Abstract
Background
Juvenile idiopathic arthritis (JIA) treatment is aimed at inducing remission to prevent joint destruction and disability. However, it is unclear what is the long-term impact on health-related outcomes of the timing of biological disease-modifying antirheumatic drug (bDMARD) initiation in JIA. Our aim was to evaluate the long-term impact of the time between JIA onset and the initiation of a bDMARD in achieving clinical remission, on physical disability and health-related quality of life (HRQoL).
Methods
Adult JIA patients registered in the Rheumatic Diseases Portuguese Register (Reuma.pt) and ever treated with bDMARD were included. Data regarding socio-demographic, JIA-related characteristics, disease activity, physical disability (HAQ-DI), HRQoL (SF-36), and treatments were collected at the last visit. Patients were divided into 3 groups (≤ 2 years, 2–5 years, or > 5 years), according to the time from disease onset to bDMARD initiation. Regression models were obtained considering remission on/off medication, HAQ-DI, SF-36, and joint surgeries as outcomes and time from disease onset to bDMARD start as an independent variable.
Results
Three hundred sixty-one adult JIA patients were evaluated, with a median disease duration of 20.3 years (IQR 12.1; 30.2). 40.4% had active disease, 35.1% were in remission on medication, and 24.4% were in drug-free remission; 71% reported some degree of physical disability. Starting a bDMARD > 5 years after disease onset decreased the chance of achieving remission off medication (OR 0.24; 95% CI 0.06, 0.92; p = 0.038). Patients who started a bDMARD after 5 years of disease onset had a higher HAQ and worse scores in the physical component, vitality, and social function domains of SF-36, and more joint surgeries when compared to an earlier start.
Conclusion
Later initiation of bDMARDs in JIA is associated with a greater physical disability, worse HRQoL, and lower chance of drug-free remission in adulthood.
Keywords: Juvenile idiopathic arthritis, Long-term outcomes, bDMARDs, Physical disability, Quality of life
Introduction
The term juvenile idiopathic arthritis (JIA) is used to designate a very heterogeneous group of chronic inflammatory diseases with childhood onset that actually correspond to distinct diseases with different prognoses [1]. In the current era of individualized treat-to-target treatment strategies [2], knowledge about the long-term outcomes of patients with JIA is essential for patient counseling and planning the transition to adult care. Since 1999, the introduction of biologics in JIA treatment has dramatically changed the long-term functional outcome of JIA, and over the last decade, the outcomes of JIA patients have been studied in cohorts with progressively longer follow-up [3–7]. Some studies have shown that the sooner treatment is begun for JIA and the more aggressive it is, the better the outcomes obtained [8–11]. However, it is less clear what is the long-term impact of the timing of biological disease-modifying antirheumatic drug (bDMARD) initiation in JIA. This timing is affected by multiple factors like the number of active joints, presence and severity of uveitis, and JIA category [12], and for this reason, the optimum time for initiating a bDMARD is difficult to establish as it varies greatly among patients.
This study aims to evaluate the long-term impact in adulthood of the time between the onset of JIA and the initiation of a bDMARD on achieving clinical remission, on physical disability, and on health-related quality of life (HRQoL).
Methods
Study design and patient selection
This is a cross-sectional analysis nested in a cohort study with the following inclusion criteria: patients with JIA according to the 2001 revised International League of Associations for Rheumatology (ILAR) criteria [1], registered in the Rheumatic Diseases Portuguese Register (Reuma.pt) [13], who at the time of data extraction (September 2021) were older than 18 years old, had ever been treated with bDMARD and have available data in adulthood.
Reuma.pt was developed by the Portuguese Society of Rheumatology, became active in June 2008, and includes patients with JIA, rheumatoid arthritis (RA), spondyloarthritis (SpA), and several other rheumatic diseases. Specifically, 2081 JIA patients with 18856 medical visits have been registered so far in Reuma.pt [14]. Data before 2008 was registered retrospectively and prospectively thereafter. Patients with disease onset before 2001 were classified retrospectively according to the ILAR classification.
Registry of patient data in Reuma.pt occurred after signed informed consent was obtained. This study was approved by the scientific committee of Reuma.pt and by the ethics committee of the Lisbon Academic Medical Centre. Reuma.pt was approved by the National Data Protection Authority and by local ethics committees of the participating centers. The study was conducted according to the Declaration of Helsinki.
Clinical data collection
The following information was obtained from the last visit available at the moment of data extraction (September 2021): sex, ethnicity, age at last visit, years of education, employment status (employed, unemployed, retired and retired due to JIA induced disability), ILAR category, age at disease onset (disease onset was defined as the date when arthritis was first documented by a physician), disease duration (years), presence of rheumatoid factor (RF), anti–citrullinated protein antibodies (ACPA), antinuclear antibodies (ANA); considered positive if titers ≥ 1/160) and human leukocyte antigen B27 (HLA B27), number of active joints, patient and physician’s global assessment of disease activity (0–10), back pain (0–10), morning stiffness intensity (0–10), erythrocyte sedimentation rate (mm/first hour) and C-reactive protein level (mg/dl), extra-articular manifestations, joint surgeries (surgical synovectomy, arthroplasty, and arthrodesis), Juvenile Arthritis Damage Index (JADI) score, current and previous therapy with corticosteroids, conventional disease modifying antirheumatic drugs (cDMARD), and bDMARDs.
Assessment of the disease activity was based on the American College of Rheumatology (ACR) provisional criteria [15] for clinical inactive disease (CID) and clinical remission (CR). Remission off drugs was defined as CID for at least 12 months without any treatment, in accordance with the criteria by Wallace et al. [16].
