Summary
Background
Multisystem inflammatory syndrome in children (MIS-C) is a severe complication of SARS-CoV-2 infection. It remains unclear how MIS-C phenotypes vary across SARS-CoV-2 variants. We aimed to investigate clinical characteristics and outcomes of MIS-C across SARS-CoV-2 eras.
Methods
We performed a multicentre observational retrospective study including seven paediatric hospitals in four countries (France, Spain, U.K., and U.S.). All consecutive confirmed patients with MIS-C hospitalised between February 1st, 2020, and May 31st, 2022, were included. Electronic Health Records (EHR) data were used to calculate pooled risk differences (RD) and effect sizes (ES) at site level, using Alpha as reference. Meta-analysis was used to pool data across sites.
Findings
Of 598 patients with MIS-C (61% male, 39% female; mean age 9.7 years [SD 4.5]), 383 (64%) were admitted in the Alpha era, 111 (19%) in the Delta era, and 104 (17%) in the Omicron era. Compared with patients admitted in the Alpha era, those admitted in the Delta era were younger (ES −1.18 years [95% CI −2.05, −0.32]), had fewer respiratory symptoms (RD −0.15 [95% CI −0.33, −0.04]), less frequent non-cardiogenic shock or systemic inflammatory response syndrome (SIRS) (RD −0.35 [95% CI −0.64, −0.07]), lower lymphocyte count (ES −0.16 × 109/uL [95% CI −0.30, −0.01]), lower C-reactive protein (ES −28.5 mg/L [95% CI −46.3, −10.7]), and lower troponin (ES −0.14 ng/mL [95% CI −0.26, −0.03]). Patients admitted in the Omicron versus Alpha eras were younger (ES −1.6 years [95% CI −2.5, −0.8]), had less frequent SIRS (RD −0.18 [95% CI −0.30, −0.05]), lower lymphocyte count (ES −0.39 × 109/uL [95% CI −0.52, −0.25]), lower troponin (ES −0.16 ng/mL [95% CI −0.30, −0.01]) and less frequently received anticoagulation therapy (RD −0.19 [95% CI −0.37, −0.04]). Length of hospitalization was shorter in the Delta versus Alpha eras (−1.3 days [95% CI −2.3, −0.4]).
Interpretation
Our study suggested that MIS-C clinical phenotypes varied across SARS-CoV-2 eras, with patients in Delta and Omicron eras being younger and less sick. EHR data can be effectively leveraged to identify rare complications of pandemic diseases and their variation over time.
Funding
None.
Keywords: Multisystem inflammatory syndrome, Paediatric inflammatory multisystem syndrome, COVID-19, SARS-CoV-2, Variants, Pediatrics, Clinical phenotypes, Outcomes
Research in context.
Evidence before this study
Multisystem inflammatory syndrome in children (MIS-C), a post-infectious vasculitis associated with SARS-CoV-2 infection, represents one of the most important complications of COVID-19 in children and young adults. While outcomes after acute COVID-19 differ based on SARS-CoV-2 variants, data on variant-specific MIS-C phenotypes and outcomes are limited. To investigate the available evidence, we searched PubMed using the following search strategy, which included both terms and controlled vocabulary terms: “(COVID-19 OR SARS-CoV-2 OR COVID19 [MeSH Terms]) AND (“multisystem inflammatory syndrome” OR MISC OR MIS-C OR “multi-system inflammatory syndrome” OR “paediatric inflammatory multisystem syndrome” OR PIMS) AND (variant OR variants)” (last search date: May 15th, 2023). The search retrieved 130 documents; of them, eight addressed the research question. Most of the studies either had a small sample size or were monocentre. Preliminary results from a large register-based study suggested that patients hospitalised during the initial period of the pandemic were at higher risk of admission to the intensive care unit and present with ventricular dysfunction compared to those hospitalised during the most recent eras.
Added value of this study
Our multicentre multinational EHR-based study, which brings together data from a large cohort of patients with MIS-C from four countries across two continents, provided evidence suggesting that MIS-C clinical and laboratory characteristics and outcomes vary according to SARS-CoV-2 variant eras. We found that patients admitted during the Delta and Omicron eras were younger and less sick than those admitted in the Alpha era. Specifically, patients admitted during the Alpha era versus subsequent variant eras had more respiratory involvement and more frequently presented with shock or systemic inflammatory response syndrome (SIRS); they also had higher C-reactive protein, absolute lymphocyte count, and troponin levels; lower albumin; and longer hospitalization.
Implications of all the available evidence
We believe our study adds valuable information in characterizing different MIS-C phenotypes across SARS-CoV-2 eras and continents. This available evidence may help in risk stratification and clinical prognostication in paediatric patients with MIS-C. Our study also showed that EHR data may be effectively leveraged to investigate rare complications of diseases in the setting of the pandemic and used to identify patterns of disease variation or severity over time.
Introduction
Multisystem inflammatory syndrome in children (MIS-C) is a post-infectious vasculitis associated with SARS-CoV-2 infection and represents one of the most important complications secondary to SARS-CoV-2 infection in children and young adults.1,2 Since the beginning of the pandemic, multiple SARS-CoV-2 variants, including Alpha, Delta, and Omicron,3 have been identified. Outcomes after acute COVID-19 in children have been reported to differ based on SARS-CoV-2 variants, with Omicron being associated with less severe illness than the Alpha and Delta variants.4 However, data on variant-specific MIS-C phenotypes and outcomes are still limited.
A report from the Centres for Disease Control and Prevention (CDC) — using U.S. voluntary national surveillance data from February 2020 to July 2021 — first observed that MIS-C clinical characteristics and outcomes appeared to vary across SARS-CoV-2 waves, with an overall decrease in the incidence of severe outcomes over time.5 This analysis was reproduced using data up to January 2022 and confirmed that MIS-C severity decreased over time.6 An analysis from Israel of data from 171 patients admitted in 12 centres showed that cardiovascular outcomes were more favourable during the Omicron wave and length of hospitalization was shorter compared to previous waves.7 A recent large cohort study from the International Kawasaki Disease Registry demonstrated that, compared to patients hospitalised during the ancestral period (pre-Alpha), the risk of intensive care unit (ICU) admission was lowest in those hospitalised during the Omicron era, and the risk of ventricular dysfunction was highest among those hospitalised during the Alpha era.8 However, other smaller single-centre and multicentre reports from South Africa, Europe, and the U.S. found no difference in MIS-C outcomes across SAS-CoV-2 variant eras.9, 10, 11, 12, 13
The Consortium for Clinical Characterization of COVID-19 (4CE) is an international consortium that brings together researchers and electronic health record (EHR) data scientists to leverage EHR data using a federated approach to address research questions related to COVID-19, while preserving data confidentiality.14 We aimed to investigate and compare clinical characteristics, laboratory data, and patient-level outcomes of patients with MIS-C who were hospitalised during different SARS-CoV-2 eras using multicentre data from the 4CE Consortium.
Methods
Study design, setting, and population
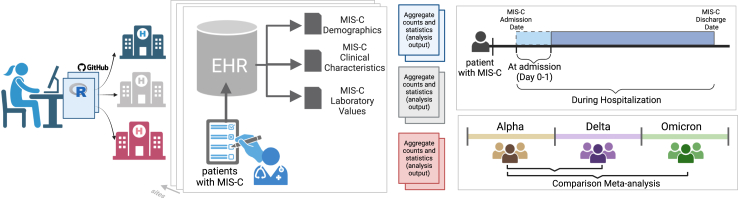
We performed a retrospective multicentre population-based study using 4CE Consortium data from 4 countries (France, Spain, U.K., and U.S.; Supplemental Table S1). The overall study methodological structure is summarized in Fig. 1. All analyses involving identifiable data were conducted at the site level; aggregate analyses were performed at the project coordinating centre (Boston Children’s Hospital, BCH). A list of consecutive patients with MIS-C was identified by MIS-C experts at each site. Inclusion criteria were age <21 years, diagnosis of MIS-C based on CDC, Royal College of Paediatrics and Child Health, or World Health Organization criteria according to institutional practice, and hospitalization for MIS-C between February 1st, 2020 and May 31st, 2022 (Supplemental Tables S1 and S2). Approval for the study was obtained from the institutional review board at each site with a waiver of informed consent since only de-identified retrospective observational data were analysed. The study was conducted following the ethical principles for medical research of the Helsinki Declaration and the quality standard required by the Strengthening the Reporting of Observational Studies in Epidemiology (STROBE) guidelines.
Fig. 1.
