Abstract
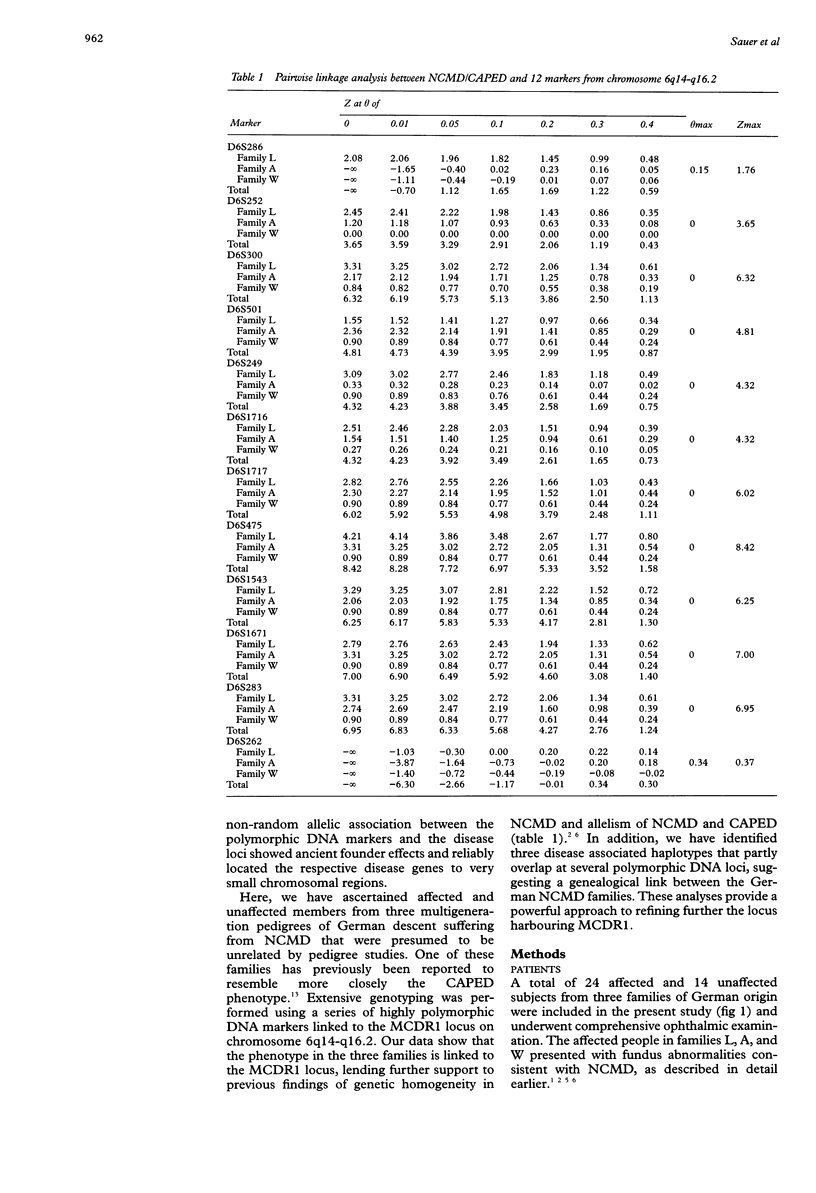
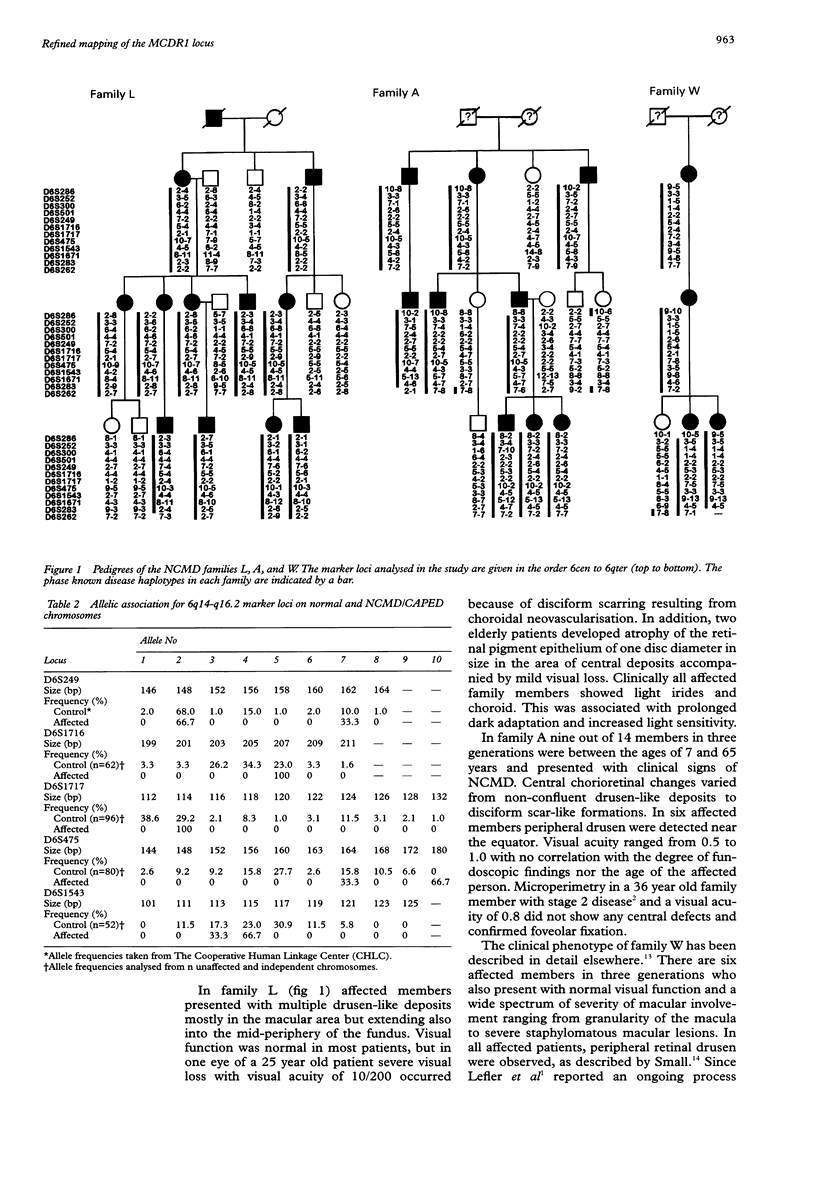
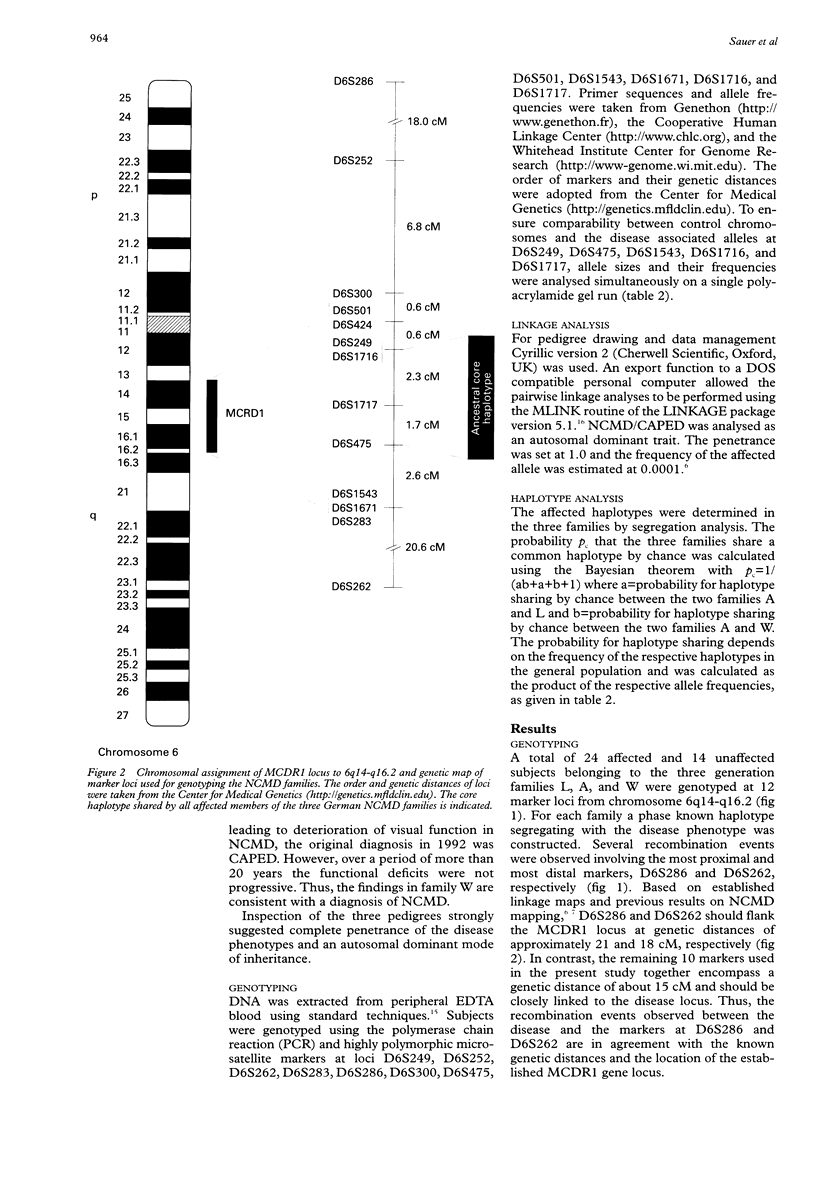
Autosomal dominant North Carolina macular dystrophy (NCMD) or central areolar pigment epithelial dystrophy (CAPED) is an allelic disorder that maps to an approximately 7.2 cM interval between DNA markers at D6S424 and D6S1671 on 6q14-q16.2. The further refinement of the disease locus has been hindered by the lack of additional recombination events involving the critical region. In this study, we have identified three multigeneration families of German descent who express the NCMD phenotype. Genotyping was carried out with a series of markers spanning approximately 53 cM around the NCMD locus, MCDR1. Genetic linkage between the markers and the disease phenotype in each of the families could be shown. Disease associated haplotypes were constructed and provide evidence for an ancestral founder for the German NCMD families. This haplotype analysis suggests that a 4.0 cM interval flanked by markers at D6S249 and D6S475 harbours the gene causing NCMD, facilitating further positional cloning approaches.
Full text
PDF


Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Fetkenhour C. L., Gurney N., Dobbie J. G., Choromokos E. Central areolar pigment epithelial dystrophy. Am J Ophthalmol. 1976 Jun;81(6):745–753. doi: 10.1016/0002-9394(76)90357-3. [DOI] [PubMed] [Google Scholar]
