in Vellore, Southern India. Clin. Infect. Dis. 2014;59:S295–S299. doi: 10.1093/cid/ciu390. [DOI] [PubMed] [Google Scholar]
- 83.Kattula D, et al. The first 1000 days of life: prenatal and postnatal risk factors for morbidity and growth in a birth cohort in southern India. BMJ Open. 2014;4:e005404. doi: 10.1136/bmjopen-2014-005404. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 84.Pathela P, et al. Diarrheal illness in a cohort of children 0–2 years of age in rural Bangladesh: I. Incidence and risk factors: risk factors for diarrhea in Bangladeshi children. Acta Paediatr. 2007;95:430–437. doi: 10.1111/j.1651-2227.2006.tb02257.x. [DOI] [PubMed] [Google Scholar]
- 85.Sarkar R, et al. Burden of childhood diseases and malnutrition in a semi-urban slum in southern India. BMC Public Health. 2013;13:87. doi: 10.1186/1471-2458-13-87. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 86.Ahmed T, et al. The MAL-ED Cohort Study in Mirpur, Bangladesh. Clin. Infect. Dis. 2014;59:S280–S286. doi: 10.1093/cid/ciu458. [DOI] [PubMed] [Google Scholar]
- 87.Colgate ER, et al. Delayed dosing of oral rotavirus vaccine demonstrates decreased risk of rotavirus gastroenteritis associated with serum zinc: a randomized controlled trial. Clin. Infect. Dis. 2016;63:634–641. doi: 10.1093/cid/ciw346. [DOI] [PubMed] [Google Scholar]
- 88.Bhandari N, et al. Food supplementation with encouragement to feed it to infants from 4 to 12 months of age has a small impact on weight gain. J. Nutr. 2001;131:1946–1951. doi: 10.1093/jn/131.7.1946. [DOI] [PubMed] [Google Scholar]
- 89.Bhandari N, et al. An educational intervention to promote appropriate complementary feeding practices and physical growth in infants and young children in rural Haryana, India. J. Nutr. 2004;134:2342–2348. doi: 10.1093/jn/134.9.2342. [DOI] [PubMed] [Google Scholar]
- 90.Korpe PS, et al. Natural history of cryptosporidiosis in a longitudinal study of slum-dwelling Bangladeshi children: association with severe malnutrition. PLoS Negl. Trop. Dis. 2016;10:e0004564. doi: 10.1371/journal.pntd.0004564. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 91.Christian P, et al. Effect of fortified complementary food supplementation on child growth in rural Bangladesh: a cluster-randomized trial. Int. J. Epidemiol. 2015;44:1862–1876. doi: 10.1093/ije/dyv155. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 92.West KP, et al. Effect of maternal multiple micronutrient vs iron–folic acid supplementation on infant mortality and adverse birth outcomes in rural Bangladesh: the JiVitA-3 randomized trial. JAMA. 2014;312:2649–2658. doi: 10.1001/jama.2014.16819. [DOI] [PubMed] [Google Scholar]
- 93.Steiner KL, et al. Species of cryptosporidia causing subclinical infection associated with growth faltering in rural and urban Bangladesh: a birth cohort study. Clin. Infect. Dis. 2018;67:1347–1355. doi: 10.1093/cid/ciy310. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 94.Mduma ER, et al. The etiology, risk factors, and interactions of enteric infections and malnutrition and the consequences for child health and development study (MAL-ED): description of the Tanzanian Site. Clin. Infect. Dis. 2014;59:S325–S330. doi: 10.1093/cid/ciu439. [DOI] [PubMed] [Google Scholar]
