SUMMARY
BACKGROUND:
Disseminated tuberculosis (TB) is a major cause of death in patients with the acquired immune-deficiency syndrome (AIDS), but its pathogenesis and clinical features have not been defined prospectively.
METHODS:
Human immunodeficiency virus (HIV) infected adults with a CD4 count ⩾ 200 cells/μl and bacille Calmette-Guérin scar underwent immunologic evaluation and subsequent follow-up.
RESULTS:
Among 20 subjects who developed disseminated TB, baseline tuberculin skin tests were ⩾15 mm in 14 (70%) and lymphocyte proliferative responses to Mycobacterium tuberculosis were positive in 14 (70%). At the time of diagnosis, fever ⩾2 weeks plus ⩾5 kg weight loss was reported in 16 (80%) patients, abnormal chest X-rays in 7/17 (41%), and positive sputum cultures in 10 (50%); median CD4 count was 30 cells/μl (range 1–122). By insertion sequence (IS) 6110 analysis, 14 (70%) blood isolates were clustered and 3/8 (37%) concurrent sputum isolates represented a different strain (polyclonal disease). Empiric TB treatment was given to eight (40%) patients; 11 (55%) died within a month.
CONCLUSIONS:
Disseminated TB in HIV occurs with cellular immune responses indicating prior mycobacterial infection, and IS6110 analysis suggests an often lethal combination of reactivation and newly acquired infection. Control will require effective prevention of both remotely and recently acquired infection, and wider use of empiric therapy in patients with advanced AIDS and prolonged fever.
Keywords: disseminated tuberculosis, HIV infection, Tanzania, malaria
RÉSUMÉ
CONTEXTE:
La tuberculose (TB) disséminée est une cause majeure de décès chez les patients atteints du syndrome d’immuno-déficience acquise (SIDA), mais sapathogénie et ses caractéristiques cliniques n’ont pas été définies de manière prospective.
MÉTHODES:
Une évaluation immunologique et un suivi ultérieur ont été menés chez des adultes infectés par le virus de l’immunodéficience humaine (VIH) dont le décompte des cellules CD4 était ⩾ 200 cellules/μl et qui étaient porteurs d’une cicatrice de bacille Calmette-Guérin.
RÉSULTATS:
Parmi 20 sujets qui ont développé ultérieurement une TB disséminée, les tests cutanés tuberculiniques étaient ⩾15 mm chez 14 patients (70%) et les réponses prolifératives lymphocytaires à l’égard de Mycobacterium tuberculosis positives chez 14 patients (70%). Au moment du diagnostic, on a signalé une fièvre durant ⩾2 semaines et une perte de poids ⩾5 kilos chez 16 patients (80%), des anomalies des clichés thoraciques chez 7/17 (41%), et des cultures de crachats positives chez 10 (50%); le décompte médian des cellules CD4 a été de 30 (extrêmes 1–122). Lors de l’analyse de sequence d’insertion (IS) 6110, on a noté un regroupement en grappes dans 14 isolats de sang (70%), et dans 3/8 (37%) isolats correspondants des crachats la souche a été différente (maladie polyclonale). Le traitement empirique de la TB a été administré à 8 patients (40%) et le décès est survenu dans le mois chez 11 patients (55%).
CONCLUSION:
En cas d’infection VIH, la TB disséminée s’accompagne de réponses immunitaires cellulaires qui indiquent une infection antérieure par les mycobactéries et d’une analyse IS6110 qui suggère une combinaison souvent létale de réactivation et de réinfection récemment acquise. La maîtrise de la maladie exigera une prévention effective des infections à la fois éloignées et récentes ainsi qu’une utilisation plus large d’une thérapie empirique chez les patients atteints d’un SIDA au stade avancé et d’une fi èvre prolongée.
RESUMEN
MARCO DE REFERENCIA:
La tuberculosis (TB) diseminada es una de las principales causas de defunción en los pacientes con el síndrome de inmunodeficiencia adquirida (SIDA), pero no se han defi nido en forma prospectiva su patogénesis y características clínicas.
MÉTODOS:
Se llevó a cabo una evaluación y seguimiento del estado inmunitario de los adultos infectados por el virus de la inmunodeficiencia humana (VIH), que presentaban un recuento de células CD4 ⩾ 200 células/μl y una cicatriz de vacunación antituberculosa.
RESULTADOS:
De las 20 personas que presentaron TB diseminada, en 14 (70%) la respuesta inicial a la tuberculina fue ⩾15 mm y la respuesta de proliferación de los linfocitos a Mycobacterium tuberculosis fue positiva en 14 pacientes (70%). En el momento del diagnóstico, 16 pacientes (80%) refirieron fiebre de ⩾2 semanas y pérdida de peso de ⩾5 kg, se observaron imágenes anormales en la radiografía de tórax en 7 de los 17 pacientes examinados (41%) y se obtuvieron cultivos de esputo positivos en 10 (50%); la mediana del recuento de células CD4 fue 30 (entre 1 y 122). Mediante el análisis del elemento de inserción (IS) 6110 se agruparon en conglomerados 14 aislados de muestras sanguíneas (70%) y 3/8 aislados simultáneos (37%) obtenidos de las muestras de esputo correspondieron a una cepa diferente (enfermedad policlonal). Se administró tratamiento antituberculoso en forma empírica a 8 pacientes (40%); 11 pacientes fallecieron en el lapso de un mes (55%).
