Abstract
Background:
Cystic echinococcosis (CE) is an important zoonotic parasitic disease caused by the larval stage or metacestode of the tapeworm Echinococcus granulosus sensu lato. Due to treatment protocols for different liver cysts, diagnosis of cyst stages is very important. Different antigens have been used for CE diagnosis. However, each one is more sensitive and effective for the diagnosis of specific CE stages is not known well. We aimed to compare Native Hydatid Cyst Fluid (HCF), Lyophilized Hydatid Cyst Fluid (LHCF), antigen B (AgB) and Lyophilized antigen B (LAgB) originated from E. granulosus sensu stricto (G1–G3) genotype, for sero- diagnosis of active, transitional and inactive human liver CE using ELISA technique.
Methods:
The HCF was collected aseptically from liver CE cysts of sheep slaughtered from 2018 to 2019 in Shiraz slaughterhouse, Southern, Iran. The cysts were characterized by PCR and sequencing for genotype specification. Four types of antigens were used: HCF, LHCF, AgB and LAgB originated from E. granulosus sensu stricto (G1–G3) genotype. Thirty-three serum samples from active, transitional, and inactive human cysts were collected. Overall, 48 samples from other parasitic diseases and 60 samples from healthy subjects as negative controls were checked using four antigens by ELISA method.
Results:
The best diagnostic sensitivity with 96.97% was observed by anti-LHCF IgG ELISA test. The best specificity with 95.37% was observed in ELISA test using LAgB.
Conclusion:
Simultaneous test of sera with anti-LHCF IgG ELISA and anti-LAgB IgG ELISA would be the best in the diagnosis of human liver cystic echinococcosis.
Keywords: Cystic echinococcosis, Ultrasonography, Echinococcus granulosus, Antigens, Human
Introduction
Cystic echinococcosis( CE) a zoonotic neglected parasitic disease, is caused by the metacestode of Echinococcus granulosus sensu lato (1). The disease is a major health problem all over the world including in Iran (2,3). Many cases of CE patients are routinely reported in different hospitals and different regions of the country (2,4).
Diagnosis of CE is mainly based on imaging methods and serological tests (5). For newly formed cysts, the efficiency of X-ray and ultrasound are not satisfactory. However, CE accompanying different imaging features, according to growth stages, relevant complications, and tissue tropism. The radiologic presentations are a variety of different features from purely cystic to completely solid mass appearance. Ultrasound is the most important imaging route used in liver CE that clearly shows the floating membranes, daughter cysts, and hydatid sands which are characteristic of purely cystic lesions (6). The radiologist’s familiarity with the CE imaging findings is very crucial for earlier diagnosis and an appropriate treatment. There are several classification schemes for liver CE based on their ultrasound appearances. The classification by the WHO is the most commonly preferred (5,7). Based on this classification, the liver cysts are classified into active (CE1 and CE2), transitional (CE3) and inactive cysts (CE4 and CE5) (5,7,8). However, this classification has changed with the long-term results of the medical and percutaneous treatment and high-field magnetic resonance spectroscopy (MRI).
While sero-negativity is observed in 20% of patients with CE (9), those with multiple cysts are usually seropositive. The rate of sero-negativity has been reported to be higher in patients with CE1, CE4, and CE5 cyst types as compared to those with CE2 and CE3 types (8,10). The transitional type of CE cysts or CE3 types, are divided into CE3a with separated endocysts and, CE3b with solid type containing daughter vesicles (6,11–13).
If CE1 or CE2 are ruptured, the risk of spoilage of protoscoleces, new cyst formation (recurrent cyst) increases (5). Therefore, diagnosis and treatment of these types of cysts are very essential. In the case of inactive cysts, watch and wait is suggested. (14). On the other hand, a number of patients’ sera may remain positive for several years after treatment (≥10years) (10). This may lead to unnecessary treatment (such as surgery) and consequently unnecessary costs (13). Clinical management of the disease is mainly based on the cyst stage according to the WHO Informal Working Group on Echinococcosis (WHOIWGE) applicable to liver cysts, ultrasound cyst classification, and other clinical factors (5,7,15) and the treatment approach is based on surgery, percutaneous drainage, benzimidazoles administration, and “watch and wait” (16).
