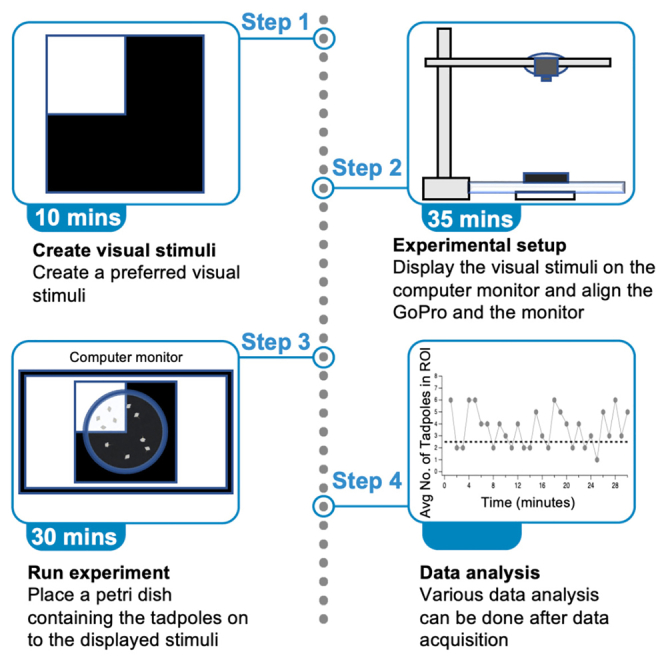
Summary
Xenopus tadpoles display innate visually guided behaviors which are thought to promote survival by guiding them toward sources of food and away from predators. Experimentally, studying these behaviors can provide insight into the formation and function of the neural circuits which underlie them. Here, we present a protocol for measuring visual preferences of freely swimming tadpoles. We describe steps to create the visual stimuli, carry out the experiments, and analyze the resulting data.
For complete details on the use and execution of this protocol, please refer to Hunt et al.1 and Bruno et al.2
Subject areas: Developmental biology, Neuroscience, Behavior
Graphical abstract
Highlights
-
•
Paradigm for testing visual preferences of freely swimming Xenopus tadpoles
-
•
Protocol describes steps to create desired visual stimuli
-
•
Explains how to carry out the experiment and perform data analysis
-
•
Can be used to test visual preferences under various conditions
Publisher’s note: Undertaking any experimental protocol requires adherence to local institutional guidelines for laboratory safety and ethics.
Xenopus tadpoles display innate visually guided behaviors which are thought to promote survival by guiding them toward sources of food and away from predators. Experimentally, studying these behaviors can provide insight into the formation and function of the neural circuits which underlie them. Here, we present a protocol for measuring visual preferences of freely swimming tadpoles. We describe steps to create the visual stimuli, carry out the experiments, and analyze the resulting data.
Before you begin
Institutional permissions
All animal husbandry and experimental procedures were approved by the University of Wyoming’s Institutional Animal Care and Use Committee (IACUC). Please acquire permission from the relevant committee at your institution before performing these procedures.
Preparing the rearing solution (Steinberg’s solution)
Timing: 30 min
-
1.
To prepare 1 L of a 100× stock of Steinberg’s solution, see the recipe provided in the materials and equipment section. Store the 100× stock at 4°C and use within 6 months from the date it was prepared.
-
2.
To prepare 1 L of the final working solution, add 10 mL of the 100× stock solution to 990 mL of Deionized (DI) Water.
Tadpole rearing
Timing: 10–18 days
-
3.
X. laevis embryos can be obtained either from gonadotropin-induced in-house mating of adult wild-type Xenopus frogs or obtained commercially. For detailed discussion of Xenopus laevis husbandry, please refer to McNamara et al.3 and Wlizla et al.4
-
4.
Embryos are raised in working Steinberg’s solution (100–150 tadpoles per 1.85 L glass bowls) and housed in an incubator maintained on a 12:12 light/dark cycle, at 22°C.
Note: We do not feed tadpoles until they reach later stage 49 (approximately 21 days postfertilization). However, if the researcher is feeding the tadpoles at the stages being tested, we advise that the feeding schedule be consistent.
-
5.
