Abstract
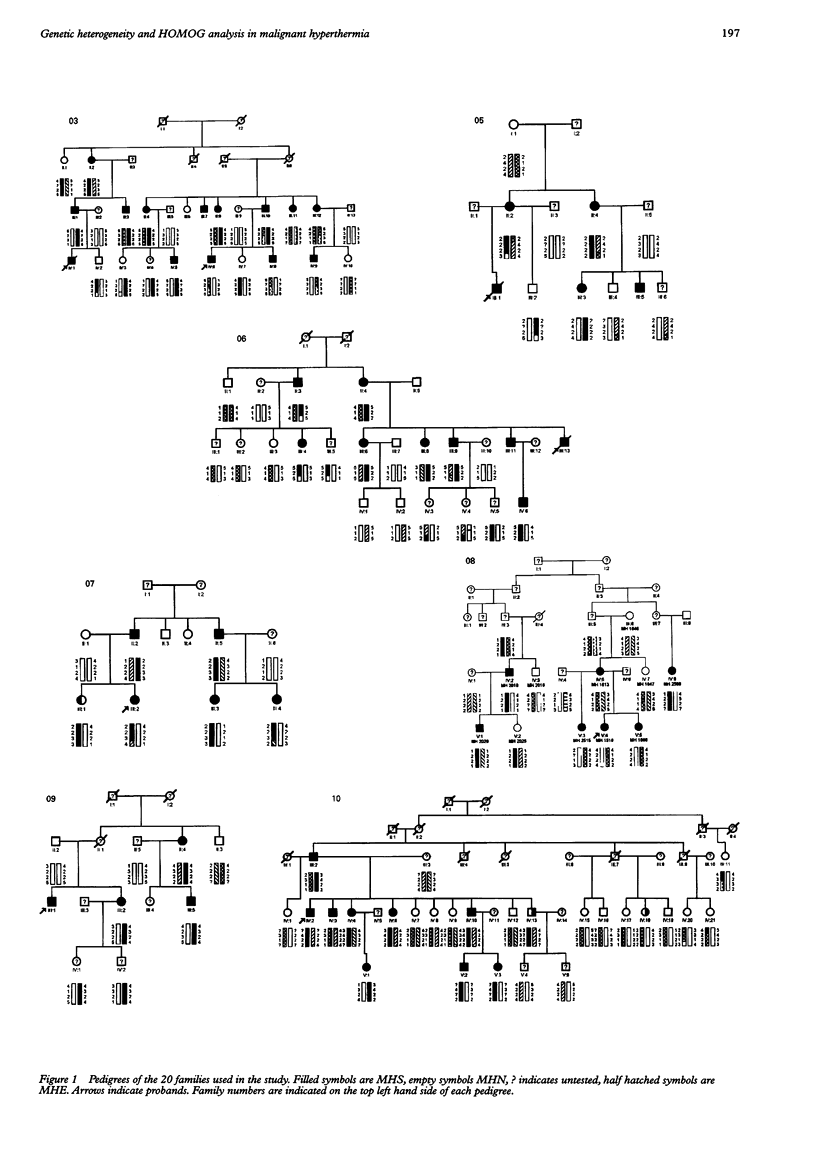
Malignant hyperthermia (MH) is an autosomal dominant genetic condition that presents in susceptible people undergoing general anaesthesia. The clinical disorder is a major cause of anaesthetic morbidity and mortality. The UK Malignant Hyperthermia Group has performed genetic linkage analysis on 20 large, well defined malignant hyperthermia families, using hypervariable markers on chromosome 19q13.1, including the candidate MH gene RYR1, the gene coding for the skeletal muscle ryanodine receptor protein. The results were analysed using LINKAGE to perform two point and multipoint lod scores, then HOMOG to calculate levels of heterogeneity. The results clearly showed genetic heterogeneity between MH families; nine of the families gave results entirely consistent with linkage to the region around RYR1 while the same region was clearly excluded in three families. In the remaining eight MHS families there were single recombinant events between RYR1 and MH susceptibility. HOMOG analysis was of little added benefit in determining the likelihood of linkage to RYR1 in these families. This confirmation of the presence of heterogeneity in the UK MH population, along with the possibility of the presence of two MH genes in some pedigrees, indicates that it would be premature and potentially dangerous to offer diagnosis of MH by DNA based methods at this time.
