Abstract
Electroencephalography (EEG) has been the primary diagnostic tool in clinical epilepsy for nearly a century. Its review is performed using qualitative clinical methods that have changed little over time. However, the intersection of higher resolution digital EEG and analytical tools developed in the past decade invites a re-exploration of relevant methodology. In addition to the established spatial and temporal markers of spikes and high-frequency oscillations, novel markers involving advanced postprocessing and active probing of the interictal EEG are gaining ground. This review provides an overview of the EEG-based passive and active markers of cortical excitability in epilepsy and of the techniques developed to facilitate their identification. Several different emerging tools are discussed in the context of specific EEG applications and the barriers we must overcome to translate these tools into clinical practice.
Keywords: cortical excitability, EEG biomarkers, epilepsy, high-frequency oscillations, interictal spikes
1 |. INTRODUCTION
Electroencephalography (EEG) has been one of the primary diagnostic tools for epilepsy since 1924, when Hans Berger first recorded human EEG. By the 1950s, clinicians had identified several patterns that correlated with epilepsy: spikes, sharp waves, seizures, et cetera. Identifying these patterns by eye became the art of the epilepsy specialist, which has continued to be handed down to each subsequent generation as the de facto standard of care. The decades that have followed have seen remarkable changes in technology and analytical tools, yet these new tools remain largely unused by clinicians. One of the primary goals of the International Conference on Technology and Analysis of Seizures (ICTALS) community is to translate these new tools into epilepsy care.
The 2022 ICTALS meeting presented several emerging techniques to identify epileptic abnormalities. In this article, we present several strategies under development to identify brain cortex that is likely to be epileptic. These strategies focus on the concept of identifying cortical excitability using tools that are not available to clinicians in their current clinical practice. After presenting an overview of these techniques, we turn our focus to several key questions that must be addressed to allow these techniques to be utilized by clinicians. We limit our scope to biomarkers to identify epileptic cortex rather than biomarkers for seizure prediction, which are discussed in a separate article in this issue.1
2 |. CORTICAL EXCITABILITY
Cortical excitability can be measured as the varying cortical response to a fixed stimulation, repeated serially. Although this is possible noninvasively (transcranial magnetic stimulation [TMS]) and invasively during intracranial EEG (iEEG) investigations, cortical excitability is mostly inferred from the passive observation of interictal epileptiform discharges (spikes, sharp-waves, high-frequency oscillations [HFOs], etc.). These discharges tend to occur frequently, multiple times per day or hour, and have long been used as markers of epilepsy, both to confirm diagnosis and to localize epileptic brain tissue. Mechanistically, these discharges are thought to reflect abnormal firing of a group of neurons,2–4 a phenomenon often referred to as “hyperexcitability” or “hypersynchronicity” in the epilepsy literature. Given that the categories referred to by the latter terminology elude clear cutoffs (e.g., When is the brain too synchronized?5), we will here simply use the term “cortical excitability,” a property of an ensemble of neurons, which individually have their own neuronal excitability. Importantly, the degree of cortical excitability can vary both in space, with focal epileptic tissue being more excitable, and in time, delineating “at-risk” periods as well as brain states (Table 1).
TABLE 1.
Overview of passive and active markers of cortical excitability.
| Passive | Active | ||||
|---|---|---|---|---|---|
|
|
|
||||
| Spikes | HFOs | Background EEG | Single pulse | Train of pulses | |
| Current use | Clinical evaluation of the irritative zone for surgical planning | Research | Research | Trigger clinical seizures for surgical planning | |
| What is measured | Visual identification of spikes Spike quantity and network |
Visual identification of HFOs HFO quantity and network |
Spectral measures Connectivity measures |
CCEP amplitude Triggered epileptiform responses Connectivity measures |
Visual identification of seizure onset |
| What is localized | Spike zone | HFO zone | Not established | Not established | Seizure onset zone |
| Monitoring over time | Strong modulation by brain states: frequency, amplitude, and propagation variable across sleep– wake, circadian, and multidien cycles | Not established | Not established | ||
| Modulation by ASMs | Expected decrease | Expected decrease | Not established | Expected decrease in excitability | Expected increase in seizure threshold |
Abbreviations: ASM, antiseizure medication; CCEP, corticocortical evoked potential; EEG, electroencephalogram; HFO, high-frequency oscillation.
