Summary
Aim
The aim of this study was to evaluate the association between the left main coronary artery (LM) bifurcation angle and the severity of the proximal left anterior descending coronary artery (LAD) stenosis.
Methods
Two hundred patients with suspected coronary artery disease who had coronary angiography were included in this observational study. The severity of coronary artery stenosis was analysed using quantitative coronary angiography software (QCA analysis). The LM–LAD and LAD–left circumflex artery (LCX) angles were measured using software (IC MEASURE) in two-dimensional axial images.
Results
The patients were divided into two groups. The first group included 100 patients with significant proximal LAD stenosis (≥ 50%) and the second, those with LAD stenosis < 50% (100 patients). Patients with significant proximal LAD stenosis were older and had a higher frequency of diabetes mellitus, and higher serum creatinine and low-density lipoprotein levels than those with non-significant LAD stenosis. The LM–LAD and LAD–LCX angles in patients with significant proximal LAD stenosis were wider than in patients with non-significant LAD stenosis (p < 0.001). The cut-off value of 42° of the LM–LAD angle had a sensitivity of 73% and specificity of 70% to predict significant proximal LAD stenosis. The cut-off value of 68° of the LAD–LCX angle had a sensitivity of 68% and specificity of 62% to predict significant proximal LAD disease. In a multivariate logistic regression analysis, LM–LAD and LAD–LCX angles were independent factors for the development of significant proximal LAD stenosis.
Conclusion
Wider LM–LAD and LAD–LCX angles were associated with the severity of proximal LAD disease. Preventative measures and close follow up are needed in such cases to improve their cardiovascular outcome.
Keywords: coronary artery disease, left main coronary artery, bifurcation angle, left anterior descending artery stenosis
Alteration of coronary wall shear stress is a major cause of coronary atherosclerosis. Maintaining a laminar shear stress is necessary for optimal vascular function, which includes the regulation of vascular diameter and inhibition of vessel wall inflammation.1,2 At the arterial bifurcation and vessel curvatures, blood flow is disturbed and becomes non-laminar, reducing shear stress (low endothelial shear stress). Atheromatous plaques are frequently developed at these sites. Non-laminar blood flow causes endothelial dysfunction as it initiates oxidative and inflammatory stress, with subsequent plaque formation.3,4
Coronary bifurcation angles have previously been investigated because a wide bifurcation angle is associated with low shear stress. This alters coronary blood flow, resulting in progression of coronary atherosclerosis and formation of atheromatous plaques.5-8
The angles of the left main coronary artery (LM)–left anterior descending coronary artery (LAD) and LAD–left circumflex artery (LCX) have previously been investigated. All these studies used multi-slice computed tomography (CT) coronary angiography.9-13 To our knowledge, these bifurcating angles have not been investigated before using invasive coronary angiography. This study aimed to determine the relationship between the LM–LAD and LAD–LCX angles and the severity of proximal LAD stenosis in individuals who had coronary angiography for suspected coronary artery disease.
Methods
This was an observational study that included 200 patients with suspected coronary artery disease who had coronary angiography at the Cardiology Department of Menoufia University Hospital in Egypt between January and July 2021. Patients with prior coronary artery bypass graft or prior percutaneous coronary intervention (PCI) and those with significant valvular heart diseases were excluded from the study.
Each patient signed an informed consent form. Menoufia University’s institutional review board evaluated and approved the study (IRB approval number: 11/2019CARD3) according to the declaration of Helsinki.
Patients were subjected to a complete medical history, including cardiovascular risk factors and a physical examination. Resting electrocardiogram and echocardiography reports were reviewed for all patients.
The procedure of coronary angiography was performed by a team of well-established cardiologists. A Siemens machine was used (AXIOM-Artis, Siemens, Forchheim, Germany). The procedure was performed according to the standard Seldinger technique. Radial arterial access was used in 44 patients and femoral arterial access in 156 patients. Images were recorded at frames per second at a resolution of 512 × 512 pixels for analytical purposes. Standard angiographic views were used in the assessment of the coronary arteries (anteroposterior, left anterior oblique, right anterior oblique) in cranial and caudal projections.