- Harley H. G., Brook J. D., Floyd J., Rundle S. A., Crow S., Walsh K. V., Thibault M. C., Harper P. S., Shaw D. J. Detection of linkage disequilibrium between the myotonic dystrophy locus and a new polymorphic DNA marker. Am J Hum Genet. 1991 Jul;49(1):68–75. [PMC free article] [PubMed] [Google Scholar]
- Hermsen V. M., Judisch G. F. Central areolar pigment epithelial dystrophy. Ophthalmologica. 1984;189(1-2):69–72. doi: 10.1159/000309388. [DOI] [PubMed] [Google Scholar]
- Holz F. G., Evans K., Gregory C. Y., Bhattacharya S., Bird A. C. Autosomal dominant macular dystrophy simulating North Carolina macular dystrophy. Arch Ophthalmol. 1995 Feb;113(2):178–184. doi: 10.1001/archopht.1995.01100020062029. [DOI] [PubMed] [Google Scholar]
- Kan Y. W., Dozy A. M. Polymorphism of DNA sequence adjacent to human beta-globin structural gene: relationship to sickle mutation. Proc Natl Acad Sci U S A. 1978 Nov;75(11):5631–5635. doi: 10.1073/pnas.75.11.5631. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kelsell R. E., Godley B. F., Evans K., Tiffin P. A., Gregory C. Y., Plant C., Moore A. T., Bird A. C., Hunt D. M. Localization of the gene for progressive bifocal chorioretinal atrophy (PBCRA) to chromosome 6q. Hum Mol Genet. 1995 Sep;4(9):1653–1656. doi: 10.1093/hmg/4.9.1653. [DOI] [PubMed] [Google Scholar]
