Abstract
Summary
It remains unclear whether the relationship between type 2 diabetes mellitus (T2DM) and bone mineral density (BMD) reflects causality in East Asian populations. Herein, a Mendelian randomization study conducted in East Asian population enhances the current clinical cognition that T2DM is not associated with reduction in BMD.
Purpose
A Mendelian randomization (MR) approach was utilized to investigate the relationship between type 2 diabetes mellitus (T2DM) and bone mineral density (BMD) in East Asian populations.
Methods
Genome-wide association study summary data from BioBank Japan were used to identify genetic variants strongly related to T2DM risk (36,614 cases and 155,150 controls) and osteoporosis (7788 cases and 204,665 controls). Heel BMD GWAS data of 1260 East Asian people from ieu open gwas project was considered as a second outcome. Inverse variance-weighted (IVW) analysis was mainly applied; MR-Egger and the weighted median were also used to obtain robust estimates. A series of sensitivity analyses including Cochran’s Q test, MR-Egger regression, and leave-one-out analysis were used to detect pleiotropy or heterogeneity.
Results
In the main analysis, IVW estimates indicated that T2DM significantly associated with the risk of osteoporosis (odds ratio = 0.92, 95% CI: 0.86–0.99, p = 0.016) and with higher BMD (OR: 1.25, 95% CI: 1.06–1.46, p = 6.49 × 10−3). Results of comprehensive sensitivity analysis were consistent with the main causality estimate. Horizontal pleiotropy and heterogeneity were absent in our MR study.
Conclusions
T2DM is not associated with reduction in BMD in terms of genetic polymorphism in East Asian populations.
Supplementary Information
The online version contains supplementary material available at 10.1007/s00198-023-06807-6.
Keywords: Bone mineral density, Mendelian randomization, Osteoporosis, Type 2 diabetes mellitus
Introduction
Osteoporosis is a metabolic bone disease characterized by loss of bone mass, damage to the microstructure of bone tissue, and a decline in bone quality, ultimately giving rise to increased bone fragility and the risk of fracture [1]. Approximately 50% of women and 20% of men are expected to have an osteoporotic fracture in their lifetime [2]. Owing to population aging, osteoporosis has become a serious public health problem. Among all osteoporotic fractures, hip fractures consistently account for the highest morbidity, mortality, and cost. By 2050, it is estimated that approximately 4.5 million to 6.26 million cases of osteoporotic hip fracture will occur worldwide, half of which will occur in Asia [3,4]. In Asian, up to one in four patients who sustain a hip fracture die within a year and 6–8% of hip fracture patients will suffer second fracture within 2 years [5,6]. Osteoporotic fractures are associated with higher rate of hospitalization and disability, decline in physical and cognitive function, and significant medical burden—projected to increase to 47.4 billion in 2030 [7]. Together, these outcomes impose a heavy social and economic burden on society.
In recent years, researchers have widely acknowledged the link between type 2 diabetes mellitus (T2DM) and osteoporosis. A meta-analysis conducted by Ma. LL et al. included 15 observational studies (3437 diabetics and 19,139 controls) with conflicting results, and concluded that people with T2DM had higher bone mineral density (BMD) than those without T2DM, regardless of the measured bone site, sex, age, body mass index (BMI), or medication use [8]. Observational studies conducted in China have also shown that T2DM was associated with the value of BMD [9–11]. However, Majima et al. found decreased BMD in the distal radius but not in the lumbar spine or femoral neck among 145 Japanese patients with T2DM [12]. In China, Chen et al. found that older men with T2DM are at greater risk of low BMD than those without T2DM [13]. These results might be owing to limitations such as small study samples, inconsistent measurement location of BMD, and confounding factors, such as metabolic diseases like hypertension and hyperlipidemia.
Mendelian randomization (MR) is a genetic epidemiological method that can uncover causal relationships between one or more genetic variations related to health outcomes, typically single-nucleotide polymorphisms (SNPs) and exposure factors [14]. MR studies have advantages over traditional observational epidemiological studies. First, confounding factors can be mitigated by the random assortment of alleles owing to Mendel’s Second Law [15]. Second, reverse causality can be avoided, with alleles randomly assigned to offspring and therefore unlikely to be interfered with by confounders; genotypes are not affected by disease, which also avoids reverse causality bias [16]. Third, because of the high accuracy of gene variation sequencing, regression dilution bias caused by measurement error can be avoided [15].
Results of the National Osteoporosis Risk Assessment have shown that Asian populations have a significantly increased likelihood of osteoporosis compared with white populations [17]. Several ethnicity phenotyping studies have provided a more detailed understanding of ethnic distinctions in the pathophysiology, prevalence, and clinical and health care system factors of T2DM and osteoporosis [18–22]. Ethnic disparities may be related to genetic risk factors, but further study is necessary. Ahmad et al. used MR to study the effects of T2DM on BMD in the European population, but there are no relevant reports in Asian populations [23]. Initially, we utilized genome-wide association study (GWAS) summary data on osteoporosis to investigate the relationship between osteoporosis and T2DM. However, in the Japanese cohort, the diagnosis of osteoporosis was dependent on physicians’ diagnoses at cooperating hospitals, rather than bone mineral density (BMD) measurements. Consequently, we attempted to confirm our findings by using GWAS data on heel BMD from the East Asian population. In this study, we aimed to provide a clinical reference for early prevention of osteoporosis and reduction of osteoporotic fracture complications.
