Abstract
This study aimed to understand the profile of hydroxychloroquine-treated patients, referral patterns, and dosing and to assess the adherence of eye care providers to the latest 2016 screening guidelines provided by the American Academy of Ophthalmology. Patients were identified using electronic health records (EHR) taking hydroxychloroquine and were seen by optometrists, retinal specialists, and non-retinal ophthalmologists. Review of EHR data includes demographic characteristics, indications, and dosing profile of hydroxychloroquine use, eye care provider managing the patient, and imaging modalities performed. A total of 166 patients were included in the study. The most common indications for screening were systemic lupus erythematosus and discoid lupus (52.4%) followed by rheumatoid arthritis (18.7%) and Sjögren’s syndrome (9.6%). Ninety-two (55.4%) patients were on a higher-than-recommended dose of > 5 mg/kg/day. Patients who weighed less (mean 63.9 kg) were taking a higher-than-recommended dose (vs. 81.5 kg, p < 0.001). Although retinal specialists adhered best to the use of all three recommended imaging modalities, visual field testing was done appropriately for only 8.3% of Asian and 71.1% of non-Asian patients. In conclusion, there is substantial variability in screening by ophthalmic providers and prescribing practices compared with the current recommendations. In particular, there is a marked deficiency in correct visual field testing in Asian patients. These findings are important to highlight potential interventions to improve screening for hydroxychloroquine toxicity.
Subject terms: Drug safety, Toxicology, Medical imaging, Retinal diseases
Introduction
Hydroxychloroquine (HCQ) is an immunomodulatory medication used to treat various autoimmune diseases. It received its United States Food and Drug Administration (FDA)-approval for treating systemic lupus erythematosus (SLE) in 19551, 2. Although it is the first-line treatment for SLE, it has also been found useful in treating Sjögren’s Syndrome (SS), Rheumatoid Arthritis (RA), and a growing number of other indications3–5.
HCQ can cause retinal toxicity, a rare but well-recognized complication from its long-term use6. While the mechanism is not fully understood, it is known that HCQ is deposited in tissues that have a high melanin content, such as skin and the retinal pigment epithelium (RPE)7. In vitro studies indicate that HCQ alters the RPE lysosome pH, resulting in higher lipofuscin levels, a byproduct of photoreceptor degeneration and, eventually, RPE and photoreceptor loss8. As such, HCQ accumulation may lead to irreversible changes to retinal architecture and vision, and progression has been noted even after discontinuation9. Patients are usually unaware of early changes resulting from toxicity, and for this reason, ophthalmic screening of patients on HCQ is important10.
In 2002, the American Academy of Ophthalmology (AAO) released its first recommendation on screening for chloroquine (CQ) and HCQ retinopathy11. With further evidence and widespread use of newer imaging modalities, these guidelines were revised in 201112, and later in 201613. Current updated guidelines recommend that HCQ dosage be limited to ≤ 5 mg/kg/day of real body weight (a change from the 2011 standard which used ideal body weight); that patients receive baseline HCQ retinopathy screening within the first year of initiating therapy and then annually following 5 years of HCQ use, except for those with high-risk associations including renal disease, prior macular disease, concurrent tamoxifen use, for whom annual screening is recommended upon initiation; and that patients are screened with a dilated fundus examination, macular spectral-domain optical coherence tomography (SD-OCT), fundus autofluorescence (FAF) imaging, and automated Humphrey Visual Field (HVF) testing using the 10–2 paradigm. The exception was for patients of Asian ethnicity, for whom updated guidelines recommended using 24–2 or 30–2 visual field testing due to evidence of peripheral macular HCQ retinopathy in this cohort of patients13.
Despite evidence-based screening guidelines and known toxicity risks, compliance with HCQ retinopathy screening and appropriate HCQ dosage remains an issue. Papers have previously investigated rheumatologists, ophthalmologists, and dermatologists’ compliance with the guidelines provided by the AAO14–19. A survey by Sulman et al.14 in 2017 showed that only 5% of rheumatologists and 15% of ophthalmologists were aware of the recommended ophthalmologic assessments for baseline and follow-up examinations. Browning17 and Semmer et al.15 noted an increased screening frequency in low-risk patients within the first 5 years of therapy, indicating overutilized healthcare resources. Alternatively, Nika et al.16 noted a lack of routine monitoring for high-risk patients. Furthermore, Braslow et al. 9/15/2023 8:31:00 AM 19noted that greater than 50% of patients on HCQ therapy were on higher-than-recommended doses (> 5 mg/kg/day).
Our retrospective chart review aimed to investigate the prescribing patterns of HCQ of referring physicians and the screening practices of eye care providers in a large academic referral center based on the current 2016 guidelines.
