Abstract
Characterizing spatial distribution of HIV outcomes is vital for targeting interventions to areas most at risk. We performed spatial analysis to identify geographic clusters and factors associated with mortality in KwaZulu-Natal, South Africa. We utilized Sizanani trial (NCT01188941) data, which enrolled participants August 2010-January 2013 and obtained vital status at 5.8 (IQR 5.0–6.4) years of follow-up. We mapped geocoded addresses to 2011 Census-defined small area layer (SAL) centroids, used Kulldorff’s spatial scan statistic to identify mortality clusters, and compared socio-demographic factors for SALs in and not in mortality clusters. We assigned 1,143 participants living with HIV (260 [23%] of whom died during follow-up) to 677 SALs. One lower mortality cluster (n=90, RR=0.23, p=0.022) was identified near a hospital outside Durban. SALs in the cluster were younger (24y vs 25y, p<0.001), had fewer bedrooms/household (3 vs 4, p<0.001), had more females (52% vs 51%, p=0.013) and residents with no schooling past age 20 (4% vs 3%, p<0.001) or no education at all (4% vs 3%, p<0.001), and had fewer residents with income >3,200 ZAR/month (5% vs 9%, p<0.001). SALs in the cluster also had reduced access to piped water (p<0.001), refuse disposal (p<0.001), and toilets (p<0.001). Targeted interventions may improve outcomes in areas with similar characteristics.
Keywords: HIV/AIDS, South Africa, mortality, geographical information systems
Introduction
South Africa has the highest HIV prevalence globally, with 7.8 million people living with HIV (PLWH) and 230,000 new infections in 2020 (UNAIDS, 2020). The scale-up of antiretroviral therapy (ART) has increased the life expectancy of PLWH; yet, HIV/AIDS remains a leading cause of death, with 83,000 deaths in 2020 (Pillay-van Wyk et al., 2016; UNAIDS, 2020). Mortality rates are exceptionally high in KwaZulu-Natal, where we and others have previously shown that mortality risk is associated with contextual factors such as mental health, social support, self-perceived barriers to care, and competing needs (Bassett et al., 2017, 2019; Pillay-van Wyk et al., 2016). Further characterization of geographical risk factors is needed to understand mortality outcomes fully.
Geographic Information Systems can map disease distributions, associated risk factors, and prevention and treatment services (Boyda et al., 2019). Many studies have used spatial clustering (i.e., classifying spatial objects based on specific characteristics, where objects in a cluster are like each other and unlike non-cluster objects) to understand HIV prevalence in sub-Saharan Africa. These studies have found HIV to be distributed in localized clusters and to be heterogeneous between provinces, districts, and finer scales (Holmes et al., 2020; Meyer-Rath et al., 2018; Tanser et al., 2018; Wabiri et al., 2016; Waruru et al., 2018). HIV prevalence and mortality in rural South Africa are concentrated in peri-urban regions, around major towns, roads, and commercial activity, indicating that urbanization may play a prominent role in cluster locations (Meyer-Rath et al., 2018; Namosha et al., 2013; Tanser et al., 2018; Tlou et al., 2017a, 2017b). Other socio-economic, mobility, and geographic factors may also influence clustering (Bulstra et al., 2020; Muttai et al., 2021; Wabiri et al., 2016).
Identifying areas with excess HIV mortality is essential to direct public health interventions appropriately. Geospatial analyses have allowed for microtargeting of prevention services based on risk, leading to declining HIV incidence and increasing equity of the HIV response (Holmes et al., 2020). Further, the United States President’s Emergency Plan for AIDS Relief, the Global Fund, and the Joint United Nations Programme on HIV/AIDS endorse focusing resources on higher-burden geographical areas and facilities (Joint United Nations Programme on HIV/AIDS [UNAIDS], 2014; The Global Fund, 2016; U.S. President’s Emergency Plan for AIDS Relief, 2022). With declining program funds and increased access to HIV services, geospatial analysis is increasingly vital for targeting service delivery to finer geographic units.
In this analysis, we used spatial statistical analysis to identify geographic clusters and area-level demographic and socio-economic factors associated with mortality in an urban South African setting with high underlying HIV prevalence and mortality.
Materials and methods
Study setting and design
The study occurred in KwaZulu-Natal, a southeast province of South Africa that is 94,361 km2 and has 11,531,628 people, making it the second-most populous province in South Africa (South African Government, n.d.). The study population was concentrated around eThekwini, an urban metropolitan municipality that contains the coastal city of Durban. eThekwini has 3,702,231 people and includes urban, peri-urban, and rural areas (Govere et al., 2021).
The analysis includes data from the Sizanani Trial (NCT01188941). This randomized controlled trial examined the efficacy of health system navigation and short messaging service reminders on linkage to and retention in HIV/TB care. From August 2010-January 2013, we enrolled adults (≥18 years) with unknown HIV status presenting for HIV testing at two hospital outpatient departments at McCord (urban) and St. Mary’s (peri-urban) Hospitals and two nurse-driven municipal primary health clinics (Tshelimnyama and Marianridge Clinics) within the service area of St. Mary’s Hospital. Study enrollment, consisting of informed consent and a baseline questionnaire, occurred before HIV testing. This trial is described elsewhere (Bassett et al., 2013, 2016, 2017). Since there was no significant difference between linkage to HIV care, TB treatment completion, or one-year mortality between study arms, we pooled data from the PLWH in intervention and control groups into a single cohort in the current study.
