Abstract
Staphylococcus lugdunensis is being increasingly reported as a pathogen with an outcome resembling that of S. aureus rather than coagulase-negative staphylococci. Recent local isolates exhibited colonial variation that delayed identification and interpretation of clinical significance. Until now previous descriptions have not emphasized colonial variation as an important identifying characteristic of S. lugdunensis.
Since its original description in 1988 (4), Staphylococcus lugdunensis has been reported in over 30 cases of endocarditis. Most reports of S. lugdunensis endocarditis have emphasised the aggressive nature of the infection and the problems associated with its identification. We made an observation (the present study and reference 13) regarding the varying colonial morphology of two invasive isolates of this species, and further data from Royal Brisbane Hospital and Gold Coast Hospital has confirmed this feature. We propose that the characteristic of colonial variation may be a common feature of S. lugdunensis, and increased awareness of this characteristic should be helpful in earlier recognition of the pathogen and appropriate management of the infection.
A 59-year-old man with Down’s Syndrome presented with a 6-week history of lethargy and weight loss and 1 week of rigors. Initial blood tests revealed a raised leukocyte count of 15.7 × 109/liter (reference range, 4.0 × 109 to 11.0 × 109/liter) with 84% neutrophils, a hemoglobin level of 121 g/liter (reference range, 132 to 180 g/liter), a platelet count of 139 × 109/liter (reference range, 150 × 109 to 400 × 109/liter), an erythrocyte sedimentation rate (ESR) of 79 mm/h (reference range, 1 to 30 mm/h), a creatinine level of 128 μmol/liter (reference range, 60 to 120 μmol/liter), and an albumin level of 29 g/liter (reference range, 35 to 50 g/liter). A midstream urine specimen showed less than 20 leucocytes/μl and was culture negative. One week later the creatinine level was 185 μmol/liter, the ESR was 106 mm/h, and the leukocyte count was within the reference range, with a normal differential. A single blood sample was collected, and both aerobic and anaerobic culture bottles yielded gram-positive cocci. A new ejection systolic cardiac murmur that radiated to the carotid arteries was noted, but no peripheral stigmata of endocarditis were seen. The patient was transferred to Royal Perth Hospital (RPH) with a provisional diagnosis of bacterial endocarditis. His oral temperature was 39.4°C, and the creatinine level had risen to 343 μmol/liter. Other investigations showed continuing hypoalbuminemia (albumin level, 27 g/liter), a hemoglobin level of 108 g/liter, a platelet count of 94 × 109/liter, a leukocyte count of 6.2 × 109/liter, an ESR of 61 mm/h, and a C-reactive protein level of 125 mg/liter (<10 mg/liter). Two more blood samples were obtained for culture. Endocarditis was suspected. Treatment with intravenous (i.v.) benzylpenicillin (1.2 g/6 h), vancomycin (1 g, stat), and gentamicin (120 mg, stat) was commenced. A transthoracic echocardiogram did not identify any valvular vegetations.
The next day, all four culture bottles contained gram-positive cocci morphologically resembling staphylococci. The results of subcultures on solid media suggested a mixed population of staphylococci. A “sweep” of colonies tested clumping factor negative, suggesting the presence of mixed coagulase-negative staphylococci (CoNS) that might be skin contaminants. On the same day, the blood culture isolate obtained prior to the patient’s admission to our institution was reported as a CoNS by another laboratory.