Methods
This study was approved by the Institutional Review Board of the University of California, San Diego (UCSD). The patients provided written informed consent as per institution protocol, and data collection and analysis were conducted according to the Principles of the Declaration of Helsinki. This study complied with the Health Insurance Portability and Accountability Act (HIPAA) of 1996.
Data source and patient selection
Analysis of electronic health record (EHR) data available at the UCSD database was performed to identify records of patients included for retrospective review from January 1, 2016 until March 31, 2022. Patients were initially identified using structured queries of the EHR data warehouse (Epic SlicerDicer, Verona, WI, USA), including “Hydroxychloroquine sulfate” ever listed as an outpatient medication and patient encounter at the UCSD Viterbi Family Department of Ophthalmology. Patients noted in the eye care provider’s chart as a screening for HCQ were included. Eligibility was confirmed with each patient via manual chart review.
Chart review
Chart review of the EHR system included review of ocular examination data, encounter notes, and ophthalmic testing data and images indexed on the Zeiss Forum software version 4.2.4.15 (Zeiss, Oberkochen, Germany) application. Variables recorded from the chart review included self-reported gender, self-reported race, indication for HCQ therapy, age at starting HCQ therapy, date of first HCQ screening, the type of eye care provider conducting the screening exams (categorized as optometrist, retinal specialist, or non-retinal ophthalmologist), daily dose at the first screening, weight (in kg), highest daily HCQ dose, date of last screening, total duration of HCQ therapy, and if the patient was still taking HCQ at the time of chart review (April 2022). Further information, collected from the patient data, included concurrent tamoxifen use, known renal disease, prior retinal or ophthalmic disease, systemic comorbidities, as well as the modalities used to screen for HCQ retinopathy, including HVF (10–2 and 24–2), SD-OCT, FAF, and multifocal electroretinogram (mfERG). The patient data were collected in a de-identified, encrypted database.
Statistical analysis
Data normality was initially assessed through the Shapiro–Wilk testing. Subject characteristics were presented as count (%) and mean (95% CI) for categorical and continuous variables, respectively. Demographic tables present p-values derived from t-tests, Fisher’s Exact test, and Chi-Squared tests for subject-level characteristics. Demographic tables consisting of a comparison of more than two cohorts present p values derived from analysis of variance (ANOVA), Fisher’s Exact test, and Chi-Squared tests for subject-level characteristics. A further analysis was conducted utilizing linear mixed-effects modeling to account for within-group variability of subjects attending the same type of eye care provider. A post hoc analysis was implemented to assess the significance of differences between pairs of group means using Tukey’s honestly significant difference (HSD) test with degrees of freedom estimated by Satterthwaite’s approximation. Mean mg/kg/day ratios were estimated for each indication of HCQ therapy, and a one-tailed t-test was implemented to evaluate if ratios were significantly greater than a mu of 5 mg/kg/day. The statistical analysis was done using the R Statistical Programming Software (Version 4.2.1). A p value less than or equal to 0.05 were considered statistically significant.
Results
The data from the EHR initially yielded 287 patients, of which 166 were noted to have eye care provider’s clinic notes on HCQ screening upon visit; hence, were included in the study. Table 1 shows the demographic characteristics of the patients included in the study. Ninety percent of patients were female and 48.8% were white. The mean duration of HCQ therapy was 5.3 years and the mean estimated daily dose was 5.2 mg/kg. The majority of the patients underwent SD-OCT, FAF, and HVF. None of the patients were noted to have signs of HCQ retinopathy.
Table 1.
Demographics of patients seen for hydroxychloroquine screening.
| Patients on HCQ (n = 166) | |
|---|---|
| Sex | |
| Female (%) | 150 (90.4%) |
| Male (%) | 16 (9.6%) |
| Race | |
| Asian (%) | 24 (14.5%) |
| Black or African American (%) | 7 (4.2%) |
| Other or mixed race (%) | 50 (30.1%) |
| Unknown (%) | 4 (2.4%) |
| White (%) | 81 (48.8%) |
| Estimated age starting HCQ in years | |
| Mean (CI) | 44.2 (41.7, 46.8) |
| Weight in kg | |
| Mean (CI) | 71.7 (68.9, 74.6) |
| Duration of therapy in years | |
| Mean (CI) | 5.3 (4.4, 6.1) |
| Estimated daily dose in mg/kg | |
| Mean (CI) | 5.2 (5.0, 5.5) |
| Cumulative dose in grams | |
| Mean (CI) | 684.4 (568.5, 800.4) |
| High-riska | |
| No (%) | 110 (66.7%) |
| Yes (%) | 55 (33.3%) |
| SD-OCT screen | |
| No (%) | 7 (4.2%) |
| Yes (%) | 159 (95.8%) |
| FAF screen | |
| No (%) | 18 (10.8%) |
| Yes (%) | 148 (89.2%) |
| HVF screen | |
| No (%) | 47 (28.3%) |
| Yes (%) | 119 (71.7%) |
HCQ Hydroxychloroquine, SD-OCT Spectral-domain optical coherence tomography, FAF Fundus autofluorescence, HVF Humphrey visual field.
aHigh-risk features including renal disease, concomitant tamoxifen use, known retinal or macular disease.