The McCord Hospital Medical Research Ethics Committee, St. Mary’s Hospital Research Ethics Committee, University of KwaZulu-Natal Biomedical Research Ethics Committee, and Mass General Brigham Institutional Review Board (Protocol 2011-P-001195, Boston, MA) approved this study.
Data elements
Residential addresses were collected at enrollment for each Sizanani trial participant and were manually geocoded by AfriGIS, a Pretoria-based technology company. We then mapped the geocoded coordinates into small area layer (SAL) spatial units defined by 2011 South African Census data (Statistics South Africa, 2011). The SAL unit comprises aggregated enumeration areas (i.e., the work area for an individual Census enumerator) at a finer scale than sub-place (i.e., named villages, suburbs, etc.) data. All individual coordinates falling within a SAL were remapped to the centroid of that SAL and characterized by SAL data. SAL-level characteristics included: median age; the median number of bedrooms per household; race, gender, education, income, and employment percentages; primary household type; main cooking, heating, and lighting methods; and availability of piped water, refuse disposal, and toilets.
The primary outcome variable for this study was mortality. We elicited five-year all-cause mortality events from the South Africa National Population Register, estimated to incorporate at least 90% of deaths nationwide (Cornell et al., 2014; Johnson et al., 2015). We used South African ID numbers obtained at enrollment to match participants to the National Population Register in November 2017; the median follow-up was 5.8 years (IQR 5.0–6.4 years).
Analysis
We used Kulldorff’s purely spatial scan statistic in SaTScan version 9.7 (https://www.satscan.org/) to detect spatial clusters of high or low mortality. As the outcome of interest was a binomial variable (alive or dead at five years), we used the Bernoulli model for scanning. The SaTScan software uses a circular window that moves across space, noting the number of observed and expected observations inside the window at each location and adjusting for heterogeneous population density and distribution. The likelihood function is maximized over all window locations and sizes, and the window with the maximum likelihood constitutes the cluster that is least likely to have occurred by chance. The p-value is obtained by comparing the rank of the maximum likelihood from the actual data set with the maximum likelihoods from 999 Monte Carlo replications of the data set generated under the null hypothesis of no clusters. We performed the Kulldorff spatial cluster detection over the 677 SAL centroid locations included in this analysis, using a maximum spatial cluster size of 25% of the population and a minimum of 2 cases per cluster for high mortality to define clusters. Clusters with a relative risk >1.0 at a p-value <0.05 were considered significant clusters of higher mortality, while those with a relative risk <1.0 and a p-value <0.05 were deemed significant clusters of lower mortality. For significant clusters, we report the number of observed and expected cases, relative risk, and p-values.
Once clusters were identified, we used the 2011 Census SAL characteristics data to compare geographic areas where mortality clusters (high or low) were present to areas without clusters. For continuous variables (e.g., age; the number of bedrooms; percentages of race, gender, education, income, and employment), we used Wilcoxon Rank Sum tests to compare population-weighted averages for SALs in and not in the clusters. We used chi-square tests for categorical variables (e.g., primary household type; main cooking, heating, and lighting methods) and we conducted Cochran-Armitage trend tests for ordered categorical variables (e.g., availability of piped water, refuse disposal, and toilets). We report results as medians and interquartile ranges or counts and percentages and provide p-values.
Results
Spatial clusters of mortality
We analyzed data from 1,143 PLWH, of whom 260 (23%) died during the follow-up period. We assigned these 1,143 PLWH to 677 SAL centroids throughout KwaZulu-Natal. One significant cluster (n=90) of lower mortality was identified, with only five mortality events observed, although 20.5 events would be expected, with a cluster relative risk of 0.23 (p=0.022) (Figure 1). The cluster was located near the St. Mary’s Hospital, Tshelimnyama Clinic, and Marianridge Clinic enrollment sites in a peri-urban area of eThekwini. We identified no significant clusters of higher mortality; the smallest p-value for a higher mortality cluster was 0.22.
Figure 1.