In the absence of a validated score for evaluation of damage in adults with JIA, we opted to use JADI, as a more comprehensive way of assessing articular damage (JADI-A) and extra-articular damage (JADI-E), [17].
The physical disability was measured by the Health Assessment Questionnaire—Disability Index (HAQ-DI), [18] obtained at the last visit registered. For the purpose of this analysis, mild disability was considered for HAQ scores > 0 and ≤ 0.5, moderate disability > 0.5 and ≤ 1.5, and severe disability > 1.5 [19].
HRQoL was assessed using the Medical Outcomes Study 36-item Short Form (SF-36) [20].
Statistical analysis
Descriptive statistics were presented through medians and interquartile range (IQR) due to the absence of Gaussian distribution evaluated by the Shapiro–Wilk test. Qualitative data were presented through absolute and relative frequencies. The former statistics were compared according to the time when the first biologic was started (< 2 years; 2–5 years; > 5 years after disease onset) using, respectively, the Kruskal–Wallis test with pairwise adjusted comparisons when justified, and the Fisher exact test. Afterwards, the variable “time when the first biologic was started” was recoded into two groups, (≤ 5 years; > 5 years) as most of the statistically significant differences were between the ones who started the first biologic for more than 5 years and one or both of the other two groups, as binary variables are more easily interpreted than variables with three or more categories, even when ordinal. These groups were compared through the Mann–Whitney U test or the Fisher exact test, according to previous considerations. This step was performed in order to select meaningful variables for further analysis.
Binary logistic and linear regression was then applied, considering joint surgeries, disease activity, physical disability, and quality of life as dependent variables, and considering the “time when the first biologic was started” as an independent variable. We performed two adjusted models to determine the association between each of the health-related outcomes (joint surgeries, clinical remission on and off medication, physical disability, and quality of life) with “time when the first biologic was started.” The first model was adjusted for gender and JIA categories and the second model adjusted for gender, JIA categories, disease duration and disease activity (for the outcomes remission on and off medication adjustment for disease activity was not applied). Due to the high variability of some of the covariates it is of extreme importance to state that models presented white noise residuals with mean zero, that is, residuals presented normal distribution — evaluated through the Shapiro–Wilk test — with mean zero and constant variance, leading to homogeneity of residuals according to predictions. Independency and absence of autocorrelation were evaluated and confirmed by the Durbin-Watson statistic.
Missing data were interpreted as random missing data without any data imputation. Analysis was performed in SPSS, version 27, and evaluated at a 5% significant level.
Results
Patient characteristics
A total of 361 adult JIA patients who had ever been treated with a bDMARD were included in the study (Fig. 1). From these 361 patients, 279 patients were diagnosed previously to 2008 when Reuma.pt became active and had data registered retrospectively until that year and prospectively afterward. For 82 patients all the data was registered prospectively. The 226 patients who had their disease onset before 2001 were classified retrospectively according to the ILAR classification. The main demographic and clinical features of the patients included in this study are shown in Table 1.
Fig. 1.
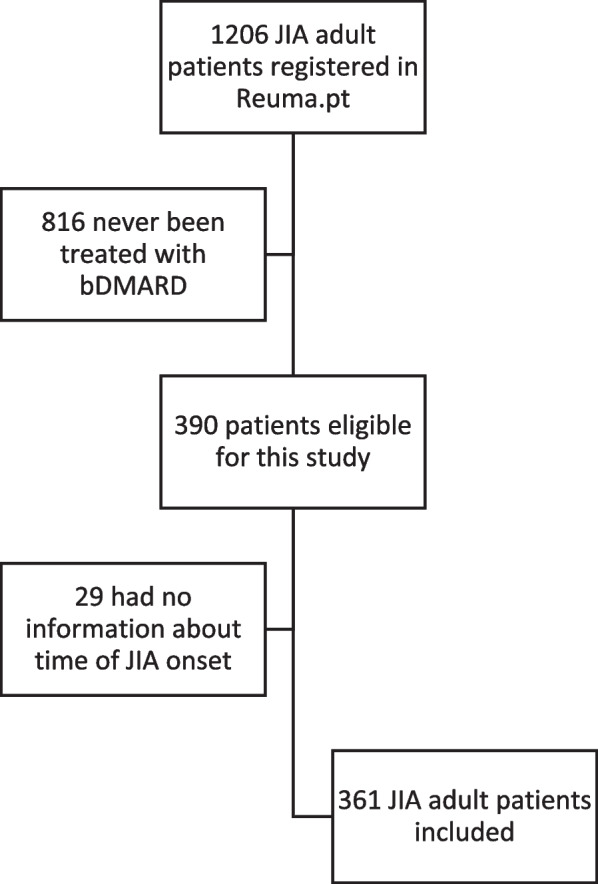
Disposition of adult JIA patients registered in Reuma.pt eligible for this study
Table 1.