We examined characteristics leading to higher-than-recommended HCQ dosage (Table 2). Of the 166 patients that were reviewed, 92 (55.4%) had daily doses greater than the AAO-recommended 5 mg/kg/day with a mean cumulative lifetime dose of 783.6 g as opposed to the mean cumulative lifetime dose of 561.2 g in the ≤ 5 mg/kg/day cohort (p = 0.06). Those at higher-than-recommended dosages were, on average, at a lower weight than those at appropriate dosage (a mean of 63.9 kg vs. 81.5 kg, respectively, p < 0.001). No statistically significant differences were observed in the likelihood of higher-than-recommended dosage based on the indication for HCQ therapy, duration of therapy, high-risk profile, and the type of eye care provider performing the screening.
Table 2.
Extended differences between patients that received doses above and below 5 mg/kg.
| ≤ 5 mg/kg (n = 74) | > 5 mg/kg (n = 92) | p value | |
|---|---|---|---|
| Weight in kg (mean [CI]) | 81.5 (76.5, 86.5) | 63.9 (61.8, 66.0) | < 0.001a |
| Duration of therapy in years (mean [CI]) | 5.0 (3.9, 6.2) | 5.5 (4.2, 6.7) | 0.594 |
| Cumulative lifetime dose in grams (mean [CI]) | 561.2 (429.2, 693.1) | 783.6 (603.5, 963.6) | 0.060 |
| Indication for HCQ therapy (total [%]) | |||
| 1. UCTD, MCTD | 3 (4.1%) | 5 (5.4%) | 0.711 |
| 2. RA | 13 (17.6%) | 18 (19.6%) | |
| 3. SLE, Discoid lupus | 41 (55.4%) | 46 (50.0%) | |
| 4. Inflammatory arthritis, uncertain | 3 (4.1%) | 8 (8.7%) | |
| 5. Sjögren | 9 (12.2%) | 7 (7.6%) | |
| 6. Otherb | 1 (1.4%) | 4 (4.3%) | |
| 7. Multiplec | 4 (5.4%) | 4 (4.3%) | |
| Attending Group (% group) | |||
| Ophthalmologist | 18 (24.3%) | 19 (20.7%) | 0.695 |
| Optometry | 15 (20.3%) | 24 (26.1%) | |
| Retina | 41 (55.4%) | 49 (53.3%) | |
| High-Riskd (% group) | |||
| No | 43 (58.9%) | 67 (72.8%) | 0.069 |
| Yes | 30 (41.1%) | 25 (27.2%) |
HCQ Hydroxychloroquine, UCTD Undifferentiated connective tissue disease, MCTD Mixed connective tissue disease, RA Rheumatoid arthritis, SLE Systemic lupus erythematosus.
aBold p-values are less than 0.05.
bOther diseases involved: Lichen planus, Dermatomyositis, Palindromic rheumatism, Unspecified dermatitis, Lichen sclerosis, Systemic sclerosis, Osteoarthritis, Autoimmune neutropenia, Psoriatic arthritis.
cMultiple autoimmune diseases involved: SLE + Dermatomyositis, Sjögren + Scleroderma, SLE + Sjögren, SLE + RA, RA + Sjögren, SLE + Scleroderma, RA + Sjögren + Lichen sclerosis, SLE + Juvenile idiopathic arthritis, SLE + Psoriatic arthritis.
dHigh-risk features including renal disease, concomitant tamoxifen use, known retinal or macular disease.