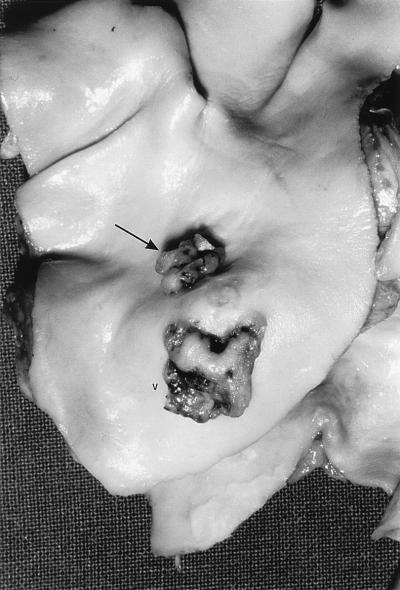
After 7 days, the patient’s renal function gradually improved after plateauing at a creatinine level of 626 μmol/liter. A transesophageal echocardiogram revealed a probable vegetation at the bifurcation of the pulmonary artery and a probable patent ductus arteriosus. In conjunction with the final identification of the blood culture isolate as S. lugdunensis in four out of four culture bottles, a diagnosis of endocarditis with renal failure secondary to immune complex glomerulonephritis was confirmed. The dosing interval of benzylpenicillin (1.2 g, i.v.) was reduced from 6 to 4 h, with a view to increasing the dose as renal function improved. After a further 7 days, the patient became markedly breathless and had abdominal distension with pain and his renal function deteriorated. A chest X ray suggested cavitation in the right lower zone with pulmonary venous congestion and right pleural effusion. Septic embolization to the lungs and possibly to the gastrointestinal tract and kidneys was suspected. The patient’s condition deteriorated rapidly, and he died 18 days after arrival at RPH. Postmortem examination revealed a patent ductus arteriosus with a luminal diameter of 4 mm and two vegetations in the pulmonary artery, with one being 10 mm in length and overlying the ostium of the patent ductus arteriosus. The second vegetation was 10 mm distal and measured 12 by 10 mm (Fig. 1). Microscopy of the vegetations revealed fibrin, gram-positive cocci, and polymorphonuclear leucocytes. Valvular material was not sent for culture. Sections of the kidneys, lungs, and skin revealed appearances consistent with thromboemboli.
FIG. 1.
Postmortem specimen of the patient in the present study. Vegetations on the pulmonary artery are shown. The larger vegetation (V) measures 12 mm in its greatest dimension. The smaller vegetation measures 10 mm in its greatest dimension and is situated around the orifice of the patent ductus arteriosus (arrow).
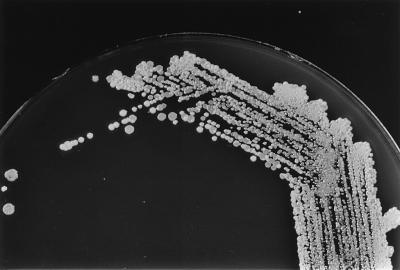
All four blood samples (cultured in BacT/Alert FAN [Organon Teknika Corporation, Durham, N.C.] aerobic and anaerobic bottles) collected at presentation (before antibiotic administration) yielded gram-positive cocci in clusters that were catalase positive, consistent with staphylococci. The results of subcultures on solid media (chocolate agar [Oxid GC agar base with growth supplement; Unipath Ltd., Basingstoke, United Kingdom] and horse blood agar) suggested a mixed population of staphylococci, with at least two different colonial morphologies evident from each blood culture (Fig. 2). Four single-colony subcultures of differing colonial morphotypes also produced colony variations that persisted in three serial subcultures of single-colony picks. All four strains tested gave identical reactions and susceptibility results. Clumping factor (coagulase rabbit plasma with EDTA; BBL Becton Dickinson, Cockeysville, Md.) was not present; the STAPH-A-LEX latex agglutination (Trinity Laboratories Inc., Raleigh, N.C.) and the tube coagulase tests were negative. The RBH-STAPH system that utilizes Rosco diagnostic tablets and susceptibilities to antibiotics for identification of staphylococci (11) showed the isolate to be furazolidone susceptible, desferrioxamine resistant, novobiocin susceptible, l-pyrrolidonyl-beta-napthylamide (PYR) positive, polymyxin resistant, and resistant to bacitracin (10 U); to exhibit a zone of inhibition with a diameter of <30 mm around the fosfomycin tablet; and to be ornithine decarboxylase (ODC) positive. These results were consistent with those for S. lugdunensis. The ID32 STAPH identification system (bioMérieux Vitek Inc., Hazelwood, Mo.) gave an identification profile of 56311460, consistent with a 99.9% positive identification of S. lugdunensis, with the key reactions being positive results for ODC, trehalose, and PYR. Susceptibility testing performed by disk diffusion (according to guidelines of the National Committee on Clinical Laboratory Standards [9]) showed the isolate to be susceptible to penicillin, oxacillin, methicillin, and vancomycin. The isolate was susceptible to all antimicrobial agents in the Vitek GPS-IX card test (bioMérieux Vitek Inc.) and was not a β-lactamase producer (when growth at the margin of the zone of inhibition around the penicillin disk was used to inoculate a nitrocefin disk [Cefinase; BBL Becton Dickinson] and inspected for chromogenic change for up to 1 h after inoculation). A nested PCR using primers specific for the S. aureus thermonuclease gene (nuc) and primers for the mecA gene encoding penicillin-binding protein 2a (1) was negative for both the nuc and mecA genes. The blood culture isolate reported as a CoNS by another laboratory was sent to our laboratory for confirmation of its identification. It gave results identical to those of our isolate in all tests, thus proving it was S. lugdunensis.
