Summary
In this protocol, we present a modified gradient coating strategy for zinc anodes. We describe steps for synthesizing electrodes, measuring electrochemistry, and assembling and testing batteries. The protocol can be applied for broadening design ideas of functional interface coating.
For complete details on the use and execution of this protocol, please refer to Chen et al. (2023).1
Subject areas: Energy, Material sciences
Graphical abstract
Highlights
-
•
Detailed protocol includes fabrication approaches for gradient-coated zinc anodes
-
•
Potential to advance zinc-ion battery applications
-
•
Gradient coating provides a design model for interface reconstruction of zinc anodes
-
•
Detailed sample preparation, instrument operation, and data analysis for precision
Publisher’s note: Undertaking any experimental protocol requires adherence to local institutional guidelines for laboratory safety and ethics.
In this protocol, we present a modified gradient coating strategy for zinc anodes. We describe steps for synthesizing electrodes, measuring electrochemistry, and assembling and testing batteries. The protocol can be applied for broadening design ideas of functional interface coating.
Before you begin
Unproductive side reactions, such as dendritic growth, are greatly restricting the deployment of aqueous zinc-ion batteries (ZIBs).2,3,4,5,6 In the Zn anode community, the introduction of interfacial coatings has proven to be a scalable strategy for the practical application of ZIBs.7
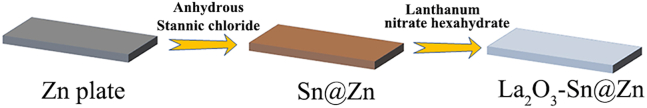
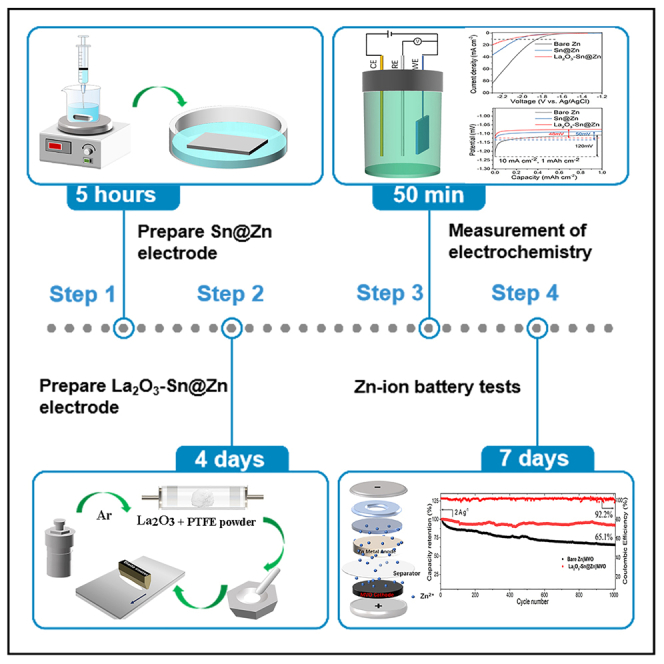
The protocol below describes steps for fabricating and evaluating Sn modified zinc (Sn@Zn) and La2O3 coated Sn@Zn (La2O3-Sn@Zn) electrodes (Scheme 1).
Scheme 1.
Fabrication steps of La2O3-Sn@Zn electrode
Preparation of raw materials and reaction bottles (for synthesis of Sn@Zn electrode)
Timing: ∼2 h
-
1.Prepare a 5 mL syringe, a 100 mL beaker, a magnetic stirrer (D = 88 mm), a 100 mL graduated cylinder, and a 200 mm diameter Petri dish.
-
a.Place them in an air blast drying oven and dry at 50°C for 1 h.
-
b.Quickly transfer the syringe to the glove box and cool to 25°C.
-
a.
-
2.
Use a syringe to aspirate 4 mL of anhydrous tin tetrachloride (SnCl4) stored in a glove box with the needle capped and stored for later use.
CRITICAL: Anhydrous stannous tetrachloride is susceptible to hydrolysis when it encounters water vapor in the air, and should be stored in a glove box.
-
3.
Take 40 mL of absolute ethanol in a graduated cylinder and pour it into a beaker.
-
4.
Inject slowly with a syringe containing 4 mL of SnCl4 into 40 mL of absolute ethanol at room temperature with constant stirring at 260 PRM to obtain 0.78 M SnCl4.
CRITICAL: When this is injected with anhydrous tin tetrachloride, the needle should be inserted below the absolute ethanol (CH3CH2OH) liquid level. The small amount of hydrochloric acid produced by the incorporation process is corrosive and needs to be performed under a fume hood, and researchers should also wear protective gloves, masks, and goggles.
Preparation of raw materials and reaction bottles (for synthesis of La2O3-Sn@Zn electrode)
Timing: ∼2 h
-
5.
Prepare a Teflon reactor, two 100 mL beakers, 2 stirring bars (B35), and a constant pressure drip funnel.