Socio-demographic and disease-related characteristics of JIA patients according to the time when biologic was started
| Total | bDMARD started < 2 years after disease onset | bDMARD started 2–5 years after disease onset | bDMARD started > 5 years after disease onset | p-value | |
|---|---|---|---|---|---|
| Patients, n (%) | 361 (100) | 52 (14.4) | 65 (18) | 244 (67.5) | |
| Female, n (%) | 194 (53.7) | 19 (36.5) | 21 (32.3) | 154 (63.1)c | 0.015* |
| JIA ILAR category, n (%) | 0.001** | ||||
| Persistent oligoarthritis | 33 (9.1) | 2 (3.8) | 12 (18.5) | 19 (7.8) | |
| Extended oligoarthritis | 58 (16.1) | 7 (13.5) | 8 (12.3) | 43 (17.6) | |
| RF-positive polyarthritis | 39 (10.8) | 3 (5.8) | 4 (6.2) | 32 (13.1) | |
| RF-negative polyarthritis | 70 (19.4) | 10 (19.2) | 11 (16.9) | 49 (20.1) | |
| Systemic | 34 (9.4) | 5 (9.6) | 8 (12.3) | 21 (8.6) | |
| Enthesitis related arthritis | 88 (24.4) | 22 (42.3) | 16 (24.6) | 50 (20.5) | |
| Psoriatic arthritis | 17 (4.7) | 3 (5.8) | 4 (6.2) | 10 (4.1) | |
| Undifferentiated arthritis | 22 (6.4) | 0 (0) | 3 (4.6) | 19 (7.8) | |
| Age at disease onset, years, median (IQR) | 11.5 (6.3–14.6) | 13.3 (11.7–15.7)a | 12.2 (9.8–14.7)b | 9.8 (5.3–14)a,b | < 0.001* |
| Age at diagnosis, years, median (IQR) | 13.3 (8.2–16.8) | 13.1 (10.4–16.1) | 13.0 (10.6–15.7) | 13.2 (6.8–18.2) | 0.392 |
| Age when first biologic was started, years, median (IQR) | 21.7 (15.8–31.9) | 15.3 (13.4–16.4)a | 15.8 (13–17.9)b | 26.3 (19.7–36)a,b | < 0.001* |
| Disease duration, years, median (IQR) | 20.3 (12.1–30.2) | 7.5 (3.8–11.6)a | 10.8 (6.4–15.9)b | 25.1 (19.3–34.1)a,b | < 0.001* |
| Age at last visit, years, median (IQR) | 29.1 (21.8–40.2) | 20.2 (18.9–23.2)a | 21.9 (19.7–25.5)b | 35.5 (27–44.8)a,b | < 0.001* |
| ANA + , n assessed: 225, n (%) | 71 (31.5) | 9 (21.4) | 11 (25.6) | 51 (36.4)c | 0,003* |
| RF + , n assessed: 267, n (%) | 73 (27.3) | 8 (20) | 5 (11.1) | 60 (33)c | 0.032* |
| ACPA + , n assessed: 175, n (%) | 47 (26.8) | 7 (22.5) | 3 (10.3) | 37 (32.2) | 0.271 |
| HLAB27 + , n assessed: 183, n (%) | 78 (42.6) | 17 (48.6) | 14 (45.2) | 47 (40.2) | 0.164 |
| Presence of uveitis, n assessed: 285, n (%) | 40 (14) | 2 (5) | 9 (20) | 29 (14.5) | 0.105 |
| Years of education, n assessed: 203; median (IQR) | 12 (9–15) | 11 (9–12) | 11 (9–14) | 12 (9–15)c | 0.24 |
| Professional situation, n assessed: 210 | 0.193 | ||||
| Employed, n (%) | 159 (75.7) | 18 (60)c | 21 (63.6) c | 120 (81.6) | |
| Unemployed, n (%) | 18 (8.6) | 0 (0) | 1 (3) | 17 (11.6)c | |
| Retired, n (%) | 7 (3.3) | 0 (0) | 0 (0) | 7 (4.8)c | |
| Retired due to JIA disability, n (%) | 17 (8.1) | 0 (0) | 0 (0) | 17 (11.6)c | |
| Past treatment | |||||
| Corticosteroids, n (%) | 181 (50.1) | 21 (40.4) | 15 (23.1) | 145 (59.4)c | 0.002 |
| cDMARDs, n (%) | 280 (77.6) | 34 (65.4) | 38 (58.5) | 208 (85.2) | 0.709 |
| Current treatment | |||||
| Corticosteroids, n assessed: 271, n (%) | 88 (32.5) | 4 (14.3) | 3 (7.3) | 81 (40.1)c | < 0,001 |
| cDMARDs, n assessed: 272, n (%) | 159 (58.5) | 13 (44.8) | 15 (36.6) | 129 (63.9) | 0.148 |
| bDMARDS, n (%) | 295 (81.7) | 33 (63.5) | 36 (55.4) | 196 (80.3) | 0.817 |
| Cumulative corticosteroid exposure, years, median (IQR) | 5.9 (2.4–14.9) | 1.8 (0.8–2.3)a | 3.9 (1.9–8.2) | 7.8 (2.9–17.1]a | < 0.001* |
Sample size is not constant due to missing data:
Patients whose bDMARD was started < 2 years after disease onset: ANA + n = 42; RF + n = 40; ACPA + n = 31; HLAB27 + n = 35; presence of uveitis n = 40; professional situation n = 30; current corticosteroids n = 28; current cDMARDs n = 29
Patients whose bDMARD was started 2–5 years after disease onset: ANA + n = 43; RF + n = 45; ACPA + n = 29; HLAB27 + n = 31; presence of uveitis n = 45; professional situation n = 33; current corticosteroids n = 41; current cDMARDs n = 41
Patients whose bDMARD was started > 5 years after disease onset: ANA + n = 140; RF + n = 182; ACPA + n = 115; HLAB27 + n = 117; presence of uveitis n = 200; professional situation n = 147; current corticosteroids n = 202; current cDMARDs n = 202
Legend: JIA juvenile idiopathic arthritis, IQR interquartile range, ANA antinuclear antibodies, RF rheumatoid factor, ACPA anti-citrullinated protein antibodies, DMARD disease-modifying antirheumatic drugs, cDMARDs conventional DMARD, bDMARDs biological DMARDs
aStatistically significant difference between “ < 2Y” and “ > 5Y” at a 5% significance level — Mann–Whitney U test adjusted for multiple comparisons
bStatistically significant difference between “2–5Y” and “ > 5Y” ate a 5% significance level — Mann–Whitney U test adjusted for multiple comparisons
cHigher frequency than expected (statistically significant at a 5% significance level — Fisher exact test and adjusted residual higher than 1.96)
*Statistically significant difference between “ < 5Y” and “ ≥ 5Y” at a 5% significance level — Mann–Whitney U test
**Fisher exact test applied using “Extended oligoarthritis,” “RF-positive polyarthritis,” and “RF-negative polyarthritis” categories of JIA grouped as one in order to perform the test with some robustness
The median age at the last registered visit was 29.1 years (IQR 21.8–40.2; range: 18–74) and the median disease duration was 20.3 years (IQR: 12.1–30.2; range: 1–71.5). Most of the patients (79%) had a disease duration of more than 10 years and 24% exceeded 30 years.
