Abstract
Gliomas are the most prevalent primary malignant brain tumors worldwide, with glioblastoma (GBM) being the most common and aggressive type. Despite two decades of relentless pursuit in exploring novel therapeutic approaches for GBM, there is limited progress in improving patients’ survival outcomes. Numerous obstacles impede the effective treatment of GBM, including the immunosuppressive tumor microenvironment (TME), the blood-brain barrier, and extensive heterogeneity. Despite these challenges, immunotherapies are emerging as a promising avenue that may offer new hope for the treatment of gliomas. There are four main types of immunotherapies for gliomas, immune checkpoint blockades, chimeric antigen receptor T-cell therapies, vaccines, and oncolytic viruses. In addition, gene therapy, bispecific antibody therapy, and combine therapy are also briefly introduced in this review. The significant role of TME in the process of immunotherapies has been emphasized in many studies. Although immunotherapy is a promising treatment for gliomas, enormous effort is required to overcome the existing barriers to its success. Owing to the rapid development and increasing attention paid to immunotherapies for gliomas, this article aims to review the recent advances in immunotherapies for gliomas.
Keywords: immunotherapy, glioma, ICB, car-t, oncolytic viruses, vaccine, tumor microenvironment
1. Introduction
Gliomas are the most prevalent primary malignant brain tumors worldwide. Gliomas often grow in the brain and come from glial tissue, yet they may form elsewhere in the central nervous system (CNS) (1–3). According to the up-to-date WHO classification of CNS tumors, diffuse gliomas in adults have been classified into: astrocytoma IDH-mutant (grade 2, 3, or 4), oligodendroglioma IDH-mutant and 1p/19q co-deleted (grade 2 or 3), and glioblastoma (GBM) IDH-wildtype (grade 4) (4). Moreover, patients diagnosed with GBM usually have a terrible prognosis, with a median overall survival (mOS) of less than two years, and a five-year survival rate of 10% (1–3).
The established treatment paradigm for GBM, known as the Stupp regimen, entails maximal surgical tumor resection followed by a combination of radiotherapy and chemotherapy (5). However, almost all patients show recurrence after receiving standard treatment. Thus, it is necessary to explore novel effective therapies for GBM. In recent years, immunotherapeutic strategies have revolutionized the treatment of various cancers, such as melanoma and lung cancer, and also bring new hope for GBM treatment (6–8).
Currently, more than 88 clinical trials on immunotherapies for GBM are being conducted worldwide (9). Moreover, the efficacy of several immunotherapeutic treatments, including the dendritic cell (DC) vaccine DCVax-L (10, 11) and oncolytic virus (OV) G47Δ (12), has been demonstrated in phase II and III clinical trials. And oncolytic virus G47Δ had also been conditionally approved in Japan for the treatment of malignant gliomas. These events have represented the potential power of immunotherapies in gliomas, and immunotherapies are worthy of our wait to change the bad prognosis of patients with GBM. However, several barriers, including blood-brain barrier (BBB), tumor microenvironment (TME), and substantial heterogeneity, broadly weaken and limit the efficacy of immunotherapies for gliomas. Therefore, novel practical therapeutic approaches are constantly being studied.
2. Immunotherapies in gliomas
Immunotherapies for cancer treatment refer to the engagement of patients’ immune systems to recognize and eliminate cancer. There are several kinds of immunotherapies used in cancer treatment, including immune checkpoint blockades (ICBs), adoptive cell therapies, therapeutic vaccines, OV therapies, etc. (13, 14). And different types of immunotherapies are applicable and suitable for different cancers. There are mainly four types of immunotherapies in treating gliomas: ICBs, Chimeric antigen receptor T (CAR-T) cell therapies, vaccines, and OVs (15, 16). ICB therapy can effectively block immune checkpoints such as PD-1/PD-L1 and thus inhibits the immunosuppressive effect. Peptide and dendritic vaccines are the immune targets of tumor-associated antigens (TAAs) and tumor-specific antigens (TSAs). CAR-T cell therapies targeting tumor surface molecules, such as EGFR variant III (EGFRvIII), IL13Rα2, and HER2, have also been explored in GBM treatment. OV therapy is an emerging treatment of GBM that has received widespread attention in recent years, with G47Δ being conditionally approved as a treatment option in Japan, opening the way for further development of immunotherapy (12). Next, we would like to analyze the recent advances in these major immunotherapeutic strategies.
2.1. ICBs
Immune checkpoints are surface molecules on immune cells that can regulate host immunity when they bind to the corresponding ligands or receptors on tumor cells or other cells (9). And tumor cells are proficient at employing this strategy to avoid the lethal effect exerted by immune cells (mainly T cells) (17). Immune checkpoint blockade (ICB) primarily refers to blocking the immunosuppressive immune checkpoints such as PD-1/PD-L1, and CTLA-4, thus inhibiting their corresponding immunosuppressive effects and playing an antitumor effect. And ICB has been proven feasible and effective in treating many cancers (18). However, there are no successful phase III clinical trials or marketing authorizations of ICBs in GBM treatment all over the world. Nevertheless, the exploration of this field is continuous and active, which can be indicated in Table 1 . With a deeper understanding of the TME, some co-stimulatory checkpoints and their specific agonists have also been studied. Unlike other cancers, such as melanoma and lung cancer, the application of ICBs seems unfavorable in treating gliomas (19). Nevertheless, the combination therapy of ICB and other treatments, such as standard chemoradiotherapy, targeted therapies, or other different kinds of immunotherapies, may find a way to success (9).
Table 1.
Recent phase II/III clinical trials of ICBs for GBM treatment.
| Target | Identifier | Title | Phase | Treatment approach | Patient | Status |
|---|---|---|---|---|---|---|
| PD-1 | NCT02017717 (CheckMate 143) | A randomized phase 3 open label study of nivolumab versus bevacizumab and multiple phase 1 safety cohorts of nivolumab or nivolumab in combination with ipilimumab across different lines of glioblastoma | 3 | Nivolumab Bevacizumab Ipilimumab |
rGBM | Completed |
| NCT02617589 (Checkmate 498) | A randomized phase 3 open label study of nivolumab vs. temozolomide each in combination with radiation therapy in newly diagnosed adult subjects with unmethylated MGMT (tumor o-6-methylguanine DNA methyltransferase) glioblastoma | 3 | Nivolumab Temozolomide Radiotherapy |
GBM (Unmethylated MGMT) | Completed | |
| NCT02667587 (Checkmate 548) | A randomized phase 3 single-blind study of temozolomide, plus radiation therapy combined with nivolumab or placebo in newly diagnosed adult subjects with MGMT-methylated glioblastoma | 3 | Nivolumab Temozolomide Radiotherapy |
nGBM | Active | |
| NCT02798406 | Combination adenovirus + pembrolizumab to trigger immune virus effects | 2 | Pembrolizumab Adenovirus |
rGBM | Completed | |
| NCT02550249 | Phase 2 study of neoadjuvant nivolumab in patients with glioblastoma multiforme | 2 | Nivolumab | nGBM and rGBM | Completed | |
| NCT02336165 | Phase 2 study to evaluate the clinical efficacy and safety of durvalumab (MEDI4736) in patients with glioblastoma (GBM) | 2 | Durvalumab | nGBM and rGBM | Completed | |
| NCT02337491 | Phase 2 study of pembrolizumab (MK-3475) with and without bevacizumab for recurrent glioblastoma | 2 | Pembrolizumab Bevacizumab |
rGBM | Completed | |
| NCT03018288 | Radiation Therapy Plus Temozolomide and Pembrolizumab With and Without HSPPC-96 in Newly Diagnosed Glioblastoma (GBM) | 2 | Pembrolizumab Temozolomide Radiotherapy HSPPC-96 |
nGBM | Completed | |
| NCT03405792 | Study Testing The Safety and Efficacy of Adjuvant Temozolomide Plus TTFields (Optune®) Plus Pembrolizumab in Patients With Newly Diagnosed Glioblastoma | 2 | Temozolomide TTFields Pembrolizumab |
nGBM | Active | |
| NCT02337686 | Pharmacodynamic study of pembrolizumab in patients with recurrent glioblastoma | 2 | Pembrolizumab | rGBM | Active | |
| NCT03452579 | A randomized phase 2 open label study of nivolumab plus standard dose bevacizumab versus nivolumab plus low dose bevacizumab in recurrent glioblastoma (GBM) | 2 | Nivolumab Bevacizumab |
rGBM | Active | |
| NCT03743662 | Nivolumab With Radiation Therapy and Bevacizumab for Recurrent MGMT Methylated Glioblastoma | 2 | Nivolumab Bevacizumab Radiotherapy |
rGBM (Methylated MGMT) | Active | |
| NCT03661723 | Pembrolizumab and Reirradiation in Bevacizumab Naïve and Bevacizumab Resistant Recurrent Glioblastoma | 2 | Pembrolizumab Bevacizumab Radiotherapy |
rGBM | Active | |
| NCT03665545 | Pembrolizumab in Association With the IMA950/Poly-ICLC for Relapsing Glioblastoma | 1/2 | Pembrolizumab IMA950/Poly-ICLC |
rGBM | Active | |
| NCT04479241 | LUMINOS-101: Lerapolturev (PVSRIPO) and Pembrolizumab in Patients With Recurrent Glioblastoma | 2 | Pembrolizumab Lerapolturev |
rGBM | Active | |
| NCT04195139 | Nivolumab and Temozolomide Versus Temozolomide Alone in Newly Diagnosed Elderly Patients With GBM (NUTMEG) | 2 | Nivolumab Temozolomide |
nGBM (Elderly) | Active | |
| NCT04013672 | Study of Pembrolizumab Plus SurVaxM for Glioblastoma at First Recurrence | 2 | Pembrolizumab SurVaxM |
rGBM (at first recurrence) | Active | |
| NCT03890952 | Translational Study of Nivolumab in Combination With Bevacizumab for Recurrent Glioblastoma | 2 | Nivolumab Bevacizumab |
rGBM | Active | |
| NCT03899857 | Pembrolizumab for Newly Diagnosed Glioblastoma (PERGOLA) | 2 | Pembrolizumab | nGBM | Active | |
| NCT03491683 | INO-5401 and INO-9012 Delivered by Electroporation (EP) in Combination With Cemiplimab (REGN2810) in Newly-Diagnosed Glioblastoma (GBM) | 1/2 | Cemiplimab INO-5401 INO-9012 |
nGBM | Active | |
| PD-L1 | NCT03174197 | Atezolizumab in Combination With Temozolomide and Radiation Therapy in Treating Patients With Newly Diagnosed Glioblastoma | 1/2 | Atezolizumab Temozolomide Radiotherapy |
nGBM | Active |
| NCT03750071 | VXM01 Plus Avelumab Combination Study in Progressive Glioblastoma | 1/2 | Avelumab VXM01 |
Progressive GBM | Active | |
| CTLA-4 | NCT04817254 | Phase II Trial Evaluating the Association of Peripheral Blood Immunologic Response to Therapeutic Response to Adjuvant Treatment With Immune Checkpoint Inhibition (ICI) in Patients With Newly Diagnosed Glioblastoma or Gliosarcoma | 2 | Ipilimumab Nivolumab TMZ |
nGBM or Gliosarcoma | Recruiting |
| NCT02794883 | A phase 2, open label, clinical trial of pre surgical and adjuvant treatment of recurrent malignant glioma with tremelimumab and durvalumab (MEDI4736) alone and in combination to determine immunologic changes from treatment | 2 | Tremelimumab Durvalumab |
Recurrent malignant glioma | Completed | |
| NCT03367715 | Nivolumab, Ipilimumab, and Short-course Radiotherapy in Adults With Newly Diagnosed, MGMT Unmethylated Glioblastoma | 2 | Ipilimumab Nivolumab Radiotherapy |
nGBM (Unmethylated MGMT) | Completed | |
| NCT04396860 | Testing the Use of the Immunotherapy Drugs Ipilimumab and Nivolumab Plus Radiation Therapy Compared to the Usual Treatment (Temozolomide and Radiation Therapy) for Newly Diagnosed MGMT Unmethylated Glioblastoma | 2/3 | Ipilimumab Nivolumab Radiotherapy |
nGBM (Unmethylated MGMT) | Active | |
| IDO-1 | NCT02052648 | A phase 1/2 study of the combination of indoximod and temozolomide for adult patients with temozolomide-refractory primary malignant brain tumors | 1/2 | Indoximod Temozolomide |
Recurrent malignant glioma | Completed |
Data from ClinicalTrials.gov.