CONCLUSIONES:
En los pacientes con infección por el VIH que presentan TB diseminada, la respuesta inmunitaria de tipo celular indica una infección micobacteriana previa y los análisis del segmento IS6110 con frecuencia demuestran una combinación mortal de reactivación y reinfección de adquisición reciente. El control de la TB exige una prevención efi caz de la enfermedad activa por evolución de las infecciones adquiridas antiguamente y las infecciones de adquisición reciente y un uso más amplio del tratamiento antituberculoso empírico en pacientes con SIDA en estado avanzado y fi ebre prolongada.
Tuberculosis (TB) is the most common cause of death from human immunodeficiency virus (HIV) infection worldwide. Autopsy studies have shown that many of these cases are not diagnosed pre-mortem.1,2 Fatal cases often represent disseminated TB, a form of disease with non-specific symptoms (e.g., persistent fever) that are often attributed to advanced acquired immune-deficiency syndrome (AIDS) alone.2–4
Cross-sectional studies among hospitalized patients with HIV infection and fever have shown that as many as 20–25% may have Mycobacterium tuberculosis bacteremia as a manifestation of disseminated TB. Many of these patients lack pulmonary manifestations and have negative sputum cultures for M. tuberculosis;4–6 short-term mortality has been high.4
No prospective studies of disseminated TB have been reported. We conducted a prospective study of disseminated TB among a large cohort of HIV-infected persons living in Dar es Salaam, Tanzania. After baseline immunologic evaluation, patients were followed every 3 months with comprehensive evaluation for pulmonary and disseminated TB, provided with empiric and therapeutic treatment for suspected and confirmed disease, and evaluated for health outcomes. Our goals were to delineate the pathophysiology and molecular epidemiology of disseminated TB to guide strategies for preventive or empiric therapy. Our findings highlight the unique pathophysiology of this disease and the importance of improving methods for both prevention and treatment.
METHODS
Participants and trial design
The DarDar Trial was designed to 1) study the pathophysiology and molecular biology of disseminated TB in HIV (present report), and 2) test the efficacy of a whole-cell mycobacterial vaccine for the prevention of disseminated TB.7 Eligible subjects were ambulatory HIV-positive residents of Dar es Salaam, Tanzania, aged ⩾18 years, with CD4 counts ⩾200 cells/μl, a visible bacille Calmette-Guérin scar from childhood immunization, a negative pregnancy test and no evidence of active TB.
At baseline all patients were interviewed, examined, and underwent the following tests: two HIV enzyme-linked immunosorbent assays (ELISAs), CD4 count, tuberculin skin test (TST; RT-23, Statens Serum Institute, Copenhagen, Denmark), phlebotomy for immunologic assays, single posterior-anterior chest X-ray, mycobacterial blood culture and collection of three sputum samples for acid-fast bacilli (AFB) stain and mycobacterial culture. Subjects with a TST result ⩾ 5 mm and no history of previous treatment for TB were given 6 months of isoniazid (INH) 300 mg daily for 6 months.8 Subjects were randomized 1:1 to receive five intradermal doses of an inactivated whole-cell mycobacterial vaccine, M. vaccae or placebo at 0, 2, 4, 6 and 12 months.9
Follow-up
After immunization, all subjects were seen routinely every 3 months and whenever they experienced new symptoms; CD4 counts were performed annually. Evaluable CD4 counts at the time of diagnosis of disseminated TB were defined nadir values within 6 months, or prior values < 100 if no CD4 count was available within 6 months. Study out-patients with CD4 counts < 200 cells/μl, new active TB, or who met other Ministry of Health (MOH) guidelines for antiretroviral therapy (ART) were referred to an MOH clinic for HIV treatment; decisions on ART for study in-patients were made by attending hospital staff.
Out-patient evaluations for active TB were conducted by study staff based on prolonged fever or cough, or unexplained weight loss, and included a physical examination, single posterior-anterior chest X-ray, three expectorated sputum samples for AFB stain and culture (one spot and two first morning samples) and a mycobacterial blood culture. Patients with suspected or confirmed TB were referred to the MOH for empiric or therapeutic TB treatment. Patients who were more than 2 weeks overdue for a study visit were followed up by phone call or home visit; patients with severe illness at home or clinic follow-up were transported to hospital for evaluation and treatment by hospital staff.
