Abstract
Objectives
NREM parasomnias also known as disorders of arousal (DOA) are characterised by abnormal motor and autonomic activation during arousals primarily from slow wave sleep. Dissociative state between sleep and wake is likely responsible for clinical symptoms of DOA. We therefore investigated potential dissociation outside of parasomnic events by using simultaneous 256-channel EEG (hdEEG) and functional magnetic resonance imaging (fMRI).
Methods
Eight DOA patients (3 women, mean age = 27.8; SD = 4.2) and 8 gender and age matched healthy volunteers (3 women, mean age = 26,5; SD = 4.0) were included into the study. They underwent 30–32 h of sleep deprivation followed by hdEEG and fMRI recording. We determined 2 conditions: falling asleep (FA) and arousal (A), that occurred outside of deep sleep and/or parasomnic event. We used multimodal approach using data obtained from EEG, fMRI and EEG-fMRI integration approach.
Results
DOA patients showed increase in delta and beta activity over postcentral gyrus and cuneus during awakening period. This group expressed increased connectivity between motor cortex and cingulate during arousals unrelated to parasomnic events in the beta frequency band. They also showed lower connectivity between different portions of cingulum. In contrast, the greater connectivity was found between thalamus and some cortical areas, such as occipital cortex.
Conclusion
Our findings suggest a complex alteration in falling asleep and arousal mechanisms at both subcortical and cortical levels in response to sleep deprivation. As this alteration is present also outside of slow wave sleep and/or parasomnic episodes we believe this could be a trait factor of DOA.
Keywords: Parasomnias, High density EEG, Functional brain imaging, Slow wave sleep, HdEEG and fMRI integration, Disorders of arousal
Highlights
-
•
NREM parasomnias occur mostly upon arousal from deep sleep stages.
-
•
Integrated hdEEG and fMRI recordings are used to study disorders of arousal.
-
•
We study arousal mechanisms on both cortical and subcortical levels in patients and in controls.
-
•
This multimodal analysis could contribute to better understanding of this disorder.
1. Introduction
Non-rapid eye movement (NREM) parasomnias are characterised by abnormal behaviours mostly arising from slow wave sleep (SWS; stage NREM 3) [1,2]. We distinguish several clinical units, such as sleep walking (SW), sleep terrors (ST) or confusional arousals and its variants [[2], [3], [4]]. These clinical units are also called 'disorders of arousal' (DOA), referring to the current pathophysiological model of NREM parasomnias, which assumes a state dissociation between wake and NREM sleep [4]. There are many factors that could increase the chance of parasomnic event, such as sleep deprivation, alcohol consumption, some types of medication and/or stress (Pressman 2007). Introducing sleep deprivation into experimental protocols to increase the chance of capturing parasomnic event have been widely used [[5], [6], [7], [8], [9]]. The state dissociation means incomplete activation after an arousing stimulus with partial awakening from NREM sleep (mainly from NREM 3 stage of sleep, less likely from NREM 2). This predominant hypothesis is supported by three lines of evidence: electroencephalographic (EEG) studies [[10], [11], [12], [13]], functional imaging studies [5,[14], [15], [16]] and structural imaging studies [17]. EEG studies prove that during the NREM parasomnia episodes, there is a fast beta activity over the motor and cingulate cortices present and concomitant slow delta activity over frontoparietal and associative cortices [18]. This is further supported by functional imaging studies, which identified NREM parasomnia patients to have a coexisting sleep and wake stages and differences of blood flow in many brain areas, such as thalamus and cingulate cortex which remained activated upon awakening [5,16].
It is well known that thalamus participates widely on sleep-wake control [[19], [20], [21], [22], [23]]. Thalamus itself might also play a crucial role in development of DOA events. Among all an abrupt occurrence of wake-like activity from otherwise slow wave sleep was reported in some portions of thalamus while stereo-EEG recording study during confusional arousal (Gibbs et al. 2016).
Furthermore, structural changes were found in NREM parasomnia patients which suggest the occurrence of specific trait factors in this sleep disorder. Heidbreder et al. [17] reported reduction in the grey matter volume in left dorsal posterior cingulate and posterior mid-cingulate cortices.
Concurrently occurring local sleep-like and wake-like activity over frontal, limbic and motor regions seem to be the hallmark of DOA episodes [4]. Our aim was to clarify whether this dissociation or the disturbed sleep-wake mechanisms are present outside the episodes themselves, already during falling asleep and whether this could be seen as a trait-factor of this sleep disorder. We were determined to answer following questions: 1) Is there a difference in thalamic connectivity in DOA patients compared to controls (thalamus-motor cortex, cingulum vs rest of the brain, thalamus-posterocentral gyrus vs. rest of the brain); 2) Is there a difference in hypothalamic connectivity (Ventrolateral preoptic area, posterolateral area) in DOA patients compared to controls (towards thalamus and/or other cortical areas). To answer the question, a series of EEG and fMRI analyses were provided. The following section summarises the working hypotheses and the neuroimaging analysis methods.
2. Methods
2.1. Participants and recording process
Patients with sleep walking and/or sleep terrors (SW/ST) [24,25] who came on their free will to the examination at the Department of Sleep Medicine, National Institute of Mental Health, Klecany, Czech Republic. The diagnosis was based on ICSD-3 criteria [1]. They underwent a medical interview with basic neurological and psychiatric assessment and overnight video-polysomnography (vPSG). We recruited 8 patients (3 women; mean age 27,8; SD = 4,2). We also recruited the group of age and gender matched paid volunteers (8 participants). These volunteers were recruited after responding to online call for volunteers. We used social media such as Facebook and Instagram, where we displayed the short screening questionnaire which tried to identify potential symptoms of sleep disorders. Those who showed potential symptoms of sleep disturbances, such as snoring, cramps and artificial feelings in lower extremities prior to sleep, artificial movement and/or behaviours in sleep, unpleasant dreams, vocalisations or else, were excluded before being invited into the sleep lab. Those who did not show any potential signs of sleep disorders and simultaneously could have been matched to our patients based on age and gender criteria were invited into the sleep lab where they were further examined by sleep specialist and the complex neurological examination along with medical and sleep history was obtained. This process led to further exclusion of some subjects. Those who were included into following phase underwent vPSG to objectively exclude any possible sleep disorder. The inclusion criteria that were mandatory for both patients and controls included 1) the age between 18 and 45 years; 2) no somatic, neurological or psychiatric illness (including claustrophobia), which could interfere with sleep; 3) no medication that could affect sleep. Exclusion criteria included: 1) gravidity or breastfeeding; 2) other sleep comorbidities (apnoe hypopnoea index above 15, periodic leg movement index above 15) and/or concomitant occurrence of rapid eye movement (REM) parasomnias. These data were obtained after analysing of vPSG data by two independent specialists. We also checked for other possible reasons, which might prevent examination in MRI, such as implantation of cardiostimulator or ferromagnetic bone implants, although due to the relatively young age of our cohort we did not exclude any participant based on this. Our subjects were free to terminate their participation in the study at any time.
Simultaneous recording of high density 256-channel electroencephalography (hd-EEG) and functional magnetic resonance imaging (fMRI) was done after 30-32-h of sleep deprivation. The ideal length of sleep deprivation was based on previous studies [[26], [27], [28], [29]]. However, as our main concern was to assure sleep and potentially to reach deep sleep stages, we decided to prolong the sleep deprivation overnight and the next day until after the lunch time to promote postsprandial sleep pressure. This in fact gave us the above mentioned 30–32 h of sleep deprivation. All participants spent this time in the sleep lab and could have used the assistance of medical personnel whenever any potential side effect, from mild to more severe (i.e. epileptic seizure) might have arisen. This also allowed us provide safe environment for them and prevent additional stress that might be caused by traveling to and from our lab. A medical check-up was done by an appointed physician prior to sleep deprivation to check for potential comorbidities that could interfere with the experiment and sleep deprivation such as recent epileptic events, exacerbation of acute or chronic disorder or else. Furthermore, practical details of the experiment were discussed again along with any additional questions. Patients spent their time in our lab in single-bed rooms. They were provided with food and regularly checked by members of personal in order to prevent unintentional napping.
After the sleep deprivation period ended, participants were escorted to another part of the building where the recording took place. They first entered the preparation room where the hd-EEG electrodes were adjusted. The EEG MR-compatible HCGSN (HydroCel Geodesic Sensor Net) 256 channels net was utilised. Due to the length of the measurement, a hydrogel-based material was used.
The MR compatible Geodesic EEG System (GES) 400 from Electrical Geodesics, Inc., Eugene, OR, USA (EGI) was used to measure EEG in the MRI scanner. The system included a Net Amps 400 amplifier, which was controlled by an iMac computer with Net Station software; synchronisation was controlled by the GES Clock Sync I/O device. EEG data were recorded with a sampling frequency of 1 kHz.