- 95.Locks LM, et al. Effect of zinc and multivitamin supplementation on the growth of Tanzanian children aged 6–84 wk: a randomized, placebo-controlled, double-blind trial. Am. J. Clin. Nutr. 2016;103:910–918. doi: 10.3945/ajcn.115.120055. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 96.Bessong PO, Nyathi E, Mahopo TC, Netshandama V. Development of the Dzimauli community in Vhembe district, Limpopo province of South Africa, for the MAL-ED cohort study. Clin. Infect. Dis. 2014;59:S317–S324. doi: 10.1093/cid/ciu418. [DOI] [PubMed] [Google Scholar]
- 97.Schoenbuchner SM, et al. The relationship between wasting and stunting: a retrospective cohort analysis of longitudinal data in Gambian children from 1976 to 2016. Am. J. Clin. Nutr. 2019;110:498–507. doi: 10.1093/ajcn/nqy326. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 98.Malaba LC, et al. Effect of postpartum maternal or neonatal vitamin A supplementation on infant mortality among infants born to HIV-negative mothers in Zimbabwe. Am. J. Clin. Nutr. 2005;81:454–460. doi: 10.1093/ajcn.81.2.454. [DOI] [PubMed] [Google Scholar]
- 99.Mangani C, et al. Effect of complementary feeding with lipid-based nutrient supplements and corn–soy blend on the incidence of stunting and linear growth among 6- to 18-month-old infants and children in rural Malawi. Matern. Child. Nutr. 2015;11:132–143. doi: 10.1111/mcn.12068. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 100.Hess SY, et al. Small-quantity lipid-based nutrient supplements, regardless of their zinc content, increase growth and reduce the prevalence of stunting and wasting in young Burkinabe children: a cluster-randomized trial. PLoS ONE. 2015;10:e0122242. doi: 10.1371/journal.pone.0122242. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 101.Valentiner-Branth P, et al. Community-based controlled trial of dietary management of children with persistent diarrhea: sustained beneficial effect on ponderal and linear growth. Am. J. Clin. Nutr. 2001;73:968–974. doi: 10.1093/ajcn/73.5.968. [DOI] [PubMed] [Google Scholar]
- 102.Yori PP, et al. Santa Clara de Nanay: the MAL-ED cohort in Peru. Clin. Infect. Dis. 2014;59:S310–S316. doi: 10.1093/cid/ciu460. [DOI] [PubMed] [Google Scholar]
- 103.Jaganath D, et al. First detected Helicobacter pylori infection in infancy modifies the association between diarrheal disease and childhood growth in Peru. Helicobacter. 2014;19:272–279. doi: 10.1111/hel.12130. [DOI] [PubMed] [Google Scholar]
- 104.Bégin F, Santizo M-C, Peerson JM, Torún B, Brown KH. Effects of bovine serum concentrate, with or without supplemental micronutrients, on the growth, morbidity, and micronutrient status of young children in a low-income, peri-urban Guatemalan community. Eur. J. Clin. Nutr. 2008;62:39–50. doi: 10.1038/sj.ejcn.1602682. [DOI] [PubMed] [Google Scholar]
- 105.Lima AAM, et al. Geography, population, demography, socioeconomic, anthropometry, and environmental status in the MAL-ED cohort and case-control study sites in Fortaleza, Ceará, Brazil. Clin. Infect. Dis. 2014;59:S287–S294. doi: 10.1093/cid/ciu438. [DOI] [PubMed] [Google Scholar]
- 106.Moore SR, et al. Early childhood diarrhoea and helminthiases associate with long-term linear growth faltering. Int. J. Epidemiol. 2001;30:1457–1464. doi: 10.1093/ije/30.6.1457. [DOI] [PubMed] [Google Scholar]
- 107.Checkley W, Epstein LD, Gilman RH, Cabrera L, Black RE. Effects of acute diarrhea on linear growth in Peruvian children. Am. J. Epidemiol. 2003;157:166–175. doi: 10.1093/aje/kwf179. [DOI] [PubMed] [Google Scholar]