3 |. PASSIVE MARKERS OF EXCITABILITY
3.1 |. Spikes
Critics say that the time of spikes has come and gone, but recent advances in engineering have given spikes new relevance. Interictal spikes (here we include “sharp waves” and other epileptiform discharges seen with standard EEG electrodes) are brief paroxysmal discharges, facilitated by cortical excitability. Spikes are an attractive biomarker of epileptic regions for surgical planning. The shared excitability underlying both spikes and seizures has led some to suggest that we think of spikes as miniseizures, providing insight into the same disordered process that leads to seizures.6 Spikes occur much more frequently than seizures, and—unlike seizures—we do not need to wean antiseizure medication (ASM) or monitor patients in the epilepsy monitoring unit to induce spikes. In the 1950s and 1960s, the epilepsy community relied heavily on spikes to guide surgery. When neurosurgeons like Penfield and Rasmussen performed open resections for epilepsy using intraoperative electrocorticography (ECoG), they would resect tissue until the spikes disappeared, reporting good outcomes with this approach.7
Later in the 20th century, spikes fell out of favor. Rasmussen noted that some patients became seizure-free despite leaving behind cortex with spikes. Clinicians increasingly saw cases of spike–seizure discordance, including patients with bitemporal spikes rendered seizure-free with a unilateral temporal lobectomy.8 This led to the belief that the “epileptogenic zone” (EZ), the cortex we must remove to stop seizures, is typically a subset of the “irritative zone,” the cortex that generates spikes.6 Epileptologists and surgeons lamented that there was no way to distinguish the spikes that needed to be resected from the innocent spikes that are better left alone. As a result, spikes now play a limited role in presurgical evaluation for drug-resistant epilepsy. The current belief that spikes localize near the EZ, but are nonspecific, suggests that they are ripe for data mining.9 The useful information is there if we can learn how to decode it.
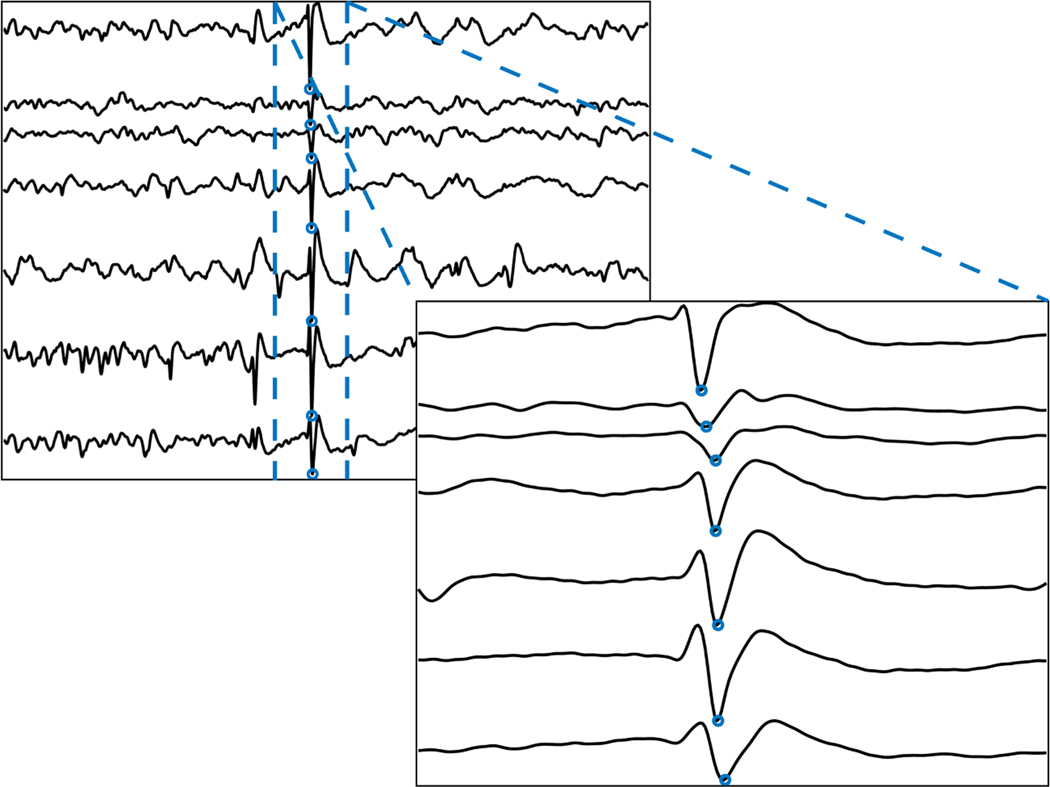
New tools have enhanced our ability to analyze spikes. Modern data storage now allows us to save the entirety of days-to-weeks-long scalp or iEEG recording at high sampling rates. Powerful computers can more quickly mine large amounts of interictal data. Sophisticated algorithms detect spikes as accurately as clinicians at speeds impossible for humans.10–12 These advances allow us to do what the human eye cannot: we can summarize the relative frequency of spikes in different brain regions, and how they change over time13; we can measure the precise timing of when a spike reaches different electrodes (Figure 1)14–17; and we can compare spike morphology across space and time.4
FIGURE 1.