The MRI data were collected by using the 3T Siemens Magneton Prisma (Erlangen, Germany) by using the 64-channel head and neck coil. The final length of the examination was no longer than 69 min. Some participants wished to terminate the examination earlier (Participants were free to terminate the recording at any time upon request without explaining the exact reason for doing so). Two of our participants actually did not want to continue the study after 40 min of recording due to unpleasant feelings in the scanner, 1 participant terminated the recording while not sleeping for many minutes). The technical aspects of the procedure as well as statistical analysis were in a great detail described by (Piorecky et al. ,2020) who focused on the method of simultaneous EEG and fMRI recording along with its specific technical features and complications [30] and they are also further specified in following subsections.
After the recording the subjects were taken to their rooms and were offered to spend some time to do personal hygiene (such as washing their hair or else) and they could take a nap before they left the lab. They were also finally checked for any discomfort or potential changes in their medical condition before leaving.
2.2. Scoring of sleep stages
In order to be able to distinguish sleep and particular sleep stages, the EEG data were also analysed by 2 independent sleep specialists in the MatLab environment (The MathWorks, Inc.). The sleep stages were staged based on the standard criteria [31]. NREM 1 stage of sleep was defined as one 30-s epoch in which alpha activity is reduced below 50% of the epoch. NREM 2 stage of sleep was defined by the occurrence of K-complexes and/or sleep spindles. NREM 3 was defined by presence of delta waves occurring in more than 20% of the epoch. These principles were applied on both vPSG data from the night sleep and data EEG data obtained during the nap in MRI scanner. We knew from vPSG data that all the subjects generated alpha rhythm in vPSG. NREM 1 was considered after alpha attenuation was detected and theta waves emerged in minimum of 15 s period. NREM 2 was defined as the period that followed the appearance of first K-complex and/or spindle in the first half of the epoch [31]. We did not find any statistically significant difference in the percentage of particular sleep stages, sleep effectivity, total sleep time or the number of periodic leg movements in sleep and apnoe/hypopnoea index in DOA patients compared to healthy individuals.
Based on the recordings from 256-channel EEG, which was measured during the nap while fMRI was also taken, we determined 2 conditions which were of our main interest. These conditions we falling asleep (FA) and Arousal (A). In our analysis we focused primarily on occipital regions as they are crucial for scoring sleep stages. Falling asleep (FA condition) was defined as a deepening of sleep by attenuation of alpha rhythm, which was generated by all the subjects. This was replaced by low amplitude, mixed frequencies mainly from the theta range lasting minimum of 5 s. The Arousal (A condition) was defined as the occurrence of faster frequencies such as alpha and/or beta frequency preceded by slower frequencies and the minimal length of this segment was 5 s. These segments were defined as such for the purposes of this particular experiment in order to allow for further analyses.
2.3. Selected neuroimaging methods and hypotheses
We used two methods to investigate the alteration of sleep-wake mechanisms during sleep initiation in superficial NREM sleep: the EEG source activation, EEG power seed based functional connectivity, fMRI (blood oxygen level desaturation; BOLD) signal analysis, and multimodal EEG-fMRI analysis. We investigated the activity of thalamus and selected hypothalamic regions.
2.4. Data preparation for multimodal analyses
2.4.1. EEG pre-processing
EEG data contains physiological and technical artefacts along with additional substantial artefacts that occurred during data acquisition in the MR machine environment. The gradient artefact (GA) was suppressed by average artefact subtraction method implemented in fully automated statistical thresholding for EEG artefact rejection (FASTR) [32]. Data corrupted by initiation scans (otherwise referred to as dummy scans) were removed from the time series. After removing GA, the signal was down-sampled from 1 kHz to 250 Hz due to the size of the data files. Hermitian polynomials were used for subsampling. The signal was filtered using a zero-phase two-way finite impulse response (FIR) filter. The lower critical frequency was 0.5 Hz and the upper one was 30.0 Hz. The ECG channel was used to create a template for the detection of cardiobalistic artefacts by the aOBS (adaptive Optimal Basis Set) method [33]. Bad time intervals with high variance values were identified by the Turkey’s method and removed. Bad channels exhibiting variance above the Tuckey’s threshold were subsequently interpolated by the average values of neighbouring electrodes. The eye movement artefacts were corrected by the ICA decomposition approach followed by our quality control clustering algorithm [34]. EEG data subjected to EEG-fMRI data integration were preprocessed in the same way as it was done in our previous methodological paper (Piorecky et al. , 2020).
2.4.2. fMRI pre-processing
As mentioned above, the MRI data were collected by using the 3T Siemens Magneton Prisma (Erlangen, Germany) by using the 64-channel head and neck coil. Before the simultaneous hdEEG and fMRI recording started, we collected anatomical scans with high resolution T1 (3D-MPRAGE with the matrix size of 256 × 256 × 192, 1 mm 3 isotropic cube, flip angel [FA] = 9°; time of repetition [TR] = 2400 ms; Echo time [TE] = 2,27 ms, and time of inversion [TI] = 900 ms). Data from fMRI (Blood Oxygen Level Desaturation; BOLD signal) were pre-processed by using SPM toolbox [35]. The main steps of pre-processing pipeline were: a bias field correction, motion correction, slice-timing correction, normalisation, and spatial smoothing. Bias field correction compensates for the inhomogeneity in signals caused by using the 64-channel coil. If this is not done directly within the MR scanner post-processing utilities, it can be performed as a first step in the pre-processing. In our case, it was done by using the segmentation tool in SPM and estimating a bias field. The bias field is then used to correct the data. The regularisation parameter for bias field correction was set to 0.001, and the FWHM parameter was set to 60. Subsequently, all volumes were realigned by a rigid body transformation to the average volume (selection corresponds to the reference volume in our case). Finally, volumes were normalised to the MNI space and smoothed by a Gaussian of 8 × 8 × 8 voxel size. Extracted parameters of translation and rotation were added as regressors to a GLM.
The head movements were minimised by specially adjusted pillows. The functional scans were afterwards collected using echo planar imaging (EPI) sequence with the following parameters: repetition time (TR) = 1000 ms, echo time (TE) = 30 ms, flip angle = 52°, matrix size = 74 × 74, resolution = 3 × 3 × 3 mm3
Since not all participants completed the whole fMRI session, we adjusted the data length to be the same for all subjects (2549 time points; TR volume). The fMRI data were first bias field corrected for inhomogeneities caused by a 64-channel head coil, and then we used a default pre-processing pipeline in the CONN toolbox (https://web.conn-toolbox.org/) in Matlab (The MathWorks, Inc.) to perform the following steps: the data were first corrected for possible head motion while in the scanner (motion correction), then the slice timing correction was performed to correct the acquisition times of the individual axial slices, then we transformed the data into the MNI space and applied spatial smoothing with 8 mm full width at half maximum (FWHM) kernel. The resulting time series were orthogonalised against signals of white matter (WM) and cerebrospinal fluid (CSF) together with motion parameters (estimated while performing the motion correction step) and then filtered using a band-pass filter with a window of 0.008–0.09 Hz followed by linear detrending.
2.4.3. EEG source localization
In order to localise the sources on EEG we firstly constructed and anonymised anatomical scan from magnetic resonance scans, which was afterwards used as a template for all the subjects in the study. The EEG signal propagation model was constructed based on the 3 data components: electrode positions on the head surface, anatomical tissue organisation and the discretisation grid of candidate EEG sources inside the brain. The electrodes were co-registered with the MRI anatomical scan by using three reference points: nasion, right and left periauricular points. The anatomical scan was segmented into 5 layers: grey matter, white matter, cerebrospinal fluid, skull bones, and scalp. The discretisation grid of EEG sources was based on MNI normalised space (Montreal Neurological Institute) and projected back into the single subject anatomical template. The EEG data were firstly filtered according to the standard EEG frequency bands: delta (1–4Hz), theta (4–8Hz), alpha (8–12 Hz), and beta (12–20 Hz). Within each band data were consequently projected onto the EEG sources area by using ELORETA (Exact Low Resolution Brain Electromagnetic Tomography) inverse algorithm [36] implemented in the Fiedltrip toolbox in MATLAB [37]. The Fieldtrip default values of parameters for the inverse projection were used. Hence, specific activation value corresponding with the data measured from scalp was allocated to each source from the discretisation grid. Moreover, based on its MNI space coordinates each source was given its specific functional area index from the AAL (Automated Anatomical Labelling) atlas [38]. In the regions of interest (see section 2.7), we also calculated the average source activation across time samples during the A condition. Hence, each subject was assigned its specific numeric value of activation for each functional area of interest and one EEG frequency band.
