Summary of recommendations.
| Topic | Recommendations |
|---|---|
| Positioning |
|
| Immobilisation |
|
| Setup |
|
| Position verification |
|
Introduction
Breast cancer is the second most common malignancy worldwide, representing 11.9% of all diagnoses [1]. A more favourable survival from breast cancer is typically observed in developed regions along with a higher incidence [1]. The meta-analysis of the Early Breast Cancer Trialists’ Collaborative Group (EBCTCG) showed that breast cancer recurrences were decreased by 50% and breast cancer death after 15 years by about 15% when using radiotherapy after breast conserving surgery in patients with breast cancer [2]. More frequently, hypofractionation schemes are used. Several randomised studies reported comparable local control rates and breast cosmesis for the 3-week hypofractionation schedule (40 Gy in 15 fractions) compared to 5-weeks of conventionally fractionated treatment (50 Gy in 25 fractions) [3], [4], [5]. According to Whelan et al., the hypofractionation schedule is more convenient for patients and less costly, which may result in an increase in the number of women receiving whole breast irradiation after breast conserving surgery [5].
With improved survival outcomes, the need to further minimise side effects is of paramount importance. While radiation treatment plans are carefully designed to spare normal tissue, accuracy of treatment delivery is fundamental to ensure that this sparing is achieved for each individual fraction. This accuracy of treatment delivery in turn relies upon the stability and reproducibility of patient positioning in combination with robust setup verification and motion management.
In many countries the five fractions schedule was introduced more rapidly due to COVID-19, based on the results of the FAST and the FAST-Forward trials [6], [7], [8], [9], [10], [11]. The speed of adoption has not given us time to reflect on this, but these hypofractionation schemes demand an increased awareness of daily variations in treatment accuracy and precision due to the higher dose per fraction. In the literature, a wide variety of studies concerning improvement in breast cancer positioning and position verification can be found. However, an overview exploring how best to meet these requirements of accuracy is lacking. This guideline was developed to analyse and discuss the positioning, immobilisation, setup and position verification strategies used for local and loco-regional photon breast cancer irradiation after lumpectomy or mastectomy. It aims to offer practical recommendations to improve the accuracy of breast cancer radiation treatment, and to inform opportunities for future research priorities. This guideline is presented in sections where the authors have distilled the literature to provide recommendations. Furthermore, the authors have included additional considerations in areas for which there is only a limited level of evidence.
Materials and methods
For the literature review the databases of PubMed, Cochrane and Google Scholar were used. The search terms were defined, and the search was performed in January 2019, see Supplementary Table 1 for all search terms. This resulted in 431 studies found in PubMed and Cochrane, and 326 studies on Google Scholar. After removing duplicates, one author selected relevant references based on their titles, the selection was verified by a second author. Pairs of authors were assigned to the following topics: “positioning”, “immobilisation”, “setup” and “position verification” for further review. Each pair selected the references for full text review based on the abstracts. If authors could not reach consensus on inclusion from initial abstract review, then the full paper was reviewed for a more comprehensive assessment. If consensus could still not be reached between the pairs of authors, then additional input from the wider author group was sought. Studies in English, German and Dutch were included. Each pair read the selected manuscripts, assessed them using risk of bias tools for randomised or non-randomised studies [12], [13] and completed evidence tables for their respective topic (Supplementary Tables 2–5). Following group review of the evidence tables, the guideline was written, and recommendations were proposed where appropriately supported by evidence. Aspects of practice considered highly relevant for practitioners but unable to be recommended due to the limitations of research were included as ‘considerations’. Literature published after January 2019 that was considered of importance for this guideline was additionally included. The literature review was complemented with the experiences and the specific knowledge of the globally distributed authors of this guideline. For a comprehensive overview of the contributions of the authors to this guideline we refer to the contribution table.
Two specific points are of importance. Firstly, the literature search term “Breathing” was initially included. It was subsequently decided that literature regarding the effect of respiratory motion on the radiation treatment plan was excluded since this is outside the scope of this guideline describing the end-to-end procedure of positioning the patient. Secondly, numeric values for setup error are reported where available. Studies that calculated relative increases or reductions in calculated Planning Target Volume (PTV) margins (considering institution's specific equipment, workflow and patient population) were acknowledged as such, however advice on specific PTV margins was beyond the scope of this guideline and not discussed. Finally, during the compilation of this guideline new immobilisation devices are in development and early studies have been performed to test these. These early pilot/feasibility studies have not been included in this guideline.
Results, recommendations and considerations
Positioning
Supine vs prone: Whole breast irradiation
From the literature search (Supplementary Table 2), it was evident that in 90% of the studies patients are positioned supine. Two randomised control trials (RCT) were carried out comparing prone with supine treatment. Mulliez et al. executed a RCT to evaluate the acute skin toxicity (dermatitis, pruritus, and pain). The latter was evaluated before treatment, weekly during irradiation and 1–2 weeks after completion of the treatment by a radiation nurse and a radiation oncologist. Prone treatment in patients with larger breasts appears to reduce desquamation, dermatitis, edema and pain significantly compared to supine treatment [14]. In the second RCT Kirby et al. included 26 patients in a cross-over trial; all were imaged in supine and prone position. The investigators found greater setup errors in the prone position, resulting in a larger Clinical Target Volume (CTV) – PTV margin (for chest-wall and clip-based translational errors in 3-dimensions: systematic errors: 1.3–1.9 mm (supine); 3.1–4.3 mm (prone); random errors: 2.6–3.2 mm (supine); 3.8–5.4 mm (prone)). Further optimizing the prone positioning and increasing experience of the staff might be of influence to reduce these larger positioning deviations [15]. A breast-volume threshold for prone radiotherapy was not defined, although both RCTs included patients with breast cup size ≥C. Several authors tried to define predictors for defining the most optimal position, supine or prone treatment. Unfortunately, a widely applicable predictor that predefines the optimal individual treatment position cannot be derived from these studies, since no overlapping predictor has been found[16], [17], [18], [19], [20]. Furthermore, the literature search included a large variety of cohort studies with various study objectives, these were assessed with a focus on comparing supine and prone treatment positions. From this it can be concluded that when the heart dose is the most important factor, supine Deep Inspiration Breath-hold (DIBH) treatment appears to be the best option. However, when lung dose is of importance as well, the prone treatment can be an option as the breast tissue falls anteriorly and away from the lung [17], [18], [21], [22]. The RCT of Bartlett et al., comparing supine voluntary breath-hold (VBH) in left-sided breast cancer with prone treatment, showed that supine VBH provided superior cardiac sparing and reproducibility than a free-breathing prone position in larger-breasted women (CTV volume >1029 cm3) [23]. Even in free-breathing, Kahán et al. reported that 1 in 5 women had higher dose to cardiac structures when positioned prone compared to supine [19]. In two systematic reviews more specific information concerning the heart and lung dose was described extensively [24], [25].
Other studies focused on different variables when performing prone breast cancer treatment. Mitchell et al. states that there is a need for a larger CTV-PTV margin when treating patients in the prone position imaged with an Electronic Portal Imaging Device in cine mode. The image analysis was therefore limited to in plane movement missing lateral or rotational errors [26]. Buijsen et al. showed that for patients with larger breasts the dose homogeneity can be improved in prone position, although a lower PTV coverage was reported [27]. A meta-analysis published in 2021 compared prone and supine treatment in free breathing, in patients with breast cancer after breast-conserving surgery without metastasis, suggesting that prone resulted in better heart sparing. Due to the low numbers of studies, the prone versus supine treatment in breath-hold was not compared [28].
Concerning the outcome of the prone treatment from the RCT performed by Vakaet et al., it appeared that cosmesis (non-blinded analysis using the BCCT.core classification [29]) was good or excellent in 92% and 75% of patients who used prone and supine positioning, respectively. The physician-assessed toxicity at 5 years was not different except for pigmentation changes measured on the LENT-SOMA scale, the 5-year overall survival was equal in both groups [30]. A better cosmesis was obtained because of a significantly better homogeneity of the isodoses in the breast in the prone position compared to supine [14]. A good cosmesis was confirmed by other studies as well. Etin-Osa et al. reported that with a median follow-up time of five years, hypo-fractionated breast radiotherapy (RT) with a simultaneous integrated boost in the prone position resulted in excellent cosmesis (patient reported) and normal tissue sparing. Longer follow-up is needed to confirm the efficacy and safety of this approach [31]. Based on the physician-assessed Harvard scale of cosmetic outcome [32] Bergom et al. found that 86% of the patients with breast volumes >1200 cm3 reported good to excellent cosmesis [33]. Finally, according to Yu et al. and Kahan et al. [19], [21] the prone position puts higher demands on staff and patient compliance. Huppert et al. described that pain from the neck and spine muscles was a common complaint. They stated that caution should be taken in women with history of neck injury or disk problems [34].
Supine vs prone: Loco-regional treatment
For loco-regional treatment, 11 articles were reviewed. Csenki et al. performed the largest study, they compared prone and supine position in free breathing in 100 patients and showed that in most cases the intended doses to axillary levels I–III and the internal mammary (IM) lymph nodes were inadequate, regardless of the treatment position. In this treatment planning study the nodal doses were significantly lower in the prone than in the supine position [35]. Alonso-Basanta et al. confirmed the latter, they compared prone or supine positioning in 20 patients for nodal treatment. On average, the mean dose to the nodal region levels I-III was 50% less in the prone as compared with the supine position [36]. However, in 2012 they reported that IMRT improved the target coverage for both positions [37]. Sethi et al. also advised that a larger cut-out in the prone breast board is needed to allow access to both breast and nodal volumes [37].
Deseyne et al. and Speleers et al. from Ghent University Hospital performed two treatment planning studies in small cohorts (5 and 6 patients respectively) and reported good target coverage (breast and nodal volumes) and less dose in the organs at risk when prone position was compared to supine treatment in free breathing [38], [39]. Deseyne et al. found significantly reduced doses for ipsilateral lung, thyroid, contralateral breast, contralateral lung and oesophagus in prone treatment [38]. Speleers et al. described that mean doses to organs at risk (OAR) were generally lower for prone crawl than for supine positions and for proton than for photon plans. Dose in the left anterior descending coronary artery, lungs, ipsilateral lung and thyroid was lower for prone photon and proton treatment [39]. Recently they described the dosimetric effect of DIBH in prone nodal treatment in 31 patients. They found that also for loco-regional treatment, the combination of prone positioning and DIBH will allow for achieving substantially lower heart (an average reduction of 2 Gy when applying DIBH) and lung doses (left mean lung dose was decreased by 13% when using DIBH in photon therapy and 21% in proton therapy) than supine or prone in shallow breathing and supine DIBH, in both photon and proton treatments [40], [41]. From an earlier study, it appeared that the patients experienced discomfort in the prone position caused by bilateral arm elevation. Therefore, the Belgian team developed a dedicated breast board in which the patients lie in a prone crawl position. The ipsilateral arm alongside the body was reported to be more comfortable, especially after axillary node dissection [42].