Graphical summary of the study methodological structure. MIS-C: multisystem inflammatory syndrome.
Data extraction, quality check, and data definition
Data were collected from the EHR of participating sites in a federated approach (Supplemental Fig. S1). Patient-level data at each site were extracted following the 4CE common data model. An R package was created for site-level analyses and shared with all sites following a step-by-step approach (Supplemental Fig. S1). Aggregated counts and statistics were then shared centrally. The code is publicly available on GitHub (GitHub Inc., San Francisco, U.S. https://github.com/covidclinical/PhaseX.2SqlDataExtraction/tree/main/Extras, https://github.com/covidclinical/Phase2.2MISCRPackage).
Data quality checks were performed at site level and across sites (Supplemental Methods). To facilitate this process, a data visualization tool was developed to compare International Classification of Disease 10th revision (ICD-10) codes, counts, laboratory values, and summary statistics across sites (R Shiny App, R Foundation for Statistical Computing, Vienna, Austria), which is publicly available (https://avillachlab.shinyapps.io/a1l1non3/). Two sites applied obfuscation thresholds for small counts to minimize disclosure risks related to small numbers of patients (Supplemental Table S1). When count values were obfuscated, a value of 1 was adopted.
Data included age, sex, clinical characteristics, laboratory data, and patient-level outcomes during the hospitalization of interest. Clinical characteristics were based on EHR-extracted ICD-10 codes. A detailed list of clinical characteristics based on ICD-10 codes is reported in the Supplemental Methods. Of note, one centre did not pass the quality check control for ICD-10 code-based data; therefore, ICD-10 code-based data of this centre were excluded from the analysis. Laboratory data at admission (day 0–1) and the worst value during hospitalization were extracted from the EHR. Definitions of worst value for each laboratory data is reported in Supplemental Table S8. Patient-level outcomes — used as a measure of severity — included ICU admission, oxygen supplementation or mechanical ventilation (MV), diuretic therapy, anticoagulation therapy, vasoactive/inotropic support, use of sedation/muscle-relaxants, cannulation to extracorporeal membrane oxygenation (ECMO), cardiac arrest, length of hospitalization, and in-hospital mortality. A composite adverse cardiovascular outcome measure was also investigated, defined as presence of at least one among ventricular dysfunction, heart failure or cardiogenic shock, inotropic/vasoactive drugs, coronary aneurysm, major arrhythmias, cardiac arrest, or veno-arterial ECMO. Definitions of outcomes based on ICD-10 codes or EHR-mapped elements is reported in the Supplemental Methods.
Definition of SARS-CoV-2 variant eras
The Global Initiative on Sharing All Influenza Data (GISAID) — an initiative developed for collecting epidemiologic data on influenza viruses that expanded its expertise on SARS-CoV-2 data during the pandemic — was used to identify SARS-CoV-2 variant eras based on variant predominance by country.15 For European sites, all cases hospitalised up to April 30, 2021 were assigned to Alpha, up to December 31, 2021 to Delta, and up to the end of the study, May 31, 2022, to Omicron. For U.S. sites, all cases hospitalised up to June 30, 2021, were assigned to Alpha, up to December 31, 2021, to Delta, and up to May 31, 2022, to Omicron. Since MIS-C may have delayed onset compared to SARS-CoV-2 infection.1,16 we conducted a sensitivity analysis by shifting the cut-off dates to two weeks later.
Statistical analysis
Data were summarized as counts and percentages for categorical variables, and means and standard deviations (SDs) for continuous variables at the site level. At the federated level, for descriptive purposes, these summary data were used to compute aggregate counts and percentages for categorical variables, as well as pooled means and pooled SDs for continuous variables. To compare patients’ characteristics and outcomes across the SARS-CoV-2 variant eras, we used meta-analysis methods based on the variable type. All comparisons were made using the Alpha era as reference. For categorical variables, we computed risk differences (RDs) with 95% confidence intervals (CIs) at the site level, and subsequently pooled RDs with 95% CIs across sites. To calculate the pooled RDs and 95% CIs, we employed a meta-analysis method specifically developed for small sample sizes.17 Unlike the conventional meta-analysis procedures, this method provides valid exact inferences under a fixed-effects framework effectively utilizing all data while not relying on the large-sample approximation or arbitrary continuity corrections.17 For continuous variables, we estimated the effect sizes (ES) using the difference in means between groups. We first computed the ES and 95% CI at the site level and subsequently pooled these across sites using fixed-effects meta-analysis. The same analyses were reproduced, as a sensitivity analysis, with the era cut-off dates shifted two weeks later. All statistical analyses were performed using R statistics (version 3.6.2., R Core Team, R Foundation for Statistical Computing, Vienna, Austria).
Role of the funding source
All authors had full access to all the data, accept full responsibility of ensuring accuracy or integrity of any part of the work, approved the final version of the manuscript and agreed to submit it for publication. There was no funding source for this study.
Results
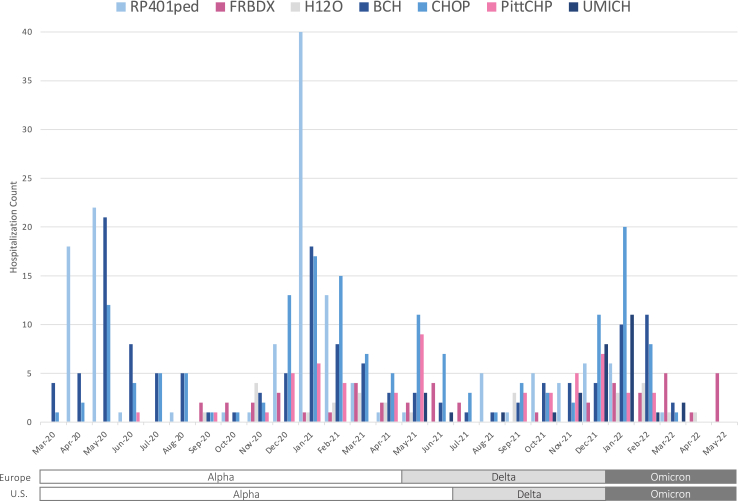
Among 598 MIS-C who were hospitalised in 7 participating sites (61% male, 39% female; pooled mean age 9.7 years [pooled SD 4.5]), 383 (64%) were admitted in the Alpha era, 111 (19%) in the Delta era, and 104 (17%) in the Omicron era. The distribution of MIS-C count over time in relation to SARS-CoV-2 variant eras is shown in Fig. 2.
Fig. 2.
Distribution of MIS-C count over time in relation to SARS-CoV-2 variant eras. Centres are showed by longitude starting from east to west, and are indicated by colours and official abbreviations as follows: RP401ped: Great Ormond Street Hospital for Children, London, U.K.; FRBDX: Bordeaux University Hospital, Bordeaux, France; H12O: Hospital Universitario 12 de Octubre, Madrid, Spain; BCH: Boston Children’s Hospital, Boston, U.S.; CHOP: The Children’s Hospital of Philadelphia, Philadelphia, U.S.; PittCHP: University of Pittsburgh Medical Center, Pittsburgh, U.S.; UMICH: University of Michigan, U.S.
Demographic and clinical characteristics
Patients’ demographic and clinical characteristics for the whole cohort and across SARS-CoV-2 eras are shown in Table 1 and Supplemental Table S3. Overall, the most common clinical manifestations were cardiovascular involvement (74%), followed by respiratory involvement (51%), and neurologic involvement (37%). Conjunctivitis was reported in 35% and a rash is 33%. A systemic inflammatory response syndrome (SIRS) was reported in 24%.
Table 1.