2.5. Neuroimaging methods and data analyses
In this section we define specific neuroimaging methods and hypotheses to evaluate the possibilities stated above. We consider 2 types of methods to achieve this: a) EEG source activation b) EEG power seed-based functional connectivity c) fMRI functional connectivity d) EEG-fMRI data integration.
2.5.1. EEG source activation
The EEG source activation is meant by the authors the EEG activity reconstructed by the EEG Source Localization method under specified conditions within a specific frequency band. Regarding the EEG source activation, we hypothesised increased delta band (1–4Hz) absolute power observed in specific brain areas listed below in patients compared to controls in A condition. Further, we expected differences between patients and controls also in other spectral bands: theta (4–8Hz), alpha (8–12 Hz), and beta (12–20 Hz) EEG bands. Based on the evidence in literature we aimed at the following regions of interest: superior parietal cortex, postcentral gyrus, cuneus, precuneus, and superior occipital cortex. Corresponding methods are described in detail in the following sections.
Statistical hypotheses were tested by using the nonparametric equivalent of t-test, Wilcoxon Rank Sum test separately for each area of interest (Parietal_Sup, Postcentral, Cuneus, Precuneus, and Occipital_Sup) and each specific frequency band (delta, theta, alpha, and beta). Our statistical analysis was also extended by testing of relative spectra, in which the power in each frequency band was divided by the sum of power values across all frequency bands. These calculations were done in the FieldTrip toolbox [37] in MATLAB.
2.5.2. EEG power seed-based connectivity
Based on the results from the literature, we assume that there is a difference in the EEG power seed-based functional connectivity between patients and controls during arousal. For that purpose, we defined three regions of interest (ROIs): motor cortex, anterior cingulate cortex, and posterior cingulate cortex, as well as two frequencies of interest (FOIs): alpha and beta.
On the subject level, we calculated ROI-to-ROI functional connectivity between selected regions based on AAL atlas: anterior cingulate cortex (AAL 31, 32), posterior cingulate cortex (AAL 35, 36), motor cortex (AAL 1, 2, 19, 20, 69, 70); hypothalamic atlas – anterior hypothalamic area, lateral hypothalamus, medial preoptic nucleus, posterior hypothalamic nucleus; Harvard-Oxford subcortical atlas – thalamus, and seed-to-voxel functional connectivity with seeds placed in the thalamus (Harvard-Oxford subcortical atlas) and postcentral gyrus (AAL 57, 58). The subject-level results were then used for the group-level statistical analysis.
To test the hypotheses regarding the difference in the seed-based functional connectivity in EEG defined in the paragraph in section 2, we performed the following analysis. Based on the co-registered AAL atlas with the source model, we defined seed regions for three previously defined ROIs as AAL areas: motor cortex (AAL 1, 2, 19, 20, 69, 70), anterior cingulate cortex (AAL 31, 32), and posterior cingulate cortex (AAL 35, 36). For each source of three orthogonal time series, we computed the first principal component, which was then used as a time series for functional connectivity calculation. As introduced in Hipp et al., 2012 the time series of each dipole was at first orthogonalised to the time series of the selected source in ROI and subsequently, the Pearson’s correlation coefficient between each dipole signal envelope of ROI and all other sources signal envelopes obtained by Hilbert transformation was calculated. In order to obtain one functional connectivity map for every subject, the frequency band and ROI, we averaged connectivity maps of each source in ROI; those maps were then utilised for further group-level statistical analysis. To test the difference in functional connectivity between healthy controls and NREM parasomnia patients we applied in each dipole position a nonparametric equivalent of the t-test, Wilcoxon Rank Sum test, separately for each frequency band and ROI, resulting in 6 statistical maps (2 FOIs x 3 ROIs). In the results section, we report masks of significant voxels with a p-value <0.05 two-tailed test, uncorrected for multiple comparisons (across voxels) because of the small sample and exploratory character of the analysis. The observed significant changes didn’t survive correction for multiple comparisons across voxels. The visualisation was performed in BrainNet Viewer [39].
2.5.3. fMRI functional connectivity
Averaged BOLD time series across each region of interest were cross-correlated between regions to form the functional connectivity matrices. The Pearson’s correlation coefficient was used here to quantify the functional connectivity [40]. The Fisher’s r-to-z transformation was applied to each correlation coefficient in order to increase the normality of the distribution of correlation values. The detailed description of the fMRI functional connectivity analysis is out of the scope of this paper and can be found elsewhere [40].
2.5.4. EEG-fMRI data integration
To further investigate relations between observed EEG and fRMI difference the EEG informed fMRI analysis was performed in EEG delta band. EEG power fluctuation in the delta band was extracted from the EEG recordings (entire measurement process) using dimension reduction methods. Subsequently, convolution was performed with a signal corresponding to the course of the hemodynamic response. In addition to spectral properties, regressors of the awakening period and transition from wake to sleep conditions were also included in the analysis. The delta band power fluctuation was treated as an EEG correlate of bold. Significant correlations between EEG and BOLD fluctuations were found using the general linear model implemented in the SPM toolbox [35]. More detailed description of the EEG informed fMRI analysis can be found in our previous methodological paper [30].
Ethical statement
This study was approved by the research ethic committee of the National Institute of Mental Health (Approval No. 185/17). Written informed consent was obtained from the participants. NREM parasomnia patients did not receive any financial compensation for their participation in our study. Healthy volunteers received 1000 CZK (approx. 41 EUR) in compensation for the time they spent in our laboratory while participating in our experiment.
3. Results
All the enrolled participants completed the study.
3.1. Polysomnographic data
As mentioned above, vPSG was done in all the subjects in order to objectively exclude any possible comorbidities in sleep. We did not find any statistically significant difference between DOA patients and healthy individuals in macrostructural sleep parameters, such as percentage of particular sleep stages, sleep onset latency, REM sleep latency, sleep effectivity and number of periodic leg movements in sleep and apnoe/hypopnoea index.
3.2. EEG source activations
The Electrical Source Imaging (ESI) method was capable of testing differences between patients and controls in their EEG source activations in brain during A condition. We found a significant yet not corrected for multiple comparison across five regions of interest. There was an increase in delta and beta activity in postcentral gyrus and cuneus during awakening periods in patients (Table 1) quantified by the absolute power. These areas were awakened slower in patients compared to controls. Along with the absolute power in all four frequency bands we also analysed relative differences in spectral power, which did not provide any statistically significant differences between NREM parasomnia patients and controls.
Table 1.
Resulting p-Values of the patients > controls difference in EEG absolute power t-Test for each ROI and frequency band.
| ROI/BAND | Delta | Theta | Alpha | Beta |
|---|---|---|---|---|
| Parietal_Sup | 0.115929 | 0.167832 | 0.231702 | 0.140482 |
| Postcentral | 0.036053 | 0.060295 | 0.060295 | 0.02704 |
| Cuneus | 0.046931 | 0.094639 | 0.347164 | 0.231702 |
| Precuneus | 0.167832 | 0.140482 | 0.198446 | 0.060295 |
| Occipital_Sup | 0.094639 | 0.167832 | 0.477545 | 0.389433 |
In Table 1, p-values of the one tailed (patientscontrols) non*parametric t-test are listed. Each p-value denotes probability that patients do not have increased EEG power compared to healthy controls.
The chart depicts p values of 1 tailed nonparametric t-test (patients > controls). The frequency bands and functional areas in which we found statistically significant changes of power in patients relative to controls are displayed in bold. P-values were not corrected for multiple testing across the five ROIs. Along with the absolute power in all four frequency bands we also analysed relative difference in spectral power, which did not provide any statistically significant differences between NREM parasomnia patients and controls.
3.3. EEG power seed-based connectivity
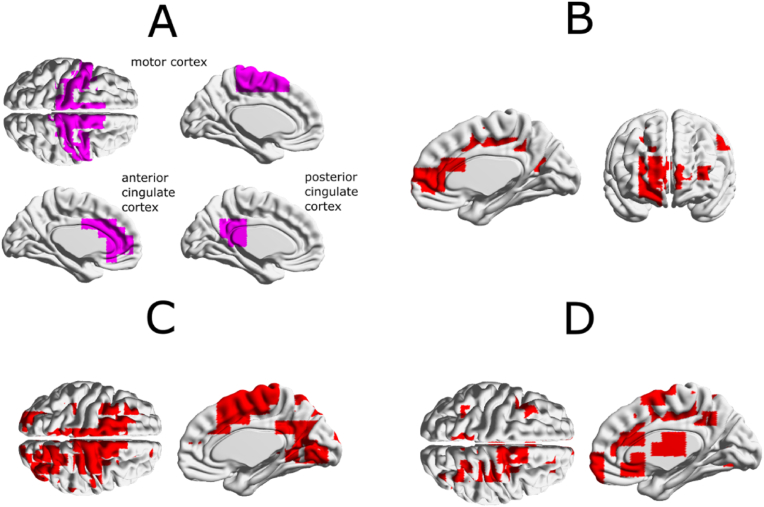
With respect to the above-mentioned stereo-EEG studies we tried to answer the question if there is a difference in a seed-based functional connectivity of selected areas (motor cortex and cingulum) outside of parasomnic events. We found the increased connectivity in parasomnia patients compared to healthy controls between the motor cortex and anterior and posterior cingulate during arousals not related to NREM parasomnia events in the beta frequency band, see Fig. 1.