-
6.
Place them in an air blast drying oven and dry at 50°C for 1 h.
-
7.
Weigh 1.46 g ethylenediaminetetraacetic acid (EDTA), 6.49 g of lanthanum nitrate hexahydrate (La(NO3)3.6H2O) into a 100 mL beaker, and add 75 mL distilled water (DI water) at room temperature in air. Subsequently, the solution was obtained by continuous stirring at 260 RPM and room temperature for 15 min (EDTA, (La(NO3)3.6H2O) and DI water in a weight ratio of 1.46:6.49:75)
-
8.
Weigh 4.21 g of potassium hydroxide (KOH), add it slowly to the above solution and stir quickly with the glass rod to adjust the solution environment (PH ∼ 8), noted as solution A.
CRITICAL: KOH is highly corrosive and tends to absorb water from the air, so weighing should be done in beakers rather than weighing paper. During the process of dissolving KOH, a large amount of heat is released. Please pay attention to safety.
Key resources table
| REAGENT or RESOURCE | SOURCE | IDENTIFIER |
|---|---|---|
| Chemicals, peptides, and recombinant proteins | ||
| Anhydrous tin tetrachloride (AR) | Aladdin | CAS:7646-78-8 |
| Absolute ethanol (AR ≥ 99.7%) | Aladdin | CAS: 64-17-5 |
| Lanthanum nitrate hexahydrate (AR 99.99%) | Macklin | CAS:10277-43-7 |
| Potassium hydrate (AR) | Macklin | CAS:1310-58-3 |
| Ethylenediaminetetraacetic acid (AR) | Macklin | CAS:60-00-4 |
| Zinc foil (Zn) (0.1 mm) | Shenzhen Kejing Zhi da Technology Co., Ltd | – |
| Polytetrafluoroethylene powder (PTFE) | Aladdin | CAS:9002-84-0 |
| Conductive carbon black (ECP-600JD) | Shenzhen Kejing Zhi da Technology Co., Ltd | ECP-600JD |
| N-methyl pyrrolidone (NMP) | Macklin | CAS:872-50-4 |
| Polyvinylidene fluoride (PVDF) | Guangdong Canrd New Energy Technology Co., Ltd | MA-EN-Bl-01 |
| N, N-dimethylformamide (DMF, 99.8%) | Macklin | CAS:68-12-2 |
| Zinc sulfate heptahydrate (AR) | Macklin | CAS:7446-20-0 |
| Vanadium pentoxide (AR) | Xiya Chemical Technology Co., Ltd | CAS:1314-62-1 |
| Hydrogen peroxide (AR, 30 wt% in H2O) | Aladdin | CAS:7722-84-1 |
| Critical commercial assays | ||
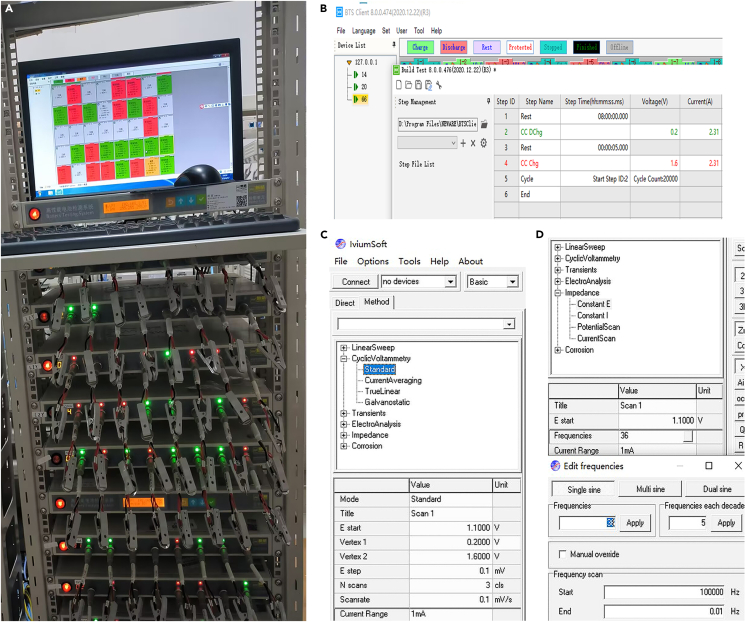
| Battery tester | Neware | https://www.neware.com.cn/ |
| Electrochemical workstation | Iviumstat | https://www.ivium.com/ |
| Software and algorithms | ||
| Microsoft Excel 2020 | Microsoft | https://www.microsoft.com/en-us/microsoft-365/excel |
| Origin Pro 2021 | OriginLab | https://www.originlab.com/ |
| Photoshop 2020 | Adobe Systems | https://www.adobe.com/ |
| Other | ||
| Analytical balance | Shanghai Sunny Hengping Scientific Instrument Co., Ltd | ME204 |
| Hydraulic button battery sealing machine | Hefei Kejing Materials Technology Co., Ltd. | MSK-110 |
| Stir plate | IKA | Model RCT B S025 |
| Dispensing funnel | Guangdong Canrd New Energy Technology Co., Ltd | – |
| Blast drying oven | Shanghai Jinghong Ltd | DHG-9053A |
| Vacuum drying oven | Shanghai Jinghong Ltd | DZF-6050 |
| Tubular furnace | HF-Kejing | OTF-1200X-S-II |
| Coin cell battery accessories | Guangdong Canrd New Energy Technology Co., Ltd | CR-2025 |
| Field Emission Scanning Electron Microscope | Hitachi High-Tech (Shanghai) Co., Ltd | SU5000 |
| Centrifuge | Sichuan Shuke Instrument Co., Ltd | TG16-WS |
| Ag/AgCl reference electrode | Shanghai Chenhua Instrument Co., Ltd | CHI112 |
| Laser Confocal Microscope | Keyence Corporation | VK-150K |
