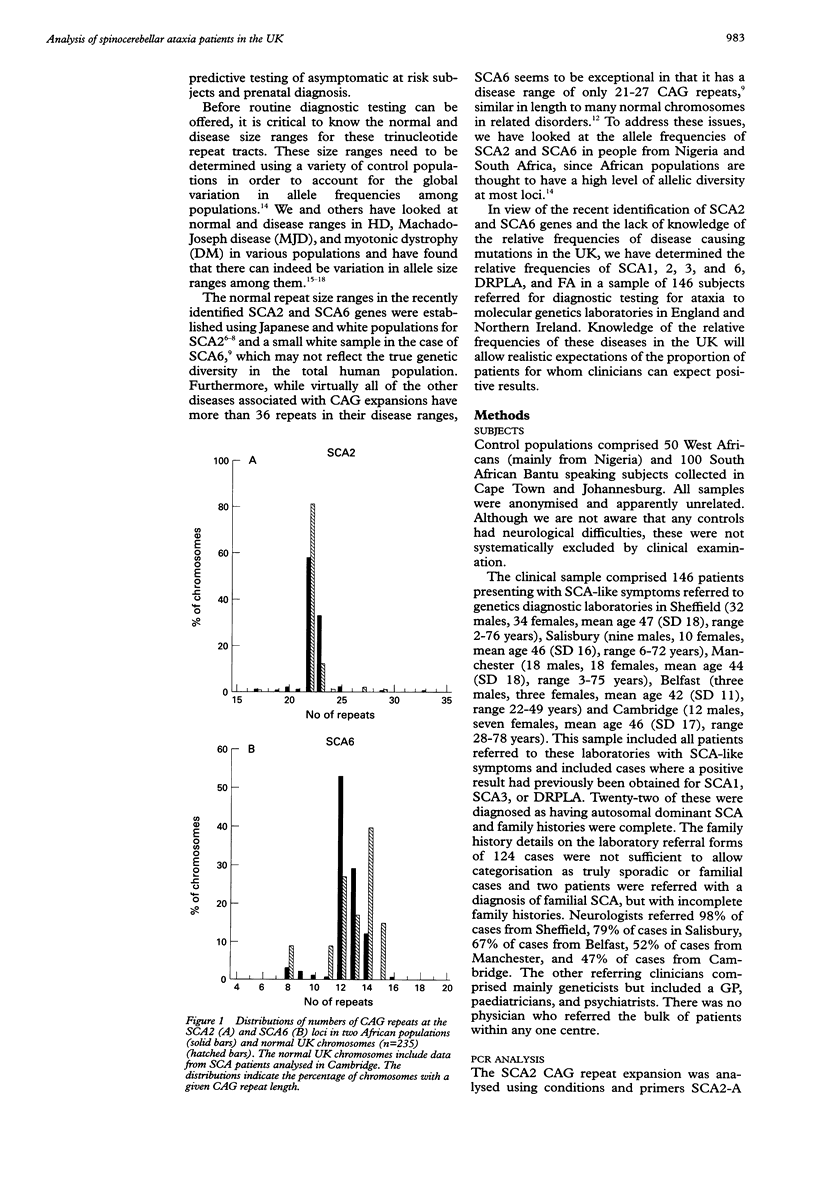
Abstract
Accurate clinical diagnosis of the spinocerebellar ataxias (SCAs) can be difficult because of overlap in phenotype with other disorders and variation in clinical manifestations. Six SCA loci have been mapped and four disease causing genes identified, in addition to the causative gene for Friedreich's ataxia (FA). All of the identified mutations are expansions of trinucleotide repeat tracts. The SCA2 and SCA6 genes were published recently. The extent of the normal CAG size ranges at these loci and the relative frequencies of the known causes of SCA in the UK are not known. This study first investigated the normal size ranges of the SCA2 and SCA6 loci by genotyping control populations of West African and South African subjects, since African populations generally show the greatest allelic diversity. We found one allele larger than the previously determined normal range for SCA2, and our results at the SCA6 locus agreed with the previously reported normal range. The second component of the study assessed the relative frequencies of the SCA1, 2, 3, and 6, DRPLA, and FA trinucleotide repeat mutations in 146 patients presenting with SCA-like symptoms referred to genetic diagnostic laboratories in the UK. We detected mutations in 14% of patients referred with a diagnosis of autosomal dominant SCA, and in 15% of patients referred with spinocerebellar ataxia where we did not have sufficient family history data available to allow categorisation as familial or sporadic cases. Friedreich's ataxia accounted for 3% of the latter category of cases in our sample, but the most common causes of SCA were SCA2 and SCA6.