There are several completed clinical trials associated with ICBs for GBM treatment. All three clinical trials used anti-PD-1 monoclonal antibodies. PD-1 is a co-repressor molecule of the CD2 family and is expressed on the activated immune cells constitutively (20). After PD-1 binding to its ligands (mainly PD-L1) on tumor cells or antigen-presenting cells (APCs), T cells’ impotence, failure, or even apoptosis can be induced (21). In addition, PD-1 can also stimulate the proliferation of regulatory T cells (Tregs) and reduce the immune responses of natural killer cells and B cells (22).
Pembrolizumab (KEYTRUDA) is a commonly used monoclonal anti-PD-1 antibody. In 2019, satisfactory results of a randomized, multi-institutional trial of neoadjuvant pembrolizumab in patients with recurrent, surgically resectable GBM were published (23). Compared with patients receiving adjuvant, post-surgical administration of pembrolizumab alone, patients receiving neoadjuvant pembrolizumab and sustained adjuvant therapy after surgery had substantially improved OS and progression-free survival (PFS). The median OS of patients in the adjuvant-only group and the neoadjuvant group were 228 days (7.5 months) and 417 days (13.7 months), respectively, with a hazard ratio (neoadjuvant/adjuvant) of 0.39 (P = 0.04). And the median PFS of patients in the adjuvant-only group and the neoadjuvant group were 72.5 days (2.4 months) and 99.5 days (3.3 months), respectively, with a hazard ratio (neoadjuvant/adjuvant) of 0.43 (P = 0.03). The frequency of the focal upregulation of PD-L1 in the TME, the downregulation of PD-1 on T cells in the peripheral blood, and the intensive clonal expansion of T cells was higher in the neoadjuvant group compared with the adjuvant-only group. And the functional activation of tumor-infiltrating lymphocytes (TILs) was induced by neoadjuvant PD-1 blockade, which produced an interferon response within the TME (23).
The other two clinical trials applied another anti-PD-1 antibody, nivolumab (OPDIVO). Nivolumab is one of the most studied anti-PD-1 antibodies in treating gliomas. CheckMate 143 was the first phase III randomized clinical trial to test the efficacy of nivolumab in patients with recurrent GBM (rGBM) (24). But this trial’s primary endpoint (median OS, mOS) was not reached. Compared with the control group (bevacizumab) (10.0 months), the mOS was similar in the nivolumab group (9.8 months) (P = 0.76). And the PFS and the ORR (overall response rate) of the bevacizumab group were better than those of the nivolumab group. Besides, the post-subgroup analysis indicated that the potential benefits from nivolumab are more probable to be obtained in patients with methylated MGMT promoters (24). NCT02550249 was another crucial phase II clinical trial of nivolumab for GBM treatment. In this single-arm phase II clinical trial, twenty-seven patients with relapsed GBM and three patients with primary GBM were included to explore the feasibility, safety, and antitumor effects of neoadjuvant nivolumab in patients with resectable GBM (25). The neoadjuvant nivolumab was found to increase the expression of chemokines, infiltration of immune cells, and clonal diversity of T cell receptors in the TME (25).
There were also two completed randomized phase III clinical trials to test the efficacy of nivolumab in patients with newly diagnosed GBM (nGBM) (26, 27). The Checkmate 498 trial aimed to test the effectiveness of nivolumab plus radiotherapy, compared with TMZ plus radiotherapy, in patients with nGBM characterized as unmethylated MGMT promoters. However, its recently published results did not reach the primary endpoint (mOS). The mOSs of the nivolumab plus radiotherapy group and TMZ plus radiotherapy were 13.4 months and 14.9 months, respectively (27). The CheckMate 548 trial was a similar phase III study to evaluate the effectiveness of nivolumab plus the Stupp regime (radiotherapy plus TMZ), compared with the Stupp regime plus placebo, in patients with nGBM with methylated MGMT promoter (26). However, the results also showed a failure in adding nivolumab to the Stupp regime could not improve the OS and PFS of the patients. The mPFSs of nivolumab plus the Stupp regime group and the Stupp regime plus placebo group were 10.6 months and 10.3 months, respectively. And the mOSs were 28.9 months and 32.1 months, respectively.
In addition, some other immune checkpoints in gliomas, such as CTLA-4 (28–30), CD47 (31–33), CD73 (34, 35), TIGIT (36), and CD137 (37), were also studied in either clinical trials and preclinical research. It is worth noting that TILs in GBM are proven to highly express a number of co-inhibitory immune checkpoints, such as TIM-3, PD-1, and LAG-3, as a result of severe exhaustion of T cells (38). And this supports the necessity of exploring other feasible immune checkpoints and combined therapies to increase the effectiveness of the ICB (26, 39).
2.2. Vaccine therapy
Vaccines have a long history of use in cancer treatment (40, 41). Several types of vaccines are used in cancer treatment, and peptide and dendritic cell (DC) vaccines are the main strategies for glioma treatment (42–44). In addition, the TAA and TSA can be used as the immune targets of vaccines to stimulate adaptive immunity (45). Table 2 lists the recent phase II/III clinical trials of vaccine therapy for GBM treatment from ClinicalTrials.gov.
Table 2.
Recent phase II/III clinical trials of vaccine therapy for GBM treatment.
| Vaccine type | Identifier | Title | Phase | Treatment approach | Patient type | Status |
|---|---|---|---|---|---|---|
| DC vaccine | NCT00045968 | A phase 3 clinical trial evaluating DCVax-L, autologous dendritic cells pulsed with tumor lysate antigen for the treatment of glioblastoma multiforme | 3 | DCVax-L | nGBM | Active |
| NCT03548571 | Open label randomized phase 2/3 trial of dendritic cell immunotherapy against cancer stem cells in glioblastoma patients receiving standard therapy (DEN-STEM) | 2/3 | DEN-STEM | GBM (IDH wild-type, Methylated MGMT) | Active | |
| NCT00639639 | Anti-tumor immunotherapy targeted against cytomegalovirus in patients with newly diagnosed glioblastoma multiforme during recovery from therapeutic temozolomide-induced lymphopenia | 1/2 | CMV-DC | nGBM | Completed | |
| NCT03688178 | DC Migration Study to Evaluate TReg Depletion In GBM Patients With and Without Varlilumab | 2 | DERIVe | nGBM | Active | |
| NCT01204684 | A Phase II Clinical Trial Evaluating Autologous Dendritic Cells Pulsed With Tumor Lysate Antigen +/- Toll-like Receptor Agonists for the Treatment of Malignant Glioma | 2 | DC vaccine Resiquimod polyICLC |
Malignant glioma | Active | |
| NCT02465268 | A Phase II Randomized, Blinded, and Placebo-controlled Trial of CMV RNA-Pulsed Dendritic Cells With Tetanus-Diphtheria Toxoid Vaccine in Patients With Newly-Diagnosed Glioblastoma | 2 | pp65 DC vaccine | nGBM | Active | |
| NCT00846456 | Phase I/II Trial of Vaccine Therapy With Tumor Stem Cell Derived mRNA- Transfected Dendritic Cells in Patients Receiving Standard Therapy for Glioblastoma | 1/2 | Tumor stem cell derived mRNA- transfected DC vaccine Stupp regimen |
GBM | Completed | |
| NCT00323115 | A Phase II Feasibility Study of Adjuvant Intra-Nodal Autologous Dendritic Cell Vaccination for Newly Diagnosed Glioblastoma Multiforme | 2 | DC vaccine | nGBM | Completed | |
| NCT01006044 | Prospective, Phase II Clinical Trial to Evaluate Efficacy and Safety of Autologous Dendritic Cell Vaccination in Glioblastoma Multiforme Patients After Complete Surgical Resection With Fluorescence Microscope | 2 | DC vaccine | GBM | Completed | |
| NCT01213407 | First Line Standard Therapy of Glioblastoma Multiforme With or Without add-on Treatment With Trivax, an Anti-tumour Immune Therapy Based on Tumour-lysate Charged Dendritic Cells | 2 | Trivax | GBM | Completed | |
| Peptide vaccine | NCT00458601 | A phase 2 study of CDX-110 with radiation and temozolomide in patients with newly diagnosed GBM | 2 | Rindopepimut (CDX 110) TMZ Radiotherapy |
nGBM | Completed |
| NCT01480479 | An international randomized double, blind, controlled study of rindopepimut/GM-CSF with adjuvant temozolomide in patients with newly diagnosed, surgically resected, EGFRvIII-positive glioblastoma | 3 | Rindopepimut (CDX 110) TMZ |
nGBM; surgically resectedGBM; EGFRvIII-positive GBM |
Completed | |
| NCT00905060 | PHASE 2, Multi-center, Single Arm Investigation of HSPPC-96 Vaccine With Temozolomide in Patients With Newly Diagnosed Glioblastoma Multiforme | 2 | HSPPC-96 Temozolomide |
nGBM | Completed | |
| NCT04280848 | Anticancer Therapeutic Vaccination Using Telomerase-derived Universal Cancer Peptides in Glioblastoma | 2 | UCPVax | GBM | Active | |
| NCT02455557 | A Phase II Study of the Safety and Efficacy of SVN53-67/M57-KLH (SurVaxM) in Survivin-Positive Newly Diagnosed Glioblastoma | 2 | SurVaxM Temozolomide |
nGBM | Active | |
| NCT00643097 | A Complementary Trial of an Immunotherapy Vaccine Against Tumor-Specific EGFRvIII | 2 | PEP-3-KLH conjugate vaccine | nGBM | Completed | |
| NCT01920191 | Phase I/II Study of Intradermal IMA950 Peptide-based Vaccine Adjuvanted With Intra Muscular Poly-ICLC in Combination With Temozolomide in Newly Diagnosed HLA-A2 Glioblastoma Patients | 1/2 | IMA950 Poly-ICLC |
GBM | Completed | |
| NCT00293423 | Phase I/II Trial of Heat Shock Protein Peptide Complex-96 (HSPPC-96) Vaccine for Patients With Recurrent High Grade Glioma | 1/2 | GP96 | Recurrent or progressive glioma | Completed | |
| NCT04116658 | A Multicenter, Open-Label, First-in-Human, Phase 1b/2a Trial of EO2401, a Novel Multipeptide Therapeutic Vaccine, With and Without Check Point Inhibitor, Following Standard Treatment in Patients With Progressive Glioblastoma | 1/2 | EO2401 | Recurrent or progressive glioma | Active | |
| NCT03665545 | Pembrolizumab in Association With the Multipeptide Vaccine IMA950 Adjuvanted With Poly-ICLC for Relapsing Glioblastoma: a Randomized Phase I/II Trial | 1/2 | IMA950/Poly-ICLC Pembrolizumab |
rGBM | Active |
Data from ClinicalTrials.gov.