Study staff provided diagnostic studies for TB on all patients who reported hospitalization with prolonged fever or cough or unexplained weight loss if such evaluation had not been conducted by hospital staff. Results were provided to hospital staff, who made decisions about empiric or therapeutic treatment for TB. Patients with a positive blood culture for M. tuberculosis were defined as having disseminated TB at the time the blood culture was obtained. Baseline and follow-up chest X-rays originally interpreted on site were read together with non-disseminated TB controls by a consultant radiologist (WB) blinded to the diagnosis. All patients treated for TB were subsequently classified as definite, probable or possible TB by an expert panel based on pre-defined study case definitions.7
Microbiology
Sputum AFB stains were performed using auraminerhodamine staining with confirmation by Ziehl-Neelsen staining. Sputum cultures were performed using standard methods on Löwenstein-Jensen (LJ) agar. Blood cultures were performed using the automated MB BacT broth system (bioMérieux, Durham, NC, USA).10 According to the manufacturer’s instructions, samples were considered negative if no growth had occurred within 42 days; positive samples were confirmed with AFB staining. Terminal subcultures were performed on LJ media and incubated in air for an additional 8 weeks. DNA probes were performed on all isolates to confirm M. tuberculosis complex (AccuProbe, Gen-Probe, San Diego, CA, USA) and insertion sequence (IS) 6110 typing performed and interpreted according to standard methods.11 The date processed was reviewed to identify clusters that may have represented cross-contamination.
Immunologic assays
Interferon-gamma (IFN-γ) release assays (IGRAs), lymphocyte proliferation assays (LPA) and assays of antibody responses to lipoarabinomannan (LAM) were performed at baseline. Peripheral blood mononuclear cells (PBMC) used in IGRAs and LPAs were isolated from fresh whole blood by ficoll density gradient centrifugation, cultured with medium, a phytohemagglutinin positive control (PHA; Sigma, St Louis, MO, USA), and Ag 85, early secreted antigenic target 6 (ESAT-6), M. vaccae sonicate, or M. tuberculosis whole-cell lysate (WCL) for 5 days.
The supernatant from antigen-stimulated PBMC cultures was frozen in Tanzania and sent to Dartmouth (NH, USA) for measurement of IFN-γ by a standard enzyme-linked immunosorbent assay (R&D Systems, Minneapolis, MN, USA). IFN-γ assays were considered valid if the IFN-γ level for the PHA condition was >300 pg/ml. For LPA, a standard tritiated thymidine incorporation assay was performed on PBMC after 5 days of incubation with study antigens. On the fourth day, 3H-thymidine was added to wells for 24 h, after which cells were harvested onto filter paper in Tanzania and sent to Finland for data acquisition on a scintillation counter. Results were expressed as a proliferation index (PI; defined as counts per minute [CPM] of antigen-stimulated cells divided by CPM of unstimulated cells). LPAs were considered valid if the PI for the PHA condition was ⩾3. Antibody responses to LAM12 were assayed via immunoglobulin ELISA.
Statistical analysis
Descriptive statistics were used to describe baseline study demographic features. Groups were compared using χ2, Fisher’s, t- or Wilcoxon-Mann-Whitney test, as appropriate. Survival was characterized using person-year rates and Kaplan-Meier estimates. Statistical software used was R 2.10 (R Foundation for Statistical Computing, Vienna, Austria) and STATA 10 (Stata Corp, College Station, TX, USA).
The study was approved by the United States Department of Health and Human Services, the Dartmouth Committee for the Protection of Human Subjects and the Ethics Committee of the Muhimbili University College of Health Sciences in Tanzania.
RESULTS
Baseline
A total of 4973 subjects were screened to identify 2013 eligible subjects; 1975 subjects returned for a first study visit and were subsequently followed for a median of 3.3 years (range 2 months to 6.3 years; Figure 1). A total of 188 (9.5%) subjects died and 366 (18.5%) were lost to follow-up. A total of 236 patients were treated for TB; expert review subsequently classified 85 cases as definite TB, 88 as probable (including 67 patients treated empirically based on clinical criteria alone) and 43 as possible (all treated empirically based on clinical criteria alone). Among the patients with definite TB, 20 had disseminated disease: seven were M. vaccae and 13 placebo recipients (hazard ratio 0.52, 95% confidence interval 0.21–1.34, P = 0.16).7 Compared to all other subjects, those with disseminated TB were older, more often male, had a lower baseline CD4 count and higher viral load (Table 1). Most subjects had positive TST reactions; 14/20 (70%) had reactions ⩾15 mm. Among the 17 subjects with reactions ⩾5 mm, 14 had no history of previous treatment for active TB, were offered INH, and completed 6 months of treatment.
Figure 1.