- 108.Checkley W, et al. Effects of Cryptosporidium parvum infection in Peruvian children: growth faltering and subsequent catch-up growth. Am. J. Epidemiol. 1998;148:497–506. doi: 10.1093/oxfordjournals.aje.a009675. [DOI] [PubMed] [Google Scholar]
- 109.Kramer MS, et al. Promotion of Breastfeeding Intervention Trial (PROBIT): a randomized trial in the Republic of Belarus. JAMA. 2001;285:413–420. doi: 10.1001/jama.285.4.413. [DOI] [PubMed] [Google Scholar]
- 110.Becquey E, et al. Comparison of preventive and therapeutic zinc supplementation in young children in Burkina Faso: a cluster-randomized, community-based trial. J. Nutr. 2016;146:2058–2066. doi: 10.3945/jn.116.230128. [DOI] [PubMed] [Google Scholar]
- 111.WHO/CHD Immunisation-Linked Vitamin A Supplementation Study Group. Randomised trial to assess benefits and safety of vitamin A supplementation linked to immunisation in early infancy. Lancet. 1998;352:1257–1263. doi: 10.1016/S0140-6736(98)02487-8. [DOI] [PubMed] [Google Scholar]
- 112.Maleta KM, et al. Provision of 10–40 g/d lipid-based nutrient supplements from 6 to 18 months of age does not prevent linear growth faltering in Malawi. J. Nutr. 2015;145:1909–1915. doi: 10.3945/jn.114.208181. [DOI] [PubMed] [Google Scholar]
- 113.Ashorn P, et al. Supplementation of maternal diets during pregnancy and for 6 months postpartum and infant diets thereafter with small-quantity lipid-based nutrient supplements does not promote child growth by 18 months of age in rural Malawi: a randomized controlled trial. J. Nutr. 2015;145:1345–1353. doi: 10.3945/jn.114.207225. [DOI] [PubMed] [Google Scholar]
- 114.Thurstans S, et al. Boys are more likely to be undernourished than girls: a systematic review and meta-analysis of sex differences in undernutrition. BMJ Glob. Health. 2020;5:e004030. doi: 10.1136/bmjgh-2020-004030. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 115.Akombi BJ, et al. Stunting, wasting and underweight in sub-Saharan Africa: a systematic review. Int. J. Environ. Res. Public. Health. 2017;14:863. doi: 10.3390/ijerph14080863. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 116.Krebs NF, et al. Birth length is the strongest predictor of linear growth status and stunting in the first 2 years of life after a preconception maternal nutrition intervention: the children of the Women First trial. Am. J. Clin. Nutr. 2022;116:86–96. doi: 10.1093/ajcn/nqac051. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 117.Villar J, et al. International standards for newborn weight, length, and head circumference by gestational age and sex: the Newborn Cross-Sectional Study of the INTERGROWTH-21st Project. Lancet. 2014;384:857–868. doi: 10.1016/S0140-6736(14)60932-6. [DOI] [PubMed] [Google Scholar]
- 118.Katoch OR. Determinants of malnutrition among children: a systematic review. Nutrition. 2021;96:111565. doi: 10.1016/j.nut.2021.111565. [DOI] [PubMed] [Google Scholar]
- 119.Li Z, Kim R, Vollmer S, Subramanian SV. Factors associated with child stunting, wasting, and underweight in 35 low- and middle-income countries. JAMA Netw. Open. 2020;3:e203386. doi: 10.1001/jamanetworkopen.2020.3386. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 120.Olusanya BO, Renner JK. Is home birth a marker for severe malnutrition in early infancy in urban communities of low-income countries? Matern. Child. Nutr. 2012;8:492–502. doi: 10.1111/j.1740-8709.2011.00330.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 121.Espo M, et al. Determinants of linear growth and predictors of severe stunting during infancy in rural Malawi. Acta Paediatr. 2002;91:1364–1370. doi: 10.1111/j.1651-2227.2002.tb02835.x. [DOI] [PubMed] [Google Scholar]
