Abstract
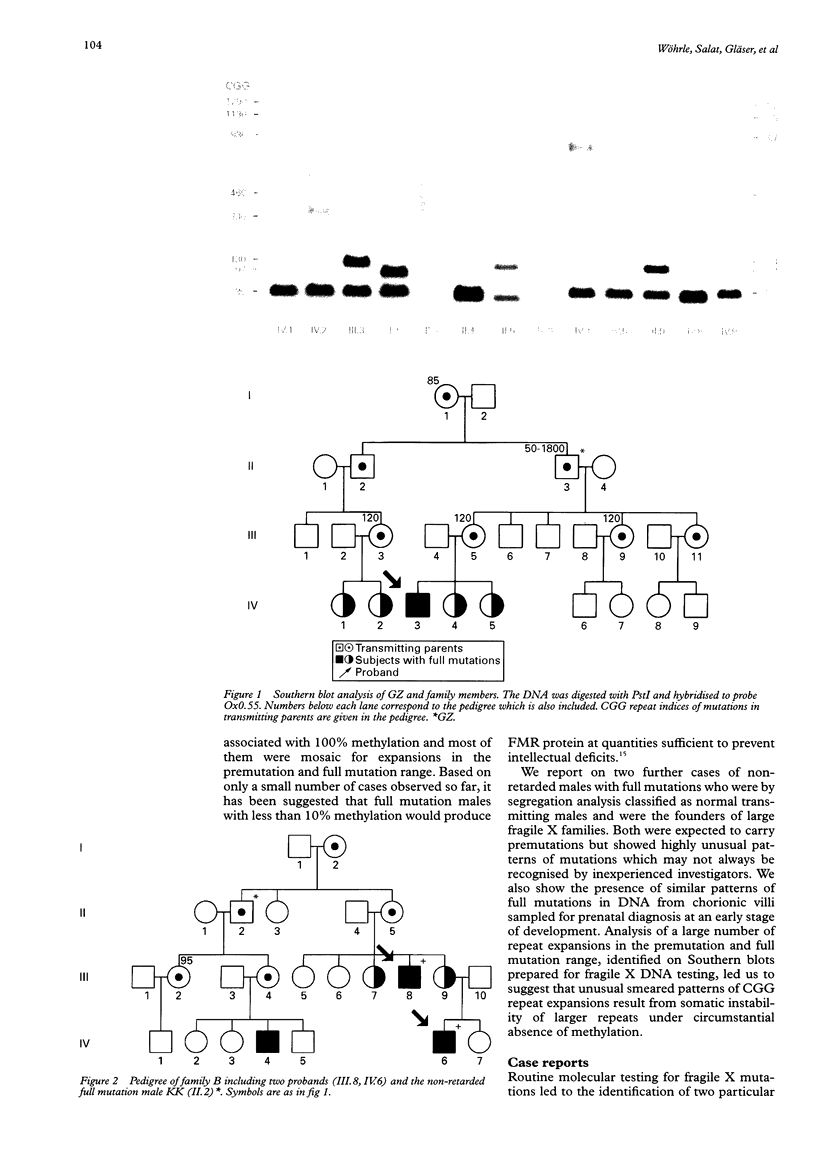
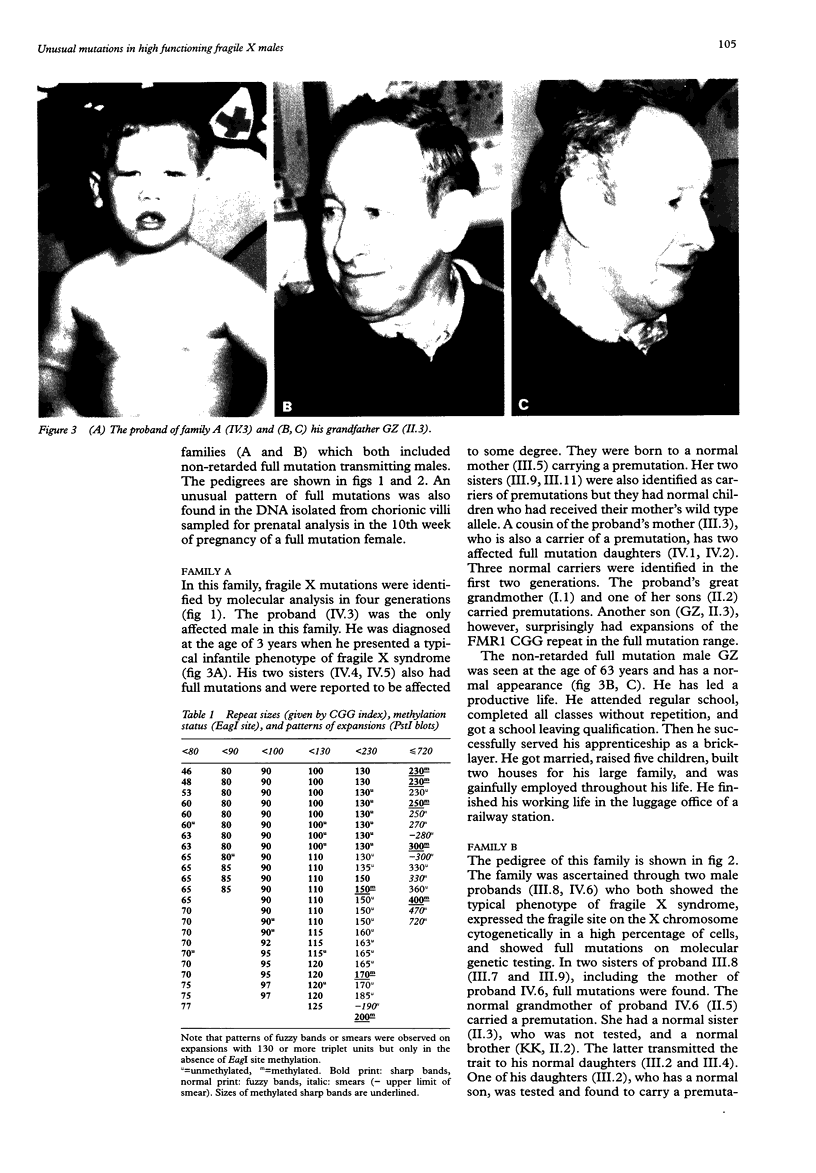
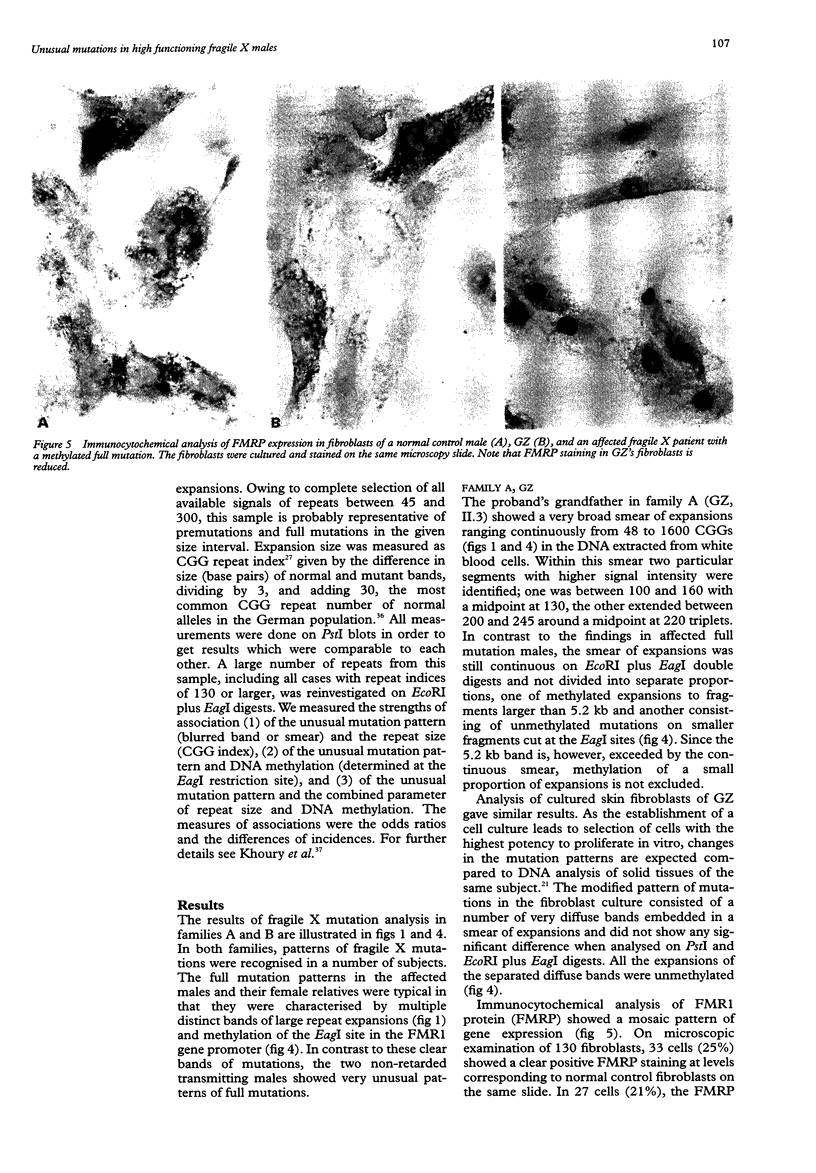
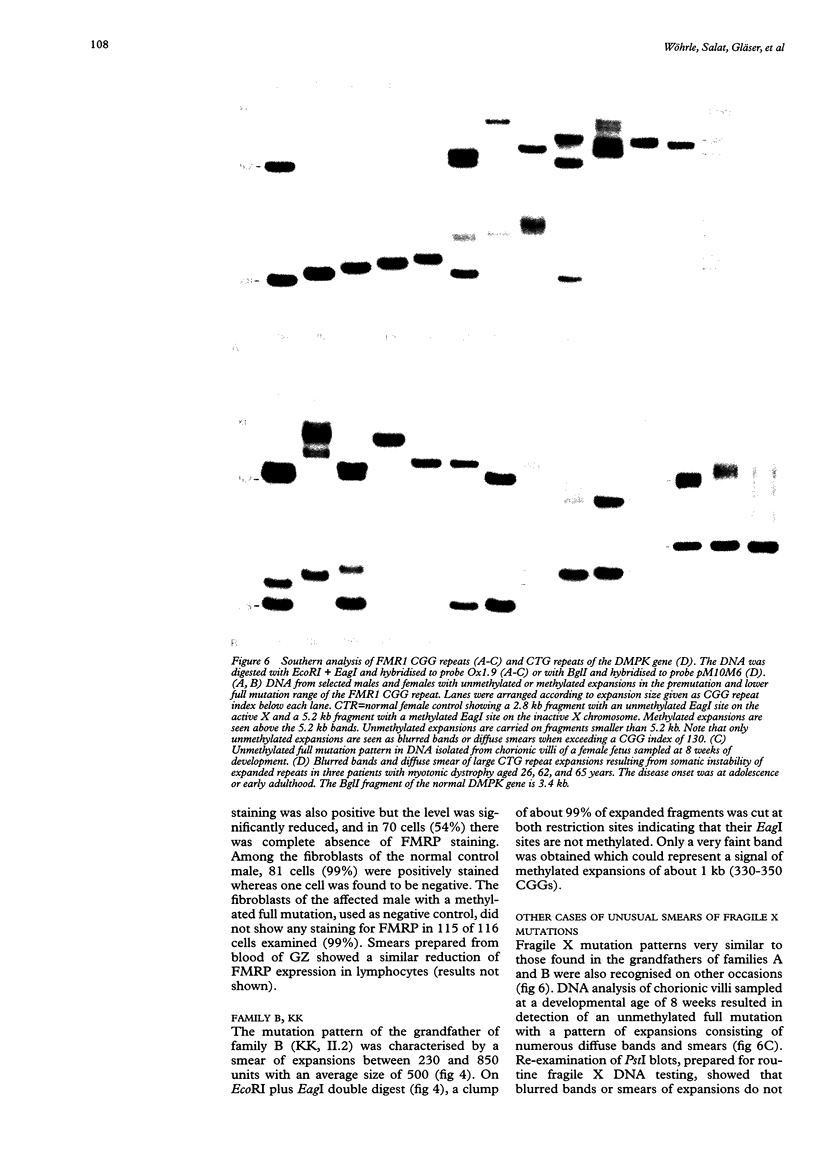
We report on further cases of high functioning fragile X males showing decreased expression of FMR1 protein, absence of detectable methylation at the EagI site in the FMR1 gene promoter, and highly unusual patterns of fragile X mutations defined as smear of expansions extending from premutation to full mutation range. Very diffuse and therefore not easily detectable patterns of full mutations were also observed on prenatal testing using DNA from chorionic villi sampled at a time of development when full mutations were still unmethylated in this particular tissue. In the search for possible determinants of such unusual patterns, repeat expansions in the premutation and in the lower full mutation range were identified on genomic PstI blots previously prepared for fragile X DNA testing. Cases with 130 or more triplets, and a number of shorter repeats, were reinvestigated on EcoRI plus EagI digests. Among the 119 expansions, there were 22 in our sample showing either blurred bands or smears on PstI blots. This particular characteristic was strongly associated with the coincidence of a repeat size of more than 130 triplets and absence of EagI site methylation. Our data set also includes cases of mosaic patterns consisting of smears of unmethylated expansions to more than 130 CGGs and of clear bands of methylated expansions. We therefore suggest that in fragile X syndrome unusual smeared patterns of mutations result from somatic instability of larger repeats under circumstantial absence of repeat methylation.