Full text
PDF




Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Adeokun A. M., West S. P., Ellis F. R., Halsall P. J., Hopkins P. M., Foroughmand A. M., Iles D. E., Robinson R. L., Stewart A. D., Curran J. L. The G1021A substitution in the RYR1 gene does not cosegregate with malignant hyperthermia susceptibility in a British pedigree. Am J Hum Genet. 1997 Apr;60(4):833–841. [PMC free article] [PubMed] [Google Scholar]
- Ball S. P., Dorkins H. R., Ellis F. R., Hall J. L., Halsall P. J., Hopkins P. M., Mueller R. F., Stewart A. D. Genetic linkage analysis of chromosome 19 markers in malignant hyperthermia. Br J Anaesth. 1993 Jan;70(1):70–75. doi: 10.1093/bja/70.1.70. [DOI] [PubMed] [Google Scholar]
- Couch F. J., Hogan K., McCarthy T. V., Gregg R. G. Dinucleotide repeat polymorphism at the RYR1 locus (19q13.1). Nucleic Acids Res. 1991 Sep 25;19(18):5094–5094. doi: 10.1093/nar/19.18.5094. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Deposition of atomic coordinates and other data for crystal structures. Nucleic Acids Res. 1992 Jan 11;20(1):1–1. [PMC free article] [PubMed] [Google Scholar]
- Deufel T., Golla A., Iles D., Meindl A., Meitinger T., Schindelhauer D., DeVries A., Pongratz D., MacLennan D. H., Johnson K. J. Evidence for genetic heterogeneity of malignant hyperthermia susceptibility. Am J Hum Genet. 1992 Jun;50(6):1151–1161. [PMC free article] [PubMed] [Google Scholar]
- Deufel T., Sudbrak R., Feist Y., Rübsam B., Du Chesne I., Schäfer K. L., Roewer N., Grimm T., Lehmann-Horn F., Hartung E. J. Discordance, in a malignant hyperthermia pedigree, between in vitro contracture-test phenotypes and haplotypes for the MHS1 region on chromosome 19q12-13.2, comprising the C1840T transition in the RYR1 gene. Am J Hum Genet. 1995 Jun;56(6):1334–1342. [PMC free article] [PubMed] [Google Scholar]
- Ellis F. R., Halsall P. J., Harriman D. G. The work of the Leeds Malignant Hyperpyrexia Unit, 1971-84. Anaesthesia. 1986 Aug;41(8):809–815. doi: 10.1111/j.1365-2044.1986.tb13122.x. [DOI] [PubMed] [Google Scholar]
- Fagerlund T., Islander G., Ranklev E., Harbitz I., Hauge J. G., Møkleby E., Berg K. Genetic recombination between malignant hyperthermia and calcium release channel in skeletal muscle. Clin Genet. 1992 May;41(5):270–272. [PubMed] [Google Scholar]
- Fujii J., Otsu K., Zorzato F., de Leon S., Khanna V. K., Weiler J. E., O'Brien P. J., MacLennan D. H. Identification of a mutation in porcine ryanodine receptor associated with malignant hyperthermia. Science. 1991 Jul 26;253(5018):448–451. doi: 10.1126/science.1862346. [DOI] [PubMed] [Google Scholar]
- Healy S. J., Heffron J. J., Lehane M., Bradley D. G., Johnson K., McCarthy T. V. Diagnosis of susceptibility to malignant hyperthermia with flanking DNA markers. BMJ. 1991 Nov 16;303(6812):1225–1228. doi: 10.1136/bmj.303.6812.1225. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hopkins P. M., Halsall P. J., Ellis F. R. Diagnosing malignant hyperthermia susceptibility. Anaesthesia. 1994 May;49(5):373–375. doi: 10.1111/j.1365-2044.1994.tb03465.x. [DOI] [PubMed] [Google Scholar]
- Iles D. E., Lehmann-Horn F., Scherer S. W., Tsui L. C., Olde Weghuis D., Suijkerbuijk R. F., Heytens L., Mikala G., Schwartz A., Ellis F. R. Localization of the gene encoding the alpha 2/delta-subunits of the L-type voltage-dependent calcium channel to chromosome 7q and analysis of the segregation of flanking markers in malignant hyperthermia susceptible families. Hum Mol Genet. 1994 Jun;3(6):969–975. doi: 10.1093/hmg/3.6.969. [DOI] [PubMed] [Google Scholar]
- Iles D. E., Segers B., Sengers R. C., Monsieurs K., Heytens L., Halsall P. J., Hopkins P. M., Ellis F. R., Hall-Curran J. L., Stewart A. D. Genetic mapping of the beta 1- and gamma-subunits of the human skeletal muscle L-type voltage-dependent calcium channel on chromosome 17q and exclusion as candidate genes for malignant hyperthermia susceptibility. Hum Mol Genet. 1993 Jul;2(7):863–868. doi: 10.1093/hmg/2.7.863. [DOI] [PubMed] [Google Scholar]