In the field of serological methods different native and recombinant antigens have been used for CE sero-diagnosis (4,17–20). Lyophilization has been used for a part of some studies (21–22). None of them has compared the effect of lyophilization on the efficacy of antigen. Moreover, application of different antigens has not clarified stage specific serodiagnosis of liver CE especially for CE1 or CE2 cases (14).
We aimed to test four types of antigens of E. granulosus sensu stricto (G1–G3 genotype) prepared from sheep hydatid cysts including native hydatid cyst fluid (HCF), lyophilized hydatid cyst fluid (LHCF), native Antigen B (AgB) and lyophilized antigen B (LAgB) that were used for sero-diagnosis of active, transitional, and inactive human liver cystic echinococcosis.
Materials and Methods
Hydatid cyst fluid
The hydatid cyst fluid (HCF) was collected aseptically from fertile sheep liver hydatid cysts from 2018 to 2019 in Shiraz slaughterhouse, Southern, Iran. The cysts were characterized by PCR and sequencing for genotype specification. The HCF of the cysts with E. granulosus sensu stricto genotype (G1–G3) were centrifuged at 3000×g in order to remove the protoscoleces and stored at −20 °C until use.
Antigen B
Antigen B was prepared from HCF as described by Oriol et al. (23). Overall, 100 mL of HCF was dialyzed overnight against 5 mM of acetate buffer (pH 5) at 4°C. Centrifugation of sample was carried out with 50000 g for 30 min followed by supernatant removal and, the sediment was dissolved in 0.2 M phosphate buffer (pH 8). To remove the globulins from the sample, saturated ammonium sulfate was used. Finally, to separate heat-stable antigen B from other components, the sample was boiled in a water bath for 15 min and centrifuged at 50 000 g for 60 min. Bradford protein assay was used to determine its concentration (24).
Lyophilization
A part of both antigens (HCF and AgB) were lyophilized according to the standard methods (24).
Sera
The study was approved by the Regional Ethics Committee of National Institute for Medical Research Development, Iran (IR.NIMAD.REC. 1397.215).
Overall, 141 serum samples were used in this study including 33 radiologically, surgically, pathologically and serologically confirmed liver CE samples (CE1=12, CE2=8, CE3=6, CE4=5, CE5=2), collected from 2019–2020 in Shiraz, Iran. Sixty sera from healthy people and 48 samples from non-CE patients were collected from Shiraz and Tehran. CE classification were done by experienced radiologists. Cystic echinococcosis stages CE1 and CE2 were grouped as “active”; CE3 as transitional and CE4 & CE5 cysts (i.e., with solid appearance) were grouped as “inactive”. Non-CE samples were collected from patients suffering from amoebiasis (E. coli) (N=1), ascariasis(N=4), blastocystosis (N=2), enterobiasis (N=1), fasciolosis (N=7), giardiasis (N=8), hymenolepiasis (N=5), leishmaniasis (N=1), malaria (N=5), strongyloidiasis (N=2), taeniasis (N=5), toxocariasis (N=4), toxoplasmosis (N=2), and trichostrongyliasis (N=1).
Enzyme-Linked Immunosorbent Assay
ELISA was carried out in flat-bottom 96-well microplates (Nunc, Maxisorp, Roskilde: Denmark) as previously described (17–18). ELISA was carried out in flat-bottom 96-well microplates according to previous studies. The absorbance was read at 492 nm after 30 min using an automatic micro-plate reader. All sera were checked using the four mentioned antigens by the ELISA method.
Statistical Analysis
The data were analyzed using SPSS software Version 22, (IBM Corp., Armonk, NY, USA). The student’s t-test was applied and a P-value of <0.05 was considered significant. The cut-off was also calculated as X±2SD.