Demographic and clinical characteristics of patients with MIS-C according to SARS-CoV-2 variant era.
| Variable | Total, N = 436a | MIS-C during Alpha era, N = 275 | MIS-C during Delta era, N = 87 | MIS-C during Omicron era, N = 74 |
Delta compared to Alpha |
Omicron compared to Alpha |
||
|---|---|---|---|---|---|---|---|---|
| Pooled RD or ES (95% CI) | P value | Pooled RD or ES (95% CI) | P value | |||||
| Age, years, pooled mean (pooled SD) [N] | 9.7 (4.5) [N = 598] | 10.3 (4.8) [N = 383] | 9.0 (3.9) [N = 111] | 8.2 (3.8) [N = 104] | −1.183 (−2.049, −0.317) | 0.007 | −1.649 (−2.535, −0.763) | <0.001 |
| Sex, N (%) | ||||||||
| Male | 365 (61.0) | 231 (60.3) | 68 (61.3) | 66 (63.5) | 0.012 (−0.097, −0.118)b | 0.884 | 0.045 (−0.178, 0.195)b | 1.000 |
| Female | 233 (39.0), [N = 598] | 152 (39.7) [N = 383] | 43 (38.7) [N = 111] | 38 (36.5) [N = 104] | ||||
| Baseline comorbidities | ||||||||
| Overweight or obesity, N (%) | 28 (6.4) | 23 (8.4) | 2 (2.2) | 3 (4.0) | −0.065 (−0.131, 0.021) | 0.176 | −0.088 (−0.145, 0.047) | 0.229 |
| Asthma, N (%) | 36 (8.3) | 23 (8.4) | 5 (5.7) | 8 (10.8) | −0.036 (−0.120, 0.042) | 0.573 | 0.032 (−0.087, 0.146) | 1.000 |
| Generalized symptoms (other than fever) or muco-cutaneous involvement, N (%) | 310 (71.1) | 201 (73.1) | 58 (66.7) | 51 (68.9) | −0.013 (−0.213, 0.102) | 1.000 | −0.047 (−0.265, 0.156) | 1.000 |
| Fatigue, asthenia | 91 (20.9) | 62 (22.5) | 15 (17.2) | 14 (18.9) | −0.035 (−0.124, 0.113) | 0.799 | −0.000 (−0.193, 0.128) | 1.000 |
| Rash | 143 (32.8) | 95 (34.5) | 24 (27.5) | 24 (32.4) | 0.036 (−0.153, 0.166) | 0.935 | −0.002 (−0.124, 0.124) | 1.000 |
| Conjunctivitis | 155 (35.5) | 99 (36.0) | 31 (35.6) | 25 (33.8) | 0.118 (−0.072, 0.234) | 0.184 | 0.008 (−0.122, 0.166) | 1.000 |
| Mucositis | 52 (11.9) | 38 (13.8) | 7 (8.1) | 7 (9.5) | −0.008 (−0.126, 0.071) | 0.961 | −0.057 (−0.120, 0.032) | 0.174 |
| Lymphadenitis/lymphadenopathy | 72 (16.5) | 47 (17.1) | 11 (12.6) | 14 (18.9) | −0.072 (−0.210, 0.057) | 0.986 | 0.000 (−0.129, 0.120) | 1.000 |
| Gastrointestinal involvement, N (%) | 248 (56.9) | 165 (60.0) | 49 (56.3) | 34 (45.9) | 0.069 (−0.133, 0.265) | 0.647 | 0.049 (−0.341, 0.273) | 1.000 |
| Abdominal pain | 148 (33.9) | 101 (36.7) | 30 (34.5) | 17 (23.0) | 0.018 (−0.143, 0.232) | 1.000 | −0.033 (−0.256, 0.184) | 1.000 |
| Nausea or vomiting | 97 (22.2) | 65 (23.6) | 18 (20.7) | 14 (18.9) | −0.011 (−0.170, 0.161) | 1.000 | −0.015 (−0.252, 0.243) | 1.000 |
| Diarrhea, enteritis, ileitis | 101 (23.2) | 75 (27.3) | 15 (17.2) | 11 (14.9) | −0.077 (−0.194, 0.137) | 0.687 | −0.014 (−0.186, 0.110) | 1.000 |
| Appendicitis, peritonitis | 3 (0.7) | 1 (0.4) | 0 (0.0) | 2 (2.7) | −0.001 (−0.030, 0.044) | 1.000 | 0.032 (−0.018, 0.111) | 0.659 |
| Respiratory involvement, N (%) | 222 (50.9) | 162 (58.9) | 29 (33.3) | 31 (41.9) | −0.148 (−0.327, −0.043) | 0.005 | −0.145 (−0.356, 0.054) | 0.376 |
| Cough | 28 (6.4) | 24 (8.7) | 2 (2.3) | 2 (2.7) | −0.037 (−0.096, 0.036) | 0.300 | −0.073 (−0.130, 0.024) | 0.116 |
| Rhinitis/Rhinorrhea | 7 (1.6) | 4 (1.4) | 1 (1.1) | 2 (2.7) | 0.000 (−0.041, 0.067) | 1.000 | 0.015 (−0.050, 0.089) | 1.000 |
| Sore throat | 26 (6.0) | 20 (7.3) | 2 (2.3) | 4 (5.4) | −0.035 (−0.085, 0.038) | 0.513 | 0.000 (−0.093, 0.099) | 1.000 |
| Respiratory failure/dyspnea | 47 (10.8) | 30 (10.9) | 9 (10.3) | 8 (10.8) | −0.023 (−0.097, 0.067) | 0.909 | −0.035 (−0.121, 0.054) | 0.924 |
| Pleural effusion | 81 (18.6) | 59 (21.4) | 11 (12.6) | 11 (14.9) | −0.040 (−0.157, 0.056) | 1.000 | −0.021 (−0.181, 0.109) | 1.000 |
| Pulmonary edema | 26 (6.0) | 17 (6.2) | 3 (3.4) | 6 (8.1) | −0.001 (−0.052, 0.064) | 1.000 | 0.051 (−0.030, 0.155) | 0.258 |
| Pneumonia | 55 (12.6) | 52 (18.9) | 1 (1.1) | 2 (2.7) | −0.129 (−0.258, −0.036) | 0.005 | −0.145 (−0.295, 0.033) | 0.223 |
| ARDS | 9 (2.1) | 6 (2.2) | 1 (1.1) | 2 (2.7) | 0.002 (−0.043, 0.060) | 1.000 | −0.025 (−0.066, 0.045) | 0.496 |
| Cardiovascular involvement, N (%) | 321 (73.6) | 223 (81.1) | 53 (60.9) | 45 (60.8) | −0.043 (−0.332, 0.075) | 0.825 | −0.020 (−0.415, 0.130) | 1.000 |
| Chest pain | 18 (4.1) | 15 (5.4) | 1 (1.1) | 2 (2.7) | −0.020 (−0.072, 0.031) | 0.511 | −0.027 (−0.095, 0.051) | 0.591 |
| Hypotension | 174 (39.9) | 117 (42.5) | 31 (35.6) | 26 (35.1) | −0.029 (−0.146, 0.224) | 1.000 | 0.003 (−0.160, 0.401) | 1.000 |
| Pre-syncope, syncope | 54 (12.4) | 36 (13.1) | 13 (14.9) | 5 (6.7) | 0.068 (−0.012, 0.156) | 0.234 | −0.010 (−0.096, 0.080) | 0.898 |
| Arrhythmias | 97 (22.2) | 69 (25.1) | 14 (16.1) | 14 (18.9) | −0.003 (−0.075, 0.076) | 1.000 | −0.032 (−0.119, 0.064) | 0.645 |
| Myocarditis | 58 (13.3) | 41 (14.9) | 7 (8.0) | 10 (13.5) | −0.075 (−0.245, 0.024) | 0.166 | −0.059 (−0.298, 0.063) | 0.585 |
| Pericarditis/pericardial effusion | 107 (24.5) | 74 (26.9) | 19 (21.8) | 14 (18.9) | 0.036 (−0.088, 0.138) | 0.898 | −0.014 (−0.146, 0.123) | 1.000 |
| Left ventricular dysfunction | 60 (13.8) | 43 (15.6) | 6 (6.9) | 11 (14.9) | −0.032 (−0.084, 0.031) | 0.318 | −0.012 (−0.103, 0.117) | 1.000 |