Shin et al. described the prone position of radiation treatment after mastectomy [43]. The outcome was promising. Prone hypofractionated breast, chest wall, and nodal radiation therapy was safe and well-tolerated in this study. 4% of the patients were rescanned in supine position to better spare the heart. None of the patients experienced grade 2 acute skin toxicity; concerning late toxicity 1 grade 3 breast retraction and no grade 2 was found. Although the initial pattern of local and regional control is encouraging, longer follow-up is warranted for efficacy and late toxicity assessment [43].
Lateral decubitus position
Another position variation is the lateral decubitus position. The group of institute Curie in Paris described their experience in large groups of around 1500 patients, in the period 1996–2014. They found a large dose reduction in the heart, ipsilateral lung and contralateral breast [44], [45], [46]. Moreover, they noted that the lateral decubitus position was well-tolerated and showed excellent dosimetric and clinical results. The cosmetic outcome was good or excellent in 81–85% of the patients [46], [47]. Davidson et al. assessed the setup accuracy of electron boosts delivered in the lateral decubitus position. The authors reported larger positioning deviations than expected in the supine position, including seven of 33 patients that demonstrated average table shifts of 2 cm or more [48]. Bronsart et al. addressed this as well. They stated that the increased complexity was a disadvantage of this positioning method, and advised for an experienced team, including a dedicated patient board [46].
Recommendations
-
•
Based on the literature and the current equipment we recommend the supine position as the standard for most treatments, see the recommendations when prone positioning is advised below. This is also in line with the commentary of Haffty: “Supine is the widely accepted norm, and simplest approach” [49].
-
•
Supine is advantageous when combined with Surface Guided Radiotherapy (SGRT) since the breast is visible for the systems.
-
•
It must be noted that prone and supine comparison studies are mostly performed more than 10 years ago, therefore research could be of added value considering technical improvements in radiotherapy treatment.
-
•
Prone holds value for improving dose homogeneity, which might result in better cosmesis, and reducing lung and skin-fold dose but can be challenging to implement and a dedicated team is needed.
-
•
For patients with larger breasts or patients that require a higher degree of lung sparing, prone may be considered if the equipment and expertise are available, and the patient can tolerate the position.
-
•
Unfortunately, a widely applicable predictor that predefines the optimal individual treatment position cannot be derived from these studies, since no overlapping predictor has been found.
-
•
For more experienced departments treatment in prone position for loco-regional radiation treatment and partial breast irradiation is achievable; outcomes reported are promising, however research is needed to confirm the findings up until now.
-
•
Concerning the variation in nodal dose coverage in the prone position compared to the supine position that are reported in the literature it is recommended to perform comparison studies with modern radiation therapy techniques in the future. The suitability of specific prone positioning devices for treatments with nodal involvement must be carefully evaluated by individual departments based on their local planning technique.
Considerations
-
•
The lateral decubitus position has been shown to be an option in a centre with considerable expertise in adopting this position. Reproducibility may be an issue and it is not certain that nodal irradiation could be delivered in this position. This treatment position is more complex and demands a dedicated team. Further research is needed including data regarding how well this position is maintained across different breast volumes.
-
•
Several studies describe the outcome of Accelerated Partial Breast Irradiation (APBI) in prone position; however, no comparison studies (supine versus prone) have been performed for APBI.
-
•
In addition to stability and comfort, patient experience should also be considered from the perspective of patient preference when evaluating patient position. While there is a lack of evidence in this area, departments are encouraged to engage with patients when evaluating new patient positioning workflows.
Supine positioning one arm up vs both arms up
Goldsworthy et al. randomised 50 patients between bilateral arm and unilateral arm abduction. They concluded that with bilateral arm abduction a reduction in the systematic error and inter-patient variability could be achieved. Bilateral arm abduction was a more stable and reproducible position (significantly lower translational displacement: 3.1 mm versus 5.3 mm; and population systematic errors 1.9 mm versus 2.7 mm) [50]. In addition, Graham et al. simulated thirty patients in a randomised trial in both an armrest and a vacuum bag. The patients were also randomised between treatment in one of the two devices. Overall, patient comfort significantly favoured the use of the armrest, although both were acceptable. Treatment times and stability of the setups were not significantly different [51].
Xiang et al. positioned patients on a supine breast bracket, using an immobilisation mould, with both arms abducted and hands either holding a single-pole or double-pole position (both hands holding separate poles). The single-pole position was perceived by patients as being more comfortable and reduced heart doses, when compared to the double-pole position [52]. However, the results might be different in a cohort of patients not using moulds. Saito et al. scanned patients with breast cancer in two arm positions: ipsilateral arm at 90°to the body axis; and both arms above the head. When the arm position changed to two arms above the head, level I lymph nodes moved anteriorly and medially and level II and III axillary nodes moved posteriorly and medially, resulting in under and overdosage of the target volumes. To note the dose distribution to each lymph node level was determined using historically designed fields in each arm position. A limitation was that the findings were based on anatomic landmarks instead of delineated lymph node levels [53]. Finally, Kapanen et al. retrospectively studied two arm positions using: the house-made rod-hold (RH) or the standard wrist-hold (WH). With the RH, the irradiated volumes of the humeral head were approximately 2 times larger than with the WH. Daily image guidance was recommended because of large random position errors obtained for the arm position with both devices [54].
Recommendations
-
•
Both arms up are considered more stable from one randomised study, in this study significantly lower translational displacements were found.
-
•
Other cohort studies conclude that the single arm position and armrest are experienced as more comfortable by patients. Therefore, one arm up may be considered for patients that cannot tolerate both arms up.
-
•
Goldsworthy et al. described the contralateral arm position as “abducted to the side of the patient or across her waist” [50].
Considerations
-
•
According to the experiences of the authors, with both arms up the patient is lying more symmetrically, which could be helpful in positioning the patient.
-
•
Of importance is that the position of the arm can influence the localisation of nodal volumes. Daily image guidance may be necessary to verify the arm position.
-
•
To note, centres might avoid a both arms up technique due to potential collision with the CT bore or the linac gantry. It might be of value to investigate whether the position of the patient can be adapted, e.g., treat the patient in an inclined or flat position.
-
•
It is important to note that none of the abovementioned studies include the patient’s Body Mass Index (BMI), therefore it is unclear whether findings are applicable to patients of larger body habitus and BMI.
-
•
Regarding the ability of the patient to adequately mobilise the shoulders, several RCTs report that physiotherapy improves shoulder function after surgery [55], [56], [57], [58], [59]. The coordination of radiotherapy and physiotherapy after the operation can be challenging in some departments, as it is resource intensive, and physiotherapy may not be readily available.
Flat vs elevated
As described in paragraph 1.1.1 and 1.1.2 patients are most often positioned in supine position lying flat or on an inclined positioning device at a fixed angle. In a cohort study, 10 patients with left-sided breast cancer were CT scanned in the flat position and the elevated position. The patients were treated with whole breast irradiation, making use of two tangential fields. It was found that the PTV moves cranially with the patient lying in the flat position. The dose outside the PTV in the nodal area was 30 Gy in the elevated position vs 23 Gy in the flat position (p < 0.01) [60]. However, flat positioning allows greater gantry clearance for a range of imaging and treatment modalities. An elevated position has been used historically for improving conformity of conventional planning techniques, which is generally no longer a consideration. When using an inclined position Jain et al. showed that a foot support is of importance to avoid the patient shifting inferiorly during the treatment process [61].
Recommendations
-
•
Based on clinical experiences both flat and elevated positions are acceptable provided collision risks are managed, and the patient is appropriately stabilised and comfortable.
-
•
It could be of benefit to some patients with larger body habitus to be slightly inclined/elevated to decrease cranial target movement and decrease the irradiation of additional healthy tissue.
Considerations
-
•
While lacking formal evidence, anecdotally the authors strongly advise the use of positioning aids, e.g., supine breast boards, which can be indexed to both the treatment couch and skin reference marks for efficient and accurate patient positioning.
-
•
As far as the authors are aware, there is a lack of studies directly comparing OAR dose, reproducibility, or comfort between flat or elevated positions.
Breast immobilisation
In addition to general patient positioning considerations discussed in the section prior, more specialised immobilisation devices can be employed with the aim of stabilising the breast in a position more advantageous for treatment planning. A total of 16 articles were reviewed in the topic of breast RT immobilisation device and the 7 articles included had low or moderate risk of bias, Supplementary Table 3.
The most common methods of breast immobilisation within the reviewed papers related to the use of an external thermoplastic mould or treatment bra in the supine position. Arenas et al. examined the impact of a plastic treatment bra on plan dosimetry in 12 patients with early-stage breast cancer with large (D cup) or pendulous breasts. Plans generated for each patient with and without the treatment bra demonstrated a significant reduction in PTV and irradiated (V95) volumes with bra use. Mean heart and lung dose were significantly reduced by 66.7% (1.4 vs 4.9 Gy) and 65.6% (3 vs 8 Gy) with bra use, respectively. Of note, this study was performed under free-breathing therefore the benefit of a treatment bra to heart-sparing together with DIBH cannot be confirmed. Conversely, phantom measurements within the study indicated that skin dose increased with bra use by a factor of approximately 1.5 [62].
Shi et al. reported similar findings from a retrospective cohort study comparing patients immobilised with an upper body thermoplastic mould to a control group standardly positioned on an elevated wing board. Significant reductions in heart and lung dose were found with the use of this immobilisation mould, at no compromise to PTV coverage. Though skin dose was not assessed, the descriptive analysis reported erythema in 9% more patients treated with a thermoplastic mould than in the group treated without a mould. Of the patients treated with a thermoplastic mould, 80% of the proportion reported pain and skin tenderness at 3-months post-radiotherapy, 9% had grade 3 symptoms [63]. A phantom study by Kelly et al. investigating skin dose from varying thicknesses of breast thermoplastic moulds and reported dose increases of up to 62% [64].
Breast setup reproducibility with immobilisation was explored in a sample of 16 patients, eight of whom had a thermoplastic mould created from the neck to the whole breast. However, no improvement in position accuracy was found based on daily Megavolt CT (MVCT) matching [65].
Kawamura et al. evaluated the setup reproducibility of 35 patients with pre-operative breast cancer in the prone position with and without a modified fabric bra. Repeated MRI scans were used to track both external breast contour and tumour location. Increased stability in tumour location was found with bra use, though differences were on average <1 mm [66].