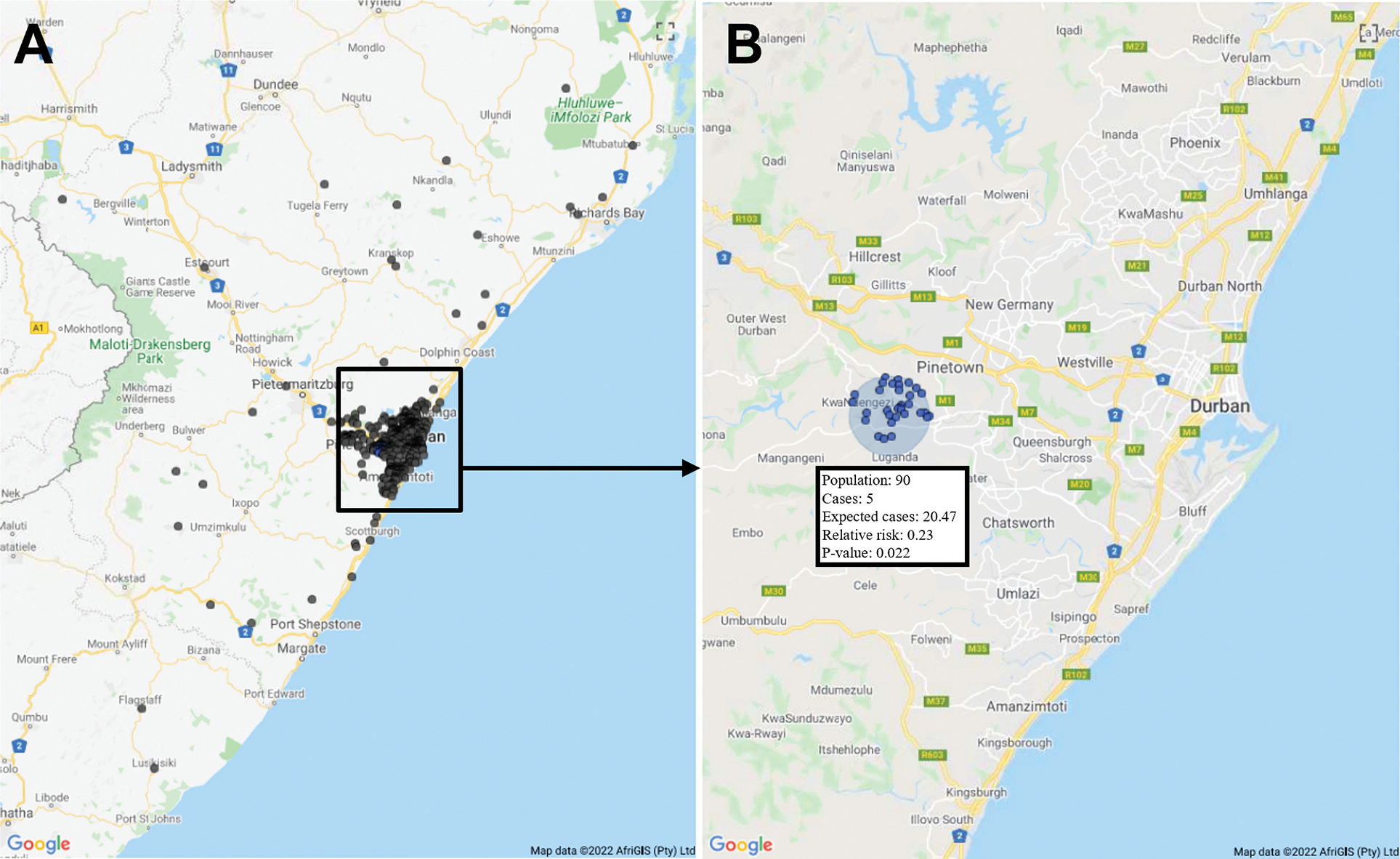
Spatial cluster of lower mortality. (A) Distribution of small area layer centroids of study participants throughout KwaZuluNatal. (B) Lower mortality cluster in relation to the greater Durban area.
Characteristics of the lower mortality cluster
SALs in the cluster were on average younger (24y vs. 25y, p<0.001), had fewer bedrooms per household (3 vs. 4, p<0.001), had higher proportions of females (52% vs. 51%, p=0.013) and residents with no schooling past age 20 (4% vs. 3%, p<0.001) or no education at all (4% vs. 3%, p<0.001), and had lower proportions of residents with monthly income >3,200 ZAR (5% vs. 9%, p<0.001) (Table 1). Additionally, there was a small but statistically significant difference in the proportion of Black African race, with a slightly lower proportion among SALs in the cluster (99.2% vs. 99.4%, p=0.007). Employment did not differ between the cluster and non-cluster areas.
Table 1.