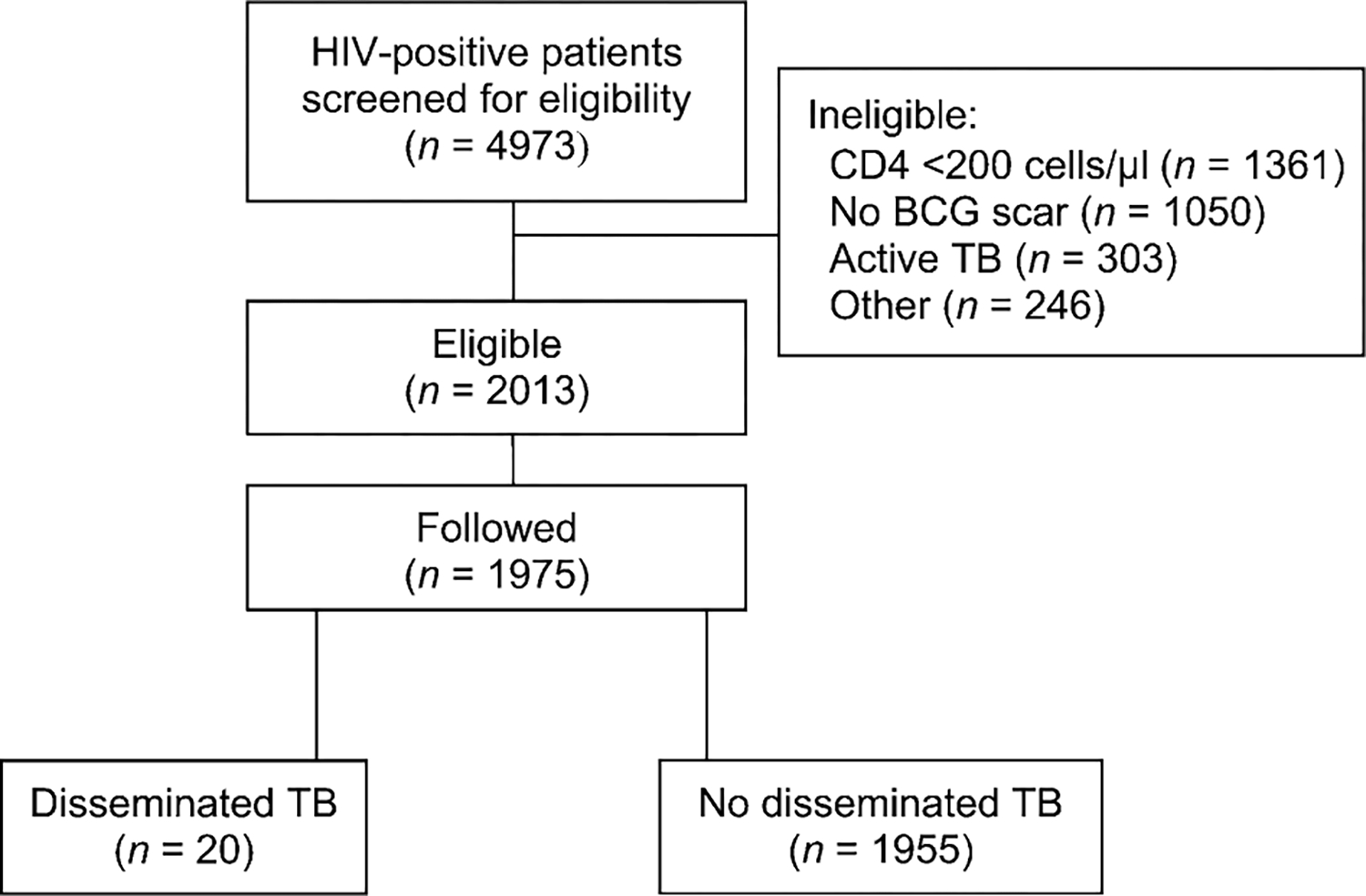
Consort diagram. HIV = human immunodeficiency virus; BCG = bacille Calmette-Guérin; TB = tuberculosis.
Table 1.
Characteristics of study subjects at baseline
| Characteristic | Disseminated TB (n = 20) | No disseminated TB (n = 1955) | P value |
|---|---|---|---|
|
| |||
| Age, years, mean | 39.2 | 33.1 | <0.001 |
| Male, n (%) | 10 (50.0) | 460 (23.5) | 0.01 |
| Median baseline CD4 count, cells/μl | 319.3 | 473.7 | 0.002 |
| Median baseline HIV viral load, log10 copies/ml* | 4.6 | 3.9 | 0.02 |
| Tuberculin skin test ≥ 5 mm, n (%)† | 17 (85.0) | 615 (32.1) | <0.001 |
| Antiretroviral treatment, n (%) | 0 | 57 (2.9) | 1.0 |
| Baseline body mass index, median (25th, 75th percentile), kg/m2 | 23.4 (21.5, 25.2) | 23.7 (21.0, 27.2) | 0.72 |
| Prior treatment for TB, n (%) | 3 (15.0) | 166 (8.5) | 0.24 |
Data available for 10 subjects with and 690 without disseminated TB.
Data available for 20 subjects with and 1914 without disseminated TB.
TB = tuberculosis; HIV = human immunodeficiency virus.
Baseline mycobacterial immune responses were performed a median of 1.56 years (range 0.51–4.45 years) before the positive blood culture. At baseline, LPA responses, but not IFN-γ responses, to all three mycobacterial antigens tested, were greater in subjects who subsequently developed disseminated TB (Figure 2). Subjects with a positive baseline LPA proliferation index (PI ⩾ 5) to WCL were more likely to develop disseminated TB than those with a negative baseline LPA to WCL (2.3% vs. 0.6%, P = 0.01).
Figure 2.