Full text
PDF




Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Aslanidis C., Jansen G., Amemiya C., Shutler G., Mahadevan M., Tsilfidis C., Chen C., Alleman J., Wormskamp N. G., Vooijs M. Cloning of the essential myotonic dystrophy region and mapping of the putative defect. Nature. 1992 Feb 6;355(6360):548–551. doi: 10.1038/355548a0. [DOI] [PubMed] [Google Scholar]
- Brook J. D., McCurrach M. E., Harley H. G., Buckler A. J., Church D., Aburatani H., Hunter K., Stanton V. P., Thirion J. P., Hudson T. Molecular basis of myotonic dystrophy: expansion of a trinucleotide (CTG) repeat at the 3' end of a transcript encoding a protein kinase family member. Cell. 1992 Feb 21;68(4):799–808. doi: 10.1016/0092-8674(92)90154-5. [DOI] [PubMed] [Google Scholar]
- Buxton J., Shelbourne P., Davies J., Jones C., Van Tongeren T., Aslanidis C., de Jong P., Jansen G., Anvret M., Riley B. Detection of an unstable fragment of DNA specific to individuals with myotonic dystrophy. Nature. 1992 Feb 6;355(6360):547–548. doi: 10.1038/355547a0. [DOI] [PubMed] [Google Scholar]
- Chen X., Mariappan S. V., Catasti P., Ratliff R., Moyzis R. K., Laayoun A., Smith S. S., Bradbury E. M., Gupta G. Hairpins are formed by the single DNA strands of the fragile X triplet repeats: structure and biological implications. Proc Natl Acad Sci U S A. 1995 May 23;92(11):5199–5203. doi: 10.1073/pnas.92.11.5199. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cleaver J. E. It was a very good year for DNA repair. Cell. 1994 Jan 14;76(1):1–4. doi: 10.1016/0092-8674(94)90165-1. [DOI] [PubMed] [Google Scholar]
- Devys D., Biancalana V., Rousseau F., Boué J., Mandel J. L., Oberlé I. Analysis of full fragile X mutations in fetal tissues and monozygotic twins indicate that abnormal methylation and somatic heterogeneity are established early in development. 1992 Apr 15-May 1Am J Med Genet. 43(1-2):208–216. doi: 10.1002/ajmg.1320430134. [DOI] [PubMed] [Google Scholar]
- Devys D., Lutz Y., Rouyer N., Bellocq J. P., Mandel J. L. The FMR-1 protein is cytoplasmic, most abundant in neurons and appears normal in carriers of a fragile X premutation. Nat Genet. 1993 Aug;4(4):335–340. doi: 10.1038/ng0893-335. [DOI] [PubMed] [Google Scholar]
- Feng Y., Zhang F., Lokey L. K., Chastain J. L., Lakkis L., Eberhart D., Warren S. T. Translational suppression by trinucleotide repeat expansion at FMR1. Science. 1995 May 5;268(5211):731–734. doi: 10.1126/science.7732383. [DOI] [PubMed] [Google Scholar]
- Fu Y. H., Kuhl D. P., Pizzuti A., Pieretti M., Sutcliffe J. S., Richards S., Verkerk A. J., Holden J. J., Fenwick R. G., Jr, Warren S. T. Variation of the CGG repeat at the fragile X site results in genetic instability: resolution of the Sherman paradox. Cell. 1991 Dec 20;67(6):1047–1058. doi: 10.1016/0092-8674(91)90283-5. [DOI] [PubMed] [Google Scholar]
- Hagerman R. J., Hull C. E., Safanda J. F., Carpenter I., Staley L. W., O'Connor R. A., Seydel C., Mazzocco M. M., Snow K., Thibodeau S. N. High functioning fragile X males: demonstration of an unmethylated fully expanded FMR-1 mutation associated with protein expression. Am J Med Genet. 1994 Jul 15;51(4):298–308. doi: 10.1002/ajmg.1320510404. [DOI] [PubMed] [Google Scholar]