Peptide vaccines comprise 8-25 amino acids and have epitopes acting as antigenic targets (43). Moreover, they are usually conjugated to a carrier protein to increase immunogenicity. These vaccines with simple structures are relatively easy to manufacture and store and have relatively lower variability when compared to other vaccines (43, 46). The frequently mutated or highly expressed proteins or antigens in GBM mainly include EGFR, EGFRvIII, NF1, TERT, PDGFRA, PTEN, RB1, IDH1, TP53, PIK3R1, and PIK3CA, some of which are regarded as ideal vaccine targets (43, 47). EGFRvIII is a deletion mutation of EGFR, which can be detected in approximately 20% of GBM (48, 49). And EGFRvIII is proven to enhance tumor growth and chemotherapy resistance. Currently, EGFRvIII is one of the most studied TSAs and an important target in vaccines for GBM treatment (50). There was an important phase III clinical trial that used the peptide vaccine rindopepimut (51). However, the results of this phase III trial of rindopepimut in combination with TMZ in patients with EGFRvIII-positive nGBM did not show a not disappoint. Compared with the TMZ group, the rindopepimut plus TMZ group did not increase the mOS. The mOS of TMZ and rindopepimut plus TMZ groups were 20.1 months and 20.0 months, respectively (P=0.93). Besides, the result also indicated the necessity of multi-peptide vaccines against several targets to overcome the antigenic heterogeneity in GBM (43, 51).
DC vaccine is another studied type of vaccine for glioma treatment (52). Dendritic cells’ function as antigen-presenting cells serves as the foundation for the main mechanism of DC vaccines (53). When immune cells, especially T cells, are activated by DCs, they can cross BBB and enter the brain tumor site to play the antitumor effects (53, 54). And DCs are believed to trigger both innate and adaptive immune responses to facilitate the transformation of immunologically cold gliomas into immunologically hot gliomas (44, 53, 55). Currently, about half of the current phase II and III trials involving vaccines are cell-based strategies, especially DC vaccines (56).
In 2023, a phase III trial of an autologous tumor lysate-loaded DC vaccine (DCVax-L) plus TMZ in patients with nGBM and rGBM reported encouraging findings (10, 11). This study’s primary and secondary endpoints were the mOSs in patients with nGBM and rGBM, respectively (10). In the nGBM part, the mOSs in the DCVax-L plus TMZ group and the TMZ control group were 19.3 and 16.5 months, respectively (P = 0.002). And the survival rates of two and five years in DCVax-L plus TMZ group and TMZ control group were 15.7% vs 9.9% and 13.0% vs 5.7%, respectively (10). In the rGBM part, the mOSs in DCVax-L plus TMZ group and TMZ control group were 13.2 and 7.8 months, respectively (P < 0.001). And the survival rates of two years and 30 months of DCVax-L plus TMZ group and TMZ control group were 15.7% vs. 9.9% and 13.0% vs. 5.7%, respectively (10). Besides, DCVax-L-treated patients with nGBM with methylated MGMT promoter survived longer (21.3 months) than those in the external control group (P = 0.03) (10). Obviously, this phase III trial advances the pace of vaccine therapy for gliomas. However, the individual patient-level data of the external control populations are not accessible. And a more credible and reasonable investigation of DCVax-L in GBM treatment is needed.
2.3. CAR-T cell therapy
CAR-T cell therapy is a typical type of adoptive T-cell therapies (57). This therapeutic approach involves collecting T cells from a patient’s peripheral blood, followed by their modification, amplification, and activation to express CAR molecules on the cell membranes. These genetically engineered T cells are then administered to the patient through injection, allowing them to target specific tumor cell antigens (57). The significance of CAR-T cell therapy in treating gliomas has been identified, although it has yet to exhibit big success (58). Table 3 lists the recent phase II/III clinical trials of CAR-T therapy for GBM treatment from ClinicalTrials.gov.
Table 3.
Recent clinical trials of CAR-T cell therapy for GBM treatment.
| Identifier | Title | Phase | Treatment approach | Patient type | Status |
|---|---|---|---|---|---|
| NCT01454596 | A phase 1/2 study of the safety and feasibility of administering T cells expressing Anti-EGFRvIII chimeric antigen receptor to patients with malignant gliomas expressing EGFRvIII | 1/2 | EGFRvIII-CARs | Malignant glioma (EGFRvIII-positive) | Completed |
| NCT02208362 | Genetically modified T-cells in treating patients with recurrent or refractory malignant glioma | 1 | IL13Rα2-CARs | Recurrent or refractory malignant glioma | Active |
| NCT01082926 | Phase 1 study of cellular immunotherapy for recurrent/refractory malignant glioma using intratumoral infusions of GRm13Z40-2, an allogenic CD8+ cytolitic T-cell line genetically modified to express the IL13-zetakine and HyTK and to be resistant to glucocorticoids in combination with interleukin-2 | 1 | GRm13Z40-2 interleukin-2 |
Recurrent/refractory malignant glioma | Completed |
| NCT01109095 | Administration of HER2 chimeric antigen receptor expressing CMV-specific cytotoxic T cells in patients with glioblastoma multiforme (HERT-GBM) | 1 | HER2-CARs | Recurrent or progressive GBM | Completed |
| NCT05063682 | A Phase 1 Study to Evaluate EGFRvIII -Targeted Chimeric Antigen Receptor (CAR) T Cells for Adult Patients With Leptomeningeal Glioblastoma | 1 | EGFRvIII-CAR T | leptomeningeal GBM | Active |
| NCT03726515 | Phase 1 Study of EGFRvIII-Directed CAR T Cells Combined With PD-1 Inhibition in Patients With Newly Diagnosed, MGMT-Unmethylated Glioblastoma | 1 | CART-EGFRvIII Pembrolizumab |
nGBM (Unmethylated MGMT, EGFRvIII-positive) | Completed |
Data from ClinicalTrials.gov.
The phase I clinical trials associated with CAR-T cell therapy in GBM treatment shows that scientists are also actively trying in this field. In these clinical trials, several commonly used targeted antigens, including EGFRvIII (59), IL13Rα2 (60), and HER2 (61), had also been indicated. NCT02208362 was a phase I clinical trial of IL13Rα2-targeted CAR-T cell therapy in GBM treatment (60). In this study, two intracranial approaches were applied to deliver CAR-T cells to a patient with rGBM, including infusions into the resected tumor cavity and the ventricular system. And it was found that intraventricular therapy can achieve the wide regression of central nervous system tumors. In comparison, the intracavitary treatment seemed only to control the local tumor recurrence. Moreover, the CAR-T cell therapy provided the patient with a response for 7.5 months. Overall, the result proved that CAR-T cell therapy can show the antitumor activity in GBM treatment, and IL13Rα2 is a valid immunotherapeutic target for CAR-T cell therapy (60). NCT02209376 was another phase I study of CAR-T cell therapy in patients with EGFRvIII-positive rGBM (59). This first-in-human experiment of EGFRvIII-targeting CAR-T cells by intravenous administration proved that CAR-T cells could transfer to the brain tumor site. It is worth noting that the EGFRvIII level on tumor cells was observed to be reduced or eliminated after the CAR-T cell administration. Moreover, the effect of CAR-T cells on the TME in GBM was also emphasized in this study. The immunosuppressive Tregs were the dominant T cell type for TILs in the TME. And many immunosuppressive molecules, including PD-L1, IL-10, and IDO1, were also observed to increase. Besides, the study also indicated the combination therapy of CAR-T cell therapy and the inhibition of immunosuppressive pathways (59). In another phase I study (NCT01109095), researchers investigated the use of HER2-targeting CAR-modified virus-specific T cells (HER2-CAR VSTs) in patients with rGBM (61). Participants in this trial were administered one or more autologous HER2-CAR VST injections across five different dosage levels. Of the 17 patients, eight exhibited clinical benefits, with one showing a partial response and seven maintaining stable disease. The median overall survival (mOS) was 11.1 months following HER2-CAR VSTs administration and 24.5 months from the time of diagnosis (61).
CAR-T cell therapy has made a step forward in both hematologic and solid tumors. However, its use for GBM treatment remains limited due to the BBB, antigen escape, tumor heterogeneity, and TME (62). Though the mentioned three phase I clinical trials indicated firm hopes for GBM treatment, more clinical trials with larger samples and more reliable examinations are needed.
2.4. OV therapy
Oncolytic virus (OV) therapy has emerged as a significant treatment strategy and has become a focus of research in the field of oncology (63). In 2021, the Japanese Ministry of Health, Labor, and Welfare (MHLW) granted conditional and time-limited approval for G47Δ to treat patients with malignant glioma in Japan (12). Table 4 presents a summary of recent phase II/III clinical trials for OV therapy in the treatment of glioblastoma (GBM), as found on ClinicalTrials.gov.
Table 4.
Recent phase II/III clinical trials of OV therapy for GBM treatment.
| Identifier | Title | Phase | Treatment approach | Patient type | Status |
|---|---|---|---|---|---|
| NCT00028158 | An Open-Label Phase Ib/II Study of the Safety, Tolerability and Efficacy of G207, a Genetically Engineered Herpes Simplex Type-1 Virus, Administered Intracerebrally to Patients With Recurrent Malignant Glioma | 1/2 | HSV G207 | Recurrent malignant glioma | Completed |
| NCT01301430 | Phase I/IIa Study of Intratumoral/Intracerebral or Intravenous/Intracerebral Administration of Parvovirus H-1 (ParvOryx) in Patients With Progressive Primary or Recurrent Glioblastoma Multiforme. | 1/2 | ParvOryx | Recurrent or progressive GBM | Completed |
| NCT01956734 | Phase I Trial of Combination of DNX-2401 (Formerly Named Delta-24-RGD) Oncolytic Adenovirus With a Short Course of Temozolomide for Treatment of Glioblastoma at First Recurrent | 1 | DNX-2401 Temozolomide |
First recurrent GBM | Completed |
| NCT02197169 | A Phase 1b, Randomized, Multi-center, Open-label Study of a Conditionally Replicative Adenovirus (DNX-2401) and Interferon Gamma (IFN-γ) for Recurrent Glioblastoma or Gliosarcoma (TARGET-I) | 1 | DNX-2401 IFN-γ |
rGBM or Gliosarcoma | Completed |
| NCT02062827 | A Phase 1 Study of M032 (NSC 733972), a Genetically Engineered HSV-1 Expressing IL-12, in Patients With Recurrent/Progressive Glioblastoma Multiforme, Anaplastic Astrocytoma, or Gliosarcoma | 1 | M032 | Recurrent/progressive GBM multiforme, anaplastic astrocytoma, or gliosarcoma | Active |
| NCT03072134 | Neural Stem Cell Oncolytic Adenoviral Virotherapy of Newly Diagnosed Malignant Glioma | 1 | NSC-CRAd-S-p7 Adiation/chemotherapy |
Newly diagnosed malignant glioma | Completed |
| NCT02798406 | A Phase II, Multi-center, Open-label Study of a Conditionally Replicative Adenovirus (DNX-2401) With Pembrolizumab (KEYTRUDA®) for Recurrent Glioblastoma or Gliosarcoma (CAPTIVE/KEYNOTE-192) | 2 | DNX-2401 pembrolizumab |
rGBM or Gliosarcoma | Completed |
| NCT02457845 | Phase I Clinical Trial of HSV G207 Alone or With a Single Radiation Dose in Children With Recurrent Supratentorial Brain Tumors | 1 | HSV G207 Radiotherapy |
Recurrent malignant glioma (Supratentorial) | Active |
| NCT00528684 | A Phase I/II Clinical Trial to Evaluate Dose Limiting Toxicity and Efficacy of Intralesional Administration of REOLYSIN® for the Treatment of Patients With Histologically Confirmed Recurrent Malignant Gliomas | 1 | REOLYSIN | Recurrent malignant glioma | Completed |
Data from ClinicalTrials.gov.