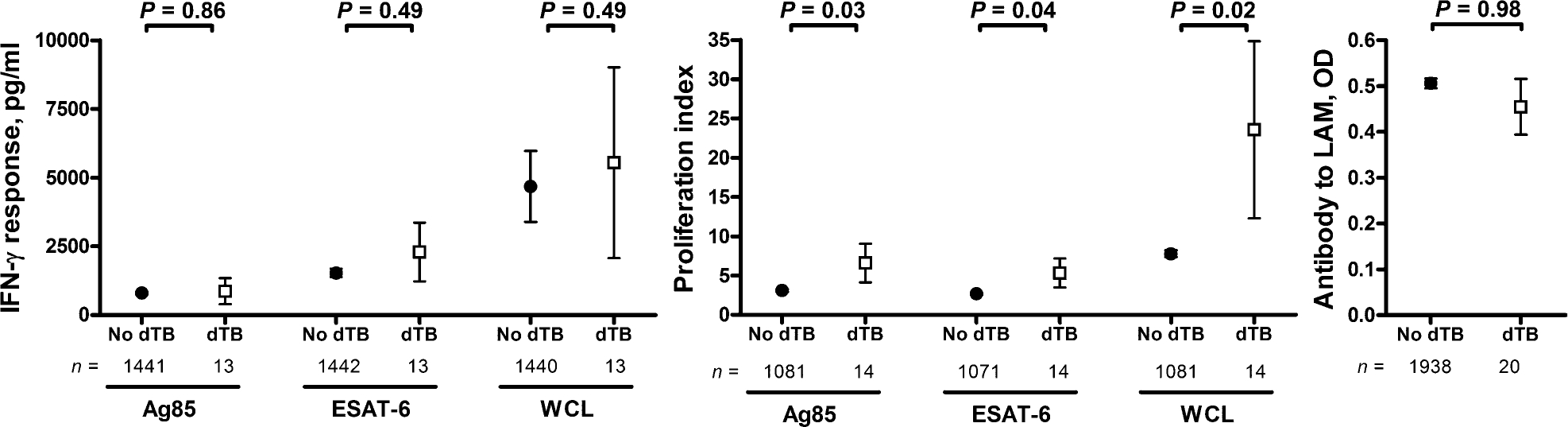
Baseline immune response to mycobacterial antigens. IFN-γ = interferon-gamma; dTB = disseminated tuberculosis; ESAT-6 = early secreted antigenic target 6; WCL = whole cell lysate; LAM = lipoarabinomannan; OD = optical density.
Clinical features
Most patients had fever ⩾2 weeks, cough ⩾2 weeks and ⩾5 kg weight loss (Table 2); 18 (90%) patients had ⩾2 of these symptoms. Seventeen (85%) subjects had been treated for malaria in the previous 3 months. CD4 counts were <75 cells/μl in 14/16 (87%) patients, with median values of 64 in M. vaccae recipients and 30 in placebo recipients (P = 0.75). Chest X-rays were abnormal in 7/17 (41%): miliary pattern was observed in 4 (1 with pleural effusion), consolidation in 2 and paratracheal adenopathy in 1. TB was considered or confirmed as the initial diagnosis within a week after the blood culture results had been obtained in five (25%) patients.
Table 2.
Characteristics of patients at the time of diagnosis of disseminated TB (n = 20)
| Characteristic | n/N (%) |
|---|---|
|
| |
| Fever ≥2 weeks | 17/20 (85) |
| Cough ≥2 weeks | 14/20 (70) |
| Weight loss ≥5 kg/year | 19/20 (95) |
| CD4 count, cells/μl median [range]* | 30 [1–122] |
| CD4 count <75 cells/μl* | 14/16 (87) |
| Anti-retroviral therapy | 3/20 (15) |
| Sputum AFB smear-positive | 5/20 (25) |
| Sputum TB culture-positive | 10/20 (50) |
| Abnormal chest X-ray† | 7/17 (41) |
| Diagnosis, n (%)‡ | |
| TB | 5 (25) |
| Malaria | 5 (25) |
| Other§ | 10 (50) |
Total of 16 evaluable CD4 counts, see text for definition.
See text for definition.
Diagnosis from 2 weeks before to 1 week after blood culture obtained.
Includes AIDS, pneumonia or Pneumocystis jirovecii infection.
TB = tuberculosis; AFB = acid-fast bacilli; AIDS = acquired immune-deficiency syndrome.
Microbiology
AFB smears were positive in five (25%) patients with disseminated TB, and sputum cultures were positive in nine (45%; one additional sputum was determined to be cross-contaminated). In the remaining 10 (50%) subjects, blood cultures were the only cultures positive for M. tuberculosis. The median time to detection of a positive blood culture was 43 days (range 19–104). IS6110 analysis was available for 20 blood isolates and eight concurrent sputum isolates, and results were compared to 295 concurrent study and community isolates. Among the blood isolates, 14 were clustered and six were unique (Table 3). Three (37%) of eight subjects with concurrent blood and sputum isolates were infected with two different strains of M. tuberculosis (polyclonal disease).
Table 3.