Results
Four antigens including HCF, LHCF, AgB and LAgB were tested and evaluated with individual sera from CE patients, patients with other parasitic infections and, healthy control individuals using anti-IgG ELISA. The results are presented in Tables 1 and 2, Figs1a–d.
Table 1:
Diagnostic performance of four different antigens (hydatid cyst fluid [HCF], lyophilized hydatid cyst fluid [LHCF], antigen B [AgB] and lyophilized antigen B [LAgB]) in IgG ELISA for sero-diagnosis of liver cystic echinococcosis (CE)
| Variable | Type of Antigen | ||||
|---|---|---|---|---|---|
| HCF [Positive/total (No/%)] | LHCF [Positive/total (No/%)] | AgB [Positive/total (No/%)] | LAgB [Positive/total (No/%)] | ||
| Type of sera | Pathologically positive sera | 30/33 (90.9) | 32/33 (96.97) | 27/33 (84.38) | 27/33 (84.38) |
| Sera of healthy people | 2/60 (3.33) | 3/60 (5) | 1/60 (1.66) | 1/60 (1.66) | |
| Sera of other diseases | 4/48 (8.33) | 5/48 (10.42) | 7/48 (14.58) | 4/48 (8.33) | |
| Statistical analysis | Sensitivity Value / 95% CI | 90.91/75.67 to 98.08 | 96.97/84.24 to 99.92 | 81.82/64.54 to 93.02 | 81.82/64.54 to 93.02 |
| Specificity Value / 95% CI | 94.44/88.30 to 97.93 | 92.59/85.93 to 96.75 | 92.59/85.93 to 96.75 | 95.37/89.53 to 98.48 | |
| Positive Likelihood Ratio Value / 95% CI | 16.36/ 7.46 to 35.88 | 13.09/6.70 to 25.57 | 11.05/ 5.56 to 21.93 | 17.67/ 7.40 to 42.22 | |
| Negative Likelihood Ratio Value / 95% CI | 0.10/0.03 to 0.28 | 0.03/0.00 to 0.23 | 0.20/0.10 to 0.41 | 0.19/0.09 to 0.39 | |
| Disease prevalence Value / 95% CI | 23.40/16.69 to 31.27 | 23.40/16.69% to 31.27 | 23.40/16.69 to 31.27 | 23.40/16.69 to 31.27 | |
| Positive Predictive Value Value / 95% CI | 83.33/69.52 to 91.64 | 80.00/67.19 to 88.65 | 77.14/62.96 to 87.02 | 84.38/ 9.33 to 92.81 | |
| Negative Predictive Value Value / 95% CI | 97.14/92.03 to 99.01 | 99.01/93.55 to 99.86 | 94.34/88.97 to 97.18 | 94.50/89.26 to 97.26 | |
| Accuracy Value / 95% CI | 93.62/88.23 to 97.04 | 93.62/88.23 to 97.04 | 90.07/83.90 to 94.46 | 92.20/86.47 to 96.04 | |
Table 2:
Results of ELISA using four different antigens (hydatid cyst fluid [HCF], lyophilized hydatid cyst fluid [LHCF], antigen B [AgB] and lyophilized antigen B [LAgB]) for sero-diagnosis of liver cystic echinococcosis (CE) according to non-relative sera (sera of other diseases)
| Variable | HCF No. reacted/’ total No. | Type of Antigens | |||
|---|---|---|---|---|---|
| LHCF No. reacted/total No. | AgB No. reacted/total No. | LAgB No. reacted/total No. | |||
| Type of sera | Amoebiasis (E.coli) | 0/1 | 0/1 | 0/1 | 0/1 |
| Ascariasis | 0/4 | 0/4 | 1/4 | 0/4 | |
| Blastocystosis | 0/2 | 0/2 | 0/2 | 0/2 | |
| Enterobiasis | 0/1 | 0/1 | 0/1 | 0/1 | |
| Fasciolosis | 3/7 | 2/7 | 2/7 | 1/7 | |
| Giardiasis | 0/8 | 0/8 | 1/8 | 1/8 | |
| Hymenolepiasis | 0/5 | 0/5 | 0/5 | 0/5 | |
| Leishmaniasis | 0/1 | 0/1 | 0/1 | 0/1 | |
| Malaria | 0/5 | 0/5 | 1/5 | 0/5 | |
| Stronyloidiasis | 0/2 | 0/2 | 0/2 | 0/2 | |
| Taeniasis | 1/5 | 2/5 | 1/5 | 1/5 | |
| Toxocariasis | 0/4 | 1/4 | 1/4 | 1/4 | |
| Toxoplasmosis | 0/2 | 0/2 | 0/2 | 0/2 | |
| Trichostrongyliasis | 0/1 | 0/1 | 0/1 | 0/1 | |
Hydatid cyst fluid [LHCF], antigen B [AgB] and lyophilized antigen B [LAgB]) for serodiagnosis of liver cystic echinococcosis (CE) according to non-relative sera (sera of other diseases)
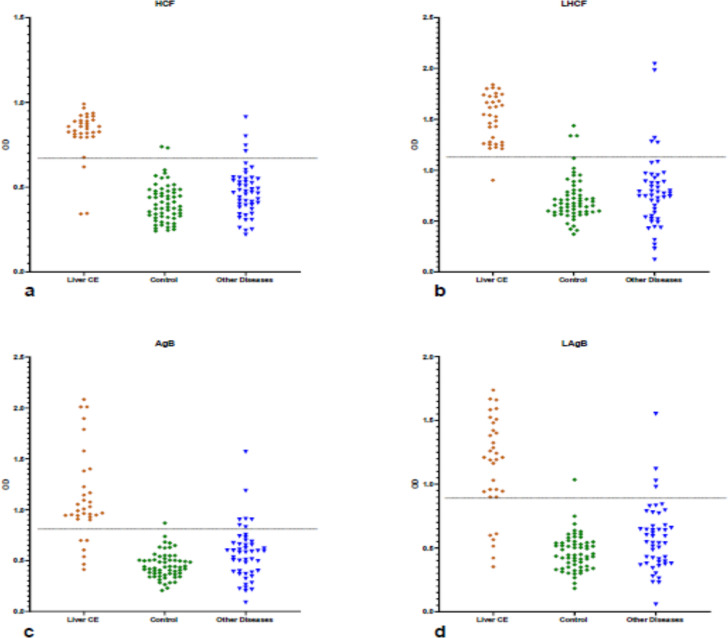
Fig. 1:
Scatter plots presentation to compare four antigens a) native hydatid cyst fluid (HCF), b) lyophilized HCF, c) antigen B (AgB) and, d) lyophilized AgB (LAgB) originated from E. granulosus sensu stricto (G1–G3) genotype of sheep hydatid cyst for sero-diagnosis of human liver cystic echinococcosis (N: 33), control (N:60), other diseases (N: 48)
Anti-LHCF IgG ELISA showed the best diagnostic sensitivity of 96.97% with a 95% confidence interval (84.24% to 99.92%) using sera obtained from 33 confirmed CE patients. The best specificity (95.37%) with a 95% confidence interval (89.53% to 98.48%) was simultaneously related to LAgB with cross-reactions by nonrelative sera including taeniasis, fascioliasis, giardiasis, and toxocariasis (Tables 1–2, Figs.1a–d).
Diagnostic performance of four antigens was compared according to the type of cysts (Table 3). LHCF had the best performance for diagnosis of all type of cysts. So that, it was able to diagnose the most cases of active cysts.