A quantitative coronary angiography software program was used to determine the severity of coronary artery stenosis (QCA Analysis, Siemens, Germany).14 The diameter of the minimum lumen was determined in projections exhibiting the most extreme narrowing. According to the absence or presence of proximal LAD stenosis, patients were divided into two groups. Significant coronary stenosis was defined by more than 50% luminal diameter reduction.15
The LM bifurcation to the LAD and LCX was assessed in spider view [left anterior oblique caudal (LAO)] at an angle of 40°, with 30° caudal projection, and in the right anterior oblique caudal (RAO) at an angle of 30°, with 20° caudal projection. The LM–LAD and LAD–LCX angles were measured using automated software (IC MEASURE) in the LAO caudal (spider) view. Still photos (screenshots) were obtained from the spider view angiographic run and lines were manually drawn corresponding to the outer edges of the LM, LAD and LCX arteries. The angles were measured twice at end-diastolic frames and the average of the angles was recorded in order to decrease inter-observer variability (Figs 1, 2).
Spider view of a 63-year-old patient with significant LAD stenosis (B). The LM–LAD angle was measured at 62.7° (A). LM: left main coronary artery, LAD: left anterior descending artery.
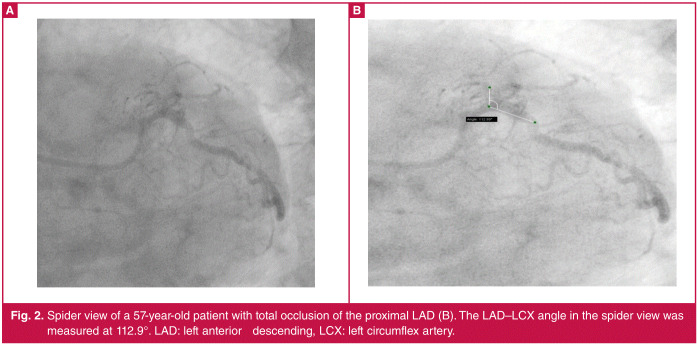
Spider view of a 57-year-old patient with total occlusion of the proximal LAD (B). The LAD–LCX angle in the spider view wasmeasured at 112.9°. LAD: left anterior descending, LCX: left circumflex artery.
Statistical analysis
Data as a whole were gathered and reviewed using the Statistical Package for Social Sciences (IBM SPSS) version 20. Qualitative data are presented as percentages and numbers; for parametric distribution, quantitative data are presented as means, standard deviations (SD) and range; and for non-parametric distribution, quantitative data are presented as medians with inter-quartile range (IQR). When comparing two groups with qualitative data, the chi-squared and Fisher’s exact tests were employed. When comparing two groups with parametric distribution of quantitative data, we used the independent t-test, whereas the Mann–Whitney test was used to compare two groups that had quantitative data but a non-parametric distribution.
A receiver operating characteristic (ROC) curve was used to calculate the diagnostic cut-off value of the bifurcating angle. Logistic regression analysis was used to detect factors associated with significant LAD stenosis. The confidence interval (CI) was set at 95% while the acceptable margin of error was set at 5%. As a result, the p-value was considered significant when < 0.05.
Results
Patients were divided into two groups; the first included patients who had proximal LAD stenosis ≥ 50% (100 patients) and the second those with LAD stenosis < 50% (100 patients). Patients with significant proximal LAD stenosis were older and had a higher frequency of diabetes mellitus, and higher serum creatinine and low-density lipoprotein cholesterol (LDL-C) levels than those with non-significant LAD stenosis. Patients’ characteristics and laboratory data are provided in Table 1.
The mean of the angles of the LM–LAD in patients with significant proximal LAD stenosis was wider than in patients with non-significant disease (60.91 ± 25.93 vs 38.91 ± 21.33°, p < 0.001). The mean of the angles of the LAD–LCX in patients with significant proximal LAD stenosis was wider than in patients with non-significant LAD stenosis (84.55 ± 32.98 vs 68.01 ± 28.12°, p < 0.001). The bifurcating coronary angles in the two groups are provided in Table 2.