- Hansen R. S., Gartler S. M., Scott C. R., Chen S. H., Laird C. D. Methylation analysis of CGG sites in the CpG island of the human FMR1 gene. Hum Mol Genet. 1992 Nov;1(8):571–578. doi: 10.1093/hmg/1.8.571. [DOI] [PubMed] [Google Scholar]
- Harley H. G., Brook J. D., Rundle S. A., Crow S., Reardon W., Buckler A. J., Harper P. S., Housman D. E., Shaw D. J. Expansion of an unstable DNA region and phenotypic variation in myotonic dystrophy. Nature. 1992 Feb 6;355(6360):545–546. doi: 10.1038/355545a0. [DOI] [PubMed] [Google Scholar]
- Hornstra I. K., Nelson D. L., Warren S. T., Yang T. P. High resolution methylation analysis of the FMR1 gene trinucleotide repeat region in fragile X syndrome. Hum Mol Genet. 1993 Oct;2(10):1659–1665. doi: 10.1093/hmg/2.10.1659. [DOI] [PubMed] [Google Scholar]
- Khandjian E. W., Fortin A., Thibodeau A., Tremblay S., Côté F., Devys D., Mandel J. L., Rousseau F. A heterogeneous set of FMR1 proteins is widely distributed in mouse tissues and is modulated in cell culture. Hum Mol Genet. 1995 May;4(5):783–789. doi: 10.1093/hmg/4.5.783. [DOI] [PubMed] [Google Scholar]
- Lachiewicz A. M., Spiridigliozzi G. A., McConkie-Rosell A., Burgess D., Feng Y., Warren S. T., Tarleton J. A fragile X male with a broad smear on Southern blot analysis representing 100-500 CGG repeats and no methylation at the EagI site of the FMR-1 gene. Am J Med Genet. 1996 Aug 9;64(2):278–282. doi: 10.1002/(SICI)1096-8628(19960809)64:2<278::AID-AJMG9>3.0.CO;2-Q. [DOI] [PubMed] [Google Scholar]
- Loesch D. Z., Huggins R., Hay D. A., Gedeon A. K., Mulley J. C., Sutherland G. R. Genotype-phenotype relationships in fragile X syndrome: a family study. Am J Hum Genet. 1993 Nov;53(5):1064–1073. [PMC free article] [PubMed] [Google Scholar]
- Malter H. E., Iber J. C., Willemsen R., de Graaff E., Tarleton J. C., Leisti J., Warren S. T., Oostra B. A. Characterization of the full fragile X syndrome mutation in fetal gametes. Nat Genet. 1997 Feb;15(2):165–169. doi: 10.1038/ng0297-165. [DOI] [PubMed] [Google Scholar]
- McConkie-Rosell A., Lachiewicz A. M., Spiridigliozzi G. A., Tarleton J., Schoenwald S., Phelan M. C., Goonewardena P., Ding X., Brown W. T. Evidence that methylation of the FMR-I locus is responsible for variable phenotypic expression of the fragile X syndrome. Am J Hum Genet. 1993 Oct;53(4):800–809. [PMC free article] [PubMed] [Google Scholar]
- McMurray C. T. Mechanisms of DNA expansion. Chromosoma. 1995 Oct;104(1):2–13. doi: 10.1007/BF00352220. [DOI] [PubMed] [Google Scholar]
- Merenstein S. A., Shyu V., Sobesky W. E., Staley L., Berry-Kravis E., Nelson D. L., Lugenbeel K. A., Taylor A. K., Pennington B. F., Hagerman R. J. Fragile X syndrome in a normal IQ male with learning and emotional problems. J Am Acad Child Adolesc Psychiatry. 1994 Nov-Dec;33(9):1316–1321. doi: 10.1097/00004583-199411000-00014. [DOI] [PubMed] [Google Scholar]
- Miller S. A., Dykes D. D., Polesky H. F. A simple salting out procedure for extracting DNA from human nucleated cells. Nucleic Acids Res. 1988 Feb 11;16(3):1215–1215. doi: 10.1093/nar/16.3.1215. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Modrich P. Mismatch repair, genetic stability, and cancer. Science. 1994 Dec 23;266(5193):1959–1960. doi: 10.1126/science.7801122. [DOI] [PubMed] [Google Scholar]