Table 3:
Diagnostic performance of four different antigens (hydatid cyst fluid [HCF], lyophilized hydatid cyst fluid [LHCF], antigen B [AgB] and lyophilized antigen B [LAgB]) for sero-diagnosis of liver cystic echinococcosis (CE) according to the cyst stages
| Variable | Type of Antigens | |||||
|---|---|---|---|---|---|---|
|
| ||||||
| HCF | LHCF | AgB | LAgB | |||
| Cyst stage [No (%)] | Active | CE1 | 11/12(91.67) | 12/12(100) | 11/12(91.67) | 11/12(91.67) |
| CE2 | 7/8 (87.5) | 7/8 (87.5) | 5/8 (62.5) | 5/8 (62.5) | ||
| Transitional | CE3 | 5/6 (83.3) | 6/6 (100) | 5/6 (83.3) | 5/6 (83.3) | |
| Inactive | CE4 | 5/5 (100) | 5/5 (100) | 5/5 (100) | 5/5 (100) | |
| CE5 | 2/2 (100) | 2/2 (100) | 1/2 (50) | 1/2 (50) | ||
Discussion
Different antigens including native, semi purified or recombinant have been used for CE diagnosis (16,19, 25–29). They have been used for both diagnostic and sero-epidemiological purposes (26, 30–34). Although, lyophilized antigens have been used in limited studies (21,22), but the validity of lyophilized HCF and AgB has not been evaluated and compared. Moreover, the use of antigens in the different stages of liver CE has not been performed, so far. In this regard, the present study was designed to compare the diagnostic efficacy of four hydatid antigens with different preparation methods; Crude and lyophilized HCF, native and lyophilized AgB in the detection of IgG antibodies using ELISA test, for diagnosis of liver CE. Moreover, these antigens were used for diagnosis of stage specific liver CE based on sonography outcomes.
Among different antigens crude, semi-purified and purified hydatid cyst fluid were evaluated in several investigations for diagnosing of CE. A 38-mer peptide (p176) of the AgB/1 subunit has been applied in an Elisa test with a higher sensitivity (80%) and specificity (94%) than native AgB, Ag5, or any other peptide antigen tested (15). In another study AgB has preferred to HCF in initial laboratory studies, with 94% sensitivity and 90.3% specificity for diagnosing of Echinococcus infections (20). Iranian AgB has higher sensitivity (96.4%) in compare with Chinese AgB, HCF and recombinant antigens that utilized (35). During a study, specific IgG ELISA AgB (antigen B-rich fraction) was the most sensitive test (96.5%) in compare to latex agglutination, immunoelectrophoresis (IEP), and specific IgE ELISA tests (36). In a preliminary comparative investigation with four commercially ELISA assays, native Ag5 showed higher sensitivity for detecting clinically positive CE patients (21). Verastegui et al. have utilized lyophilized HCF from different host and compared the accuracy of the antigens in ELISA, EITB (enzyme-linked immunoelectrotransfer blot) and DD5 (double-diffusion). According their results, the EITB assay offers greater sensitivity and specificity than do the ELISA and the DD5 test (22).
Our results showed that sensitivity of HCF (lyophilized/non-lyophilized) is high and is parallel to the results of Nasrieh and Abdel-Hafez (37) but in contrast to the earlier findings that showed purified antigens to be preferable to crude cyst fluid (38). Moreover, lyophilized antigens using IgG ELISA provided more accurate than non-lyophilized ones. As respect, the carriage and keeping of LHCF is simpler than other types of antigens, and according to our reliable results, lyophilization could be an effective method for providing antigens in different laboratories. Cross-reactivity of crude HCF antigen with human fascioliasis, which is endemic in certain areas of Iran and in parts of the world with endemic fasciolosis, is important. LAgB showed less cross-reactivity, particularly with fascioliasis and other prevalent helminthic diseases in Iran. This could be an additional advantage of using LAgB over other E. granulosus antigens, especially where CE and fascioliasis coexist. The concentration and purity of antigen as well as the quality and quantity of host immune responses in various endemic regions and in different patient groups also may effect on the sensitivity and specificity of the tests (27). On the other hand, at different stages of the metacestode development a variation in the composition of AgB is observed which could not be ruled out (38). Fascioliasis, taeniasis, and toxocariasis are the most important helminthic diseases which have a cross-reactivity with sera of CE considered well (27,39).