In addition to treatment bras and thermoplastic moulds, several studies described the use of more specialised devices for other radiation treatment technologies. A pre-clinical feasibility study by Arimura et al. reported the development of a hybrid breast immobilisation system for proton therapy. Combining whole body immobilisation with a 3D-printed breast cup has been shown to achieve a high level of breast stability, including mitigation of respiratory motion in preliminary results [67]. In a similarly specialised context, Snider et al. carried out a planning study of 15 patients testing a breast-specific stereotactic treatment machine, the GammaPod. Patients were positioned in the prone position on a custom treatment couch with a vacuum-assisted breast cup, which the authors report as validated for delivering a treatment with a PTV margin of 3 mm [68]. Both technologies are of interest for continued research but are not yet applicable in general clinical contexts.
Recommendations
-
•
There is currently insufficient evidence to support the widespread adoption of any specific type of immobilisation device of the breast.
-
•
Treatment bras or thermoplastic moulds may be beneficial for selected patients with large/pendulous breasts in stabilising breast tissue in a position that enables more effective organs at risk sparing. Studies using moulds in prone treatment or comparing the use of moulds in supine with prone treatment have not been performed yet in patients with large breasts.
-
•
The impact of any immobilisation device on skin dose and subsequent risk of increased toxicity must be carefully evaluated by the local department prior to clinical implementation, and closely monitored thereafter.
Considerations
-
•
Breast immobilisation methods can be complex to reproduce during treatment if they are not implemented with extensive training and clear documentation, i.e., documentation for application and troubleshooting.
-
•
While some methods of immobilisation can give patients more dignity by covering their breasts, immobilisation devices that require the treatment staff to manipulate or position the patient’s breast within the immobilisation device itself can diminish the patient’s experience and make the procedure less dignified and may cause additional discomfort if the patient has developed radiation dermatitis.
-
•
When applying a breast immobilisation device together with SGRT, in-house testing should be undertaken to identify how positioning of the device and its impact on the patient surface is managed within the SGRT workflow.
Setup
A total of sixteen articles were reviewed in relation to setup for breast cancer radiotherapy (Supplementary Table 4). Only studies that included a comparator within the context of the setup process were included, resulting in four articles related to treatments delivered in the supine position. Setup here is defined as the process of reproducing the patient’s planned position prior to each treatment fraction. This is distinguished from initial patient positioning established at CT simulation (discussed in the previous section), and the verification of patient setup during treatment (discussed in the following section). During CT, simulation reference marks are standardly placed on the patient’s skin surface which may be tattoos or non-permanent skin marks. This was studied in an RCT (176 vs 166 patients) to investigate the treatment accuracy of both types of skin marks [69]. Based on weekly portal imaging, no significant difference in random and systematic errors could be identified between the two groups. Additional to considerations regarding setup accuracy, the SuPPORT 4All study reported that permanent tattoos may impact patients’ well-being [70]. Petillion et al. [71] found that the skin mobility makes the lateral skin marks less reliable for anteroposterior patient setup. Setting a calculated vertical couch position was seen to reduce random setup error in the anteroposterior direction from 4.6 mm to 2.2 mm. Furthermore, Gonzalez et al. recently showed that SGRT resulted in a significant increase in the accuracy of surgical clip localisation within the breast compared to skin marker-based setup [72]. SGRT is further discussed in the position verification section of this guideline, and its comparability to other IGRT modalities further supports its potential to replace the role of skin marks.
Recommendations
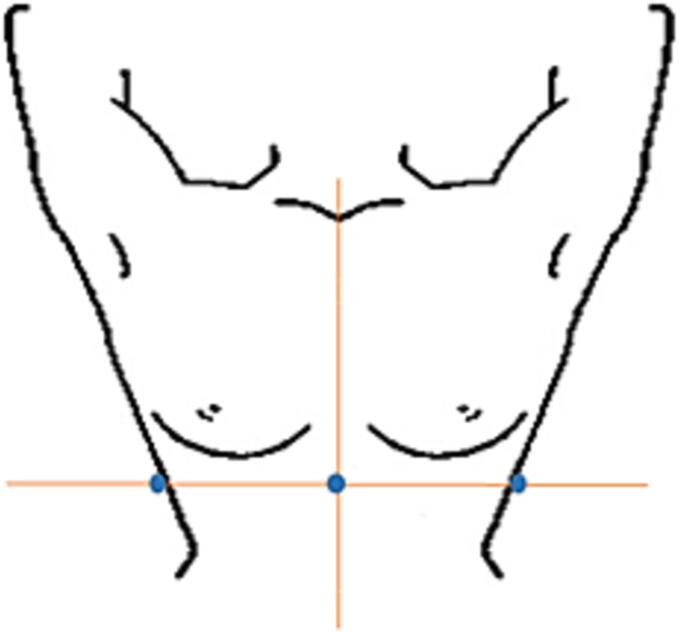
Given the limited published data available, there is similarly limited evidence to guide practice recommendations. In general, skin marks are needed to setup the patient before performing a position verification procedure. In the absence of relevant evidence, the guideline authors [70] advise the following configuration of skin marks, Fig. 1:
-
·
Caudal: one skin mark at patient sagittal mid-line;
-
·
Lateral: two points at each side of the patient halfway the chest since these are stable points.
Fig. 1.
Configuration of the skin marks for patient setup.
Considerations
-
•
Setting a calculated couch vertical position rather than shifting from lateral skin mark height (for offline position verification) could be helpful to improve setup accuracy.
-
•
Temporary skin marks may be an alternative to permanent tattoos with a lesser impact on patient well-being [70].
-
•
SGRT may improve setup accuracy and enable the omission of skin marks entirely, though this must be validated in the context of a department’s local workflow.
Position verification
Position verification encompasses the imaging modality utilised, the frequency with which the modality is applied, and the matching structures that are prioritised when evaluating setup errors and applying corrections. For the purposes of this guideline, data relating to intrafractional position verification, and the impact of respiratory motion were excluded.
Fifty-two studies were identified as relating to position verification, Supplementary Table 5. Table 1 shows the distribution of studies by imaging modality utilised. Importantly, 39 studies (75%) included only a single imaging modality. Such studies were considered to be at high risk of bias and of limited value when considering the value of one imaging modality over another as variations in patient positioning and image matching practice cannot be readily accounted for. Of the 13 studies comparing two or more imaging modalities, seven [73], [74], [75], [76], [77], [78], [79] related to the validation of surface-guided RT (SGRT), with the remaining six [61], [80], [81], [82], [83], [84] involving some combination of 2D, 2D-2D and 3D modalities. A similarly limited number of studies directly evaluated different imaging frequencies or matching processes.
Table 1.
The distribution of studies by imaging modality.
| Imaging modality | Number of studies (%)* | References |
|---|---|---|
| 2D (e.g., kV, MV) | 19 (37%) | [26], [61], [75], [76], [81], [82], [84], [85], [86], [87], [88], [89], [90], [91], [92], [93], [94], [95], [96] |
| 2D-2D (e.g., kV-kV, MV-kV) | 18 (35%) | [77], [79], [80], [81], [83], [84], [86], [97], [98], [99], [100], [101], [102], [103], [104], [105], [106], [107] |
| 3D (e.g., CBCT, MVCT) | 17 (33%) | [61], [73], [74], [78], [80], [82], [83], [84], [108], [109], [110], [111], [112], [113], [114], [115], [116] |
| SGRT | 8 (15%) | [73], [74], [75], [76], [77], [78], [79], [117] |
| Other (e.g., ultrasound, MRI) | 4 (8%) | [118], [119], [120], [121] |
| Total | 52 (100%) |
The combined modality numbers exceed the total number of studies assessed due to 13 studies including multiple imaging modalities.
2D imaging has been a long-established approach to breast position verification, based primarily on MV portal imaging of treatment field(s) and evaluation of the chest wall and anterior breast contour. A wide range of 2D imaging frequencies were reported across the selected studies from weekly to daily. In the absence of daily imaging, random setup error cannot be accounted for, though systematic errors can be somewhat mitigated using action-level protocols [122], [123]. Importantly, systematic errors require comparatively larger PTV margin expansions to reduce the risk of geometric miss of the tumour volume over the course of treatment [124]. Among the 19 2D imaging studies, 12 included no comparator modality, and reported systematic and random errors ranged from 1.5 to 23.4 mm and 1.5–7.6 mm, respectively [26], [85], [86], [87], [88], [89], [90], [91], [92], [93], [94], [95], [96]. While these values are primarily indicative of setup reproducibility between studies, they also highlight the need to validate setup errors locally to ensure that the accuracy achieved by departmental workflows is adequate for the PTV margins applied.
2D imaging is limited in that ‘out of plane’ (i.e., perpendicular to the image acquired) setup errors cannot be assessed. Jain et al. [61] evaluated the setup errors of 10 patients using post-treatment Cone Beam CT (CBCT) following initial 2D imaging. All patients were found to have systematic errors exceeding 5 mm in at least one direction, though this was most frequently observed in the lateral plane. Plans were recalculated based on these errors and demonstrated reduced target volume coverage and homogeneity. Similarly, Topolnjak et al. [82] compared CBCT and portal images for 20 patients and found 2D imaging to underestimate both systematic and random errors.
2D-2D imaging enables localisation of the patient in all three planes through the acquisition of two images typically acquired at orthogonal angles. Petillion et al. [107] compared two methods of orthogonal imaging at cardinal (i.e., 0°, 90°, 180°, 270°) and non-cardinal angles (derived from the tangential treatment field). The non-cardinal technique was found to have significantly reduced residual error based on intrafactional 2D imaging and would enable whole-breast PTV margins to be reduced by 3–4 mm. 2D-2D residual errors have been similarly assessed but based on image match prioritisation by Laaksooma et al. [100]. Using cardinal imaging angles, matching to a combination of the sternum, ribs and vertebrae was found to be optimal, while the vertebrae alone were the least accurate. A PTV margin reduction of 1.2 mm in the posterior tangential plane was calculated to be feasible from the reduction in residual error. Studies involving CBCT following initial 2D-2D match have shown residual errors of 3–5 mm [83] and the need for additional PTV margins of approximately 2 mm [80].