Fig. 1.
Functional connectivity masks of statistical maps interpolated on the cortical surface for the beta frequency band. (A) ROIs defined according to the AAL atlas: motor cortex, anterior cingulate cortex, posterior cingulate cortex. (B) Statistical mask of difference in „seed-based“ connectivity from motor areas for parasomnias > healthy controls (p<0.05, no multiple testing corrections). (C) Statistical mask of difference in „seed-based“ connectivity from anterior cingulate cortex for parasomnias > healthy controls (p<0.05, no multiple testing correction). (D) Statistical mask of difference in „seed-based“ connectivity from posterior cingulate cortex for parasomnias > healthy controls (p<0.05, no multiple testing corrections). The tested functional connectivity in source space was measured by the Pearson’s correlation coefficient between envelopes of two orthogonalised dipole moment time series (seesection 2.5b).
We analysed functional connectivity in patients compared to healthy controls using both ROI-to-ROI and Seed-to-voxel approaches.
3.4. fMRI functional connectivity
We analysed functional connectivity in patients compared to healthy controls using both ROI-to-ROI and Seed-to-voxel approach.
3.4.1. ROI-to-ROI functional connectivity
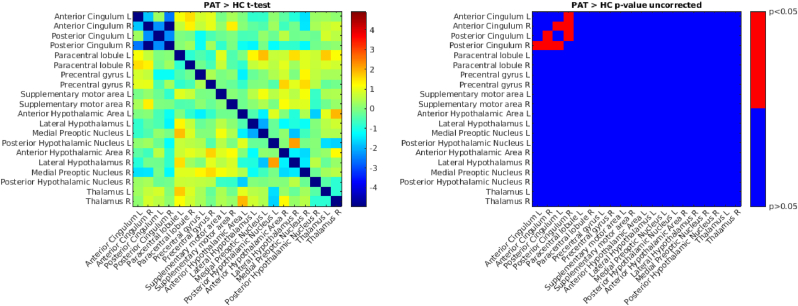
We found lower connectivity in patients compared to healthy controls between right anterior cingulum and right posterior cingulum, left anterior cingulum and right posterior cingulum, left posterior cingulum and right posterior cingulum, and left posterior cingulum and right anterior cingulum (Fig. 2) (see Fig. 3).
Fig. 2.
Comparison of ROI-to-ROI functional connectivity (FC) in patients vs. healthy controls. We used a two sample t-test to compare functional connectivity in patients vs. healthy controls (on the left), on the right is the corresponding matrix of p-values thresholded at p < 0.05 (uncorrected for multiple comparisons). b) Seed-to-voxel Functional Connectivity.
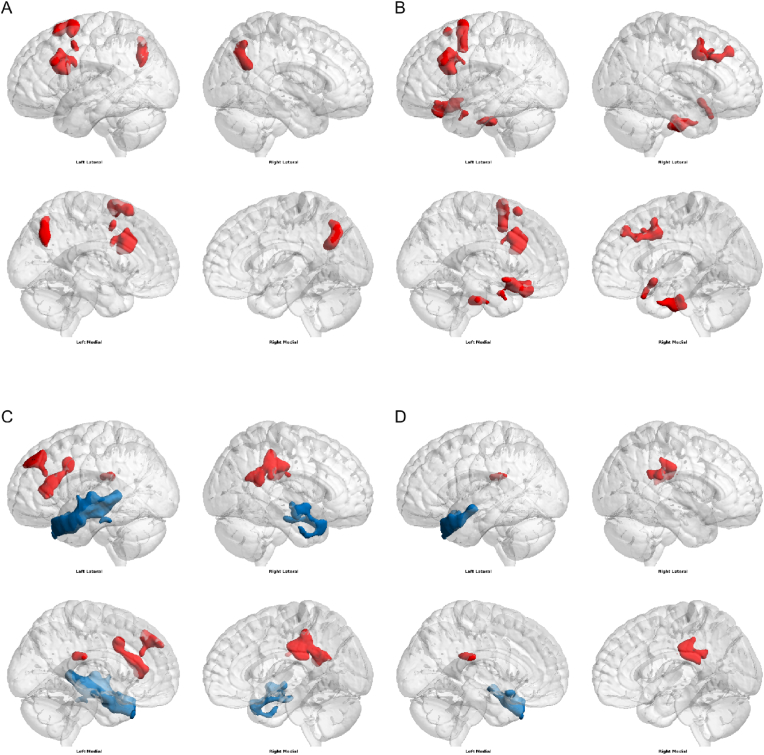
Fig. 3.
Comparison of Seed-to-voxel functional connectivity (FC) in patients vs. healthy controls - greater FC in patients vs. healthy controls (red), lower FC in patients vs. healthy controls (blue). (A) seed in left thalamus, (B) seed in right thalamus, (C) seed in left postcentral gyrus, (D) seed in right postcentral gyrus. Voxel threshold p < 0.05 cluster threshold p < 0.05 (uncorrected for multiple comparisons). The brain networks were visualised with the BrainNet Viewer (http://www.nitrc.org/projects/bnv/) [39]. (For interpretation of the references to colour in this figure legend, the reader is referred to the Web version of this article.)
We found statistically significant differences in connectivity between several regions. See Table 2.
Table 2.
Comparison of Seed-to-voxel functional connectivity (FC) in patients vs. healthy controls.
| Greater connectivity in patients | Lower connectivity in patients | |
|---|---|---|
| Left thalamus | Precuneous Cortex, Middle Frontal Gyrus Left, Superior Frontal Gyrus Left, Inferior Frontal Gyrus, pars opercularis Left, Precentral Gyrus Left, Inferior Frontal Gyrus, pars triangularis Left, Lateral Occipital Cortex, superior division Left | |
| Right thalamus | Middle Frontal Gyrus Left, Middle Frontal Gyrus Right, Brain-Stem, Frontal Orbital Cortex Left, Subcallosal Cortex, Superior Frontal Gyrus Left, Inferior Frontal Gyrus, pars opercularis Left, Precentral Gyrus Right, Frontal Pole Right, Frontal Orbital Cortex Right, Parahippocampal Gyrus, anterior division Left, Precentral Gyrus Left, Inferior Frontal Gyrus, pars triangularis Left, Parahippocampal Gyrus, anterior division Right, Accumbens Left, Frontal Medial Cortex, Accumbens Right, Frontal Pole Left, Temporal Pole Left, Putamen Left. | |
| Left posterior gyrus | Cingulate Gyrus, posterior division, Precentral Gyrus Right, and Postcentral Gyrus Right, Frontal Pole Left, Middle Frontal Gyrus Left, Paracingulate Gyrus Left, Cingulate Gyrus, anterior division, Frontal Orbital Cortex Left, and Insular Cortex Left | Temporal Pole Left, Middle Temporal Gyrus, posterior division Left, Middle Temporal Gyrus, anterior division Left, Superior Temporal Gyrus, anterior division Left, Superior Temporal Gyrus, posterior division Left, Planum Temporale Left, Middle Temporal Gyrus, temporooccipital part Left, Inferior Temporal Gyrus, posterior division Left, Planum Polare Left, Frontal Orbital Cortex Left, Inferior Temporal Gyrus, anterior division Left, Precentral Gyrus Left, Temporal Pole Right, Middle Temporal Gyrus, anterior division Right, Superior Temporal Gyrus, anterior division Right, Middle Temporal Gyrus, posterior division Right, Superior Temporal Gyrus, posterior division Right, Inferior Temporal Gyrus, posterior division Right, Planum Polare Right, and Inferior Temporal Gyrus, anterior division Right. |
| Right postcentral gyrus | Cingulate Gyrus, posterior division | Temporal Pole Left, Middle Temporal Gyrus, anterior division Left, Frontal Orbital Cortex Left, Superior Temporal Gyrus, anterior division Left, Insular Cortex Left, Middle Temporal Gyrus, posterior division Left, Planum Polare Left. |
3.5. EEG-fMRI data integration
During FA condition we found significant activation of thalamus compared to resting state activity in patients, which was not found in healthy controls. Thalamic activation was also observed in both patients and controls during arousals. The condition of FA and delta fluctuation greatly reduced the activations, due to the large number of voxels, we limited the display of activations only to the size of the cluster of activated voxels (n > 5), not by standard statistical correction. The reported results are not statistically significant, but show an interesting trend.