Methods
GWAS data sources
We obtained GWAS summary data for both T2DM and osteoporosis from BioBank Japan (BBJ). GWAS summary data of T2DM were obtained from a meta-analysis of 36,614 cases and 155,150 controls with Japanese ancestry [24]. GWAS summary data of osteoporosis were obtained for 7788 cases and 204,665 controls with Japanese ancestry [25]. As BBJ project registered not only patients with newly developed diseases but also patients who were diagnosed and treated before starting the project, some participants were enrolled several years after disease onset or diagnosis. Patients with T2DM or osteoporosis were diagnosed by physicians at the cooperating hospitals [26,27]. We also include another GWAS summary data for heel BMD of 1260 East Asian people from ieu open gwas project (https://gwas.mrcieu.ac.uk/) (Dataset: ukb-e-3148_EAS) as a second outcome.
Instrumental variables selection process
Candidate genetic instrumental variables (IVs) robustly associated with the exposure of interest (p < 5 × 10−8) were obtained from GWAS of T2DM [28]. Linkage disequilibrium clumping with a clumping window of 10 MB was applied to eliminate SNPs with larger p values at a threshold of linkage disequilibrium R2 > 0.001, using the Asian population reference to ensure independence among IVs. SNPs that were significantly associated with the outcome (p < 5 × 10−8) were discarded. For missed SNPs in the outcome GWAS dataset, proxies were identified at the cutoff of R2 > 0.8. If no suitable proxy was available, SNPs were discarded. The F-statistic was used to verify the strength of IVs, using the following formula: R2 × (N − 2)/(1 − R2). Here, R2 indicates the proportion of variance in educational attainment explained by a given SNP and N indicates sample size. More specifically, R2 was calculated with the following formula: R2 = [2 × Beta2 × (1 − EAF) × EAF]/[2 × Beta2 × (1 − EAF) × EAF + 2 × SE2 × N × (1 − EAF) × EAF]. Here, Beta indicates the genetic effect of SNP on educational attainment, EAF is effect allele frequency, SE is standard error, and N is sample size; only strong IVs (F-statistic > 10) for each of the exposures of interest were retained [29,30]. Fourth, we excluded ambiguous and palindromic SNPs (minor allele frequency > 0.42) for which the effect cannot be corrected in the harmonizing process. The MR-pleiotropy residual sum and outlier (MR-PRESSO) test was conducted to discard SNPs with potential pleiotropy.
Mendelian randomization
To perform robust and reliable causal inference of the effect of T2DM on BMD, in the main analysis, we performed multiplicative random-effect inverse variance-weighted (MRE-IVW) analysis [31]. Sensitivity analyses were performed using weighted median [32] and MR-Egger methods [33]. MR-Egger regression is not restricted to a zero intercept, which can determine a genotype–outcome dose–response relationship in which pleiotropic effects are taken into account [34]. However, the MR-Egger method is more sensitive than other methods for detecting unobserved associations of genetic variants with confounders of the exposure–outcome association, and a larger sample size is required for the same underlying exposure variants [33]. The weighted median method can provide consistent effect estimates when at least 50% of the information in the analysis comes from valid instruments. The Cochran Q test for the IVW method was used to detect heterogeneity [35]. No heterogeneity was detected if the p value of Cochran’s Q was > 0.05. The intercept term derived from MR-Egger regression was used to examine horizontal pleiotropy. The leave-one-out test was then performed to assess whether the IVW estimate was biased by the influence of single SNPs.
We looked up each SNP in Phenoscanner (http://www.phenoscanner.medschl.cam.ac.uk/) to assess whether the estimate was violated by potential risk factors, including age, BMI, sex, history of fragility fracture, unhealthy lifestyle (smoking, alcohol, high-sodium diet), endocrine factors (estrogen, glucocorticoids, vitamin D, parathyroid hormone, calcitonin), concomitant chronic diseases (liver and kidney diseases, cerebrovascular diseases, rheumatoid diseases), drugs (chemotherapy drugs, steroids), and malnutrition. All the statistical analyses were performed using R software (version 4.0.2, TwoSampleMR package 0.5.5).
Gene ontology (GO) enrichment analysis
To further explore the biological role underlying T2DM on the osteoporosis development, we performed a gene ontology (GO) and KEGG enrichment analysis using the nearest genes for each lead SNP. Comprehensive gene list annotation and analysis resource were performed in Metascape (http://metascape.org/gp/index.html), a customer-friendly web-based portal [36]. Enrichment dot bubble was plotted by https://www.bioinformatics.com.cn, a free online platform for data analysis and visualization.
Results
Eighty-two SNPs robustly associated with T2DM were remained after clumping method. And as shown in Supplementary table S1, 18 SNPs were found to correlate with potential risk factors and removed. In the harmonizing process, palindromic SNP (rs2057565) was removed. Thus, after rigorous SNP filtering steps in quality control, 63 IVs remained for further analysis (see Supplementary table S2). All the SNPs used in our analyses together explained 1.66% of the variance in type 2 diabetes mellitus. No weak IVs (F-statistic ≤ 10) for our exposure of interest were detected. No potentially pleiotropic variants were identified in the MR-PRESSO outlier test. As seen in Table 1, in the main analysis, IVW estimates indicated that T2DM significantly decreased the risk of osteoporosis (odds ratio [OR] = 0.92, 95% confidence interval [CI]: 0.86–0.99, p = 0.016). Results of MR-Egger and weighted median were consistent with IVW, though insignificant. The p value derived from MR-Egger intercept was 0.729 (Egger_intercept = 0.0025), suggesting absence of horizontal pleiotropy. The value of Cochran’s Q was 65.106, indicating an absence of heterogeneity (p = 0.369).
Table 1.