- Nakahori Y., Knight S. J., Holland J., Schwartz C., Roche A., Tarleton J., Wong S., Flint T. J., Froster-Iskenius U., Bentley D. Molecular heterogeneity of the fragile X syndrome. Nucleic Acids Res. 1991 Aug 25;19(16):4355–4359. doi: 10.1093/nar/19.16.4355. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nolin S. L., Glicksman A., Houck G. E., Jr, Brown W. T., Dobkin C. S. Mosaicism in fragile X affected males. Am J Med Genet. 1994 Jul 15;51(4):509–512. doi: 10.1002/ajmg.1320510444. [DOI] [PubMed] [Google Scholar]
- Pieretti M., Zhang F. P., Fu Y. H., Warren S. T., Oostra B. A., Caskey C. T., Nelson D. L. Absence of expression of the FMR-1 gene in fragile X syndrome. Cell. 1991 Aug 23;66(4):817–822. doi: 10.1016/0092-8674(91)90125-i. [DOI] [PubMed] [Google Scholar]
- Razin A., Shemer R. DNA methylation in early development. Hum Mol Genet. 1995;4(Spec No):1751–1755. doi: 10.1093/hmg/4.suppl_1.1751. [DOI] [PubMed] [Google Scholar]
- Reyniers E., Vits L., De Boulle K., Van Roy B., Van Velzen D., de Graaff E., Verkerk A. J., Jorens H. Z., Darby J. K., Oostra B. The full mutation in the FMR-1 gene of male fragile X patients is absent in their sperm. Nat Genet. 1993 Jun;4(2):143–146. doi: 10.1038/ng0693-143. [DOI] [PubMed] [Google Scholar]
- Richards R. I., Sutherland G. R. Simple repeat DNA is not replicated simply. Nat Genet. 1994 Feb;6(2):114–116. doi: 10.1038/ng0294-114. [DOI] [PubMed] [Google Scholar]
- Rousseau F., Heitz D., Biancalana V., Blumenfeld S., Kretz C., Boué J., Tommerup N., Van Der Hagen C., DeLozier-Blanchet C., Croquette M. F. Direct diagnosis by DNA analysis of the fragile X syndrome of mental retardation. N Engl J Med. 1991 Dec 12;325(24):1673–1681. doi: 10.1056/NEJM199112123252401. [DOI] [PubMed] [Google Scholar]
- Rousseau F., Robb L. J., Rouillard P., Der Kaloustian V. M. No mental retardation in a man with 40% abnormal methylation at the FMR-1 locus and transmission of sperm cell mutations as premutations. Hum Mol Genet. 1994 Jun;3(6):927–930. doi: 10.1093/hmg/3.6.927. [DOI] [PubMed] [Google Scholar]
- Sherman S. L., Jacobs P. A., Morton N. E., Froster-Iskenius U., Howard-Peebles P. N., Nielsen K. B., Partington M. W., Sutherland G. R., Turner G., Watson M. Further segregation analysis of the fragile X syndrome with special reference to transmitting males. Hum Genet. 1985;69(4):289–299. doi: 10.1007/BF00291644. [DOI] [PubMed] [Google Scholar]
- Sherman S. L., Morton N. E., Jacobs P. A., Turner G. The marker (X) syndrome: a cytogenetic and genetic analysis. Ann Hum Genet. 1984 Jan;48(Pt 1):21–37. doi: 10.1111/j.1469-1809.1984.tb00830.x. [DOI] [PubMed] [Google Scholar]
- Smeets H. J., Smits A. P., Verheij C. E., Theelen J. P., Willemsen R., van de Burgt I., Hoogeveen A. T., Oosterwijk J. C., Oostra B. A. Normal phenotype in two brothers with a full FMR1 mutation. Hum Mol Genet. 1995 Nov;4(11):2103–2108. doi: 10.1093/hmg/4.11.2103. [DOI] [PubMed] [Google Scholar]