OVs are genetically modified, weakly pathogenic viruses that enhance the antitumor effects without harming normal cells (63). OV therapy hasadvantage in this era of mature genetic engineering. There are three purposes for genetic modification of OVs: 1) delete virulence genes to improve the safety of OVs, 2) enhance the tumor cell tropism and targeting of OVs to tumor cells, and 3) modify OVs with different therapeutic genes to enhance anti-tumor effects (64). On the one hand, OVs can self-replicate in host cancer cells, leading to direct lysis of the cancer cells (63). On the other hand, OVs help activate innate and adaptive immune responses by the releases of damage-related molecular patterns (DAMPs), viral pathogen-associated molecular patterns (PAMPs), and TAAs, to improve the immunosuppressive TME (64–67). Besides, OVs can selectively target and inhibit glioma stem cells (GSCs), which is an essential factor for drug resistance, tumor blood vessel formation, and immunosuppressive glioma microenvironment (68–70).
Since gliomas develop predominantly in the brain and lack distant metastases, which allows for the viruses that need an active cell cycle for reproduction, gliomas are particularly well-suited for OV treatment (71). The viruses used for OV therapy in gliomas include Oncolytic H-1 Parvovirus (72), Oncolytic Reovirus (73, 74), Oncolytic measles virus (75), Newcastle disease virus (74), Oncolytic vaccinia virus (76), Poliovirus (77), Oncolytic adenovirus (78), Oncolytic zika virus (79), Oncolytic herpes simplex virus (64, 80). Moreover, of all the OVs, oHSV progresses furthest in the clinical practice, including G47Δ, G207, HSV1716, and rQNestin-34.5 (80).
UMIN000015995 was a phase II trial of G47Δ in Japan for patients with residual or recurrent GBM (12). The third-generation as well as the triple-mutated oncolytic herpes simplex virus type 1 (HSV-1) G47 was created by deleting the US11 promoter from its parental G207 and overlapping the US11 gene (81). The primary endpoint (1-year survival rate) was achieved ahead of schedule, which was 84.2%. Additionally, the secondary endpoints of OS and PFS were 20.2 months and 4.7 months, respectively, after the G47 initiation (12). Based on the exciting results, G47Δ obtained conditional and time-limited approval in Japan for malignant glioma patients (12).
As a promising cancer treatment approach, OV therapy has a significant effect on gliomas. Moreover, the successful application of G47Δ in Japan also indicates the huge potential of OV therapy. Nevertheless, further research and phase III trials with large samples are needed to verify the real efficacy of this novel treatment approach.
2.5. Other therapies
2.5.1. Gene therapy
Gene therapy, a rapidly evolving field in oncology, encompasses the techniques used to introduce exogenous genes into targeted cells or tissues. These interventions are aimed at correcting or compensating for genetic defects and abnormalities for therapeutic purposes (82). Vectors in gene therapy can deliver entire genes, gene regulatory elements, or oligonucleotides and are categorized into viral and non-viral types (83). Viral vectors, including adenovirus vectors (AdV), adeno-associated viral vectors (AAV), retrovirus vectors (RV), and lentiviral vectors (LV), are non-toxic purified viruses designed to deliver genetic payloads without causing infections (84–88). Recently, non-viral vector-mediated gene therapy, such as nanoparticles with low toxicity and immunogenicity, has emerged as a promising approach in GBM treatment. These non-viral vectors can efficiently traverse the BBB and deliver gene drugs for glioma therapy (89, 90). The primary strategies in gene therapy are suicide gene therapy, tumor suppressor gene therapy, gene target therapy, and immunomodulatory gene therapy (91). Suicide gene therapy involves the delivery of genes coding specific enzymes that convert or activate non-toxic prodrugs into cytotoxic drugs at tumor sites, resulting in oncolytic action and tumor cell apoptosis (92). Tumor suppressor gene therapy focuses on restoring normal tumor suppressor gene function in tumor cells, thereby inhibiting tumor growth through genes like TP53, p16, and PTEN (93). Gene-targeted therapy binds specific tumor antigens and blocks carcinogenic pathways (91), while immunomodulatory gene therapy regulates the immunosuppressive tumor microenvironment (TME) and enhances immune cell effects. This therapeutic approach can be either categorized into gene therapy or immunotherapy. Moreover, with the development of both gene therapy and immunotherapy, the two approaches have become more closely linked. One good example is OV-based immunotherapy. Natural viruses can be genetically modified through genetic engineering into special OVs, which can specifically recognize, infect, and replicate in tumor cells, thereby destroying the tumor cells (71). Besides, tumor vaccines and CAR-T therapies can also be regarded as gene therapies in some situations.
2.5.2. Bispecific antibody therapy
In recent years, bispecific antibodies (BsAbs) have emerged as a promising therapeutic approach in oncology, with notable applications in cancer treatment (94, 95). Unlike conventional antibodies, BsAbs possess two distinct antigen-binding sites, allowing them to function through various mechanisms, including immune cell activation, co-inhibitory receptor blockade, co-stimulatory molecule triggering, signaling pathway suppression, and collaborative targeting of cancer-related antigens (96). Several BsAbs, such as blinatumomab, mosunetuzumab, teclistamab, epcoritamab, and glofitamab, have gained approval for cancer treatment (95). However, the development of BsAbs for glioma therapy has been relatively slow, with most investigations confined to preclinical stages (97–101). One particular focus of this review is AG596, a bispecific T-cell engager (BiTE) that has entered phase I clinical trials, offering insight into the mechanism of BsAbs in oncological applications (102, 103). BiTEs represent a distinct class of BsAbs, comprised of two single-chain variable fragments (scFv) connected by a short peptide linker. Each scFv serves a specific function: one targets a tumor-associated antigen on tumor cells, while the other binds to CD3 expressed on T cells. The design of BiTEs in glioma treatment often involves EGFRvIII, similar to vaccine and CAR-T therapies (98–101). AG596, for instance, can simultaneously engage the tumor-specific antigen (EGFRvIII) on glioblastoma (GBM) cells and CD3 on T cells, thereby activating T cell proliferation and cytotoxic secretion. This process leads to T cell-mediated GBM cell destruction. Preclinical studies have demonstrated that AG596 effectively mediates the lysis of EGFRvIII-positive GBM cell lines, improving overall survival (OS) rates in mice bearing EGFRvIII-expressing GBM cells (100). Moreover, the selectivity of AMG 596 has been confirmed through testing. Its binding was specific to EGFRvIII-positive GBM cells, with no observed T cell activity in EGFRvIII-negative cells and no toxicity detected in normal tissues (EGFRvIII-negative) in cynomolgus monkeys (100). A phase I, first-in-human, sequential dose-escalation/expansion study of AMG 596 in patients with EGFRvIII-positive GBM or malignant glioma (either recurrent or newly diagnosed) (NCT03296696) provided preliminary evidence for its safety, tolerability, and anti-tumor activity in recurrent GBM (104). However, further research is required to fully understand and harness the potential of AMG 596 in GBM therapy. Other ongoing clinical trials of BiTEs for glioma treatment include NCT04903795 and NCT03344250 (phase I).
2.5.3. Combined therapies
GBM is recognized as a highly malignant brain tumor, characterized by pronounced heterogeneity, an immunosuppressive TME, and a propensity for recurrence (105, 106). This complexity has necessitated the adoption of combined therapies, a strategy that neurosurgeons have embraced early on. The Stupp regimen, combining surgery with chemotherapy and radiotherapy, is a prototypical example (5). The emergence of novel treatments, including immunotherapy, targeted therapy, and tumor treatment fields, has given rise to innovative combinations, showing promise in both preclinical and clinical settings. Several tables from sections 2.1 to 2.4, along with most of our discussions, touch upon these combined therapies. This section would like to briefly introduce several prevalent combined therapeutic modes that incorporate immunotherapies in both clinical practice and trials.
Immunotherapy combined with chemotherapy (especially temozolomide (TMZ)). This approach is commonly used in clinical trials, and immunotherapy combined with current standard therapy (Stupp Regimen) can also be categorized into this category. Examples of clinical trials are Checkmate 498 and Checkmate 548 (using nivolumab, TMZ, and radiotherapy), NCT03018288 (Pembrolizumab, Temozolomide Radiotherapy, and HSPPC-96), NCT03548571, NCT00458601 (Rindopepimut, TMZ, and radiotherapy), NCT01480479 (Rindopepimut and TMZ), NCT02455557 (SurVaxM and TMZ), NCT01956734 (DNX-2401 and TMZ), etc.
Immunotherapy combined with targeted therapy (frequently paired with bevacizumab). This combined approach has also been explored in clinical trials such as CheckMate 143 (nivolumab and bevacizumab), NCT02337491 (Pembrolizumab and Bevacizumab), and NCT03452579 (Nivolumab and Bevacizumab), etc.
Combination of different types of immunotherapies or immunotherapeutic drugs (in the same type). Examples of combined immunotherapies in the same categories mainly include NCT02794883 (Tremelimumab and Durvalumab), NCT04396860 (Ipilimumab, Nivolumab, and Radiotherapy), etc. And examples of combined immunotherapies in different categories include NCT02798406 (Pembrolizumab and Adenovirus), NCT03750071 (Avelumab and VXM01), NCT01082926 (GRm13Z40-2 and IL-2), NCT03726515 (CART-EGFRvIII and Pembrolizumab), NCT02798406 (DNX-2401 and pembrolizumab), etc.
Given the current landscape of immunotherapies in gliomas, it is anticipated that more combined therapies yielding convincing results will emerge in the near future. These combined modalities hold the potential to address the multifaceted challenges posed by GBM, offering a more comprehensive approach to treatment.