MR estimates of the causal association and compensatory sensitivity analyses between T2DM and osteoporosis and BMD
| Exposure | Outcome | Main analysis | Compensatory sensitivity analyses | ||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|
| MRE-IVW | Weighted median | MR-Egger | MR-Egger regression | Cochran’s Q test | |||||||
| OR (95% CI) | p | OR (95% CI) | p | OR (95% CI) | p | Egger_intercept | p | Q value | p | ||
| T2DM | Osteoporosis | 0.92 (0.86, 0.99) | 0.016 | 0.92 (0.83, 1.03) | 0.144 | 0.89 (0.74, 1.07) | 0.221 | 0.0025 | 0.729 | 65.107 | 0.369 |
| BMD | 1.247 (1.064, 1.463) | 6.49 × 10−3 | 1.425 (1.11, 1.83) | 5.50 × 10−3 | 1.196 (0.762, 1.879) | 0.440 | 0.0034 | 0.846 | 56.633 | 0.563 | |
MR Mendelian randomization, MRE-IVW multiplicative random effects-inverse variance-weighted, OR odds ratio, CI confidence interval, T2DM type 2 diabetes mellitus, BMD bone mineral density
Three missed SNPs without available proxies (rs149692182, rs4793326, and rs5874792) in the Heel BMD GWAS dataset were discarded, leaving 60 IVs remained for further analysis between T2DM and BMD (see Supplementary table S2). As shown in Table 1, based on IVW method, T2DM was identified to be causally associated with higher BMD (OR: 1.25, 95% CI: 1.06–1.46, p = 6.49 × 10−3). The causal association remained significant based on weighted median method (OR: 1.43, 95% CI: 1.11–1.83, p = 5.50 × 10−3). Results of MR-Egger were consistent with IVW, though insignificant (OR: 1.20, 95% CI: 0.76–1.88, p = 0.440). The p value derived from MR-Egger intercept was 0.846 (Egger_intercept = 0.0034), suggesting absence of horizontal pleiotropy. The value of Cochran’s Q was 56.633, indicating an absence of heterogeneity (p = 0.563).
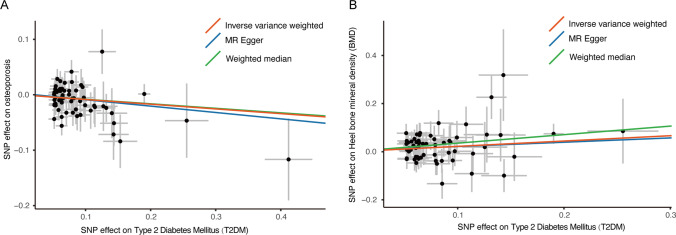
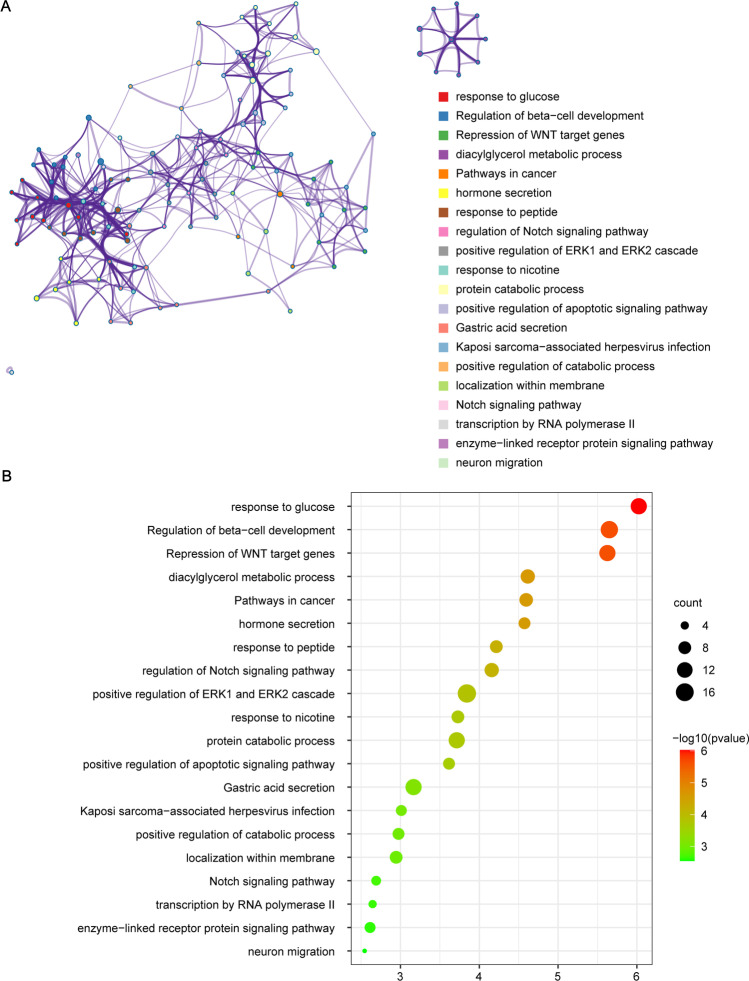
Figure 1 shows the scatter plot of the above three methods for the association of T2DM with risk of osteoporosis (Fig. 1A) and BMD (Fig. 1B). And in the leave-one-out test, no SNPs were found to violate the causality estimates (Supplemental Fig. S1). GO and KEGG pathway enrichment analysis found significant enrichment of top 20 crucial regulation pathways (Fig. 2). Among them, several pathways may be involved in osteoporosis pathogenesis, like repression of WNT target genes, diacylglycerol metabolic process, and regulation of Notch signaling pathway.
Fig. 1.
Scatter plot of Mendelian randomization analyses for the association of T2DM with risk of osteoporosis (A) and heel bone mineral density (B). MR, Mendelian randomization; SNP, single-nucleotide polymorphism; T2DM, type 2 diabetes mellitus
Fig. 2.
Gene ontology enrichment analysis of nearest genes for single-nucleotide polymorphisms used on significant MR analysis results (A) enriched ontology clusters, where every cluster is represented in a single color and shown as a circle; (B) GO enrichment dot bubble plot where count means enriched number of genes
Discussion
To the best of our knowledge, this is the first MR study among East Asian individuals using multiple genetic variants for T2DM and analyzing their effect on osteoporosis/BMD. This study provides robust genetic evidence in support of the hypothesis that T2DM is not associated with reduction in BMD in terms of genetic polymorphism. No evidence of directional pleiotropy or heterogeneity was observed in our study.