Table 1. Patients’ characteristics and laboratory data of the population studied.
| Characteristics | pLAD 50% (n=100) | pLAD < 50% n=100) | Test of significance | p-value |
| Age, years | 54.47 + 11.68 | 47.30 + 12.78 | t=4.142 | <0.001 |
| Males, n, % | 58 (58) | 51 (51) | x2=0.988 | 0.320 |
| Diabetes mellitus, n, % | 37 (37) | 21 (21) | x2=6.217 | 0.013 |
| Hypertension, n, % | 23 (23) | 13 (13.0) | x2 =3.388 | 0.066 |
| Smoking, n, % | 23 (23) | 17 (17) | x2=1.125 | 0.289 |
| Dyslipidaemia, n, % | 34 (34) | 28 (28) | x2=0.842 | 0.359 |
| Creatinine, mg/dl | 1.02 + 0.26 | 0.89 + 0.19 | t=3.915 | < 0.001 |
| Total cholesterol, mg/dl | 193.50 + 31.1 | 203.24 + 34.34 | t=2.103 | 0.037 |
| [mmol/l] | [5.01 + 0.81] | [5.26 + 0.89] | ||
| LDL-C, mg/dl | 127.24 + 29.67 | 136 + 32.57 | t=1.988 | 0.048 |
| [mmol/l] | [3.30 + 0.77] | [3.52 + 0.84] | ||
| HDL-C, mg/dl | 38.82 + 6.91 | 38.78 + 7.89 | t=0.038 | 0.970 |
| [mmol/l] | [1.01 + 0.18] | [1.00 + 0.20] | ||
| Triglycerides, mg/dl | 138.36 + 49.45 | 145.35 + 58.69 | U=4476.5 | 0.201 |
| [mmol/l] | [1.56 + 0.56] | [1.64 + 0.66] | ||
| LVEF, % | 53.31 + 8.83 | 59.63 + 6.46 | 5.777 | < 0.001 |
pLAD: proximal left anterior descending artery, HDL-C: high-density lipoprotein cholesterol, LDL-C: low-density lipoprotein cholesterol, LVEF: left ventricular ejection fraction.
Table 2. Bifurcating coronary angles in the two groups studied.
| Angles | pLAD 50% (n = 100) | pLAD < 50% (n = 100) | Test of significance | p-value |
| LM-LAD angle, O | 60.91 + 25.93 | 38.91 + 21.33 | U = 2533.5 | < 0.001 |
| LAD-LCX angle, O | 84.55 + 32.98 | 68.01 + 28.12 | U = 3453.5 | < 0.001 |
Data are described as mean ± SD. LM: left main coronary artery, pLAD: proximal left anterior descending artery, LCX: left circumflex artery.
The validity for LM–LAD and LAD–LCX angles to predict significant proximal LAD stenosis is demonstrated in Table 3. The ROC curve for the LM–LAD angle had an area under the curve (AUC) of 0.747. The cut-off value of 42° of the LM–LAD angle had a sensitivity of 73%, specificity of 70%, positive predictive value of 70% and negative predictive value of 72.2% to predict significant proximal LAD stenosis. The ROC curve of the LAD–LCX angle had an AUC of 0.655. The cut-off value of 68° of the LAD–LCX angle had a sensitivity of 68%, specificity of 62%, positive predictive value of 64.3% and negative predictive value of 66% to predict significant proximal LAD stenosis (Fig. 3).
Table 3. Validity (AUC, sensitivity, specificity) for the LM–LAD and LAD–LCX angles to predict significant coronary artery stenosis.
| Angles | AUC | p-value | 95% CI | Cut off | Sensi- tivity | Speci- ficity | PPV | NPV |
| LM-LAD angle | 0.747 | < 0.001* | 0.677-0.816 | > 42 | 73.0 | 70.0 | 70.9 | 72.2 |
| LAD-LCX angle | 0.655 | < 0.001* | 0.578-0.731 | > 68 | 68.0 | 62.0 | 64.3 | 66.0 |
AUC: area under a curve, CI: confidence interval, PPV: positive predictive value, NPV: negative predictive value. *Significant as p < 0.05

ROC curve for the LM–LAD and LAD–LCX angles for prediction of LAD stenosis.