Small Area Layer Characteristics
| Characteristics, median [IQR] or n (%) | In Low Mortality Cluster (n = 90) |
Not In Low Mortality Cluster (n = 1053) |
p-value |
|---|---|---|---|
|
| |||
| Age, years | 24.0 [23.0–25.0] | 25.0 [23.0–27.0] | <0.001 |
| Median bedrooms per household | 3.0 [2.0–4.0] | 4.0 [2.0–5.0] | <0.001 |
| Percent Black African | 99.2 [66.8–99.5] | 99.4 [86.7–99.8] | 0.007 |
| Percent female | 52.0 [50.8–53.2] | 51.4 [49.5–53.1] | 0.013 |
| Percent no school past age 20 | 4.0 [3.0–7.0] | 3.0 [1.0–5.0] | <0.001 |
| Percent no school at all | 4.0 [3.0–7.0] | 3.0 [1.0–5.0] | <0.001 |
| Percent earning >3,200 ZAR/month | 5.1 [3.5–6.9] | 9.2 [5.3–18.7] | <0.001 |
| Percent employed | 38.7 [31.3–45.3] | 41.0 [30.3–51.1] | 0.143 |
| Main household type | |||
| Formal | 90 (100.0) | 967/1046 (92.5) | 0.063 |
| Informal | 0 (0.0) | 40/1046 (3.8) | |
| Traditional | 0 (0.0) | 37/1046 (3.5) | |
| Other | 0 (0.0) | 2/1046 (0.2) | |
| Main cooking method | |||
| Electricity | 90 (100.0) | 1012/1046 (96.8) | 0.221 |
| Wood | 0 (0.0) | 25/1046 (2.4) | |
| Paraffin | 0 (0.0) | 9/1046 (0.9) | |
| Main heating method | |||
| Electricity | 90 (100.0) | 995/1046 (95.1) | 0.332 |
| Wood | 0 (0.0) | 40/1046 (3.8) | |
| Paraffin | 0 (0.0) | 7/1046 (0.7) | |
| Coal | 0 (0.0) | 2/1046 (0.2) | |
| Gas | 0 (0.0) | 2/1046 (0.2) | |
| Main lighting method | |||
| Electricity | 90 (100.0) | 1008/1046 (96.4) | 0.184 |
| Candles | 0 (0.0) | 37/1046 (3.5) | |
| Paraffin | 0 (0.0) | 1/1046 (0.1) | |
| Piped water | |||
| Piped (tap) water inside dwelling/institution | 43 (47.8) | 712/1051 (67.8) | <0.001 |
| Piped (tap) water inside yard | 16 (17.8) | 256/1051 (24.4) | |
| Piped (tap) water on community stand: distance less than 200m from dwelling/institution | 30 (33.3) | 52/1051 (5) | |
| Piped (tap) water on community stand: distance between 200m and 500m from dwelling/institution | 1 (1.1) | 14/1051 (1.3) | |
| Piped (tap) water on community stand: distance greater than 1000m (1km) from dwelling/institution | 0 (0.0) | 6/1051 (0.6) | |
| No access to piped (tap) water | 0 (0.0) | 11/1051 (1.1) | |
| Refuse | |||
| Removed by local authority/private company at least once a week | 66 (73.3) | 921/1046 (88.1) | 0.005 |
| Removed by local authority/private company less often | 4 (4.4) | 5/1046 (0.5) | |
| Own refuse dump | 20 (22.2) | 106/1046 (10.1) | |
| Communal refuse dump | 0 (0.0) | 2/1046 (0.2) | |
| No rubbish disposal/Other | 0 (0.0) | 12/1046 (1.1) | |
| Toilet | |||
| Flush toilet (sewage system) | 41 (45.6) | 783/1046 (74.9) | <0.001 |
| Flush toilet (septic tank) | 8 (8.9) | 57/1046 (5.5) | |
| Chemical toilet | 24 (26.7) | 59/1046 (5.6) | |
| Pit toilet with ventilation | 3 (3.3) | 67/1046 (6.4) | |
| Pit toilet without ventilation | 11 (12.2) | 51/1046 (4.9) | |
| Bucket toilet/None/Other | 3 (3.3) | 29/1046 (2.8) | |
100% of the SALs in the cluster had formal (e.g., houses, flats, apartments, or townhouses) instead of informal or traditional households as the primary household type and electricity as the main cooking, heating, and lighting method. These factors were not significantly different between the cluster and non-cluster areas.
SALs both in and not in the cluster most often accessed piped water inside a dwelling; however, the cluster tended towards piped water that was further away from households (p<0.001), with a higher proportion accessing piped water from a nearby community stand. Refuse was often removed by a local authority or private company at least once a week, both in and not in the cluster; however, SALs in the cluster tended towards less refuse disposal (p<0.001), with a higher proportion maintaining their own refuse dump. The most common toilet was a flush toilet connected to a sewage system both in and not in the cluster; however, the cluster tended toward lower quality toilets (p<0.001), with a higher proportion of chemical, pit, and bucket toilets.
Discussion
This study found a single cluster of lower 5-year all-cause mortality among PLWH in the Sizanani trial, in a peri-urban area near Durban, despite socio-demographic risk factors including lower education, income, and poorer access to water, refuse disposal, and toilets. The cluster was in the catchment area of St. Mary’s Hospital, a district hospital enrollment site serving a relatively poor population approximately 20 kilometers west of Durban. The municipal clinic enrollment sites, Tshelimnyama and Marianridge, also referred to St. Mary’s for ART initiation after HIV testing. The area is semi-rural, surrounded by townships and informal settlements.
The lower mortality cluster near the peri-urban study enrollment sites indicates that people who tested for HIV near their residence experienced more favorable health outcomes in this study. We have previously found pre-treatment losses to care to be more likely among people that lived ≥10 kilometers from the McCord or St. Mary’s testing centers (Losina et al., 2010). Further, people who lived ≥5 kilometers from the test sites had higher odds of late-stage HIV disease presentation, which is associated with higher mortality (Drain et al., 2013). Thus, participants in the cluster near the peri-urban testing sites may have received more timely HIV care, resulting in lower mortality.