Type 2 diabetes mellitus (T2DM) is well-known to be associated with normal or elevated bone density but, concurrently, low bone turnover and increased risk for fracture [37–39]. Through observational studies, there is still no unified conclusion regarding the effect of T2DM on BMD in Asian populations. Some observational studies have indeed found that patients with T2DM have higher BMD compared to non-diabetics [9,40], while others have found no association [41] or the opposite [12,13,42,43]. The conflicting results may be due to differences in study design, use of medications, and confounding factors, such as BMI. Therefore, in our study, we adopted an MR method to avoid bias so as to explain the contradiction in previous observational studies and confirm that T2DM is not associated with reduction in BMD. Results from the National Osteoporosis Risk Assessment have shown that Asian populations are associated with a significantly increased likelihood of osteoporosis compared with white populations [17]. In an MR study, Ahmad et al. reported that genetically influenced increases in T2DM risk and fasting plasma glucose have weak positive effects on BMD [23]. Another MR study among non-diabetic individuals of European descent by Mitchell et al. demonstrated that a genetically predicted 1-mmol/L increment in fasting glucose was associated with a 4% higher total hip BMD, albeit without reaching statistical significance (p = 0.06) [44]. A two-sample MR study using GWAS summary statistics obtained from the Meta-analyses of the Glucose and Insulin-related traits Consortium and Genetic Factors for Osteoporosis Consortium showed that lumbar spine BMD increased by 0.49 g/cm2 (95% CI: 0.01–0.97) in response to a per-unit increase in fasting insulin, revealing a higher BMD in patients with T2DM than in those without diabetes [45]. Consistent with studies conducted among individuals of European descent, the observed association with an elevated BMD was confirmed in our study among individuals of Asian descent.
Despite a normal to high BMD compared with non-diabetic individuals, patients with T2DM more often have an increased fracture risk [37–39]. Fragility fractures in patients with T2DM may be explained by the presence of impaired structural properties, including abnormalities in dynamic, material, and microarchitectural bone properties, which ultimately lead to bone fragility [46–48]. In a study among Japanese men, those with T2DM had a hazard ratio of 2.76 for fragility fractures compared with normoglycemic men [49]. Both longer diabetes duration and poor glycemic control are associated with a higher fracture risk [50]. Losada-Grande et al. investigated the association between insulin use and fracture risk and found that insulin use appeared to be associated with a 38% excess fracture risk among patients with T2DM in the early stages of disease [38]. Studies have shown that higher glycated hemoglobin (HbA1C)-poor blood glucose control is positively correlated with higher BMD in patients with diabetes [8]. Long-term high blood glucose levels lead to retinopathy and peripheral neuropathy, which further affect the patient’s balance and result in an increased risk of falling [51].
Given the cross-talk between osteoblasts and osteoclasts with abnormal microstructural repair in these T2DM patients, it is expected that reduction in bone resorption follows with resultant low bone turnover — the dynamic process of resorption followed by replacement by new bone [52,53]. A balance between osteoclast-dependent bone resorption and osteoblast-dependent bone formation is essential for the maintenance of bone material quality. High glucose levels may interfere with osteoclast and matrix differentiation and inhibit osteoclast-mediated bone matrix degradation, resulting in elevated BMD [54]. Another possible explanation is that patients with T2DM have an increased number of osteoblast precursor cells and elevated levels of Dickkopf-related protein-1 (DKK-1), a regulator that inhibits osteoblast maturation; this phenomenon can increase the number of immature osteoblasts in patients with T2DM in comparison with controls [55]. β-Cell failure and low levels of insulin-like growth factor-1 negatively affect osteoblast function [56]. The possibility of a preventive or therapeutic role for thiazides and statins in osteoporosis has been emphasized in some studies because users of these anti-diabetic drugs have significantly greater bone mineral content than non-users [57,58]. The reductase inhibitor 3-hydroxy-3-methylglutaryl coenzyme, the main component of statins, can stimulate bone formation by increasing the expression of the bone morphogenetic protein-2 gene in bone cells. Thiazides can significantly elevate the levels of circulating calcium, thus promoting bone formation. Furthermore, high circulating insulin levels in patients with insulin resistance could explain the high BMD in patients with T2DM because insulin is known to exert anabolic effects on bone [59,60]. It is speculated that low turnover of bone in diabetes may lead to defective microfracture repairs and, hence, to their accumulation, contributing to decreased bone quality.
GO and KEGG pathway enrichment analysis also found significant enrichment of several crucial regulation pathways, which observed to be involved in osteoporosis. Among them, diacylglycerol (DAG) metabolic process and regulation of Notch signaling pathway might be remarkable. Recent lipidomics studies have shown significant dysregulation of lipids in aging-related bone mineral density loss and the occurrence of osteoporosis, including alterations in DAG [61,62]. In response to stimulations, activated phospholipase C (PLC) hydrolyzes PIP2 to DAG. DAG activates protein kinase C (PKC)[63], leading to GSK-3β inactivation and NFATc1 induction, which has a key role in the RANKL-induced osteoclast differentiation [64]. Furthermore, Notch signaling is implicated in governing cell fate determination, proliferation, differentiation, and apoptosis of skeletal cells, including osteocytes, chondrocytes, osteoblasts, and osteoclasts [65]. The deletion of Notch receptors in mouse bone marrow macrophages increased osteoclastogenesis as well as osteoclast precursor proliferation, exerting its effect both in osteoclast precursors and indirectly via osteoblast lineage cells directly, which raise caution that Notch signaling inhibition may be one of the osteoporosis pathogenesis [66].