FIG. 2.
Marked colonial variation suggestive of mixed culture. Shown is a primary subculture of a positive blood culture (subcultured on chocolate agar) after overnight incubation at 35°C in 5% CO2.
This case adds to the growing literature of serious invasive S. lugdunensis infections. Most reports of S. lugdunensis infections comment on the problems in identifying this CoNS since it often gives positive results for clumping factor and other rapid agglutination test results suggestive of S. aureus. Some automated identification systems are unable to identify S. lugdunensis due to insufficient discriminatory biochemical reactions (especially insufficient ODC) or an inadequate database (6, 12, 13). S. lugdunensis appears to be an aggressive pathogen; similar to S. aureus, it causes an aggressive form of endocarditis with a poor clinical outcome. Including this case, there have been 34 cases of S. lugdunensis endocarditis reported; of the patients involved in these cases, 19 (of which 7 died) had valve replacement surgery, and 13 (of which 11 died) did not have surgery. Reports on the remaining two patients did not indicate whether they had valvular surgery or not; one died, and the outcome of the other was not given. The poor outcomes of S. lugdunensis endocarditis are in marked contrast to the better outcome generally associated with endocarditis caused by other CoNS species (2, 7, 11, 12).
We believe that previous studies of S. lugdunensis infections may have underreported the characteristic of colony variation seen in this species. The original description of the species (4) and only three other reports have described colony variation in S. lugdunensis isolates (3, 5, 6). The initial description of the species mentioned colony variation for 3 of the 11 strains reported, so different colony morphotypes may not be a consistent feature of the species. However, we suspect that other reports may not have mentioned colony variation. This is likely, since the feature was omitted in a previous report from Royal Perth Hospital (13).
Isolates of CoNS species collected from Royal Brisbane Hospital and Gold Coast Hospital exhibit different colony morphology characteristics depending on the species and the duration of incubation and subculture (Table 1). A summary of the findings is as follows. (i) S. lugdunensis, S. capitis, and S. hominis have a significant percentage of isolates with mixed morphotypes on both horse and sheep blood agar. Other CoNS species have a lower percentage of mixed morphotypes or no colonial variation at all. (ii) Colony variation essentially disappears for S. capitis and S. hominis after extended incubation or subculture. Mixed morphotypes were noted to be more persistent through incubation and subculture in S. lugdunensis strains. (iii) Preceding antimicrobial therapy may play a role in producing colony variation in S. lugdunensis (although in the present case, antimicrobials were not administered before the blood samples from which S. lugdunensis was isolated were collected). Insufficient clinical information regarding preceding antimicrobial exposure was collected for other CoNS species exhibiting colony variation.
TABLE 1.
CoNS species and colony variation
| CoNS species | Total no. of isolates | Isolates exhibiting colony variation after:
|
|||
|---|---|---|---|---|---|
| 24-h incubation (n [% of total]) | 48-h incubation (n) | Subculture and 24-h incubation (n) | Subculture and 48-h incubation (n) | ||
| S. lugdunensis | 9 | 4a (44.4) | 3 | 3 | 2 |
| S. epidermidis | 83 | 6 (7.2) | 1 | 0 | 0 |
| S. hominis | 16 | 5 (33.3) | 2 | 1 | 1 |
| S. capitis | 13 | 4 (30.8) | 1 | 0 | 0 |
| S. haemolyticus | 31 | 1 (3.2) | 0 | 0 | 0 |
| S. warneri | 18 | 1 (5.5) | 0 | 0 | 0 |
| S. saprophyticus | 30 | 0 | 0 | 0 | 0 |
| S. schleiferi | 3 | 0 | 0 | 0 | 0 |
| S. xylosus | 3 | 0 | 0 | 0 | 0 |
| S. simulans | 7 | 0 | 0 | 0 | 0 |
| S. auricularis | 1 | 0 | 0 | 0 | 0 |
Seven of the nine S. lugdunensis isolates, including these four strains exhibiting colony variation, had been exposed to antimicrobials before the specimen was collected.
We would be interested to learn whether previous authors have noted colony variations in their S. lugdunensis isolates but did not report it. We wish to draw attention to this characteristic in order to facilitate the rapid diagnosis of S. lugdunensis infections. It is hoped that quicker recognition of this species will lead to earlier administration of appropriate therapy with a better outcome (6, 8).