3D imaging, most commonly in the form of CBCT, offers the benefit of soft tissue visualisation throughout all three planes of the patient. As reported above, studies have indicated the value of 3D imaging in identifying residual error from 2D and 2D-2D imaging modalities, further enabling more accurate validation of PTV margins. Such data is however complicated by the range of structures that can be used to determine the ‘ideal’ matched position of 3D images. Studies involving partial-breast irradiation often focus on the localisation of surgical clips [80] or the surgical bed [83], which may not be representative of the wider target volume treated in whole-breast, or locoregional, irradiation. Penninkhof et al. [84] evaluated the variation in surgical clip position throughout treatment in a cohort of 30 patients treated on the whole-breast with simultaneously integrated boosts using MV, orthogonal kV and CBCT imaging. Clip position was seen to be relatively stable for most patients, with a mean agreement of 1–2 mm with the chest wall and external breast contour. A trend towards increased clip displacement was seen over the course of treatment, with three of 30 patients requiring repeat CT and replanning. Significant changes in the seroma can also be detected by 3D imaging earlier in treatment as evidenced by Troung et al. [111], who reported a 13.7% mean reduction in seroma volume between planning CT and first treatment CBCT. Assessment of whole-breast target volumes using CBCT has also shown more than 15% variation in volume over the course of treatment [61]. The information gained by 3D imaging must also be considered alongside its limitations. Increased dose to larger volumes of normal tissue, time of acquisition and limited scan field of view and length are important factors. Additionally, CBCT modalities often bring increased collision risk with the patient, couch, or positioning equipment.
SGRT has gained interest over recent years due to its avoidance of ionising radiation and ability to track intrafractional movement. It is a modality well-suited to supine breast position verification as it relies on the external body contour as a surrogate for the treatment volume. Of the seven studies involving SGRT, three involved a comparison with 3D imaging [73], [74], [78], two with 2D-2D imaging [77], [79], and a further two with 2D imaging [75], [76]. SGRT has been reported to have a mean agreement within 2 mm in all directions of CBCT imaging matched to soft tissue [73], [74] or bony anatomy [78]. When evaluated against 2D-2D imaging matched to surgical clips, Gierga et al. [77] reported median residual errors of 3 mm and 6 mm for gated and free-breathing SGRT, respectively. Chang et al. [79] similarly found mean residual setup errors of approximately 2 mm in all directions when comparing surface alignment with clip matching for partial breast irradiation. Of note, SGRT was shown to correlate better with clip location than matching to bony anatomy. SGRT comparisons with 2D imaging described good agreement, though neither study reported residual error values [75], [76], and the limitations of 2D imaging accuracy must be taken into consideration. An added benefit of SGRT is its ability to be used in real-time to guide patient setup, and its speed of acquisition and automated assessment compared to other imaging modalities. Ma et al. [78] reported a mean duration of setup, registration and correction of 1 min using SGRT compared to 6 min with CBCT.
Recommendations
From the limited number of studies available, and the small sample sizes observed, only limited guidance on clinical practice can be offered. Larger clinical studies comparing methods of position verification using clearly defined positioning and matching workflows are required in this area. The position verification recommendations from the authors are as follows:
-
•
Where available, 2D-2D or 3D imaging daily is recommended for online position verification.
-
•
If 2D-2D or 3D position verification is not available, the limitations of 2D position verification (online or offline) in visualising out-of-plane setup errors should be considered and appropriate target volume margins employed.
-
•
Image-matching should evaluate bony anatomy directly underlying the treated volume as well as breast tissue or external breast contour.
-
•
SGRT should not be used as a sole means of position verification without centres first conducting a local study to validate consistent agreement with the pre-existing IGRT modality. Particular caution is advised in the use of SGRT alone for partial-breast or integrated boost treatments, as changes in the surgical bed (or surgical clips as a surrogate) may go undetected.
Considerations
-
•
3D imaging is advantageous for the assessment of soft tissue displacement and change over the course of treatment; however, collision risk must be carefully assessed based on equipment, patient position and isocentre location.
-
•
The dose contribution from 3D imaging should also be considered, however this is likely to be limited for patients receiving hypofractionated treatment regimes.
Discussion and future work
In this guideline, we described the specific requirements and possibilities in the photon radiation therapy workflow for patients with breast cancer. However, we have not covered some specific items. We did not describe the various techniques for performing Deep Inspiration Breath-hold. This has been thoroughly described in the ESTRO-ACROP guideline: recommendations on implementation of breath-hold techniques in radiotherapy [125]. Furthermore, we did not describe the workflow and necessities of immobilisation and positioning in proton therapy, upright radiotherapy and MR-Linac [126]. These emerging technologies require their own specific considerations, which are beyond the scope of a general guideline.
Apart from the workflow of patient positioning and position verification in patients with breast cancer one should realise that the choice of a specific treatment technique has certain effects as well. For example, studies have reported conflicting findings regarding IMRT plans as having greater or lesser sensitivity to changes in patient position and contour compared to 3DCRT plans [61], [127]. As well as being beyond the scope of the current guideline, the variation and complexity in modern treatment planning approaches requires that departments must have their own internal workflows for evaluating the impact of positioning errors and anatomical changes on delivered dose.
The image guidance approach adopted should consider the following important factors; a modelling study by Batumalai et al. [128] estimated an increased lifetime attributable risk of developing secondary contralateral breast cancer of between 0.4% and 1.5% from daily MV image guidance. Alvadaro et al. obtained the organ doses from the standard low-dose mode CBCT and proposed methods to reduce this dose [129]. Recently Borm et al. found that daily versus weekly CBCT did not affect the target coverage and dose in the organs at risk in VMAT breast cancer radiation treatment [130]. This highlights the important interplay between patient positioning and position verification, whereby positioning workflows with a high level of reproducibility reduce the perceived benefit of higher frequency IGRT. It is however important to note that, particularly in the context of increasingly conformal and complex planning modalities, validation of patient position on a daily basis becomes increasingly important to ensure the accurate delivery of the planned dose.
In this guideline we included several studies concerning the use of SGRT. However, we did not include the workflow of SGRT in breast positioning. Validation of SGRT as a sole method of setup and position verification for distinct treatment indications (e.g., whole breast, loco-regional breast cancer, partial-breast) needs to be investigated more thoroughly. In the ESTRO-ACROP SGRT guideline it was recommended that SGRT should be verified by an established x-ray modality of IGRT at least weekly [131].
Alongside the recommendations and considerations offered within this guideline, it is important to acknowledge the influence of clinical hardware and software on position verification practice. Staff must be appropriately trained in workflows adapted to the locally available technology to ensure IGRT is performed accurately and consistently. While rarely investigated within the literature reviewed, systematic and random interobserver errors of 2 mm or larger has been reported across IGRT modalities [100], [110]. Hardware limitations can also be a key determinant of position verification workflow due to factors such as collision risk between the gantry and patient or couch top. This is particularly relevant for CBCT workflows, which is anecdotally a frequent challenge reported by departments. Developing this guideline, we noted that there is a future opportunity for a technical guideline on CBCT implementation for breast position verification.
For researchers studying the field of positioning and setup accuracy we would recommend considering the following design characteristics at the outset in order that the study findings can be used to inform and improve future radiotherapy practice.
-
•
In general, low sample sizes made the ability to draw definitive, generalisable conclusions in this guideline impossible. Where possible, researchers should estimate the study sample size using an appropriate power calculation either based on a pilot study or literature where a similar technique has been studied.
-
•
Where possible new setup approaches should be tested against the current gold standard using a randomised comparison. Single (non-randomised) cohort design studies do not allow a suitable assessment of accuracy and it becomes difficult to assess whether levels of accuracy achieved are an improvement on existing methods, or whether the magnitude of the benefit obtained with the new setup method is clinically significant.
-
•
Possible confounding variables should be measured, reported and included in multi-variate analysis to enable accurate assessment of setup variations. Confounding variables would include patient BMI, breast volume, whether an immobilisation device was used, or use of a breath-hold technique. Performing these analyses demands larger patient cohorts which may only be met by promoting collaborative multi-centre studies.
-
•
Within the literature no specific variables have been given to determine which treatment position will be best for each individual patient. Prone could be better for patients with larger breasts. However, the variable “large-breasted” was not described at all or was defined differently in the performed studies. For example, Zhao et al. [20] and Bergom et al. [33] described ml breast volume; Mulliez et al. [14], Buijsen et al. [27] and Kirby et al. [15] used cup size as a unit. For comparing studies, it would be beneficial to use one entity. Ooi et al. found that BMI may be causally linked to larger breast size, but not the reverse, it seems that BMI is a less reliable unit [132]. Therefore, we suggest that breast volume in ml (1 ml = 1 cubic centimetre) would be the best unit. Cup size is an inappropriate unit to use as cup size can differ per country or bra manufacturer and each bra cup size covers a large range of breast volumes. For example, women with a breast volume of 1000–1099 ml could be fitted to four different Australian bra sizes [133]. Furthermore, Ringberg et al. found that a C-cup size could measure breast volumes with a range of 350 ml to 1800 ml [134].
-
•
Thorough documentation of all positioning variables and position verification workflow (e.g., modality, matching prioritisation) is of importance to ensure any findings can be replicated and applied to practice. This is also required for findings to be combined in reviews or meta-analyses.
Declaration of Competing Interest
The authors declare that they have no known competing financial interests or personal relationships that could have appeared to influence the work reported in this paper.
Acknowledgments
Thomas Vissers (help with setting up the literature review) and Christa Rooken (help with collecting specific articles), librarians Haaglanden Medical Center, The Hague, NL.
Deborah Harrop, librarian Sheffield Hallam University, Sheffield, UK.
Footnotes
Supplementary data to this article can be found online at https://doi.org/10.1016/j.tipsro.2023.100219.