| Platinum electrodes | Guangdong Canrd New Energy Technology Co., Ltd | Pt230 |
| Ultrasonic Cleaner | Kunshan Ultrasonic Instrument Co., Ltd | KQ2200W |
| X-Ray diffractometer | PANalytical B.V. | X′Pert-pro |
| Atomic force microscopy | Germany BRUKER | Dimension Icon |
| Thermogravimetric analyzer | Shimadzu Corporation | SDT-Q600 |
CRITICAL: Tin tetrachloride solution is easy to spontaneously hydrolyze with water vapor in the air and should be stored in an anhydrous environment.
Alternatives: There are no special requirements on the source of reagents for this protocol. Chemicals from other suppliers, such as Sigma-Aldrich and Alfa Aesar can also be used. Instruments and software with similar functions can also be used.
Materials and equipment
| Stock solution of SnCl4 solution (storage: 25°C) | ||
|---|---|---|
| Reagent | Final concentration | Amount |
| Anhydrous tin tetrachloride | N/A | 4 mL |
| Absolute ethanol | N/A | 40 mL |
| Total | 0.78 M | 44 mL |
Note: The tin tetrachloride solution can be stored at 25°C for 2 weeks.
| Solution for the preparation of lanthanum oxide (storage: 25°C) | ||
|---|---|---|
| Reagent | Final concentration | Amount |
| La (NO3)3·6H2O | N/A | 6.49 g |
| KOH | N/A | 4.21 g |
| EDTA | N/A | 1.46 g |
| DI water | N/A | 75 mL |
| Total | N/A | 75 mL |
Note: The solution for the preparation of lanthanum oxide can be stored at 25°C for 3 months.
Step-by-step method details
Part 1: Synthesis of Sn@Zn electrode
Timing: ∼5 h
-
1.Cut zinc foil (5 cm × 10 cm), sandpaper, wash and air dry.
-
a.Flatten the zinc foil with a glass plate load.
-
b.Moisten zinc foil with water and sand it with 2000 mesh sandpaper.
-
a.
-
2.
Immerse zinc foil into (0.78 M) 40 mL SnCl4 for 15 min.
CRITICAL: During the reaction with the zinc foil, gently shake the surface dish to ensure that the concentration of 0.78 M SnCl4 around the zinc foil is uniform, and the surface dish can be selected according to actual needs, and put on the lab coat to prevent splashing.
-
3.
Clean reacted zinc foil using alcohol, and then dry it under vacuum at 60°C for 3 h.
Part 2: Synthesis of La2O3-Sn@Zn electrode
Timing: ∼4 days
Timing: ∼2 days (for step 4)
Timing: ∼6 h (for step 5)
Timing: ∼2 h (for step 6)
Timing: ∼20 h (for step 7)
In this part, the preparation process is mainly divided into four steps, namely the preparation of lanthanum oxide (La2O3), preparation of hydrophobic lanthanum oxide, the preparation of coating slurry, and the preparation of coating by scraper method.
-
4.Synthesis of lanthanum oxide.
-
a.Transfer 32 mL solution A to a stainless steel autoclave (50 mL) and hold at 180°C for 12 h.
-
b.Transfer reacted solution to a 50 mL centrifuge tube and centrifuge at 6000 rpm for 5 min
 CRITICAL: Centrifuge tubes should be placed symmetrically to prevent damage to the centrifuge, and researchers should standardize the use of laboratory equipment.
CRITICAL: Centrifuge tubes should be placed symmetrically to prevent damage to the centrifuge, and researchers should standardize the use of laboratory equipment. -
c.Centrifuge the sample at least three times with DI water and CH3CH2OH, and dry it at 100°C in the vacuum drying oven.
-
d.Transfer dried samples into a tube furnace for annealing.
-
i.Turn on the vacuum pump, open the inlet and outlet valves of the tube furnace and pump out the air inside the tube.
