Abstract
Four hepatitis C virus transmission chains at three dialysis units were disclosed by limited sequencing; three of these were disclosed by analysis of the NS5-B region of the genome. Dialysis on the same shift as that during which infected patients were dialyzed was the common factor for seven patients in two chains. Two nurses exposed to needle sticks and their sources of infection constituted two other chains. The strains of three chains belonged to subtype 1a and formed clusters with an intrachain variability of 0 to 6 nucleotides compared to 8 to 37 nucleotides for unrelated strains within this subtype. The clusters were supported by bootstrap values ranging from 89 to 100%.
Hepatitis C virus (HCV) is a major agent of transfusion-transmitted hepatitis (9). Nosocomial spread of HCV has, however, been reported to occur during dialysis (2, 4, 11, 14, 20) and in hematological wards (1), and this explains the high HCV prevalence in nontransfused patients in these settings (16). There are six genotypes of HCV designated by Arabic numerals and an increasing number of subgenotypes or subtypes designated by letters (15, 17). In the Western world genotypes 1, 2, and 3 make up the majority of HCV strains, with genotype 1 being most frequent (17). Within a genotype, sequence variability has been used to characterize strains to identify nosocomial transmission (1, 2, 4, 9, 11, 14, 20) and transmission from batches of immunoglobulins (6, 7, 13) and through needle sticks (19).
We have investigated the association between cases of infection of patients and staff with HCV at three different dialysis units in different towns in central Sweden by sequencing within the NS5-B region (Table 1). Twenty-one other HCV-infected individuals provided strains representative for Sweden (Table 2).
TABLE 1.
Data on patients and genotypes of recovered HCV strains in the three investigations
| Patient no. | Sex | Yr born | Origin of serum sample | Epidemiological backgrounda | Date of onset of HCV infection | Serum samplee
|
HCV genotype | |
|---|---|---|---|---|---|---|---|---|
| Date taken | Identification | |||||||
| 1:1 | M | 1946 | Karlstad | DP | Dec. 1989b | Dec. 1991 | 1182/92d | 3a |
| 1:2 | F | 1971 | Karlstad | DP | Jan. 1992b | May 1992 | 7394/93 | 1a |
| 1:3 | F | 1921 | Karlstad | DP | Aug. 1992c | Aug. 1992 | 7398/93 | 1a |
| 1:4 | M | 1915 | Karlstad | DP | Aug. 1992c | Nov. 1992 | 7400/93 | 1a |
| 1:5 | M | 1919 | Karlstad | DP | Aug. 1992c | Nov. 1993 | 316/94 | 1a |
| 1:6 | F | 1966 | Karlstad | DP | Nov. 1992b | Jan. 1995 | 286/95 | 1a |
| 1:7 | M | 1923 | Karlstad | DP | Apr. 1993b | Apr. 1993 | 7395/93d | 1a |
| 1:8 | M | 1941 | Karlstad | DP | June 1994c | June 1994 | 974/94 | 2b |
| 1:9 | F | 1945 | Karlstad | Nurse | Oct. 1994b | Oct. 1994 | 1047/94 | 3a |
| 2:1 | M | 1919 | Stockholm/Avesta | DP | 1990b | Dec. 1995 | 699/95 | 1a |
| 2:2 | M | 1924 | Stockholm/Avesta | DP | 1990b | Dec. 1995 | 700/95 | 1a |
| 2:3 | M | 1943 | Avesta | DP | Nov. 1990b | Oct. 1994 | 681/95 | 1a |
| 2:4 | M | 1972 | Avesta | DP | Sep. 1993 | Nov. 1995 | 695/95 | 1a |
| 2:5 | M | 1955 | Avesta | DP | June 1995 | Nov. 1995 | 694/95 | 1a |
| 2:6 | F | 1926 | Avesta | DP | June 1995 | Nov. 1995 | 696/95 | 1a |
| 3:1 | F | 1934 | Uppsala | Nurse | June 1993c | June 1993 | 6260/93 | 1a |
| 3:2 | M | 1923 | Uppsala | DP | Dec. 1991b | Jul. 1992 | 406/94 | 1a |
DP, dialysis patient.
