Abstract
Senescent cells accumulate following oncogene induction, but their role in transformation remains unclear. Prieto et al. and Haston et al. found that senescent cells in premalignant lung lesions are mainly macrophages that promote lung tumorigenesis, and removing them through senolytic approaches can prevent malignant progression.
Cellular senescence is a chronic damage response to genotoxic and oncogenic stress leading to durable cell cycle arrest mediated by the P16INK4A/RB1 and P53/P21CIP1 tumor suppressor pathways and a senescence-associated secretory phenotype (SASP) that communicates these damage signals to the surrounding tissue. First described as a phenomenon during cellular and organismal aging in the 1960s by Hayflick and Moorhead, senescence is now understood to be involved in a variety of physiological processes, including development, wound healing, inflammation, and cancer1. In 2005, a series of groundbreaking studies showed that markers of senescence were present in human and mouse premalignant cells in the lungs and other tissues following oncogene induction but not in fully developed tumors2. These findings suggested that senescence could act as a tumor suppressive barrier to malignancy by limiting the outgrowth of damaged cells. However, the development of genetic mouse models and pharmacological compounds (known as senolytics) allowing selective depletion of senescent cells over the past decade has demonstrated that senescence can not only contribute to age-related pathologies but also to tumorigenesis itself3,4.
The mechanisms underlying the dual role of senescence in tumor suppression and promotion are not yet fully understood. Heterogeneity in senescent states and cell types may contribute to the context-dependent outcomes of senescence in cancer. Indeed, senescent fibroblasts in the tumor microenvironment (TME) and their SASP have been shown to promote immune suppression and tumor progression5. Macrophages, which are abundant in many solid tumors, can also express senescence markers and phenotypes that impact their polarization6. Therefore, dissecting the contribution of various senescent cell populations to tumor development and progression in physiological cancer contexts is needed to uncover the underlying mechanisms driving transformation and develop effective cancer interception strategies.
Haston et al. and Prieto et al., in two papers published in this issue of Cancer Cell7,8, generated KRAS-driven lung cancer models in senescence reporter mice to track and selectively deplete senescent cells using the canonical senescence marker P16INK4a during spontaneous lung tumorigenesis. Consistent with previous reports2, they observed that p16+ senescent cells accumulated in early stage premalignant lesions in the lungs following oncogene-induced senescence and persisted, albeit at lower frequencies, during adenocarcinoma formation. However, they also found that pharmacogenetic elimination of P16INK4a-expressing cells or senolytic treatment with BCL2 pathway inhibitors reduced tumor progression, reversed immune suppression, and prolonged animal survival, suggesting that oncogene-induced senescence contributed unexpectedly to tumor promotion (Figure 1). Surprisingly, Prieto et al. found that mice harboring an activating KRASG12D allele in combination with germline knockout of the p16 or p21 tumor suppressors also displayed a reduction rather than an acceleration in lung tumor progression.
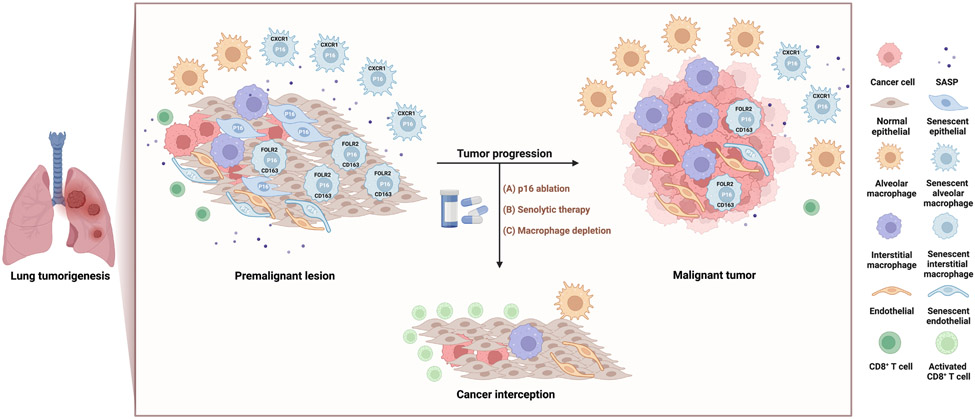
Figure 1. Senescent macrophages that accrue following oncogene-induced senescence promote lung tumorigenesis, and their clearance delays tumor progression.