Appendix A. Supplementary data
The following are the Supplementary data to this article:
References
- 1.Ferlay J., Soerjomataram I., Dikshit R., Eser S., Mathers C., Rebelo M., et al. Cancer incidence and mortality worldwide: sources, methods and major patterns in GLOBOCAN 2012. Int J Cancer. 2015;136:E359–E386. doi: 10.1002/ijc.29210. [DOI] [PubMed] [Google Scholar]
- 2.Breast Cancer Trialists E, Group C. Effect of radiotherapy after breast-conserving surgery on 10-year recurrence and 15-year breast cancer death: meta-analysis of individual patient data for 10â€̂801 women in 17 randomised trials. The Lancet 2011;378:1707–16. 10.1016/S0140. [DOI] [PMC free article] [PubMed]
- 3.Haviland J.S., Owen J.R., Dewar J.A., Agrawal R.K., Barrett J., Barrett-Lee P.J., et al. The UK Standardisation of Breast Radiotherapy (START) trials of radiotherapy hypofractionation for treatment of early breast cancer: 10-year follow-up results of two randomised controlled trials. Lancet Oncol. 2013;14:1086–1094. doi: 10.1016/S1470-2045(13)70386-3. [DOI] [PubMed] [Google Scholar]
- 4.The START Trialists’ Group. The UK Standardisation of Breast Radiotherapy (START) Trial B of radiotherapy hypofractionation for treatment of early breast cancer: a randomised trial. Lancet 2008;9:1098–107. 10.1016/S0140. [DOI] [PMC free article] [PubMed]
- 5.Whelan T.J., Pignol J.-P., Levine M.N., Julian J.A., MacKenzie R., Parpia S., et al. Long-term results of hypofractionated radiation therapy for breast cancer. N Engl J Med. 2010;(362):513–520. doi: 10.1056/NEJMoa0906260. [DOI] [PubMed] [Google Scholar]
- 6.Meattini I, Becherini C, Boersma L, Kaidar-Person O, Marta GN, Montero A, et al. European Society for Radiotherapy and Oncology Advisory Committee in Radiation Oncology Practice consensus recommendations on patient selection and dose and fractionation for external beam radiotherapy in early breast cancer. Rev Lancet Oncol 2022;23:21–31. 10.1016/S1470-2045(21)00539-8. [DOI] [PubMed]
- 7.Krug D., Baumann R., Combs S.E., Duma M.N., Dunst J., Feyer P., et al. Moderate hypofractionation remains the standard of care for whole-breast radiotherapy in breast cancer: considerations regarding FAST and FAST-Forward. Strahlenther Onkol. 2021;197:269–280. doi: 10.1007/s00066-020-01744-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Coles C.E., Aristei C., Bliss J., Boersma L., Brunt A.M., Chatterjee S., et al. International guidelines on radiation therapy for breast cancer during the COVID-19 pandemic. Clin Oncol. 2020;32:279–281. doi: 10.1016/j.clon.2020.03.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Murray Brunt A., Haviland J.S., Wheatley D.A., Sydenham M.A., Alhasso A., Bloomfield D.J., et al. Hypofractionated breast radiotherapy for 1 week versus 3 weeks (FAST-Forward): 5-year efficacy and late normal tissue effects results from a multicentre, non-inferiority, randomised, phase 3 trial. Lancet. 2020;(395):1613–1626. doi: 10.1200/JCO.19.02750. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Murray Brunt A., Haviland J.S., Sydenham M., Agrawal R.K., Algurafi H., Alhasso A., et al. Ten-year results of FAST: a randomized controlled trial of 5-fraction whole-breast radiotherapy for early breast cancer. J Clin Oncol. 2020;(38):3261–3272. doi: 10.1200/JCO.19.02750. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.The FAST Trialists group. First results of the randomised UK FAST Trial of radiotherapy hypofractionation for treatment of early breast cancer (CRUKE/04/015). Radiother Oncol 2011;100:93–100. 10.1016/j.radonc.2011.06.026. [DOI] [PubMed]
- 12.Sterne JA, Hernán MA, Reeves BC, Savović J, Berkman ND, Viswanathan M, et al. ROBINS-I: a tool for assessing risk of bias in non-randomised studies of interventions. BMJ (Online) 2016;355. 10.1136/bmj.i4919. [DOI] [PMC free article] [PubMed]
- 13.Higgins JPT, Altman DG, Gøtzsche PC, Jüni P, Moher D, Oxman AD, et al. The Cochrane Collaboration’s tool for assessing risk of bias in randomised trials. BMJ (Online) 2011;343. 10.1136/bmj.d5928. [DOI] [PMC free article] [PubMed]
- 14.Mulliez T., Veldeman L., van Greveling A., Speleers B., Sadeghi S., Berwouts D., et al. Hypofractionated whole breast irradiation for patients with large breasts: a randomized trial comparing prone and supine positions. Radiother Oncol. 2013;108:203–208. doi: 10.1016/j.radonc.2013.08.040. [DOI] [PubMed] [Google Scholar]
- 15.Kirby A.M., Evans P.M., Helyer S.J., Donovan E.M., Convery H.M., Yarnold J.R. A randomised trial of Supine versus Prone breast radiotherapy (SuPr study): Comparing set-up errors and respiratory motion. Radiother Oncol. 2011;100:221–226. doi: 10.1016/j.radonc.2010.11.005. [DOI] [PubMed] [Google Scholar]
- 16.Varga Z., Hideghéty K., Mezo T., Nikolényi A., Thurzó L., Kahán Z. Individual positioning: a comparative study of adjuvant breast radiotherapy in the prone versus supine position. Int J Radiat Oncol Biol Phys. 2009;75:94–100. doi: 10.1016/j.ijrobp.2008.10.045. [DOI] [PubMed] [Google Scholar]
- 17.Wang X., Fargier-Bochaton O., Dipasquale G., Laouiti M., Kountouri M., Gorobets O., et al. Is prone free breathing better than supine deep inspiration breath-hold for left whole-breast radiotherapy? A dosimetric analysis. Strahlenther Onkol. 2021;197:317–331. doi: 10.1007/s00066-020-01731-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Lin H., Liu T., Shi C., Petillion S., Kindts I., Weltens C., et al. Feasibility study of individualized optimal positioning selection for left-sided whole breast radiotherapy: DIBH or prone. J Appl Clin Med Phys. 2018;19:218–229. doi: 10.1002/acm2.12283. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Kahán Z., Rárosi F., Gaál S., Cserháti A., Boda K., Darázs B., et al. A simple clinical method for predicting the benefit of prone vs. supine positioning in reducing heart exposure during left breast radiotherapy. Radiother Oncol. 2018;126:487–492. doi: 10.1016/j.radonc.2017.12.021. [DOI] [PubMed] [Google Scholar]
- 20.Zhao X., Wong E.K., Wang Y., Lymberis S., Wen B., Formenti S., et al. A support vector machine (SVM) for predicting preferred treatment position in radiotherapy of patients with breast cancer. Med Phys. 2010;37:5341–5350. doi: 10.1118/1.3483264. [DOI] [PubMed] [Google Scholar]
- 21.Yu T., Xu M., Sun T., Shao Q., Zhang Y.J., Liu X.J., et al. External-beam partial breast irradiation in a supine versus prone position after breast-conserving surgery for Chinese breast cancer patients. Sci Rep. 2018;8 doi: 10.1038/s41598-018-33741-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Krengli M., Masini L., Caltavuturo T., Pisani C., Apicella G., Negri E., et al. Prone versus supine position for adjuvant breast radiotherapy: A prospective study in patients with pendulous breasts. Radiat Oncol. 2013;8 doi: 10.1186/1748-717X-8-232. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Bartlett F.R., Colgan R.M., Donovan E.M., McNair H.A., Carr K., Evans P.M., et al. The UK HeartSpare Study (Stage IB): randomised comparison of a voluntary breath-hold technique and prone radiotherapy after breast conserving surgery. Radiother Oncol. 2015;114:66–72. doi: 10.1016/j.radonc.2014.11.018. [DOI] [PubMed] [Google Scholar]
- 24.Taylor C.W., Zhe W., Macaulay E., Jagsi R., Duane F., Darby S.C. Exposure of the heart in breast cancer radiation therapy: a systematic review of heart doses published during 2003 to 2013. Int J Radiat Oncol Biol Phys. 2015;93:845–853. doi: 10.1016/j.ijrobp.2015.07.2292. [DOI] [PubMed] [Google Scholar]
- 25.Aznar M.C., Duane F.K., Darby S.C., Wang Z., Taylor C.W. Exposure of the lungs in breast cancer radiotherapy: a systematic review of lung doses published 2010–2015. Radiother Oncol. 2018;126:148–154. doi: 10.1016/j.radonc.2017.11.022. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Mitchell J., Formenti S.C., DeWyngaert J.K. Interfraction and intrafraction setup variability for prone breast radiation therapy. Int J Radiat Oncol Biol Phys. 2010;76:1571–1577. doi: 10.1016/j.ijrobp.2009.07.1683. [DOI] [PubMed] [Google Scholar]
- 27.Buijsen J., Jager J.J., Bovendeerd J., Voncken R., Borger J.H., Boersma L.J., et al. Prone breast irradiation for pendulous breasts. Radiother Oncol. 2007;82:337–340. doi: 10.1016/j.radonc.2006.08.014. [DOI] [PubMed] [Google Scholar]
- 28.Lai J., Zhong F., Deng J., Hu S., Shen R., Luo H., et al. Prone position versus supine position in postoperative radiotherapy for breast cancer: a meta-analysis. Medicine. 2021;100:e26000. doi: 10.1097/MD.0000000000026000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Cardoso J.S., Cardoso M.J. Towards an intelligent medical system for the aesthetic evaluation of breast cancer conservative treatment. Artif Intell Med. 2007;40:115–126. doi: 10.1016/j.artmed.2007.02.007. [DOI] [PubMed] [Google Scholar]
- 30.Vakaet V., van Hulle H., Vergotte M., Schoepen M., Deseyne P., van Greveling A., et al. 5-Year outcomes of a randomized trial comparing prone and supine whole breast irradiation in large-breasted women. Int J Radiat Oncol Biol Phys. 2021;110:766–771. doi: 10.1016/j.ijrobp.2021.01.026. [DOI] [PubMed] [Google Scholar]
- 31.Osa E.O.O., Dewyngaert K., Roses D., Speyer J., Guth A., Axelrod D., et al. Prone breast intensity modulated radiation therapy: 5-year results. Int J Radiat Oncol Biol Phys. 2014;89:899–906. doi: 10.1016/j.ijrobp.2014.03.036. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Ann Rose M, Olivotto I, Cady B, Koufman C, Osteen R, Silver B, et al. conservative surgery and radiation therapy for early breast cancer long-term cosmetic results. 1989. 10.1001/archsurg.1989.01410020023002. [DOI] [PubMed]