- 122.Özaltin E, Hill K, Subramanian SV. Association of maternal stature with offspring mortality, underweight, and stunting in low- to middle-income countries. JAMA. 2010;303:1507–1516. doi: 10.1001/jama.2010.450. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 123.Astuti FD, Azka A, Rokhmayanti R. Maternal age correlation of stunting in children: systematics review. J. Matern. Child Health. 2022;7:479–448. doi: 10.26911/thejmch.2022.07.04.11. [DOI] [Google Scholar]
- 124.Verma P, Prasad JB. Stunting, wasting and underweight as indicators of under-nutrition in under five children from developing countries: a systematic review. Diabetes Metab. Syndr. Clin. Res. Rev. 2021;15:102243. doi: 10.1016/j.dsx.2021.102243. [DOI] [PubMed] [Google Scholar]
- 125.Vollmer S, Bommer C, Krishna A, Harttgen K, Subramanian S. The association of parental education with childhood undernutrition in low- and middle-income countries: comparing the role of paternal and maternal education. Int. J. Epidemiol. 2017;46:312–323. doi: 10.1093/ije/dyw133. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 126.Vaivada T, et al. Stunting in childhood: an overview of global burden, trends, determinants, and drivers of decline. Am. J. Clin. Nutr. 2020;112:777S–791S. doi: 10.1093/ajcn/nqaa159. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 127.Mensch BS, Chuang EK, Melnikas AJ, Psaki SR. Evidence for causal links between education and maternal and child health: systematic review. Trop. Med. Int. Health. 2019;24:504–522. doi: 10.1111/tmi.13218. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 128.Rachmi CN, Agho KE, Li M, Baur LA. Stunting, underweight and overweight in children aged 2.0–4.9 years in Indonesia: prevalence trends and associated risk factors. PLoS ONE. 2016;11:e0154756. doi: 10.1371/journal.pone.0154756. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 129.Shah PS, Zao J, Ali S. Maternal marital status and birth outcomes: a systematic review and meta-analyses. Matern. Child Health J. 2011;15:1097–1109. doi: 10.1007/s10995-010-0654-z. [DOI] [PubMed] [Google Scholar]
- 130.Abate, K., Assen, W., Ali, R., Kalkidan, H. & Waseyehon, A.w.h. Varying relationship of wealth of households and stunting of under five children in sub-Saharan Africa: a meta-analysis of demographic health surveys. EC Nutrition14.8, 616–623 (2019).
- 131.Manley J, et al. Cash transfers and child nutritional outcomes: a systematic review and meta-analysis. BMJ Glob. Health. 2020;5:e003621. doi: 10.1136/bmjgh-2020-003621. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 132.Ballard, T., Coates, J., Swindale, A. & Deitchler, M. Household Hunger Scale: Indicator Definition and Measurement Guide (USAID, FANTA, FHI 360, 2011).
- 133.Coates, J., Webb, P. & Houser, R. Measuring Food Insecurity: Going Beyond Indicators of Income and Anthropometry (USAID, FANTA, FHI 360, 2003).
- 134.Coates, J., Swindale, A. & Bilinsky, P. Household Food Insecurity Access Scale (HFIAS) for Measurement of Food Access: Indicator Guide Version 3 (USAID, FANTA, FHI 360, 2007).