3. TME in gliomas
As introduced above, immunotherapies have demonstrated promising outcomes in preclinical and clinical studies, especially the successful phase III trial of DCVax-L and the approval of G47Δ in Japan. However, there are also many obstructions on the way to success. The tumor microenvironment (TME) is regarded as one of the most critical factors in various treatments, including immunotherapy. On the one hand, the immunosuppressive TME in gliomas can result in drug resistance and tumor recurrence. On the other hand, understanding deeply and making great use of the TME can also promote the progress of immunotherapy in gliomas.
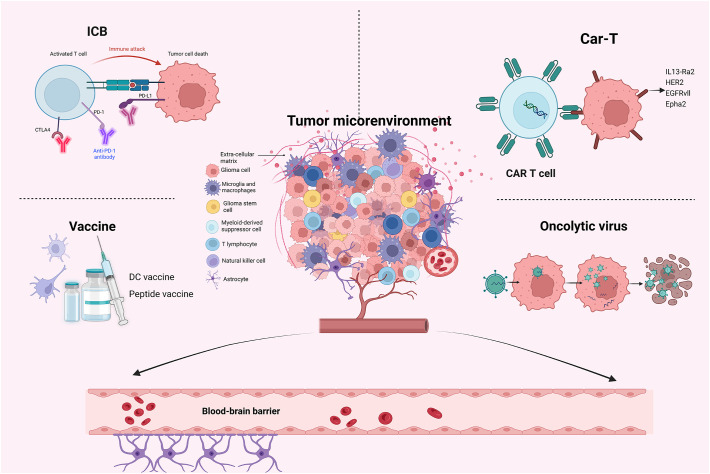
The TME of gliomas is complex and heterogeneous, consisting of various components, including astrocytes, pericytes, endothelial cells, GSCs, blood vessels, glioma-associated stromal cells, immune cells including myeloid-derived suppressor cells (MDSCs), glioma-associated microglia/macrophages (GAMs), CD4+ T cells, Tregs and NK cells, and extracellular matrix (ECM) (107–109) ( Figure 1 ). These elements may interact with one another to promote the spread and proliferation of glioma cells. It is widely acknowledged that TME is one of the main reasons for the unsatisfactory immunotherapeutic effect of gliomas. Additionally, more and more immunotherapy-related research pays much attention to TME in gliomas (23, 25, 59).
Figure 1.
The illustration of four main immunotherapies and TME in gliomas.
The powerful immunosuppressive effect in the TME of gliomas is caused by several mechanisms, including the abnormal function of cells such as the existence of immunosuppressive cells (M2 GAMs, Tregs, and MDSCs) and immunosuppressive cytokines (TGF-β, IL-10), low number of TILs, and the high expression of inhibitory immune checkpoint molecules such as PD-1, TIM-3, and LAG-3 (38, 109–112). The most abundant cells in GBM are GAMs, which are divided into the M1 phenotype (proinflammatory) and M2 phenotype (immunosuppressive) (107). These GAMs contribute to tumor heterogeneity and progression. On the one hand, M2 GAMs can produce a lot of IL-10 and TGF-β and low levels of IL-12, which can play an immunosuppressive role in the TME (109). GAMs can also promote the proliferation of GSCs, which is closely related to drug resistance (113).
Compared with other tumors, research on TILs in gliomas is relatively less (110). After T cells infiltrate the TME, dysfunction occurs through different mechanisms, including aging, tolerance, and incompetence (111). Additionally, glioma cells and certain immune cells emit a variety of immunosuppressive substances into the TME, including TGF-β and IL-10. Then these factors attract and stimulate immunosuppressive cells such as TAMs and Treg cells and inhibit the activation of APCs and effector immune cells (114, 115).
Glioma stem cells (GSCs), namely cancer stem cells in gliomas, also play a vital role in the TME. GSCs have the ability of self-renewal and multi-differentiation, which are regarded as the primary cause of tumor occurrence, development, drug resistance and recurrence, and heterogeneity (116–118). GSCs can regulate cell metabolism in the TME of gliomas to increase resistance to challenging conditions in addition to reprogramming associated cells to promote tissue remodeling (116–118). The intimate interaction between GSCs and glioma TME is a critical factor to the resistance of immunotherapy, and targeting both GSCs and TME can enhance the immunotherapeutic effect.
The BBB is also found closely connected with the immune response in gliomas. BBB is a semi-permeable physiological boundary formed by parenchymal capillary endothelial cells, capillary astrocytic endfeet, and capillary basal membrane pericytes (119, 120). The presence of BBB benefits the central nervous system’s special immune privilege, which is characterized by the lack of traditional lymphatic structures, a dearth of APC, low levels of MHC molecule expression, and the constitutive expression of immunosuppressive cytokines like IL10 and TGF-β (121–123). Moreover, BBB can also largely limit the delivery of most therapeutic drugs in treating gliomas (124). In addition to being a barrier to both immunity and drug delivery, several studies on tumor brain metastasis proved that the properties of BBB can be fundamentally regulated by some components of the TME (125).
4. Conclusion and prospect
This review analyzed the present landscape of immunotherapies for gliomas, focusing on four main therapeutic approaches: ICBs, CAR-T cell therapy, vaccine therapy, and OV therapy. Among these, the efficacy of the dendritic cell vaccine DCVax-L and OV G47Δ has been validated in phase II and III clinical trials, respectively. Notably, G47Δ received conditional and time-limited approval from the Japanese Ministry of Health, Labor, and Welfare (MHLW) in 2021 for the treatment of malignant glioma. This review also briefly introduces gene therapy and bispecific antibody therapy, both closely aligned with immunotherapy, as well as combined therapies incorporating immunotherapy. The critical role of the tumor microenvironment (TME) in the effectiveness of immunotherapies for gliomas has been emphasized in this review.
Immunotherapy, given its present standing and rapid evolution, is heralded as a promising avenue for glioma treatment. However, significant challenges must be overcome to realize its full potential. These include:
Understanding Molecular Biology: Investigations into the molecular biology of GBM, TME, and the BBB are vital for designing innovative immunotherapeutic drugs with potential antitumor effects.
Exploring TME-Immunotherapy Relationships: Continued and profound exploration of the relationship between TME and immunotherapy is necessary for the success of immunotherapies in gliomas.
Combination Therapies: Active and rational exploration of combined therapies is likely to become the focal point in this field, offering synergistic advantages.
Personalized Treatment: Tailoring treatments to individual patients with distinct molecular characteristics may be essential to optimize therapeutic outcomes.
In conclusion, while the rapid advancement of immunotherapy provides hope, the complexities of gliomas necessitate an equally nuanced approach to treatment. We ardently hope that immunotherapy, with continued research and development, will become a formidable tool in the fight against GBM in the foreseeable future.
Author contributions
FY: Data curation, Formal Analysis, Funding acquisition, Investigation, Methodology, Writing – original draft, Writing – review & editing. YX: Funding acquisition, Writing – review & editing, Data curation, Formal Analysis, Investigation, Methodology, Writing – original draft. HG: Data curation, Investigation, Methodology, Writing – original draft. RG: Supervision, Writing – review & editing, Conceptualization, Supervision. LY: Supervision, Writing – review & editing, Conceptualization, Supervision. ZL: Supervision, Writing – review & editing, Conceptualization, Supervision. HW: Conceptualization, Funding acquisition, Resources, Supervision, Writing – review & editing.
Acknowledgments
We greatly appreciate Professor Jifan Hu from Stanford University for modifying the language of this article.
Funding Statement
This work was supported by Natural Science Foundation of China grants (81500116) to HW, Jilin Science and Technique development grants (20200201472JC) to HW, Jilin Science and Technique development grants (YDZJ202301ZYTS092) to RG, National College Students’ innovation and entrepreneurship training program (202210183300) to YX, and College Students’ innovation and entrepreneurship training program of Jilin University (X202210183572) to FY.
Conflict of interest
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Publisher’s note
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article, or claim that may be made by its manufacturer, is not guaranteed or endorsed by the publisher.
References
- 1. Ostrom QT, Patil N, Cioffi G, Waite K, Kruchko C, Barnholtz-Sloan JS. CBTRUS statistical report: primary brain and other central nervous system tumors diagnosed in the United States in 2013-2017. Neuro Oncol (2020) 22(12 Suppl 2):iv1–iv96. doi: 10.1093/neuonc/noaa200 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2. Ostrom QT, Cioffi G, Waite K, Kruchko C, Barnholtz-Sloan JS. CBTRUS statistical report: primary brain and other central nervous system tumors diagnosed in the United States in 2014-2018. Neuro Oncol (2021) 23(12 Suppl 2):iii1–iii105. doi: 10.1093/neuonc/noab200 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. Ostrom QT, Price M, Neff C, Cioffi G, Waite KA, Kruchko C, et al. CBTRUS statistical report: primary brain and other central nervous system tumors diagnosed in the United States in 2015-2019. Neuro Oncol (2022) 24(Suppl 5):v1–v95. doi: 10.1093/neuonc/noac202 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Louis DN, Perry A, Wesseling P, Brat DJ, Cree IA, Figarella-Branger D, et al. The 2021 WHO classification of tumors of the central nervous system: a summary. Neuro Oncol (2021) 23(8):1231–51. doi: 10.1093/neuonc/noab106 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Stupp R, Mason WP, van den Bent MJ, Weller M, Fisher B, Taphoorn MJ, et al. Radiotherapy plus concomitant and adjuvant temozolomide for glioblastoma. N Engl J Med (2005) 352(10):987–96. doi: 10.1056/NEJMoa043330 [DOI] [PubMed] [Google Scholar]
- 6. Redman JM, Gibney GT, Atkins MB. Advances in immunotherapy for melanoma. BMC Med (2016) 14:20. doi: 10.1186/s12916-016-0571-0 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Reck M, Remon J, Hellmann MD. First-line immunotherapy for non-small-cell lung cancer. J Clin Oncol (2022) 40(6):586–97. doi: 10.1200/JCO.21.01497 [DOI] [PubMed] [Google Scholar]