T2DM is often combined with obesity, which can also have detrimental effects on bone. Bioactive lipids play important structural and functional roles and directly relate to bone homeostasis. The increased bone marrow fat (BMF) is generating growing interest as a possible explanation to the bone loss in diabetes patients. Patsch and his colleagues [67] found that altered bone marrow fat composition (specifically the proportion of saturated versus unsaturated lipid) is linked with fragility fractures and diabetes. Stem cell differentiation to adipocytes involves the transcription factor known as peroxisome proliferator-activated receptor (PPARγ2) and is viewed as competing with osteoblastogenesis [68]. Adipose tissue can release a number of adipokines (e.g., leptin, adiponectin, and resistin), which can regulate bone formation by affecting osteoblasts or osteoclasts [69].
Our study has the following limitations.
Diagnoses of these diseases were based on the physicians’ diagnoses at cooperating hospitals. First of all, diagnosis of osteoporosis in the Japanese cohort was based on the physicians’ diagnoses at cooperating hospitals without mention of BMD measurement or history of fracture, implying that the findings might have lacked precision and could be somewhat unreliable. Somehow, measurement error in using the outcome of “osteoporosis” as a measure for low BMD may limit the strength of these conclusions and prevent a causal interpretation. However, we also conducted additional analysis using heel bone mineral density GWAS, which will provide more solid evidence for the causal relationship between type 2 diabetes mellitus and bone mineral density in an East Asian population. This approach will help to overcome the limitations of the initial diagnosis and strengthen the validity of the findings. Second, the SNPs in this study were derived from a Japanese GWAS database, which does not completely represent all genetic characteristics of the Asian population. Third, summary-level data from the BBJ GWAS studies might be potentially overlapping, which has the potential to bias causal effect estimates in MR studies. Fourth, the number of SNPs as IVs was relatively small, which can only explain a limited causal relationship. Fifth, the use of publicly available data means that it was not possible to conduct subgroup analyses by age, sex, and disease duration. Despite these weaknesses, to the best of our knowledge, this is the first MR study with the largest GWAS dataset to focus on the effect of T2DM traits on the risk of osteoporosis in Asian individuals. Our study findings provide evidence for causal inference in the absence of a randomized, controlled trial. We also applied a variety of methods based on different assumptions in our study, making the causal findings more reliable.
Conclusion
Our MR study among East Asian populations provides further evidence that T2DM is not genetically associated with reduction in BMD. In clinical practice, it is important to regulate blood glucose levels to achieve a balance between increasing bone intensity and reducing diabetes-related complications.
Supplementary Information
Below is the link to the electronic supplementary material.
Plots of leave-one-out analyses for Mendelian randomization analysis of the causal effects of T2DM on risk of osteoporosis (A) and Heel bone mineral density(B). MR, Mendelian randomization; T2DM, type 2 diabetes mellitus. (PNG 117 kb)
Acknowledgements
We thank Japan Biobank for providing GWAS data and making the GWAS summary data publicly available.
Funding
This study was supported by the Medical Research Foundation of Guangdong Province (A2021280), Natural Science Foundation of Guangdong Province (2022A1515012334), Sun Yat-sen Clinical Research Cultivation Program (SYS-Q-202105, SYS-Q-202202) and Sun Yat-sen Scientific Research Project (YXQH202202, YXQH202213), and Guangzhou Women and Children’s Medical Center for Clinical Doctor (KTa377a204193688).
Data availability
The data used to generate the results in this study were obtained from genome-wide association study summary statistics which were publicly released by genetic consortia.
Declarations
Ethical approval
We used publicly available aggregate data in this study; therefore, no separate ethical approval was required.
Conflicts of interest
None.
Footnotes
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Guiwu Huang and Xiong Chen contributed equally to this work.
Contributor Information
Weidong Song, Email: songwd@mail.sysu.edu.cn.
Gang Zeng, Email: zengg5@mail.sysu.edu.cn.
References
- 1.Nelson HD, Haney EM, Dana T, Bougatsos C, Chou R. Screening for osteoporosis: an update for the U.S. Preventive Services Task Force. Ann Intern Med. 2010;153(2):99–111. doi: 10.7326/0003-4819-153-2-201007200-00262. [DOI] [PubMed] [Google Scholar]
- 2.Greenblatt MB, Tsai JN, Wein MN. Bone turnover markers in the diagnosis and monitoring of metabolic bone disease. Clin Chem. 2017;63(2):464–474. doi: 10.1373/clinchem.2016.259085. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Gullberg B, Johnell O, Kanis JA. World-wide projections for hip fracture. Osteoporos Int. 1997;7(5):407–413. doi: 10.1007/PL00004148. [DOI] [PubMed] [Google Scholar]
- 4.Cooper C, Campion G, Melton LJ., 3rd Hip fractures in the elderly: a world-wide projection. Osteoporos Int. 1992;2(6):285–289. doi: 10.1007/BF01623184. [DOI] [PubMed] [Google Scholar]