We investigated the thalamic activation during the power fluctuation in the delta frequency band. We found increased activity in thalamic structures in controls but not in NREM parasomnia patients during detection of delta waves on EEG. The amount of delta waves detected in the recording however did not meet the criteria of NREM 3 stage of sleep, yet these waves were captured as a part of mainly NREM 2 stage.
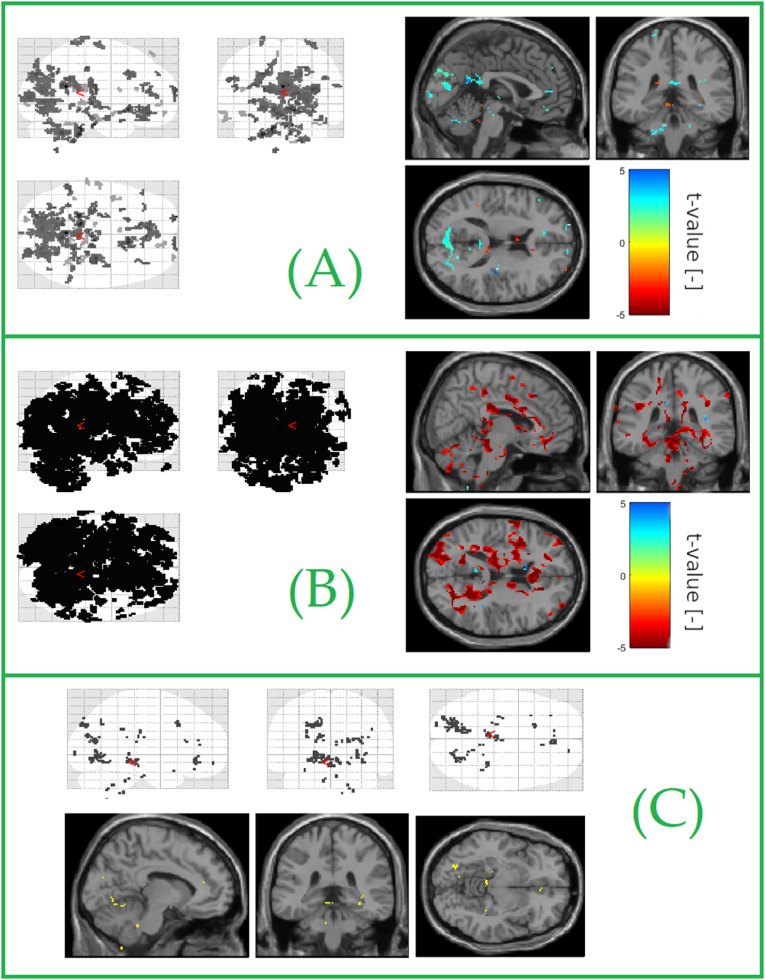
These results were already published by Ref. [30] (Fig. 4). Power fluctuations in delta frequency band in EEG were also accompanied with the BOLD activations of other cortical areas in patients, such as frontal eye field, dorsolateral prefrontal cortex, secondary visual cortex and associated visual cortex, supramarginal gyrus and parahippocampus. Control subjects presented with the activation of secondary visual cortex, retrosplenial cortex and dorsal anterior cingulate cortex.
Fig. 4.
Statistically significant clusters identified by one-sample t-test for: (A) difference of delta covariate versus spontaneous BOLD fluctuation in the control group; glassbrain view (left), orthogonal slices view (right). (B) difference of delta covariate versus spontaneous BOLD fluctuation in patients. (C) Statistically significant clusters identified separately by a single-sample t-test in patients and controls that were common to both groups. Significant activations correspond to voxels correlated with the covariate representing the average change (selected electrodes) of power in the delta band. The figure shows all detected common locations.
In the case of covariates based analysis, we found significant activity in Brodman area 7 and precuneus for the theta average covariate after correction in the control group. In the group of patients, there was significant activity in the lingual gyrus and Brodmann's area 39. These results survived correction for multiple comparison.
No significant activity was identified for the delta band covariates in the control group. In patients, there was significant change in the area of the fusiform gyrus. Hence a large number of voxels we applied FDR and FWE correction.
4. Discussion
Our findings show a large-scale alteration of sleep initiation in NREM parasomnias at both cortical and subcortical levels.
4.1. Dissociative state outside the NREM parasomnia episodes
Several lines of focused research have confirmed the presence of dissociation state during NREM parasomnia episodes [4,41]. The dissociation state which is known to be a pathophysiological basis of NREM parasomnia episodes was convincingly defined based on stereo-EEG studies in patients with epilepsy before the epileptosurgery in which parasomnic episodes were accidently recorded [10,42,43]. The authors found an ongoing delta activity over frontoparietal association network and current occurrence of alpha activity over motor cortex and limbic structures, such as cingulate and temporopolar cortex, insula and amygdala. Our results show increased connectivity of motor cortex and cingulum and thus are in line with these findings. The frequently observed affective response and amnesia on the event are probably the results of limbic structures activation, which are disconnected from the prefrontal cortex. Moreover, parallel deactivation of frontal association areas and hippocampal cortex during the episodes further strengthens this process. Along with these findings, changes of the activity of ventromedial thalamus were observed during these episodes by mild decrease in delta activity and profound increase of beta activity which is similar to the one observed in wakefulness in terms of frequency and amplitude [43]. Although this general topographic pattern is similar in all NREM parasomnic episodes, it could be inferred that each episode presents its specific activity in relevant brain areas which could partly explain the variable clinical presentation. Terzaghi et al. [42] found this dissociative pattern also outside the NREM parasomnic event (outside the behaviour presentation of the episodes). These findings together with described structural changes in NREM parasomnias [17] lead us to the possibility that such dissociative state could be found also outside of these episodes or even outside slow wave sleep. We therefore focused on the possibility to find corresponding changes already during sleep-wake transition, namely falling asleep (FA) and waking up (A) by using simultaneous hd-EEG and fMRI methods which allowed us great spatio-temporal discrimination as well as metabolic functions of both cortical and subcortical brain structures. The measurement was purposefully done after sleep deprivation which itself predisposes to occurrence of parasomnic episodes [44].
During the sleep-wake transition, we found significantly increased delta activity over postcentral gyrus and cuneus as part of the arousals in patients compared to healthy controls. We interpret these findings as slower awakening process in NREM parasomnia patients compared to healthy individuals. It is well known that the sleep-wake transition is not an immediate binary change, but a process of gradual activation of different brain regions; our results suggest that this process is significantly impaired in patients with NREM parasomnias. We cannot rule out an abnormal neuronal excitability of various cortical areas in this group of patients. Postcentral gyrus is functionally related to multisensoric integration and spatial map construction which is then used for spatial orientation and planning of motor activity; it also plays a role in mediation and processing of sensoric inputs with directed motor response [45]. Some of SPECT studies also report decreased regional perfusion in these areas in NREM parasomnic patients [5,15].
Although the time sequence could be debated, many recent studies have confirmed the increase in slow wave activity (SWA) preceding the parasomnic event compared to awakening from NREM 3 without the parasomnic episode and behavioural presentation [13,[46], [47], [48]]. Another spectral analysis study found the coexistence of arousal and deep sleep during 20 s immediately preceding the NREM parasomnia event compared to the same length period 2 min prior to the event [12]. Furthermore, the authors found decreased local functional connectivity for delta frequency band in parieto-occipital regions along with increased connectivity in alpha and beta frequency band over fronto-parietal regions [12]. Our results further confirm the increased connectivity in beta frequency band in motor cortex and cingulum, therefore they are in line with these findings.
To our knowledge only a very limited number of studies have focused on sleep of NREM parasomnia patients outside of parasomnic event or even outside of NREM 3 stage of sleep. Although the decrease of SWA was observed over central regions in these patients also in superficial NREM sleep stages, the authors only focused on the analysis of one or only few channels data [46,49]. Until now there is only one hd-EEG study which was done over the whole night [4]. This study found the exponential decrease of slow wave activity that was slower in patients than in control subjects [4]. The data showed decrease in SWA which was not global but was localised mainly in centro-parietal regions (motor area, premotor area and cingulate areas). According to that study, the changes in SWA topography are present both in NREM and REM sleep and also in wakefulness. Although we did not see the degree of connectivity in delta frequency range, we could argue that our data contribute to the importance of these regions for the NREM parasomnias’ clinical presentation.