Characteristics of patients with clustered or unique blood isolates as resolved by IS6110 analysis
| Characteristic | Blood isolate clustered (n = 14) | Blood isolate unique (n = 6) | P value |
|---|---|---|---|
|
| |||
| TST ≥15 mm, n (%) | 11 (79) | 3 (50) | 0.20 |
| Received placebo vaccine, n (%) | 8 (57) | 5 (83) | 0.26 |
| Median CD4 at baseline, cells/μl | 271 | 292 | 0.74 |
| Median CD4 at time of disseminated tuberculosis, cells/μl | 54* | 18* | 0.04 |
| Polyclonal disease, n/N (%) | 2/5 (40) | 1/3 (33) | 0.85 |
Value based on comparison of 16 evaluable CD4 counts, see text for definition.
IS = insertion sequence; TST = tuberculin skin test.
Outcomes
Eight (40%) patients received treatment for TB, with initiation ranging from 5 days before until 22 days after the blood culture was obtained. These included 4/5 patients with positive AFB smears and 4/7 with abnormal chest X-rays. Seventeen (85%) patients died at a median of 26 days (range 4–244); all but one of the deaths occurred within 6 months of the time of documented dissemination. At the time the positive blood cultures were reported, only five subjects were still alive.
The three long-term survivors were all treated for TB within 16 days of obtaining the blood culture and prior to any being reported positive. All three had an abnormal chest X-ray based on on-site interpretation (only one abnormal by subsequent reading by the consultant radiologist); one had a positive AFB smear. The most recent CD4 counts were 1, 30 and 64 cells/μl. One patient had been receiving ART for 1 year at the time the blood culture was obtained; the other two were started on ART at respectively 7 months and 3 years later. All three survivors had unique isolates using IS6110.
DISCUSSION
This prospective study demonstrates that disseminated TB occurs among HIV-infected patients with prior mycobacterial infection, chronic but ineffective mycobacterial cellular immune responses, low CD4 counts and disease due to one or more strains of M. tuberculosis. Non-specific clinical findings, coupled with frequently negative microbiologic and radiologic studies, contribute to missed diagnosis and misdiagnosis, undertreatment for TB and a high mortality rate.
Consistent with previous studies, we observed that disseminated TB was typically seen in patients with low CD4 counts characteristic of advanced HIV infection. This unique prospective study, in which all subjects had CD4 > 200 cells/μl, at enrollment provides additional new insights into the pathogenesis of this problem. At baseline, subjects who subsequently developed disseminated TB typically had evidence of prior mycobacterial infection, including a positive TST and positive LPA for mycobacterial antigens, consistent with reactivation. However, strain typing indicated that the majority of blood isolates were clustered, consistent with recent infection.
Previous studies using IS6110 to analyze isolates of HIV-associated pulmonary TB in endemic regions have similarly distinguished reactivation and new infection.13–17 In addition, these studies have also documented re-infection, i.e., sequential infection with two different strains. However, simultaneous infection with two different strains, i.e., polyclonal infection, has been described only rarely: in Spain 3/50 (6%) patients with HIV-associated TB had polyclonal infection.18 We found polyclonal disease in 3/8 (37%) disseminated TB patients in Tanzania with available sputum isolates; all six isolates were drug-susceptible. This rate of polyclonal disease may represent an underestimate, as in this study we only had one isolate per culture to analyze. This limitation also meant we could not resolve whether both strains were disseminated or whether only one was disseminated and the second only pulmonary. Previous studies in which multiple colonies from each positive culture were typed demonstrated that AIDS patients can have polyclonal disseminated infection with M. avium complex, a ubiquitous environmental organism.19 Polyclonal disease with M. tuberculosis is likely to be more common in settings with high rates of endemic pulmonary TB, such as Tanzania, than in regions with lower incidence, such as Europe and the United States.
Baseline LPA responses to WCL were also associated with an increased risk of disseminated TB. However, baseline IFN-γ responses to mycobacterial antigens were not elevated in subjects who subsequently developed disseminated TB. In an analysis of placebo recipients from the entire DarDar cohort, we found that increased baseline IFN-γ responses to multiple mycobacterial antigens were associated with a decreased risk of subsequent definite culture-confirmed TB.20 These prospective observations are collectively consistent with the hypothesis that IFN-γ responses to broad and shared mycobacterial antigens are critical in protection against TB. In contrast, IFN-γ responses to ESAT-6 alone have been associated with risk of TB in HIV-negative subjects.21 The vaccine-induced reduction in the risk of disseminated TB observed in the parent study, although underpowered for statistical significance, suggests that even in patients with HIV infection, mycobacterial immune responses can be effectively boosted to protect against disseminated TB.7
We found that HIV-infected patients with disseminated TB were often undiagnosed or misdiagnosed and had a high fatality rate. These findings are consistent with studies showing that TB, often disseminated TB, is the most common cause of death in patients with HIV infection living in TB-endemic countries.1,2 Particularly disturbing was the frequency with which patients with HIV and fever of several weeks’ duration were diagnosed and treated for malaria, a disease associated with days, not weeks, of fever, and were consequently not treated for TB. Malaria smears may be positive in asymptomatic persons living in endemic areas,22,23 and false-positive malaria diagnoses are to be expected in HIV-infected patients with fever due to TB. Furthermore, the failure of many subjects to initiate recommended ART contributed substantially to the risk of disseminated TB.
