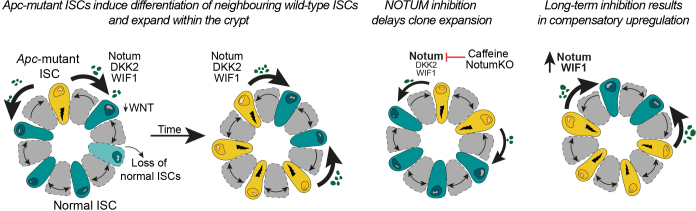
Colorectal cancer (CRC) development is characterized by stepwise accumulation of mutations, of which the majority display early mutations in tumor suppressor gene APC.1 Previous work revealed that Apc loss confers a competitive advantage to mutant intestinal stem cells (ISCs), which consequently replace all normal ISCs and drive crypt fixation in vivo.2 Recent studies demonstrate that this advantage can be attributed to the secretion of Wnt antagonists (eg, NOTUM/WIF1/DKK2) that drive normal ISC differentiation.3,4 In particular, NOTUM, which functions as negative regulator of Wnt activity by deacylating Wnt ligands,5 poses an interesting chemoprevention target because it is highly up-regulated in Apc-mutant murine and human cells.3,4,6 Interestingly, recent work has identified caffeine as a potent NOTUM inhibitor by binding its catalytic pocket and thereby inhibiting its function.7 Therefore, in this study, we investigate the chemopreventive effects of caffeine on the expansion of Apc-mutant clones in the intestine (see also Supplementary Methods).
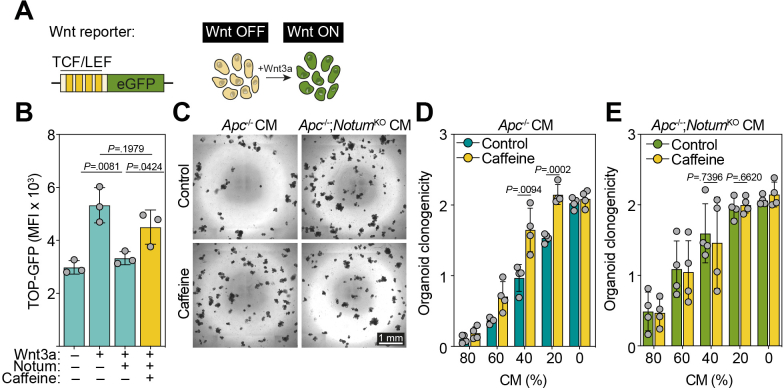
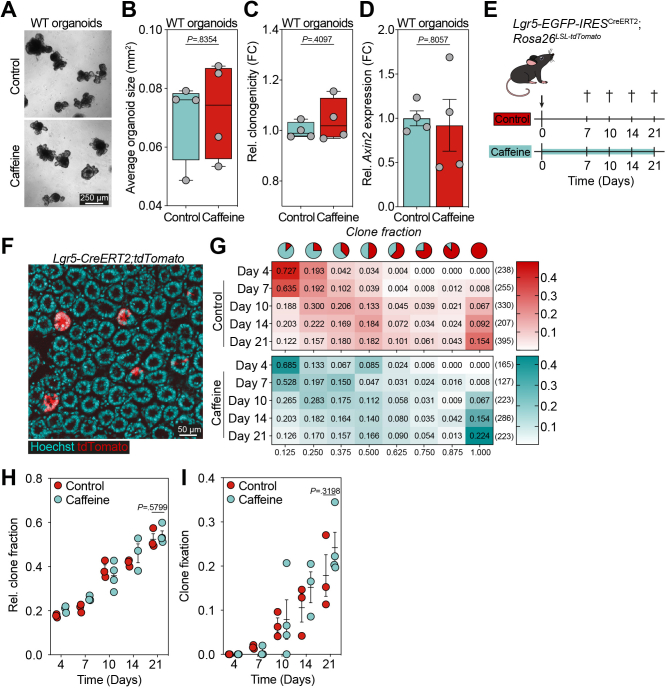
To assess the role of caffeine on Wnt signaling in vitro, we first validated the recently described inhibitory effect of caffeine on NOTUM7 by using a Wnt reporter cell line (Supplementary Figure 1A and B). Administration of recombinant NOTUM decreased Wnt pathway activity, an effect that is alleviated by supplementing 200 μmol/L caffeine (Supplementary Figure 1B). Next, we investigated the effect of caffeine on intestinal organoids. We previously demonstrated that incubation of wild-type organoids with conditioned medium (CM) of Apc-/- organoids resulted in loss of clonogenic potential.3,4 Moreover, we demonstrated that dilution of CM results in a dose-dependent rescue of clonogenicity, an effect that occurred at lower dilution using CM derived from Apc-/-;NotumKO organoids,3 highlighting the importance of NOTUM in executing the inhibitory effect.3 Here, we reveal that addition of 200 μmol/L caffeine to Apc-/- CM improves wild-type clonogenicity to a similar extent as previously reported using Apc-/-;NotumKO CM3 (Figure 1A, Supplementary Figure 1C and D). Importantly, no effect was observed between organoids incubated with Apc-/-;NotumKO CM with or without caffeine (Figure 1A, Supplementary Figure 1E). Together, these data indicate that caffeine reduces the Wnt inhibiting effects of Apc-mutant cells on their wild-type counterparts. Of note, caffeine treatment alone did not affect wild-type organoid growth, size, clonogenicity, and expression of Wnt target gene Axin2 (Supplementary Figure 2A–D).
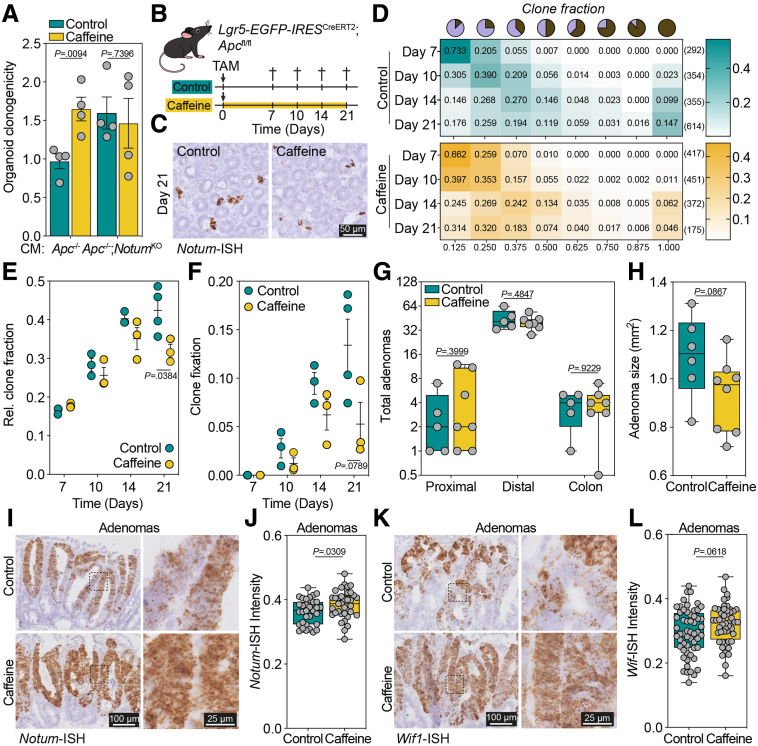
Figure 1.
(A) Clonogenicity of WT organoids incubated with 40% Apc-/-CM or Apc-/-;NotumKOCM with/without caffeine (n = 4). (B) Schematic illustration of in vivo experiment. (C) Detection of Apc-mutant clones using Notum-ISH. (D–F) Clone fraction distributions (n = no. of crypts) (D), average clone fraction (E), and crypt fixation (F) of Apc-mutant clones in the absence/presence of caffeine (n = 3–4 mice per group). (G and H) Adenoma number per intestinal region (G), average adenoma size in the distal SI (H) (n = 5–7 mice per group). (I–L) Notum (I and J) and Wif1 (K and L) expression in adenomas of mice treated with/without caffeine (n = 3 mice, each dot is an ISH-positive region).