The possible genotype variation of CE cysts, host immune response, CE cyst location in the body, the number and cyst stage, and role of immune complexes should be considered (39). In addition, false-negative results may be related to small size cysts, and CE1, CE4, and CE5 cyst stages (40). An attempt has also been used for different serological tests based on liver cysts stages for diagnosis of CE by ultrasonography (7). The results were promising such that application of HCF (Crude or L lyophilized) and LAgB presented higher sensitivity and specificity for diagnosis of liver CE respectively. However, according present information, more samples are needed to evaluate the diagnostic performance of antigens based on cyst stage, which is the main limitation of such studies.
Conclusion
Using anti-LHCF IgG ELISA for initial screening of liver CE is recommended. However, as the LAgB has higher specificity, application of both antigens for better performance in the diagnosis of liver CE stages in an ELISA test is suggested.
Journalism Ethics considerations
Ethical issues (Including plagiarism, informed consent, misconduct, data fabrication and/or falsification, double publication and/or submission, redundancy, etc.) have been completely observed by the authors.
Acknowledgements
Research reported in this publication was supported by Elite Researcher Grant Committee under award number [963476] from the National Institutes for Medical Research Development (NIMAD), Tehran, Iran.
Footnotes
Conflict of interest
The authors declare that there is no conflict of interest.
References
- 1.Thompson A, Deplazes P, Lymbery A. (2017). Preface. Adv Parasitol, 95: xiii–xiv. [DOI] [PubMed] [Google Scholar]
- 2.Rokni MB. (2009). Echinococcosis/hydatidosis in Iran. Iran J Parasitol, 4 (2): 1–16. [Google Scholar]
- 3.Sadjjadi SM. (2006). Present situation of echinococcosis in the Middle East and Arabic North Africa. Parasitol Int, 55: 197–202. [DOI] [PubMed] [Google Scholar]
- 4.Sadjjadi SM, Ardehali S, Noman-Pour B, et al. (2001). Diagnosis of cystic echinococcosis: ultrasound imaging or countercurrent immuno-electrophoresis? East Mediterr Health J, 7 (6): 907–11. [PubMed] [Google Scholar]
- 5.Brunetti E, Kern P, Vuitton DA. (2010). Writing Panel for the WHO-IWGE. Expert consensus for the diagnosis and treatment of cystic and alveolar echinococcosis in humans. Acta Trop, 114 (1): 1–16. [DOI] [PubMed] [Google Scholar]
- 6.Turgut AT, Akhan O, Bhatt S, et al. (2008). Sonographic spectrum of hydatid disease. Ultrasound Q, 24 (1): 17–29. [DOI] [PubMed] [Google Scholar]
- 7.WHO Informal Working Group (2003). International classification of ultrasound images in cystic echinococcosis for application in clinical and field epidemiological settings. Acta Trop, 85 (2): 253–61. [DOI] [PubMed] [Google Scholar]
- 8.Tamarozzi F, Akhan O, Cretu CM, et al. (2019). Epidemiological factors associated with human cystic echinococcosis: a semi-structured questionnaire from a large population-based ultrasound cross-sectional study in Eastern Europe and Turkey. Parasit Vectors, 12 (1): 371. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Brunetti E, Tamarozzi F, Macpherson C, et al. (2018). Ultrasound and cystic echinococcosis. Ultrasound Int Open, 4(3): E70–E78. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Rinaldi F, Brunetti E, Neumayr A, et al. (2014). Cystic echinococcosis of the liver: A primer for hepatologists. World J Hepatol, 6 (5): 293–305. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Tamarozzi F, Nicoletti GJ, Neumayr A, et al. (2014). Acceptance of standardized ultrasound classification, use of albendazole, and long-term follow-up in clinical management of cystic echinococcosis: a systematic review. Curr Opin Infect Dis, 27 (5): 425–31. [DOI] [PubMed] [Google Scholar]