- 33.Bergom C., Kelly T., Morrow N., Wilson J.F., Walker A., Xiang Q., et al. Prone whole-breast irradiation using three-dimensional conformal radiotherapy in women undergoing breast conservation for early disease yields high rates of excellent to good cosmetic outcomes in patients with large and/or pendulous breasts. Int J Radiat Oncol Biol Phys. 2012;83:821–828. doi: 10.1016/j.ijrobp.2011.08.020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Huppert N., Jozsef G., DeWyngaert K., Formenti S.C. The role of a prone setup in breast radiation therapy. Front Oncol. 2011;1 doi: 10.3389/fonc.2011.00031. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Csenki M., Újhidy D., Cserháti A., Kahán Z., Varga Z. Radiation dose to the nodal regions during prone versus supine breast irradiation. Ther Clin Risk Manag. 2014;10:367–372. doi: 10.2147/TCRM.S59483. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Alonso-Basanta M., Ko J., Babcock M., Dewyngaert J.K., Formenti S.C. Coverage of axillary lymph nodes in supine vs. prone breast radiotherapy. Int J Radiat Oncol Biol Phys. 2009;73:745–751. doi: 10.1016/j.ijrobp.2008.04.040. [DOI] [PubMed] [Google Scholar]
- 37.Sethi R.A., No H.S., Jozsef G., Ko J.P., Formenti S.C. Comparison of three-dimensional versus intensity-modulated radiotherapy techniques to treat breast and axillary level III and supraclavicular nodes in a prone versus supine position. Radiother Oncol. 2012;102:74–81. doi: 10.1016/j.radonc.2011.09.008. [DOI] [PubMed] [Google Scholar]
- 38.Deseyne P., Speleers B., De Neve W., Boute B., Paelinck L., Van H.T., et al. Whole breast and regional nodal irradiation in prone versus supine position in left sided breast cancer. Radiat Oncol. 2017;12 doi: 10.1186/s13014-017-0828-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Speleers B.A., Belosi F.M., De Gersem W.R., Deseyne P.R., Paelinck L.M., Bolsi A., et al. Comparison of supine or prone crawl photon or proton breast and regional lymph node radiation therapy including the internal mammary chain. Sci Rep. 2019;9 doi: 10.1038/s41598-019-41283-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Speleers B., Schoepen M., Belosi F., Vakaet V., de Neve W., Deseyne P., et al. Effects of deep inspiration breath hold on prone photon or proton irradiation of breast and regional lymph nodes. Sci Rep. 2021;11 doi: 10.1038/s41598-021-85401-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Mulliez T., Veldeman L., Speleers B., Mahjoubi K., Remouchamps V., Van Greveling A., et al. Heart dose reduction by prone deep inspiration breath hold in left-sided breast irradiation. Radiother Oncol. 2015;114:79–84. doi: 10.1016/j.radonc.2014.11.038. [DOI] [PubMed] [Google Scholar]
- 42.Boute B., De Neve W., Speleers B., Van Greveling A., Monten C., Van Hoof T.V., et al. Potential benefits of crawl position for prone radiation therapy in breast cancer. J Appl Clin Med Phys. 2017;18:200–205. doi: 10.1002/acm2.12118. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Shin S.M., No H.S., Vega R.M., Fenton-Kerimian M., Maisonet O., Hitchen C., et al. Breast, chest wall, and nodal irradiation with prone set-up: Results of a hypofractionated trial with a median follow-up of 35 months. Pract Radiat Oncol. 2016;6:e81–e88. doi: 10.1016/j.prro.2015.10.022. [DOI] [PubMed] [Google Scholar]
- 44.Campana F., Kirova Y.M., Rosenwald J.C., Dendale R., Vilcoq J.R., Dreyfus H., et al. Breast radiotherapy in the lateral decubitus position: a technique to prevent lung and heart irradiation. Int J Radiat Oncol, Biol, Phys. 2005;61:1348–1354. doi: 10.1016/j.ijrobp.2004.08.051. [DOI] [PubMed] [Google Scholar]
- 45.Kirova Y.M., Hijal T., Campana F., Fournier-Bidoz N., Stilhart A., Dendale R., et al. Whole breast radiotherapy in the lateral decubitus position: a dosimetric and clinical solution to decrease the doses to the organs at risk (OAR) Radiother Oncol. 2014;110:477–481. doi: 10.1016/j.radonc.2013.10.038. [DOI] [PubMed] [Google Scholar]
- 46.Bronsart E., Dureau S., Xu H.P., Bazire L., Chilles A., Costa E., et al. Whole breast radiotherapy in the lateral isocentric lateral decubitus position: long-term efficacy and toxicity results. Radiother Oncol. 2017;124:214–219. doi: 10.1016/j.radonc.2017.07.001. [DOI] [PubMed] [Google Scholar]
- 47.Krhili S., Costa E., Xu H.P., Kirova Y.M. Whole breast radiotherapy in the isocentric lateral decubitus position: role of the immobilization device and table on clinical results. Cancer/Radiotherapie. 2019;23:209–215. doi: 10.1016/j.canrad.2018.09.001. [DOI] [PubMed] [Google Scholar]
- 48.Davidson S., Kirsner S., Mason B., Kisling K., Barrett R.D., Bonetati A., et al. Dosimetric impact of setup accuracy for an electron breast boost technique. Pract Radiat Oncol. 2015;5:e499–e504. doi: 10.1016/j.prro.2015.02.011. [DOI] [PubMed] [Google Scholar]
- 49.Haffty B.G. Supine or prone breast radiation: upsides and downsides. Int J Radiat Oncol Biol Phys. 2018;101:510–512. doi: 10.1016/j.ijrobp.2018.03.023. [DOI] [PubMed] [Google Scholar]
- 50.Goldsworthy S.G.S., Sinclair N.S.N., Tremlett J.T.J., Chalmers A.C.A., Francis M.F.M., Simcock R.S.R. Abducting both arms improves stability during breast radiotherapy: the Bi Arm study in radiotherapy. J Radiother Pract. 2011;10:250–259. doi: 10.1017/S1460396910000452. [DOI] [Google Scholar]
- 51.Graham P, Elomari F, Brownde L. Armrest versus vacuum bag immobilization in the treatment of breast cancer by radiation therapy:A randomized comparison. Australas Radiol 2000;44:193–7. 10.1046/j.1440-1673.2000.00804.x. [DOI] [PubMed]
- 52.Xiang Q., Jie W., Zhu K.K., Wang Q., Cheng J. Which technique of positioning and immobilization is better for breast cancer patients in postmastectomy IMRT, single-pole or double-pole immobilization? J Appl Clin Med Phys. 2019;20:168–174. doi: 10.1002/acm2.12506. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Saito A.I., Vargas C., Morris C.G., Lightsey J., Mendenhall N.P. Differences between current and historical breast cancer axillary lymph node irradiation based on arm position: Implications for radiation oncologists. Am J Clin Oncol: Cancer Clin Trials. 2009;32:381–386. doi: 10.1097/COC.0b013e318191718d. [DOI] [PubMed] [Google Scholar]
- 54.Kapanen M., Laaksomaa M., Skyttä T., Haltamo M., Pehkonen J., Lehtonen T., et al. Residual position errors of lymph node surrogates in breast cancer adjuvant radiotherapy: comparison of two arm fixation devices and the effect of arm position correction. Med Dosim. 2016;41:47–52. doi: 10.1016/j.meddos.2015.08.001. [DOI] [PubMed] [Google Scholar]
- 55.Lauridsen M.C., Christiansen P., Hessov I. The effect of physiotherapy on shoulder function in patients surgically treated for breast cancer: a randomized study. Acta Oncol (Madr) 2005;44:449–457. doi: 10.1080/02841860510029905. [DOI] [PubMed] [Google Scholar]
- 56.Leal NFB da S, de Oliveira HF, Carrara HHA. Fisioterapia supervisionada nas mulheres em radioterapia para o câncer de mama. Rev Lat Am Enfermagem 2016;24. 10.1590/1518-8345.0702.2755.
- 57.Yang A., Sokolof J., Gulati A. The effect of preoperative exercise on upper extremity recovery following breast cancer surgery: a systematic review. Int J Rehabil Res. 2018;41:189–196. doi: 10.1097/MRR.0000000000000288. [DOI] [PubMed] [Google Scholar]
- 58.Brahmbhatt P., Sabiston C.M., Lopez C., Chang E., Goodman J., Jones J., et al. Feasibility of prehabilitation prior to breast cancer surgery: a mixed-methods study. Front Oncol. 2020;10 doi: 10.3389/fonc.2020.571091. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Wu F., Laza-Cagigas R., Pagarkar A., Olaoke A., el Gammal M., Rampal T. The feasibility of prehabilitation as part of the breast cancer treatment pathway. PM and R. 2021;13:1237–1246. doi: 10.1002/pmrj.12543. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Roos J., Mast M., Egmond van E., Petoukhova A., Struikmans H. Van wig naar plat: wat is het effect op de dosis in het normale weefsel bij borstsparende bestraling. Gamma Professional. 2013;63:22–25. [Google Scholar]
- 61.Jain P., Marchant T., Green M., Watkins G., Davies J., McCarthy C., et al. Inter-fraction motion and dosimetric consequences during breast intensity-modulated radiotherapy (IMRT) Radiother Oncol. 2009;90:93–98. doi: 10.1016/j.radonc.2008.10.010. [DOI] [PubMed] [Google Scholar]
- 62.Arenas M., Hernández V., Farrús B., Müller K., Gascón M., Pardo A., et al. Do breast cups improve breast cancer dosimetry? A comparative study for patients with large or pendulous breasts. Acta Oncol (Madr) 2014;53:795–801. doi: 10.3109/0284186X.2014.893062. [DOI] [PubMed] [Google Scholar]
- 63.Shi L., He C., Guo X., Zhao L., Fu T., Han B. Original Article A new method to deliver breast and chest wall radiation in breast radiotherapy for lung and heart sparing. Int J Clin Exp Med. 2016;9:13484–13492. [Google Scholar]
- 64.Kelly A., Hardcastle N., Metcalfe P., Cutajar D., Quinn A., Foo K., et al. Surface dosimetry for breast radiotherapy in the presence of immobilization cast material. Phys Med Biol. 2011;56:1001–1013. doi: 10.1088/0031-9155/56/4/008. [DOI] [PubMed] [Google Scholar]
- 65.Agostinelli S., Garelli S., Bellini A., Pupillo F., Guenzi M., Bosetti D., et al. Helical Tomotherapy of the breast: can thermoplastic immobilization improve the reproducibility of the treatment setup and the accuracy of the delivered dose? Phys Med. 2015;31:49–53. doi: 10.1016/j.ejmp.2014.09.007. [DOI] [PubMed] [Google Scholar]
- 66.Kawamura M., Maeda Y., Yamamoto K., Takamatsu S., Sato Y., Minami H., et al. Development of the breast immobilization system in prone setup: the effect of bra in prone position to improve the breast setup error. J Appl Clin Med Phys. 2017;18:155–160. doi: 10.1002/acm2.12116. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Arimura T., Ogino T., Yoshiura T., Matsuyama M., Kondo N., Miyazaki H., et al. A feasibility study of a hybrid breast-immobilization system for early breast cancer in proton beam therapy. Med Phys. 2017;44:1268–1274. doi: 10.1002/mp.12166. [DOI] [PubMed] [Google Scholar]