- 135.Moradi S, et al. Food insecurity and the risk of undernutrition complications among children and adolescents: a systematic review and meta-analysis. Nutrition. 2019;62:52–60. doi: 10.1016/j.nut.2018.11.029. [DOI] [PubMed] [Google Scholar]
- 136.Ayelign A, Zerfu T. Household, dietary and healthcare factors predicting childhood stunting in Ethiopia. Heliyon. 2021;7:e06733. doi: 10.1016/j.heliyon.2021.e06733. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 137.Benjamin-Chung J, et al. Household finished flooring and soil-transmitted helminth and Giardia infections among children in rural Bangladesh and Kenya: a prospective cohort study. Lancet Glob. Health. 2021;9:e301–e308. doi: 10.1016/S2214-109X(20)30523-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 138.Bruce NG, et al. Control of household air pollution for child survival: estimates for intervention impacts. BMC Public Health. 2013;13:S8. doi: 10.1186/1471-2458-13-S3-S8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 139.Woolley KE, et al. Effectiveness of interventions to reduce household air pollution from solid biomass fuels and improve maternal and child health outcomes in low- and middle-income countries: a systematic review and meta-analysis. Indoor Air. 2022;32:e12958. doi: 10.1111/ina.12958. [DOI] [PubMed] [Google Scholar]
- 140.Wong HJ, Moy FM, Nair S. Risk factors of malnutrition among preschool children in Terengganu, Malaysia: a case control study. BMC Public Health. 2014;14:785. doi: 10.1186/1471-2458-14-785. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 141.Aiga H, et al. Risk factors for malnutrition among school-aged children: a cross-sectional study in rural Madagascar. BMC Public Health. 2019;19:773. doi: 10.1186/s12889-019-7013-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 142.Abatzoglou JT, Dobrowski SZ, Parks SA, Hegewisch KC. TerraClimate, a high-resolution global dataset of monthly climate and climatic water balance from 1958–2015. Sci. Data. 2018;5:170191. doi: 10.1038/sdata.2017.191. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 143.Lieber M, Chin-Hong P, Kelly K, Dandu M, Weiser SD. A systematic review and meta-analysis assessing the impact of droughts, flooding, and climate variability on malnutrition. Glob. Public Health. 2022;17:68–82. doi: 10.1080/17441692.2020.1860247. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 144.Le K, Nguyen M. In-utero exposure to rainfall variability and early childhood health. World Dev. 2021;144:105485. doi: 10.1016/j.worlddev.2021.105485. [DOI] [Google Scholar]
- 145.Brown ME, et al. Empirical studies of factors associated with child malnutrition: highlighting the evidence about climate and conflict shocks. Food Secur. 2020;12:1241–1252. doi: 10.1007/s12571-020-01041-y. [DOI] [Google Scholar]
- 146.Phalkey RK, Aranda-Jan C, Marx S, Höfle B, Sauerborn R. Systematic review of current efforts to quantify the impacts of climate change on undernutrition. Proc. Natl Acad. Sci. USA. 2015;112:E4522–E4529. doi: 10.1073/pnas.1409769112. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 147.Susianto, S. C., Suprobo, N. R. & Maharani, M. Early breastfeeding initiation effect in stunting: a systematic review. Asian J. Health Res.10.55561/ajhr.v1i1.11 (2022).
- 148.Smith ER, et al. Delayed breastfeeding initiation is associated with infant morbidity. J. Pediatr. 2017;191:57–62.e2. doi: 10.1016/j.jpeds.2017.08.069. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 149.Mardani RAD, Wu W-R, Nhi VT, Huang H-C. Association of breastfeeding with undernutrition among children under 5 years of age in developing countries: a systematic review and meta-analysis. J. Nurs. Scholarsh. 2022;54:692–703. doi: 10.1111/jnu.12799. [DOI] [PubMed] [Google Scholar]
- 150.Richard SA, et al. Diarrhea in early childhood: short-term association with weight and long-term association with length. Am. J. Epidemiol. 2013;178:1129–1138. doi: 10.1093/aje/kwt094. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
This file contains Supplementary Notes 1–7
Data Availability Statement
The data that support the findings of this analysis are a combination of data from multiple principal investigators and institutions. The data are available, upon reasonable request, to the requestor by contacting the individual principal investigators. The individuals and the contact information to help the requestor obtain access to the data are listed at https://www.synapse.org/#!Synapse:syn51570682/wiki/. The analysis dataset is at https://www.synapse.org/#!Synapse:syn51570682/datasets/. This dataset is access controlled and not available publicly for privacy reasons.
Code used in the study has been deposited at Zenodo: https://zenodo.org/record/793781177.