Tadpoles are ready for testing when they have reached developmental stage 48/49, approximately 10–18 days post fertilization (dpf). Developmental stages are identified according to Nieuwkoop and Faber.5
Note: This test may not be suitable for measuring the visual preference of tadpoles younger than stage 47 as they do not consistently display robust swimming behaviors (See limitations section)
Creating the visual stimuli
Timing: 10 min
Using Adobe Photoshop.
-
6.
To achieve a device-independent color model, create stimuli in Lab color mode.
Note: In Lab color mode, the values or coordinates used to specify the color will produce the same color independent of any output device, leading to perceptually uniform color expression [i.e., the color will be appearing the same across different devices].
-
7.
Create a square such that one quadrant of the square is the color and/or luminance of interest, and the remaining 3 quadrants are the color or luminance being tested against.
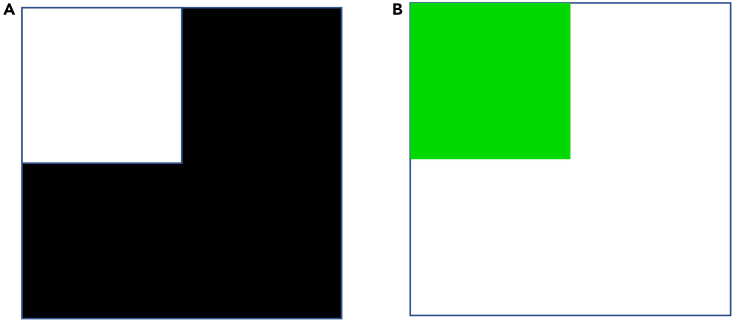
Note: For example, if we wish to test the preference for green when green is pitted against light (white), one quadrant of the test dish is green, and the remaining 3 quadrants are light. Examples are shown in Figure 1.
-
8.
Within Photoshop, set the white (or light) stimulus to a Lightness Channel (L) value of 100 while the black (or dark) stimulus is set to an L value of 0.
-
9.
Any color stimulus can be created by altering the Lab values. For example, the green stimulus has Lab values of L: 75, a: −75, b: 75 (closest RGB: 0, 216, 0), blue stimulus has Lab values of L: 25, a: 50, b: −100 (closest RGB: 0, 33, 221) and red stimulus had Lab values of L: 50, a: 75, b: 50 (closest RGB: 232, 20, 42).
-
10.
To avoid loss of image quality each time it is opened and saved again, save stimulus image in a lossless compression file format, such as png.
Figure 1.
Examples of color/luminance stimulus
(A) Example of a stimulus created to test the preference for light when light (shown as white) is pitted against dark.
(B) Example of stimulus created to test the preference for green when green is pitted against light.
Using Microsoft PowerPoint.
-
11.
PowerPoint can also be used to create light vs. dark stimulus. Do not use PowerPoint to create color stimulus due to difficulty in creating device independent color models.
-
12.
Create the light stimulus using the Grayscale Slider with a brightness set at 100% and set the brightness at 0% for the dark stimulus. Control luminance by varying the brightness settings.
Preparing the test dish
Timing: 10 min
-
13.
Choose a 14 cm-diameter, clear, and transparent petri dish.
-
14.
Cover the outside edge of the dish with black construction paper to obscure extraneous visual stimuli.
Note: The height of the construction paper should correspond to the height of the petri dish.