The LM–LAD and LAD–LCX angles and other factors associated with development of significant proximal LAD stenosis [age, diabetes mellitus, left ventricular ejection fraction, LDL-C, total cholesterol and serum creatinine] were evaluated in a multivariate logistic regression analysis. The LM–LAD and LAD–LCX angles were independent factors for the development of significant proximal LAD stenosis. The odds ratio for the LM–LAD angle was 1.094 and 95% CI was 1.053–1.137, with p < 0.001. The odds ratio for the LAD–LCX angle was 0.951 and 95% CI was 0.924–0.978, with p = 0.001 (Table 4).
Table 4. Univariate and multivariate logistic regression analysis for the parameters affecting coronary artery disease.
| Univariate | Multivariate | |||
| Parameters | p-value | OR (95% CI) | p-value | OR (95% CI) |
| Age, years | < 0.001 | 1.048 (1.024-1.073) | 0.001 | 1.054 (1.022-1.088) |
| Females | 0.321 | 0.754 (0.431-1.317) | ||
| Diabetes mellitus | 0.014 | 2.209 (1.177-4.147) | 0.366 | 1.502 (0.621-3.629) |
| Hypertension | 0.069 | 1.999 (0.948-4.215) | ||
| Dyslipidaemia | 0.360 | 1.325(0.726-2.417) | ||
| Smoking | 0.290 | 1.458 (0.725-2.935) | ||
| Total cholesterol | 0.039 | 0.991 (0.982-1.0) | 0.831 | 0.996 (0.965-1.029) |
| LDL-C | 0.049 | 0.991 (0.982-1.0) | 0.681 | 0.993 (0.960-1.027) |
| HDL-C | 0.969 | 1.001 (0.964-1.039) | ||
| Triglycerides | 0.366 | 0.998 (0.992-1.0) | ||
| Creatinine, mg/dl | < 0.001 | 0.090 (0.025-0.326) | ||
| LVEF, % | < 0.001 | 0.895 (0.855-0.936) | 0.001 | 0.913 (0.866-0.963) |
| LM-LAD angle | < 0.001 | 1.039 (1.025-1.053) | < 0.001 | 1.094 (1.053-1.137) |
| LAD-LCX angle | < 0.001 | 1.018 (1.008-1.028) | 0.001 | 0.951 (0.924-0.978) |
HDL-C: high-density lipoprotein cholesterol, LDL-C: low density lipoprotein cholesterol, LVEF: left ventricular ejection fraction, LM: left main coronary artery, LAD: left anterior descending artery, LCX: left circumflex artery, OR: odds ratio, CI: confidence interval, LL: lower limit, UL: upper limit.
Discussion
Wide LM–LAD and LAD–LCX angles cause low wall shear stress, with subsequent progression of LAD atherosclerosis. As the bifurcation angle increased, the blood turbulence increased. Turbulence induced endothelial dysfunction, enhanced adhesion molecule expression, foam cell production and proliferation of smooth muscle cells. All these mechanisms contribute to the progression of coronary plaques in the area near to the bifurcation angle.16,17
The relationship between bifurcation angle and wall shear stress was previously investigated by multi-slice coronary computed tomography. Chaichana et al.18 reported a significant relationship between the LAD–LCX angle measured by coronary CT angiography and the severity of LAD coronary stenosis. Patients with angles more than 75° had more significant LAD stenosis than those with smaller angles. Sun et al.19 also used CT coronary angiography and they reported an LAD–LCX angle of 94 ± 19° in patients with significant LAD stenosis, versus 75 ± 19° in patients with a normal LAD. Similarly, Rodriguez- Granillo et al.20 reported a cut-off value of 88.5° could predict significant ostial to mid LAD and LCX disease, and they concluded that the left main bifurcation angle and severity of the plaques are closely related.
In our study, we assessed the LM bifurcation angle during coronary angiography using automated software (IC MEASURE), and to our knowledge, the association between bifurcation angle and the severity of proximal LAD stenosis has not been investigated before by invasive coronary angiography in patients who had no previous PCI.