The distribution of the JIA categories over the 3 different timings of bDMARD initiation groups (< 2 years; 2–5 years; > 5 years) is shown in Table 1.
The first bDMARD treatment was started in 14.4% within two years of JIA onset, 18% after two to five years of disease, and 67.5% more than 5 years after disease onset. The median age at bDMARD start was 15.5 years (IQR 13.4–16.5) in patients that started this treatment in the first 2 years after disease onset and 15.7 years (IQR 13.1–17.8) in the group that started bDMARD 2–5 years after disease onset. The patients who started bDMARD after 5 years of disease onset had a median age at bDMARD start of 26.28 years (IQR 19.7–35.6), were older at the last visit than the patients from the other groups, and had longer disease duration, and 72% had the disease onset before the year 2000. In the subgroup of this cohort that had the disease onset after the year 2000 (n = 169) the distribution according to the 3 groups was 29.4% in both groups that started bDMARD before 5 years after disease onset and 41.6% in the group that started later, after 5 years of disease onset.
Forty percent of the studied patients still had active disease and 80.6% were on a cDMARD or bDMARD. The most frequently used cDMARD was methotrexate in 70.9% of the cases and the most frequently used bDMARD was etanercept in 54.8% followed by adalimumab in 29.6% of the cases. Only 14.9% of the total cohort was in remission off medication. 61.5% of the patients had no or mild disability and just 12.9% had severe disability. Cumulative corticosteroid exposure was significantly higher in the > 5 years group when compared to the patients who started bDMARD earlier in the disease course (7.8 [IQR 2.9–17.1] vs 3.9 [IQR 1.9–8.2] and 1.8 [IQR 0.8–2.9]; p < 0.001).
Patient health-related outcomes according to the timing of bDMARD initiation
Patients who started bDMARD after 5 years of disease onset were more likely to have active disease when compared to the patients who started bDMARD earlier (85.7% vs 8.8% and 5.5.% respectively, p < 0.001; Table 2). This group of patients with a later start of bDMARD was also 76% less likely to be on remission off medication, irrespectively of JIA category, gender, and disease duration (OR 0.24; 95% CI 0.06, 0.92; p = 0.038; Table 3).
Table 2.
Outcomes at the last follow-up according to the time when bDMARD was started
| Total | bDMARD started < 2 years after disease onset | bDMARD started 2–5 years after disease onset | bDMARD started > 5 years after disease onset | p-value | |
|---|---|---|---|---|---|
| Disease activity (n assessed: 225) | |||||
| Active (N/%) | 91 (40.4) | 5 (5.5) | 8 (8.8) | 78 (85.7)c | < 0.001* |
| Remission on medication (N/%) | 79 (35.1)c | 10 (12.6) | 12 (15.1) | 57 (72.1) | 0.156 |
| Remission off medication (N/%) | 54 (24)c | 8 (14.8)c | 14 (25.9) | 32 (59.2) | < 0.001* |
| JADI-A (n assessed: 158; median (IQR)) | 1 (0–14.5) | 0 (0–1)a | 0 (0–1)b | 3 (0–28)a,b | 0.002* |
| JADI-E (n assessed: 158; median (IQR)) | 0 (0–1) | 0 (0–0)a | 0 (0–0) | 0 (0–2)a | 0.013* |
| HAQ (n assessed: 283; median (IQR)) | 0.25 (0–1) | 0 (0–0.25)c | 0 (0–0.09)b | 0.5 (0–1.25)a,b | < 0.001* |
| SF36 physical component (n assessed: 218; median (IQR)) | 41.9 (29.9–53.2) | 56.2 (44.2–58.6)c | 49.5(44.0–57.8)b | 40.2 (32.9–64.4)a,b | 0.001 |
| SF36 PF (median (IQR)) | 67.5 (40–95) | 97.5 (82.5–100) | 92.5 (70–100)b | 55 (30–90)a,b | < 0.001* |
| SF36 RP (median (IQR)) | 93.8 (25–100) | 100 (64.1–100) | 100 (87.5–100)b | 75 (25–100)b | 0.013* |
| SF36 BP (median (IQR)) | 62 (41–84) | 73 (38.8–88) | 82 (61.3–96)b | 52 (41–84)b | 0.019* |
| SF36 GH (median (IQR)) | 47 (32.8–67) | 73,5 (50.8–87)c | 45 (37–67) | 47 (30–67)a | 0.046 |
| SF36 mental component (n assessed: 217; median (IQR)) | 48.1 (37.3–65.8) | 54.0 (32.9–64.4) | 58.4 (43.4–72.1) | 47.4 (34.7–66.6) | 0.165 |
| SF36 VT (median (IQR)) | 50 (40–75) | 65.6 (50–82.2) | 63.8 (55.3–79.7)b | 50 (40–69.4)b | 0.008* |
| SF36 SF (median (IQR)) | 87.5 (51–100) | 87.5 (71.9–100) | 100 (78.1–100)b | 75 (50–100)b | 0.017* |
| SF36 RE (median (IQR)) | 100 (64.6–100) | 100 (91.7–100) | 100 (100–100) | 100 (33.3–100) | 0.154 |
| SF36 MH (median (IQR)) | 76 (60–88) | 86.5 (77.5–91.3) | 75 (61.4–90) | 76 (60–88) | 0.198 |
| Joint surgery ever (n assessed: 361; N/%) | 61 (16.9) | 0 (0.0) | 1 (2.2) | 57 (24.1) | < 0.001* |
Legend: IQR interquartile range, bDMARD biological disease-modifying antirheumatic drugs, JADI-A Juvenile Arthritis Damage Index – articular, JADI-E Juvenile Arthritis Damage Index – extra-articular, HAQ Health Assessment Questionnaire, SF36 Medical Outcomes Study 36-item Short Form, SF36 PC physical component of the Short Form 36, SF36 MC mental component of the Short Form 36, PF physical function, RP role limitations due to physical problems, BP intensity and discomfort caused by pain, GH general health, VT vitality, SF social function, RE role limitations due to emotional problems, MH mental health
aStatistically significant difference between “ < 2Y” and “ > 5Y” at a 5% significance level — Mann–Whitney U test adjusted for multiple comparisons
bStatistically significant difference between “2–5Y” and “ > 5Y” ate a 5% significance level — Mann–Whitney U test adjusted for multiple comparisons
cHigher frequency than expected (Statistically significant at a 5% significance level — Fisher exact test and adjusted residual higher than 1.96)
*Statistically significant difference between “ < 5Y” and “ ≥ 5Y” at a 5% significance level — Mann–Whitney U test
Table 3.