We also found the residential addresses for Sizanani participants to be distributed across KwaZulu-Natal, indicating that some participants traveled further than their closest clinic to access HIV testing. Studies have commonly found that PLWH travel to more distant clinics to avoid the stigma of being recognized by community members or because they perceive remote clinics to provide higher quality HIV care (Akullian et al., 2016; Bassett et al., 2015; Mee et al., 2020). However, the logistical challenges of getting to a faraway clinic can limit ongoing care receipt, such that increased ART uptake and decreased mortality risk are associated with a shorter distance to the clinic (Cooke et al., 2010; Hannaford et al., 2021; Hendrickson et al., 2018; Sartorius, 2013). Differentiated HIV treatment models may improve outcomes by tailoring care to individual patients, potentially reducing stigma and the distance traveled to access care (Boyda et al., 2019; Ehrenkranz et al., 2019; Grimsrud et al., 2017; Sharer et al., 2019).
We did not observe a lower mortality cluster near the urban enrolment site (McCord Hospital), potentially reflecting differences between the McCord and St. Mary’s Hospital areas. St. Mary’s Hospital received higher state subsidies during the study period, allowing them to charge lower fees than McCord. Further, the approximately 20 alternative accredited ART initiation centers in greater Durban were concentrated around McCord, allowing more anonymity in HIV testing and care initiation. However, a higher number of alternative locations may have contributed to a higher loss to care rate, as there may inherently be less community or healthcare support among clinic populations that are not as clearly defined (Losina et al., 2010).
Conversely, St. Mary’s Hospital had an accompanying church, children’s school, and monastery and conducted extensive community outreach to other clinics and individuals in their catchment area through efforts such as community health messaging and involvement of community health workers. These community services and connections may have encouraged increased healthcare engagement and positive health benefits among patients living in the area. We have also found that receiving HIV testing at McCord versus St. Mary’s was correlated with poorer social support (Drain et al., 2015). Targeted expansion of interventions such as support groups or adherence clubs may improve outcomes in similarly urban areas as social support is a major facilitator of linkage and adherence to HIV care (Croome et al., 2017; Fox et al., 2019; Kave et al., 2019).
Prior geospatial studies of mortality in South Africa have focused on rural areas. In contrast to our research, they have identified peri-urban regions as higher risk, primarily due to high underlying HIV prevalence relative to the study area (Namosha et al., 2013; Tlou et al., 2017a, 2017b). Our lower mortality cluster was characterized by traits typical of a poorer, more rural area, with smaller households and relatively low education, income, and sanitation. However, numerous other studies have shown lower socio-economic status as a significant mortality risk factor among PLWH (Probst et al., 2016; Sartorius, 2013; Sartorius et al., 2010; Sartorius & Sartorius, 2013). Further, improved access to water and sanitation significantly reduces diarrheal deaths due to inadequate water, sanitation, and hygiene (World Health Organization, n.d.). Therefore, it is likely that community services, cohesion, social support, or other factors contributed more to the lower mortality than our observed socio-demographic trends. South Africa should continue efforts toward income equality and access to education, piped water, and sanitation; still, our study indicates that health services targeting relatively poor areas can also encourage positive health outcomes (Mayosi et al., 2012).
Our study has several limitations. The first is that we recruited participants in and around Durban, so findings may not be generalizable to the entirety of KwaZulu-Natal, particularly more rural areas. Second, since we relied on area-level characteristics obtained from the Census data, we did not include individual contextual factors that may have contributed to our observed outcomes. Finally, we could not determine the cause of our participants’ deaths, so we cannot confirm that they were HIV-related; however, data suggest that HIV/AIDS-related deaths drove mortality in South Africa during the study period (Pillay-van Wyk et al., 2016).
In conclusion, we found a lower mortality area in KwaZulu-Natal to be younger, with smaller households, with more females and residents with limited/no schooling, and fewer residents in the high-income category or with optimal access to sanitation (water, refuse, toilets). A local district hospital providing tailored outreach, HIV testing, community-related interventions, and ongoing care may have mitigated these socio-demographic risk factors. Targeted interventions that improve access to and acceptability of HIV care may reduce deaths among PLWH in similar peri-urban areas with high HIV prevalence. Adherence clubs, decentralized medication delivery in community venues (e.g, schools, churches), and self-forming client-managed groups can improve retention in care and long-term virologic suppression, while leveraging community strengths (Fox et al., 2019, Fatti et al., 2021, Jobarteh et al., 2016).
Acknowledgments
This work will be presented in part as an abstract at AIDS 2022, the 24th International AIDS Conference (July 29-August 2, 2022). We thank the clinical sites for their dedication to research, and we gratefully acknowledge the study participants.
Funding
This work was supported by the National Institute of Mental Health under Grant R01MH090326 and R01MH108427 (IVB) and the Weissman Family MGH Research Scholar Award (IVB). Its contents are solely the authors’ responsibility and do not necessarily represent the official views of the National Institutes of Health or the Massachusetts General Hospital Executive Committee on Research.
Footnotes
Declaration of interest statement
The authors declare that they have no competing interests.
Data availability statement
The data supporting this study’s findings are available from the corresponding author (IVB) upon reasonable request.