-
ii.Adjust the rotameter, and pass argon gas inside the tube furnace.
-
iii.Press the heat up button and heat at 5 °C min−1 to 750°C for 2 h.Note: To ensure that the tube furnace is air-free, the researchers need to perform more than three vacuums and argon fillings in sequence.
 CRITICAL: La2O3 tends to absorb moisture from the air. Therefore, it should be quickly removed and poured into a sealed vial during the annealing and cooling process (at about 80°C). Researchers should be careful to wear gloves to prevent burns.
CRITICAL: La2O3 tends to absorb moisture from the air. Therefore, it should be quickly removed and poured into a sealed vial during the annealing and cooling process (at about 80°C). Researchers should be careful to wear gloves to prevent burns.
-
i.
-
a.
-
5.Synthesis of hydrophobic lanthanum oxide.
-
a.Weigh 2 g of La2O3 and quickly add to a 20 mL sample bottle.
-
b.Weigh 0.02 g PTFE powder and add to above bottle.
-
c.Add 15 mL of CH3CH2OH into the above bottle (La2O3, PTFE and C2H5OH in a weight ratio of 100:1:13) and place it in an ultrasonic cleaner and sonicate for 2 h.
-
d.Transfer mixed solution d to the corundum boat and place in a tube furnace for sintering.
-
i.Nitrogen filling and vacuuming were performed three times alternately.
-
ii.Set up 2 stages of heating program, room temperature at 5°C /min to 80°C, holding temperature for 2 h, 80 °C at 5 °C/min to 350°C holding temperature for 30 min.
-
iii.Adjust the rotameter until a continuous bubble appears in the waste gas bottle at the end of the tube furnace (about one per second).Note: La2O3 and PTFE powder need to be mixed under absolute ethanol to exclude the interference experiment of lanthanum hydroxide generated after deterioration.
 CRITICAL: Do not pour the solids at the bottom of the sample bottle into the porcelain boat, and only take the supernatant to prevent impurities from causing experimental interference.
CRITICAL: Do not pour the solids at the bottom of the sample bottle into the porcelain boat, and only take the supernatant to prevent impurities from causing experimental interference.
-
i.
-
a.
-
6.Preparation of lanthanum oxide slurry.
-
a.Wash the mortar and pestle, and dry it in a blast oven at 105°C.
-
b.Weigh 200 mg of La2O3 with on an analytical balance.
-
c.Weigh 22 mg of PVDF (La2O3: PVDF, 9:1).
-
d.Add 2 mL of NMP, and grind for 1 h.
-
a.
CRITICAL: Grind the slurry in one direction scraping solids off of the agate mortar walls and push them back to the center every 5 min before continuing to grind.
-
7.Prepare La2O3-Sn@Zn electrode by the scraper method.
-
a.Aspirate the as-prepared slurry with a 1 mL pipette and place it 3 cm from one end of the Sn@Zn electrode.
-
b.Scrapes the paste well on Sn@Zn electrode with the applicator.
-
c.Dry prepared electrode under vacuum (−0.1 MPa) at 50°Cfor 12 h drying oven.
-
a.
Note: Adjust the thickness of the scraper to 10 μm.
CRITICAL: Spray a little alcohol on the glass plate in advance to increase the adsorption force of the electrode sheet and the glass plate to prevent shaking during preparation process.
Part 3: Measurement of electrochemistry
Timing: ∼50 min
To confirm electrochemical properties of La2O3-Sn@Zn anode, we assembled a three-electrode system for hydrogen evolution reaction and nucleation overpotential tests to investigate its stable electrochemical window. To study the cycling stability of La2O3-Sn@Zn anodes, the Zn plating/stripping was measured in a symmetric cell.
-
8.Hydrogen evolution testing-Linear sweep voltammetry (LSV).
-
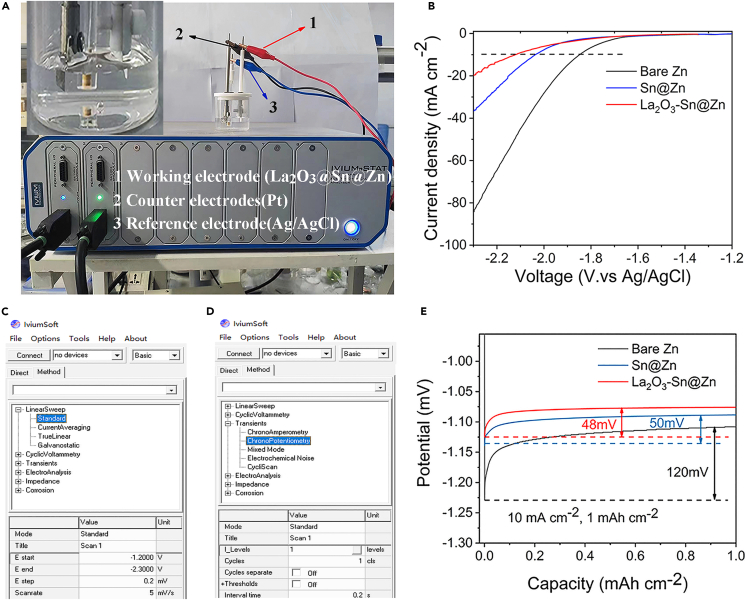
a.Prepare three electrodes (Figure 1A).