Multivariate regression to identify the association of the timing of bDMARD start and remission on and off medication, physical disability, HRQoL, and joint surgeries as outcomes
| Outcomes | > 5 years from disease-onset to bDMARD start, adjusted for JIA categoryc and gender | > 5 years from disease-onset to bDMARD start, adjusted for JIA categoryc, gender, disease duration, and disease activity | ||
|---|---|---|---|---|
| Beta/OR (95% CI) | p-value | Beta/OR (95% CI) | p-value | |
| Remission on medicationa | 0.61 (0.25; 1.49) | 0.278 | 2.47 (0.55; 11.12)d | 0.240 |
| Remission off medicationa | 0.28 (0.12; 1.66) | 0.003 | 0.24 (0.06; 0.92)d | 0.038 |
| HAQ total scoreb | 0.48 (0.27; 0.68) | < 0.001 | 0.05 (− 0.18; 0,28) | 0.687 |
| SF 36 physical componentb | − 10.63 (− 16.00; − 5.27) | < 0.001 | − 0.01 (− 8.76; 6.85) | 0.997 |
| PFb | − 29.29 (− 41.79; − 16.79) | < 0.001 | − 2.88 (.17.70; 11.94) | 0.700 |
| RPb | − 25.57 (− 40.82; − 10.32) | 0.001 | 0.53 (− 19.38; 20.44) | 0.958 |
| BPb | − 14.82 (− 25.62; − 4.03) | 0.007 | 2.63 (− 10.59; 15.84) | 0.694 |
| GHb | − 7.59 (− 16.86; 1.69) | 0.108 | − 0.26 (− 12.68; 12.17) | 0.967 |
| SF36 mental componentb | − 6.16 (− 14.50; 2.18) | 0.147 | − 0.41 (− 11.83; 11.00) | 0.944 |
| VTb | − 10.73 (− 19.05; − 2.41) | 0.012 | − 3.81 (− 14.98; 7.36) | 0.500 |
| SFb | − 15.49 (− 26.15; − 4.84) | 0.005 | − 8.00 (− 22.39; 6.40) | 0.273 |
| REb | − 14.63 (− 28.63; − 0.64) | 0.041 | − 3.61 (− 16.91; 24.14) | 0.727 |
| MHb | − 5.95 (− 14.27; 2.46) | 0.165 | − 1.60 (− 12.76; 9.56) | 0.776 |
| Joint surgeriesa | 26.6 (3.62; 195.34) | 0.001 | 7.33 (0.82; 65.62) | 0.075 |
Legend: JIA juvenile idiopathic arthritis, bDMARD biological disease-modifying antirheumatic drugs, HAQ Health Assessment Questionnaire, SF36 Medical Outcomes Study 36-item Short Form, SF36 PC physical component of the Short Form 36, SF36 MC mental component of the Short Form 36, PF physical function, RP role limitations due to physical problems, BP intensity and discomfort caused by pain, GH general health, VT vitality, SF social function, RE role limitations due to emotional problems, MH mental health
aLogistic regression (regression coefficients presented as odds ratio (OR))
bLinear regression (regression coefficients presented as beta)
cHaving JIA Persistent oligoarthritis as reference
dAdjustment for disease activity does not apply
The group of patients who started bDMARD after 5 years of disease onset had also a higher HAQ (in < 2 years group — median of 0 [IQR 0–0.25], 2–5 years group — median of 0 [IQR 0–0.09], > 5 years group — median 0.5 [IQR 0–1.25]; p < 0.001) and scored worse on the physical component of SF-36 (in < 2 years group — median of 56.2 [IQR 44.2–58.6], 2–5 years group — median of 49.5 [IQR 44.0–57.8] and > 5 years group — median of 40.2 [IQR 32.9–64.4]; p = 0.001). Also, in the mental domains of vitality and social function, the scores were likely to be worse if the bDMARD was started later than 5 years (median of 50 [IQR 40–69.4]; p = 0.008 and 75 [IQR 50–100]; p = 0.017, respectively) when compared to an earlier start (Table 2). These associations were independent of the JIA category or gender but not of disease duration or disease activity (Table 3).