- 5.Chen FP, Shyu YC, Fu TS, et al. Secular trends in incidence and recurrence rates of hip fracture: a nationwide population-based study. Osteoporos Int. 2017;28(3):811–818. doi: 10.1007/s00198-016-3820-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Hagino H, Sawaguchi T, Endo N, Ito Y, Nakano T, Watanabe Y. The risk of a second hip fracture in patients after their first hip fracture. Calcif Tissue Int. 2012;90(1):14–21. doi: 10.1007/s00223-011-9545-6. [DOI] [PubMed] [Google Scholar]
- 7.Borgström F, Karlsson L, Ortsäter G, et al. Fragility fractures in Europe: burden, management and opportunities. Arch Osteoporos. 2020;15(1):59. doi: 10.1007/s11657-020-0706-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Ma L, Oei L, Jiang L, et al. Association between bone mineral density and type 2 diabetes mellitus: a meta-analysis of observational studies. Eur J Epidemiol. 2012;27(5):319–332. doi: 10.1007/s10654-012-9674-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Lau EM, Leung PC, Kwok T, et al. The determinants of bone mineral density in Chinese men–results from Mr. Os (Hong Kong), the first cohort study on osteoporosis in Asian men. Osteoporos Int. 2006;17(2):297–303. doi: 10.1007/s00198-005-2019-9. [DOI] [PubMed] [Google Scholar]
- 10.Shan PF, Wu XP, Zhang H, et al. Bone mineral density and its relationship with body mass index in postmenopausal women with type 2 diabetes mellitus in mainland China. J Bone Miner Metab. 2009;27(2):190–197. doi: 10.1007/s00774-008-0023-9. [DOI] [PubMed] [Google Scholar]
- 11.Khoo CC, Woo J, Leung PC, Kwok A, Kwok T. Determinants of bone mineral density in older postmenopausal Chinese women. Climacteric. 2011;14(3):378–383. doi: 10.3109/13697137.2010.548566. [DOI] [PubMed] [Google Scholar]
- 12.Majima T, Komatsu Y, Yamada T, et al. Decreased bone mineral density at the distal radius, but not at the lumbar spine or the femoral neck, in Japanese type 2 diabetic patients. Osteoporos Int. 2005;16(8):907–913. doi: 10.1007/s00198-004-1786-z. [DOI] [PubMed] [Google Scholar]
- 13.Chen HL, Deng LL, Li JF. Prevalence of osteoporosis and its associated factors among older men with type 2 diabetes. Int J Endocrinol. 2013;2013:285729. doi: 10.1155/2013/285729. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Haycock PC, Burgess S, Wade KH, Bowden J, Relton C, Davey SG. Best (but oft-forgotten) practices: the design, analysis, and interpretation of Mendelian randomization studies. Am J Clin Nutr. 2016;103(4):965–978. doi: 10.3945/ajcn.115.118216. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Larsson SC, Michaëlsson K, Burgess S. Mendelian randomization in the bone field. Bone. 2019;126:51–58. doi: 10.1016/j.bone.2018.10.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Zheng J, Baird D, Borges MC, et al. Recent developments in mendelian randomization studies. Curr Epidemiol Rep. 2017;4(4):330–345. doi: 10.1007/s40471-017-0128-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Siris ES, Miller PD, Barrett-Connor E, et al. Identification and fracture outcomes of undiagnosed low bone mineral density in postmenopausal women: results from the National Osteoporosis Risk Assessment. JAMA. 2001;286(22):2815–2822. doi: 10.1001/jama.286.22.2815. [DOI] [PubMed] [Google Scholar]
- 18.Goff LM. Ethnicity and type 2 diabetes in the UK. Diabet Med. 2019;36(8):927–938. doi: 10.1111/dme.13895. [DOI] [PubMed] [Google Scholar]
- 19.Kakoly NS, Khomami MB, Joham AE, et al. Ethnicity, obesity and the prevalence of impaired glucose tolerance and type 2 diabetes in PCOS: a systematic review and meta-regression. Hum Reprod Update. 2018;24(4):455–467. doi: 10.1093/humupd/dmy007. [DOI] [PubMed] [Google Scholar]
- 20.Mathur R, Palla L, Farmer RE, Chaturvedi N, Smeeth L. Ethnic differences in the severity and clinical management of type 2 diabetes at time of diagnosis: a cohort study in the UK Clinical Practice Research Datalink. Diabetes Res Clin Pract. 2020;160:108006. doi: 10.1016/j.diabres.2020.108006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Goff LM, Ladwa M, Hakim O, Bello O. Ethnic distinctions in the pathophysiology of type 2 diabetes: a focus on black African-Caribbean populations. Proc Nutr Soc. 2020;79(2):184–193. doi: 10.1017/S0029665119001034. [DOI] [PubMed] [Google Scholar]
- 22.Zheng XY, Zhou Z, Gao Y, et al. Racial differences and factors associated with low femoral neck bone mineral density: an analysis of NHANES 2005–2014 data. Arch Osteoporos. 2021;16(1):9. doi: 10.1007/s11657-020-00850-0. [DOI] [PubMed] [Google Scholar]
- 23.Ahmad OS, Leong A, Miller JA, et al. A Mendelian randomization study of the effect of type-2 diabetes and glycemic traits on bone mineral density. J Bone Miner Res. 2017;32(5):1072–1081. doi: 10.1002/jbmr.3063. [DOI] [PubMed] [Google Scholar]
- 24.Suzuki K, Akiyama M, Ishigaki K, et al. Identification of 28 new susceptibility loci for type 2 diabetes in the Japanese population. Nat Genet. 2019;51(3):379–386. doi: 10.1038/s41588-018-0332-4. [DOI] [PubMed] [Google Scholar]