| Heart failure | 56 (12.8) | 41 (14.9) | 10 (11.5) | 5 (6.8) | −0.022 (−0.084, 0.089) | 1.000 | 0.067 (−0.178, 0.043) | 0.553 |
| Cardiogenic shock | 84 (19.3) | 52 (18.9) | 16 (18.4) | 16 (21.6) | −0.076 (−0.169, 0.103) | 0.652 | 0.017 (−0.201, 0.192) | 1.000 |
| Shock (non-cardiogenic)/SIRS, N (%) | 187 (42.9) | 146 (53.1) | 16 (18.4) | 23 (31.1) | −0.348 (−0.645, −0.067) | 0.006 | −0.101 (−0.288, 0.041) | 0.207 |
| Septic shock | 45 (10.3) | 36 (13.1) | 5 (5.7) | 4 (5.4) | −0.107 (−0.175, 0.021) | 0.106 | −0.011 (−0.110, 0.057) | 1.000 |
| Hypovolemic shock | 8 (1.83) | 4 (1.4) | 2 (2.3) | 2 (2.7) | −0.004 (−0.037, 0.060) | 1.000 | 0.047 (−0.029, 0.129) | 0.266 |
| Shock, others (non-cardiogenic) | 90 (20.6) | 63 (22.9) | 14 (16.1) | 13 (17.6) | −0.135 (−0.281, 0.064) | 0.342 | −0.001 (−0.179, 0.175) | 1.000 |
| SIRS | 104 (23.8) | 93 (33.8) | 3 (3.4) | 8 (10.8) | −0.278 (−0.463, −0.024) | 0.010 | −0.181 (−0.304, −0.050) | 0.004 |
| Neurologic involvement, N (%) | 163 (37.4) | 130 (47.3) | 19 (21.8) | 14 (18.9) | −0.130 (−0.297, 0.026) | 0.317 | −0.048 (−0.371, 0.104) | 0.958 |
| Headache | 54 (12.4) | 43 (15.6) | 4 (4.6) | 7 (9.5) | −0.088 (−0.168, 0.002) | 0.059 | 0.002 (−0.188, 0.117) | 1.000 |
| Disorientation/Confusion | 27 (6.2) | 23 (8.4) | 2 (2.3) | 2 (2.7) | −0.044 (0.120, 0.044) | 0.437 | −0.047 (−0.106, 0.044) | 0.317 |
| Seizures | 9 (2.1) | 6 (2.2) | 2 (2.3) | 1 (1.3) | 0.013 (−0.037, 0.065) | 0.948 | −0.021 (−0.063, 0.042) | 0.509 |
| Muscle weakness/myalgia/myositis | 47 (10.8) | 35 (12.7) | 9 (10.3) | 3 (4.0) | 0.031 (−0.070, 0.134) | 0.935 | 0.021 (−0.146, 0.095) | 1.000 |
| Encephalopathy/meningoencephalitis | 9 (2.1) | 6 (2.2) | 2 (2.3) | 1 (1.3) | −0.047 (−0.186, 0.071) | 0.841 | −0.003 (−0.167, 0.048) | 1.000 |
| Stroke | 3 (0.7) | 2 (0.7) | 0 (0) | 1 (1.3) | 0.000 (−0.032, 0.047) | 1.000 | 0.017 (−0.039, 0.086) | 0.749 |
| Kidney dysfunction, N (%) | 92 (21.1) | 66 (24.0) | 14 (16.1) | 12 (16.2) | −0.048 (−0.233, 0.058) | 0.540 | 0.000 (−0.273, 0.161) | 1.000 |
| Liver dysfunction, N (%) | 50 (11.5) | 32 (11.6) | 7 (8.0) | 11 (14.9) | −0.013 (−0.297, 0.026) | 0.460 | 0.045 (−0.011, 0.217) | 0.950 |
A detailed definition of the variables based on EHR data or ICD-10 codes is reported as Supplementary Material.
Aggregate counts and summary statistics for the total sample and the MIS-C era subgroups were calculated for descriptive purposes only. Meta-analyses were computed by pooling risk differences (RD, categorical variables) or effect sizes (ES, continuous variables) and their 95% confidence intervals (CIs) previously calculated at site-level. A detailed definition of the variables based on EHR data or ICD-10 codes is reported as Supplementary Material.
ES: effect size; MIS-C: multisystem inflammatory syndrome; RD: risk difference; SD: standard deviation; SIRS: systemic inflammatory response syndrome.
ICD-10-code-based data from one centre, which did not pass the quality check, were excluded from analysis, thereby reducing sample size.
Male as reference category.
Compared with patients admitted during the Alpha era, those admitted during the Delta era were younger (pooled ES −1.18 years [95% CI −2.05, −0.32]), had fewer respiratory symptoms (pooled RD −0.15 [95% CI −0.33, −0.04]), particularly pneumonia (pooled RD −0.13 [95% CI −0.26, −0.04]), and less frequently presented with non-cardiogenic shock or SIRS (pooled RD −0.35 [95% CI −0.64, −0.07]), particularly SIRS (pooled RD −0.28 [95% CI −0.46, −0.02]). Compared with patients admitted during the Alpha era, patients admitted during the Omicron era were also younger (pooled ES −1.6 years [95% CI −2.5, −0.8]) and less frequently presented with SIRS (pooled RD −0.18 [95% CI −0.30, −0.05]).
Laboratory data
Laboratory characteristics for the whole cohort and across SARS-CoV-2 eras are shown in Fig. 3, Fig. 4, as well as Supplemental Tables S3 and S4. Overall, complete blood count abnormalities included abnormal white blood count (29%), lymphopenia (10%), anaemia (27%), and thrombocytopenia (22%). Coagulation abnormalities were seen in 25% and electrolyte abnormalities in 34%, including hyponatremia in 22%. Kidney dysfunction occurred in 21% and liver dysfunction in 11%.
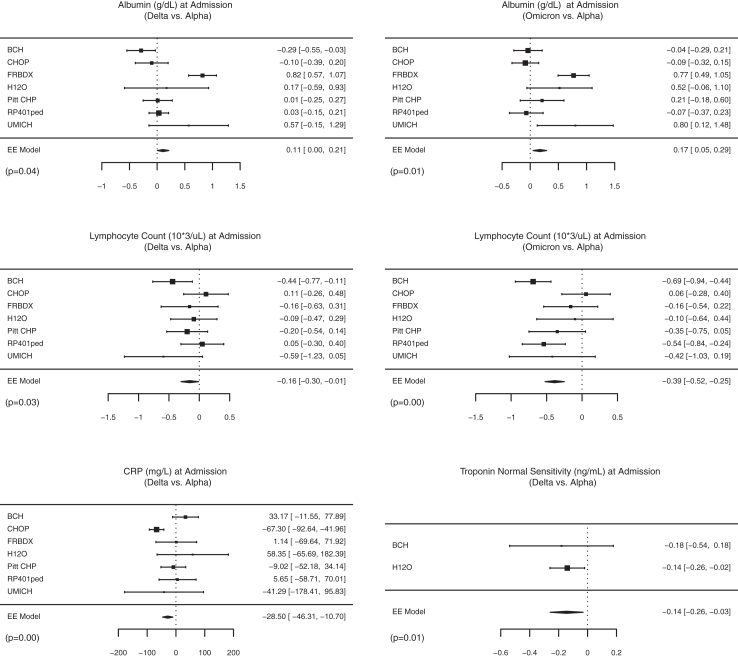
Fig. 3.
Significant differences in laboratory characteristics at admission according to SARS-CoV-2 variant eras by forest plots. Effect sizes and 95% confidence intervals are showed for each site using squared points and bars, respectively. Diamonds represent the pooled effect size and 95% confidence intervals. CRP: C-reactive protein; EE: exact effect.
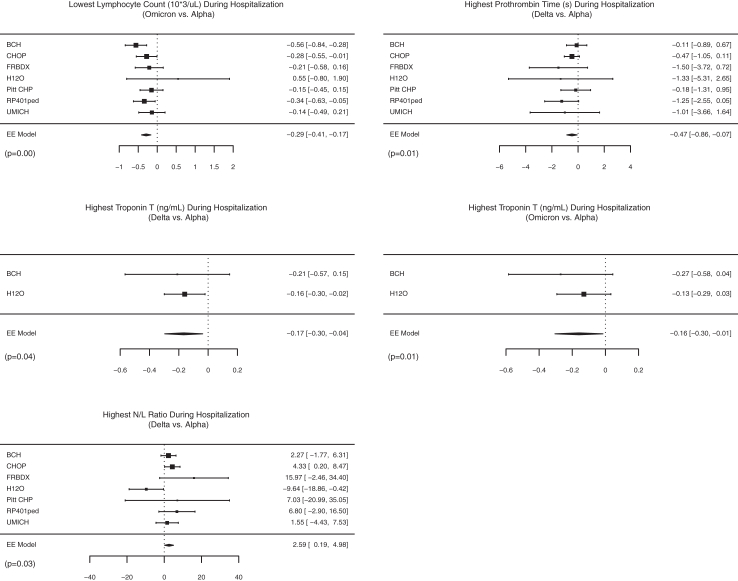
Fig. 4.
Significant differences in laboratory characteristics during hospitalization according to SARS-CoV-2 variant eras by forest plots. Effect sizes and 95% confidence intervals are showed for each site using squared points and bars, respectively. Diamonds represent the pooled effect size and 95% confidence intervals. EE: exact effect; N/L: neutrophile/lymphocyte ratio.
