Abstract
This abstract provides an overview of metachromatic leukodystrophy (MLD), an autosomal recessive disorder stemming from arylsulfatase A deficiency. MLD leads to cerebroside sulfate accumulation, causing central and peripheral demyelination. Clinical manifestations vary by age group: late-infantile (rapid progression), juvenile (slower progression), and adult-onset (psychiatric symptoms). A case study details a 23-year-old with progressive vision impairment, motor weakness, and cognitive changes. Examination and MRI findings led to suspicion of MLD, later confirmed by enzyme testing. Optic nerve involvement is emphasized, along with diagnostic criteria involving enzyme assays, imaging, and urinary sulfatide excretion tests. While no cure exists, symptomatic and supportive care, including hematopoietic stem cell transplantation, remains key in MLD management.
Keywords: Adult onset, Metachromatic leukodystrophy (MLD), Optic atrophy, Motor weakness
Introduction and importance
Metachromatic leukodystrophy is an autosomal recessive, inherited metabolic disease caused by the deficient activity of arylsulfatase A (ASA) [1]. ASA is a lysosomal enzyme responsible for the de-sulfation of cerebroside sulfate, a major glycolipid of myelin. Decreased ASA activity leads to the accumulation of cerebroside sulfate in the CNS and peripheral nerves (as well as the kidneys and other visceral organs), resulting in central and peripheral demyelination [2]. MLD is named from the presence of metachromatic granules in the affected cells, formed as a result of the accumulation of sulfatide and sphingolipids in myelin [3]. The type and severity of the symptoms of MLD correlate with the levels of residual ASA activity [4,5]. There are 3 main clinical types of MLD which are as follows: late-infantile (age of onset before 30 months), juvenile (age of onset between 2.5 and 16 years), and adult-onset (age of onset after 16 years) [6]. Peripheral neuropathy occurs in all forms of MLD. Late infantile onset is associated with poor prognosis (death typically occurs within 5-6 years) and manifests as regression of motor skills, gait abnormalities, seizures, ataxia, hypotonia, extensor plantar and optic atrophy. Juvenile type of MLD usually presents with the same pattern but with slower progression, with the possibility of survival until early adulthood. Adult onset MLD is associated with dementia, behavioral difficulties, and psychosis [7]. Here we present a case of adult-onset Metachromatic leukodystrophy with optic atrophy and motor symptoms.
Case details
A 23-year-old lady presented to our center in need of diagnostic imaging and further evaluation as a referral. She was in her usual state of health 4 months back when she started having problems with her vision. The patient experienced the blurring of vision which was gradual, progressive, and affected both her eyes. The symptom was painless, was not associated with redness in the eye, discharge, or abnormal sensation in the eye, and was not preceded by any trauma or intervention involving the eye. She had a history of similar visual disturbances remitting and relapsing intermittently over the last 3 years. The symptoms were limited to the right eye in the past, unlike this episode which involved both eyes. She visited an eye hospital, where an evaluation of her symptoms unveiled bilateral optic nerve atrophies. Subsequently, she was referred to our center for potential magnetic resonance imaging (MRI) of the head. A comprehensive patient history was acquired to gather additional insights into the diagnosis of her peculiar symptoms. The patient described that she had been experiencing unusual symptoms for many years which were intermittent but had frequent occurrences. She recounted an episode of inability to move both her legs approximately 12 years back. These symptoms coincided with slurring of her speech which led to difficulty speaking. The symptoms improved over time without any medical interventions only to resurface periodically. At present, she is experiencing weakness in her right leg, leading to challenges in walking. She was unable to recall any potential triggers for her symptoms. There is no record of previous headaches, loss of consciousness, irregular body movements, abnormal sensations, or sensory loss throughout her body. Her hearing has never posed any issues. No instances of past trauma were identified. Bowel and bladder control have never been compromised. The systemic review revealed no notable findings. She was delivered at home through a spontaneous vaginal birth and did not receive standard postnatal care. Her immunizations were administered by the prevailing national immunization schedule. Her parents observed her developmental milestones aligning with those of her siblings. Menarche occurred at the age of 13, and she has been experiencing regular menstrual cycles. There are no documented diagnosed comorbidities, and her family history does not indicate any comorbidities. The patient has never undergone surgical procedures. According to her parents, when her symptoms emerged, she exhibited withdrawal from other family members, displayed signs of anxiety, and frequently experienced mood fluctuations. Additionally, she displayed forgetfulness and struggled with maintaining focus. Consequently, she discontinued her studies, which the family believed to be a way to avoid potential embarrassment due to her motor symptoms at school.