-
i.Work electrode: Punch the sample to obtain La2O3-Sn@Zn electrode with 12 mm diameter.
-
ii.Counter electrode: Cut Pt foil (2.5 cm × 2.5 cm).
-
iii.Reference electrode: Ag/AgCl (3.5 M KCl)
-
i.
-
b.Assemble the electrochemical cell and add 1 M Na2SO4 aqueous solution as a supporting electrolyte.
 CRITICAL: Before each experiment, the solution was treated with argon for about 5 min, which avoids any disturbance due to dissolved oxygen.
CRITICAL: Before each experiment, the solution was treated with argon for about 5 min, which avoids any disturbance due to dissolved oxygen. -
c.Set initial potential to −1.2 V, set the end potential to −2.3 V, and set the scan rate to 5 mV s−1 (Figure 1C).
-
d.Record the LSV curve (Figure 1B).Note: It is necessary to keep the working electrode inserted into the liquid level at the same height for each test.
-
a.
-
9.Nucleation overpotential testing.
-
a.Prepare electrodes in air at room temperature (Figure 1A).
-
i.Working electrode: Punch the sample to obtain La2O3-Sn@Zn electrode with 12 mm diameter.
-
ii.Counter electrode: Cut Pt foil (2.5 cm × 2.5 cm).
-
iii.Reference electrode: Ag/AgCl (3.5 M KCl)
-
i.
-
b.Assemble the electrochemical cell at room temperature, and add 2 M ZnSO4 aqueous solution as a supporting electrolyte.
-
c.Record the zinc nucleation overpotential curves (Figure 1E).
-
a.
Note: Prepare and assemble cells at room temperature in air.
-
10.Symmetrical battery test.
-
a.Prepare two same electrodes.
-
i.Work and counter electrode: La2O3-Sn@Zn electrode with 12 mm diameter.
-
i.
-
b.Assemble symmetrical battery (La2O3-Sn@Zn//La2O3-Sn@Zn) and add 40 μL of electrolyte.
-
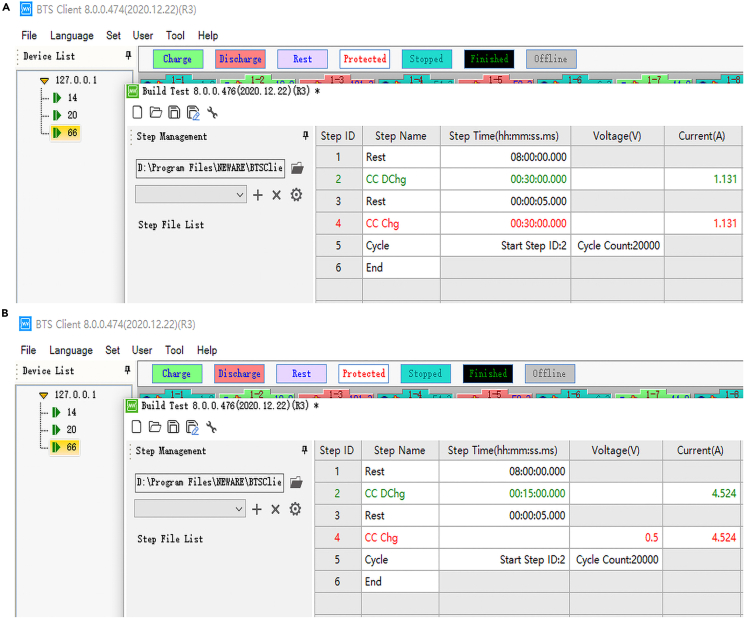
c.Set the symmetric battery program (Figure 2A).
-
d.Record the voltage change curve.
-
a.
-
11.Asymmetrical battery test.
-
a.Prepare two electrodes.
-
i.Anode: La2O3-Sn@Zn electrode with 12 mm diameter.
-
ii.Cathode: Cu electrode with 12 mm diameter.
-
i.
-
b.Assemble half battery (La2O3-Sn@Zn//Cu), and add 40 μL of electrolyte.
-
c.Set the half battery program (Figure 2B).
-
d.Record the voltage change curve.
-
a.
Figure1.
Measurement of electrochemistry
(A) Workstations and three electrode testing systems.
(B) LSV curves of electrodes in 1 M Na2SO4 at a rate of 5 mV s−1.
(C) Detailed hydrogen evolution test procedures.
(D) Detailed nucleation overpotential test procedure.
(E) Nucleation overpotential curves of Zn at a current density 10 mA cm−2 with a capacity of 1 mAh cm−2.