First known anti-HCV-positive sample.
First anti-HCV-negative and HCV PCR-positive sample.
Analysis of the C (core) region.
For the HCV strain used for genetic analysis.
TABLE 2.
Data on Swedish background HCV strains used in the phylogenetic analyses
| Serum sample
|
HCV genotype | ||
|---|---|---|---|
| Identification | Origin | Background | |
| 484/94 | Jönköping | Immunoglobulin | 1a |
| 586/96 | Jönköping | Immunoglobulin | 1a |
| 504/94 | Halmstad | Immunoglobulin | 1a |
| 531/94 | Avesta | Immunoglobulin | 1a |
| 3817/91 | Stockholm | Chronic hepatitis | 1a |
| 506/96 | Uppsala | Dialysis | 1a |
| 906/96 | Västerås | Dialysis | 1a |
| 911/96 | Ängelholm | Sexual transmission | 1a |
| 6723/91 | Uppsala | Dialysis | 1b |
| 7457/91 | Karlstad | Blood donor | 1b |
| 7458/91 | Karlstad | Blood donor | 1b |
| 6099/91 | Stockholm | Karolinska Hospital | 1b |
| 7739/91 | Stockholm | Karolinska Hospital | 1b |
| 4917/91 | Stockholm | Karolinska Hospital | 1b |
| 7458/93 | Stockholm | Karolinska Hospital | 1b |
| 6097/91 | Stockholm | Karolinska Hospital | 1b |
| 912/96 | Ängelholm | Sexual transmission | 1b |
| 3453/91 | Stockholm | Blood donor | 2b |
| 6716/91 | Uppsala | Dialysis | 2b |
| 1109/94 | Uppsala | Dialysis | 2b |
| 1041/91 | Uppsala | Chronic hepatitis | 2b |
| 460/94a | Uddevalla | Sexual transmission | 3a |
| 461/94a | Uddevalla | Sexual transmission | 3a |
Analysis of the C (core) region.
One investigation comprised 8 of 10 anti-HCV-positive patients at a unit where 60 patients were dialyzed. Patients 1:1 and 1:2 were known seropositives. Since 1991 all patients have been screened for anti-HCV every third month. In November 1992 patients 1:3, 1:4, 1:5, and 1:6 seroconverted. Sera taken in August from patients 1:3, 1:4, and 1:5 were retrospectively found to be HCV RNA positive by PCR. Patients 1:3, 1:4, and 1:5 had been dialyzed in the same room and during the same shift as patient 1:2 in May. There was no earlier serum available from patient 1:6, who had not been dialyzed with patients 1:2, 1:3, 1:4, and 1:5. In April 1993 patient 1:7 seroconverted, and in June 1994 patient 1:8 seroconverted. During September 1994 all staff members were screened for anti-HCV, and a nurse, 1:9, was found to be positive. It then came to light that she had been exposed to patient 1:1 by a needle stick in 1989.
Another investigation comprised all 6 anti-HCV-positive patients at another unit where 43 patients were dialyzed. Patients 2:1 and 2:2, known to be anti-HCV positive, had been dialyzed at this unit during the summer since 1990. In August 1990 patient 2:3 was dialyzed in the same room and during the same shift as patient 2:2. In November patient 2:3 was found to be anti-HCV positive. In July 1993 patients 2:4 and 2:5 were also dialyzed with patient 2:2, and patient 2:4 seroconverted in August. In April 1995 patients 2:5 and 2:6 were dialyzed with patient 2:4, and in June patients 2:5 and 2:6 both seroconverted.
The third investigation dealt with another nurse, 3:1, who in April 1993 was exposed to anti-HCV-positive patient 3:2 by a needle stick. In June she was HCV RNA positive, and in September she seroconverted.