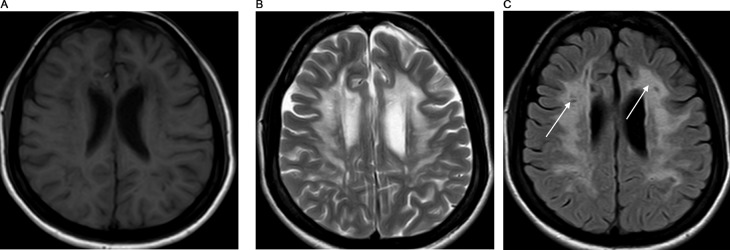
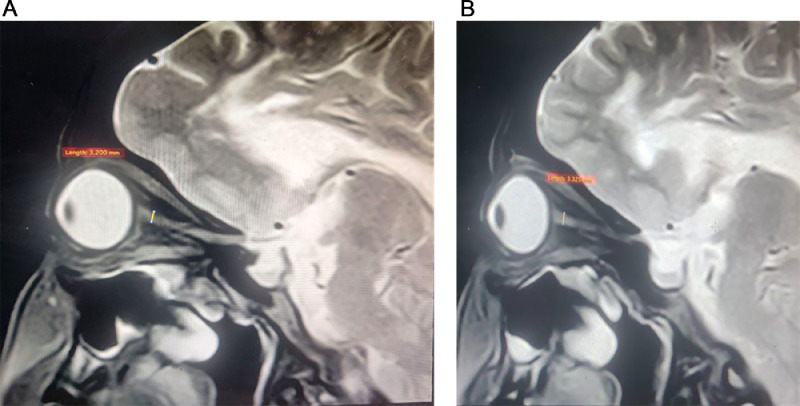
During the examination, the patient displayed a slender physique and exhibited a clear orientation to time, place, and person. No facial dysmorphism was observed. There were no indications of pallor, jaundice, lymph node enlargement, or dehydration. Moreover, the patient maintained stable vital signs. The assessment of visual acuity revealed a measurement of 6/60 in both eyes. Color vision demonstrated impairment, and the visual fields appeared narrowed. The corneas exhibited clarity, while bilateral optic disc pallor was observed. Sensations were found to be within normal limits across all 4 limbs; however, hypotonia and reduced power (graded as 3/5) were observed in the right hip and knee muscles. The patient displayed emotional instability. Given the history and the findings from the examination, there arose a suspicion of a progressive neurodegenerative disorder. Subsequently, routine investigations were conducted, including an MRI. MRI revealed T2/FLAIR confluent hyperintensity in the bilateral cerebral periventricular and deep white matter of frontoparietal lobes giving a tigroid appearance of the lesion (Figs. 1A–C). The reduced diameter of the bilateral optic nerve sheath diameter is noted measuring 3.2 mm on the right and 3.3 mm on the left side (Figs. 2A and B). These findings strongly indicated a rare diagnosis of metachromatic leukodystrophy. This diagnosis was corroborated by evaluating the arylsulfatase A (ARSA) enzyme level in the leukocytes, which displayed a discernible reduction in concentration. Additional investigation regarding any history of consanguinity was conducted, yet the family denied its presence.
Fig. 1.
(A) T1 weighted axial MRI image at the lateral ventricle level showing low signal intensity in the periventricular and deep white matter of the bilateral frontal lobe. (B) T2 weighted axial image at the level of lateral ventricle showing high signal intensity in the bilateral periventricular and deep white matter with low signal stripes in between giving a “tigroid” pattern. (C) FLAIR weighted axial image at the level of the lateral ventricle showing the high signal intensity in the periventricular and deep white matter of the bilateral frontoparietal lobe with low signal stripes within ( white arrow).
Fig. 2.
(A) Oblique coronal T2 weighted image of right orbit showing the measurement of optic nerve sheath diameter. (B) Oblique coronal T2 weighted image of left orbit showing the measurement of the left optic nerve sheath diameter.
The patient and her family were told about the diagnosis, possible genetic implications, and its prognosis. The family was made aware of the modalities of management of the condition and was explained that a “cure” was not to be expected. Physiotherapy and regular follow-up were suggested to facilitate the family as the disease progressed. Both the patient and her family understood that she had a disease that had impaired several aspects of her life and nothing in their lifestyle was responsible for its causation. They came to terms with the irreversible nature of the disease and were willing to follow up to understand any new symptoms and comfort the patient in the best way possible.