Full text
PDF


Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Burke J. R., Ikeuchi T., Koide R., Tsuji S., Yamada M., Pericak-Vance M. A., Vance J. M. Dentatorubral-pallidoluysian atrophy and Haw River syndrome. Lancet. 1994 Dec 17;344(8938):1711–1712. doi: 10.1016/s0140-6736(94)90497-9. [DOI] [PubMed] [Google Scholar]
- Campuzano V., Montermini L., Moltò M. D., Pianese L., Cossée M., Cavalcanti F., Monros E., Rodius F., Duclos F., Monticelli A. Friedreich's ataxia: autosomal recessive disease caused by an intronic GAA triplet repeat expansion. Science. 1996 Mar 8;271(5254):1423–1427. doi: 10.1126/science.271.5254.1423. [DOI] [PubMed] [Google Scholar]
- Cancel G., Dürr A., Didierjean O., Imbert G., Bürk K., Lezin A., Belal S., Benomar A., Abada-Bendib M., Vial C. Molecular and clinical correlations in spinocerebellar ataxia 2: a study of 32 families. Hum Mol Genet. 1997 May;6(5):709–715. doi: 10.1093/hmg/6.5.709. [DOI] [PubMed] [Google Scholar]
- Connarty M., Dennis N. R., Patch C., Macpherson J. N., Harvey J. F. Molecular re-investigation of patients with Huntington's disease in Wessex reveals a family with dentatorubral and pallidoluysian atrophy. Hum Genet. 1996 Jan;97(1):76–78. doi: 10.1007/BF00218837. [DOI] [PubMed] [Google Scholar]
- Dürr A., Cossee M., Agid Y., Campuzano V., Mignard C., Penet C., Mandel J. L., Brice A., Koenig M. Clinical and genetic abnormalities in patients with Friedreich's ataxia. N Engl J Med. 1996 Oct 17;335(16):1169–1175. doi: 10.1056/NEJM199610173351601. [DOI] [PubMed] [Google Scholar]
- Filla A., De Michele G., Cavalcanti F., Pianese L., Monticelli A., Campanella G., Cocozza S. The relationship between trinucleotide (GAA) repeat length and clinical features in Friedreich ataxia. Am J Hum Genet. 1996 Sep;59(3):554–560. [PMC free article] [PubMed] [Google Scholar]
- Genis D., Matilla T., Volpini V., Rosell J., Dávalos A., Ferrer I., Molins A., Estivill X. Clinical, neuropathologic, and genetic studies of a large spinocerebellar ataxia type 1 (SCA1) kindred: (CAG)n expansion and early premonitory signs and symptoms. Neurology. 1995 Jan;45(1):24–30. doi: 10.1212/wnl.45.1.24. [DOI] [PubMed] [Google Scholar]
- Geschwind D. H., Perlman S., Figueroa C. P., Treiman L. J., Pulst S. M. The prevalence and wide clinical spectrum of the spinocerebellar ataxia type 2 trinucleotide repeat in patients with autosomal dominant cerebellar ataxia. Am J Hum Genet. 1997 Apr;60(4):842–850. [PMC free article] [PubMed] [Google Scholar]
- Goldman A., Ramsay M., Jenkins T. Absence of myotonic dystrophy in southern African Negroids is associated with a significantly lower number of CTG trinucleotide repeats. J Med Genet. 1994 Jan;31(1):37–40. doi: 10.1136/jmg.31.1.37. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Imbert G., Saudou F., Yvert G., Devys D., Trottier Y., Garnier J. M., Weber C., Mandel J. L., Cancel G., Abbas N. Cloning of the gene for spinocerebellar ataxia 2 reveals a locus with high sensitivity to expanded CAG/glutamine repeats. Nat Genet. 1996 Nov;14(3):285–291. doi: 10.1038/ng1196-285. [DOI] [PubMed] [Google Scholar]
- Kawaguchi Y., Okamoto T., Taniwaki M., Aizawa M., Inoue M., Katayama S., Kawakami H., Nakamura S., Nishimura M., Akiguchi I. CAG expansions in a novel gene for Machado-Joseph disease at chromosome 14q32.1. Nat Genet. 1994 Nov;8(3):221–228. doi: 10.1038/ng1194-221. [DOI] [PubMed] [Google Scholar]
- Kidd K. K. Can we find genes for schizophrenia? Am J Med Genet. 1997 Feb 21;74(1):104–111. doi: 10.1002/(sici)1096-8628(19970221)74:1<104::aid-ajmg21>3.0.co;2-u. [DOI] [PubMed] [Google Scholar]
- Li S. H., McInnis M. G., Margolis R. L., Antonarakis S. E., Ross C. A. Novel triplet repeat containing genes in human brain: cloning, expression, and length polymorphisms. Genomics. 1993 Jun;16(3):572–579. doi: 10.1006/geno.1993.1232. [DOI] [PubMed] [Google Scholar]