The angles of LM–LAD and LAD–LCX in our report were wider in patients with significant proximal LAD disease than in those with non-significant disease (60.91 ± 25.93 vs 38.91 ± 21.33° and 84.55 ± 32.98 vs 68.01 ± 28.12°, respectively). The cut-off value of 42° of the LM–LAD angle had a sensitivity of 73% and specificity of 70% to predict significant proximal LAD stenosis. The cut-off value of 68° of the LAD–LCX angle had a sensitivity of 68% and specificity of 62% to predict significant proximal LAD stenosis. The bifurcation angles retained statistical significance in a multivariate logistic regression analysis.
In this study, patients with significant proximal LAD stenosis were older than those with non-significant disease. Age has a role in worsening of vascular functionality, increasing the risk of cardiovascular disease. There is a direct relationship between age and prevalence of cardiovascular disease.21,22 In addition, in this study, patients with significant LAD stenosis demonstrated higher LDL-C values and higher prevalence of diabetes mellitus than those with non-significant LAD stenosis.
Diabetes mellitus and dyslipidaemia are risk factors for cardiovascular disorders.23 Studies have shown that the administration of statins and thiazolidinedione (pioglitazone and rosiglitazone) causes a significant regression of the coronary atherosclerotic plaques and they can be used in the secondary prevention of cardiovascular disease.24-26
A wide angle between the LM and LAD could predict in-stent restenosis after stenting of the proximal LAD, as reported by Konishi et al.27 The effect of the bifurcation angle on 12-month outcome after stenting of the LM–LAD was investigated by Amemiya et al.28 The angle was evaluated by three-dimensional quantitative coronary angiography analysis. They reported a significant relationship between the pre-procedure bifurcation angle and adverse cardiac events.
In a sub-study of the SYNTAX trial, the bifurcation angle had an impact on five-year outcome after stenting of the main left coronary artery.29 A LM–LAD angle of more than 152° during LM bifurcation stenting was associated with a greater risk of target lesion failure in the crush technique.30
Our study had some limitations. It had a limited sample size and was conducted at a single medical centre. Its findings should be confirmed in additional centres with a larger sample size. Assessment of the severity of LAD stenosis by intravascular ultrasound was unavailable. The results should be confirmed in further investigations employing intravascular ultrasound.
Conclusion
Wider LM–LAD and LAD–LCX angles were associated with severity of proximal LAD stenosis. Preventative measures and close follow up are needed in such cases to improve the cardiovascular outcome.
References
- 1.Cunningham KS, Gotlieb AI. The role of shear stress in the pathogenesis of atherosclerosis. Lab Invest. 2005;85(1):9–23. doi: 10.1038/labinvest.3700215. [DOI] [PubMed] [Google Scholar]
- 2.Siasos G, Sara JD, Zaromytidou M. Local low shear stress and endothelial dysfunction in patients with nonobstructive coronary atherosclerosis. J Am Coll Cardiol. 2018;71(19):2092–2102. doi: 10.1016/j.jacc.2018.02.073. [DOI] [PubMed] [Google Scholar]
- 3.Wentzel JJ, Chatzizisis YS, Gijsen FJ, Giannoglou GD, Feldman CL, Stone PH. Endothelial shear stress in the evolution of coronary atherosclerotic plaque and vascular remodelling: current understanding and remaining questions. Cardiovasc Res. 2012;96(2):234–243. doi: 10.1093/cvr/cvs217. [DOI] [PubMed] [Google Scholar]
- 4.Rodriguez-Granillo GA, García-García HM, Wentzel J. Plaque composition and its relationship with acknowledged shear stress patterns in coronary arteries. J Am Coll Cardiol. 2006;47(4):884–885. doi: 10.1016/j.jacc.2005.11.027. [DOI] [PubMed] [Google Scholar]