Clinical discussion
Metachromatic leukodystrophy (MLD) is a rare, autosomal recessive disorder caused by the deficient activity of Arylsulfatase A (ASA), leading to the accumulation of cerebroside sulfate in the central nervous system (CNS) and peripheral nerves, resulting in central and peripheral neuropathy [1]. The accumulation of cerebroside sulfate (also termed metachromatic material) occurs in Schwann cells and endo-neural macrophages and is termed sulfatides. These accumulated products lower the cerebroside-sulfatide ratio in myelin composition disrupting the myelin metabolism. Ultimately, the Schwann cells and phagocytes die, and the demyelination of myelin CNS, and peripheral nerves occurs [8]. MLD occurs with an estimated frequency of 1:40,000 [9,10]. Up to 16% of the general population may have an ARSA deficiency [9].
There are 3 main clinical types of MLD which are as follows: late-infantile (age of onset before 30 months), juvenile (age of onset between 2.5 and 16 years), and adult onset (age of onset after 16 years) [6]. The type and severity of the symptoms of MLD correlate with the levels of residual ASA activity [4,5].
The most common type of MLD is late infantile, marked by ataxia, walking loss, hypotonia, and abnormal reflexes. Progression includes tetraplegia, bulbar paralysis, optic nerve atrophy, CNS involvement, and mental impairment, often leading to death by age 4 or 5. ASA activity is markedly low. Juvenile MLD involves mental changes, euphoria, behavioral shifts, language difficulties, and slower motor symptoms with spastic tetraplegia, ataxia, seizures, and optic nerve atrophy. ASA activity remains lower, though not as in the late infantile type [8,9]. The adult type of MLD may present with 2 sets of manifestations: either motor (pyramidal cerebellar syndrome) or psychiatric manifestations. Psychiatric manifestations may include psychosis, auditory hallucinations, delusions, altered thought processes, and catatonia. Once the disease progresses, it may manifest as seizures, chorea, and dystonia [11]. Adult onset MLD is the least common form of MLD, and it is often mistakenly diagnosed as early-onset dementia or schizophrenia because of its slow progression. Slow disease progression with periods of relative stability and regression is typical of an adult-onset MLD [12]. The presence of psychiatric manifestations is due to the impairment in the process of myelination, especially in the frontotemporal area of the brain leading to alterations in the structure and function of the central nervous system, thus showing symptoms similar to schizophrenia [11].
Optic nerve involvement is usually seen in the late-infantile and juvenile types of MLD (hernnadez-1,2,6). Optic nerve atrophy in adult-onset MLD is rare and there are very few case reports on the same [13,14]. A certain degree of optic nerve involvement is reported in MLD. It is probably due to the accumulation of metachromatic deposits in the retinal ganglionic cells, and optic and ciliary nerves resulting in optic nerve atrophy and blindness, later as the disease progresses [15]. Besides this, other ocular manifestations include skew deviation, retinal degeneration, and cherry red spots. Abnormal grayness in the parafoveal area is also reported [16]. Besides the accumulation of sulfatides, other factors also play a role in the neuronal cell degradation and apoptosis in the optic nerve like mislocalization of abnormal lipids and inflammatory processes [17]. There have been reports of Light microscopic and Ultrastructural studies of internal structures of the eye, demonstrating accumulates in the retinal ganglion, inner plexiform, internal granular, and neuroepithelial layers of the retina. Various cytoplasmic inclusions have also been demonstrated in the optic nerve and ciliary nerves [16].
Diagnosis of MLD is established by demonstrating the deficient activity of ASA enzyme in leucocytes (white cell enzyme testing) or cultured skin fibroblasts. One must also consider the pseudo-deficiency state, where the activity of the ASA enzyme is low but there are no manifestations of MLD. In a pseudo-deficiency state, the activity of the ASA enzyme is typically 5%-20% of the controls [18]. In patients of MLD, the urine excretion of sulfatides is increased from 10-fold to 100-fold, a phenomenon that does not occur in a pseudo-deficiency state. So, the urine sulfatide excretion test can be used to differentiate actual deficiency from the pseudo-deficiency state. However, this test is obsolete nowadays [18]. Besides this, supportive evidence can be obtained from MRI of the brain, nerve conduction velocity studies (to demonstrate slowed conduction from peripheral nerves), lumbar puncture (to test for high protein concentrates), and sural nerve biopsy (to demonstrate the deposition of metachromatic lipids) for establishing the diagnosis of MLD [10,19].