Figure 2.
La2O3-Sn@Zn electrode performance evaluation test procedure
(A) Detailed symmetrical battery test procedures.
(B) Detailed asymmetrical battery test procedures.
Part 4: Assembly and testing of La2O3-Sn@Zn//MVO batteries
Timing: ∼7 days
Fabrication of multivalent vanadium-based oxide (MVO) cathodes
-
12.Preparation of raw materials and reaction bottles.
-
a.Prepare a 500 mesh stainless steel mesh with a diameter of 12 mm (weigh and record the mass of each disk), conductive carbon black (ECP-600JD), the polyvinylidene fluoride (PVDF) binder and N-methyl pyrrolidone (NMP) solvent.
-
b.Preparation of MVO materials.
-
i.Weigh 2.27 g of V2O5 and add to 187 mL of DI water, stirring continuously.
-
ii.Add 31 mL of H2O2 dropwise at room temperature and stir well (V2O5, DI water and H2O2 with a mass ratio of 1:831:81)
-
iii.Transfer mix solution to a 300 mL Teflon-lined autoclave and hold at 200°C for 72 h.
-
iv.Wash 3 times with DI water, centrifuge, and dry overnight at 80°C under vacuum.
-
v.Above solid was removed and calcined in a nitrogen atmosphere at 400°C for 1 h.
-
i.
-
a.
-
13.Preparation of MVO electrode.
-
a.Weighing and Mixing (MVO, carbon black and PVDF with a mass ratio of 8:1:1).
-
i.Weigh 0.2 g MVO into the agate mortar and grind it into delicate powder (5 min).
-
ii.Weigh 0.025 g conductive carbon black and continue grinding (30 min).
-
iii.Weigh 0.025 g PVDF and continue grinding (10 min).
 CRITICAL: Grind the slurry in one direction scraping solids off of the agate mortar walls and push them back to the center every 5 min before continuing to grind.
CRITICAL: Grind the slurry in one direction scraping solids off of the agate mortar walls and push them back to the center every 5 min before continuing to grind.
-
i.
-
b.Adding NMP.
 CRITICAL: NMP should be added drop by drop during the grinding process to avoid adding large amounts of NMP at one time. And slowly add about 200 mL of NMP to form well-dispersed slurry within under continuous grinding for 15–30 min.
CRITICAL: NMP should be added drop by drop during the grinding process to avoid adding large amounts of NMP at one time. And slowly add about 200 mL of NMP to form well-dispersed slurry within under continuous grinding for 15–30 min. -
c.Electrode coating.
 CRITICAL: Aspirate 2 mL of slurry, and use a 100 μm thickness spatula to evenly apply it to the stainless-steel mesh.
CRITICAL: Aspirate 2 mL of slurry, and use a 100 μm thickness spatula to evenly apply it to the stainless-steel mesh. -
d.Dry the electrodes.
 CRITICAL: Make sure to fully dry the prepared working electrodes in a vacuum drying oven at 60°C for 12 h to remove any solvent residues.
CRITICAL: Make sure to fully dry the prepared working electrodes in a vacuum drying oven at 60°C for 12 h to remove any solvent residues. -
e.Calculate the mass loading.Note: Weigh the mass of the dried electrode sheet and calculate the mass of the MVO. The mass loading of active material is about 2 mg in this protocol.
-
f.Configure the electrolyte.
-
i.Weight 14.38 g of ZnSO4·7H2O dissolve in 25 mL DI water to obtain 2 M ZnSO4.Note: To ensure the accuracy of concentration, the volumetric flask should be configured with a volumetric flask, with the solution shaken and set to stand.
-
i.
-
a.
-
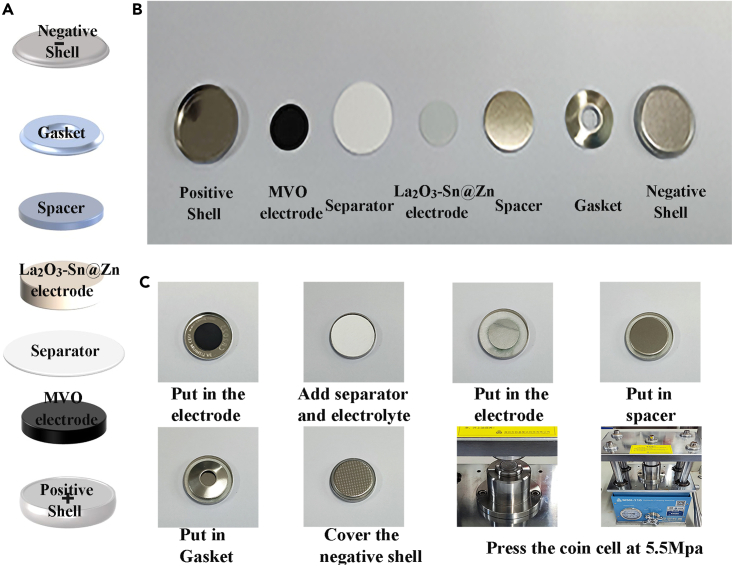
14.Assemble CR2025 coin cells in the atmosphere (Figure 3).