- Levitt R. C., Nouri N., Jedlicka A. E., McKusick V. A., Marks A. R., Shutack J. G., Fletcher J. E., Rosenberg H., Meyers D. A. Evidence for genetic heterogeneity in malignant hyperthermia susceptibility. Genomics. 1991 Nov;11(3):543–547. doi: 10.1016/0888-7543(91)90061-i. [DOI] [PubMed] [Google Scholar]
- Levitt R. C., Olckers A., Meyers S., Fletcher J. E., Rosenberg H., Isaacs H., Meyers D. A. Evidence for the localization of a malignant hyperthermia susceptibility locus (MHS2) to human chromosome 17q. Genomics. 1992 Nov;14(3):562–566. doi: 10.1016/s0888-7543(05)80152-1. [DOI] [PubMed] [Google Scholar]
- MacLennan D. H., Duff C., Zorzato F., Fujii J., Phillips M., Korneluk R. G., Frodis W., Britt B. A., Worton R. G. Ryanodine receptor gene is a candidate for predisposition to malignant hyperthermia. Nature. 1990 Feb 8;343(6258):559–561. doi: 10.1038/343559a0. [DOI] [PubMed] [Google Scholar]
- McCarthy T. V., Healy J. M., Heffron J. J., Lehane M., Deufel T., Lehmann-Horn F., Farrall M., Johnson K. Localization of the malignant hyperthermia susceptibility locus to human chromosome 19q12-13.2. Nature. 1990 Feb 8;343(6258):562–564. doi: 10.1038/343562a0. [DOI] [PubMed] [Google Scholar]
- Otsu K., Khanna V. K., Archibald A. L., MacLennan D. H. Cosegregation of porcine malignant hyperthermia and a probable causal mutation in the skeletal muscle ryanodine receptor gene in backcross families. Genomics. 1991 Nov;11(3):744–750. doi: 10.1016/0888-7543(91)90083-q. [DOI] [PubMed] [Google Scholar]
- Phillips M. S., Fujii J., Khanna V. K., DeLeon S., Yokobata K., de Jong P. J., MacLennan D. H. The structural organization of the human skeletal muscle ryanodine receptor (RYR1) gene. Genomics. 1996 May 15;34(1):24–41. doi: 10.1006/geno.1996.0238. [DOI] [PubMed] [Google Scholar]
- Robinson R. L., Monnier N., Wolz W., Jung M., Reis A., Nuernberg G., Curran J. L., Monsieurs K., Stieglitz P., Heytens L. A genome wide search for susceptibility loci in three European malignant hyperthermia pedigrees. Hum Mol Genet. 1997 Jun;6(6):953–961. doi: 10.1093/hmg/6.6.953. [DOI] [PubMed] [Google Scholar]
- Saiki R. K., Gelfand D. H., Stoffel S., Scharf S. J., Higuchi R., Horn G. T., Mullis K. B., Erlich H. A. Primer-directed enzymatic amplification of DNA with a thermostable DNA polymerase. Science. 1988 Jan 29;239(4839):487–491. doi: 10.1126/science.2448875. [DOI] [PubMed] [Google Scholar]
- Sudbrak R., Golla A., Hogan K., Powers P., Gregg R., Du Chesne I., Lehmann-Horn F., Deufel T. Exclusion of malignant hyperthermia susceptibility (MHS) from a putative MHS2 locus on chromosome 17q and of the alpha 1, beta 1, and gamma subunits of the dihydropyridine receptor calcium channel as candidates for the molecular defect. Hum Mol Genet. 1993 Jul;2(7):857–862. doi: 10.1093/hmg/2.7.857. [DOI] [PubMed] [Google Scholar]
- Sudbrak R., Procaccio V., Klausnitzer M., Curran J. L., Monsieurs K., van Broeckhoven C., Ellis R., Heyetens L., Hartung E. J., Kozak-Ribbens G. Mapping of a further malignant hyperthermia susceptibility locus to chromosome 3q13.1. Am J Hum Genet. 1995 Mar;56(3):684–691. [PMC free article] [PubMed] [Google Scholar]
- Weber J. L., May P. E. Abundant class of human DNA polymorphisms which can be typed using the polymerase chain reaction. Am J Hum Genet. 1989 Mar;44(3):388–396. [PMC free article] [PubMed] [Google Scholar]
- Weissenbach J., Gyapay G., Dib C., Vignal A., Morissette J., Millasseau P., Vaysseix G., Lathrop M. A second-generation linkage map of the human genome. Nature. 1992 Oct 29;359(6398):794–801. doi: 10.1038/359794a0. [DOI] [PubMed] [Google Scholar]