References
- Akullian AN, Mukose A, Levine GA, & Babigumira JB (2016). People living with HIV travel farther to access healthcare: A population-based geographic analysis from rural Uganda. Journal of the International AIDS Society, 19(1), 20171. 10.7448/IAS.19.1.20171 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bassett IV, Coleman SM, Giddy J, Bogart LM, Chaisson CE, Ross D, Flash MJE, Govender T, Walensky RP, Freedberg KA, & Losina E (2017). Barriers to care and 1-year mortality among newly diagnosed HIV-infected people in Durban, South Africa. Journal of Acquired Immune Deficiency Syndromes (1999), 74(4), 432–438. 10.1097/QAI.0000000000001277 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bassett IV, Coleman SM, Giddy J, Bogart LM, Chaisson CE, Ross D, Jacobsen MM, Robine M, Govender T, Freedberg KA, Katz JN, Walensky RP, & Losina E (2016). Sizanani: A randomized trial of health system navigators to improve linkage to HIV and TB care in South Africa. Journal of Acquired Immune Deficiency Syndromes (1999), 73(2), 154–160. 10.1097/QAI.0000000000001025 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bassett IV, Giddy J, Chaisson CE, Ross D, Bogart LM, Coleman SM, Govender T, Robine M, Erlwanger A, Freedberg KA, Katz JN, Walensky RP, & Losina E (2013). A randomized trial to optimize HIV/TB care in South Africa: Design of the Sizanani trial. BMC Infectious Diseases, 13, 390. 10.1186/1471-2334-13-390 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bassett IV, Regan S, Mbonambi H, Blossom J, Bogan S, Bearnot B, Robine M, Walensky RP, Mhlongo B, Freedberg KA, Thulare H, & Losina E (2015). Finding HIV in hard to reach populations: Mobile HIV testing and geospatial mapping in Umlazi township, Durban, South Africa. AIDS and Behavior, 19(10), 1888–1895. 10.1007/s10461-015-1012-3 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bassett IV, Xu A, Giddy J, Bogart LM, Boulle A, Millham L, Losina E, & Parker RA (2019). Assessing rates and contextual predictors of 5-year mortality among HIV-infected and HIV-uninfected individuals following HIV testing in Durban, South Africa. BMC Infectious Diseases, 19(1), 751. 10.1186/s12879-019-4373-9 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Boyda DC, Holzman SB, Berman A, Grabowski MK, & Chang LW (2019). Geographic Information Systems, spatial analysis, and HIV in Africa: A scoping review. PloS One, 14(5), e0216388. 10.1371/journal.pone.0216388 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bulstra CA, Hontelez JAC, Giardina F, Steen R, Nagelkerke NJD, Bärnighausen T, & de Vlas SJ (2020). Mapping and characterising areas with high levels of HIV transmission in sub-Saharan Africa: A geospatial analysis of national survey data. PLoS Medicine, 17(3), e1003042. 10.1371/journal.pmed.1003042 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cooke GS, Tanser FC, Bärnighausen TW, & Newell M-L (2010). Population uptake of antiretroviral treatment through primary care in rural South Africa. BMC Public Health, 10, 585. 10.1186/1471-2458-10-585 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cornell M, Lessells R, Fox MP, Garone DB, Giddy J, Fenner L, Myer L, Boulle A, & IeDEA-Southern Africa Collaboration. (2014). Mortality among adults transferred and lost to follow-up from antiretroviral therapy programmes in South Africa: A multicenter cohort study. Journal of Acquired Immune Deficiency Syndromes (1999), 67(2), e67–75. 10.1097/QAI.0000000000000269 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Croome N, Ahluwalia M, Hughes LD, & Abas M (2017). Patient-reported barriers and facilitators to antiretroviral adherence in sub-Saharan Africa. AIDS (London, England), 31(7), 995–1007. 10.1097/QAD.0000000000001416 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Drain PK, Losina E, Coleman SM, Bogart L, Giddy J, Ross D, Katz JN, & Bassett IV (2015). Social support and mental health among adults prior to HIV counseling and testing in Durban, South Africa. AIDS Care, 27(10), 1231–1240. 10.1080/09540121.2015.1046417 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Drain PK, Losina E, Parker G, Giddy J, Ross D, Katz JN, Coleman SM, Bogart LM, Freedberg KA, Walensky RP, & Bassett IV (2013). Risk factors for late-stage HIV disease presentation at initial HIV diagnosis in Durban, South Africa. PloS One, 8(1), e55305. 10.1371/journal.pone.0055305 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ehrenkranz P, Grimsrud A, & Rabkin M (2019). Differentiated service delivery: Navigating the path to scale. Current Opinion in HIV and AIDS, 14(1), 60–65. 10.1097/COH.0000000000000509 [DOI] [PubMed] [Google Scholar]