- 25.Ishigaki K, Akiyama M, Kanai M, et al. Large-scale genome-wide association study in a Japanese population identifies novel susceptibility loci across different diseases. Nat Genet. 2020;52(7):669–679. doi: 10.1038/s41588-020-0640-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Hirata M, Kamatani Y, Nagai A, et al. Cross-sectional analysis of BioBank Japan clinical data: a large cohort of 200,000 patients with 47 common diseases. J Epidemiol. 2017;27(3s):S9–s21. doi: 10.1016/j.je.2016.12.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Nagai A, Hirata M, Kamatani Y, et al. Overview of the BioBank Japan Project: study design and profile. J Epidemiol. 2017;27(3s):S2–s8. doi: 10.1016/j.je.2016.12.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Chen X, Kong J, Pan J, et al. Kidney damage causally affects the brain cortical structure: a Mendelian randomization study. EBioMedicine. 2021;72:103592. doi: 10.1016/j.ebiom.2021.103592. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Staiger DO, Stock JH. Instrumental variables regression with weak instruments. Cambridge: National Bureau of Economic Research; 1994. [Google Scholar]
- 30.Pierce BL, Burgess S. Efficient design for Mendelian randomization studies: subsample and 2-sample instrumental variable estimators. Am J Epidemiol. 2013;178(7):1177–1184. doi: 10.1093/aje/kwt084. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Burgess S, Dudbridge F, Thompson SG. Combining information on multiple instrumental variables in Mendelian randomization: comparison of allele score and summarized data methods. Stat Med. 2016;35(11):1880–1906. doi: 10.1002/sim.6835. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Bowden J, Davey Smith G, Haycock PC, Burgess S. Consistent estimation in Mendelian randomization with some invalid instruments using a weighted median estimator. Genet Epidemiol. 2016;40(4):304–314. doi: 10.1002/gepi.21965. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Bowden J, Davey Smith G, Burgess S. Mendelian randomization with invalid instruments: effect estimation and bias detection through Egger regression. Int J Epidemiol. 2015;44(2):512–525. doi: 10.1093/ije/dyv080. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Burgess S, Bowden J (2015) Integrating summarized data from multiple genetic variants in Mendelian randomization: bias and coverage properties of inverse-variance weighted methods. arXiv preprint arXiv:151204486
- 35.Greco MF, Minelli C, Sheehan NA, Thompson JR. Detecting pleiotropy in Mendelian randomisation studies with summary data and a continuous outcome. Stat Med. 2015;34(21):2926–2940. doi: 10.1002/sim.6522. [DOI] [PubMed] [Google Scholar]
- 36.Zhou Y, Zhou B, Pache L, et al. Metascape provides a biologist-oriented resource for the analysis of systems-level datasets. Nat Commun. 2019;10(1):1523. doi: 10.1038/s41467-019-09234-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Cipriani C, Colangelo L, Santori R, et al. The interplay between bone and glucose metabolism. Front Endocrinol (Lausanne) 2020;11:122. doi: 10.3389/fendo.2020.00122. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Losada-Grande E, Hawley S, Soldevila B, et al. Insulin use and excess fracture risk in patients with type 2 diabetes: a propensity-matched cohort analysis. Sci Rep. 2017;7(1):3781. doi: 10.1038/s41598-017-03748-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Vestergaard P. Discrepancies in bone mineral density and fracture risk in patients with type 1 and type 2 diabetes–a meta-analysis. Osteoporos Int. 2007;18(4):427–444. doi: 10.1007/s00198-006-0253-4. [DOI] [PubMed] [Google Scholar]
- 40.Iki M, Fujita Y, Kouda K, et al. Hyperglycemia is associated with increased bone mineral density and decreased trabecular bone score in elderly Japanese men: the Fujiwara-kyo osteoporosis risk in men (FORMEN) study. Bone. 2017;105:18–25. doi: 10.1016/j.bone.2017.08.007. [DOI] [PubMed] [Google Scholar]
- 41.Liu M, Lu Y, Cheng X, et al. Relationship between abnormal glucose metabolism and osteoporosis in Han Chinese men over the age of 50 years. Clin Interv Aging. 2019;14:445–451. doi: 10.2147/CIA.S164021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Jang M, Kim H, Lea S, Oh S, Kim JS, Oh B. Effect of duration of diabetes on bone mineral density: a population study on East Asian males. BMC Endocr Disord. 2018;18(1):61. doi: 10.1186/s12902-018-0290-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Cui R, Zhou L, Li Z, Li Q, Qi Z, Zhang J. Assessment risk of osteoporosis in Chinese people: relationship among body mass index, serum lipid profiles, blood glucose, and bone mineral density. Clin Interv Aging. 2016;11:887–895. doi: 10.2147/CIA.S103845. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Mitchell A, Larsson SC, Fall T, Melhus H, Michaëlsson K, Byberg L. Fasting glucose, bone area and bone mineral density: a Mendelian randomisation study. Diabetologia. 2021;64(6):1348–1357. doi: 10.1007/s00125-021-05410-w. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Zhou H, Li C, Song W, et al. Increasing fasting glucose and fasting insulin associated with elevated bone mineral density-evidence from cross-sectional and MR studies. Osteoporos Int. 2021;32(6):1153–1164. doi: 10.1007/s00198-020-05762-w. [DOI] [PubMed] [Google Scholar]
- 46.Farr JN, Drake MT, Amin S, Melton LJ, 3rd, McCready LK, Khosla S. In vivo assessment of bone quality in postmenopausal women with type 2 diabetes. J Bone Miner Res. 2014;29(4):787–795. doi: 10.1002/jbmr.2106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Srikanthan P, Crandall CJ, Miller-Martinez D, et al. Insulin resistance and bone strength: findings from the study of midlife in the United States. J Bone Miner Res. 2014;29(4):796–803. doi: 10.1002/jbmr.2083. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Leslie WD, Rubin MR, Schwartz AV, Kanis JA. Type 2 diabetes and bone. J Bone Miner Res. 2012;27(11):2231–2237. doi: 10.1002/jbmr.1759. [DOI] [PubMed] [Google Scholar]