- 5.Mollet NR, Cademartiri F, van Mieghem F. High-resolution spiral computed tomography coronary angiography in patients referred for diagnostic conventional coronary angiography. Circulation. 2005;112(15):2318–2323. doi: 10.1161/CIRCULATIONAHA.105.533471. [DOI] [PubMed] [Google Scholar]
- 6.Schuijf JD, Beck T, Burgstahler C. Differences in plaque composition and distribution in stable coronary artery disease versus acute coronary syndromes; non-invasive evaluation with multi-slice computed tomography. Acute Card Care. 2007;9(1):48–53. doi: 10.1080/17482940601052648. [DOI] [PubMed] [Google Scholar]
- 7.Moon SH, Byun JH, Kim JW. Clinical usefulness of the angle between left main coronary artery and left anterior descending coronary artery for the evaluation of obstructive coronary artery disease. PLoS One. 2018;13(9):e0202249. doi: 10.1371/journal.pone.0202249. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Juan YH, Tsay PK, Shen WC. Comparison of the left main coronary bifurcating angle among patients with normal, non-significantly and significantly stenosed left coronary arteries. Sci Rep. 2017;7(1):1515. doi: 10.1038/s41598-017-01679-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Temov K, Sun Z. Coronary computed tomography angiography investigation of the association between left main coronary artery bifurcation angle and risk factors of coronary artery disease. Int J Cardiovasc Imaging. 2016;32:129–137. doi: 10.1007/s10554-016-0884-2. [DOI] [PubMed] [Google Scholar]
- 10.Cademartiri F, Mollet NR, Runza G. Diagnostic accuracy of multislice computed tomography coronary angiography is improved at low heart rates. Int J Cardiovasc Imaging. 2006;22(1):101–105. doi: 10.1007/s10554-005-9010-6. [DOI] [PubMed] [Google Scholar]
- 11.Kimura BJ, Russo RJ, Bhargava V, McDaniel MB, Peterson KL, DeMaria AN. Atheroma morphology and distribution in proximal left anterior descending coronary artery: in vivo observations. J Am Coll Cardiol. 1996;27(4):825–831. doi: 10.1016/0735-1097(95)00551-x. [DOI] [PubMed] [Google Scholar]
- 12.Pflederer T, Ludwig J, Ropers D, Daniel WG, Achenbach S. Measurement of coronary artery bifurcation angles by multidetector computed tomography. Invest Radiol. 2006;41:793–798. doi: 10.1097/01.rli.0000239318.88270.9f. [DOI] [PubMed] [Google Scholar]
- 13.Sun Z, Xu L, Fan Z. Coronary CT angiography in calcified coronary plaques: Comparison of diagnostic accuracy between bifurcation angle measurement and coronary lumen assessment for diagnosing significant coronary stenosis. Int J Cardiol. 2016;203:78–86. doi: 10.1016/j.ijcard.2015.10.079. [DOI] [PubMed] [Google Scholar]
- 14.Tu S, Jing J, Holm NR. In vivo assessment of bifurcation optimal viewing angles and bifurcation angles by three-dimensional (3D) quantitative coronary angiography. Int J Cardiovasc Imaging. 2012;28:1617–1625. doi: 10.1007/s10554-011-9996-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Fihn SD, Blankenship JC, Alexander KP. 2014 ACC/AHA/AATS/PCNA/SCAI/STS focused update of the guideline for the diagnosis and management of patients with stable ischemic heart disease: a report of the American College of Cardiology/American Heart Association Task Force on Practice Guidelines, and the American Association for Thoracic Surgery, Preventive Cardiovascular Nurses Association, Society for Cardiovascular Angiography and Interventions, and Society of Thoracic Surgeons. J Thorac Cardiovasc Surg. 2015;149(3):e5–23. doi: 10.1016/j.jtcvs.2014.11.002. [DOI] [PubMed] [Google Scholar]
- 16.Cecchi E, Giglioli C, Valente S. Role of hemodynamic shear stress in cardiovascular disease. Atherosclerosis. 2011;214(2):249–256. doi: 10.1016/j.atherosclerosis.2010.09.008. [DOI] [PubMed] [Google Scholar]
- 17.VanderLaan PA, Reardon CA, Getz GS. Site specificity of atherosclerosis: site-selective responses to atherosclerotic modulators. Arterioscler Thromb Vasc Biol. 2004;24:12–22. doi: 10.1161/01.ATV.0000105054.43931.f0. [DOI] [PubMed] [Google Scholar]