Quantitative analysis reveals the latency of spike detection across electrodes.
Applying these tools has taught us that not all spikes are equal as surgical biomarkers. Combining analysis of time-varying spike rates with automated sleep staging demonstrated that spikes in non-rapid eye movement sleep are more frequent and better localize seizure generators than spikes occurring in wakefulness.18,19 Also, the earliest spike in a spike train better localizes the seizure onset zone than the later spikes.20 Combining EEG with functional magnetic resonance imaging reveals the area of maximal hemodynamic response to spikes, which also corresponds to the seizure onset zone.21 Spikes with co-occurring HFOs have different single-neuron correlates than spikes without HFOs, and these HFO-spikes better localize the seizure onset zone.4 We can now do what Rasmussen could not: we can say which spikes are informative of the EZ.
Within the limitations of retrospective studies, quantitative spike data appear to localize the EZ as well as clinical designations of the seizure onset zone.18 These advances in spike research are concurrent with increasing recognition of the limits of seizure data. For instance, chronic iEEG has revealed that independent bilateral seizures are common, and it often takes longer to record the first contralateral seizure than is feasible in the hospital setting.22 Together, these results argue that spikes should be more than ancillary data in surgical planning. The advances in engineering presented at ICTALS are elevating spikes to the same level as seizures.
3.2 |. High-frequency oscillations
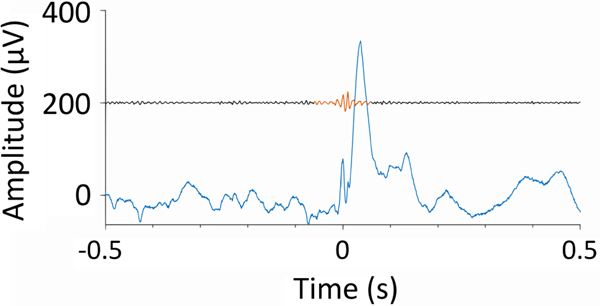
Research over the past years has shown HFOs to be a specific interictal biomarker of epileptic tissue in various patient cohorts, be it in presurgical iEEG to help assess the EZ23–25 (Figure 2), intraoperative ECoG,26 or noninvasive scalp EEG.27–33 Each modality addresses different clinical questions. For example, HFOs in noninvasive recordings hold promise for their widespread application in measuring seizure propensity, disease severity, and treatment response,27 thus improving prognostication of the disease state. These studies suggest that HFO-based information may improve current electrophysiological practice, which relies on the detection of seizures and interictal spikes. However, multiple studies challenge the overly optimistic view that HFOs can identify epileptogenic tissue using only a few minutes of interictal slow-wave sleep data.24,34–38 To meet these challenges, strategies have been proposed on how to validate biomarkers based on HFOs.39,40
FIGURE 2.
An interictal epileptic discharge (blue) recorded in human epileptic hippocampus and associated with a high-frequency oscillation (red, bandpass filtered 80–500 Hz and shifted by 200 μV) on its rising flank.
However, one aspect that has hindered the acceptance of HFOs is the inconsistency in not just how HFOs are analyzed but also in what goals we expect them to accomplish. For example, whether HFOs can be used intraoperatively to adjust resection boundaries34 is a different question than whether HFOs can predict surgery outcome.41 Part of the confusion is that HFOs themselves are not a biomarker; part of a biomarker’s definition is the specific clinical context, and HFOs are an electrographic element that may be useful in multiple clinical contexts. Even just considering HFOs measured during interictal periods of iEEG, at least three potential biomarkers may be considered: a diagnostic biomarker of the EZ (here defined as the cortical region necessary and sufficient for seizure initiation), a prognostic biomarker of surgery outcome, or a diagnostic biomarker of seizure onset zone. Of these three, only the latter two can be validated, as the EZ cannot be directly and objectively assessed. It can be argued that a biomarker of seizure onset zone is not needed, as it already is determined by standard clinical procedures and there is need to find unique information to improve outcomes, not merely the same information that often fails. Each HFO-based biomarker may have different optimal choices for data collection, HFO detection and analysis, and biomarker validation. Thus, as applies for all markers of cortical excitability, future progress will be facilitated by clearly demarcating which biomarker is being discussed in each paper and presentation. This will help prevent results from one HFO-based biomarker being falsely attributed to a different HFO-based biomarker.