Brain MRI in MLD reveals hyperintense areas of diffuse, bilateral, and often symmetrical demyelination in the periventricular white matter and cerebellum that may converge with disease progression, with frontal predominance in the late stages (juvenile forms and in adults) [11]. The basal ganglia and thalamus are also involved later in the course of the disease [13]. On computed tomography (CT), hyper-densities are observed in white matter, particularly in the frontal and parietal regions with cortical and subcortical atrophy accompanied by ventricular dilatation as the disease progresses. Areas of hypodensity reflect the loss of myelination and cerebroside accumulation [9].
MLD is a noncurable disorder. However, allogenic hematopoietic stem cell transplantation (HSCT) is known to show a positive response in slowing the progression of the disease. Other options include gene therapy and enzyme replacement therapy, but the results are inconclusive [18]. So, the indicated treatment for MLD is symptomatic and supportive which includes physiotherapy, respiratory therapy, nutrition, nursing care, adaptation to the environment, and maintaining social support. Antiepileptics and antispasmodics can be used when indicated [10].
Conclusion
This case report highlights the diverse clinical presentations and diagnostic challenges of MLD, focusing on adult-onset and optic atrophy. It emphasizes the need for a comprehensive approach, including clinical evaluation, enzyme assays, imaging, and supportive tests. Adult-onset MLD is rare, highlighting the need for heightened awareness and consideration. Supportive care, including symptomatic management and potential interventions like hematopoietic stem cell transplantation, is crucial. Further research and collaboration are needed for better understanding and management of this complex disorder.
Financial support and sponsorship
None.
Author contributions
Shailendra Katwal: Conceptualization, as mentor and reviewer for this case report and for data interpretation. Sundar Suwal: Contributed to performing literature review and editing. Suman Lamichhane: Contributed to writing the paper and reviewer for this case. Amrit Bhusal: Contributed to writing the paper. Aastha Ghimire: Contributed to writing the paper. All authors have read and approved the manuscript.
Registration of research studies
Not applicable.
Guarantor
Shailendra Katwal.
Provenance and peer review
Not commissioned, externally peer reviewed.
Ethical approval
This case report did not require review by the ethical committee.
Patient consent
Written informed consent was obtained from the patient for publication of this case report and accompanying images. A copy of the written consent is available for review by the Editor-in-Chief of this journal on request.
Footnotes
Acknowledgments: None.
Competing Interests: The authors declare that they have no known competing financial interests or personal relationships that could have appeared to influence the work reported in this paper.
References
- 1.Austin JH, Balasubramanian AS, Pattabiraman TN, Saraswathi S, Basu DK, Bachhawat BK. A controlled study of Enzymic activities in three human disorders of glycolipid metabolism. J Neurochem. 1963;10(11):805–816. doi: 10.1111/j.1471-4159.1963.tb11905.x. [DOI] [PubMed] [Google Scholar]
- 2.Sedel F, Tourbah A, Fontaine B, Lubetzki C, Baumann N, Saudubray J-M, et al. Leukoencephalopathies associated with inborn errors of metabolism in adults. J Inherit Metab Dis. 2008;31:295–307. doi: 10.1007/s10545-008-0778-0. [DOI] [PubMed] [Google Scholar]
- 3.Shaimardanova AA, Chulpanova DS, Solovyeva VV, Mullagulova AI, Kitaeva KV, Allegrucci C, et al. Metachromatic leukodystrophy: diagnosis, modeling, and treatment approaches. Front Med (Lausanne) 2020;7 doi: 10.3389/fmed.2020.576221. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Luijten JA, Straks W, Blikkendaal-Lieftinck LF, Staal GE, Willemse J. Metachromatic leukodystrophy: a comparative study of the ultrastructural findings in the peripheral nervous system of three cases, one of the late infantile, one of the juvenile and one of the adult form of the disease. Neuropadiatrie. 1978;9(4):338–350. doi: 10.1055/s-0028-1091493. [DOI] [PubMed] [Google Scholar]