- 68.Snider J.W., Mutaf Y., Nichols E., Hall A., Vadnais P., Regine W.F., et al. Dosimetric improvements with a novel breast stereotactic radiotherapy device for delivery of preoperative partial-breast irradiation. Oncology (Switzerland) 2017;92:21–30. doi: 10.1159/000449388. [DOI] [PubMed] [Google Scholar]
- 69.Probst H., Dodwell D., Gray J.C., Holmes M. An evaluation of the accuracy of semi-permanent skin marks for breast cancer irradiation. Radiography. 2006;12:186–188. doi: 10.1016/j.radi.2005.07.001. [DOI] [Google Scholar]
- 70.Probst H., Rosbottom K., Crank H., Stanton A., Reed H. The patient experience of radiotherapy for breast cancer: a qualitative investigation as part of the SuPPORT 4 All study. Radiography. 2021;27:352–359. doi: 10.1016/j.radi.2020.09.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Petillion S., Verhoeven K., Weltens C., van den Heuvel F. Efficacy and workload analysis of a fixed vertical couch position technique and a fixed-action-level protocol in whole-breast radiotherapy. J Appl Clin Med Phys. 2015;(16) doi: 10.1120/jacmp.v16i2.5265. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.González-Sanchis A., Brualla-González L., Fuster-Diana C., Gordo-Partearroyo J.C., Piñeiro-Vidal T., García-Hernandez T., et al. Surface-guided radiation therapy for breast cancer: more precise positioning. Clin Transl Oncol. 2021;23:2120–2126. doi: 10.1007/s12094-021-02617-6. [DOI] [PubMed] [Google Scholar]
- 73.Wei X., Liu M., Ding Y., Li Q., Cheng C., Zong X., et al. Setup errors and effectiveness of optical laser 3D surface imaging system (Sentinel) in postoperative radiotherapy of breast cancer. Sci Rep. 2018;8 doi: 10.1038/s41598-018-25644-w. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74.Cravo Sá A., Fermento A., Neves D., Ferreira S., Silva T., Marques Coelho C., et al. Radiotherapy setup displacements in breast cancer patients: 3D surface imaging experience. Rep Pract Oncol Radiother. 2018;23:61–67. doi: 10.1016/j.rpor.2017.12.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75.Li S., de Weese T., Movsas B., Frassica D., Liu D., Kim J., et al. Initial validation and clinical experience with 3D optical-surface-guided whole breast irradiation of breast cancer. Technol Cancer Res Treat. 2012;(11):57–68. doi: 10.7785/tcrt.2012.500235. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76.Deantonio L., Masini L., Loi G., Gambaro G., Bolchini C., Krengli M. Detection of setup uncertainties with 3D surface registration system for conformal radiotherapy of breast cancer. Rep Pract Oncol Radiother. 2011;16:77–81. doi: 10.1016/j.rpor.2011.02.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77.Gierga D.P., Riboldi M., Turcotte J.C., Sharp G.C., Jiang S.B., Taghian A.G., et al. Comparison of target registration errors for multiple image-guided techniques in accelerated partial breast irradiation. Int J Radiat Oncol Biol Phys. 2008;70:1239–1246. doi: 10.1016/j.ijrobp.2007.11.020. [DOI] [PubMed] [Google Scholar]
- 78.Ma Z, Zhang W, Su Y, Liu P, Pan Y, Zhang G, et al. Optical surface management system for patient positioning in interfractional breast cancer radiotherapy. Biomed Res Int 2018;2018. 10.1155/2018/6415497. [DOI] [PMC free article] [PubMed]
- 79.Chang A.J., Zhao H., Wahab S.H., Moore K., Taylor M., Zoberi I., et al. Video surface image guidance for external beam partial breast irradiation. Pract Radiat Oncol. 2012;2:97–105. doi: 10.1016/j.prro.2011.06.013. [DOI] [PubMed] [Google Scholar]
- 80.Feng C.H., Gerry E., Chmura S.J., Hasan Y., Al-Hallaq H.A. An image-guided study of setup reproducibility of postmastectomy breast cancer patients treated with inverse-planned intensity modulated radiation therapy. Int J Radiat Oncol Biol Phys. 2015;91:58–64. doi: 10.1016/j.ijrobp.2014.09.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 81.Thomsen M.S., Harrov U., Fledelius W., Poulsen P.R. Inter- and intra-fraction geometric errors in daily image-guided radiotherapy of free-breathing breast cancer patients measured with continuous portal imaging. Acta Oncol (Madr) 2014;53:802–808. doi: 10.3109/0284186X.2014.905700. [DOI] [PubMed] [Google Scholar]
- 82.Topolnjak R., Sonke J.J., Nijkamp J., Rasch C., Minkema D., Remeijer P., et al. Breast patient setup error assessment: Comparison of electronic portal image devices and cone-beam computed tomography matching results. Int J Radiat Oncol Biol Phys. 2010;78:1235–1243. doi: 10.1016/j.ijrobp.2009.12.021. [DOI] [PubMed] [Google Scholar]
- 83.Fatunase T., Wang Z., Yoo S., Hubbs J.L., Prosnitz R.G., Yin F.F., et al. Assessment of the residual error in soft tissue setup in patients undergoing partial breast irradiation: results of a prospective study using cone-beam computed tomography. Int J Radiat Oncol Biol Phys. 2008;70:1025–1034. doi: 10.1016/j.ijrobp.2007.07.2344. [DOI] [PubMed] [Google Scholar]
- 84.Penninkhof J., Quint S., de Boer H., Mens J.W., Heijmen B., Dirkx M. Surgical clips for position verification and correction of non-rigid breast tissue in simultaneously integrated boost (SIB) treatments. Radiother Oncol. 2009;90:110–115. doi: 10.1016/j.radonc.2008.10.009. [DOI] [PubMed] [Google Scholar]
- 85.Kanae M., Masahiro K., Yuuki Y., Hideki A., Masataka O., Irfan S., et al. Evaluation of setup errors at the skin surface position for whole breast radiotherapy of breast cancer patients. Acta Medica Okayamag. 2018;331–6 doi: 10.18926/AMO/56167. [DOI] [PubMed] [Google Scholar]
- 86.Jensen C.A., Roa A.M.A., Johansen M., Lund J.Å., Frengen J. Robustness of VMAT and 3DCRT plans toward setup errors in radiation therapy of locally advanced left-sided breast cancer with DIBH. Phys Med. 2018;45:198–204. doi: 10.1016/j.ejmp.2017.11.019. [DOI] [PubMed] [Google Scholar]
- 87.Jones S., Fitzgerald R., Owen R., Ramsay J. Quantifying intra- and inter-fractional motion in breast radiotherapy. J Med Radiat Sci. 2015;62:40–46. doi: 10.1002/jmrs.61. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 88.Lutz C.M., Poulsen P.R., Fledelius W., Offersen B.V., Thomsen M.S. Setup error and motion during deep inspiration breath-hold breast radiotherapy measured with continuous portal imaging. Acta Oncol (Madr) 2016;55:193–200. doi: 10.3109/0284186X.2015.1045625. [DOI] [PubMed] [Google Scholar]
- 89.Yue N.J., Goyal S., Kim L.H., Khan A., Haffty B.G. Patterns of intrafractional motion and uncertainties of treatment setup reference systems in accelerated partial breast irradiation for right- and left-sided breast cancer. Pract Radiat Oncol. 2014;4:6–12. doi: 10.1016/j.prro.2012.12.003. [DOI] [PubMed] [Google Scholar]
- 90.Yang D.S., Yoon W.S., Chung S.Y., Lee J.A., Lee S., Park Y.J., et al. Set-up uncertainty during breast radiotherapy: image-guided radiotherapy for patients with initial extensive variation. Strahlenther Onkol. 2013;189:315–320. doi: 10.1007/s00066-012-0271-4. [DOI] [PubMed] [Google Scholar]
- 91.Kinoshita R., Shimizu S., Taguchi H., Katoh N., Fujino M., Onimaru R., et al. Three-dimensional intrafractional motion of breast during tangential breast irradiation monitored with high-sampling frequency using a real-time tumor-tracking radiotherapy system. Int J Radiat Oncol Biol Phys. 2008;70:931–934. doi: 10.1016/j.ijrobp.2007.10.003. [DOI] [PubMed] [Google Scholar]
- 92.Morrow N v., Stepaniak C, White J, Wilson JF, Li XA. Intra- and Interfractional Variations for Prone Breast Irradiation: An Indication for Image-Guided Radiotherapy. Int J Radiat Oncol Biol Phys 2007;69:910–7. 10.1016/j.ijrobp.2007.06.056. [DOI] [PubMed]
- 93.Koseoglu F.G., Tuncel N., Kizildag A.U., Garipagaoglu M., Adli M., Andic C. Assessment of setup accuracy in patients receiving postmastectomy radiotherapy using electronic portal imaging. Radiat Med - Med Imag Radiat Oncol. 2007;25:45–52. doi: 10.1007/s11604-006-0102-6. [DOI] [PubMed] [Google Scholar]
- 94.Mukundan H., Mukherjee D., Tyagi K., Taneja S., Ranjan S., Sahu S. Dosimetric and isocentric variations due to patient setup errors in CT-based treatment planning for breast cancer by electronic portal imaging. Med J Armed Forces India. 2020;76:51–57. doi: 10.1016/j.mjafi.2018.07.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 95.Murthy K., Al-Rahbi Z., Sivakumar S., Davis C., Ravichandran R., el Ghamrawy K. Verification of setup errors in external beam radiation therapy using electronic portal imaging. J Med Phys. 2008;33:49–53. doi: 10.4103/0971-6203.41192. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 96.Jacob J., Heymann S., Borget I., Dumas I., Riahi E., Maroun P., et al. Dosimetric effects of the interfraction variations during whole breast radiotherapy: a prospective study. Front Oncol. 2015;5 doi: 10.3389/fonc.2015.00199. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 97.Hwang J.M., Hung J.Y., Tseng Y.H., Chang Y.K., Wang Y.N., Chang C.S. Use of electronic portal images to evaluate setup error and intra-fraction motion during free-breathing breast IMRT treatment. Med Dosim. 2019;44:233–238. doi: 10.1016/j.meddos.2018.08.001. [DOI] [PubMed] [Google Scholar]
- 98.Hirata K., Yoshimura M., Mukumoto N., Nakamura M., Inoue M., Sasaki M., et al. Three-dimensional intrafractional internal target motions in accelerated partial breast irradiation using three-dimensional conformal external beam radiotherapy. Radiother Oncol. 2017;124:118–123. doi: 10.1016/j.radonc.2017.04.023. [DOI] [PubMed] [Google Scholar]
- 99.Strom E.A., Amos R.A., Shaitelman S.F., Kerr M.D., Hoffman K.E., Smith B.D., et al. Proton partial breast irradiation in the supine position: treatment description and reproducibility of a multibeam technique. Pract Radiat Oncol. 2015;5:e283–e290. doi: 10.1016/j.prro.2015.01.010. [DOI] [PubMed] [Google Scholar]