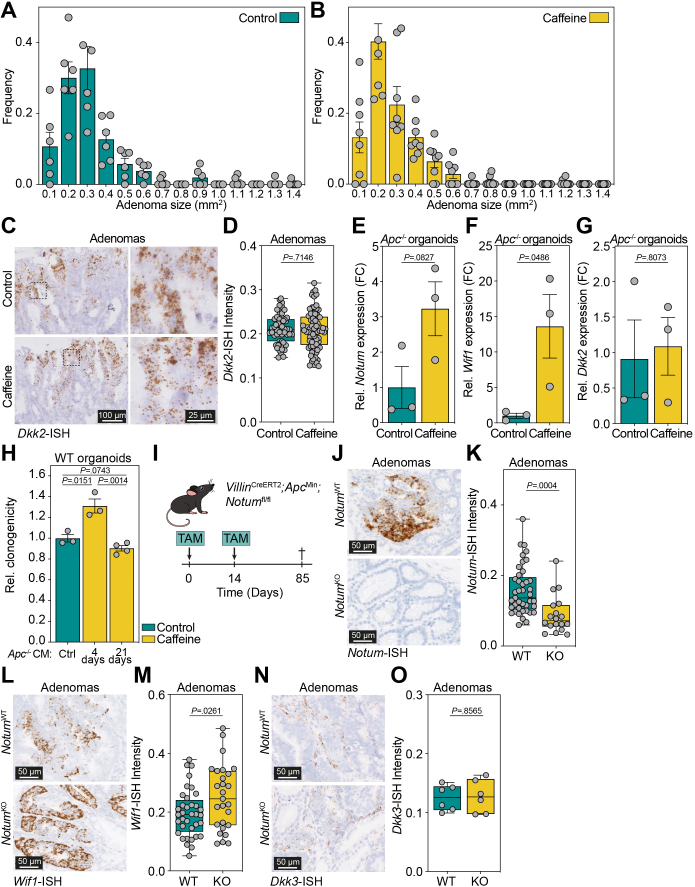
We next assessed the influence of caffeine on normal and mutant ISC dynamics in vivo. We induced Apc loss and traced the expansion of Apc-deficient clones over time in the absence or presence of caffeine administered in the drinking water (400 mg/L) (Figure 1B). As previously reported, Apc-mutant clones can be visualized by Notum expression (Figure 1C),6 and clone fraction distributions were followed over time (Figure 1D). We detected a significant reduction in the average clone fraction (Figure 1E) and observed a reduced number of fixed mutant clones (Figure 1F) in caffeine-treated mice. In line with our in vitro findings, wild-type ISC dynamics did not significantly change upon caffeine administration (Supplementary Figure 2E–I). Although the reduction in crypt fixation of Apc-mutant clones would suggest a subsequent reduction in adenoma development, long-term administration of caffeine does not impact the number and location of adenomas (Figure 1G). However, average adenoma size (Figure 1H) and the corresponding size distributions per animal (Supplementary Figure 3A and B) suggest that adenomas in caffeine-treated mice are generally smaller, pointing toward a delay in adenoma development. Closer analysis of these adenomas reveals a significant increase in Notum and elevated Wif1 expression in response to long-term caffeine treatment (Figure 1I–L), whereas Dkk2 expression is unaltered (Supplementary Figure 3C and D). These findings suggest that long-term NOTUM inhibition results in a compensatory up-regulation of Wnt antagonists. In line with this observation, long-term (21 days) in vitro caffeine administration to Apc-mutant organoids resulted in elevated Notum expression and significant Wif1 up-regulation (Supplementary Figure 3E–G). Moreover, CM transfer of Apc-mutant organoids pretreated with caffeine for 21 days failed to rescue wild-type clonogenicity compared with CM of short-term (4 days) treated Apc-mutant organoids (Supplementary Figure 3H). To strengthen these findings, we analyzed adenomas from NotumKO mice (Supplementary Figure 3I).4 Previous work revealed that concomitant Apc and Notum loss reduces adenoma size and number but does not completely prevent adenoma formation, suggesting that Notum loss also delays tumor initiation.4 To assess whether this is caused by compensatory up-regulation of Wnt antagonists, we quantified the expression of the 3 most up-regulated Wnt antagonists detected in this model4: Notum, Wif1, and Dkk3. As expected, we observed a marked decrease in Notum expression in NotumKO mice (Supplementary Figure 3J and K). Furthermore, NotumKO adenomas display increased Wif1 expression (Supplementary Figure 3L and M), whereas Dkk3 expression remains unchanged (Supplementary Figure 3N and O). Together, our findings reveal that long-term NOTUM inhibition by caffeine or conditional Notum loss activates a feedback loop that facilitates increased expression of Wnt antagonists resulting in progression