Patients who started bDMARD after 5 years of disease onset also underwent joint surgery more often than those who started bDMARDs earlier (24.1% vs 2.2%, p < 0.001). The odds of having a joint surgery were increased (odds ratio (OR) = 26.6, 95% CI: 3.62–195.34; p = 0.001) if the patients started bDMARDs after 5 years of disease onset in an independent way of JIA category and gender but not of the disease duration or disease activity (Table 3).
Discussion
The early start of biologic therapy in pediatric patients with JIA is crucial when the control of the disease with conventional DMARDs is not achievable [12, 21]. This is reflected in the more recent recommendations for JIA treatment where bDMARDs, according to a treat-to-target strategy, should be considered in an early phase of the disease, in order to achieve remission if this is not reached with cDMARDs [2, 22–24]. However, it is less clear what is the long-term impact of the timing of bDMARD initiation as an independent factor on JIA outcomes.
In our study, we found that only a minority of the patients started bDMARDs early in the disease course (14.4% in the first 2 years after disease onset). One possible reason for this observation is that more than half of these patients (53.3%) had the disease onset before the year 2000 and thus did not have access to bDMARDs during the first years of their disease course.
This adult JIA population had a predominance of polyarticular and ERA categories, which reflects the JIA population that prevails in adult rheumatology care [25]. The distribution of the JIA categories according to the 3 groups, showed for all of them a higher prevalence in the > 5 years group, even for the systemic-onset JIA where the bDMARDS are now recommended in the early stages of the disease and even in first-line [22]. However, also in this JIA category, more than half of the patients (55.9%) had the disease onset before the year 2000 which explains the distribution over the 3 study groups. Nevertheless, even in the subgroup of this cohort that had the disease onset after the year 2000, 41.6% started the bDMARD later than 5 years of disease onset. This is in line with other registries like Biker and Biobadaser, which showed that in 2000, only around 25% of patients received the first biologic at a pediatric age, with this percentage increasing linearly until reaching 65% in 2015 [8, 26].
To our knowledge, besides our study, only Minden et al. [8] analyzed the relation of an early versus late start of bDMARD in the JIA course and the likelihood of having a drug-free remission, full functional capability, and the need for joint surgery. Similarly to what was found in this previous study [8], in our cohort patients who started bDMARD after 5 years of disease onset were less likely to achieve remission off medication, when compared to the patients who started bDMARD earlier, irrespectively of the JIA category, gender, and disease duration. Up to 5 years we could not find any significant differences regarding the timing of an earlier start (less than 2 years and between 2 and 5 years). However, the evidence that a later bDMARD start decreases the chance of achieving remission off medication supports the concept of the “window of opportunity” that has been defended also for JIA and guides the treat-to-target strategy proposed for JIA [2].
As expected, given the fact the patients in our study who started bDMARD after 5 years of disease onset were older, had longer disease duration, and most of them had their disease onset before the biological era, these patients had more physical disability and worse scores regarding the physical component of SF-36, and vitality and social function evaluated by the mental component of the SF-36. In fact, the association between these worse results regarding physical disability and HRQoL and the timing of bDMARD initiation was shown to be independent of JIA category or gender but not independent of disease duration or disease activity, which could have more weight in these outcomes than the timing of bDMARDs initiation by itself. This is in line with several studies that had shown that disease duration and disease activity (especially pain) are important predictors for worse HRQoL and physical disability [27–29].
Recently data from BiKeR/JuMBO registers showed that patients who were refractory or intolerant to conventional treatment and started bDMARDs within the first two years of JIA onset had a significantly lower likelihood of requiring joint surgery [8]. Similarly, we found that the odds of having a joint surgery were increased if the patients started bDMARDs after 5 years of disease onset in an independent way of JIA category and gender. However, in our study, this association was not independent of the disease duration or disease activity that seem to be more important contributors to this outcome than the moment of bDMARD initiation.
Our study has some limitations. First, its cross-sectional design may not accurately estimate the evolution over time of disease activity and HRQoL in JIA patients. As this is a long-term study the effect of different treatment strategies might have also had an impact on these outcomes at different time points. Additionally, selection bias of the registry may overrepresent more severe cases and some categories of JIA, like the ones with polyarticular involvement, as many patients in remission could have been lost for follow-up and patients with milder disease could have been less motivated to be enrolled. Another factor is that the moment of starting the first cDMARD was not considered since most patients were enrolled in Reuma.pt at the start of a bDMARD therapy. The number of patients in each JIA category, grouped according to the timing of bDMARD initiation, was too small to allow the study of the impact of bDMARD start on the outcomes for each category.
This study’s strength lies in the large dimension of the adult JIA patient cohort, in its long follow-up, and in the fact that it reflects real-world evidence and clinical practice. This is one of the first studies to address the influence of the timing of biological treatment initiation on JIA long-term health-related outcomes.
Conclusion
In conclusion, our results document that in JIA patients, the late start of bDMARDs increases the likelihood of having more physical disability and worse HRQoL and decreases the chances of achieving remission off medication in adulthood.
Acknowledgements
This study would not have been possible without the collaboration of numerous clinicians, patients, and their parents.
Abbreviations
- ACR
American College of Rheumatology
- ACPA
Anti-citrullinated protein antibodies
- ANA
Antinuclear antibodies
- bDMARD
Biological disease-modifying antirheumatic drug
- cDMARD
Conventional disease-modifying antirheumatic drugs
- CID
Clinical inactive disease
- CR
Clinical remission
- HRQoL
Health-related quality of life
- HAQ-DI
Health Assessment Questionnaire—Disability Index
- HLAB27
Human leukocyte antigen B27
- JADI-A
Juvenile Arthritis Damage Index – articular
- JADI-E
Juvenile Arthritis Damage Index – extra-articular
- JIA
Juvenile idiopathic arthritis
- SF-36
Medical Outcomes Study 36-item Short Form
- Reuma.pt
Rheumatic Diseases Portuguese Register
- RA
Rheumatoid arthritis
- RF
Rheumatoid factor
- SpA
Spondyloarthritis
Authors’ contributions
FOR, AMR, MJS and JEF contributed to the conceptualization and methodology of the study. ATM, FA, LB, SA, ACD, JAMG, CF, AFM, GS, IC and RF contributed to the data acquisition. FOR and AMR performed the statistical analysis. FOR performed data interpretation, wrote the main manuscript, and prepared the figures and tables. All authors reviewed and approved the final manuscript.