- 12.Junghanss T, da Silva AM, Horton J, et al. (2008). Clinical management of cystic echinococcosis: state of the art, problems, and perspectives. Am J Trop Med Hyg, 79 (3): 301–11. [PubMed] [Google Scholar]
- 13.Mihmanli M, Idiz UO, Kaya C, et al. (2016). Current status of diagnosis and treatment of hepatic echinococcosis. World J Hepatol, 8 (28): 1169–1181. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Tamarozzi F, Silva R, Fittipaldo VA, et al. (2021). Serology for the diagnosis of human hepatic cystic echinococcosis and its relation with cyst staging: A systematic review of the literature with meta-analysis. PLoS Negl Trop Dis, 15 (4): e0009370. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.González-Sapienza G, Lorenzo C, Nieto A. (2000). Improved immunodiagnosis of cystic hydatid disease by using a synthetic peptide with higher diagnostic value than that of its parent protein, Echinococcus granulosus antigen B. J Clin Microbiol, 38 (11): 3979–83. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Piccoli L, Tamarozzi F, Cattaneo F, et al. (2014). Long-term sonographic and serological follow-up of inactive echinococcal cysts of the liver: hints for a “watch-and-wait” approach. PLoS Negl Trop Dis, 8 (8): e3057. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Sadjjadi SM, Abidi H, Sarkari B, et al. (2007). Evaluation of enzyme linked immunosorbent assay, utilizing native antigen B for serodiagnosis of human hydatidosis. Iran J Immunol, 4 (3): 167–72. [PubMed] [Google Scholar]
- 18.Virginio VG, Hernández A, Rott MB, et al. (2003). A set of recombinant antigens from Echinococcus granulosus with potential for use in the immunodiagnosis of human cystic hydatid disease. Clin Exp Immunol, 132 (2): 309–15. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Carmena D, Benito A, Eraso E. (2006). Antigens for the immunodiagnosis of Echinococcus granulosus infection: An update. Acta Trop, 98 (1): 74–86. [DOI] [PubMed] [Google Scholar]
- 20.Sadjjadi SF, Mohammadzadeh T, Hafezi F, et al. (2022) Evaluation of the Ability of Antigen B Originated from Echinococcus granulosus Sensu Stricto and E. canadensis for the Diagnosis of Confirmed Human Cystic Echinococcosis Using ELISA. Iran J Parasitol, 17(3):358–65. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Pagnozzi D, Biosa G, Addis MF, et al. (2014). An easy and efficient method for native and immunoreactive Echinococcus granulosus antigen 5 enrichment from hydatid cyst fluid. PLoS One, 9 (8): e104962. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Verastegui M, Moro P, Guevara A, et al. (1992). Enzyme-linked immunoelectrotransfer blot test for diagnosis of human hydatid disease. J Clin Microbiol, 30 (6): 1557–61. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Oriol R, Williams JF, Pérez Esandi MV, et al. (1971). Purification of lipoprotein antigens of Echinococcus granulosus from sheep hydatid fluid. Am J Trop Med Hyg, 20 (4): 569–74. [DOI] [PubMed] [Google Scholar]
- 24.Bradford MM. (1976). A rapid and sensitive method for the quantitation of microgram quantities of protein utilizing the principle of protein-dye binding. Anal Biochem, 72 (1–2): 248–54. [DOI] [PubMed] [Google Scholar]
- 25.Sadjjadi FS, Ahmadi N, Rezaie-Tavirani M, et al. (2021). Following up of Surgical Treated Human Liver Cystic Echinococcosis: A Proteomics Approach. Iran J Parasitol. 16(1):11–22. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Haniloo A, Massoud J, Rokni MB. (2005). Evaluation and comparison of antigen B-ELISA and antigen B-immunoblotting in immunodiagnosis of cystic hydatid disease. Pak J Med Sci, 21 (3): 352–356. [Google Scholar]