- Orr H. T., Chung M. Y., Banfi S., Kwiatkowski T. J., Jr, Servadio A., Beaudet A. L., McCall A. E., Duvick L. A., Ranum L. P., Zoghbi H. Y. Expansion of an unstable trinucleotide CAG repeat in spinocerebellar ataxia type 1. Nat Genet. 1993 Jul;4(3):221–226. doi: 10.1038/ng0793-221. [DOI] [PubMed] [Google Scholar]
- Pulst S. M., Nechiporuk A., Nechiporuk T., Gispert S., Chen X. N., Lopes-Cendes I., Pearlman S., Starkman S., Orozco-Diaz G., Lunkes A. Moderate expansion of a normally biallelic trinucleotide repeat in spinocerebellar ataxia type 2. Nat Genet. 1996 Nov;14(3):269–276. doi: 10.1038/ng1196-269. [DOI] [PubMed] [Google Scholar]
- Ranum L. P., Lundgren J. K., Schut L. J., Ahrens M. J., Perlman S., Aita J., Bird T. D., Gomez C., Orr H. T. Spinocerebellar ataxia type 1 and Machado-Joseph disease: incidence of CAG expansions among adult-onset ataxia patients from 311 families with dominant, recessive, or sporadic ataxia. Am J Hum Genet. 1995 Sep;57(3):603–608. [PMC free article] [PubMed] [Google Scholar]
- Ross C. A. When more is less: pathogenesis of glutamine repeat neurodegenerative diseases. Neuron. 1995 Sep;15(3):493–496. doi: 10.1016/0896-6273(95)90138-8. [DOI] [PubMed] [Google Scholar]
- Rubinsztein D. C., Amos W., Leggo J., Goodburn S., Ramesar R. S., Old J., Bontrop R., McMahon R., Barton D. E., Ferguson-Smith M. A. Mutational bias provides a model for the evolution of Huntington's disease and predicts a general increase in disease prevalence. Nat Genet. 1994 Aug;7(4):525–530. doi: 10.1038/ng0894-525. [DOI] [PubMed] [Google Scholar]
- Rubinsztein D. C., Leggo J., Amos W., Barton D. E., Ferguson-Smith M. A. Myotonic dystrophy CTG repeats and the associated insertion/deletion polymorphism in human and primate populations. Hum Mol Genet. 1994 Nov;3(11):2031–2035. [PubMed] [Google Scholar]
- Rubinsztein D. C., Leggo J., Coetzee G. A., Irvine R. A., Buckley M., Ferguson-Smith M. A. Sequence variation and size ranges of CAG repeats in the Machado-Joseph disease, spinocerebellar ataxia type 1 and androgen receptor genes. Hum Mol Genet. 1995 Sep;4(9):1585–1590. doi: 10.1093/hmg/4.9.1585. [DOI] [PubMed] [Google Scholar]
- Rubinsztein D. C., Leggo J., Coles R., Almqvist E., Biancalana V., Cassiman J. J., Chotai K., Connarty M., Crauford D., Curtis A. Phenotypic characterization of individuals with 30-40 CAG repeats in the Huntington disease (HD) gene reveals HD cases with 36 repeats and apparently normal elderly individuals with 36-39 repeats. Am J Hum Genet. 1996 Jul;59(1):16–22. [PMC free article] [PubMed] [Google Scholar]
- Sanpei K., Takano H., Igarashi S., Sato T., Oyake M., Sasaki H., Wakisaka A., Tashiro K., Ishida Y., Ikeuchi T. Identification of the spinocerebellar ataxia type 2 gene using a direct identification of repeat expansion and cloning technique, DIRECT. Nat Genet. 1996 Nov;14(3):277–284. doi: 10.1038/ng1196-277. [DOI] [PubMed] [Google Scholar]
- Silveira I., Lopes-Cendes I., Kish S., Maciel P., Gaspar C., Coutinho P., Botez M. I., Teive H., Arruda W., Steiner C. E. Frequency of spinocerebellar ataxia type 1, dentatorubropallidoluysian atrophy, and Machado-Joseph disease mutations in a large group of spinocerebellar ataxia patients. Neurology. 1996 Jan;46(1):214–218. doi: 10.1212/wnl.46.1.214. [DOI] [PubMed] [Google Scholar]
- Zhuchenko O., Bailey J., Bonnen P., Ashizawa T., Stockton D. W., Amos C., Dobyns W. B., Subramony S. H., Zoghbi H. Y., Lee C. C. Autosomal dominant cerebellar ataxia (SCA6) associated with small polyglutamine expansions in the alpha 1A-voltage-dependent calcium channel. Nat Genet. 1997 Jan;15(1):62–69. doi: 10.1038/ng0197-62. [DOI] [PubMed] [Google Scholar]
- Zoghbi H. Y. The expanding world of ataxins. Nat Genet. 1996 Nov;14(3):237–238. doi: 10.1038/ng1196-237. [DOI] [PubMed] [Google Scholar]