A few other issues are worth noting. (1) HFO-based biomarkers should be validated against the measure appropriate for the specific biomarker. In some cases, this may require studies at the group level and use of surrogate benchmarks like the seizure onset zone or HFO defined by human experts. (2) Data collection and processing standards need to be developed for each biomarker. If interictal, intracranial data are used, we recommend analyzing multiday recordings to account for temporal variability.24,35,38,42 Many laboratories choose segments of slow wave sleep for HFO analysis,42–44 whereas others report HFO detection also at other states of vigilance.35,45 The low amplitude and high frequency of HFOs compared to spikes make it challenging to distinguish HFOs from artifacts. This requires appropriate hardware and highly standardized processing and detection standards. (3) HFOs should be detected by an automated algorithm, mitigating the influence of human bias and variability, while facilitating reproducible results and eliminating the prohibitive time constraints of human marking. Several software packages perform fast HFO detection.46–50 (4) It should be recognized that HFOs can be produced by physiological processes, and there is yet no consensus about how to identify a pathological versus physiological HFO. Development of methods that can disambiguate pathological from physiological HFOs is expected to greatly enhance HFO-based biomarkers. For clinical application, the focus should be on detecting HFOs that serve the intended purpose of the HFO, for example, as a biomarker for seizure outcome prediction. Here, it is not the morphology of the single HFO that defines its predictive power46 but rather the consistent appearance of HFOs over time24,38 or the association of an HFO with an interictal epileptic discharge.28,38 (5) Sharing benchmark datasets24 or multicenter studies36,42 serve to validate HFO detectors. (6) Hardware with sufficiently high sampling rate and low noise level must become widely available. Solving these issues will pave the way for HFO detection systems with CE/US Food and Drug Administration approval, which is a prerequisite for the broad application of HFO as a biomarker in epilepsy.
3.3 |. Interictal background EEG
Beyond clearcut discrete epileptic events such as spikes and HFOs, it is possible that the ongoing brain activity recorded in the interictal EEG is altered in epileptic parenchyma. One quantitative and objective computational approach is to measure EEG band power, although other approaches have been considered.51 The idea behind studying EEG band power is that abnormal cortical excitability is expected to have some spectral signature that deviates from normal spectral properties of a brain region. By quantifying this deviation, or abnormality, we capture abnormal cortical excitability.
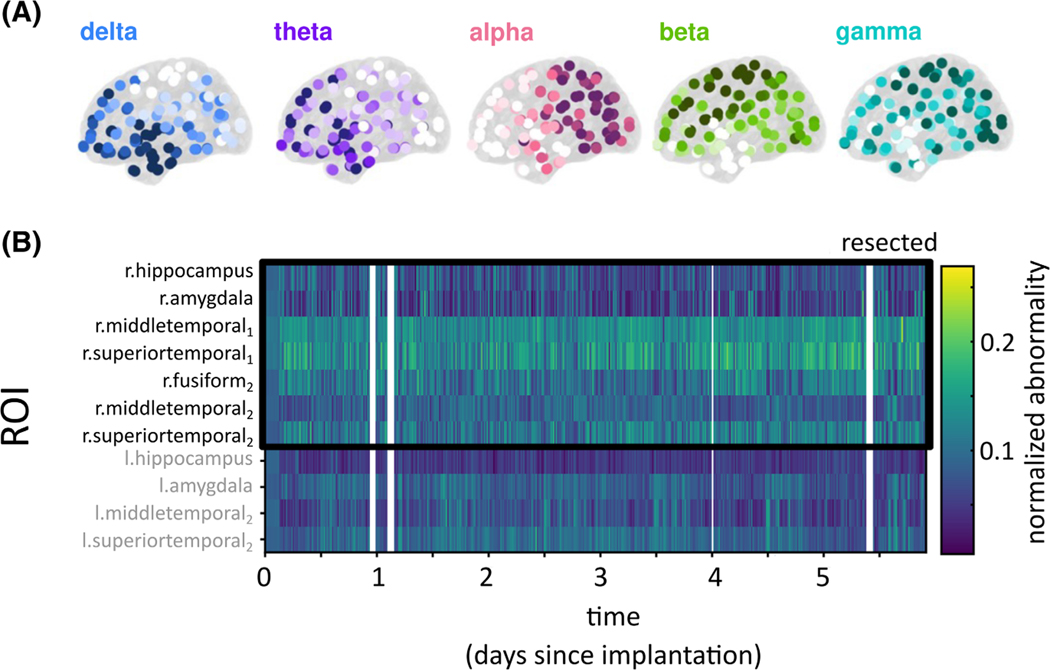
The normal spectral properties of different brain regions have been consistently described for decades. Properties of various modalities such as scalp EEG and magnetoencephalography show prominent patterns, or gradients including strong posterior alpha power, and high temporal delta power (see Markello et al.52 for the most recent maps). Past and recent work using iEEG has been able to reproduce those maps in a normative mapping approach (Figure 3A), where only “normal” interictal recording channels far away from the EZ were used.53–56 Furthermore, Taylor et al.55 reported that comparing a new patient’s interictal data to this normative map yields patient-specific abnormalities that can localize the epileptogenic tissue. Finally, in preliminary data, we also demonstrate that this approach can be applied over long time scales to highlight abnormal cortical excitability as a function of brain regions and time (Figure 3B), thus opening the possibility of timed interventions in the future. The utility of the described and similar computational approaches remains to be demonstrated in large multicenter studies retrospectively and prospectively, ideally with community-defined assessment approaches and outcome measures.