- Kerem B., Rommens J. M., Buchanan J. A., Markiewicz D., Cox T. K., Chakravarti A., Buchwald M., Tsui L. C. Identification of the cystic fibrosis gene: genetic analysis. Science. 1989 Sep 8;245(4922):1073–1080. doi: 10.1126/science.2570460. [DOI] [PubMed] [Google Scholar]
- Kunkel L. M., Smith K. D., Boyer S. H., Borgaonkar D. S., Wachtel S. S., Miller O. J., Breg W. R., Jones H. W., Jr, Rary J. M. Analysis of human Y-chromosome-specific reiterated DNA in chromosome variants. Proc Natl Acad Sci U S A. 1977 Mar;74(3):1245–1249. doi: 10.1073/pnas.74.3.1245. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Käsmann B., Blankenagel A., Daus W. Die zentrale areoläre Pigmentepitheldystrophie. Ihre Abgrenzung zu anderen dominaten Makuladystrophien. Ophthalmologe. 1992 Feb;89(1):60–66. [PubMed] [Google Scholar]
- Lathrop G. M., Lalouel J. M., Julier C., Ott J. Multilocus linkage analysis in humans: detection of linkage and estimation of recombination. Am J Hum Genet. 1985 May;37(3):482–498. [PMC free article] [PubMed] [Google Scholar]
- Small K. W., Hermsen V., Gurney N., Fetkenhour C. L., Folk J. C. North Carolina macular dystrophy and central areolar pigment epithelial dystrophy. One family, one disease. Arch Ophthalmol. 1992 Apr;110(4):515–518. doi: 10.1001/archopht.1992.01080160093040. [DOI] [PubMed] [Google Scholar]