- Smith S. S., Laayoun A., Lingeman R. G., Baker D. J., Riley J. Hypermethylation of telomere-like foldbacks at codon 12 of the human c-Ha-ras gene and the trinucleotide repeat of the FMR-1 gene of fragile X. J Mol Biol. 1994 Oct 21;243(2):143–151. doi: 10.1006/jmbi.1994.1640. [DOI] [PubMed] [Google Scholar]
- Steinbach P., Wöhrle D., Tariverdian G., Kennerknecht I., Barbi G., Edlinger H., Enders H., Götz-Sothmann M., Heilbronner H., Hosenfeld D. Molecular analysis of mutations in the gene FMR-1 segregating in fragile X families. Hum Genet. 1993 Nov;92(5):491–498. doi: 10.1007/BF00216457. [DOI] [PubMed] [Google Scholar]
- Sutcliffe J. S., Nelson D. L., Zhang F., Pieretti M., Caskey C. T., Saxe D., Warren S. T. DNA methylation represses FMR-1 transcription in fragile X syndrome. Hum Mol Genet. 1992 Sep;1(6):397–400. doi: 10.1093/hmg/1.6.397. [DOI] [PubMed] [Google Scholar]
- Verkerk A. J., Pieretti M., Sutcliffe J. S., Fu Y. H., Kuhl D. P., Pizzuti A., Reiner O., Richards S., Victoria M. F., Zhang F. P. Identification of a gene (FMR-1) containing a CGG repeat coincident with a breakpoint cluster region exhibiting length variation in fragile X syndrome. Cell. 1991 May 31;65(5):905–914. doi: 10.1016/0092-8674(91)90397-h. [DOI] [PubMed] [Google Scholar]
- Wang Z., Taylor A. K., Bridge J. A. FMR1 fully expanded mutation with minimal methylation in a high functioning fragile X male. J Med Genet. 1996 May;33(5):376–378. doi: 10.1136/jmg.33.5.376. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Willemsen R., Mohkamsing S., de Vries B., Devys D., van den Ouweland A., Mandel J. L., Galjaard H., Oostra B. Rapid antibody test for fragile X syndrome. Lancet. 1995 May 6;345(8958):1147–1148. doi: 10.1016/s0140-6736(95)90979-6. [DOI] [PubMed] [Google Scholar]
- Wöhrle D., Hennig I., Vogel W., Steinbach P. Mitotic stability of fragile X mutations in differentiated cells indicates early post-conceptional trinucleotide repeat expansion. Nat Genet. 1993 Jun;4(2):140–142. doi: 10.1038/ng0693-140. [DOI] [PubMed] [Google Scholar]
- Wöhrle D., Hirst M. C., Kennerknecht I., Davies K. E., Steinbach P. Genotype mosaicism in fragile X fetal tissues. Hum Genet. 1992 Apr;89(1):114–116. doi: 10.1007/BF00207057. [DOI] [PubMed] [Google Scholar]
- Wöhrle D., Kennerknecht I., Wolf M., Enders H., Schwemmle S., Steinbach P. Heterogeneity of DM kinase repeat expansion in different fetal tissues and further expansion during cell proliferation in vitro: evidence for a casual involvement of methyl-directed DNA mismatch repair in triplet repeat stability. Hum Mol Genet. 1995 Jul;4(7):1147–1153. doi: 10.1093/hmg/4.7.1147. [DOI] [PubMed] [Google Scholar]
- de Graaff E., Willemsen R., Zhong N., de Die-Smulders C. E., Brown W. T., Freling G., Oostra B. Instability of the CGG repeat and expression of the FMR1 protein in a male fragile X patient with a lung tumor. Am J Hum Genet. 1995 Sep;57(3):609–618. [PMC free article] [PubMed] [Google Scholar]
- de Vries B. B., Jansen C. C., Duits A. A., Verheij C., Willemsen R., van Hemel J. O., van den Ouweland A. M., Niermeijer M. F., Oostra B. A., Halley D. J. Variable FMR1 gene methylation of large expansions leads to variable phenotype in three males from one fragile X family. J Med Genet. 1996 Dec;33(12):1007–1010. doi: 10.1136/jmg.33.12.1007. [DOI] [PMC free article] [PubMed] [Google Scholar]