Compared with patients admitted during the Alpha era, patients admitted during the Delta era had significantly lower absolute lymphocyte count (pooled ES –0.16 × 109/uL [95% CI −0.30, −0.01]), C-reactive protein (CRP, pooled ES −28.5 mg/L [95% CI −46.3, −10.7]), and normal-sensitivity troponin-T (pooled ES −0.14 ng/mL [95% CI −0.26, −0.03]), as well as higher albumin at admission (pooled ES 0.1 g/dL [95% CI 0.01, −0.2]). The highest troponin-T and highest prothrombin time during hospitalization were also significantly lower in Delta patients (pooled ES −0.17 ng/mL [95% CI −0.30, −0.04] and pooled ES −0.5 s [95% CI −0.8, −0.1]). Compared with patients admitted during the Alpha era, those admitted during the Omicron era had lower lymphocyte counts (pooled ES −0.39 × 109/uL [95% CI −0.52, −0.25]) and higher albumin at admission (pooled ES 0.2 g/dL [95% CI 0.1, 0.3]). During hospitalization, patients admitted during the Omicron era also had lower lymphocyte count (pooled ES –0.29 × 109/uL [95% CI −0.41, −0.17]), higher neutrophil/lymphocyte ratio (pooled ES 2.5 [95% CI 0.2, 5.0]), and lower normal-sensitivity troponin-T (pooled ES −0.16 ng/mL [95% CI −0.30, −0.01]).
Patient-level outcomes
Patient-level outcomes are shown in Table 2. Half of the cohort (51%) was admitted to the ICU. Diuretics were administered in 24% of patients, and anticoagulation therapy in 53%. Vasoactive/inotropic support was initiated in 12%, and 8% received supplemental oxygen or MV in 8%. Cardiac arrest occurred in three patients (1%), and two patients (1%) required ECMO. Coronary aneurysms were observed in 10% and coronary artery thrombosis in 1%. Forty-four percent had the composite adverse cardiovascular outcome. The pooled mean length of hospitalization was 7.3 days (pooled SD 6.8). No patients died during the study period.
Table 2.
Patient-level outcomes in patients with MIS-C according to SARS-CoV-2 era.
| Variable | Total, N = 436a | MIS-C during Alpha era, N = 275 | MIS-C during Delta era, N = 87 | MIS-C during Omicron era, N = 74 |
Delta compared to Alpha |
Omicron compared to Alpha |
||
|---|---|---|---|---|---|---|---|---|
| Pooled RD or ES (95% CI) | P value | Pooled RD or ES (95% CI) | P value | |||||
| ICU admission, N (%) | 222 (50.9) | 151 (54.9) | 40 (45.98) | 31 (41.89) | −0.028 (−0.119, 0.042) | 0.456 | 0.026 (−0.186, 0.139) | 1.000 |
| Diuretic therapy, N (%) | 107 (24.5) | 70 (25.5) | 17 (19.5) | 20 (27.0) | −0.051 (−0.142, 0.023) | 0.201 | 0.083 (0.048, 0.232) | 0.454 |
| Anticoagulation therapy, N (%) | 233 (53.4) | 175 (63.6) | 38 (43.7) | 20 (27.0) | 0.013 (−0.208, 0.085) | 1.000 | −0.190 (−0.370, −0.037) | 0.008 |
| Sedation or muscle relaxant, N (%) | 111 (25.5) | 87 (31.6) | 16 (18.4) | 8 (10.1) | −0.021 (−0.110, 0.045) | 0.524 | −0.025 (−0.257, 0.053) | 0.588 |
| Vasoactive/inotropic support, N (%) | 53 (12.2) | 33 (12.0) | 11 (12.6) | 9 (12.2) | −0.008 (−0.058, 0.059 | 1.000 | 0.000 (−0.076, 0.105) | 1.000 |
| O2 supplementation or MV, N (%) | 33 (7.6) | 22 (8.0) | 6 (6.9) | 5 (6.8) | 0.002 (−0.060, 0.065) | 1.000 | −0.048 (−0.149, 0.057) | 0.632 |
| Cardiac arrest, N (%) | 3 (0.7) | 3 (1.1) | 0 (0.0) | 0 (0.0) | −0.007 (−0.038, 0.041) | 0.858 | −0.007 (−0.046, 0.055) | 0.964 |
| ECMO, N (%) | 2 (0.5) | 2 (0.7) | 0 (0.0) | 0 (0.0) | 0.000 (−0.034, 0.046) | 1.000 | −0.004 (−0.042, 0.058) | 1.000 |
| Coronary aneurysm, N (%) | 42 (9.6) | 27 (9.8) | 6 (6.9) | 9 (12.2) | −0.030 (−0.080, 0.057) | 0.612 | 0.069 (−0.031, 0.193) | 0.338 |
| Coronary artery thrombosis or myocardial infarct, N (%) | 3 (0.7) | 3 (1.1) | 0 (0.0) | 0 (0.0) | −0.009 (−0.037, 0.042) | 0.825 | −0.009 (−0.046, 0.056) | 0.864 |
| Composite adverse cardiovascular outcomeb, N (%) | 193 (44.3) | 130 (47.3) | 33 (38.6) | 30 (40.5) | −0.079 (−0.215, 0.062) | 0.321 | −0.013 (−0.257, 0.147) | 1.000 |
| Length of hospitalization, days, pooled mean (pooled SD) [N] | 7.7 (6.6) [598] | 8.1 (7.5) [383] | 7.1 (4.4) [111] | 7.1 (4.7) [104] | −1.345 (−2.287, −0.403) | 0.005 | −0.988 (−1.992, 0.016) | 0.054 |
| Mortality, N (%) | 0 (0.0) | 0 (0.0) | 0 (0.0) | 0 (0.0) | – | – | – | – |
A detailed definition of the variables based on EHR data or ICD-10 codes is reported as Supplementary Material.
Aggregate counts and summary statistics for the total sample and the MIS-C era subgroups were calculated for descriptive purposes only. Meta-analyses were computed by pooling risk differences (RD, categorical variables) or effect sizes (ES, continuous variables) and their 95% confidence intervals (CIs) previously calculated at site-level.
ECMO: Extracorporeal Membrane Oxygenation; ES: effect size; ICU: intensive care unit; MIS-C: multisystem inflammatory syndrome; MV: mechanical ventilation; O2: oxygen; RD: risk difference; SD: standard deviation.
ICD-10-code-based data from one centre, which did not pass the quality check, were excluded from analysis, thereby reducing sample size.
Composite cardiovascular outcome: ventricular dysfunction, heart failure or cardiogenic shock (ICD-10 codes), inotropic/vasoactive drugs (EHR data), coronary aneurysm (ICD-10 codes), major arrhythmias (ICD-10 codes) cardiac arrest (EHR data and ICD-codes), VA-ECMO (EHR data).
Compared with patients admitted during the Alpha era, those admitted during the Omicron era less frequently received anticoagulation therapy (pooled RD −0.17 [95% CI −0.34, −0.03]). Length of hospitalization was shorter in patients admitted during the Delta versus Alpha eras (−1.3 days [95% CI −2.3, −0.4]). There were no other significant differences in patient-level outcome across eras.
Sensitivity analyses
Sensitivity analyses confirmed the above-mentioned statistically significant differences, with the addition that prothrombin time at admission was lower in Delta versus Alpha (pooled ES −0.4 s [95% CI −0.8, −0.01]), and white blood count and D-dimer were lower in Omicron versus Alpha (pooled ES −1.79 × 109/uL [95% CI −3.49, −0.09] pooled ES −884 ng/mL [95% CI −1747, −21]) (Supplemental Tables S5–S7).
Discussion
With evolution of SARS-CoV-2 variants, therapeutic approaches, and population immunity, better understanding of the changing manifestations of COVID-19-associated diseases may improve clinical risk-stratification. This EHR-based study, which brings together data from a large cohort of patients with MIS-C from four countries across two continents, showed that MIS-C clinical and laboratory characteristics vary according to SARS-CoV-2 variant eras. We found that patients admitted during the Delta and Omicron eras were younger and less sick than those admitted in the Alpha era. Specifically, patients admitted during the Alpha era versus subsequent variant eras had more respiratory involvement, shock, and SIRS; higher CRP, absolute lymphocyte count, and troponin levels; lower albumin; and longer hospitalization.