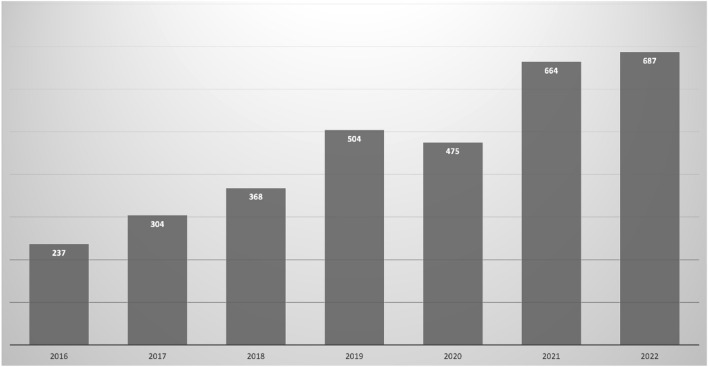
We characterized individual indications for HCQ therapy as seen in Table 3. The most common indications for HCQ use were SLE and discoid lupus (87/166 [52.4%]), followed by RA (31/166 [18.7%]) and Sjögren’s Syndrome (16/166 [9.6%]). Interestingly, there was an increasingly diverse range of conditions being treated with HCQ in the other category including lichen planus, dermatomyositis, dermatitis, lichen sclerosis, systemic sclerosis, osteoarthritis, autoimmune neutropenia and psoriatic arthritis. There was a progressive increase in HCQ retinopathy screening annually (Fig. 1). Taken together, this suggests that the number of patients prescribed and conditions treated with HCQ is increasing, therefore increasing the risk of an increase in absolute numbers of HCQ toxicity cases.
Table 3.
Differences between indications for hydroxychloroquine therapy.
| UCTD, MCTD (n = 8) | RA (n = 31) | SLE, Discoid lupus (n = 87) | Inflammatory arthritis, uncertain (n = 11) | Sjögren (n = 16) | Otherb (n = 5) | Multiplec (n = 8) | p value | |
|---|---|---|---|---|---|---|---|---|
| Estimated age starting HCQ (mean [CI]) | 54.5 (39.7, 69.3) | 58.9 (54.3, 63.6) | 36.1 (33.3, 38.9) | 50.5 (39.2, 61.9) | 50.3 (41.1, 59.5) | 40.2 (20.5, 59.9) | 46.9 (36.2, 57.5) | < 0.001a |
| Duration of therapy (mean [CI]) | 5.4 (2.0, 8.7) | 4.1 (2.8, 5.5) | 6.3 (4.9, 7.8) | 3.5 (1.1, 5.9) | 4.2 (2.7, 5.6) | 3.4 (− 0.2, 7.0) | 3.8 (1.1, 6.5) | 0.288 |
| Cumulative lifetime dose (mean [CI]) | 684.4 (162.7, 1206.0) | 541.6 (350.3, 733.0) | 835.2 (638.3, 1032.0) | 484.5 (146.8, 822.1) | 438.0 (302.7, 573.3) | 408.8 (− 42.6, 860.2) | 538.4 (133.6, 943.1) | 0.239 |
HCQ Hydroxychloroquine, UCTD Undifferentiated connective tissue disease, MCTD Mixed connective tissue disease, RA Rheumatoid arthritis, SLE Systemic lupus erythematosus.
aBold p values are less than 0.05.
bOther diseases involved: Lichen planus, Dermatomyositis, Palindromic rheumatism, Unspecified dermatitis, Lichen sclerosis, Systemic sclerosis, Osteoarthritis, Autoimmune neutropenia, Psoriatic arthritis.
cMultiple autoimmune diseases involved: SLE + Dermatomyositis, Sjögren + Scleroderma, SLE + Sjögren, SLE + RA, RA + Sjögren, SLE + Scleroderma, RA + Sjögren + Lichen sclerosis, SLE + Juvenile idiopathic arthritis, SLE + Psoriatic arthritis.
Figure 1.
Annual visits of patients taking hydroxychloroquine sulfate seen at our eye institution.
The age at which HCQ therapy was started was different among the disease groups (p < 0.001), as the SLE group started on average the earliest (36.1 years), and the RA group started on average the latest (58.9 years). The SLE group had the longest mean duration of therapy (6.3 years) and the highest cumulative dose (835.2 g) on initial screening. However, there was no statistically significant difference among the comparison of all cohorts.
We examined differences in patient characteristics and screening practices based on eye care provider type, focusing on retinal specialists, non-retinal ophthalmologists, and optometrists, as seen in Table 4. There was no statistically significant difference between patient characteristics seen by the different eye care providers in terms of duration of therapy, cumulative dose, and high-risk profile, suggesting that the groups saw similar patients. Optometrists were less likely to employ FAF (vs. ophthalmologists [p = 0.019], retina [p < 0.001]) and ophthalmologists were less likely to employ HVF (vs. optometrist [p = 0.031], retina [p = 0.016]) on screening visit when compared to their counterparts (Table 5). Of note, only 2/24 (8.3%) Asian patients underwent the recommended 24–2 test pattern and only 101/142 (71.1%) non-Asian patients underwent the recommended 10–2 test pattern. Baseline mfERG, which is an optional test, was performed on only 5 patients by a single retinal specialist. One-hundred fifty-eight (158/166) patients underwent at least two structural and functional screening modalities (HVF, SD-OCT, FAF), the most common of which was the aforementioned three modalities (105/158), followed by SD-OCT and FAF (41/158), then SD-OCT and HVF (11/158). Only one patient underwent simultaneous FAF and HVF without SD-OCT.