Key resources table
| REAGENT or RESOURCE | SOURCE | IDENTIFIER |
|---|---|---|
| Chemicals, peptides, and recombinant proteins | ||
| Potassium chloride | Fisher Scientific | P217-500 |
| Sodium chloride | Fisher Scientific | S271-3 |
| Magnesium sulfate, 7-hydrate | Mallinckrodt Chemicals | MAL 6066-04 |
| Calcium nitrate tetrahydrate | Sigma-Aldrich | 237124-500G |
| HEPES | Sigma-Aldrich | H3375-1KG |
| Sodium hydroxide | Fisher Scientific | S318-500 |
| Experimental models: Organisms/strains | ||
|
Xenopus laevis tadpoles Stage 48/49, Gender indeterminate at these early stages. |
In-house mating of adult Xenopus frogs | NCBI:txid8355 |
| Software and algorithms | ||
| Excel | Microsoft | RRID:SCR_016137 |
| Powerpoint | Microsoft | N/A |
| iMovie | Apple | N/A |
| Quicktime Player | Apple | N/A |
| Adobe Photoshop | Adobe | RRID:SCR_014199 |
| Other | ||
| GoPro camera | GoPro Inc. | N/A |
| Clear plastic petri dish | Fisher Scientific | CAT# FB0875714 |
| Laboratory clamp stand | Humboldt MFG. Co | # H-21217 |
| LCD computer monitor | Any type | |
| Transfer pipette | Fisher Scientific | CAT# 13-711-7M |
| 50 mL Conical tube | Corning Science | REF# 352098 |
Materials and equipment
Steinberg’s solution (Stock)
| Reagent | Final Concentration(mM) | Amount(g) |
|---|---|---|
| KCl | 6.7 | 0.5 |
| Ca(NO3)2.4H20 | 3.4 | 0.8 |
| MgSO4.7H20 | 8.3 | 2.0 |
| NaCl | 580 | 34.0 |
| HEPES | 499.37 | 119.0 |
| Deionized (DI) Water | N/A | 1 L |
| Total | N/A | 1 L |
Note: Adjust pH to 7.5 using 10 N NaOH, store at 4°C. and use within 6 months.
Step-by-step method details
Measuring color/luminance preferences of freely swimming tadpoles
Timing: 35 min
This section describes the experimental setup (Figure 2A) and provides detailed procedures for recording the visual preferences of freely swimming Xenopus laevis tadpoles.
-
1.
Position the LCD monitor, Clamp stand and GoPro camera as shown in Figure 2A.
-
2.
Add 75 mL of working Steinberg’s solution into the plastic petri dish (test dish).
-
3.
Use a transfer pipette to transfer ten randomly chosen tadpoles from the rearing bowl to the test dish.
Note: If testing the light or color preference of tadpoles previously exposed to pharmacological agents, be sure to first transfer the tadpoles from the exposure bowl to a holding bowl containing working Steinberg’s solution before transfer to the test dish to prevent any drug from contaminating the test dish.
CRITICAL: This test has been optimized using ten tadpoles in a 14 cm diameter test dish. We have determined that, with ten tadpoles in the test dish, there appears to be no schooling behavior that would interfere with the display of individual preferences.1 If more than 10 tadpoles are used, it would be necessary to determine whether the group displays schooling behavior. If so, this would not be optimal.
-
4.
Turn on the computer monitor to display the stimulus on the screen.
-
5.
Place the test dish containing the tadpoles over the stimulus on the monitor such that one-quarter of the test dish displays the test stimulus, or region of interest (ROI) (Figure 2B). Tadpoles usually swim freely in the test dish.
Note: Monitor brightness can be adjusted to a certain set point in the computer display settings and maintained across different experiments/ trials. If using multiple monitors, ensure that the monitor brightness is the same for each computer. For measuring the accurate display properties of a computer monitor, we suggest downloading the “DisplayHDR Test” software.
Note: Monitor temperature generally is not a concern for backlit LCD monitors with IPS panel, OLED monitors, or mini-LED monitors because these types do not heat up; however, CRT monitors, older backlit LCD monitors with VA/TN panel that don’t have an active cooling solution (fans built in the back of the monitor chassis) heat up, and so should not be used for this experiment.
-
6.
Turn on the GoPro camera and record the freely swimming tadpoles for any desired amount of time. The recording is carried out at room temperature (approximately 22°C). Once the recording is completed, turn off the GoPro and retrieve the video for offline analysis.
Note: Do not place a lid over the test dish during imaging as it may interfere with image acquisition.
CRITICAL: The strength in the preference for light has been observed to oscillate over the 24-h light/dark cycle.2 Thus, the experiments should be carried out at the same time of the day to avoid potential variations.