Our retrospective multicentre study, using data extracted from the EHR from sites in the U.S. and Europe, adds to the evidence that COVID-19 phenotypes differ across SARS-CoV-2 eras.4,18, 19, 20 One recent study showed that paediatric patients with acute COVID-19 and laboratory-proven Delta or Omicron variants were more likely to have fever and respiratory symptoms than patients affected by other strains.20 A large multicentre study in children with acute COVID-19 showed that severe illness was significantly less common during the Omicron era versus the Alpha and Delta eras.4 This study also showed that MIS-C occurred more frequently during the Alpha era,4 consistent with other reports.7,21,22 In this context, recent studies have focused on investigating if MIS-C phenotypes may vary as well, based on SARS-CoV-2 variants.6, 7, 8,10, 11, 12, 13 Compared with EHR studies that selected patients with COVID-19-related conditions using diagnostic codes as the inclusion criteria, our study design had the advantage of patient selection using lists of patients with MIS-C validated by MIS-C experts at the site level.
Consistent with early reports,8 we showed that patients with MIS-C who were admitted during Delta and Omicron eras were younger than those admitted in the Alpha era. This may be related to differences in variant pathogenicity with predilection for younger patients for the recent variants, differences in the host immune response to the variant, and vaccination status.23,24 Particularly in the U.S. at the time of Delta and Omicron eras, older children may have received SARS-CoV-2 vaccination, which has been proven to protect against MIS-C.25,26 Data from Israel first showed that receiving even one dose of vaccine was associated with a lower risk of MIS-C.25 A large multicentre case-control study subsequently showed that patients with MIS-C were less likely to have been fully vaccinated compared with hospitalised controls.26 Another recent study showed that all but one MIS-C patient during the Delta period were found to be unvaccinated, with an estimated vaccine effectiveness of 94% (95% CI 55–99%). Interestingly, data from South Africa, which included all unvaccinated individuals, showed no difference in age among MIS-C groups based on variant.9 Further studies will likely be needed to clarify this aspect.
Analysis of clinical characteristics showed that patients admitted during the Delta era less frequently had respiratory involvement, in particular pneumonia. Whereas smaller studies showed inconsistent data on respiratory symptoms,9, 10, 11, 12 our findings are similar to those reported by a large CDC-based study6 and a large recent North American report.8 In our study, patients admitted during the Delta or Omicron eras were also less likely to present with non-cardiogenic shock or SIRS; interestingly, the prevalence of cardiogenic shock did not change across eras. This represents novel results compared to previous analyses, which pooled all types of shock together.6,8,10 Changes in cardiorespiratory symptoms over time may be due to different host immune responses to different strains, with milder inflammation response to more recent variants,23,24 potentially modulated by the vaccination status,23,24 or may be a consequence of earlier and more effective treatment approaches in the more recent era, such as an increased use of steroids.8
In terms of illness severity and outcomes, previous studies showed less frequent ICU admission and shorter hospital length of stay among patients admitted in the post-Alpha eras.6,7 Our study was consistent in showing a shorter length of hospitalization for patients admitted in the post-Alpha eras, but did not find any significant difference in the rates of ICU admission. This may be due to differences in institutional practices on the criteria for ICU admission, or to changes in clinical practice following the first era. Cardiovascular outcomes, when analysed as dichotomous measures of the presence or absence of ventricular dysfunction, major arrhythmias, coronary aneurysm, cardiac arrest, ECMO, or as a composite measure, did not significantly differ across eras. However, we showed that patients admitted during the Delta or Omicron eras had significantly lower troponin levels compared with those admitted during the Alpha era. This finding is consistent with results of a smaller study from Israel7 and a larger CDC data-based study,6 suggesting that cardiovascular outcomes may be milder in post-Alpha eras.
The reason why patients admitted during Alpha were more frequently treated with anticoagulants is unclear. It may be related to less severe cardiovascular outcomes in post-Alpha eras, and to lower D-dimer levels as suggested by the sensitivity analyses. In addition, changes in thromboprophylaxis over time may have been affected by refinement of criteria for the use of anticoagulants versus acetylsalicylic acid (aspirin) in national and international guidelines.27,28 The possibility of reduced practice variation due to publication of guidelines is supported by early reports showing wide variability in use of anticoagulation or antiplatelet therapy, with some of the centres administering anticoagulation to most of the patients.1,16,29,30
Our study also showed how EHR data may be leveraged effectively to answer clinical questions without the need for sharing protected health information and while considering centre-level variability. Laboratory test results and the other EHR-based variables have the advantage of being directly extracted without any human involvement, limiting any typo-errors, and enabling all results to be considered for our analysis. This collaborative multicentre infrastructure may be used to identify trends in epidemiology, disease phenotype, and treatment of diverse diseases requiring hospitalization. Once algorithms have been created and validated across sites, they may be deployed rapidly and serve as an effective easily-available surveillance tool to inform clinical care and public health policy.
Our study has limitations. Its retrospective design carries risks of missing data and reporting bias. Although the EHR-based automatic data extraction might have helped in decreasing the amount of missing data, we could not control for data not entered in the EHR and for reporting bias such as more severe symptoms possibly being entered more frequently than those less severe. Additionally, our study used the 4CE infrastructure, developed for COVID-19 research, which did not include specific data on MIS-C including treatments such as intravenous immunoglobulin or corticosteroids. Moreover, it was not possible to reliably extract vaccination status from the EHR, since ICD-10 codes were available only for the last part of the study period. Coding practices likely vary at each site; however, to avoid missing codes (e.g., European-specific ICD-10 codes), we performed an extensive quality check by manually reviewing all ICD-10 codes attributed to patients with MIS-C at each site, and–to partially control for a site-effect–we first calculated the risk difference within institution and only as a second step we computed a meta-analysis across sites. Microbiology data on variants were not available; thus, SARS-CoV-2 eras were defined based on epidemiological assumptions; however, this approach has been widely used by previous studies,6, 7, 8, 9, 10, 11, 12 and sensitivity analyses were performed to improve the reliability of our inferences. Moreover, although we included international data across two continents, representativeness of and within countries is limited and generalizability to other geographic areas will need to be confirmed. An additional limitation was the use of different criteria for MIS-C based on the nation of the institution. Finally, the meta-analysis method we employed to account for small sample sizes might have been overly conservative, as stated by the authors17; however, we preferred a conservative approach to avoid overstatements. Given the small size of the subgroups, further stratification analyses—e.g., based on age-subgroups—were not possible.
Despite these limitations, we believe our study using real-world data adds valuable information in characterizing different MIS-C phenotypes across SARS-CoV-2 eras and continents. MIS-C clinical and laboratory characteristics vary according to SARS-CoV-2 variant eras, with patients in the Delta and Omicron eras being younger and having a less severe presentation than those in the Alpha era as indicated by clinic manifestations, laboratory data, and patient-level-outcome. EHR-based aggregate data may be effectively leveraged to investigate rare complications of diseases in the setting of the pandemic and used to identify patterns of disease variation or severity over time.
Contributors
FS, AGS, SM, TC, FTB, GSO, KM, JWN, and PA contributed to the design and conceptualization of the study. FS, AGS, SM, DAH, CLB, NGB, TGG, RI, KFK, SEM, KDM, TMA, CMR, MM, KM, MPJ, MAS, AS, DMT, GV, SV, ZX, and PA contributed to data collection and verified the underlying data reported in the manuscript. FS, AGS, SM, XL, VNR, TC, FTB, GSO, CS, EB, AD, ME, KFK, JLG, SEM, TMA, MM, MAS, AMS, DMT, XW, JMZ, and PA contributed to data analysis or interpretation. SEM supplied grant funding for the work. All authors contributed to drafting the work or revising it critically for important intellectual content and approved the final version. All authors had full access to all the data, accept full responsibility of ensuring accuracy or integrity of any part of the work, approved the final version of the manuscript and agreed to submit it for publication.
Data sharing statement
Summary data according to site and SARS-CoV-2 era are publicly available through the study visualization tool (https://avillachlab.shinyapps.io/a1l1non3/). The analytic code is publicly available on GitHub (https://github.com/covidclinical/PhaseX.2SqlDataExtraction/tree/main/Extras, https://github.com/covidclinical/Phase2.2MISCRPackage).