- 8. Ferrall L, Lin KY, Roden RBS, Hung CF, Wu TC. Cervical cancer immunotherapy: facts and hopes. Clin Cancer Res (2021) 27(18):4953–73. doi: 10.1158/1078-0432.CCR-20-2833 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Mahmoud AB, Ajina R, Aref S, Darwish M, Alsayb M, Taher M, et al. Advances in immunotherapy for glioblastoma multiforme. Front Immunol (2022) 13:944452. doi: 10.3389/fimmu.2022.944452 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Liau LM, Ashkan K, Brem S, Campian JL, Trusheim JE, Iwamoto FM, et al. Association of autologous tumor lysate-loaded dendritic cell vaccination with extension of survival among patients with newly diagnosed and recurrent glioblastoma: A phase 3 prospective externally controlled cohort trial. JAMA Oncol (2023) 9(1):112–21. doi: 10.1001/jamaoncol.2022.5370 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Liau LM, Ashkan K, Tran DD, Campian JL, Trusheim JE, Cobbs CS, et al. First results on survival from a large Phase 3 clinical trial of an autologous dendritic cell vaccine in newly diagnosed glioblastoma. J Transl Med (2018) 16(1):142. doi: 10.1186/s12967-018-1507-6 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Todo T, Ito H, Ino Y, Ohtsu H, Ota Y, Shibahara J, et al. Intratumoral oncolytic herpes virus G47Δ for residual or recurrent gli oblastoma: a phase 2 trial. Nat Med (2022) 28(8):1630–9. doi: 10.1038/s41591-022-01897-x [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Dagher OK, Schwab RD, Brookens SK, Posey AD, Jr. Advances in cancer immunotherapies. Cell (2023) 186(8):1814–.e1. doi: 10.1016/j.cell.2023.02.039 [DOI] [PubMed] [Google Scholar]
- 14. Shi T, Song X, Wang Y, Liu F, Wei J. Combining oncolytic viruses with cancer immunotherapy: establishing a new generation of cancer treatment. Front Immunol (2020) 11:683. doi: 10.3389/fimmu.2020.00683 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Lim M, Xia Y, Bettegowda C, Weller M. Current state of immunotherapy for glioblastoma. Nat Rev Clin Oncol (2018) 15(7):422–42. doi: 10.1038/s41571-018-0003-5 [DOI] [PubMed] [Google Scholar]
- 16. Sampson JH, Gunn MD, Fecci PE, Ashley DM. Brain immunology and immunotherapy in brain tumours. Nat Rev Cancer (2020) 20(1):12–25. doi: 10.1038/s41568-019-0224-7 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. Rong L, Li N, Zhang Z. Emerging therapies for glioblastoma: current state and future directions. J Exp Clin Cancer Res (2022) 41(1):142. doi: 10.1186/s13046-022-02349-7 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. Korman AJ, Garrett-Thomson SC, Lonberg N. The foundations of immune checkpoint blockade and the ipilimumab approval decennial. Nat Rev Drug Discovery (2022) 21(7):509–28. doi: 10.1038/s41573-021-00345-8 [DOI] [PubMed] [Google Scholar]
- 19. Ott PA, Hodi FS, Robert C. CTLA-4 and PD-1/PD-L1 blockade: new immunotherapeutic modalities with durable clinical benefit in melanoma patients. Clin Cancer Res (2013) 19(19):5300–9. doi: 10.1158/1078-0432.CCR-13-0143 [DOI] [PubMed] [Google Scholar]
- 20. Han Y, Liu D, Li L. PD-1/PD-L1 pathway: current researches in cancer. Am J Cancer Res (2020) 10(3):727–42. [PMC free article] [PubMed] [Google Scholar]
- 21. Berger KN, Pu JJ. PD-1 pathway and its clinical application: A 20year journey after discovery of the complete human PD-1 gene. Gene (2018) 638:20–5. doi: 10.1016/j.gene.2017.09.050 [DOI] [PubMed] [Google Scholar]
- 22. Taube JM, Klein A, Brahmer JR, Xu H, Pan X, Kim JH, et al. Association of PD-1, PD-1 ligands, and other features of the tumor immune microenvironment with response to anti-PD-1 therapy. Clin Cancer Res (2014) 20(19):5064–74. doi: 10.1158/1078-0432.CCR-13-3271 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23. Cloughesy TF, Mochizuki AY, Orpilla JR, Hugo W, Lee AH, Davidson TB, et al. Neoadjuvant anti-PD-1 immunotherapy promotes a survival benefit with intratumoral and systemic immune responses in recurrent glioblastoma. Nat Med (2019) 25(3):477–86. doi: 10.1038/s41591-018-0337-7 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24. Reardon DA, Brandes AA, Omuro A, Mulholland P, Lim M, Wick A, et al. Effect of nivolumab vs bevacizumab in patients with recurrent glioblastoma: the checkMate 143 phase 3 randomized clinical trial. JAMA Oncol (2020) 6(7):1003–10. doi: 10.1001/jamaoncol.2020.1024 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25. Schalper KA, Rodriguez-Ruiz ME, Diez-Valle R, Lopez-Janeiro A, Porciuncula A, Idoate MA, et al. Neoadjuvant nivolumab modifies the tumor immune microenvironment in resectable glioblastoma. Nat Med (2019) 25(3):470–6. doi: 10.1038/s41591-018-0339-5 [DOI] [PubMed] [Google Scholar]
- 26. Lim M, Weller M, Idbaih A, Steinbach J, Finocchiaro G, Raval RR, et al. Phase III trial of chemoradiotherapy with temozolomide plus nivolumab or placebo for newly diagnosed glioblastoma with methylated MGMT promoter. Neuro Oncol (2022) 24(11):1935–49. doi: 10.1093/neuonc/noac116 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27. Omuro A, Brandes AA, Carpentier AF, Idbaih A, Reardon DA, Cloughesy T, et al. Radiotherapy combined with nivolumab or temozolomide for newly diagnosed glioblastoma with unmethylated MGMT promoter: An international randomized phase III trial. Neuro Oncol (2023) 25(1):123–34. doi: 10.1093/neuonc/noac099 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28. Duerinck J, Schwarze JK, Awada G, Tijtgat J, Vaeyens F, Bertels C, et al. Intracerebral administration of CTLA-4 and PD-1 immune checkpoint blocking monoclonal antibodies in patients with recurrent glioblastoma: a phase I clinical trial. J Immunother Cancer (2021) 9(6):e002296. doi: 10.1136/jitc-2020-002296 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29. Brown NF, Ng SM, Brooks C, Coutts T, Holmes J, Roberts C, et al. A phase II open label, randomised study of ipilimumab with temozolomide versus temozolomide alone after surgery and chemoradiotherapy in patients with recently diagnosed glioblastoma: the Ipi-Glio trial protocol. BMC Cancer (2020) 20(1):198. doi: 10.1186/s12885-020-6624-y [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30. Omuro A, Vlahovic G, Lim M, Sahebjam S, Baehring J, Cloughesy T, et al. Nivolumab with or without ipilimumab in patients with recurrent glioblastoma: results from exploratory phase I cohorts of CheckMate 143. Neuro Oncol (2018) 20(5):674–86. doi: 10.1093/neuonc/nox208 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31. Zen K, Guo Y, Bian Z, Lv Z, Zhu D, Ohnishi H, et al. Inflammation-induced proteolytic processing of the SIRPalpha cytoplasmic ITIM in neutrophils propagates a proinflammatory state. Nat Commun (2013) 4:2436. doi: 10.1038/ncomms3436 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32. Zhang M, Hutter G, Kahn SA, Azad TD, Gholamin S, Xu CY, et al. Anti-CD47 treatment stimulates phagocytosis of glioblastoma by M1 and M2 polarized macrophages and promotes M1 polarized macrophages in vivo. PLoS One (2016) 11(4):e0153550. doi: 10.1371/journal.pone.0153550 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33. Gholamin S, Youssef OA, Rafat M, Esparza R, Kahn S, Shahin M, et al. Irradiation or temozolomide chemotherapy enhances anti-CD47 treatment of glioblastoma. Innate Immun (2020) 26(2):130–7. doi: 10.1177/1753425919876690 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34. Azambuja JH, Schuh RS, Michels LR, Iser IC, Beckenkamp LR, Roliano GG, et al. Blockade of CD73 delays glioblastoma growth by modulating the immune environment. Cancer Immunol Immunother (2020) 69(9):1801–12. doi: 10.1007/s00262-020-02569-w [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35. Goswami S, Walle T, Cornish AE, Basu S, Anandhan S, Fernandez I, et al. Immune profiling of human tumors identifies CD73 as a combinatorial target in glioblastoma. Nat Med (2020) 26(1):39–46. doi: 10.1038/s41591-019-0694-x [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36. Hung AL, Maxwell R, Theodros D, Belcaid Z, Mathios D, Luksik AS, et al. TIGIT and PD-1 dual checkpoint blockade enhances antitumor immunity and survival in GBM. Oncoimmunology (2018) 7(8):e1466769. doi: 10.1080/2162402X.2018.1466769 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37. Puigdelloses M, Garcia-Moure M, Labiano S, Laspidea V, Gonzalez-Huarriz M, Zalacain M, et al. CD137 and PD-L1 targeting with immunovirotherapy induces a potent and durable antitumor immune response in glioblastoma models. J Immunother Cancer (2021) 9(7):e002644. doi: 10.1136/jitc-2021-002644 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38. Woroniecka K, Chongsathidkiet P, Rhodin K, Kemeny H, Dechant C, Farber SH, et al. T-cell exhaustion signatures vary with tumor type and are severe in glioblastoma. Clin Cancer Res (2018) 24(17):4175–86. doi: 10.1158/1078-0432.CCR-17-1846 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39. Woroniecka KI, Rhodin KE, Dechant C, Cui X, Chongsathidkiet P, Wilkinson D, et al. 4-1BB agonism averts TIL exhaustion and licenses PD-1 blockade in glioblastoma and other intracranial cancers. Clin Cancer Res (2020) 26(6):1349–58. doi: 10.1158/1078-0432.CCR-19-1068 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40. Morton DL, Barth A. Vaccine therapy for Malignant melanoma. CA Cancer J Clin (1996) 46(4):225–44. doi: 10.3322/canjclin.46.4.225 [DOI] [PubMed] [Google Scholar]
- 41. Cunto-Amesty G, Monzavi-Karbassi B, Luo P, Jousheghany F, Kieber-Emmons T. Strategies in cancer vaccines development. Int J Parasitol (2003) 33(5-6):597–613. doi: 10.1016/S0020-7519(03)00054-7 [DOI] [PubMed] [Google Scholar]
- 42. Zhao B, Wu J, Li H, Wang Y, Wang Y, Xing H, et al. Recent advances and future challenges of tumor vaccination therapy for recurrent glioblastoma. Cell Commun Signal (2023) 21(1):74. doi: 10.1186/s12964-023-01098-0 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43. Swartz AM, Batich KA, Fecci PE, Sampson JH. Peptide vaccines for the treatment of glioblastoma. J Neurooncol (2015) 123(3):433–40. doi: 10.1007/s11060-014-1676-y [DOI] [PubMed] [Google Scholar]
- 44. Li L, Zhou J, Dong X, Liao Q, Zhou D, Zhou Y. Dendritic cell vaccines for glioblastoma fail to complete clinical translation: Bottlenecks and potential countermeasures. Int Immunopharmacol (2022) 109:108929. doi: 10.1016/j.intimp.2022.108929 [DOI] [PubMed] [Google Scholar]
- 45. Sayegh ET, Oh T, Fakurnejad S, Bloch O, Parsa AT. Vaccine therapies for patients with glioblastoma. J Neurooncol (2014) 119(3):531–46. doi: 10.1007/s11060-014-1502-6 [DOI] [PubMed] [Google Scholar]
- 46. Purcell AW, McCluskey J, Rossjohn J. More than one reason to rethink the use of peptides in vaccine design. Nat Rev Drug Discovery (2007) 6(5):404–14. doi: 10.1038/nrd2224 [DOI] [PubMed] [Google Scholar]