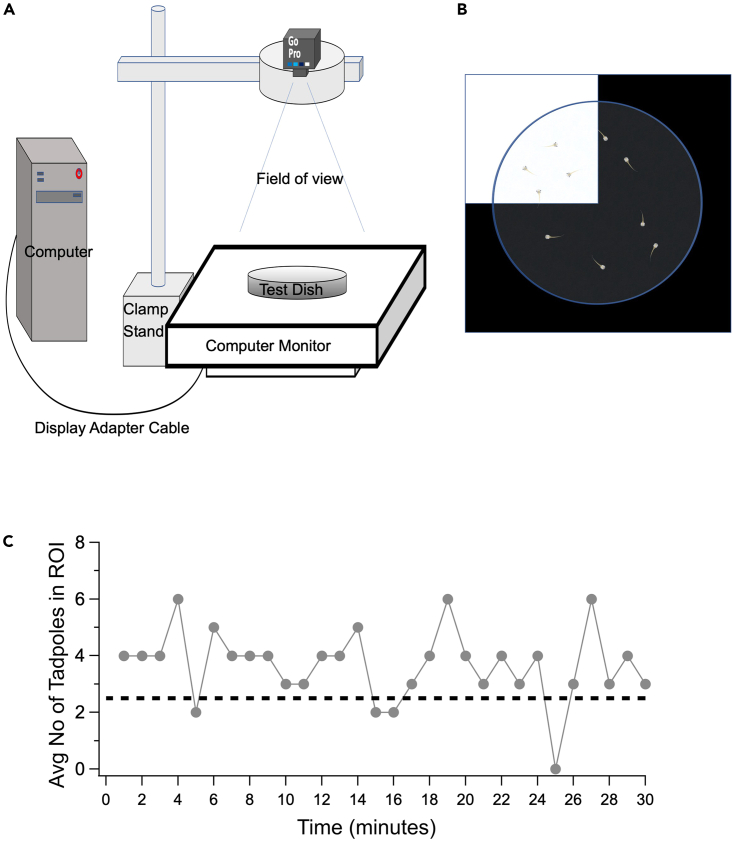
Figure 2.
Experimental set-up and analysis of color/luminance preference test
(A) Equipment set-up: The computer monitor is set to display the test stimulus.
(B) The test dish is placed on the displayed stimulus such that one quarter of the dish represents the region of interest (ROI).
(C) Graph represents the visual preference of tadpoles across a 30-min trial. The preference is determined by counting every minute, on the minute the number of tadpoles that reside in the ROI. The overall preference for the ROI during the trial is determined by averaging the preference across the 30 time-points. Dashed line represents the average number of tadpoles in the ROI that would be expected if there is no preference for, or avoidance of, the ROI.
Expected outcomes
The experimental paradigm described in this protocol is designed to study long-term visual preferences of freely swimming Xenopus tadpoles. The visual preference (or avoidance) is determined by the number of tadpoles that reside in the region of interest (ROI) at any given time during the test trial. Given that there are 10 tadpoles per group, an average score of 2.5 tadpoles (25%) in the ROI indicates no preference for that ROI. A score greater than 25% indicates a preference for the ROI; below 25%, an avoidance of the ROI (Figure 2C). Tadpoles typically display an even distribution across an all-white test dish, reflecting a lack of schooling behavior that could otherwise interfere with the display of each individual’s preference.1
A preference for an ROI (color based or luminance based) could arise due to a change in swimming patterns upon entering the ROI, a decrease in swimming speed while in the ROI, an increase in the frequency with which tadpoles enter the ROI, or a combination of any of these. Video analysis of tadpole swimming behavior can offer insight into the mechanistic basis for the tadpole visual preference. For example, the Xenopus tadpole’s preference for green is manifested mainly by significant slowing down of the tadpoles on entering the green ROI1 while the preference for light over dark manifests mainly through longer swimming bouts in the lighter ROI, manifested by changes in swimming patterns.2
Quantification and statistical analysis
This section describes procedures for quantification of Xenopus tadpole’s group/ individual visual preferences as well as their behavioral dynamics that could account for such preferences.
-
1.
Open-Field Group Color/Luminance Preference Assay.
To quantify tadpole color/luminance preference over time, utilize a scan sampling methodology to analyze videos of freely swimming tadpoles.-
a.Open the GoPro videos in QuickTime player, or any other compatible video player.Note: Some GoPro models may break up videos of experimental trials into multiple files. If this happens, use iMovie, or any other compatible software to combine the individual files into one continuous video.