The NS5-B region was chosen for analysis, since it is not considered to be subject to a high level of immune pressure. Viral RNA was extracted from serum mainly as described by Garson et al. (6). cDNA synthesis was performed with Moloney murine leukemia virus reverse transcriptase and primer hep102 (5′-AGCATGATGTTATCAGCTCC-3′) at positions 8681 to 8700 (3). The cDNA was amplified by PCR with hep101 (5′-ATACCCGCTGCTTTGACTC-3′) at positions 8258 to 8276 and hep102. Nesting and sequencing were performed with hep101 and hep105 (5′-ATACCTAGTCATAGCCTCCGTGA-3′) at positions 8616 to 8638. The obtained sequences were aligned with the corresponding parts of 116 HCV sequences from GenBank, and that of the GB virus B (GBV-B) genome (10) by using Tree-Align software (8). A dendrogram was created with DNADIST and NEIGHBOR, PHYLIP package, version 3.53 (5) by using GBV-B as the outgroup. SEQBOOT was used to bootstrap 1,000 data sets.
For some strains the NS5-B region could not be amplified. In these cases part of the core region was amplified with the primers NCR3 (6) and 186 (12). Primers 256 (12) and hep140 (5′-TGAGCACGAATCCTAAACCTCA-3′) at positions 343 to 364 were used for nesting and sequencing. The obtained sequences were aligned with 49 HCV sequences from GenBank. Neighbor joining and bootstrapping were performed as described above.
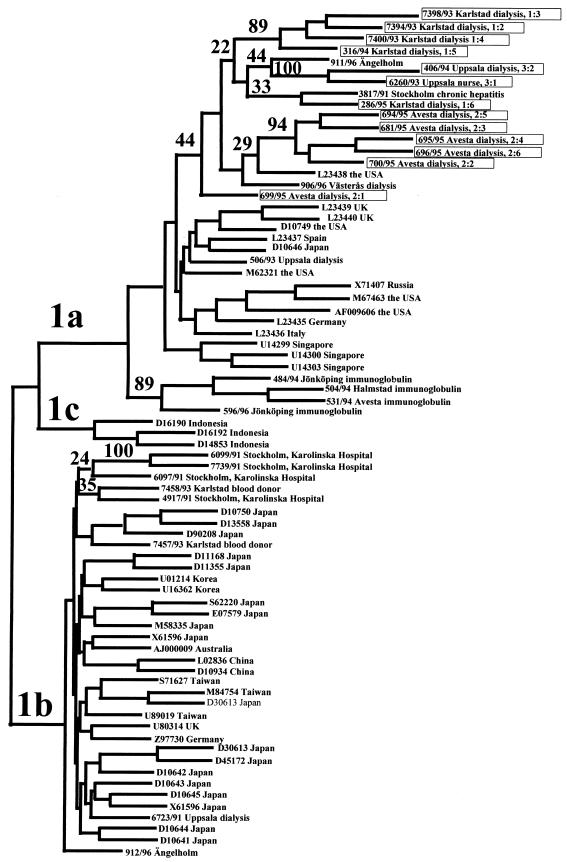
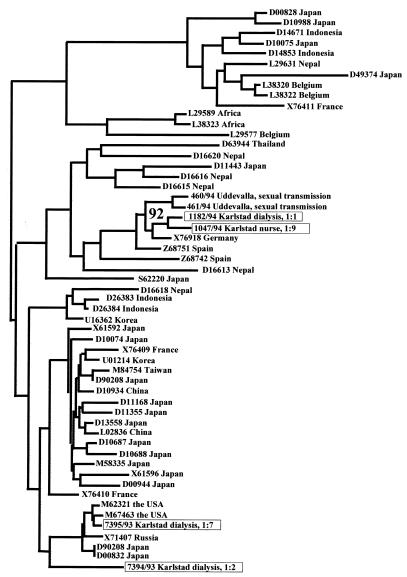
The genotypes of 17 different HCV strains recovered from the three units are shown in Table 1. Dendrograms based on NS5-B and core regions are shown in Fig. 1 and 2, respectively.
FIG. 1.