- 47. Sturm D, Bender S, Jones DT, Lichter P, Grill J, Becher O, et al. Paediatric and adult glioblastoma: multiform (epi)genomic culprits emerge. Nat Rev Cancer (2014) 14(2):92–107. doi: 10.1038/nrc3655 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48. Brennan CW, Verhaak RG, McKenna A, Campos B, Noushmehr H, Salama SR, et al. The somatic genomic landscape of glioblastoma. Cell (2013) 155(2):462–77. doi: 10.1016/j.cell.2013.09.034 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49. Weller M, Kaulich K, Hentschel B, Felsberg J, Gramatzki D, Pietsch T, et al. Assessment and prognostic significance of the epidermal growth factor receptor vIII mutation in glioblastoma patients treated with concurrent and adjuvant temozolomide radiochemotherapy. Int J Cancer (2014) 134(10):2437–47. doi: 10.1002/ijc.28576 [DOI] [PubMed] [Google Scholar]
- 50. Huang PH, Mukasa A, Bonavia R, Flynn RA, Brewer ZE, Cavenee WK, et al. Quantitative analysis of EGFRvIII cellular signaling networks reveals a combinatorial therapeutic strategy for glioblastoma. Proc Natl Acad Sci U S A (2007) 104(31):12867–72. doi: 10.1073/pnas.0705158104 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51. Weller M, Butowski N, Tran DD, Recht LD, Lim M, Hirte H, et al. Rindopepimut with temozolomide for patients with newly diagnosed, EGFRvIII-expressing glioblastoma (ACT IV): a randomised, double-blind, international phase 3 trial. Lancet Oncol (2017) 18(10):1373–85. doi: 10.1016/S1470-2045(17)30517-X [DOI] [PubMed] [Google Scholar]
- 52. Lee-Chang C, Lesniak MS. Next-generation antigen-presenting cell immune therapeutics for gliomas. J Clin Invest (2023) 133(3):e163449. doi: 10.1172/JCI163449 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53. Bregy A, Wong TM, Shah AH, Goldberg JM, Komotar RJ. Active immunotherapy using dendritic cells in the treatment of glioblastoma multiforme. Cancer Treat Rev (2013) 39(8):891–907. doi: 10.1016/j.ctrv.2013.05.007 [DOI] [PubMed] [Google Scholar]
- 54. Wylie B, Macri C, Mintern JD, Waithman J. Dendritic cells and cancer: from biology to therapeutic intervention. Cancers (Basel) (2019) 11(4):521. doi: 10.3390/cancers11040521 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55. Frederico SC, Hancock JC, Brettschneider EES, Ratnam NM, Gilbert MR, Terabe M. Making a cold tumor hot: the role of vaccines in the treatment of glioblastoma. Front Oncol (2021) 11:672508. doi: 10.3389/fonc.2021.672508 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56. Zhang M, Choi J, Lim M. Advances in immunotherapies for gliomas. Curr Neurol Neurosci Rep (2022) 22(1):1–10. doi: 10.1007/s11910-022-01176-9 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57. Chen D, Yang J. Development of novel antigen receptors for CAR T-cell therapy directed toward solid Malignancies. Transl Res (2017) 187:11–21. doi: 10.1016/j.trsl.2017.05.006 [DOI] [PubMed] [Google Scholar]
- 58. Li L, Zhu X, Qian Y, Yuan X, Ding Y, Hu D, et al. Chimeric antigen receptor T-cell therapy in glioblastoma: current and future. Front Immunol (2020) 11:594271. doi: 10.3389/fimmu.2020.594271 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59. O'Rourke DM, Nasrallah MP, Desai A, Melenhorst JJ, Mansfield K, Morrissette JJD, et al. A single dose of peripherally infused EGFRvIII-directed CAR T cells mediates antigen loss and induces adaptive resistance in patients with recurrent glioblastoma. Sci Transl Med (2017) 9(399):eaaa0984. doi: 10.1126/scitranslmed.aaa0984 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60. Brown CE, Alizadeh D, Starr R, Weng L, Wagner JR, Naranjo A, et al. Regression of glioblastoma after chimeric antigen receptor T-cell therapy. N Engl J Med (2016) 375(26):2561–9. doi: 10.1056/NEJMoa1610497 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61. Ahmed N, Brawley V, Hegde M, Bielamowicz K, Kalra M, Landi D, et al. HER2-specific chimeric antigen receptor-modified virus-specific T cells for progressive glioblastoma: A phase 1 dose-escalation trial. JAMA Oncol (2017) 3(8):1094–101. doi: 10.1001/jamaoncol.2017.0184 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62. Bagley SJ, Desai AS, Linette GP, June CH, O'Rourke DM. CAR T-cell therapy for glioblastoma: recent clinical advances and future challenges. Neuro Oncol (2018) 20(11):1429–38. doi: 10.1093/neuonc/noy032 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63. Liu P, Wang Y, Wang Y, Kong Z, Chen W, Li J, et al. Effects of oncolytic viruses and viral vectors on immunity in glioblastoma. Gene Ther (2022) 29(3-4):115–26. doi: 10.1038/s41434-020-00207-9 [DOI] [PubMed] [Google Scholar]
- 64. Qi Z, Long X, Liu J, Cheng P. Glioblastoma microenvironment and its reprogramming by oncolytic virotherapy. Front Cell Neurosci (2022) 16:819363. doi: 10.3389/fncel.2022.819363 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65. Martikainen M, Essand M. Virus-based immunotherapy of glioblastoma. Cancers (Basel) (2019) 11(2):186. doi: 10.3390/cancers11020186 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66. Aurelian L. Oncolytic viruses as immunotherapy: progress and remaining challenges. Onco Targets Ther (2016) 9:2627–37. doi: 10.2147/OTT.S63049 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67. Chiocca EA, Abbed KM, Tatter S, Louis DN, Hochberg FH, Barker F, et al. A phase I open-label, dose-escalation, multi-institutional trial of injection with an E1B-Attenuated adenovirus, ONYX-015, into the peritumoral region of recurrent Malignant gliomas, in the adjuvant setting. Mol Ther (2004) 10(5):958–66. doi: 10.1016/j.ymthe.2004.07.021 [DOI] [PubMed] [Google Scholar]
- 68. Zhu Z, Gorman MJ, McKenzie LD, Chai JN, Hubert CG, Prager BC, et al. Zika virus has oncolytic activity against glioblastoma stem cells. J Exp Med (2017) 214(10):2843–57. doi: 10.1084/jem.20171093 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69. Jiang H, Gomez-Manzano C, Aoki H, Alonso MM, Kondo S, McCormick F, et al. Examination of the therapeutic potential of Delta-24-RGD in brain tumor stem cells: role of autophagic cell death. J Natl Cancer Inst (2007) 99(18):1410–4. doi: 10.1093/jnci/djm102 [DOI] [PubMed] [Google Scholar]
- 70. Kanai R, Wakimoto H, Martuza RL, Rabkin SD. A novel oncolytic herpes simplex virus that synergizes with phosphoinositide 3-kinase/Akt pathway inhibitors to target glioblastoma stem cells. Clin Cancer Res (2011) 17(11):3686–96. doi: 10.1158/1078-0432.CCR-10-3142 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71. Wollmann G, Ozduman K, van den Pol AN. Oncolytic virus therapy for glioblastoma multiforme: concepts and candidates. Cancer J (2012) 18(1):69–81. doi: 10.1097/PPO.0b013e31824671c9 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72. Angelova AL, Barf M, Geletneky K, Unterberg A, Rommelaere J. Immunotherapeutic potential of oncolytic H-1 parvovirus: hints of glioblastoma microenvironment conversion towards immunogenicity. Viruses (2017) 9(12):382. doi: 10.3390/v9120382 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73. Samson A, Scott KJ, Taggart D, West EJ, Wilson E, Nuovo GJ, et al. Intravenous delivery of oncolytic reovirus to brain tumor patients immunologically primes for subsequent checkpoint blockade. Sci Transl Med (2018) 10(422):eaam7577. doi: 10.1126/scitranslmed.aam7577 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74. Fan X, Lu H, Cui Y, Hou X, Huang C, Liu G. Overexpression of p53 delivered using recombinant NDV induces apoptosis in glioma cells by regulating the apoptotic signaling pathway. Exp Ther Med (2018) 15(5):4522–30. doi: 10.3892/etm.2018.5935 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75. Aref S, Bailey K, Fielding A. Measles to the rescue: A review of oncolytic measles virus. Viruses (2016) 8(10):294. doi: 10.3390/v8100294 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76. Vasileva N, Ageenko A, Dmitrieva M, Nushtaeva A, Mishinov S, Kochneva G, et al. Double recombinant vaccinia virus: A candidate drug against human glioblastoma. Life (Basel) (2021) 11(10):1084. doi: 10.3390/life11101084 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77. Brown MC, Gromeier M. Cytotoxic and immunogenic mechanisms of recombinant oncolytic poliovirus. Curr Opin Virol (2015) 13:81–5. doi: 10.1016/j.coviro.2015.05.007 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78. Ulasov IV, Zhu ZB, Tyler MA, Han Y, Rivera AA, Khramtsov A, et al. Survivin-driven and fiber-modified oncolytic adenovirus exhibits potent antitumor activity in established intracranial glioma. Hum Gene Ther (2007) 18(7):589–602. doi: 10.1089/hum.2007.002 [DOI] [PubMed] [Google Scholar]
- 79. Su KY, Balasubramaniam V. Zika virus as oncolytic therapy for brain cancer: myth or reality? Front Microbiol (2019) 10:2715. doi: 10.3389/fmicb.2019.02715 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 80. Nguyen HM, Saha D. The current state of oncolytic herpes simplex virus for glioblastoma treatment. Oncolytic Virother (2021) 10:1–27. doi: 10.2147/OV.S268426 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 81. Todo T, Martuza RL, Rabkin SD, Johnson PA. Oncolytic herpes simplex virus vector with enhanced MHC class I presentation and tumor cell killing. Proc Natl Acad Sci U S A (2001) 98(11):6396–401. doi: 10.1073/pnas.101136398 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 82. Natsume A, Yoshida J. Gene therapy for high-grade glioma: current approaches and future directions. Cell Adh Migr (2008) 2(3):186–91. doi: 10.4161/cam.2.3.6278 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 83. Varela ML, Comba A, Faisal SM, Argento A, Franson A, Barissi MN, et al. Gene therapy for high grade glioma: the clinical experience. Expert Opin Biol Ther (2022) 23(2):145-161. doi: 10.1080/14712598.2022.2157718 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 84. Caffery B, Lee JS, Alexander-Bryant AA. Vectors for glioblastoma gene therapy: viral & Non-viral delivery strategies. Nanomaterials (Basel) (2019) 9(1):105. doi: 10.3390/nano9010105 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 85. Lowenstein PR, Castro MG. Evolutionary basis of a new gene- and immune-therapeutic approach for the treatment of Malignant brain tumors: from mice to clinical trials for glioma patients. Clin Immunol (2018) 189:43–51. doi: 10.1016/j.clim.2017.07.006 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 86. AAV-mediated gene delivery of LIGHT prolongs survival in glioblastoma. Cancer Discovery (2023) 13(7):1511. doi: 10.1158/2159-8290.CD-RW2023-078 [DOI] [Google Scholar]