To our knowledge only a very few studies used MRI techniques in NREM parasomnia research. In the morphometric study by Heidbreder et al. [17] it was found that there is a significant decrease in the grey matter volume in left dorsal cingulum and intermediate cingulate cortex in NREM parasomnia patients [17]. Although we did not analyse specific morphological parameters in our study sample, we did not detect any clinically significant differences between NREM parasomnia patients and controls in terms of morphological changes or even pathological findings on MRI scans.
As mentioned above, we measured our data under the sleep deprivation protocol which is the one of the most important factors to promote parasomnic events [50]. Somnambulic patients were found to exhibit increased hypersynchronous delta activity in their sleep after sleep deprivation [9], which lead us to the possibility to further strengthened the suspected disturbance in homeostatic regulation of sleep and wake in NREM parasomnia patients by exposing them to prolonged sleep deprivation.
4.2. Potential contribution of different connectivity of thalamus and other structures to the DOA pathology
The central role of thalamus in sleep has been well known. Since the early stages of sleep research it was suggested that stimulation of specific thalamic regions might have initiated sleep in experimental animals [51] ad it is now clear that this happens under the high degree of spatial and temporal organisation [21]. The authors stated that the cortical slow waves observed in EEG in sleep originate in both cortex and thalamus [21]. They are synchronised in the wide brain scale and terminated based on the activity of thalamus relay and local cortical inhibition. Thalamus also receives inputs from hypothalamus and brain stem nuclei and summation of these inputs might play a role in controlling cortical tone and arousal state [52].
NREM sleep is characterised by synchronised electric activity amongst thalamo-cortical network which leads to increased slow wave activity. These oscillations than reflects the recurrent cortical neurons oscillations [53]. Sherman et al. [54] suggested that thalamus plays a role in wide-scale synchronisation of the cortex by providing trans-thalamic link and hence the control mechanism for this synchronisation [54]. The integrative model has also been proposed by Gent et al. (2018) who suggests that cortical active state could be triggered by midline thalamus which receives ascending arousal inputs and the wide-scale cortical synchronisation regulated by higher order thalamus [21]. Evidence also suggests that some thalamic parts, such as midline thalamus or pulvinar might be advanced to the cortex and sensory thalamus in slow waves and sleep onset [19,20,22]. Thalamus might also regulate local sleep by phase-locking of somatosensory thalamus to somatosensory but not frontal cortex, as was found in mouse model [19,20,22]. Motor thalamus might drive a local sleep states in sensory thalamus and thus contribute to attention deficits in sleep deprivation [23].
Considering the physiological background and current knowledge about the role of thalamus in sleep and wake regulation [21], one of these causes could be impaired phase lock between thalamic activity and transition from one stage to another in NREM parasomnia patients that leads to impairment in coordination of sleep and wake transition. Another explanation might be an increased role of local sleep and its effect on various thalamic regions along with the impairment of inputs from arousing areas such as brainstem and/or hypothalamus in NREM parasomnia patients. It has already been suggested that sleep deprivation might lead to increased activation of motor thalamus allowing the sensory thalamus to remain in local sleep leading to commonly seen loss of concentration in sleep deprived individuals [21]. The previously reported deactivation of thalamus while arousals NREM parasomnia patients [14] was not found in our participants. However, the reason for this could be preceding sleep deprivation and also the fact, that our subjects were not awaken from deep sleep stages during parasomnic episode as it was in the study of Bassetti et al. [14].
It is therefore possible that our results indicate an altered homeostatic regulation of sleep and wake with increased role of cortical delta waves generators after sleep deprivations in NREM parasomnia patients. This is further proven by our results that show increased thalamo-cortical connectivity.
Cingulate cortex is a major structure connecting the two brain hemispheres [55]. Anterior cingulate cortex is activated in REM sleep and along with amygdala this could contribute to memory processing during REM sleep [56]. Cerebral blood flow is even more decreased in this area during slow wave sleep [16]. Modern neuroimaging methods based on fMRI showed that sleep deprivation reduces functional connectivity between anterior cingulate cortex and posterior cingulate cortex in healthy individuals [57]. We found different level of connectivity to various brain structures in this area in controls compared to NREM parasomnia patients. It is possible that NREM parasomnia patients react differently to sleep deprivation compared to healthy individuals. Furthermore, some degree of disturbed interhemispheric connectivity could also be suspected.
Parahippocampal activity has been related to REM-like activity that emerges during sleep onset and might be responsible for vivid hypnagogic images [58]. REM-like parahippocampal slow activity occurs as a response to hypothalamic switch off effect during falling asleep and thus seems to further inhibit hippocampal activity during transition from wake to sleep. We found increased connectivity between thalamus and parahippocampal gyrus in DOA patients compared to controls. This could suggest increased tendency of NREM parasomnia patients to generate vivid visual imagery during sleep. It is well known that vivid dreams with very complex content are one of the symptoms of NREM parasomnias [59]. The fact that we did not see this activation in healthy controls who did not report vivid dreams in their medical history could be an explanation of this phenomenon.
4.3. Conclusion
Our results suggest an alteration of the sleep-wake transition in patients with NREM parasomnias in response to sleep deprivation. This alteration results from changes in neuronal activation and connectivity at both subcortical and cortical levels. Although we did not measure morphological parameters of the regions of interest, we suggest that the underlying causes of this alteration of sleep initiation lie in the disruption of the homeostatic regulation of sleep and wakefulness controlled by the thalamus and the thalamic response to certain stimuli, such as sleep deprivation, which may be altered in patients with NREM parasomnias.
It could be debated whether the dissociation that we observed during falling asleep is a characteristic symptom of this sleep disorder as this might also be an increased tendency of local motor network to reduce the arousal threshold as an adaptive mechanism. We know that such a mechanism is found in nature, for example dolphins can perform a complex motor tasks such as swimming even while sleeping [60]. Thus, similar mechanism in humans might have been an evolutionary advantage allowing immediate motor response in life threatening situations.
4.4. Implications for further research
The role of thalamus and other structures that we saw to be specifically activated in patients and controls during production of delta waves needs further examination by using the combination of EEG and fMRI methods in order to confirm their role in this mechanism and possibly by increasing the sample size also show the degree of this difference by more precise statistical analysis. Further research should therefore focus on these analyses in both transitional sleep stages and slow wave sleep. In relation to NREM parasomnias, these observations should be done also during and along the parasomnic events.
4.5. Limitations
The chosen combination of modern neuroimaging methods, hd-EEG and fMRI recording, present a number of technical limitations, taken both separately and combined. The detailed description of these issues was published by our previous methodological paper (Piorecky et al. , 2020).
From a clinical point of view, some limitations were brought up by extended sleep deprivation. Although we did not experience any major side effects of the procedure, this might have been challenging for our participants. This was also one of the reasons to complete the pilot study with a limited sample size. As a result, we were not able to always achieve adequate statistical power to provide further statistical comparisons. Confirmation of our findings with increased sample size would certainly be helpful in greater understanding of NREM parasomnias.
Finally, the main limitations can be found in combination of the above-mentioned issues. Due to the extremely noisy environment for EEG registration within an active MRI scanner and the limited number of patients involved in the pilot study we observed only a negligible number of significant differences which survived the correction for multiple comparison in the EEG-fMRI data integration approach. All other results turned out to be insignificant after correction.
Disclosure statement
The project was supported from Programme Cooperatio, Neuroscience Charles University. The authors declare no conflict of interests.
CRediT authorship contribution statement
E. Miletínová: Conceptualization, Methodology, Formal analysis, Data curation, Writing – original draft, Writing – review & editing, Project administration. M. Piorecký: Conceptualization, Methodology, Software, Validation, Formal analysis, Writing – review & editing, Visualization. V. Koudelka: Conceptualization, Validation, Software, Resources, Writing – original draft, Writing – review & editing. S. Jiříček: Software, Validation, Data curation, Writing – original draft, Visualization. D. Tomeček: Software, Data curation, Writing – original draft, All authors have read and agreed to the published version of the manuscript. M. Brunovský: Investigation, Supervision, Funding acquisition. J. Horáček: Conceptualization, Supervision, Funding acquisition. J. Bušková: Conceptualization, Methodology, Formal analysis, Investigation, Data curation, Writing – original draft, Writing – review & editing, Supervision, Project administration, Funding acquisition.
Declaration of competing interest
The authors declare that they have no known competing financial interests or personal relationships that could have appeared to influence the work reported in this paper.
Acknowledgements
We would like to thank to all the contributors to our work who allowed this study to be done. Namely we thank Gabriela Řimnáčová who helped with the project management and recruitment of volunteers. We also thank to the technicians who helped with technical site of the project such as patients' monitoring, EEG and MRI preparation and recording.