- Fatti G, Ngorima-Mabhena N, Tiam A, Tukei BB, Kasu T, Muzenda T, Maile K, Lombard C, Chasela C, & Grimwood A (2021). Community-based differentiated service delivery models incorporating multi-month dispensing of antiretroviral treatment for newly stable people living with HIV receiving single annual clinical visits: A pooled analysis of two cluster-randomized trials in southern Africa. Journal of the International AIDS Society, 24(S6). 10.1002/jia2.25819 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fox MP, Pascoe S, Huber AN, Murphy J, Phokojoe M, Gorgens M, Rosen S, Wilson D, Pillay Y, & Fraser-Hurt N (2019). Adherence clubs and decentralized medication delivery to support patient retention and sustained viral suppression in care: Results from a cluster-randomized evaluation of differentiated ART delivery models in South Africa. PLoS Medicine, 16(7), e1002874. 10.1371/journal.pmed.1002874 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Govere SM, Kalinda C, & Chimbari MJ (2021). Factors influencing rapid antiretroviral therapy initiation at four eThekwini clinics, KwaZulu-Natal, South Africa. AIDS and Behavior. 10.1007/s10461-021-03530-3 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Grimsrud A, Barnabas RV, Ehrenkranz P, & Ford N (2017). Evidence for scale up: The differentiated care research agenda. Journal of the International AIDS Society, 20(Suppl 4), 22024. 10.7448/IAS.20.5.22024 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hannaford A, Moll AP, Madondo T, Khoza B, & Shenoi SV (2021). Mobility and structural barriers in rural South Africa contribute to loss to follow up from HIV care. AIDS Care, 33(11), 1436–1444. 10.1080/09540121.2020.1808567 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hendrickson CJ, Pascoe SJS, Huber AN, Moolla A, Maskew M, Long LC, & Fox MP (2018). “My future is bright…I won’t die with the cause of AIDS”: Ten-year patient ART outcomes and experiences in South Africa. Journal of the International AIDS Society, 21(10), e25184. 10.1002/jia2.25184 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Holmes CB, Rabkin M, Ford N, Preko P, Rosen S, Ellman T, & Ehrenkranz P (2020). Tailored HIV programmes and universal health coverage. Bulletin of the World Health Organization, 98(2), 87–94. 10.2471/BLT.18.223495 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jobarteh K, Shiraishi RW, Malimane I, Samo Gudo P, Decroo T, Auld AF, Macome V, & Couto A (2016). Community ART Support Groups in Mozambique: The Potential of Patients as Partners in Care. PLOS ONE, 11(12), e0166444. 10.1371/journal.pone.0166444 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Johnson LF, Dorrington RE, Laubscher R, Hoffmann CJ, Wood R, Fox MP, Cornell M, Schomaker M, Prozesky H, Tanser F, Davies M-A, Boulle A, & International Epidemiologic Databases to Evaluate AIDS Southern Africa (IeDEA-SA) Collaboration. (2015). A comparison of death recording by health centres and civil registration in South Africans receiving antiretroviral treatment. Journal of the International AIDS Society, 18, 20628. 10.7448/IAS.18.1.20628 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Joint United Nations Programme on HIV/AIDS (UNAIDS). (2014). Local Epidemics Issues Brief. https://www.unaids.org/sites/default/files/media_asset/JC2559_local-epidemics_en.pdf
- Kave S, Khuzwayo NF, Hatcher A, & Sikweyiya Y (2019). The role of support groups in linking and retaining newly diagnosed clients in HIV care in a peri-urban location in South Africa. African Journal of AIDS Research: AJAR, 18(1), 9–17. 10.2989/16085906.2018.1551233 [DOI] [PubMed] [Google Scholar]
- Losina E, Bassett IV, Giddy J, Chetty S, Regan S, Walensky RP, Ross D, Scott CA, Uhler LM, Katz JN, Holst H, & Freedberg KA (2010). The “ART” of linkage: Pre-treatment loss to care after HIV diagnosis at two PEPFAR sites in Durban, South Africa. PloS One, 5(3), e9538. 10.1371/journal.pone.0009538 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mayosi BM, Lawn JE, van Niekerk A, Bradshaw D, Abdool Karim SS, Coovadia HM, & Lancet South Africa team. (2012). Health in South Africa: Changes and challenges since 2009. Lancet (London, England), 380(9858), 2029–2043. 10.1016/S0140-6736(12)61814-5 [DOI] [PubMed] [Google Scholar]
- Mee P, Rice B, Kabudula CW, Tollman SM, Gómez-Olivé FX, & Reniers G (2020). The impact of HIV status on the distance traveled to health facilities and adherence to care. A record-linkage study from rural South Africa. Journal of Global Health, 10(2), 020435. 10.7189/jogh.10.020435 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Meyer-Rath G, McGillen JB, Cuadros DF, Hallett TB, Bhatt S, Wabiri N, Tanser F, & Rehle T (2018). Targeting the right interventions to the right people and places: The role of geospatial analysis in HIV program planning. AIDS (London, England), 32(8), 957–963. 10.1097/QAD.0000000000001792 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Muttai H, Guyah B, Achia T, Musingila P, Nakhumwa J, Oyoo R, Olweny W, Odeny R, Ohaga S, Agot K, Oruenjo K, Awino B, Joseph RH, Miruka F, & Zielinski-Gutierrez E (2021). Mapping geographic clusters of new HIV diagnoses to inform granular-level interventions for HIV epidemic control in western Kenya. BMC Public Health, 21(1), 1926. 