- 87. Kushiya H, Hiraoka K, Suzuki T, Inoko K, Inagaki A, Niwa H, et al. Retroviral replicating vector toca 511 (Vocimagene amiretrorepvec) for prodrug activator gene therapy of lung cancer. Cancers (Basel) (2022) 14(23):5820. doi: 10.3390/cancers14235820 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 88. Birocchi F, Cusimano M, Rossari F, Beretta S, Rancoita PMV, Ranghetti A, et al. Targeted inducible delivery of immunoactivating cytokines reprograms glioblastoma microenvironment and inhibits growth in mouse models. Sci Transl Med (2022) 14(653):eabl4106. doi: 10.1126/scitranslmed.abl4106 [DOI] [PubMed] [Google Scholar]
- 89. Tian T, Liang R, Erel-Akbaba G, Saad L, Obeid PJ, Gao J, et al. Immune checkpoint inhibition in GBM primed with radiation by engineered extracellular vesicles. ACS Nano (2022) 16(2):1940–53. doi: 10.1021/acsnano.1c05505 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 90. Janjua TI, Rewatkar P, Ahmed-Cox A, Saeed I, Mansfeld FM, Kulshreshtha R, et al. Frontiers in the treatment of glioblastoma: Past, present and emerging. Adv Drug Delivery Rev (2021) 171:108–38. doi: 10.1016/j.addr.2021.01.012 [DOI] [PubMed] [Google Scholar]
- 91. Giotta Lucifero A, Luzzi S. Against the resilience of high-grade gliomas: gene therapies (Part II). Brain Sci (2021) 11(8):976. doi: 10.3390/brainsci11080976 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 92. Karjoo Z, Chen X, Hatefi A. Progress and problems with the use of suicide genes for targeted cancer therapy. Adv Drug Delivery Rev (2016) 99(Pt A):113–28. doi: 10.1016/j.addr.2015.05.009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 93. Vahabi M, Dehni B, Antomás I, Giovannetti E, Peters GJ. Targeting miRNA and using miRNA as potential therapeutic options to bypass resistance in pancreatic ductal adenocarcinoma. Cancer Metastasis Rev (2023). doi: 10.1007/s10555-023-10127-w [DOI] [PMC free article] [PubMed] [Google Scholar]
- 94. van de Donk N, Zweegman S. T-cell-engaging bispecific antibodies in cancer. Lancet (2023) 402(10396):142–58. doi: 10.1016/S0140-6736(23)00521-4 [DOI] [PubMed] [Google Scholar]
- 95. Tapia-Galisteo A, Álvarez-Vallina L, Sanz L. Bi- and trispecific immune cell engagers for immunotherapy of hematological Malignancies. J Hematol Oncol (2023) 16(1):83. doi: 10.1186/s13045-023-01482-w [DOI] [PMC free article] [PubMed] [Google Scholar]
- 96. Farhangnia P, Ghomi SM, Akbarpour M, Delbandi AA. Bispecific antibodies targeting CTLA-4: game-changer troopers in cancer immunotherapy. Front Immunol (2023) 14:1155778. doi: 10.3389/fimmu.2023.1155778 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 97. Fan R, Chen C, Mu M, Chuan D, Liu H, Hou H, et al. Engineering MMP-2 activated nanoparticles carrying B7-H3 bispecific antibodies for ferroptosis-enhanced glioblastoma immunotherapy. ACS Nano (2023) 17(10):9126–39. doi: 10.1021/acsnano.2c12217 [DOI] [PubMed] [Google Scholar]
- 98. Huang L, He H, Wang K, Ma X, Chen X, Chen W, et al. EGFRvIII-targeted immunotoxin combined with temozolomide and bispecific antibody for the eradication of established glioblastoma. BioMed Pharmacother (2022) 155:113659. doi: 10.1016/j.biopha.2022.113659 [DOI] [PubMed] [Google Scholar]
- 99. Iurlaro R, Waldhauer I, Planas-Rigol E, Bonfill-Teixidor E, Arias A, Nicolini V, et al. A novel EGFRvIII T-cell bispecific antibody for the treatment of glioblastoma. Mol Cancer Ther (2022) 21(10):1499–509. doi: 10.1158/1535-7163.MCT-22-0201 [DOI] [PubMed] [Google Scholar]
- 100. Sternjak A, Lee F, Thomas O, Balazs M, Wahl J, Lorenczewski G, et al. Preclinical assessment of AMG 596, a bispecific T-cell engager (BiTE) immunotherapy targeting the tumor-specific antigen EGFRvIII. Mol Cancer Ther (2021) 20(5):925–33. doi: 10.1158/1535-7163.MCT-20-0508 [DOI] [PubMed] [Google Scholar]
- 101. Yin Y, Rodriguez JL, Li N, Thokala R, Nasrallah MP, Hu L, et al. Locally secreted BiTEs complement CAR T cells by enhancing killing of antigen heterogeneous solid tumors. Mol Ther (2022) 30(7):2537–53. doi: 10.1016/j.ymthe.2022.05.011 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 102. Ma J, Mo Y, Tang M, Shen J, Qi Y, Zhao W, et al. Bispecific antibodies: from research to clinical application. Front Immunol (2021) 12. doi: 10.3389/fimmu.2021.626616 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 103. Rosenthal M, Balana C, Van Linde ME, Sayehli C, Fiedler WM, Wermke M, et al. Novel anti-EGFRvIII bispecific T cell engager (BiTE) antibody construct in glioblastoma (GBM): Trial in progress of AMG 596 in patients with recurrent or newly diagnosed disease. J Clin Oncol (2019) 37(15_suppl):TPS2071–TPS. doi: 10.1200/JCO.2019.37.15_suppl.TPS2071 [DOI] [Google Scholar]
- 104. Rosenthal MA, Balana C, van Linde ME, Sayehli C, Fiedler WM, Wermke M, et al. ATIM-49 (LTBK-01). AMG 596, A NOVEL ANTI-EGFRVIII BISPECIFIC T CELL ENGAGER (BITE®) MOLECULE FOR THE TREATMENT OF GLIOBLASTOMA (GBM): PLANNED INTERIM ANALYSIS IN RECURRENT GBM (RGBM). Neuro-Oncology (2019) 21(Supplement_6):vi283–vi. doi: 10.1093/neuonc/noz219.1195 [DOI] [Google Scholar]
- 105. Akhavan D, Alizadeh D, Wang D, Weist MR, Shepphird JK, Brown CE. CAR T cells for brain tumors: Lessons learned and road ahead. Immunol Rev (2019) 290(1):60–84. doi: 10.1111/imr.12773 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 106. Wang X, Lu J, Guo G, Yu J. Immunotherapy for recurrent glioblastoma: practical insights and challenging prospects. Cell Death Disease (2021) 12(4):299. doi: 10.1038/s41419-021-03568-0 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 107. Bikfalvi A, da Costa CA, Avril T, Barnier JV, Bauchet L, Brisson L, et al. Challenges in glioblastoma research: focus on the tumor microenvironment. Trends Cancer (2023) 9(1):9–27. doi: 10.1016/j.trecan.2022.09.005 [DOI] [PubMed] [Google Scholar]
- 108. De Vleeschouwer S, Bergers G. Glioblastoma: to target the tumor cell or the microenvironment? In: De Vleeschouwer S, editor. Glioblastoma. Brisbane (AU: (2017). doi: 10.15586/codon.glioblastoma.2017.ch16 [DOI] [PubMed] [Google Scholar]
- 109. Gieryng A, Pszczolkowska D, Walentynowicz KA, Rajan WD, Kaminska B. Immune microenvironment of gliomas. Lab Invest (2017) 97(5):498–518. doi: 10.1038/labinvest.2017.19 [DOI] [PubMed] [Google Scholar]
- 110. Maddison K, Graves MC, Bowden NA, Fay M, Vilain RE, Faulkner S, et al. Low tumour-infiltrating lymphocyte density in primary and recurrent glioblastoma. Oncotarget (2021) 12(21):2177–87. doi: 10.18632/oncotarget.28069 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 111. Woroniecka KI, Rhodin KE, Chongsathidkiet P, Keith KA, Fecci PE. T-cell dysfunction in glioblastoma: applying a new framework. Clin Cancer Res (2018) 24(16):3792–802. doi: 10.1158/1078-0432.CCR-18-0047 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 112. Hussain SF, Yang D, Suki D, Aldape K, Grimm E, Heimberger AB. The role of human glioma-infiltrating microglia/macrophages in mediating antitumor immune responses. Neuro Oncol (2006) 8(3):261–79. doi: 10.1215/15228517-2006-008 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 113. Zhang C, Cheng W, Ren X, Wang Z, Liu X, Li G, et al. Tumor purity as an underlying key factor in glioma. Clin Cancer Res (2017) 23(20):6279–91. doi: 10.1158/1078-0432.CCR-16-2598 [DOI] [PubMed] [Google Scholar]
- 114. Da Ros M, De Gregorio V, Iorio AL, Giunti L, Guidi M, de Martino M, et al. Glioblastoma chemoresistance: the double play by microenvironment and blood-brain barrier. Int J Mol Sci (2018) 19(10):2879. doi: 10.3390/ijms19102879 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 115. Nduom EK, Weller M, Heimberger AB. Immunosuppressive mechanisms in glioblastoma. Neuro Oncol (2015) 17 Suppl 7(Suppl 7):vii9–vii14. doi: 10.1093/neuonc/nov151 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 116. Biserova K, Jakovlevs A, Uljanovs R, Strumfa I. Cancer stem cells: significance in origin, pathogenesis and treatment of glioblastoma. Cells (2021) 10(3):621. doi: 10.3390/cells10030621 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 117. Gimple RC, Bhargava S, Dixit D, Rich JN. Glioblastoma stem cells: lessons from the tumor hierarchy in a lethal cancer. Genes Dev (2019) 33(11-12):591–609. doi: 10.1101/gad.324301.119 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 118. Lathia JD, Mack SC, Mulkearns-Hubert EE, Valentim CL, Rich JN. Cancer stem cells in glioblastoma. Genes Dev (2015) 29(12):1203–17. doi: 10.1101/gad.261982.115 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 119. Brown LS, Foster CG, Courtney JM, King NE, Howells DW, Sutherland BA. Pericytes and neurovascular function in the healthy and diseased brain. Front Cell Neurosci (2019) 13:282. doi: 10.3389/fncel.2019.00282 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 120. Serlin Y, Shelef I, Knyazer B, Friedman A. Anatomy and physiology of the blood-brain barrier. Semin Cell Dev Biol (2015) 38:2–6. doi: 10.1016/j.semcdb.2015.01.002 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 121. Louveau A, Harris TH, Kipnis J. Revisiting the mechanisms of CNS immune privilege. Trends Immunol (2015) 36(10):569–77. doi: 10.1016/j.it.2015.08.006 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 122. Jackson CM, Lim M, Drake CG. Immunotherapy for brain cancer: recent progress and future promise. Clin Cancer Res (2014) 20(14):3651–9. doi: 10.1158/1078-0432.CCR-13-2057 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 123. Louveau A, Smirnov I, Keyes TJ, Eccles JD, Rouhani SJ, Peske JD, et al. Structural and functional features of central nervous system lymphatic vessels. Nature (2015) 523(7560):337–41. doi: 10.1038/nature14432 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 124. Griffith JI, Rathi S, Zhang W, Zhang W, Drewes LR, Sarkaria JN, et al. Addressing BBB heterogeneity: A new paradigm for drug delivery to brain tumors. Pharmaceutics (2020) 12(12):1205. doi: 10.3390/pharmaceutics12121205 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 125. Quail DF, Joyce JA. The microenvironmental landscape of brain tumors. Cancer Cell (2017) 31(3):326–41. doi: 10.1016/j.ccell.2017.02.009 [DOI] [PMC free article] [PubMed] [Google Scholar]