- 49.Iki M, Fujita Y, Kouda K, et al. Hyperglycemic status is associated with an elevated risk of osteoporotic fracture in community-dwelling elderly Japanese men: the Fujiwara-kyo osteoporosis risk in men (FORMEN) cohort study. Bone. 2019;121:100–106. doi: 10.1016/j.bone.2019.01.005. [DOI] [PubMed] [Google Scholar]
- 50.Compston J. Type 2 diabetes mellitus and bone. J Intern Med. 2018;283(2):140–153. doi: 10.1111/joim.12725. [DOI] [PubMed] [Google Scholar]
- 51.Napoli N, Strollo R, Paladini A, Briganti SI, Pozzilli P, Epstein S. The alliance of mesenchymal stem cells, bone, and diabetes. Int J Endocrinol. 2014;2014:690783. doi: 10.1155/2014/690783. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Ballato E, Deepika F, Prado M, et al. Circulating osteogenic progenitors and osteoclast precursors are associated with long-term glycemic control, sex steroids, and visceral adipose tissue in men with type 2 diabetes mellitus. Front Endocrinol. 2022;13:936159. doi: 10.3389/fendo.2022.936159. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Purnamasari D, Puspitasari MD, Setiyohadi B, Nugroho P, Isbagio H. Low bone turnover in premenopausal women with type 2 diabetes mellitus as an early process of diabetes-associated bone alterations: a cross-sectional study. BMC Endocr Disord. 2017;17(1):72. doi: 10.1186/s12902-017-0224-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Hu Z, Ma C, Liang Y, Zou S, Liu X. Osteoclasts in bone regeneration under type 2 diabetes mellitus. Acta Biomater. 2019;84:402–413. doi: 10.1016/j.actbio.2018.11.052. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Sassi F, Buondonno I, Luppi C, et al. Type 2 diabetes affects bone cells precursors and bone turnover. BMC Endocr Disord. 2018;18(1):55. doi: 10.1186/s12902-018-0283-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Hough FS, Pierroz DD, Cooper C, Ferrari SL. MECHANISMS IN ENDOCRINOLOGY: mechanisms and evaluation of bone fragility in type 1 diabetes mellitus. Eur J Endocrinol. 2016;174(4):R127–R138. doi: 10.1530/EJE-15-0820. [DOI] [PubMed] [Google Scholar]
- 57.Wasnich RD, Benfante RJ, Yano K, Heilbrun L, Vogel JM. Thiazide effect on the mineral content of bone. N Engl J Med. 1983;309(6):344–347. doi: 10.1056/NEJM198308113090605. [DOI] [PubMed] [Google Scholar]
- 58.Pasco JA, Kotowicz MA, Henry MJ, Sanders KM, Nicholson GC. Statin use, bone mineral density, and fracture risk: Geelong Osteoporosis Study. Arch Intern Med. 2002;162(5):537–540. doi: 10.1001/archinte.162.5.537. [DOI] [PubMed] [Google Scholar]
- 59.Conte C, Epstein S, Napoli N. Insulin resistance and bone: a biological partnership. Acta Diabetol. 2018;55(4):305–314. doi: 10.1007/s00592-018-1101-7. [DOI] [PubMed] [Google Scholar]
- 60.Hamann C, Kirschner S, Günther KP, Hofbauer LC. Bone, sweet bone–osteoporotic fractures in diabetes mellitus. Nat Rev Endocrinol. 2012;8(5):297–305. doi: 10.1038/nrendo.2011.233. [DOI] [PubMed] [Google Scholar]
- 61.Mo C, Du Y, O'Connell TM. Applications of lipidomics to age-related musculoskeletal disorders. Curr Osteoporos Rep. 2021;19(2):151–157. doi: 10.1007/s11914-021-00656-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Zamani A, Decker C, Cremasco V, Hughes L, Novack DV, Faccio R. Diacylglycerol kinase ζ (DGKζ) is a critical regulator of bone homeostasis via modulation of c-Fos levels in osteoclasts. J Bone Miner Res. 2015;30(10):1852–1863. doi: 10.1002/jbmr.2533. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Berridge MJ. The inositol trisphosphate/calcium signaling pathway in health and disease. Physiol Rev. 2016;96(4):1261–1296. doi: 10.1152/physrev.00006.2016. [DOI] [PubMed] [Google Scholar]
- 64.Shin J, Jang H, Lin J, Lee SY. PKCβ positively regulates RANKL-induced osteoclastogenesis by inactivating GSK-3β. Mol Cells. 2014;37(10):747–752. doi: 10.14348/molcells.2014.0220. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Zieba JT, Chen YT, Lee BH, Bae Y (2020) Notch signaling in skeletal development, homeostasis and pathogenesis. Biomolecules 19;10(2):332 [DOI] [PMC free article] [PubMed]
- 66.Bai S, Kopan R, Zou W, et al. NOTCH1 regulates osteoclastogenesis directly in osteoclast precursors and indirectly via osteoblast lineage cells. J Biol Chem. 2008;283(10):6509–6518. doi: 10.1074/jbc.M707000200. [DOI] [PubMed] [Google Scholar]
- 67.Patsch JM, Li X, Baum T, et al. Bone marrow fat composition as a novel imaging biomarker in postmenopausal women with prevalent fragility fractures. J Bone Miner Res. 2013;28(8):1721–1728. doi: 10.1002/jbmr.1950. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Syversen U, Stunes AK, Gustafsson BI, et al. Different skeletal effects of the peroxisome proliferator activated receptor (PPAR)alpha agonist fenofibrate and the PPARgamma agonist pioglitazone. BMC Endocr Disord. 2009;9:10. doi: 10.1186/1472-6823-9-10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Li Y, Cao S, Gaculenko A, Zhan Y, Bozec A, Chen X. Distinct Metabolism of Bone Marrow Adipocytes and their Role in Bone Metastasis. Front Endocrinol (Lausanne) 2022;13:902033. doi: 10.3389/fendo.2022.902033. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Plots of leave-one-out analyses for Mendelian randomization analysis of the causal effects of T2DM on risk of osteoporosis (A) and Heel bone mineral density(B). MR, Mendelian randomization; T2DM, type 2 diabetes mellitus. (PNG 117 kb)
Data Availability Statement
The data used to generate the results in this study were obtained from genome-wide association study summary statistics which were publicly released by genetic consortia.