Table 4.
Extended differences between patients seen by each eye care providers.
| Ophthalmology (n = 37) | Optometry (n = 39) | Retina (n = 90) | p value | |
|---|---|---|---|---|
| Duration of therapy in years | 0.634 | |||
| Mean (CI) | 5.2 (3.0, 7.4) | 6.0 (3.9, 8.1) | 5.0 (4.0, 5.9) | |
| Cumulative dose in grams | 0.668 | |||
| Mean (CI) | 697.4 (376.0, 1018.9) | 771.2 (523.2, 1019.2) | 641.5 (504.0, 778.9) | |
| High-riskb | 0.296 | |||
| No | 23 (62.2%) | 30 (76.9%) | 57 (64.0%) | |
| Yes | 14 (37.8%) | 9 (23.1%) | 32 (36.0%) | |
| SD-OCT screen | 0.121 | |||
| No | 1 (2.7%) | 4 (10.3%) | 2 (2.2%) | |
| Yes | 36 (97.3%) | 35 (89.7%) | 88 (97.8%) | |
| FAF screen | < 0.001a | |||
| No | 2 (5.4%) | 13 (33.3%) | 3 (3.3%) | |
| Yes | 35 (94.6%) | 26 (66.7%) | 87 (96.7%) | |
| HVF screen | 0.010a | |||
| No | 18 (48.6%) | 8 (20.5%) | 21 (23.3%) | |
| Yes | 19 (51.4%) | 31 (79.5%) | 69 (76.7%) |
SD-OCT Spectral-domain optical coherence tomography, FAF Fundus autofluorescence, HVF Humphrey visual field.
aBold p values are less than 0.05.
bHigh-risk features including renal disease, concomitant tamoxifen use, known retinal or macular disease.
Table 5.
Pairwise comparison between imaging modalities performed by eye care providers.
| SD-OCT | FAF | HVF | |
|---|---|---|---|
| General ophthalmology versus Optometry | 0.431 | 0.019a | 0.031a |
| General ophthalmology versus Retina | 0.986 | 0.851 | 0.016a |
| Optometry versus Retina | 0.164 | < 0.001a | 0.934 |
SD-OCT Spectral-domain optical coherence tomography, FAF Fundus autofluorescence, HVF Humphrey visual field.
aBold p values are less than 0.05.
Discussion
Several FDA-approved oral, topical, and intravitreal medications have been demonstrated to cause toxicity to the retina20. For over two decades, HCQ toxicity has been carefully scrutinized, and evidence-based data documenting the toxicity have led to continually updating screening guidelines. However, despite the different disciplines' efforts to provide care for patients on HCQ, screening remains poor. Our study explored the patient and physician characteristics leading to differences in HCQ screening practices since the 2016 AAO screening guidelines. A survey by Winebrake et al.21 in 2020 regarding the perspective of rheumatologists on the 2016 AAO guidelines cited that limited dosing options, lack of supporting evidence, and low patient adherence were the main obstacles to implementing the guidelines. However, in 2020, the American College of Rheumatology, American Academy of Dermatology, Rheumatologic Dermatology Society, and the American Academy of Ophthalmology provided a joint statement on HCQ use with respect to retinal toxicity22.
The most common indications for HCQ therapy screening in our cohort were SLE and RA, consistent with a previous 2013 study17. Other indications for HCQ use were also seen in our study, which comprised 30.7% (51/166) of our cohort. Several researchers investigated screening and retinal toxicity identified in patients with SLE and RA2, 5, 16, 23–27. However, screening for HCQ retinopathy from indications other than SLE and RA has not been carefully studied. In our academic center, we found that the rate of patients prescribed with HCQ from all indications appeared to be increasing annually. This will likely lead to an increase in the number of patients at risk of toxicity if screening and dosing are not performed appropriately. Awareness of the use of HCQ, aside from SLE and RA, should warrant eye care providers of a more comprehensive assessment to identify changes for possible HCQ retinopathy, even though the clinic visit was not primarily set up to address HCQ screening.