of adenoma formation (Supplementary Figure 4). We specifically observe up-regulation of Notum and Wif1, which work at the Wnt-ligand level, but not Dkk2 and Dkk3, which interfere with co-receptors LRP5/6, suggesting that this feedback loop could function to control the bioavailability of Wnt ligands. Our findings are especially relevant because of the vast global consumption of caffeinated beverages and the high prevalence of APC mutations in sporadic CRC. This study emphasizes the inherent difficulty of targeting the Wnt pathway as a cancer prevention strategy, and future research should consider the duration of caffeine administration as well as dosing schedules to avoid compensation. Although it remains unclear whether caffeine is the only putative cancer protective ingredient in coffee,8 our results could potentially explain why coffee intake is associated with a reduced risk of CRC development9 and progression.10
Footnotes
Conflicts of interest This author discloses the following: LV received consultancy fees from Bayer, Genentech, MSD, Servier, and Pierre Fabre, but these had no relation to the content of this publication. Currently LV is an employee of Genentech-Roche. The remaining authors disclose no conflicts.
Funding S.M.v.N. is supported by ZonMw (Rubicon 452021320) and an EMBO Postdoctoral Fellowship (122-2022, non-stipendiary). This work is supported by Oncode Institute, the New York Stem Cell Foundation and grants from the European Research Council (ERG-CoG 101045612 - NIMICRY) and ZonMw (Vici 09-15018-21-10029) to L.V. L.V. is a New York Stem Cell Foundation–Robertson Stem Cell Investigator.
Contributor Information
Louis Vermeulen, Email: l.vermeulen@amsterdamumc.nl.
Sanne M. van Neerven, Email: s.m.vanneerven@amsterdamumc.nl.
Supplementary Material
Supplementary Figure.
Supplementary Figure.
Supplementary Figure.
Supplementary Figure.
References
- 1.Fearon E.R., et al. Cell. 1990;61:759–767. doi: 10.1016/0092-8674(90)90186-i. [DOI] [PubMed] [Google Scholar]
- 2.Vermeulen L., et al. Science. 2013;342:995–998. doi: 10.1126/science.1243148. [DOI] [PubMed] [Google Scholar]
- 3.van Neerven S.M., et al. Nature. 2021;594:1–6. [Google Scholar]
- 4.Flanagan D.J., et al. Nature. 2021;594:430–435. doi: 10.1038/s41586-021-03525-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Kakugawa S., et al. Nature. 2015;519:187–192. doi: 10.1038/nature14259. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Kleeman S.O., et al. Gut. 2020;69:1092–1103. doi: 10.1136/gutjnl-2019-319126. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Zhao Y., et al. Commun Biol. 2020;3:1–8. [Google Scholar]
- 8.Sartini M., et al. Nutrients. 2019;11:694. doi: 10.3390/nu11030694. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Wang Y., et al. Eur J Clin Nutr. 2019;74:297–306. doi: 10.1038/s41430-019-0467-0. [DOI] [PubMed] [Google Scholar]
- 10.Mackintosh C., et al. JAMA Oncol. 2020;6:1713–1721. doi: 10.1001/jamaoncol.2020.3938. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.