-
a.Prepare battery assembly equipment.
-
b.Assembling coin cell batteries.Note: Use insulated tweezers to clamp the positive electrode, diaphragm, electrolyte, working electrode, gasket (1.1 mm), and shrapnel into the positive case in turn, and cover the negative case.
-
c.Use the battery sealing machine to pressurize the battery to 5.5 MPa for sealing. Place the assembled battery in a box and let it rest for 8 h before testing.
-
a.
-
15.
Battery tests.
To characterize ZIBs performance, they were connected to Neware battery-testing system to record galvanostatic charge-discharge (GCD) profiles, Coulombic efficiency (CE) and cycle stability. Besides, the cyclic voltammetry curves (CV) and electrochemical impedance spectra (EIS) of ZIBs were tested on an IviumStat electrochemical workstation. The main key parameters settings and results of the ZIBs tests are shown in Figure 4. The detail characterization of the ZIBs in this protocol is shown in Figure 5.-
a.Test ZIBs performance using the electrochemical workstation and the Neware battery-testing system.
-
i.Detailed parameter setting for GCD profiles at a current density of 2 A g−1 (Figure 4B).
-
ii.Detailed parameter setting for CV curves in the range from 0.2 to 1.6 V (vs. Zn/Zn2+) at scan rate of 0.1 mV s−1 (Figure 4C).
-
iii.Detailed parameter settings for EIS spectra at the frequency range from 100 kHz to 0.01 Hz (Figure 4D).
-
i.
-
a.
Figure 3.
Assembly of full cell (La2O3-Sn@Zn//MVO)
(A) Assembly schematic of full battery.
(B) Pictures of individual battery components.
(C) Pictures of the battery assembly process.
Figure 4.
Battery test
(A) Neware battery-testing system.
(B) Detailed cycling performance test protocol.
(C) Detailed cyclic voltammetry test procedure.
(D) Detailed impedance test procedures.
Figure 5.
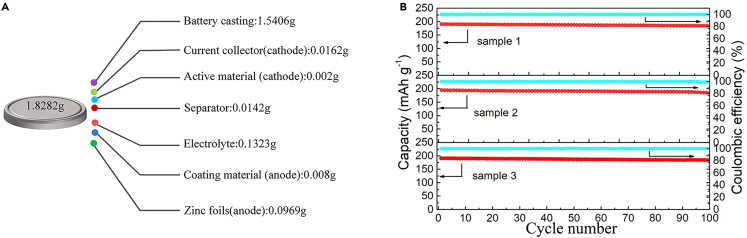
The true status of the protocol
(A) Detail weight of each accessory (error control within 2%).
(B) Repeatability GCD profiles for 3 full cells.
Expected outcomes
This protocol provides a gradient coating design strategy for zinc anode, focusing on its preparation process and detailed characterization of electrochemistry. In addition, the full cells assembly process and its performance evaluation are also described in detail. The assembled (La2O3-Sn@Zn//MVO) full cells exhibit remarkable capacity retention about 92.2% after 1000 charge/discharge cycles. Finally, the protocol was analyzed by weighing and calculating the actual mass of each component of this full cells for the practicality of the protocol (Figure 5A), and reproducibility studies with were performed with three identical full cells. Notably, all full cells exhibited good and consistent cycling stability, as shown in (Figure 5B).
Limitations
There are three main limitations to this protocol: 1) anhydrous tin tetrachloride is easily hydrolyzed when it encounters water vapor in the air, so it should be stored in anhydrous environment during storage, and added below the level of anhydrous solvent during use; 2) lanthanum oxide is very easy to react with water in the air, so it needs to be purified in the process of using it; 3) When preparing lanthanum oxide coatings by the scraper method, it is important to consider whether the contact between the lanthanum oxide and zinc surfaces is consistent. Otherwise, the mass distribution of the active material is uneven, leading to large differences in ZIBs performance.
Troubleshooting
Problem 1
Anhydrous tin tetrachloride is very sensitive to water vapor, affecting the thickness of the tin layer (step 2 in the before you begin section).
Potential solution
When taking anhydrous tin tetrachloride, a syringe should be used, and the needle should be inserted below the liquid level when configuring the solution.
Problem 2
Lanthanum oxide is very sensible to water vapor and deterioration causes impurity of the chemical and affects the electrochemical properties (step 1d in the part 2: synthesis of lanthanum oxide).
Potential solution
The lanthanum oxide obtained is removed in time when the sintering cooling temperature is 80°C, and a certain amount of absolute ethanol is injected into the vial in advance, and the impurities are layered with the drug by sonication.