Six years following the presentation of the most updated AAO screening guidelines, adherence to the HCQ dosing guidelines remains a major concern. Our group observed that 55.4% of the patients in our cohort were taking HCQ at a daily dose greater than the recommended 5 mg/kg/day, a finding similar to a previous 2017 study (56%), based on the 2011 AAO screening guidelines19. The patients in our cohort were likelier to weigh less than their counterparts at a ≤ 5 mg/kg/day recommended dosage, and the mode daily dose in this cohort was 400 mg. As new guidelines have shifted towards a basis of real (or actual) weight13, some patients on higher-than-recommended dosages may still have been taking the medication based on the prior-recommended basis of ideal body weight12. As of 2022, smaller incremental dosages (100 mg, 200 mg, 300 mg, and 400 mg) of HCQ are available, which may provide more options of more optimized dosing. Another way to overcome higher-than-recommended dosing was suggested in a study by Chen et al.28 that analyzed the impact of an EHR-based clinical decision support (CDS) tool by alerting the prescribing clinician of an HCQ dose greater than 5 mg/kg of actual body weight or 400 mg daily dose, whichever is lower. The study noted a significant decrease in the prevalence of excessive dosing based on the 2016 AAO guidelines.
Although cumulative dose had been thought to be a strong indicator of risk, the new AAO guidelines cited that the risk is more accurately assessed based on the duration of use relative to daily per-weight dosage13. If HCQ is taken at 4.0–5.0 mg/kg/day, the 15-years cumulative risk of retinopathy is around 5–7.5% but can be as high as 20–25% if taken at > 5.0 mg/kg/day29. This means that, in our cohort, 55.4% of patients are at risk for developing HCQ retinopathy if they continue to use the higher-than-recommended dose.
Regarding eye care providers’ adherence to the latest guidelines, we observed that retinal specialists were most compliant in screening their patients, including screening with all three recommended modalities. This increased compliance is likely related to a keener awareness of retinal specialists to the updated AAO recommendations and further dedication of retinal specialists to HCQ screening during the patient encounter. However, a deficiency was adhering to the recommended test patterns for Asians (24–2) for all eye care providers. A study by Kim, et al. noted a higher sensitivity of the 30–2 (90.6%) test pattern in detecting early pericentral retinopathy compared to 10–2 (53.1%) test pattern and a higher specificity of the 10–2 (89.6%) test pattern compared to 30–2 (84.8%) test pattern in detecting overall retinopathy among Asian patients taking HCQ30. This shows an important use of a broader test pattern to detect early changes within the peripheral macular areas in the Asian population.
There are a few limitations to our study. Given the retrospective nature of this study, records obtained from the electronic medical system may generate inaccuracies regarding the different data collected particularly for the cumulative dose of HCQ. However, as described previously, based on updated guidelines, the duration of therapy relative to the per-weight dose was a more accurate risk prediction; thus, identifying the initiation of treatment would provide less error than computing the total regimen dose. Also, since this was a single-institution study, our conclusions are less generalizable.
Based on our data, our group recommends and suggests the following with respect to HCQ retinopathy screening: 1. Increasing awareness of existing and upcoming screening guidelines not only for eye care providers but also for the HCQ-prescribing clinicians; 2. Increasing awareness to eye care providers that diseases other than SLE and RA might be treated with HCQ and thus require screening; 3. Highlight that Asian patients should be screened using 24–2 or 30–2 HVF rather than using the standard 10–2 testing. 4. Using EHR resources (such as a CDS tool) to flag that high-risk medications, such as HCQ or tamoxifen, are being prescribed to the eye care provider’s patient, to avoid missed screening and to promote discussion for non-toxic use as well as implementation to alert prescribing clinicians of a possible toxic dose of > 5 mg/kg/day may be helpful; 5. Developing streamlined eye care providers or HCQ-prescribing clinician’s order sets to facilitate compliant screening; 6. Communication and collaboration between physicians to prevent under- or overtreatment; and 7. Consideration for prescribers to use tablets of all currently available dosage forms (100 mg, 200 mg, 300 mg, and 400 mg) to facilitate physician prescribing per guidelines.
In conclusion, adherence to the updated screening guidelines remains a concern. Our study demonstrated that there is still room for improvement in adhering to the current AAO guidelines for HCQ retinopathy screening in an academic setting, as the trends and practices elucidated in the present study highlight specific deficiencies and potential initiatives for improvement. Proper dosing and imaging modalities should be reviewed to manage patients with chronic HCQ therapy carefully. Eye care providers should be aware of the updated criteria, particularly using the suggested wider visual testing patterns when screening Asian patients.
Acknowledgements
The authors would like to acknowledge the following: National Institutes of Health grant OT2OD032644 (Kalaw), Foundation Fighting Blindness grant CD-GT-0918-0746-SE (Borooah), Nixon Visions Foundation grant (Borooah), National Institutes of Health grant DP5OD29610 (Baxter).