FIGURE 3.
Normative band power maps and abnormality mapping in an example patient. (A) Intracranial electroencephalographic (iEEG) normative band power maps in five frequency bands derived from >200 patients and >21 000 electrode contacts.55 (B) iEEG band power abnormality for an example patient calculated as a function of brain region and time. Band power abnormality is persistently higher in the resected tissue; the patient was seizure-free after surgery. However, band power abnormality can also fluctuate, for example, in the midtemporal area in a circadian rhythm. l, left; r, right; ROI, region of interest.
4 |. ACTIVE MARKERS OF EXCITABILITY
The idea to actively “probe” the epileptic brain and gauge its dynamical properties is not new,57,58 but much work remains to be done to determine the true potential of this approach. Tools to probe cortical excitability include noninvasive TMS and direct cortical stimulation.
TMS coupled with electromyography can evaluate the excitability of the corticospinal tracts (through motor-evoked potentials), whereas TMS coupled with EEG can evaluate the excitability within one lobe (e.g., frontal) or between lobes (e.g., frontoparietal through TMS-evoked potentials). This macroscopic view has confirmed that neuroactive drugs, including benzodiazepines, other ASMs, and antipsychotics, can broadly alter cortical excitability.59,60
Direct cortical stimulations are discussed in depth in another article in this special issue entitled “Stimulation to Probe, Excite, and Inhibit the Epileptic Brain” but are succinctly mentioned here to contrast them with passive markers of epilepsy. Corticocortical evoked potentials (CCEPs) evoked by single-pulse electrical stimulation of the cortex via intracranial electrodes offer a more refined spatial resolution at the level of individual gyri and sulci and of specific long-range connections. This mesoscopic view could in theory enable a more precise delineation of epileptic tissue, based on putatively enhanced excitability. Whether CCEPs to mild stimulations are increased within the EZ is yet unclear, despite a number of reports postulating this view.58,61–63 Advanced analyses of CCEPs include characterizing them as short oscillatory perturbations. Theory-based approaches have characterized CCEPs in terms of complexity and resonance properties of the underlying cortex.64
In the epileptic parenchyma, another phenomenon was found 20 years ago; in addition to eliciting CCEPs, stronger single-pulse stimulations can elicit delayed, epileptiform responses, an induced spike. Despite a landmark publication,65 and a few replications,66 this finding has not gained much traction in clinical practice, and the question of whether it applies more broadly to delineating epileptic cortex remains open.
On the other hand, seizure induction protocols have now been adopted by a number of hospitals. Here, a train of pulses is applied over a few seconds to entrain the cortex into a triggered seizure, and confirm its expected, patient-typical clinical correlates.67 The ability to trigger a seizure at the suspected seizure onset zone and not in other cortices contributes to the localizing value of actively probing the epileptic brain.
The advantage of direct cortical stimulation over TMS is clear from a spatial resolution perspective. However, an untapped diagnostic advantage is that of repeated stimulations that can offer nearly continuous probing of the epileptic brain (e.g., every 10–20 s). Beyond its therapeutic use in deep or responsive neurostimulation, repeated brain stimulation may open the path to true active monitoring of the time-varying function of a given circuit. Given recent advances in understanding pharmacoresistant epilepsy as cycles of enhanced seizure likelihood,68,69 monitoring cortical excitability may identify at-risk phases.