- 5.van Rappard DF, Boelens JJ, Wolf NI. Metachromatic leukodystrophy: disease spectrum and approaches for treatment. Best Pract Res Clin Endocrinol Metab. 2015;29(2):261–273. doi: 10.1016/j.beem.2014.10.001. [DOI] [PubMed] [Google Scholar]
- 6.Bubis JJ, Adlesberg L. Congenital metachromatic leukodystrophy. Report of a case. Acta Neuropathol. 1966;6(3):298–302. doi: 10.1007/BF00687860. [DOI] [PubMed] [Google Scholar]
- 7.Rauschka H, Colsch B, Baumann N, Wevers R, Schmidbauer M, Krammer M, et al. Late-onset metachromatic leukodystrophy: genotype strongly influences phenotype. Neurology. 2006;67:859–863. doi: 10.1212/01.wnl.0000234129.97727.4d. [DOI] [PubMed] [Google Scholar]
- 8.Beerepoot S, Nierkens S, Boelens JJ, Lindemans C, Bugiani M, Wolf NI. Peripheral neuropathy in metachromatic leukodystrophy: current status and future perspective. Orphanet J Rare Dis. 2019;14:240. doi: 10.1186/s13023-019-1220-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Black DN, Taber KH, Hurley RA. Metachromatic leukodystrophy: a model for the study of psychosis. J Neuropsychiatry Clin Neurosci. 2003;15:3. doi: 10.1176/jnp.15.3.289. [DOI] [PubMed] [Google Scholar]
- 10.Espejo LM, de la Espriella R, Hernandez JF. Metachromatic leukodystrophy. Case presentation. Rev Colomb Psiquiat. 2017;46(1):44–49. doi: 10.1016/j.rcp.2016.05.001. [DOI] [PubMed] [Google Scholar]
- 11.Walterfang M, Bonnot O, Mocellin R, Velakoulis D. The neuropsychiatry of inborn errors of metabolism. J Inherit Metab Dis. 2013;36:687–702. doi: 10.1007/s10545-013-9618-y. [DOI] [PubMed] [Google Scholar]
- 12.Shaimardanova AA, Chulpanova DS, Solovyeva VV, Mullagulova AI, Kitaeva KV, Allegrucci C, et al. Metachromatic leukodystrophy: diagnosis, modeling, and treatment approaches. Front Med. 2020;7 doi: 10.3389/fmed.2020.576221. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Roi D, Mankad K, Kaliakatsos M, Cleary M, Manzur A, D'Arco F. Thickening of the optic nerves in metachromatic leucodystrophy: a new MRI finding. Neuroradiol J. 2016;29(2):134–136. doi: 10.1177/1971400916633479. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Goebel HH, Shimokawa K, Argyrakis A, Pilz H. The ultrastructure of the retina in adult metachromatic leukodystrophy. Am J Ophthalmol. 1978;85:841–849. doi: 10.1016/s0002-9394(14)78115-2. [DOI] [PubMed] [Google Scholar]
- 15.Libert J, Van Hoof F, Toussaint D, Roozitalab H, Kenyon KR, Green WR. Ocular findings in metachromatic leukodystrophy. An electron microscopic and enzyme study in clinical and genetic variants. Arch Ophthalmol. 1979;97:1495–1504. doi: 10.1001/archopht.1979.01020020157015. [DOI] [PubMed] [Google Scholar]
- 16.Scott IU, Green WR, Goyal AK, de la Cruz Z, Naidu S, Moser H. New sites of ocular involvement in late-infantile metachromatic leukodystrophy revealed by histopathologic studies. Graefe's Arch Clin Exp Ophthalmol. 1993;231 doi: 10.1007/BF00920946. [DOI] [PubMed] [Google Scholar]
- 17.Sevin C, Aubourg P, Cartier N. Enzyme, cell and gene based therapies for metachromatic leukodystrophy. J Inherit Metab Dis. 2007;30:175–183. doi: 10.1007/s10545-007-0540-z. [DOI] [PubMed] [Google Scholar]
- 18.Lynch DS, Wade C, de Paiva ARB, John N, Kinsella JA, Merwick Á, et al. Practical approach to the diagnosis of adult-onset leukodystrophies: an updated guide in the genomic era. J Neurol Neurosurg Psychiatry. 2019;90:543–555. doi: 10.1136/jnnp-2018-319481. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Quigley HA, Green WR. Clinical and ultrastructural ocular histopathologic studies of adult-onset metachromatic leukodystrophy. Am J Ophthalmol. 1976;82:472–479. doi: 10.1016/0002-9394(76)90497-9. [DOI] [PubMed] [Google Scholar]