In premalignant hyperplastic and adenoma lesions in the lungs of mouse models and patient samples, Prieto et al. observe the accumulation of senescent CXCR1+ alveolar macrophages, and Haston et al. senescent FOLR2+/CD163+ interstitial macrophages and PECAM+ endothelial cells. Depletion of these senescent cells via pharmacogenetic p16 ablation, senolytic compounds, or macrophage depleting agents delays tumor onset and progression, and remodels the immune suppressive lung TME to activate cytotoxic CD8+ T cells. These findings suggests that senolytic therapy that targets senescent macrophage populations may be a novel approach to intercept lung cancer. Figure created with Biorender.com.
To investigate how senescence blockade could suppress lung tumorigenesis, the groups used flow cytometry, co-immunofluorescence, and single cell RNA-sequencing techniques to identify and characterize senescent cell types in the lung at different stages of tumorigenesis. They found that while a small subset of premalignant epithelial cells displayed senescence markers, the majority of senescent cells during oncogene-induced senescence were found in non-tumor macrophage and endothelial cell compartments (Figure 1). Macrophage populations sharing similar senescence and SASP markers were also observed in the lungs of aged mice, suggesting senescent macrophages are a conserved senescent population during both tumorigenesis and aging in the lung. Importantly, senolytic treatment primarily targeted these senescent macrophage populations, and treatment with non-selective macrophage depleting agents also led to a similar decrease in tumor onset and progression. These results indicate that senescent macrophages may play a crucial role in lung tumorigenesis.
It is interesting to note that the two studies uncovered distinct senescent macrophage and endothelial populations. Prieto et al. identified CXCR1+ alveolar macrophages as a unique senescent population during lung tumorigenesis and aging. On the other hand, Haston et al. discovered that FOLR2+/CD163+ interstitial macrophages, as well as PECAM+ endothelial cells, were the predominant senescent cell types in the lung. Despite these differences, both groups could identify macrophage populations exhibiting these unique markers and senescent features in premalignant hyperplasic and adenoma lesions in human patients, demonstrating the translational relevance of their findings.
Although the senescence ablation experiments in lung cancer mouse models demonstrated a functional role for senescent cells in promoting tumorigenesis, whether this can be attributed solely to senescent macrophage populations is limited by the current lack of tools to specifically target them. Senolytic approaches targeting p16+ cells or the anti-apoptotic BCL-2 pathway used here and widely in the field could remove other senescent cell types beyond macrophages, including endothelial cells as shown by Haston and colleagues, as well as non-senescent cells expressing these markers. Moreover, as the bulk removal of macrophages also leads to lung tumor suppression, it remains unclear how senescent and non-senescent macrophages differ in their phenotypes and tumor-promoting properties, and if there is a selective advantage to targeting senescent macrophages specifically. The identification of unique cell surface markers on senescent macrophages and other senescent cell types through single cell technologies employed by the authors will facilitate the development of more accurate senescence biomarkers and senolytic tools to address these limitations in the future.
The findings from these studies also raise several thought-provoking questions. First, how does oncogene-induced senescence in epithelial cells lead to macrophage senescence? Given that the SASP is known to drive “paracrine” senescence in adjacent bystander cells9, it is interesting to consider whether oncogene-induced senescent cells, through secretion of the SASP factors such as IL-8, could directly attract and senesce CXCR1+ macrophages. Second, how do senescent macrophages and their SASP support tumorigenesis? Preliminary experiments in these studies suggest that senescent macrophages could facilitate tumor progression directly through SASP factors that promote tumor cell proliferation or indirectly by suppressing cytotoxic CD8+ T cell responses and inducing angiogenesis. However, further mechanistic interrogation is needed to define their exact pro-tumorigenic functions in cancer. Third, do senescent macrophage populations exist and play a role in other cancer settings? The recent identification of a senescent TREM2+ neutrophil population that promoted tumorigenesis in the prostate following oncogene-induced senescence10 suggests that multiple senescent myeloid cell types could contribute to tumorigenesis and be potentially targeted for therapeutic intervention. It will be important to investigate how differences in tissue background or senescence-inducing stimuli impact heterogeneity in senescent stromal cell types and their functions within the TME. Finally, the striking finding from both papers that these same senescent macrophage populations and markers are found in normal lung tissue of aged mice suggests an intriguing link between senescence and the increased incidence of carcinogenesis in the older individuals that warrants further investigation.