10.1186/s12889-021-11890-7 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Namosha E, Sartorius B, & Tanser F (2013). Spatial clustering of all-cause and HIV-related mortality in a rural South African population (2000–2006). PloS One, 8(7), e69279. 10.1371/journal.pone.0069279 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pillay-van Wyk V, Msemburi W, Laubscher R, Dorrington RE, Groenewald P, Glass T, Nojilana B, Joubert JD, Matzopoulos R, Prinsloo M, Nannan N, Gwebushe N, Vos T, Somdyala N, Sithole N, Neethling I, Nicol E, Rossouw A, & Bradshaw D (2016). Mortality trends and differentials in South Africa from 1997 to 2012: Second National Burden of Disease Study. The Lancet. Global Health, 4(9), e642–653. 10.1016/S2214-109X(16)30113-9 [DOI] [PubMed] [Google Scholar]
- Probst C, Parry CDH, & Rehm J (2016). Socio-economic differences in HIV/AIDS mortality in South Africa. Tropical Medicine & International Health: TM & IH, 21(7), 846–855. 10.1111/tmi.12712 [DOI] [PubMed] [Google Scholar]
- Sartorius B (2013). Modelling determinants, impact, and space-time risk of age-specific mortality in rural South Africa: Integrating methods to enhance policy relevance. Global Health Action, 6, 19239. 10.3402/gha.v6i0.19239 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sartorius B, Kahn K, Vounatsou P, Collinson MA, & Tollman SM (2010). Space and time clustering of mortality in rural South Africa (Agincourt HDSS), 1992–2007. Global Health Action, 3. 10.3402/gha.v3i0.5225 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sartorius B, & Sartorius K (2013). Identifying and targeting mortality disparities: A framework for sub-saharan Africa using adult mortality data from South Africa. PloS One, 8(8), e71437. 10.1371/journal.pone.0071437 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sharer M, Davis N, Makina N, Duffy M, & Eagan S (2019). Differentiated antiretroviral therapy delivery: Implementation barriers and enablers in South Africa. The Journal of the Association of Nurses in AIDS Care: JANAC, 30(5), 511–520. 10.1097/JNC.0000000000000062 [DOI] [PMC free article] [PubMed] [Google Scholar]
- South African Government. (n.d.). South Africa’s provinces. Retrieved March 29, 2022, from https://www.gov.za/about-sa/south-africas-provinces#kzn
- Statistics South Africa. (2011). 2011 Census | Statistics South Africa. http://www.statssa.gov.za/?page_id=3839
- Tanser F, Bärnighausen T, Dobra A, & Sartorius B (2018). Identifying “corridors of HIV transmission” in a severely affected rural South African population: A case for a shift toward targeted prevention strategies. International Journal of Epidemiology, 47(2), 537–549. 10.1093/ije/dyx257 [DOI] [PMC free article] [PubMed] [Google Scholar]
- The Global Fund. (2016). The Global Fund Strategy 2017–2022: Investing to end epidemics. https://www.theglobalfund.org/media/2531/core_globalfundstrategy2017-2022_strategy_en.pdf
- Tlou B, Sartorius B, & Tanser F (2017a). Space-time variations in child mortality in a rural South African population with high HIV prevalence (2000–2014). PloS One, 12(8), e0182478. 10.1371/journal.pone.0182478 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tlou B, Sartorius B, & Tanser F (2017b). Space-time patterns in maternal and mother mortality in a rural South African population with high HIV prevalence (2000–2014): Results from a population-based cohort. BMC Public Health, 17(1), 543. 10.1186/s12889-017-4463-9 [DOI] [PMC free article] [PubMed] [Google Scholar]
- UNAIDS. (2020). South Africa. https://www.unaids.org/en/regionscountries/countries/southafrica
- U.S. President’s Emergency Plan for AIDS Relief. (2022). PEPFAR 2022 Country and Regional Operational Plan (COP/ROP) Guidance for all PEPFAR-Supported Countries. https://www.state.gov/wp-content/uploads/2022/02/COP22-Guidance-Final_508-Compliant-3.pdf
- Wabiri N, Shisana O, Zuma K, & Freeman J (2016). Assessing the spatial nonstationarity in relationship between local patterns of HIV infections and the covariates in South Africa: A geographically weighted regression analysis. Spatial and Spatio-Temporal Epidemiology, 16, 88–99. 10.1016/j.sste.2015.12.003 [DOI] [PubMed] [Google Scholar]
- Waruru A, Achia TNO, Tobias JL, Ngʼangʼa J, Mwangi M, Wamicwe J, Zielinski-Gutierrez E, Oluoch T, Muthama E, & Tylleskär T (2018). Finding hidden HIV clusters to support geographic-oriented H IV interventions in Kenya. Journal of Acquired Immune Deficiency Syndromes (1999), 78(2), 144–154. 10.1097/QAI.0000000000001652 [DOI] [PMC free article] [PubMed] [Google Scholar]
- World Health Organization. (n.d.). Water, sanitation and hygiene (WASH). Retrieved April 18, 2022, from https://www.who.int/health-topics/water-sanitation-and-hygiene-wash
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
The data supporting this study’s findings are available from the corresponding author (IVB) upon reasonable request.