References
- 1.American Association Of Sleep Medicine . disorders—third ed. 2014. International classification of sleep. ICSD-3) [Google Scholar]
- 2.Howell M.J. Parasomnias: an updated review. Neurotherapeutics. 2012;9:753–775. doi: 10.1007/s13311-012-0143-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Lopez R., Jaussent I., Dauvilliers Y. Objective daytime sleepiness in patients with somnambulism or sleep terrors. Neurology. 2014;83:2070–2076. doi: 10.1212/WNL.0000000000001019. [DOI] [PubMed] [Google Scholar]
- 4.Castelnovo A., Lopez R., Proserpio P., Nobili L., Dauvilliers Y. NREM sleep parasomnias as disorders of sleep-state dissociation. Nat Rev Neurol. 2018;14:470–481. doi: 10.1038/s41582-018-0030-y. [DOI] [PubMed] [Google Scholar]
- 5.Dang-Vu T.T., Zadra A., Labelle M.A., Petit D., Soucy J.P., Montplaisir J. Sleep deprivation reveals altered brain perfusion patterns in somnambulism. PLoS One. 2015;10 doi: 10.1371/journal.pone.0133474. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Joncas S., Zadra A., Montplaisir J. Paquet A.J. The value of sleep deprivation as a diagnostic tool in adult sleepwalkers. Neurology. 2002;58(6):936–940. doi: 10.1212/wnl.58.6.936. [DOI] [PubMed] [Google Scholar]
- 7.Labelle M.A., Dang-Vu T.T., Petit D., Desautels A., et al. Sleep deprivation impairs inhibitory control during wakefulness in adult sleepwalkers. J Sleep Res. 2015;24(6):658–665. doi: 10.1111/jsr.12315. [DOI] [PubMed] [Google Scholar]
- 8.Pilon M., Montplaisir J., Zadra A.A. Precipitating factors of somnambulism: impact of sleep deprivation and forced arousals. Neurology. 2008;70(24):2284–2290. doi: 10.1212/01.wnl.0000304082.49839.86. [DOI] [PubMed] [Google Scholar]
- 9.Pilon M., Zadra A., Joncas S., Montplaisir J. Hypersynchronous delta waves and somnambulism: brain topography and effect of sleep deprivation. Sleep. 2006;29:77–84. doi: 10.1093/sleep/29.1.77. [DOI] [PubMed] [Google Scholar]
- 10.Terzaghi M., Sartori I., Tassi L., Didato G., Rustioni V., Lorusso G., Manni R., Nobili L. Evidence of dissociated arousal states during NREM parasomnia from an intracerebral neurophysiological study. Sleep. 2009;32:409–412. doi: 10.1093/sleep/32.3.409. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Januszko P., Niemcewicz S., Gajda T., Wolynczyk-Gmaj D., Piotrowska A.J., Gmaj B., Piotrowski T., Szelenberger W. Sleepwalking episodes are preceded by arousal-related activation in the cingulate motor area: EEG current density imaging. Clin Neurophysiol. 2016;127:530–536. doi: 10.1016/j.clinph.2015.01.014. [DOI] [PubMed] [Google Scholar]
- 12.Desjardins M.E., Carrier J., Lina J.M., Fortin M., Gosselin N., Montplaisir J., Zadra A. EEG functional connectivity prior to sleepwalking: evidence of interplay between sleep and wakefulness. Sleep. 2017;40 doi: 10.1093/sleep/zsx024. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Castelnovo A., Riedner B.A., Smith R.F., Tononi G., Boly M., Benca R.M. Scalp and source power topography in sleepwalking and sleep terrors: a high-density EEG study. Sleep. 2016;39:1815–1825. doi: 10.5665/sleep.6162. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Bassetti C., Vella S., Donati F., Wielepp P., Weder B. SPECT during sleepwalking. Lancet. 2000;356:484–485. doi: 10.1016/S0140-6736(00)02561-7. [DOI] [PubMed] [Google Scholar]
- 15.Desjardins M.E., Baril A.A., Soucy J.P., Dang-Vu T.T., Desautels A., Petit D., Montplaisir J., Zadra A. Altered brain perfusion patterns in wakefulness and slow-wave sleep in sleepwalkers. Sleep. 2018;41 doi: 10.1093/sleep/zsy039. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Maquet P., Degueldre C., Delfiore G., Aerts J., Peters J.M., Luxen A., Franck G. Functional neuroanatomy of human slow wave sleep. J Neurosci. 1997;17:2807–2812. doi: 10.1523/JNEUROSCI.17-08-02807.1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Heidbreder A., Stefani A., Brandauer E., Steiger R., Kremser C., Gizewski E.R., Young P., Poewe W., Hogl B., Scherfler C. Gray matter abnormalities of the dorsal posterior cingulate in sleep walking. Sleep Med. 2017;36:152–155. doi: 10.1016/j.sleep.2017.05.007. [DOI] [PubMed] [Google Scholar]
- 18.Hrozanova M., Morrison I., Riha R.L. Adult NREM parasomnias: an update. Clocks Sleep. 2019;1:87–104. doi: 10.3390/clockssleep1010009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Baker R., Gent T.C., Yang Q., Parker S., Vyssotski A.L., Wisden W., Brickley S.G., Franks N.P. Altered activity in the central medial thalamus precedes changes in the neocortex during transitions into both sleep and propofol anesthesia. J Neurosci. 2014;34:13326–13335. doi: 10.1523/JNEUROSCI.1519-14.2014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Gent T.C., Bandarabadi M., Herrera C.G., Adamantidis A.R. Thalamic dual control of sleep and wakefulness. Nat Neurosci. 2018;21:974–984. doi: 10.1038/s41593-018-0164-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Gent T.C., Bassetti C., Adamantidis A.R. Sleep-wake control and the thalamus. Curr Opin Neurobiol. 2018;52:188–197. doi: 10.1016/j.conb.2018.08.002. [DOI] [PubMed] [Google Scholar]
- 22.Magnin M., Rey M., Bastuji H., Guillemant P., Mauguiere F., Garcia-Larrea L. Thalamic deactivation at sleep onset precedes that of the cerebral cortex in humans. Proc Natl Acad Sci U S A. 2010;107:3829–3833. doi: 10.1073/pnas.0909710107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Nir Y., Andrillon T., Marmelshtein A., Suthana N., Cirelli C., Tononi G., Fried I. Selective neuronal lapses precede human cognitive lapses following sleep deprivation. Nat Med. 2017;23:1474–1480. doi: 10.1038/nm.4433. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Pressman M.R., Bornemann M.C. The ICSD-3 NREM parasomnia section is evidence based resulting from international collaboration, consensus and best practices. J Clin Sleep Med. 2015;11:187–188. doi: 10.5664/jcsm.4474. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Gauld C., Lopez R., Geoffroy P.A., Morin C.M., Guichard K., Giroux E., Dauvilliers Y., Dumas G., Philip P., Micoulaud-Franchi J.A. A systematic analysis of ICSD-3 diagnostic criteria and proposal for further structured iteration. Sleep Med Rev. 2021;58 doi: 10.1016/j.smrv.2021.101439. [DOI] [PubMed] [Google Scholar]
- 26.Siclari F., Bernardi G., Riedner B.A., Larocque J.J., Benca R.M., Tononi G. Two distinct synchronization processes in the transition to sleep: a high-density electroencephalographic study. Sleep. 2014;37:1621–1637. doi: 10.5665/sleep.4070. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Redcay E., Kennedy D.P., Courchesne E. fMRI during natural sleep as a method to study brain function during early childhood. Neuroimage. 2007;38:696–707. doi: 10.1016/j.neuroimage.2007.08.005. [DOI] [PubMed] [Google Scholar]
- 28.Horovitz S.G., Fukunaga M., De Zwart J.A., Van Gelderen P., Fulton S.C., Balkin T.J., Duyn J.H. Low frequency BOLD fluctuations during resting wakefulness and light sleep: a simultaneous EEG-fMRI study. Hum Brain Mapp. 2008;29:671–682. doi: 10.1002/hbm.20428. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Huster R.J., Debener S., Eichele T., Herrmann C.S. Methods for simultaneous EEG-fMRI: an introductory review. J Neurosci. 2012;32:6053–6060. doi: 10.1523/JNEUROSCI.0447-12.2012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Piorecky M., Koudelka V., Miletinova E., Buskova J., Strobl J., Horacek J., Brunovsky M., Jiricek S., Hlinka J., Tomecek D., Piorecka V. Simultaneous fMRI-EEG-based characterisation of NREM parasomnia disease: methods and limitations. Diagnostics. 2020;10 doi: 10.3390/diagnostics10121087. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Berry R.B., Brooks R., Gamaldo C., Harding S.M., Lloyd R.M., Quan S.F., Troester M.T., Vaughn B.V. AASM scoring manual updates for 2017 (version 2.4) J Clin Sleep Med. 2017;13:665–666. doi: 10.5664/jcsm.6576. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Niazy R.K., Beckmann C.F., Iannetti G.D., Brady J.M., Smith S.M. Removal of FMRI environment artifacts from EEG data using optimal basis sets. Neuroimage. 2005;28:720–737. doi: 10.1016/j.neuroimage.2005.06.067. [DOI] [PubMed] [Google Scholar]