Blood cultures permitted detection of disseminated TB in the study; however, positive reports were usually available only after death. Improved diagnostic methods such as PCR offer promise in the more rapid detection of mycobacteremia,24 but until better diagnostics are available we believe that empiric treatment for TB should be used more widely in TB-endemic regions. Our recommendation would be to begin empiric therapy for TB in patients with advanced HIV infection and >2 weeks of fever, especially patients with CD4 counts < 100 cells/μl. This would include patients with positive malaria smears. Therapy could be withdrawn in 6–8 weeks if diagnostic studies for TB were negative and there was no response to empiric therapy.
Prevention of disseminated TB in HIV infection may be more challenging than prevention of pulmonary TB, given the pathophysiology we have described in the present cohort. For patients who already have advanced AIDS with CD4 counts < 75 cells/μl and who live in TB-endemic countries, consideration should be given to long-term INH prophylaxis after thorough exclusion of active TB. For patients with early-stage HIV infection, standard INH prophylaxis, early diagnosis and treatment of pulmonary TB, institution of ART at CD4 counts < 350 cells/μl and development of a TB vaccine strategy that is safe and effective in HIV infection are all important.25–28
In summary, this prospective study has demonstrated that HIV-associated disseminated TB is associated with large baseline TST reactions, a chronic ineffective cellular immune response to prior mycobacterial infection and the development of dissemination in a setting of advanced AIDS with CD4 counts < 75 cells/μl. Disease is frequently polyclonal and may represent the concurrence of reactivation disease and newly acquired infection. Current diagnostics fail to identify many patients, resulting in misdiagnosis and missed diagnosis with high mortality. Empiric therapy for TB should be considered for patients in TB-endemic countries with advanced AIDS and fever lasting ⩾2 weeks.
Acknowledgements
The authors thank the following for assistance with the study: H Soini, J Vuola, W Wieland-Alter, B Mchaki, O Rautio, other laboratory and clinical staff and the many Tanzanian subjects who volunteered for the study. Funding was provided by the National Institutes of Health, Division of Acquired Immunodeficiency Syndrome, AI 45407, and Fogarty International Center, D43-TW006807. ClinicalTrials.gov number NCT00052195.
Footnotes
Potential conflicts of interest: No authors have financial interests that pose a conflict of interest. SR Pharma (London, UK) manufactured and provided vaccine and one vehicle for the study site, but did not contribute to the study in any other way.
References
- 1.Lucas SB, Hounnou A, Peacock C, et al. The mortality and pathology of HIV infection in a West African city. AIDS 1993; 7: 1569–1579. [DOI] [PubMed] [Google Scholar]
- 2.Ansari NA, Kombe AH, Kenyon TA, et al. Pathology and causes of death in a group of 128 predominantly HIV-positive patients in Botswana, 1997–1998. Int J Tuberc Lung Dis 2002; 6: 55–63. [PubMed] [Google Scholar]
- 3.von Reyn C F The significance of bacteremic tuberculosis among persons with HIV infection in developing countries. AIDS 1999; 13: 2193–2195. [DOI] [PubMed] [Google Scholar]
- 4.Archibald LK, den Dulk M O, Pallangyo KJ, Reller LB. Fatal Mycobacterium tuberculosis bloodstream infections in febrile hospitalized adults in Dar es Salaam, Tanzania. Clin Infect Dis 1998; 26: 290–296. [DOI] [PubMed] [Google Scholar]
- 5.Talbot EA, Hay Burgess D C, Hone NM, et al. Tuberculosis serodiagnosis in a predominantly HIV-infected population of hospitalized patients with cough, Botswana, 2002. Clin Infect Dis 2004; 39: e1–7. [DOI] [PubMed] [Google Scholar]
- 6.Waddell RD, Lishimpi K, von Reyn C F, et al. Bacteremia due to Mycobacterium tuberculosis or M. bovis, bacille Calmette-Guerin (BCG) among HIV-positive children and adults in children. AIDS 2001; 15: 55–60. [DOI] [PubMed] [Google Scholar]
- 7.von Reyn C F, Mtei L, Arbeit RD, et al. Prevention of tuberculosis in bacille Calmette-Guerin-primed, HIV-infected adults boosted with an inactivated whole-cell mycobacterial vaccine. AIDS 2010; 24: 675–685. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Munseri P, Talbot E, Mtei L, von Reyn C F. Completion of isoniazid preventive therapy among HIV-infected patients in Tanzania. Int J Tuberc Lung Dis 2008; 12: 1037–1041. [PubMed] [Google Scholar]