Funding
The authors have not declared a specific grant for this research from any funding agency in the public, commercial, or not-for-profit sectors.
Availability of data and materials
Data is available upon request.
Declarations
Ethics approval and consent to participate
This study was approved by the scientific committee of Reuma.pt and by the ethics committee of the Lisbon Academic Medical Centre. Reuma.pt was approved by the National Data Protection Authority and by local ethics committees of the participating centers. The study was conducted according to the Declaration of Helsinki.
Consent for publication
Not required.
Competing interests
The authors declare no competing interests.
Footnotes
Publisher’s Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
References
- 1.Petty RE, Southwood TR, Manners P, Baum J, Glass DN, Goldenberg J, et al. International League of Associations for Rheumatology classification of juvenile idiopathic arthritis: second revision, Edmonton, 2001. J Rheumatol. 2004;31(2):390–392. [PubMed] [Google Scholar]
- 2.Ravelli A, Consolaro A, Horneff G, Laxer RM, Lovell DJ, Wulffraat NM, et al. Treating juvenile idiopathic arthritis to target: recommendations of an international task force. Ann Rheum Dis. 2018;77(6):819–828. doi: 10.1136/annrheumdis-2018-213030. [DOI] [PubMed] [Google Scholar]
- 3.Tollisen A, Selvaag AM, Aasland A, Lerdal A, Flatø B. Longitudinal health status from early disease to adulthood and associated prognostic factors in juvenile idiopathic arthritis. J Rheumatol. 2019;46(10):1335–1344. doi: 10.3899/jrheum.180948. [DOI] [PubMed] [Google Scholar]
- 4.Glerup M, Rypdal V, Arnstad ED, Ekelund M, Peltoniemi S, Aalto K, et al. Long-term outcomes in juvenile idiopathic arthritis: 18 years of follow-up in the population-based Nordic Juvenile Idiopathic Arthritis (JIA) cohort. Arthritis Care Res (Hoboken). 2019. [DOI] [PubMed]
- 5.Glerup M, Rypdal V, Arnstad ED, Ekelund M, Peltoniemi S, Aalto K, et al. Long-term outcomes in juvenile idiopathic arthritis: eighteen years of follow-up in the population-based nordic juvenile idiopathic arthritis cohort. Arthritis Care Res (Hoboken) 2020;72(4):507–516. doi: 10.1002/acr.23853. [DOI] [PubMed] [Google Scholar]
- 6.Oliveira-Ramos F, Eusébio M, Martins FM, Mourão AF, Furtado C, Campanilho-Marques R, et al. Juvenile idiopathic arthritis in adulthood: fulfilment of classification criteria for adult rheumatic diseases, long-term outcomes and predictors of inactive disease, functional status and damage. RMD Open. 2016;2(2):e000304. 10.1136/rmdopen-2016-000304. [DOI] [PMC free article] [PubMed]
- 7.Oliveira Ramos F, Rodrigues A, Magalhaes Martins F, Melo AT, Aguiar F, Brites L, et al. Health-related quality of life and disability in adults with juvenile idiopathic arthritis: comparison with adult-onset rheumatic diseases. RMD Open. 2021;7(3):e001766. doi: 10.1136/rmdopen-2021-001766. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Minden K, Horneff G, Niewerth M, Seipelt E, Aringer M, Aries P, et al. Time of disease-modifying antirheumatic drug start in juvenile idiopathic arthritis and the likelihood of a drug-free remission in young adulthood. Arthritis Care Res (Hoboken) 2019;71(4):471–481. doi: 10.1002/acr.23709. [DOI] [PubMed] [Google Scholar]
- 9.Tynjälä P, Vähäsalo P, Tarkiainen M, Kröger L, Aalto K, Malin M, et al. Aggressive combination drug therapy in very early polyarticular juvenile idiopathic arthritis (ACUTE-JIA): a multicentre randomised open-label clinical trial. Ann Rheum Dis. 2011;70(9):1605–1612. doi: 10.1136/ard.2010.143347. [DOI] [PubMed] [Google Scholar]
- 10.Wallace CA, Giannini EH, Spalding SJ, Hashkes PJ, O’Neil KM, Zeft AS, et al. Trial of early aggressive therapy in polyarticular juvenile idiopathic arthritis. Arthritis Rheum. 2012;64(6):2012–2021. doi: 10.1002/art.34343. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Hissink Muller PCE, Brinkman DMC, Schonenberg D, Koopman-Keemink Y, Brederije ICJ, Bekkering WP, et al. A comparison of three treatment strategies in recent onset non-systemic juvenile idiopathic arthritis: initial 3-months results of the BeSt for Kids-study. Pediatr Rheumatol Online J. 2017;15(1):11. doi: 10.1186/s12969-017-0138-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Yue X, Huang B, Hincapie AL, Wigle PR, Qiu T, Li Y, et al. Prescribing patterns and impact of factors associated with time to initial biologic therapy among children with non-systemic juvenile idiopathic arthritis. Paediatr Drugs. 2021;23(2):171–182. doi: 10.1007/s40272-021-00436-4. [DOI] [PubMed] [Google Scholar]
- 13.Canhão H, Faustino A, Martins F, Fonseca JE. Rheumatic Diseases Portuguese register board coordination, portuguese society of rheumatology. Reuma. pt - the rheumatic diseases portuguese register. Acta Reumatol Port. 2011;36(1):45–56. [PubMed] [Google Scholar]
- 14.Totais de doentes e consultas registados no Reuma.pt - Área dos doentes. Cited 2022 Jul 27. Available from: https://app.reuma.pt/reuma/TotaisDoentesConsultas.aspx?tipoBD=&lang=pt_PT.