Part of a dendrogram, representing the branches formed by genotype 1 strains, obtained by neighbor joining of 300 nucleotides within the NS5-B regions of 156 sequences. The strains recovered in the three investigations are identified by the numbers of the patient from which they were taken (see Table 1). The accession numbers of sequences from GenBank are given at the nodes. Bootstrap values obtained from 1,000 replicas are given as percentages at the branching forks. Boxed strains are those investigated in this study.
FIG. 2.
Dendrogram obtained by neighbor joining based on 256 nucleotides within the C (core) regions of four HCV strains recovered in the investigations, two Swedish background strains, and 49 strains from GenBank. Genotypes are not given, since the analysis did not discriminate between strains belonging to different genotypes. The bootstrap value for the association of strains from patients 1:1 and 1:9 obtained from 1,000 replicas is given as a percentage at the fork formed by these strains. The strains investigated in this study (boxes) are identified by the numbers of the patients from which they were taken (see Table 1).
The HCV strains from patients 1:2, 1:3, 1:4, and 1:5 differed by three to six nucleotides and clustered within subtype 1a with 89% bootstrap support, confirming that patient 1:2 was the source of infection for patients 1:3, 1:4, and 1:5. The strain from patient 1:6 differed from the strains from patients 1:2, 1:3, 1:4, and 1:5 by 14 to 18 nucleotides, while that from patient 1:8 belonged to subtype 2b.
The NS5-B regions of the HCV strains from patients 1:1 and 1:7 could not be amplified. Therefore, the core regions of these strains were sequenced along with those of strains from patient 1:2 and from patient 1:9, the nurse presumed to be infected by patient 1:1. The strain from patient 1:7 was more related to HCV-1 of U.S. origin than to the strain from patient 1:2. Strains from patients 1:1 and 1:9 belonged to subtype 3a and differed by three nucleotides.
All six strains recovered from the second dialysis unit belonged to subtype 1a. Five of these strains from patients 2:2, 2:3, 2:4, 2:5, and 2:6, showed a maximum divergence of three nucleotides and formed a cluster supported by 93% bootstrap. The strain from patient 2:1 differed by 8 to 10 nucleotides from those from patients 2:2, 2:3, 2:4, 2:5, and 2:6.
The HCV strains from the nurse (3:1) and her presumed source of infection (patient 3:2) differed by two nucleotides and clustered with 100% bootstrap support. One of four background strains from this unit belonged to subtype 1a, one belonged to subtype 1b, and two related strains belonged to subtype 2b.
In our investigations four independent chains of HCV transmission were confirmed, two of which involved the transmission of HCV between patients. At one unit four of the strains were closely related, and at a second unit five were closely related, although the strains from different units were unrelated. Comparing the sequences of strains within the same transmission chains showed that the divergence was 0 to 6 nucleotides, while it was 8 to 37 nucleotides for unrelated strains within the same subtype.
That the dialysis patients were the sources of infection for the two nurses confirms that HCV-infected dialysis patients have a virus load compatible with needle stick transmission, which may also explain the frequent horizontal non-transfusion-related transmission of HCV at dialysis centers reported herein and in other articles (2, 14, 18). The exact mode of transmission between the dialysis patients was not clarified. There were no known violations of the infection control precautions, but the finding that transmission resulted from dialysis of noninfected and infected patients on the same shift, although the patients did not use the same dialysis machine, confirmed a previous report of transmission between dialysis patients that also could be related to treatment during the same shift (2).
We have compared only one sequence from each case. HCV strains in dialysis patients have, however, been shown to be heterogeneous by several techniques (14, 18, 20). It may be argued that several HCV strains from each individual should have been sequenced. However, sequencing PCR-generated amplimers provides the consensus sequence for the infected individual, an estimate of which by cloning would have been laborious. In conclusion it was shown that sequencing a PCR fragment within the NS5-B region and phylogenetic analysis of the sequences provided a powerful instrument for investigating nosocomial HCV transmission, despite the fact that most of the strains belonged to the same subtype.
Nucleotide sequence accession numbers.
The nucleotide sequence data reported in this paper will appear in the EMBL, GenBank, and DDBJ nucleotide sequence databases under accession no. AF071953 to AF071987.