Problem 3
The scraper process has certain requirements for the coating slurry, which can affect the coating distribution and its properties (step 2c in the part 2: synthesis of hydrophobic lanthanum oxide).
Potential solution
Longer sonication time for lanthanum oxide and longer grinding time for PVDF and lanthanum oxide were chosen.
Problem 4
The HER test results are easily affected by the presence of oxygen in the DI water (step 1b in the part 3: measurement of electrochemistry).
Potential solution
Before the hydrogen evolution test, the solution is first treated with argon gas for 5 min to remove the residual oxygen from the DI water.
Problem 5
There are differences in the electrochemical performance of batteries produced in the same production batch (step 3 in the part 4: assembly and testing of La2O3-Sn@Zn//MVO batteries).
Potential solution
Battery assembly techniques, unevenness of the slurry may lead to differences in battery performance. Firstly, when assembling the battery, the two electrodes should be aligned and assembled, and the pressure of the pressure of the pressure battery should be controlled around 5.5Mpa as much as possible. Secondly, during the whole process of grinding the slurry, care should be taken to always grind in one direction, grinding every 5 min to scrape it to the bottom of the mortar. Finally, differences in the mass load of the active material pasted on the stainless steel mesh can lead to differences in battery performance. The best way to prepare the cell is by applying it uniformly and selecting working electrodes with similar mass loadings of active substance (differences of no more than 0.05 mg).
Resource availability
Lead contact
Further information and requests for resources and reagents should be directed to and will be fulfilled by the lead contact, Dr. Bo-Tian Liu (btliu2018@glut.edu.cn).
Materials availability
This study did not generate new unique reagents.
Acknowledgments
This work was supported by the National Natural Science Foundation of China (21805056), the Technology Base and Special Talents Development Foundation of Guangxi Province (GUIKE-AD19110039, GUIKE-AD21220108), the Scientific Research Foundation of Guilin University of Technology (GUTQDJJ2018007), and the Guangxi Key Laboratory of Electrochemical and Magneto chemical Functional Materials (EMFM20221104).
Author contributions
B.-T.L. conceived and designed the experiments. B.-T.L., W.C., J.T., S.G., Y.X., J.S., and Q.Z., carried out the fabrication of materials and performed the electrochemical and microstructural characterizations. B.-T.L. and W.C. wrote the paper, and all authors discussed the results and commented on the manuscript.
Declaration of interests
The authors declare no competing interests.
Data and code availability
No unique datasets or codes were generated in this study.
References
- 1.Chen W., Tang J., Ji F., Sun J., Zhu Q., Liu B.T. A multifunctional gradient coating enables dendrite-free and side reaction-free zinc anodes for stable zinc ion batteries. Cell Rep. Phys. Sci. 2023;4:101344. doi: 10.1016/j.xcrp.2023.101344. [DOI] [Google Scholar]
- 2.Hao J., Li X., Zeng X., Li D., Mao J., Guo Z. Deeply understanding the Zn anode behaviour and corresponding improvement strategies in different aqueous Zn-based batteries. Energy Environ. Sci. 2020;13:3917–3949. doi: 10.1039/D0EE02162H. [DOI] [Google Scholar]
- 3.Wang S.-B., Ran Q., Yao R.-Q., Shi H., Wen Z., Zhao M., Lang X.-Y., Jiang Q. Lamella-nanostructured eutectic zinc-aluminum alloys as reversible and dendrite-free anodes for aqueous rechargeable batteries. Nat. Commun. 2020;11:1634. doi: 10.1038/s41467-020-15478-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Zhao Z., Wang R., Peng C., Chen W., Wu T., Hu B., Weng W., Yao Y., Zeng J., Chen Z., et al. Horizontally arranged zinc platelet electrodeposits modulated by fluorinated covalent organic framework film for high-rate and durable aqueous zinc ion batteries. Nat. Commun. 2021;12:6606. doi: 10.1038/s41467-021-26947-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Ge H., Feng X., Liu D., Zhang Y. Recent advances and perspectives for Zn-based batteries: Zn anode and electrolyte. Nano Res. Energy. 2023;2:e9120039. doi: 10.1016/j.ensm.2019.04.022. [DOI] [Google Scholar]
- 6.Zou Y., Yang X., Shen L., Su Y., Chen Z., Gao X., Zhou J., Sun J. Emerging strategies for steering orientational deposition toward high-performance Zn metal anodes. Energy Environ. Sci. 2022;15:5017–5038. doi: 10.1039/d2ee02416k. [DOI] [Google Scholar]
- 7.Li T.-C., Lim Y.V., Xie X., Li X.-L., Li G., Fang D., Li Y., Ang Y.-S., Ang L.-K., Yang H.-Y. ZnSe modified Zinc metal anodes: toward enhanced zincophilicity and ionic diffusion. Small. 2021;17:2101728. doi: 10.1012/smll.202101728. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
No unique datasets or codes were generated in this study.