- Small K. W. North Carolina macular dystrophy, revisited. Ophthalmology. 1989 Dec;96(12):1747–1754. doi: 10.1016/s0161-6420(89)32655-8. [DOI] [PubMed] [Google Scholar]
- Small K. W., Puech B., Mullen L., Yelchits S. North Carolina macular dystrophy phenotype in France maps to the MCDR1 locus. Mol Vis. 1997 Jan 2;3:1–1. [PubMed] [Google Scholar]
- Small K. W., Weber J. L., Roses A., Lennon F., Vance J. M., Pericak-Vance M. A. North Carolina macular dystrophy is assigned to chromosome 6. Genomics. 1992 Jul;13(3):681–685. doi: 10.1016/0888-7543(92)90141-e. [DOI] [PubMed] [Google Scholar]
- Small K. W., Weber J., Roses A., Pericak-Vance P. North Carolina macular dystrophy (MCDR1). A review and refined mapping to 6q14-q16.2. Ophthalmic Paediatr Genet. 1993 Dec;14(4):143–150. doi: 10.3109/13816819309042913. [DOI] [PubMed] [Google Scholar]
- Theilmann J., Kanani S., Shiang R., Robbins C., Quarrell O., Huggins M., Hedrick A., Weber B., Collins C., Wasmuth J. J. Non-random association between alleles detected at D4S95 and D4S98 and the Huntington's disease gene. J Med Genet. 1989 Nov;26(11):676–681. doi: 10.1136/jmg.26.11.676. [DOI] [PMC free article] [PubMed] [Google Scholar]