Taken together, both studies provide a more detailed understanding of the complex role of senescence in cancer and reveal a mechanism by which senescent macrophages contribute to carcinogenesis. While further research is needed to optimize specific senescence biomarkers and targets to translate these findings to clinical use, these studies underscore the potential of senolytic therapy as a treatment intervention for lung cancer.
ACKNOWLEDGEMENTS
We thank K. Murphy and L. Chibaya for their helpful feedback. This work was supported by a R00 CA241110 grant from the National Cancer Institute (NCI) and a Prostate Cancer Research Program (PCRP) Idea Development Award (W81XWH-22-1-0505) from the Department of Defense (DoD) office of the Congressionally Directed Medical Research Programs (CDMRP).
Footnotes
DECLARATIONS OF INTEREST
M.R. is a consultant for Boehringer Ingelheim.
REFERENCES
- 1.Gorgoulis V, Adams PD, Alimonti A, Bennett DC, Bischof O, Bishop C, Campisi J, Collado M, Evangelou K, Ferbeyre G, et al. (2019). Cellular Senescence: Defining a Path Forward. Cell 179, 813–827. 10.1016/j.cell.2019.10.005. [DOI] [PubMed] [Google Scholar]
- 2.Braig M, and Schmitt CA (2006). Oncogene-induced senescence: putting the brakes on tumor development. Cancer Res 66, 2881–2884. 10.1158/0008-5472.CAN-05-4006. [DOI] [PubMed] [Google Scholar]
- 3.Alimirah F, Pulido T, Valdovinos A, Alptekin S, Chang E, Jones E, Diaz DA, Flores J, Velarde MC, Demaria M, et al. (2020). Cellular Senescence Promotes Skin Carcinogenesis through p38MAPK and p44/42MAPK Signaling. Cancer Res 80, 3606–3619. 10.1158/0008-5472.CAN-20-0108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Baker DJ, Childs BG, Durik M, Wijers ME, Sieben CJ, Zhong J, Saltness RA, Jeganathan KB, Verzosa GC, Pezeshki A, et al. (2016). Naturally occurring p16(Ink4a)-positive cells shorten healthy lifespan. Nature 530, 184–189. 10.1038/nature16932. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Ruhland MK, Loza AJ, Capietto AH, Luo X, Knolhoff BL, Flanagan KC, Belt BA, Alspach E, Leahy K, Luo J, et al. (2016). Stromal senescence establishes an immunosuppressive microenvironment that drives tumorigenesis. Nat Commun 7, 11762. 10.1038/ncomms11762. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Liu JY, Souroullas GP, Diekman BO, Krishnamurthy J, Hall BM, Sorrentino JA, Parker JS, Sessions GA, Gudkov AV, and Sharpless NE (2019). Cells exhibiting strong p16(INK4a) promoter activation in vivo display features of senescence. Proc Natl Acad Sci U S A 116, 2603–2611. 10.1073/pnas.1818313116. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Prieto LI, Sturmlechner I, Graves SI, Zhang C, Goplen NP, Yi ES, Sun J, Li H, and Baker DJ (2023). Senescent alveolar macrophages promote early-stage lung tumorigenesis. Cancer Cell 41, 1261–1275 e1266. 10.1016/j.ccell.2023.05.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Haston S, Gonzalez-Gualda E, Morsli S, Ge J, Reen V, Calderwood A, Moutsopoulos I, Panousopoulos L, Deletic P, Carreno G, et al. (2023). Clearance of senescent macrophages ameliorates tumorigenesis in KRAS-driven lung cancer. Cancer Cell 41, 1242–1260 e1246. 10.1016/j.ccell.2023.05.004. [DOI] [PubMed] [Google Scholar]
- 9.Acosta JC, Banito A, Wuestefeld T, Georgilis A, Janich P, Morton JP, Athineos D, Kang TW, Lasitschka F, Andrulis M, et al. (2013). A complex secretory program orchestrated by the inflammasome controls paracrine senescence. Nat Cell Biol 15, 978–990. 10.1038/ncb2784. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Bancaro N, Cali B, Troiani M, Elia AR, Arzola RA, Attanasio G, Lai P, Crespo M, Gurel B, Pereira R, et al. (2023). Apolipoprotein E induces pathogenic senescent-like myeloid cells in prostate cancer. Cancer Cell 41, 602–619 e611. 10.1016/j.ccell.2023.02.004. [DOI] [PubMed] [Google Scholar]