Declaration of interests
The authors have no conflicts of interests to declare related to the content of this manuscript. JNW has research grant funding from National Heart, Lung, and Blood Institute (NHLBI), the Department of Defense, the Centres for Disease Control (CDC), and Pfizer; has been a consultant for Pfizer; chaired the Independent Events Adjudication Committees for Novartis, Pfizer, and Bristol-Myer-Squibb; and received honoraria from Daiichi Sankyo for service on the Steering Committee of the ENNOBLE-ATE Trial and from UpToDate. GSO has research grant funding from the National Institute of Environmental Health Sciences (NIEHS), National Institutes of Health (NIH), and from the National Cancer Institute (NCI). DAH has research grant funding from the National Center for Advancing Translational Sciences (NCATS). TGG has research grant funding from the Institute of Health Carlos III, the European Regional Development Fund (ERDF), the National Institute of Environmental Health Sciences (NIEHS), National Institutes of Health (NIH), and National Cancer Institute (NCI). KFK has research grant funding from the National Institute of Child Health and Human Development (NIHCD). SEM has research grant funding from the National Center for Advancing Translational Sciences (NCATS). AD has research grant funding from Pfizer. DMT has research grant funding from NIH. AMS has research grant funding from the National Heart, Lung, and Blood Institute (NHLBI) and from the National Center for Advancing Translational Sciences (NCATS). GV has internal research funding from the Centre Hospitalier Universitaire de Bordeaux. ZX has research grant funding from the National Institute of Neurological Disorders and Stroke (NINDS). None of these funding sources had any role in supporting the study design; in the collection, analysis, and interpretation of data; in the writing of the report; or in the decision to submit the paper for publication. All the other authors have no conflicts of interests to declare.
Footnotes
Supplementary data related to this article can be found at https://doi.org/10.1016/j.eclinm.2023.102212.
Contributor Information
Francesca Sperotto, Email: francesca.sperotto@childrens.harvard.edu.
Paul Avillach, Email: paul_avillach@hms.harvard.edu.
The Consortium for Clinical Characterization of COVID-19 by EHR (4CE):
James R. Aaron, Atif Adam, Giuseppe Agapito, Adem Albayrak, Giuseppe Albi, Mario Alessiani, Anna Alloni, Danilo F. Amendola, François Angoulvant, Li LLJ. Anthony, Bruce J. Aronow, Fatima Ashraf, Andrew Atz, Paul Avillach, Vidul Ayakulangara Panickan, Paula S. Azevedo, Rafael Badenes, James Balshi, Ashley Batugo, Brendin R. Beaulieu-Jones, Brett K. Beaulieu-Jones, Douglas S. Bell, Antonio Bellasi, Riccardo Bellazzi, Vincent Benoit, Michele Beraghi, José Luis Bernal-Sobrino, Mélodie Bernaux, Romain Bey, Surbhi Bhatnagar, Alvar Blanco-Martínez, Martin Boeker, Clara-Lea Bonzel, John Booth, Silvano Bosari, Florence T. Bourgeois, Robert L. Bradford, Gabriel A. Brat, Stéphane Bréant, Nicholas W. Brown, Raffaele Bruno, William A. Bryant, Mauro Bucalo, Emily Bucholz, Anita Burgun, Tianxi Cai, Mario Cannataro, Aldo Carmona, Anna Maria Cattelan, Charlotte Caucheteux, Julien Champ, Jin Chen, Krista Y. Chen, Luca Chiovato, Lorenzo Chiudinelli, Kelly Cho, James J. Cimino, Tiago K. Colicchio, Sylvie Cormont, Sébastien Cossin, Jean B. Craig, Juan Luis Cruz-Bermúdez, Jaime Cruz-Rojo, Arianna Dagliati, Mohamad Daniar, Christel Daniel, Priyam Das, Batsal Devkota, Audrey Dionne, Rui Duan, Julien Dubiel, Scott L. DuVall, Loic Esteve, Hossein Estiri, Shirley Fan, Robert W. Follett, Thomas Ganslandt, Noelia García-Barrio, Lana X. Garmire, Nils Gehlenborg, Emily J. Getzen, Alon Geva, Rachel SJ. Goh, Tomás González González, Tobias Gradinger, Alexandre Gramfort, Romain Griffier, Nicolas Griffon, Olivier Grisel, Alba Gutiérrez-Sacristán, Pietro H. Guzzi, Larry Han, David A. Hanauer, Christian Haverkamp, Derek Y. Hazard, Bing He, Darren W. Henderson, Martin Hilka, Yuk-Lam Ho, John H. Holmes, Jacqueline P. Honerlaw, Chuan Hong, Kenneth M. Huling, Meghan R. Hutch, Richard W. Issitt, Anne Sophie Jannot, Vianney Jouhet, Mundeep K. Kainth, Kernan F. Kate, Ramakanth Kavuluru, Mark S. Keller, Chris J. Kennedy, Kate F. Kernan, Daniel A. Key, Katie Kirchoff, Jeffrey G. Klann, Isaac S. Kohane, Ian D. Krantz, Detlef Kraska, Ashok K. Krishnamurthy, Sehi L'Yi, Judith Leblanc, Guillaume Lemaitre, Leslie Lenert, Damien Leprovost, Molei Liu, Ne Hooi Will Loh, Qi Long, Sara Lozano-Zahonero, Yuan Luo, Kristine E. Lynch, Sadiqa Mahmood, Sarah E. Maidlow, Adeline Makoudjou, Simran Makwana, Alberto Malovini, Kenneth D. Mandl, Chengsheng Mao, Anupama Maram, Monika Maripuri, Patricia Martel, Marcelo R. Martins, Jayson S. Marwaha, Aaron J. Masino, Maria Mazzitelli, Diego R. Mazzotti, Arthur Mensch, Marianna Milano, Marcos F. Minicucci, Bertrand Moal, Taha Mohseni Ahooyi, Jason H. Moore, Cinta Moraleda, Jeffrey S. Morris, Michele Morris, Karyn L. Moshal, Sajad Mousavi, Danielle L. Mowery, Douglas A. Murad, Shawn N. Murphy, Thomas P. Naughton, Carlos Tadeu Breda Neto, Antoine Neuraz, Jane Newburger, Kee Yuan Ngiam, Wanjiku FM. Njoroge, James B. Norman, Jihad Obeid, Marina P. Okoshi, Karen L. Olson, Gilbert S. Omenn, Nina Orlova, Brian D. Ostasiewski, Nathan P. Palmer, Nicolas Paris, Lav P. Patel, Miguel Pedrera-Jiménez, Ashley C. Pfaff, Emily R. Pfaff, Danielle Pillion, Sara Pizzimenti, Tanu Priya, Hans U. Prokosch, Robson A. Prudente, Andrea Prunotto, Víctor Quirós-González, Rachel B. Ramoni, Maryna Raskin, Siegbert Rieg, Gustavo Roig-Domínguez, Pablo Rojo, Nekane Romero-Garcia, Paula Rubio-Mayo, Paolo Sacchi, Carlos Sáez, Elisa Salamanca, Malarkodi Jebathilagam Samayamuthu, L. Nelson Sanchez-Pinto, Arnaud Sandrin, Nandhini Santhanam, Janaina C.C. Santos, Fernando J. Sanz Vidorreta, Maria Savino, Emily R. Schriver, Petra Schubert, Juergen Schuettler, Luigia Scudeller, Neil J. Sebire, Pablo Serrano-Balazote, Patricia Serre, Arnaud Serret-Larmande, Mohsin A. Shah, Zahra Shakeri Hossein Abad, Domenick Silvio, Piotr Sliz, Jiyeon Son, Charles Sonday, Andrew M. South, Francesca Sperotto, Anastasia Spiridou, Zachary H. Strasser, Amelia LM. Tan, Bryce W.Q. Tan, Byorn W.L. Tan, Suzana E. Tanni, Deanne M. Taylor, Ana I. Terriza-Torres, Valentina Tibollo, Patric Tippmann, Emma MS. Toh, Carlo Torti, Enrico M. Trecarichi, Andrew K. Vallejos, Gael Varoquaux, Margaret E. Vella, Guillaume Verdy, Jill-Jênn Vie, Shyam Visweswaran, Michele Vitacca, Kavishwar B. Wagholikar, Lemuel R. Waitman, Xuan Wang, Demian Wassermann, Griffin M. Weber, Martin Wolkewitz, Scott Wong, Zongqi Xia, Xin Xiong, Ye Ye, Nadir Yehya, William Yuan, Joany M. Zachariasse, Janet J. Zahner, Alberto Zambelli, Harrison G. Zhang, Daniela Zöller, Valentina Zuccaro, and Chiara Zucco
Appendix A. Supplementary data
References