- 33.Marino M., Liu Q., Koudelka V., Porcaro C., Hlinka J., Wenderoth N., Mantini D. Adaptive optimal basis set for BCG artifact removal in simultaneous EEG-fMRI. Sci Rep. 2018;8:8902. doi: 10.1038/s41598-018-27187-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Piorecky M., Koudelka V., Strobl J., Brunovsky M., Krajca V. Artifacts in simultaneous hdeeg/fmri imaging: a nonlinear dimensionality reduction approach. Sensors. 2019;19(20):4454. doi: 10.3390/s19204454. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Keator D.B., Gadde S., Grethe J.S., Taylor D.V., Potkin S.G., First B. A general XML schema and SPM toolbox for storage of neuro-imaging results and anatomical labels. Neuroinformatics. 2006;4:199–212. doi: 10.1385/ni:4:2:199. [DOI] [PubMed] [Google Scholar]
- 36.Pascual-Marqui R.D. 2007. Discrete, 3D distributed, linear imaging methods of electric neuronal activity. Part 1: exact, zero error localization. arXiv preprint arXiv:0710.3341. [Google Scholar]
- 37.Oostenveld R., Fries P., Maris E., Schoffelen J.M. FieldTrip: open source software for advanced analysis of MEG, EEG, and invasive electrophysiological data. Comput Intell Neurosci. 2011 doi: 10.1155/2011/156869. 2011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Tzourio-Mazoyer N., Landeau B., Papathanassiou D., Crivello F., Etard O., Delcroix N., Mazoyer B., Joliot M. Automated anatomical labeling of activations in SPM using a macroscopic anatomical parcellation of the MNI MRI single-subject brain. Neuroimage. 2002;15:273–289. doi: 10.1006/nimg.2001.0978. [DOI] [PubMed] [Google Scholar]
- 39.Xia M., Wang J., He Y. BrainNet Viewer: a network visualization tool for human brain connectomics. PLoS One. 2013;8 doi: 10.1371/journal.pone.0068910. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Jajcay L., et al. Brain functional connectivity asymmetry: left hemisphere is more modular. Symmetry. 2022;14(4):833. [Google Scholar]
- 41.Camaioni M., Scarpelli S., Gorgoni M., Alfonsi V., De Gennaro L. EEG patterns prior to motor activations of parasomnias: a systematic review. Nat Sci Sleep. 2021;13:713–728. doi: 10.2147/NSS.S306614. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Terzaghi M., Ratti P.L., Manni F., Manni R. Sleep paralysis in narcolepsy: more than just a motor dissociative phenomenon? Neurol Sci. 2012;33:169–172. doi: 10.1007/s10072-011-0644-y. [DOI] [PubMed] [Google Scholar]
- 43.Sarasso S., Pigorini A., Proserpio P., Gibbs S.A., Massimini M., Nobili L. Fluid boundaries between wake and sleep: experimental evidence from Stereo-EEG recordings. Arch Ital Biol. 2014;152:169–177. doi: 10.12871/0002982920142311. [DOI] [PubMed] [Google Scholar]
- 44.Pressman M.R. Factors that predispose, prime and precipitate NREM parasomnias in adults: clinical and forensic implications. Sleep Med Rev. 2007;11:5–30. doi: 10.1016/j.smrv.2006.06.003. discussion 31-3. [DOI] [PubMed] [Google Scholar]
- 45.Chen T.L., Babiloni C., Ferretti A., Perrucci M.G., Romani G.L., Rossini P.M., Tartaro A., Del Gratta C. Human secondary somatosensory cortex is involved in the processing of somatosensory rare stimuli: an fMRI study. Neuroimage. 2008;40:1765–1771. doi: 10.1016/j.neuroimage.2008.01.020. [DOI] [PubMed] [Google Scholar]
- 46.Espa F., Ondze B., Deglise P., Billiard M., Besset A. Sleep architecture, slow wave activity, and sleep spindles in adult patients with sleepwalking and sleep terrors. Clin Neurophysiol. 2000;111:929–939. doi: 10.1016/s1388-2457(00)00249-2. [DOI] [PubMed] [Google Scholar]
- 47.Guilleminault C., Poyares D., Aftab F.A., Palombini L. Sleep and wakefulness in somnambulism: a spectral analysis study. J Psychosom Res. 2001;51:411–416. doi: 10.1016/s0022-3999(01)00187-8. [DOI] [PubMed] [Google Scholar]
- 48.Perrault R., Carrier J., Desautels A., Montplaisir J., Zadra A. Electroencephalographic slow waves prior to sleepwalking episodes. Sleep Med. 2014;15:1468–1472. doi: 10.1016/j.sleep.2014.07.020. [DOI] [PubMed] [Google Scholar]
- 49.Gaudreau H., Joncas S., Zadra A., Montplaisir J. Dynamics of slow-wave activity during the NREM sleep of sleepwalkers and control subjects. Sleep. 2000;23:755–760. [PubMed] [Google Scholar]
- 50.Pressman M.R. Factors that predispose, prime and precipitate NREM parasomnias in adults: clinical and forensic implications. Sleep Med Rev. 2007;11:5–30. doi: 10.1016/j.smrv.2006.06.003. [DOI] [PubMed] [Google Scholar]
- 51.Akert K. The anatomical substrate of sleep. Prog Brain Res. 1965;18:9–19. doi: 10.1016/s0079-6123(08)63581-5. [DOI] [PubMed] [Google Scholar]
- 52.Herrera C.G., Cadavieco M.C., Jego S., Ponomarenko A., Korotkova T., Adamantidis A. Hypothalamic feedforward inhibition of thalamocortical network controls arousal and consciousness. Nat Neurosci. 2016;19:290–298. doi: 10.1038/nn.4209. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Timofeev I., Grenier F., Bazhenov M., Sejnowski T.J., Steriade M. Origin of slow cortical oscillations in deafferented cortical slabs. Cerebr Cortex. 2000;10:1185–1199. doi: 10.1093/cercor/10.12.1185. [DOI] [PubMed] [Google Scholar]
- 54.Sherman S.M. Thalamus plays a central role in ongoing cortical functioning. Nat Neurosci. 2016;19:533–541. doi: 10.1038/nn.4269. [DOI] [PubMed] [Google Scholar]
- 55.Wang L., Li K., Zhang Q.E., Zeng Y.W., Jin Z., Dai W.J., Su Y.A., Wang G., Tan Y.L., Yu X., Si T.M. Interhemispheric functional connectivity and its relationships with clinical characteristics in major depressive disorder: a resting state fMRI study. PLoS One. 2013;8 doi: 10.1371/journal.pone.0060191. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Maquet P., Peters J., Aerts J., Delfiore G., Degueldre C., Luxen A., Franck G. Functional neuroanatomy of human rapid-eye-movement sleep and dreaming. Nature. 1996;383:163–166. doi: 10.1038/383163a0. [DOI] [PubMed] [Google Scholar]
- 57.Bosch O.G., Rihm J.S., Scheidegger M., Landolt H.P., Stampfli P., Brakowski J., Esposito F., Rasch B., Seifritz E. Sleep deprivation increases dorsal nexus connectivity to the dorsolateral prefrontal cortex in humans. Proc Natl Acad Sci U S A. 2013;110:19597–19602. doi: 10.1073/pnas.1317010110. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Bodizs R., Sverteczki M., Lazar A.S., Halasz P. Human parahippocampal activity: non-REM and REM elements in wake-sleep transition. Brain Res Bull. 2005;65:169–176. doi: 10.1016/j.brainresbull.2005.01.002. [DOI] [PubMed] [Google Scholar]
- 59.Siclari F., Valli K., Arnulf I. Dreams and nightmares in healthy adults and in patients with sleep and neurological disorders. Lancet Neurol. 2020;19:849–859. doi: 10.1016/S1474-4422(20)30275-1. [DOI] [PubMed] [Google Scholar]
- 60.Mukhametov L.M., Supin A.Y., Polyakova I.G. Interhemispheric asymmetry of the electroencephalographic sleep patterns in dolphins. Brain Res. 1977;134:581–584. doi: 10.1016/0006-8993(77)90835-6. [DOI] [PubMed] [Google Scholar]