5 |. NETWORK ANALYSIS
Epilepsy is increasingly viewed as a brain network disorder,70–72 which makes visual inspection of iEEG data to identify epileptogenic regions challenging. As such, several studies have described computational approaches to view iEEG recordings from a network perspective and aim to offer fast and objective measures of epileptogenicity.73,74
One approach is to apply network-based measures to capture pairwise dependencies in the iEEG window of interest. Specifically, correlation or coherence between each pair of iEEG channels is computed and organized into an adjacency matrix, on which summary statistics are derived, including degree distribution and variants of nodal centrality.75–84 Such network-based measures have provided insights into the role of the EZ in the iEEG network, but researchers recognize that many different networks (adjacency matrices) can have identical summary statistics. Additionally, as iEEG sampling is not spatially uniform, and together with the brain’s complex morphology, network “hubs” may arise through higher spatial sampling or in areas of dense gray matter tissue. Finally, the sampling of the brain via iEEG is patient-specific, making direct comparisons of network properties across patients challenging. Wang et al.85 have thus proposed spatial normalizations and normative approaches (as in the above case of band power abnormality), which have recently shown some success.56
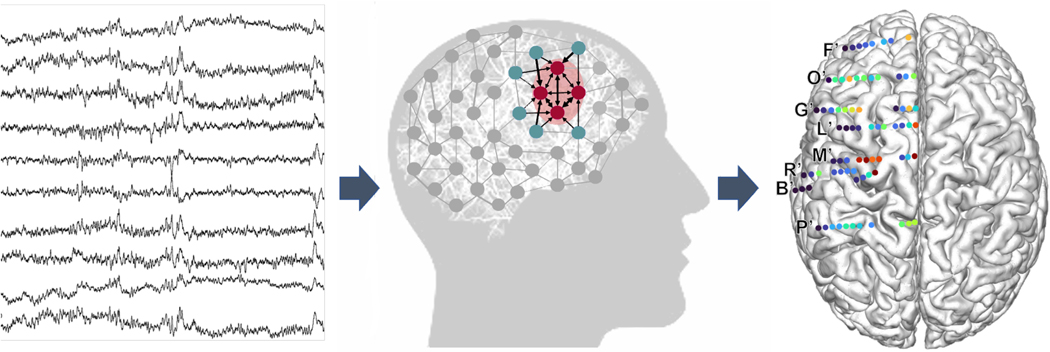
Another approach to studying epileptic networks is to assume that the iEEG recordings are observations of a dynamic networked “system.” These recordings are then used to perform “system identification” to create a generative model that can produce the observed iEEG data. The model parameters describe internal properties of the system, including bandwidth, stability, controllability, connectivity, fragility, and system gain.86 One recent study introduced the notion of “sources” and “sinks,” where sources are brain regions that significantly influence sink regions, and between seizures EZs are sinks (Figure 4).87 Sources and sinks are quantified by dynamic model parameters. Transfer functions and directed transfer functions64,88–91 (to name a few) and linear time varying models87,92 are two classes of dynamic models proposed to assist in localizing the EZ.
FIGURE 4.
Network analysis from intracranial EEG (iEEG) recordings, showing an example of data that could be shown to a clinician. Raw iEEG (left) is converted to network models (middle) with nodes quantified as “sinks” and “sources”. The pink nodes represent “sinks” as they are heavily influenced by other nodes (inward arrows) and are not influential themselves (no outgoing arrows). The blue nodes are “sources”. They heavily influence other nodes (only outgoing arrows). (Right) Source-sink index overlayed on implantation map of a patient. Letters represent the clinical labels of each electrode track. Colors represent values of the source-sink index, one of many possible iEEG markers. Here, red indicates the index is a stronger source; blue is a stronger sink.
6 |. EPILEPSY BIOMARKERS: QUESTIONS AND CONTROVERSIES
Epilepsy biomarkers are defined as objectively measurable characteristics of a normal or pathological process that can be measured and used to provide actionable clinical information, for example, to predict epilepsy development (epileptogenesis) or to monitor seizure propensity (ictogenesis). By identifying these biomarkers, we may be able to predict epilepsy development, to identify the tissue capable of generating spontaneous seizures, to measure disease progression, and to prognosticate pharmacoresistance.93 Although epilepsy is characterized by the presence of seizures, their occurrence alone does not necessarily satisfy the criteria for epilepsy diagnosis, as single seizures as the result of an acute brain insult are no rarity. Therefore, there is an urgent need for additional, more valid epilepsy markers, to allow for the identification and differentiation of epilepsy. EEG is the most accessible and established modality in epilepsy diagnostics, and signal changes, even in the absence of seizures, have been related to distinct disease states. Because no EEG biomarker has proven reliable in detecting and predicting epilepsy so far, it is imperative to develop novel approaches to extract more advanced biomarkers from the EEG signal.