Author contributions
F.G.P.K.—designed the study, interpreted the data, interpreted the data, drafted, completed, and revised the manuscript; J.A.—designed the study, acquired and interpreted the data, drafted and completed the manuscript; E.W.—analyzed and interpreted the data, completed the manuscript; B.P.—analyzed the data, reviewed the manuscript; S.L.B.—acquired the data, reviewed the manuscript; S.B.—conceptualized and designed the study, interpreted the data, reviewed, revised, and finalized the manuscript. All authors listed have contributed to the work and approved the final version of the manuscript.
Data availability
All data in the current study are available from the corresponding author (SB) upon reasonable request.
Competing interests
The authors declare no competing interests.
Footnotes
Publisher's note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
These authors contributed equally: Fritz Gerald P. Kalaw and Justin Arnett.
References
- 1.Ben-Zvi I, Kivity S, Langevitz P, Shoenfeld Y. Hydroxychloroquine: From Malaria to Autoimmunity. Clin. Rev. Allergy Immunol. 2012;42:145–153. doi: 10.1007/s12016-010-8243-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Dima A, Jurcut C, Chasset F, Felten R, Arnaud L. Hydroxychloroquine in systemic lupus erythematosus: Overview of current knowledge. Ther. Adv. Musculoskelet. Dis. 2022;14:1759720x2110730. doi: 10.1177/1759720X211073001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Hao Y, Feng L, Teng Y, Cheng Y, Feng J. Management of multiple neurological complications in mixed connective tissue disease: A case report. Medicine. 2018;97:e11360. doi: 10.1097/MD.0000000000011360. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Wang X, et al. The Efficiency of hydroxychloroquine for the treatment of primary Sjögren’s syndrome: A systematic review and meta-analysis. Front. Pharmacol. 2021;12:693796. doi: 10.3389/fphar.2021.693796. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Nazir AM, et al. Evaluating the use of hydroxychloroquine in treating patients with rheumatoid arthritis. Cureus. 2021 doi: 10.7759/cureus.19308. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Skorupa T, Shmerling RH. Adherence to weight-based dosing guidelines in patients receiving hydroxychloroquine for systemic lupus erythematosus and rheumatoid arthritis: Results of a quality improvement initiative. ACR Open Rheumatol. 2021;3:733–737. doi: 10.1002/acr2.11320. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Ding HJ, Denniston AK, Rao VK, Gordon C. Hydroxychloroquine-related retinal toxicity. Rheumatology. 2016;55:957–967. doi: 10.1093/rheumatology/kev357. [DOI] [PubMed] [Google Scholar]
- 8.Sundelin SP, Terman A. Different effects of chloroquine and hydroxychloroquine on lysosomal function in cultured retinal pigment epithelial cells. APMIS. 2002;110:481–489. doi: 10.1034/j.1600-0463.2002.100606.x. [DOI] [PubMed] [Google Scholar]
- 9.Souza Botelho M, et al. Systematic review and meta-analysis of the safety of chloroquine and hydroxychloroquine from randomized controlled trials on malarial and non-malarial conditions. Syst. Rev. 2021;10:294. doi: 10.1186/s13643-021-01835-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Parrulli S, et al. Quantitative autofluorescence findings in patients undergoing hydroxychloroquine treatment. Clin. Exp. Ophthalmol. 2022;50:500–509. doi: 10.1111/ceo.14090. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Marmor MF, Carr RE, Easterbrook M, Farjo AA, Mieler WF. Recommendations on screening for chloroquine and hydroxychloroquine retinopathy. Ophthalmology. 2002;109:1377–1382. doi: 10.1016/S0161-6420(02)01168-5. [DOI] [PubMed] [Google Scholar]
- 12.Marmor MF, Kellner U, Lai TYY, Lyons JS, Mieler WF. Revised recommendations on screening for chloroquine and hydroxychloroquine retinopathy. Ophthalmology. 2011;118:415–422. doi: 10.1016/j.ophtha.2010.11.017. [DOI] [PubMed] [Google Scholar]
- 13.Marmor MF, Kellner U, Lai TYY, Melles RB, Mieler WF. Recommendations on screening for chloroquine and hydroxychloroquine retinopathy (2016 revision) Ophthalmology. 2016;123:1386–1394. doi: 10.1016/j.ophtha.2016.01.058. [DOI] [PubMed] [Google Scholar]