-
b.At every minute of the recording, on the minute, the number of tadpoles in the ROI is counted (Figure 2C). The overall group preference is obtained by averaging the individual data point across the entire trial.Note: A tadpole is considered to be in the ROI if both of its eyes are in the ROI or if one eye and the majority of its head are in the ROI.Note: While the analysis of the behavior (counting the number of tadpoles in the ROI) is highly objective, analysis may be performed blind to condition to minimize any bias.
-
c.This method can also be used to quantify the visual preferences of individual tadpoles by counting every minute, on the minute, whether the tadpole resides in the ROI, and calculating the percentage of time spent in the ROI.Note: To track individual tadpoles in the dark requires a night vision GoPro.
-
a.
-
2.Behavioral dynamics.
-
a.Swimming speed.
-
i.To quantify tadpole swimming speed, convert the mp4 video of the test trial to AVI file format. Open the AVI file in ImageJ. This will display the original video as still frames.
-
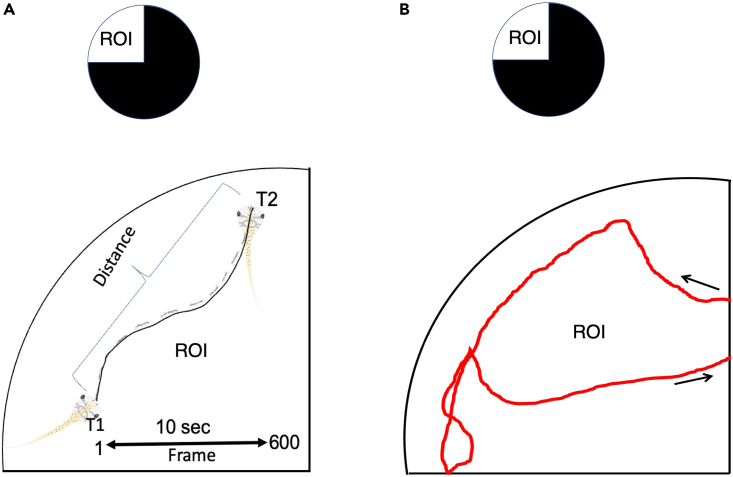
ii.In ImageJ, use the free hand selection tool to trace the tadpole swimming trajectory and measure the distance of the trajectory the tadpole swims in a set amount of time (for example 10 s, which will be 600 frames for a GoPro that records images at 60 frames per second) immediately after entering the ROI (Figure 3A).Note: Swimming speeds are typically measured mid-trial (i.e. not at the very beginning or very end). For tracing of the tadpole trajectory, use the most anterior point of the tadpole head as the measuring point (Figure 3A).
-
iii.Swimming distance is measured using the measure function in ImageJ and converted from pixels to millimeter. For this conversion, set the known distance to be the diameter of the test dish.
-
iv.The swimming speed is calculated by dividing the distance by the time (mm/s).
-
i.
-
b.Swimming bouts.To quantify individual swimming bouts – the amount of time the tadpole stayed in the ROI from when the tadpole first entered the ROI to when the tadpole exited the ROI – track the time in seconds from when the tadpole entered the ROI until the tadpole exited.
-
c.Swimming Pattern.To determine the swimming patterns of individual tadpoles while in the ROI, use line drawings to trace individual tadpole as it swims from the time the tadpole enters the ROI to when it exits the ROI (Figure 3B).
-
d.Cross-over frequency.To quantify the frequency with which tadpoles cross over into the ROI, count the number of tadpoles that enter the ROI within an arbitrarily set time frame (e.g., 1 min) of the trial.
-
a.
Figure 3.
Determining tadpole speed and swimming patterns
(A) (top) Example of stimulus for testing the preference for light (ROI) when light is pitted against dark. (bottom) Schematic showing the tracing of tadpole trajectory within a set time (10 s) in the ROI. The speed is calculated as swimming distance/time.
(B) (top) Example of stimulus for testing the preference for light (ROI) when light is pitted against dark. (bottom) Schematic showing a single swimming bout displayed by a tadpole. ROI: Region of interest, T1 and T2: Time points 1 and 2, arrows indicate the points of entry and exit of tadpole from the ROI.