- 27.Khalilpour A, Sadjjadi SM, Kazemi-Moghadam Z, et al. (2014). Lateral flow test using Echinococcus granulosus native antigen B and comparison of IgG and IgG4 dipsticks for detection of human cystic echinococcosis. Am J Trop Med Hyg, 91 (5): 994–999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Zibaei M, Azargoon A, Ataie-Khorasgani M, et al. (2013). The serological study of cystic echinococcosis and assessment of surgical cases during 5 years (2007–2011) in Khorram Abad, Iran. Niger J Clin Pract, 16 (2): 221–5. [DOI] [PubMed] [Google Scholar]
- 29.Mamuti W, Sako Y, Nakao M, et al. (2006). Recent advances in characterization of Echinococcus antigen B. Parasitol Int, 55 Suppl:S57–62. [DOI] [PubMed] [Google Scholar]
- 30.Rokni MB, Lesan S, Massoud J, et al. (2006). Comparative evaluation of Fast enzyme linked immunosorbent assay (FastELISA) and standard-ELISA for the diagnosis of human hydatidosis. Iran J Public Health, 35 (2): 29–32. [Google Scholar]
- 31.Asghari M, Mohebali M, Kia EB, et al. (2013). Seroepidemiology of Human Hydatidosis Using AgB-ELISA Test in Arak, Central Iran. Iran J Public Health, 42(9): 391–396. [PMC free article] [PubMed] [Google Scholar]
- 32.Dabaghzadeh H, Bairami A, Kia EB, et al. (2018). Seroprevalence of human cystic echinococcosis in Alborz Province, Central Iran in 2015. Iran J Public Health, 47: 561–566. [PMC free article] [PubMed] [Google Scholar]
- 33.Darabi E, Motevaseli E, Khorramizadeh MR, et al. (2019). Design and Construction of a Fusion Peptide Containing B1, B2, B4, and EPC1 Epitopes for Diagnosis of Human Cystic Echinococcosis. Iran J Public Health, 48 (9): 1671–1680. [PMC free article] [PubMed] [Google Scholar]
- 34.Hafezi F, Mohammadzadeh T, Pazoki R, et al. (2022). Sero-Epidemiological Study of Human Hydatidosis in Semnan and Sorkheh, Semnan Province, Iran. Iran J Public Health, 51 (6): 1411–1418. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Mohammadzadeh T, Sako Y, Sadjjadi SM, et al. (2012). Comparison of the usefulness of hydatid cyst fluid, native antigen B and recombinant antigen B8/1 for serological diagnosis of cystic echinococcosis. Trans R Soc Trop Med Hyg, 106(6): 371–5. [DOI] [PubMed] [Google Scholar]
- 36.Sbihi Y, Rmiqui A, Rodriguez-Cabezas MN, et al. (2001). Comparative sensitivity of six serological tests and diagnostic value of ELISA using purified antigen in hydatidosis. J Clin Lab Anal, 15 (1): 14–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Nasrieh MA, Abdel-Hafez SK. (2004). Echinococcus granulosus in Jordan: assessment of various antigenic preparations for use in the serodiagnosis of surgically confirmed cases using enzyme immuno assays and the indirect haemagglutination test. Diagn Microbiol Infect Dis, 48(2): 117–23. [DOI] [PubMed] [Google Scholar]
- 38.Tawfeek GM, Elwakil HS, Awad NS, et al. (2009). Genetic variability of antigen B among Echinococcus granulosus Egyptian isolates. Korean J Parasitol, 47 (3): 259–64. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Siles-Lucas M, Casulli A, Conraths FJ, et al. (2017). Laboratory diagnosis of Echinococcus spp. in human patients and infected animals. Adv Parasitol, 96: 159–257. [DOI] [PubMed] [Google Scholar]
- 40.Manzano-Román R, Sánchez-Ovejero C, Hernández-González A, et al. (2015). Serological diagnosis and follow-up of human cystic echinococcosis: A new hope for the future? Biomed Res Int, 2015:428205. [DOI] [PMC free article] [PubMed] [Google Scholar]