Biomarker research has rapidly evolved in the past decades, and rigorous standards have been defined to facilitate the development of tools deriving from and dedicated to a specific clinical context. Biomarker performance should be judged depending on standards of statistical verification and validation conferring clinical utility. Biomarker research should also follow the new BEST (Biomarkers, Endpoints, and Other Tools) guidelines with a standardized description and a defined context of use.94 Although most studies measure biomarkers retrospectively, in clinical data collected and stored for later research use, it is clear that these conditions do not suffice to determine clinical usefulness. As described above in the HFO section, choice of biomarker has significant implications for rigor, reproducibility, and translation. Biomarkers should be easily, accurately, and reproducibly detectable but may be specific for a certain time window, condition, or syndrome. Furthermore, it cannot be ruled out that a single biomarker will prove insufficient and multiple biomarkers will be required to secure a reliable detection or prediction.1
How do we translate new epilepsy biomarkers into clinical practice? First, we must validate proposed biomarkers in large external datasets, which will require improved methods of data-sharing, or federated approaches to sharing code across centers.95,96 Next, one approach to translation is to build an easy-to-use tool for clinicians to predict important clinical outcomes, such as the 5-SENSE score, which incorporates scalp EEG spikes and other features to predict focality of the seizure onset zone.97 We can also package quantitative algorithms into submodules of commonly used commercial software, putting sophisticated techniques into the hands of clinicians. We may ultimately need prospective, randomized controlled trials, testing whether incorporating these biomarkers into surgical decision-making improves outcomes.34 Recent advances in translational work have given hope in the quest for EEG-based epilepsy biomarkers. Now we need to put the tools for mining them in the hands of epileptologists, and overcome the last barriers to translation by implementing technical advances in clinical practice. We need to persuade policy-makers that these “fancier tools” are needed and that the extra cost/work/devices are worth it for the benefit to our patients. Another barrier to overcome is the “black box problem,” which is met with skepticism by most clinicians. Earning their trust will require demonstrating clinical benefit in human trials and possibly also providing some physiological insight and/or normative values from the outputs of these tools, rather than simply presenting an abstract result.
7 |. CONCLUSIONS
Novel EEG-based passive and active markers of cortical excitability, as identified by emerging techniques, have the potential to open new avenues in epilepsy diagnosis and treatment and ultimately revolutionize patient care. Given the recent technological advances, this multidisciplinary area of research is currently located in the spotlight of human neuroscience. Further prospective, large-scale research is clearly needed to answer open questions and pave the way for the transfer of these biomarkers from research to clinical practice and implement them to the benefit of individual patients.
Key Points.
Advances in computation and engineering have enabled the development of novel quantitative biomarkers of cortical excitability in epilepsy
We discuss developments in passive (recorded in the resting state of the EEG) and active (stimulation-induced) biomarkers of excitability
Several barriers must be overcome to permit translation of these biomarkers to clinical practice
ACKNOWLEDGMENTS
We acknowledge the generous sponsors of the ICTALS 2022 conference in Bern, including the University of Bern, the Inselspital, University Hospital Bern, the Alliance for Epilepsy Research, the Swiss National Science Foundation, UCB, FHC, the Wyss Center for Bio and Neuro Engineering, the American Epilepsy Society, CURE Epilepsy foundation, Ripple Neuro, Sintetica, DIXI Medical, UNEEG Medical, and NeuroPace. We also thank Max for the amazing raclette on the mountainside. Let us do that again.
FUNDING INFORMATION
This work was supported by the SNF (208184, G.R.; Eccellenza 203339, M.O.B), Velux Stiftung (1232, M.O.B.), Vontobel Foundation (G.R.), EMDO Foundation (G.R.), Anna Mueller-Grocholski Foundation (G.R.), National Institutes of Health (K01ES026839, S.G.; R01NS094399, S.G., W.S.; K23NS121401, E.C.C.; R01NS125897, S.S.; R01NS125897, S.S.; R01NS102190, B.W.; R01NS102574, B.W.; R01NS107291, B.W.; RF1AG064312, B.W.; RF1NS120947, B.W.; R01AG073410, B.W.; R01HL161253, B.W.; R01NS126282, B.W.; R01AG073598, B.W.), NSF (2014431, B.W.), SNF (204651, J.S.), and Burroughs Wellcome Fund (E.C.C.).
Funding information
Anna Mueller-Grocholski Foundation; Burroughs Wellcome Fund; EMDO Stiftung; Velux Stiftung; National Heart, Lung, and Blood Institute, Grant/Award Number: R01HL161253; National Institute of Environmental Health Sciences, Grant/Award Number: K01ES026839; National Institute of Neurological Disorders and Stroke, Grant/Award Number: K23NS121401, R01NS094399, R01NS102190, R01NS102574, R01NS107291, R01NS125897, R01NS126282 and RF1NS120947; National Institute on Aging, Grant/Award Number: R01AG073410, R01AG073598 and RF1AG064312; National Science Foundation, Grant/Award Number: 2014431; Schweizerischer Nationalfonds zur Förderung der Wissenschaftlichen Forschung, Grant/Award Number: 204651 and SNSF 208184; Vontobel-Stiftung
Footnotes
CONFLICT OF INTEREST STATEMENT
S.G. and W.S. have a licensing agreement with Natus Medical. W.S. has a consulting agreement with Neuronostics. B.W. is a cofounder of Beacon Biosignals and receives royalties for authoring Pocket Neurology from Wolters Kluwer and Atlas of Intensive Care Quantitative EEG from Demos Medical. M.O.B. is a shareholder of Epios, a medical device company based in Geneva, Switzerland. E.C.C. has a consulting agreement with Epiminder. The remaining authors report no conflicts of interest. We confirm that we have read the Journal’s position on issues involved in ethical publication and affirm that this report is consistent with those guidelines.