- 18.Chaichana T, Sun Z, Jewkes J. Computation of hemodynamics in the left coronary artery with variable angulations. J Biomech. 2011;44:1869–1878. doi: 10.1016/j.jbiomech.2011.04.033. [DOI] [PubMed] [Google Scholar]
- 19.Sun Z, Cao Y. Multislice CT angiography assessment of left coronary artery: correlation between bifurcation angle and dimensions and development of coronary artery disease. Eur J Radiol. 2011;79:e90–e95. doi: 10.1016/j.ejrad.2011.04.015. [DOI] [PubMed] [Google Scholar]
- 20.Rodriguez-Granillo GA, Rosales MA, Degrossi E, Durbano I, Rodriguez AE. Multislice CT coronary angiography for the detection of burden, morphology and distribution of atherosclerotic plaques in the left main bifurcation. Int J Cardiovasc Imaging. 2007;23:389–392. doi: 10.1007/s10554-006-9144-1. [DOI] [PubMed] [Google Scholar]
- 21.North BJ, Sinclair DA. The intersection between aging and cardiovascular disease. Circ Res. 2012;110:1097–1108. doi: 10.1161/CIRCRESAHA.111.246876. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Rodgers JL, Jones J, Bolleddu SI. Cardiovascular risks associated with gender and aging. J Cardiovasc Dev Dis. 2019;6(2):19. doi: 10.3390/jcdd6020019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Malakar AK, Choudhury D, Halder B. A review on coronary artery disease, its risk factors, and therapeutics. J Cell Physiol. 2019;234(10):16812–16823. doi: 10.1002/jcp.28350. [DOI] [PubMed] [Google Scholar]
- 24.Takayama T, Hiro T, Yamagishi M. Effect of rosuvastatin on coronary atheroma in stable coronary artery disease: multicenter coronary atherosclerosis study measuring effects of rosuvastatin using intravascular ultrasound in Japanese subjects (COSMOS). Circ J. 2009;73:2110–2117. doi: 10.1253/circj.cj-09-0358. [DOI] [PubMed] [Google Scholar]
- 25.Nakayama T, Komiyama N, Yokoyama M. Pioglitazone induces regression of coronary atherosclerotic plaques in patients with type 2 diabetes mellitus or impaired glucose tolerance: a randomized prospective study using intravascular ultrasound. Int J Cardiol. 2010;138(2):157–165. doi: 10.1016/j.ijcard.2008.08.031. [DOI] [PubMed] [Google Scholar]
- 26.Gerstein HC, Ratner RE, Cannon CP. Effect of rosiglitazone on progression of coronary atherosclerosis in patients with type 2 diabetes mellitus and coronary artery disease: the assessment on the prevention of progression by rosiglitazone on atherosclerosis in diabetes patients with cardiovascular history trial. Circulation. 2010;121(10):1176–1187. doi: 10.1161/CIRCULATIONAHA.109.881003. [DOI] [PubMed] [Google Scholar]
- 27.Konishi T, Yamamoto T, Funayama N. Relationship between left coronary artery bifurcation angle and restenosis after stenting of the proximal left anterior descending artery. Coronary Artery Dis. 2016;27(6):449–459. doi: 10.1097/MCA.0000000000000381. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Amemiya K, Domei T, Iwabuchi M. Impact of the bifurcation angle on major cardiac events after cross-over single stent strategy in unprotected left main bifurcation lesions: 3-dimensional quantitative coronary angiographic analysis. Am J Cardiovasc Dis. 2014;4(4):168–176. [PMC free article] [PubMed] [Google Scholar]
- 29.Girasis C, Farooq V, Diletti R. Impact of 3-dimensional bifurcation angle on 5-year outcome of patients after percutaneous coronary intervention for left main coronary artery disease: a substudy of the SYNTAX trial (synergy between percutaneous coronary intervention with taxus and cardiac surgery). J Am Coll Cardiol Cardiovasc Interv. 2013;6(12):1250–1260. doi: 10.1016/j.jcin.2013.08.009. [DOI] [PubMed] [Google Scholar]
- 30.Ki YJ, Jung JH, Han JK. Clinical implications of bifurcation angles in left main bifurcation intervention using a two-stent technique. J Interv Cardiol. 2020;11 doi: 10.1155/2020/2475930. 2475930. [DOI] [PMC free article] [PubMed] [Google Scholar]