- 15.Wallace CA, Giannini EH, Huang B, Itert L, Ruperto N, Childhood Arthritis Rheumatology Research Alliance et al. American College of Rheumatology provisional criteria for defining clinical inactive disease in select categories of juvenile idiopathic arthritis. Arthritis Care Res (Hoboken). 2011;63(7):929–36. doi: 10.1002/acr.20497. [DOI] [PubMed] [Google Scholar]
- 16.Wallace CA, Ruperto N, Giannini E, Childhood Arthritis and Rheumatology Research Alliance, Pediatric Rheumatology International Trials Organization, Pediatric Rheumatology Collaborative Study Group Preliminary criteria for clinical remission for select categories of juvenile idiopathic arthritis. J Rheumatol. 2004;31(11):2290–4. [PubMed] [Google Scholar]
- 17.Viola S, Felici E, Magni-Manzoni S, Pistorio A, Buoncompagni A, Ruperto N, et al. Development and validation of a clinical index for assessment of long-term damage in juvenile idiopathic arthritis. Arthritis Rheum. 2005;52(7):2092–2102. doi: 10.1002/art.21119. [DOI] [PubMed] [Google Scholar]
- 18.Fries JF, Spitz P, Kraines RG, Holman HR. Measurement of patient outcome in arthritis. Arthritis Rheum. 1980;23(2):137–145. doi: 10.1002/art.1780230202. [DOI] [PubMed] [Google Scholar]
- 19.Ruperto N, Ravelli A, Levinson JE, Shear ES, Murray K, Link Tague B, et al. Long-term health outcomes and quality of life in American and Italian inception cohorts of patients with juvenile rheumatoid arthritis. II. Early predictors of outcome. J Rheumatol. 1997;24(5):952–8. [PubMed] [Google Scholar]
- 20.Ferreira PL. Development of the Portuguese version of MOS SF-36. Part II –Validation tests. Acta Med Port. 2000;13(3):119–27. [PubMed] [Google Scholar]
- 21.Goettel AM, DeClercq J, Choi L, Graham TB, Mitchell AA. Efficacy and safety of abatacept, adalimumab, and etanercept in pediatric patients with Juvenile idiopathic arthritis. J Pediatr Pharmacol Ther. 2021;26(2):157–162. doi: 10.5863/1551-6776-26.2.157. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Onel KB, Horton DB, Lovell DJ, Shenoi S, Cuello CA, Angeles-Han ST, et al. 2021 American College of rheumatology guideline for the treatment of Juvenile idiopathic arthritis: therapeutic approaches for oligoarthritis, temporomandibular joint arthritis, and systemic Juvenile idiopathic arthritis. Arthritis Rheumatol. 2022;74(4):553–569. doi: 10.1002/art.42037. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Ringold S, Angeles-Han ST, Beukelman T, Lovell D, Cuello CA, Becker ML, et al. 2019 American College of rheumatology/arthritis foundation guideline for the treatment of Juvenile idiopathic arthritis: therapeutic approaches for non-systemic polyarthritis, sacroiliitis, and enthesitis. Arthritis Care Res (Hoboken) 2019;71(6):717–734. doi: 10.1002/acr.23870. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.2016 update of the Portuguese recommendations for the use of biological therapies in children and adolescents with Juvenile Idiopathic Arthritis - PubMed. Cited 2022 Sep 15. Available from: https://pubmed.ncbi.nlm.nih.gov/27770754/. [PubMed]
- 25.Raab A, Sengler C, Niewerth M, Klotsche J, Horneff G, Zink A, et al. Comorbidity profiles among adult patients with juvenile idiopathic arthritis: results of a biologic register. Clin Exp Rheumatol. 2013;31(5):796–802. [PubMed] [Google Scholar]
- 26.BethencourtBaute JJ, Sanchez-Piedra C, Ruiz-Montesinos D, Medrano San Ildefonso M, Rodriguez-Lozano C, Perez-Pampin E, et al. Persistence and adverse events of biological treatment in adult patients with juvenile idiopathic arthritis: results from BIOBADASER. Arthritis Res Ther. 2018;20(1):227. doi: 10.1186/s13075-018-1728-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Amine B, Rostom S, Benbouazza K, Abouqal R, Hajjaj-Hassouni N. Health related quality of life survey about children and adolescents with juvenile idiopathic arthritis. Rheumatol Int. 2009;29(3):275–279. doi: 10.1007/s00296-008-0672-y. [DOI] [PubMed] [Google Scholar]
- 28.Barth S, Haas JP, Schlichtiger J, Molz J, Bisdorff B, Michels H, et al. Long-term health-related quality of life in German patients with Juvenile idiopathic arthritis in comparison to German general population. PLoS One. 2016;11(4):e0153267. doi: 10.1371/journal.pone.0153267. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Oen K, Guzman J, Dufault B, Tucker LB, Shiff NJ, Duffy KW, et al. Health-related quality of life in an inception cohort of children with Juvenile idiopathic arthritis: a longitudinal analysis. Arthritis Care Res (Hoboken) 2018;70(1):134–144. doi: 10.1002/acr.23236. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
Data is available upon request.