Acknowledgments
We thank Marsali Newman and Cecily Metcalf for the postmortem specimens and descriptions. We also thank the Medical Illustrations Department of Royal Perth Hospital for excellent photographic work.
REFERENCES
- 1.Coombs G W, Kay I D, Pearman J W, Christiansen K J. Programme and abstracts of the 20th International Congress of Chemotherapy. Sydney, Australia: International Congress of Chemotherapy; 1997. The role of multiplex mecA/nuc PCR for routine detection of methicillin resistance in staphylococci, abstr. 2292; p. 38. [Google Scholar]
- 2.De Hondt G, Ieven M, Vandermersch C, Colaert J. Destructive endocarditis caused by Staphylococcus lugdunensis. Case report and review of the literature. Acta Clin Belg. 1997;52:27–30. doi: 10.1080/17843286.1997.11718547. [DOI] [PubMed] [Google Scholar]
- 3.Fleurette J, Bes M, Brun Y, Freney J, Forey F, Coulet M, Reverdy M E, Etienne J. Clinical isolates of Staphylococcus lugdunensis and S. schleiferi: bacteriological characteristics and susceptibility to antimicrobial agents. Res Microbiol. 1989;140:107–118. doi: 10.1016/0923-2508(89)90044-2. [DOI] [PubMed] [Google Scholar]
- 4.Freney J, Brun Y, Bes M, Meugnier H, Grimont F, Grimont P A D, Nervi C, Fleurette J. Staphylococcus lugdunensis sp. nov. and Staphylococcus schleiferi sp. nov., two species from human clinical specimens. Int J Syst Bacteriol. 1988;38:168–172. [Google Scholar]
- 5.Herchline T E, Ayers L W. Occurrence of Staphylococcus lugdunensis in consecutive clinical cultures and relationship of isolation to infection. J Clin Microbiol. 1991;29:419–421. doi: 10.1128/jcm.29.3.419-421.1991. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Koh T W, Brecker S J D, Leyton C A. Successful treatment of Staphylococcus lugdunensis endocarditis complicated by multiple emboli: a case report and review of the literature. Int J Cardiol. 1996;55:193–197. doi: 10.1016/0167-5273(96)02679-4. [DOI] [PubMed] [Google Scholar]
- 7.Kralovic S M, Melin-Aldana H, Smith K K, Linnemann C C., Jr Staphylococcus lugdunensis endocarditis after tooth extraction. Clin Infect Dis. 1995;20:715–716. doi: 10.1093/clinids/20.3.715-a. [DOI] [PubMed] [Google Scholar]
- 8.Lessing M P A, Crook D W M, Bowler I C J, Gribbin B. Native-valve endocarditis caused by Staphylococcus lugdunensis. Q J Med. 1996;89:855–858. doi: 10.1093/qjmed/89.11.855. [DOI] [PubMed] [Google Scholar]
- 9.National Committee for Clinical Laboratory Standards. Performance standards for antimicrobial susceptibility testing. Sixth informational supplement. NCCLS document M100-S8. Villanova, Pa: National Committee for Clinical Laboratory Standards; 1998. [Google Scholar]
- 10.Nuttall N. Programme and abstracts of the 8th International Symposium on Staphylococci and Staphylococcal Infections. Aix-Les-Bains, France: Société Français de Microbiologie; 1996. RBH-STAPH: a simple, effective method to identify coagulase negative staphylococci of clinical significance, abstr. O-18; p. 67. [Google Scholar]
- 11.Paterson D L, Nuttall N. Serious infections due to Staphylococcus lugdunensis. Aust N Z J Med. 1997;27:591. doi: 10.1111/j.1445-5994.1997.tb00969.x. [DOI] [PubMed] [Google Scholar]
- 12.Vandenesch F, Etienne J, Reverdy M E, Eykyn S J. Endocarditis due to Staphylococcus lugdunensis: report of 11 cases and review. Clin Infect Dis. 1993;17:871–876. doi: 10.1093/clinids/17.5.871. [DOI] [PubMed] [Google Scholar]
- 13.Waterer G, Wilson R, Dimmitt S, Watson M. Staphylococcus lugdunensis endocarditis. Aust N Z J Med. 1997;27:84–85. doi: 10.1111/j.1445-5994.1997.tb00925.x. [DOI] [PubMed] [Google Scholar]