Acknowledgments
Noel McCarthy is acknowledged for having proofread the manuscript.
This work was supported by the Swedish Medical Research Council grant K97-16P-11019-04B and K97-06X-10385-05.
REFERENCES
- 1.Allander T, Gruber A, Naghavi M, Beyene A, Söderström T, Björkholm M, Grillner L, Persson M A A. Frequent patient-to-patient transmission of hepatitis C virus in a haematology ward. Lancet. 1995;345:603–607. doi: 10.1016/s0140-6736(95)90518-9. [DOI] [PubMed] [Google Scholar]
- 2.Allander T, Medin C, Jakobson S H, Grillner L, Persson M A A. Hepatitis C transmission in a hemodialysis unit: molecular evidence for spread of virus among patients not sharing equipment. J Med Virol. 1994;43:415–419. doi: 10.1002/jmv.1890430417. [DOI] [PubMed] [Google Scholar]
- 3.Choo Q-L, Richman K, Han J H, Berger K, Lee C, Dong C, Gallegos C, Coit D, Medina-Selby A, Barr P J, Weiner A, Bradley D W, Kuo G, Houghton M. Genetic organization and diversity of the hepatitis C virus. Proc Natl Acad Sci USA. 1991;88:2451–2455. doi: 10.1073/pnas.88.6.2451. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.de Lamballerie X, Olmer M, Bouchouareb D, Zandotti C, de Micco P. Nosocomial transmission of hepatitis C virus in haemodialysis patients. J Med Virol. 1996;49:296–302. doi: 10.1002/(SICI)1096-9071(199608)49:4<296::AID-JMV7>3.0.CO;2-Z. [DOI] [PubMed] [Google Scholar]
- 5.Felsenstein J. PHYLIP: phylogeny inference package, version 3.52c. Seattle, Wash: University of Washington; 1993. [Google Scholar]
- 6.Garson J A, Ring C, Tuke P, Tedder R S. Enhanced detection by PCR of hepatitis C virus RNA. Lancet. 1990;336:878–879. doi: 10.1016/0140-6736(90)92384-t. [DOI] [PubMed] [Google Scholar]
- 7.Healey C J, Sabharwal N K, Daub J, Davidson F, Yap P L, Fleming K A, Chapman R W, Simmonds P, Capel H. Outbreak of acute hepatitis C following the use of anti-hepatitis C virus-screened intravenous immunoglobulin therapy. Gastroenterology. 1996;110:1120–1126. doi: 10.1053/gast.1996.v110.pm8613001. [DOI] [PubMed] [Google Scholar]
- 8.Hein J. Unified approach to alignment and phylogenies. Methods Enzymol. 1990;183:626–645. doi: 10.1016/0076-6879(90)83041-7. [DOI] [PubMed] [Google Scholar]
- 9.Kuo G, Choo Q-L, Alter H J, Gitnik G L, Redker A G, Purcell R H, Miyamura T, Dienstag J L, Alter M J, Stevens C E, Tegtmeiter G E, Bonino F, Colombo M, Lee W-S, Kuo C, Berger K, Shuster J R, Overby L R, Bradley D W, Houghton M. An assay for circulating antibodies to a major etiologic agent of human non-A, non-B hepatitis. Science. 1989;244:362–364. doi: 10.1126/science.2496467. [DOI] [PubMed] [Google Scholar]
- 10.Muerhoff A S, Leary T P, Simons J N, Pilot-Matias T J, Dawson G J, Erker J C, Chalmers M L, Schlauder G G, Desai S M, Mushahwar I K. Genomic organization of GB viruses A and B: two new members of the Flaviviridae associated with GB agent hepatitis. J Virol. 1995;69:5621–5630. doi: 10.1128/jvi.69.9.5621-5630.1995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Munro J, Briggs J D, McCruden E A B. Detection of a cluster of hepatitis C infections in a renal transplant unit by analysis of sequence variation of the NS5a gene. J Infect Dis. 1996;174:177–180. doi: 10.1093/infdis/174.1.177. [DOI] [PubMed] [Google Scholar]