- 14.Shulman S, et al. Implementation of recommendations for the screening of hydroxychloroquine retinopathy: Poor adherence of rheumatologists and ophthalmologists. Lupus. 2017;26:277–281. doi: 10.1177/0961203316660204. [DOI] [PubMed] [Google Scholar]
- 15.Semmer AE, Lee MS, Harrison AR, Olsen TW. Hydroxychloroquine retinopathy screening. Br. J. Ophthalmol. 2008;92:1653–1655. doi: 10.1136/bjo.2008.144402. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Nika M, Blachley TS, Edwards P, Lee PP, Stein JD. Regular examinations for toxic maculopathy in long-term chloroquine or hydroxychloroquine users. JAMA Ophthalmol. 2014;132:1199. doi: 10.1001/jamaophthalmol.2014.1720. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Browning DJ. Impact of the revised American academy of ophthalmology guidelines regarding hydroxychloroquine screening on actual practice. Am. J. Ophthalmol. 2013;155:418–428.e1. doi: 10.1016/j.ajo.2012.09.025. [DOI] [PubMed] [Google Scholar]
- 18.Au A, et al. Hydroxychloroquine screening practice patterns within a large multispecialty ophthalmic practice. Am. J. Ophthalmol. 2015;160:561–568.e2. doi: 10.1016/j.ajo.2015.06.009. [DOI] [PubMed] [Google Scholar]
- 19.Braslow RA, Shiloach M, Macsai MS. Adherence to hydroxychloroquine dosing guidelines by rheumatologists. Ophthalmology. 2017;124:604–608. doi: 10.1016/j.ophtha.2016.12.021. [DOI] [PubMed] [Google Scholar]
- 20.Hsu ST, Ponugoti A, Deaner JD, Vajzovic L. Update on retinal drug toxicities. Curr. Ophthalmol. Rep. 2021;9:168–177. doi: 10.1007/s40135-021-00277-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Winebrake J, et al. Rheumatologists’ perspective on hydroxychloroquine guidelines. Lupus Sci. Med. 2020;7:e000427. doi: 10.1136/lupus-2020-000427. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Rosenbaum JT, et al. American College of Rheumatology, American Academy of Dermatology, Rheumatologic Dermatology Society, and American Academy of Ophthalmology 2020 Joint Statement on Hydroxychloroquine Use With Respect to Retinal Toxicity. Arthritis Rheumatol. 2021;73:908–911. doi: 10.1002/art.41683. [DOI] [PubMed] [Google Scholar]
- 23.Abdelbaky MSE, El Mamoun TA, Mabrouk FI, Hassan RM. Frequency and risk factors for hydroxychloroquine retinopathy among patients with systemic lupus erythematosus, Egypt. J. Intern. Med. 2021;33:18. doi: 10.1186/s43162-021-00047-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Wolfe F, Marmor MF. Rates and predictors of hydroxychloroquine retinal toxicity in patients with rheumatoid arthritis and systemic lupus erythematosus. Arthritis Care Res. 2010;62:775–784. doi: 10.1002/acr.20133. [DOI] [PubMed] [Google Scholar]
- 25.Daftarian N, et al. RetINal toxicity and hydroxychloroquine therapy (INTACT): Protocol for a prospective population-based cohort study. BMJ Open. 2022;12:e053852. doi: 10.1136/bmjopen-2021-053852. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Kao J-H, et al. Characteristics and potential risk factors of hydroxychloroquine retinopathy in patients with systemic lupus erythematosus: Focusing on Asian population. J. Ocul. Pharmacol. Ther. 2022;38:728–733. doi: 10.1089/jop.2022.0060. [DOI] [PubMed] [Google Scholar]
- 27.Osmani Z, et al. Hydroxychloroquine treatment in European patients with lupus erythematosus: Dosing, retinopathy screening and adherence. Lupus Sci. Med. 2021;8:e000478. doi: 10.1136/lupus-2021-000478. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Chen RI, et al. Impact of an electronic decision support tool to improve ophthalmic safety in hydroxychloroquine prescribing practices. Ophthalmic Surg. Lasers Imaging Retina. 2022;53:310–316. doi: 10.3928/23258160-20220601-02. [DOI] [PubMed] [Google Scholar]
- 29.Melles RB, Marmor MF. The risk of toxic retinopathy in patients on long-term hydroxychloroquine therapy. JAMA Ophthalmol. 2014;132:1453. doi: 10.1001/jamaophthalmol.2014.3459. [DOI] [PubMed] [Google Scholar]
- 30.Kim KE, Ryu SJ, Kim YH, Seo Y, Ahn SJ. Visual field examinations using different strategies in Asian patients taking hydroxychloroquine. Sci. Rep. 2022;12:14778. doi: 10.1038/s41598-022-19048-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
All data in the current study are available from the corresponding author (SB) upon reasonable request.