Limitations
This test measures the visual preferences of freely swimming tadpoles. If tadpoles are immobile for an extended period for any reason (for example due to age or effects of pharmacological or other manipulations), then in such case, the immobility of the tadpoles means that any analysis done would no longer be quantifying tadpole swimming behaviors. Because the normal swimming behavior of tadpoles is established around developmental stage 47, the experiments described in this protocol may not be accurate for measuring the visual preferences of tadpoles younger than stage 47.
Troubleshooting
Problem 1
The computer screen goes off before conclusion of video recording (step 6, step-by-step method details).
Potential solution
Set the computer screen timeout to be longer than the duration of the test trial. Additionally, turn off the monitor’s power-saving mode if active.
Problem 2
The GoPro turns off in the middle of a recording session (step 6, step-by-step method details).
Potential solution
Make sure the GoPro is fully charged before each recording. GoPros display an estimated recording time for each level of battery life. Ensure that your chosen trial time is several minutes less than the estimated recording time.
Problem 3
Video recordings are not successfully saved in GoPro (step 6, step-by-step method details).
Potential solution
This is usually due to either the capacity of GoPro SD card being exceeded, or the SD card has failed or has been damaged. To preserve capacity for future recordings, delete videos from the GoPro after transferring them to a computer. Replace failed or damaged SD cards.
And have backup, appropriately formatted SD cards on hand.
Problem 4
The stimulus displayed on the monitor has a shifted color representation compared to the originally created file (step 4, step-by-step method details).
Potential solution
Make sure the display adapter cable is plugged in correctly in both the monitor and the computer; make sure the monitor is set to normal/factory setting; make sure the display adapter cable is plugged into the discrete graphics card if the computer has one and update the graphics driver to the newest version available, without enabling any display color enhancement or color deficiency correction settings. If none of the above works, make sure the original stimulus file is in a compatible file form.
Problem 5
The GoPro video recordings cannot be opened (step 6, step-by-step method details).
Potential solution
Make sure that your computer has the appropriate hardware and software required to open the videos in the format saved by the GoPro. For example, newer GoPro series save the recorded video in MOV file format, and old devices may not be compatible with it. In this case, it is recommended to use video format converters to convert the GoPro videos into more common formats, such as MP4 format.
Resource availability
Lead contact
Further information and requests for resources and reagents should be directed to and will be fulfilled by the lead contact, [Kara G. Pratt] (kpratt4@uwyo.edu).
Materials availability
This study did not generate new unique reagents.
Data and code availability
This study did not generate or analyze datasets or code.
Acknowledgments
This work was supported by the National Science Foundation (2212591) to K.G.P. U.G.U. was supported by the National Institute of General Medical Sciences (P20GM103432).
Author contributions
U.G.U. wrote original manuscript draft. U.G.U., K.Z., and K.G.P. prepared figures. All authors reviewed, edited, and approved the final manuscript.
Declaration of interests
The authors have no competing interests.
Contributor Information
Uwemedimo G. Udoh, Email: uudoh@uwyo.edu.
Kara G. Pratt, Email: kpratt4@uwyo.edu.
References
- 1.Hunt J.E., Bruno J.R., Pratt K.G. An innate color preference displayed by Xenopus tadpoles is persistent and requires the tegmentum. Front. Behav. Neurosci. 2020;14:71. doi: 10.3389/fnbeh.2020.00071. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Bruno J.R., Udoh U.G., Landen J.G., Osborn P.O., Asher C.J., Hunt J.E., Pratt K.G. A circadian-dependent preference for light displayed by Xenopus tadpoles is modulated by serotonin. iScience. 2022;25:105375. doi: 10.1016/j.isci.2022.105375. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.McNamara S., Wlizla M., Horb M.E. Husbandry, general care, and transportation of Xenopus laevis and Xenopus tropicalis. Methods Mol. Biol. 2018;1865:1–17. doi: 10.1007/978-1-4939-8784-9_1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Wlizla M., McNamara S., Horb M.E. Generation and care of Xenopus laevis and Xenopus tropicalis embryos. Methods Mol. Biol. 2018;1865:19–32. doi: 10.1007/978-1-4939-8784-9_2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Nieuwkoop P.D., Faber J. Routledge; 1994. Normal Table of Xenopus Laevis (Daudin): A Systematical & Chronological Survey of the Development from the Fertilized Egg till the End of Metamorphosis. [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
This study did not generate or analyze datasets or code.