- 12.Okamoto H, Sugiuama Y, Okada S-I, Urai K K, Akhane Y, Sugai Y, Tanaka T, Sato K, Tsuda F, Miyakawa Y, Mayumi M. Typing hepatitis C virus by polymerase chain reaction with type specific primers: application to clinical surveys and tracing infectious sources. J Gen Virol. 1992;73:673–679. doi: 10.1099/0022-1317-73-3-673. [DOI] [PubMed] [Google Scholar]
- 13.Power J P, Lawlor E, Daviudson F, Holmes E C, Yap P L, Simmonds P. Molecular epidemiology of an outbreak of infection with hepatitis C virus in recipients of anti-D immunoglobulin. Lancet. 1995;345:1211–1213. doi: 10.1016/s0140-6736(95)91993-7. [DOI] [PubMed] [Google Scholar]
- 14.Sampietro M, Badalamenti S, Salvadori S, Corbetta N, Graziani G, Como G, Fiorelli G, Ponticelli C. High prevalence of a rare hepatitis C virus in patients treated in the same hemodialysis unit: evidence for nosocomial transmission of HCV. Kidney Int. 1995;47:911–917. doi: 10.1038/ki.1995.136. [DOI] [PubMed] [Google Scholar]
- 15.Simmonds P, Alberti A, Alter H J, Bonino F, Bradley D W, Brechot C, Brouwer J T, Chan S-W, Chayama K, Chen D-S, Choo Q-L, Colombo M, Cuypers H T M, Date T, Dusheiko G M, Esteban J I, Fay O, Hadziyannis S J, Han J, Hatzakis A, Holmes E C, Hotta H, Houghton M, Irvine B, Kohara M, Kolberg J A, Kuo G, Lau J Y N, Lelie P N, Maertens G, McOmish F, Miyamura T, Mizokami M, Nomoto A, Prince A M, Reesink H W, Rice C, Roggendorf M, Schalm S W, Shikata T, Shimotohno K, Stuyver L, Trepo C, Weiner A, Yap P L, Urdea M S. A proposed system for nomenclature of hepatitis C virus genotypes. Hepatology. 1994;19:1321–1324. [PubMed] [Google Scholar]
- 16.Simon N, Couroucé A-M, Lemerec N, Trépo C, Ducamp S. A twelve year natural history of hepatitis C virus infection in hemodialyzed patients. Kidney Int. 1994;46:504–511. doi: 10.1038/ki.1994.301. [DOI] [PubMed] [Google Scholar]
- 17.Stuyver L, van Arnhem W, Wyseur A, Hernandez F, De laporte E, Maertens G. Classification of hepatitis C viruses based on phylogenetic analysis of the envelope 1 and nonstructural 5B regions and identification of five additional subtypes. Proc Natl Acad Sci USA. 1994;91:10134–10138. doi: 10.1073/pnas.91.21.10134. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Stuyver L, Claeys H, Wyseur A, van Arnhem W, de Beenhouwer H, Uytendaele S, Beckers J, Matthijs D, Leroux-Roels G, Maertens G, de Paepe M. Hepatitis C virus in a hemodialysis unit: molecular evidence for nosocomial transmission. Kidney Int. 1996;49:889–895. doi: 10.1038/ki.1996.122. [DOI] [PubMed] [Google Scholar]
- 19.Suzuki K, Mizokami M, Lau J Y N, Mizoguchi N, Kato K, Mizuno Y, Sodeyama T, Kiyosawa K, Gojobori T. Confirmation of hepatitis C virus transmission through needlestick accidents by molecular evolutionary analysis. J Infect Dis. 1994;170:1575–1578. doi: 10.1093/infdis/170.6.1575. [DOI] [PubMed] [Google Scholar]
- 20.Zeuzem S, Scheuermann E H, Waschk D, Lee J-H, Blaser C, Franke A, Roth K W. Phylogenetic analysis of hepatitis C virus isolates from hemodialysis patients. Kidney Int. 1996;49:896–902. doi: 10.1038/ki.1996.123. [DOI] [PubMed] [Google Scholar]