- 9.Vuola J, Ristola M, Cole B, et al. Immunogenicity of an inactivated mycobacterial vaccine for the prevention of HIV-associated tuberculosis: a randomized, controlled trial. AIDS 2003; 17: 2351–2355. [DOI] [PubMed] [Google Scholar]
- 10.Crump JA, Tanner DC, Mirrett S, McKnight CM, Reller LB. Controlled comparison of BACTEC 13 A, MYCO/F LYTIC, BacT/ALERT MB, and ISOLATOR 10 systems for detection of mycobacteremia. J Clin Microbiol 2003; 41: 1987–1990. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Bifani PJ, Mathema B, Liu Z, et al. Identification of a W variant outbreak of Mycobacterium tuberculosis via population based molecular epidemiology. JAMA 1999; 24: 2321–2327. [DOI] [PubMed] [Google Scholar]
- 12.Nigou J, Gilleron M, Puzo G. Lipoarabinomannans: from structure to biosynthesis. Biochimie 2003; 85: 153–166. [DOI] [PubMed] [Google Scholar]
- 13.Small PM, Shafer RW, Hopewell PC, et al. Exogenous reinfection with multidrug resistant Mycobacterium tuberculosis in patients with advanced HIV infection. N Engl J Med 1993; 328: 1137–1143. [DOI] [PubMed] [Google Scholar]
- 14.Godfrey-Faussett P, Githui W, Batchelor B, et al. Recurrence of HIV-related tuberculosis in an endemic area may be due to relapse or reinfection. Tubercle Lung Dis 1994; 75: 199–202. [DOI] [PubMed] [Google Scholar]
- 15.Sonnenberg P, Murray J, Glynn JR, Shearer S, Kambashi B, Godfrey-Faussett P. HIV-1 and recurrence, relapse, and reinfection of tuberculosis after cure: a cohort study in South African mineworkers. Lancet 2001; 358: 1687–1693. [DOI] [PubMed] [Google Scholar]
- 16.van Rie A, Warren R, Richardson M, et al. Exogenous reinfection as a cause of recurrent tuberculosis after curative treatment. N Engl J Med 1999; 341: 1174–1179. [DOI] [PubMed] [Google Scholar]
- 17.Andrews JR, Gandhi NR, Moodley P, et al. Exogenous reinfection as a cause of multidrug-resistant and extensively drug-resistant tuberculosis in rural South Africa. J Infect Dis 2008; 198: 1582–1589. [DOI] [PubMed] [Google Scholar]
- 18.de Viedma D G, Marin M, Serrano MJR, Alcala L, Bouza E. Polyclonal and compartmentalized infection by Mycobacterium tuberculosis in patients with both respiratory and extra-respiratory involvement. J Infect Dis 2003; 187: 695–699. [DOI] [PubMed] [Google Scholar]
- 19.von Reyn C F, Jacobs NJ, Arbeit RD, Maslow JN, Niemczyk S. Polyclonal Mycobacterium avium infections in patients with AIDS: variations in antimicrobial susceptibilities of different strains of M. avium isolated from the same patient. J Clin Microbiol 1995; 33: 1008–1010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Lahey T, Sheth S, Matee M, et al. Interferon gamma responses to mycobacterial antigens protect against subsequent HIV-associated tuberculosis. J Infect Dis 2010; 202: 1265–1272. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Doherty TM, Demissie A, Olobo J, et al. Immune responses to the Mycobacterium tuberculosis-specific antigen ESAT-6 signal subclinical infection among contacts of tuberculosis patients. J Clin Microbiol 2002; 40: 704–706. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Dal-Bianco MP, Koster KB, Kombila UD, et al. High prevalence of asymptomatic Plasmodium falciparum infection in Gabonese adults. Am J Trop Med Hyg 2007; 77: 939–942. [PubMed] [Google Scholar]
- 23.Imperato P J Malaria parasitemia in healthy Africans in North Mara, Tanzania. J Community Health 1986; 11: 92–97. [DOI] [PubMed] [Google Scholar]
- 24.Tissari P, Zumla A, Tarkka E, et al. Accurate and rapid identification of bacterial species from positive blood cultures with a DNA-based microarray platform: an observational study. Lancet 2010; 375: 224–230. [DOI] [PubMed] [Google Scholar]
- 25.Lawn SD, Myer L, Bekker LG, Wood R. Burden of tuberculosis in an antiretroviral treatment programme in sub-Saharan Africa: impact on treatment outcomes and implications for tuberculosis control. AIDS 2006; 20: 1605–1612. [DOI] [PubMed] [Google Scholar]
- 26.Lawn SD, Myer L, Edwards D, Bekker LG, Wood R. Short-term and long-term risk of tuberculosis associated with CD4 cell recovery during antiretroviral therapy in South Africa. AIDS 2009; 23: 1717–1725. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.von Reyn C F, Vuola J. New vaccines for the prevention of tuberculosis. Clin Infect Dis 2002; 35: 465–474. [DOI] [PubMed] [Google Scholar]
- 28.Harries AD, Zachariah R, Lawn SD. Providing HIV care for co-infected tuberculosis patients: a perspective from sub-Saharan Africa. Int J Tuberc Lung Dis 2009; 13: 6–16. [PubMed] [Google Scholar]