- 1.Feldstein L.R., Rose E.B., Horwitz S.M., et al. Multisystem inflammatory syndrome in U.S. Children and Adolescents. N Engl J Med. 2020;383(4):334–346. doi: 10.1056/NEJMoa2021680. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Son M.B.F., Murray N., Friedman K., et al. Multisystem inflammatory syndrome in children — initial therapy and outcomes. N Engl J Med. 2021;385(1):23–34. doi: 10.1056/nejmoa2102605. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Lambrou A.S., Shirk P., Steele M.K., et al. Morbidity and mortality weekly report genomic surveillance for SARS-CoV-2 variants: predominance of the delta (B.1.617.2) and Omicron (B.1.1.529) variants-United States. Morb Mortal Wkly Rep. 2022;71(6):206–211. doi: 10.15585/mmwr.mm7106a4. https://www.cdc.gov/mmwr/volumes/71/wr/mm7106a4.htm#F1_down [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Bahl A., Mielke N., Johnson S., Desai A., Qu L. Severe COVID-19 outcomes in pediatrics: an observational cohort analysis comparing Alpha Delta, and Omicron variants. Lancet Reg Health Am. 2023;18 doi: 10.1016/j.lana.2022.100405. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Miller A., Zambrano L., Yousaf A., et al. Multisystem inflammatory syndrome in children United States, February 2020–July 2021. Clin Infect Dis. 2021;51(SI-1):3273–3283. doi: 10.3906/sag-2105-342. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Miller A.D., Yousaf A.R., Bornstein E., et al. Multisystem inflammatory syndrome in children during severe acute respiratory syndrome coronavirus 2 (SARS-CoV-2) delta and Omicron variant circulation-United States, July 2021-January 2022. Clin Infect Dis. 2022;75(2):S303–S307. doi: 10.1093/cid/ciac471. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Levy N., Koppel J., Kaplan O., et al. Severity and incidence of multisystem inflammatory syndrome in children during 3 SARS-CoV-2 PandemicWaves in Israel. JAMA. 2022;327(24):2452–2454. doi: 10.1093/cid/ciab1007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.McCrindle B., Harahsheh A., Handoko R., et al. SARS-CoV-2 variants and multisystem inflammatory syndrome in children. N Engl J Med. 2023;388(17):1624–1626. doi: 10.1056/NEJMc2215074. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Abraham D.R., Butters C., Abdulbari Yunis N., et al. The impact of SARS-CoV-2 variants on the clinical phenotype and severity of multisystem inflammatory syndrome in children in South Africa. Pediatr Infect Dis J. 2022;41(12):E510–E512. doi: 10.1097/INF.0000000000003691. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Pino R., Antoñanzas J.M., Paredes-Carmona F., et al. Multisystem inflammatory syndrome in children and SARS-CoV-2 variants: a two-year ambispective multicentric cohort study in Catalonia, Spain. Eur J Pediatr. 2023;182(4):1897–1909. doi: 10.1007/s00431-023-04862-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Ptak K., Szymońska I., Olchawa-Czech A., Kukla K., Cisowska M., Kwinta P. Comparison of the course of multisystem inflammatory syndrome in children during different pandemic waves. Eur J Pediatr. 2023;182(4):1647–1656. doi: 10.1007/s00431-022-04790-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Laird-Gion J., Dionne A., Gauvreau K., et al. MIS-C across three SARS-CoV-2 variants: changes in COVID-19 testing and clinical characteristics in a cohort of U. S. Children. Eur J Pediatr. 2023;182(6):2865–2872. doi: 10.1007/s00431-023-04968-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Nygaard U., Holm M., Hartling U.B., et al. Incidence and clinical phenotype of multisystem inflammatory syndrome in children after infection with the SARS-CoV-2 delta variant by vaccination status: a Danish nationwide prospective cohort study. Lancet Child Adolesc Health. 2022;6(7):459–465. doi: 10.1016/S2352-4642(22)00100-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Brat G.A., Weber G.M., Gehlenborg N., et al. International electronic health record-derived COVID-19 clinical course profiles: the 4CE consortium. NPJ Digit Med. 2020;3(1):109. doi: 10.1038/s41746-020-00308-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Elbe S., Buckland-Merrett G. Data, disease and diplomacy: GISAID’s innovative contribution to global health. Glob Challenges. 2017;1(1):33–46. doi: 10.1002/gch2.1018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Sperotto F., Friedman K.G., Son M.B.F., VanderPluym C.J., Newburger J.W., Dionne A. Cardiac manifestations in SARS-CoV-2-associated multisystem inflammatory syndrome in children: a comprehensive review and proposed clinical approach. Eur J Pediatr. 2021;180(2):307–322. doi: 10.1007/s00431-020-03766-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Tian L., Cai T., Pfeffer M.A., Piankov N., Cremieux P.Y., Wei L.J. Exact and efficient inference procedure for meta-analysis and its application to the analysis of independent 2 × 2 tables with all available data but without artificial continuity correction. Biostatistics. 2009;10(2):275–281. doi: 10.1093/biostatistics/kxn034. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Aznar-Gimeno R., Paño-Pardo J.R., Esteban L.M., et al. Changes in severity, mortality, and virus genome among a Spanish cohort of patients hospitalized with SARS-CoV-2. Sci Rep. 2021;11(1):1–15. doi: 10.1038/s41598-021-98308-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.de Prost N., Audureau E., Heming N., et al. Clinical phenotypes and outcomes associated with SARS-CoV-2 variant Omicron in critically ill French patients with COVID-19. Nat Commun. 2022;13(1):1–12. doi: 10.1038/s41467-022-33801-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Quintero A.M., Eisner M., Sayegh R., et al. Differences in SARS-CoV-2 clinical manifestations and disease severity in children and adolescents by infecting variant. Emerg Infect Dis. 2022;28(11):2270–2280. doi: 10.3201/eid2811.220577. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Eleftheriou I., Maritsi D., Lampidi S., et al. Decreasing incidence of the multisystem inflammatory syndrome in children over 3 pandemic waves. Pediatr Infect Dis J. 2023;42(2):122–124. doi: 10.1097/INF.0000000000003766. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Cohen J., Carter M., Cheung C., Ladhani S. Lower risk of multisystem inflammatory syndrome in children (MIS-C) with the delta and Omicron variants of SARS-CoV-2. Clin Infect Dis. 2014;76(3):e518–e521. doi: 10.1093/cid/ciac553. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Koutsakos M., Lee W.S., Reynaldi A., et al. The magnitude and timing of recalled immunity after breakthrough infection is shaped by SARS-CoV-2 variants. Immunity. 2022;55(7):1316–1326.e4. doi: 10.1016/j.immuni.2022.05.018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Minervina A.A., Pogorelyy M.V., Kirk A.M., et al. SARS-CoV-2 antigen exposure history shapes phenotypes and specificity of memory CD8 T cells. Nat Immunol. 2022;23(5):781–790. doi: 10.1038/s41590-022-01184-4.SARS-CoV-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Levy M., Recher M., Hubert H., et al. Multisystem inflammatory syndrome in children by COVID-19 vaccination status of adolescents in France. JAMA. 2021;373(3):281–282. doi: 10.1136/bmj.n1087. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Zambrano L.D., Newhams M.M., Olson S.M., Halasa N.B., Price A.M., Orzel A.O. Effectiveness of BNT162b2 (Pfizer-BioNTech) mRNA vaccination against multisystem inflammatory syndrome in children among persons aged 12–18 Years. MMWR Morb Mortal Wkly Rep. 2021;71(2):52–58. doi: 10.15585/mmwr.mm7102e1. https://www.cdc.gov/mmwr/volumes/71/wr/mm7102e1.htm [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Goldenberg N.A., Sochet A., Albisetti M., et al. Consensus-based clinical recommendations and research priorities for anticoagulant thromboprophylaxis in children hospitalized for COVID-19–related illness. J Thromb Haemost. 2020;18(11):3099–3105. doi: 10.1111/jth.15073. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Henderson L.A., Canna S.W., Friedman K.G., et al. American College of rheumatology clinical guidance for multisystem inflammatory syndrome in children associated with SARS–CoV-2 and hyperinflammation in pediatric COVID-19: version 3. Arthritis Rheumatol. 2022;74(4):e1–e20. doi: 10.1002/art.42062. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Belhadjer Z., Méot M., Bajolle F., Khraiche D., Legendre A. Acute heart failure in multisystem inflammatory syndrome in children (MIS-C) in the context of global SARS-CoV-2 pandemic. Circulation. 2020;142:429–436. doi: 10.1161/CIRCULATIONAHA.120.048360. [DOI] [PubMed] [Google Scholar]
- 30.Kaushik S., Aydin S.I., Derespina K.R., et al. Multisystem inflammatory syndrome in children (MIS-C) associated with SARS-CoV-2 infection: a multi-institutional study from New York city. J Pediatr. 2020;224:24–29. doi: 10.1016/j.jpeds.2020.06.045. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.