- 100.Laaksomaa M., Kapanen M., Skyttä T., Peltola S., Hyödynmaa S., Kellokumpu-Lehtinen P.L. Estimation of optimal matching position for orthogonal kV setup images and minimal setup margins in radiotherapy of whole breast and lymph node areas. Rep Pract Oncol Radiother. 2014;19:369–375. doi: 10.1016/j.rpor.2014.05.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 101.Harris E.J., Donovan E.M., Coles C.E., de Boer H.C.J., Poynter A., Rawlings C., et al. How does imaging frequency and soft tissue motion affect the PTV margin size in partial breast and boost radiotherapy? Radiother Oncol. 2012;103:166–171. doi: 10.1016/j.radonc.2012.03.015. [DOI] [PubMed] [Google Scholar]
- 102.Park C.K., Pritz J., Zhang G.G., Forster K.M., Harris E.E.R. Validating fiducial markers for image-guided radiation therapy for accelerated partial breast irradiation in early-stage breast cancer. Int J Radiat Oncol Biol Phys. 2012:82. doi: 10.1016/j.ijrobp.2011.07.027. [DOI] [PubMed] [Google Scholar]
- 103.Lozano E.M., Pérez L.A., Torres J., Carrascosa C., Sanz M., Mendicote F., et al. Correction of systematic set-up error in breast and head and neck irradiation through a no-action level (NAL) protocol. Clin Transl Oncol. 2011;13:34–42. doi: 10.1007/s12094-011-0614-0. [DOI] [PubMed] [Google Scholar]
- 104.Yue N.J., Goyal S., Zhou J., Khan A.J., Haffty B.G. Intrafractional target motions and uncertainties of treatment setup reference systems in accelerated partial breast irradiation. Int J Radiat Oncol Biol Phys. 2011;79:1549–1556. doi: 10.1016/j.ijrobp.2010.05.034. [DOI] [PubMed] [Google Scholar]
- 105.Lawson J.D., Fox T., Elder E., Nowlan A., Davis L., Keller J., et al. Early clinical experience with kilovoltage image-guided radiation therapy for interfraction motion management. Med Dosim. 2008;33:268–274. doi: 10.1016/j.meddos.2007.06.002. [DOI] [PubMed] [Google Scholar]
- 106.Semaniak A., Kukołowicz P. Set-up uncertainty during postmastectomy radiotherapy with segmented photon beams technique. Rep Pract Oncol Radiother. 2015;20:181–187. doi: 10.1016/j.rpor.2015.01.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 107.Petillion S., Verhoeven K., Weltens C., van den Heuvel F. Accuracy of a new paired imaging technique for position correction in whole breast radiotherapy. J Appl Clin Med Phys. 2015;16:22–31. doi: 10.1120/jacmp.v16i1.4796. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 108.Chung M.J., Lee G.J., Suh Y.J., Lee H.C., Lee S.W., Jeong S., et al. Setup error and effectiveness of weekly image-guided radiation therapy of tomodirect for early breast cancer. Cancer Res Treat. 2015;47:774–780. doi: 10.4143/crt.2014.189. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 109.van Mourik A., van Kranen S., den Hollander S., Sonke J.J., van Herk M., van Vliet-Vroegindeweij C. Effects of setup errors and shape changes on breast radiotherapy. Int J Radiat Oncol Biol Phys. 2011;79:1557–1564. doi: 10.1016/j.ijrobp.2010.07.032. [DOI] [PubMed] [Google Scholar]
- 110.White E.A., Cho J., Vallis K.A., Sharpe M.B., Lee G., Blackburn H., et al. Cone beam computed tomography guidance for setup of patients receiving accelerated partial breast irradiation. Int J Radiat Oncol Biol Phys. 2007;68:547–554. doi: 10.1016/j.ijrobp.2007.01.048. [DOI] [PubMed] [Google Scholar]
- 111.Truong M.T., Hirsch A.E., Kovalchuk N., Qureshi M.M., Damato A., Schuller B., et al. Cone-beam computed tomography image guided therapy to evaluate lumpectomy cavity variation before and during breast radiotherapy. J Appl Clin Med Phys. 2013:14. doi: 10.1120/jacmp.v14i2.4243. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 112.Jung J.H., Cho K.H., Moon S.K., Bae S.H., Min C.K., Kim E.S., et al. Rotation errors of breast cancer on 3D-CRT in TomoDirect. Prog Med Phys. 2015;26:6. doi: 10.14316/pmp.2015.26.1.6. [DOI] [Google Scholar]
- 113.Hasan Y., Kim L., Wloch J., Chi Y., Liang J., Martinez A., et al. Comparison of planned versus actual dose delivered for external beam accelerated partial breast irradiation using cone-beam CT and deformable registration. Int J Radiat Oncol Biol Phys. 2011;80:1473–1476. doi: 10.1016/j.ijrobp.2010.04.013. [DOI] [PubMed] [Google Scholar]
- 114.Jung J.H., Jung J.Y., Bae S.H., Moon S.K., Cho K.H. Setup deviations for whole-breast radiotherapy with TomoDirect: a comparison of weekly and biweekly image-guided protocols. J Korean Phys Soc. 2016;69:1247–1253. doi: 10.3938/jkps.69.1247. [DOI] [Google Scholar]
- 115.Alderliesten T., Heemsbergen W.D., Betgen A., Topolnjak R., Elkhuizen P.H.M., van Vliet-Vroegindeweij C., et al. Breast-shape changes during radiation therapy after breast-conserving surgery. Phys Imaging Radiat Oncol. 2018;6:71–76. doi: 10.1016/j.phro.2018.05.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 116.Sánchez-Rubio P., Rodríguez-Romero R., Castro-Tejero P. A retrospective tomotherapy image-guidance study: analysis of more than 9,000 MVCT scans for ten different tumor sites. J Appl Clin Med Phys. 2014;15:30–45. doi: 10.1120/jacmp.v15i6.4663. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 117.Barbés B., Azcona J.D., Prieto E., de Foronda J.M., García M., Burguete J. Development and clinical evaluation of a simple optical method to detect and measure patient external motion. J Appl Clin Med Phys. 2015;16:306–321. doi: 10.1120/jacmp.v16i5.5524. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 118.Acharya S., Fischer-Valuck B.W., Mazur T.R., Curcuru A., Sona K., Kashani R., et al. Magnetic resonance image guided radiation therapy for external beam accelerated partial-breast irradiation: evaluation of delivered dose and intrafractional cavity motion. Int J Radiat Oncol Biol Phys. 2016;96:785–792. doi: 10.1016/j.ijrobp.2016.08.006. [DOI] [PubMed] [Google Scholar]
- 119.van Heijst T.C.F., Philippens M.E.P., Charaghvandi R.K., den Hartogh M.D., Lagendijk J.J.W., Desirée Van Den Bongard H.J.G., et al. Quantification of intra-fraction motion in breast radiotherapy using supine magnetic resonance imaging. Phys Med Biol. 2016;61:1352–1370. doi: 10.1088/0031-9155/61/3/1352. [DOI] [PubMed] [Google Scholar]
- 120.Zhang R., Andreozzi J.M., Gladstone D.J., Hitchcock W.L., Glaser A.K., Jiang S., et al. Cherenkoscopy based patient positioning validation and movement tracking during post-lumpectomy whole breast radiation therapy. Phys Med Biol. 2015;60:L1–L. doi: 10.1088/0031-9155/60/1/L1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 121.Chadha M., Young A., Geraghty C., Masino R., Harrison L. Image guidance using 3D-ultrasound (3D-US) for daily positioning of lumpectomy cavity for boost irradiation. Radiat Oncol. 2011:6. doi: 10.1186/1748-717X-6-45. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 122.de Boer HCJ, And MS, Heijmen BJM. A protocol for the reduction of systematic patient setup errors with minimal portal imaging workload. Int J Radiat Oncol Biol Phys 2001;50:1350–65. 10.1016/s0360-3016(01)01624-8. [DOI] [PubMed]
- 123.de Boer H.C.J., Heijmen B.J.M. eNAL: an extension of the NAL setup correction protocol for effective use of weekly follow-up measurements. Int J Radiat Oncol Biol Phys. 2007;67:1586–1595. doi: 10.1016/j.ijrobp.2006.11.050. [DOI] [PubMed] [Google Scholar]
- 124.van Herk M. Errors and margins in radiotherapy. Semin Radiat Oncol. 2004;14:52–64. doi: 10.1053/j.semradonc.2003.10.003. [DOI] [PubMed] [Google Scholar]
- 125.Aznar MC, Carrasco de fez P, Corradini S, Mast M, McNair H, Meattini I, et al. ESTRO-ACROP guideline: Recommendations on implementation of breath-hold techniques in radiotherapy. Radiother Oncol 2023;185. 10.1016/j.radonc.2023.109734. [DOI] [PubMed]
- 126.Cuccia F., Alongi F., Belka C., Boldrini L., Hörner-Rieber J., McNair H., et al. Patient positioning and immobilization procedures for hybrid MR-Linac systems. Radiat Oncol. 2021;16 doi: 10.1186/s13014-021-01910-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 127.Fung W.W.K., Wu V.W.C. Image-guided radiation therapy using computed tomography in radiotherapy. J Radiother Pract. 2011;10:121–136. doi: 10.1017/S1460396910000270. [DOI] [Google Scholar]
- 128.Batumalai V., Quinn A., Jameson M., Delaney G., Holloway L. Imaging dose in breast radiotherapy: Does breast size affect the dose to the organs at risk and the risk of secondary cancer to the contralateral breast? J Med Radiat Sci. 2015;62:32–39. doi: 10.1002/jmrs.91. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 129.Alvarado R., Booth J.T., Bromley R.M., Gustafsson H.B. An investigation of image guidance dose for breast radiotherapy. J Appl Clin Med Phys. 2013;14:25–38. doi: 10.1120/jacmp.v14i3.4085. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 130.Borm K.J., Junker Y., Düsberg M., Devečka M., Münch S., Dapper H., et al. Impact of CBCT frequency on target coverage and dose to the organs at risk in adjuvant breast cancer radiotherapy. Sci Rep. 2021:11. doi: 10.1038/s41598-021-96836-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 131.Freislederer P., Batista V., Öllers M., Buschmann M., Steiner E., Kügele M., et al. ESTRO-ACROP guideline on surface guided radiation therapy. Radiother Oncol. 2022;173:188–196. doi: 10.1016/j.radonc.2022.05.026. [DOI] [PubMed] [Google Scholar]
- 132.Ooi B.N.S., Loh H., Ho P.J., Milne R.L., Giles G., Gao C., et al. The genetic interplay between body mass index, breast size and breast cancer risk: a Mendelian randomization analysis. Int J Epidemiol. 2019;48:781–794. doi: 10.1093/ije/dyz124. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 133.McGhee D.E., Steele J.R. Breast volume and bra size. Int J Clothing Sci Technol. 2011;23:351–360. doi: 10.1108/09556221111166284. [DOI] [Google Scholar]
- 134.Ringberg A., Bågeman E., Rose C., Ingvar C., Jernström H. Of cup and bra size: reply to a prospective study of breast size and premenopausal breast cancer incidence [1] Int J Cancer. 2006;119:2242–2243. doi: 10.1002/ijc.22104. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.