REFERENCES
- 1.Andrzejak RG, Zaveri H, Schulze-Bonhage A, Leguia MG, Stacey WC, Richardson MP, et al. Seizure forecasting: where do we stand? Epilepsia. 2023. 10.1111/epi.17546 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Keller CJ, Truccolo W, Gale JT, Eskandar E, Thesen T, Carlson C, et al. Heterogeneous neuronal firing patterns during interictal epileptiform discharges in the human cortex. Brain. 2010;133(Pt 6):1668–81. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Curot J, Barbeau E, Despouy E, Denuelle M, Sol JC, Lotterie J-A, et al. Local neuronal excitation and global inhibition during epileptic fast ripples in humans. Brain. 2023;146(2):561–75. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Guth TA, Kunz L, Brandt A, Dümpelmann M, Klotz KA, Reinacher PC, et al. Interictal spikes with and without high-frequency oscillation have different single-neuron correlates. Brain. 2021;144(10):3078–88. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Jiruska P, de Curtis M, Jefferys JGR, Schevon CA, Schiff SJ, Schindler K. Synchronization and desynchronization in epilepsy: controversies and hypotheses. J Physiol. 2013;591(4):787–97. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Lüders HO, Najm I, Nair D, Widdess-Walsh P, Bingman W. The epileptogenic zone: general principles. Epileptic Disord. 2006;8(Suppl 2):S1–9. [PubMed] [Google Scholar]
- 7.Penfield W, Jasper H. Epilepsy and the functional anatomy of the human brain. Vol xv. Oxford: Little, Brown & Co.; 1954. p. 896 (Epilepsy and the functional anatomy of the human brain). [Google Scholar]
- 8.Hamer HM, Najm I, Mohamed A, Wyllie E. Interictal epileptiform discharges in temporal lobe epilepsy due to hippocampal sclerosis versus medial temporal lobe tumors. Epilepsia. 1999;40(9):1261–8. [DOI] [PubMed] [Google Scholar]
- 9.Thomas J, Kahane P, Abdallah C, Avigdor T, Zweiphenning WJEM, Chabardes S, et al. A subpopulation of spikes predicts successful epilepsy surgery outcome. Ann Neurol. 2023;93(3):522–35. [DOI] [PubMed] [Google Scholar]
- 10.Kural MA, Jing J, Fürbass F, Perko H, Qerama E, Johnsen B, et al. Accurate identification of EEG recordings with interictal epileptiform discharges using a hybrid approach: artificial intelligence supervised by human experts. Epilepsia. 2022;63(5):1064–73. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Thomas J, Jin J, Thangavel P, Bagheri E, Yuvaraj R, Dauwels J, et al. Automated detection of interictal epileptiform discharges from scalp electroencephalograms by convolutional neural networks. Int J Neural Syst. 2020;30(11):2050030. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Fürbass F, Kural MA, Gritsch G, Hartmann M, Kluge T, Beniczky S. An artificial intelligence-based EEG algorithm for detection of epileptiform EEG discharges: validation against the diagnostic gold standard. Clin Neurophysiol. 2020;131(6):1174–9. [DOI] [PubMed] [Google Scholar]
- 13.Conrad EC, Tomlinson SB, Wong JN, Oechsel KF, Shinohara RT, Litt B, et al. Spatial distribution of interictal spikes fluctuates over time and localizes seizure onset. Brain. 2020;143(2):554–69. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Tomlinson SB, Wong JN, Conrad EC, Kennedy BC, Marsh ED. Reproducibility of interictal spike propagation in children with refractory epilepsy. Epilepsia. 2019;60(5):898–910. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Maharathi B, Wlodarski R, Bagla S, Asano E, Hua J, Patton J, et al. Interictal spike connectivity in human epileptic neocortex. Clin Neurophysiol. 2019;130(2):270–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Maharathi B, Patton J, Serafini A, Slavin K, Loeb JA. Highly consistent temporal lobe interictal spike networks revealed from foramen ovale electrodes. Clin Neurophysiol. 2021;132(9):2065–74. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Smith EH, Liou J, Merricks EM, Davis T, Thomson K, Greger B, et al. Human interictal epileptiform discharges are bidirectional traveling waves echoing ictal discharges. Elife. 2022;11:e73541. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Klimes P, Cimbalnik J, Brazdil M, Hall J, Dubeau F, Gotman J, et al. NREM sleep is the state of vigilance that best identifies the epileptogenic zone in the interictal electroencephalogram. Epilepsia. 2019;60(12):2404–15. [DOI] [PubMed] [Google Scholar]
- 19.von Ellenrieder N, Peter-Derex L, Gotman J, Frauscher B. SleepSEEG: automatic sleep scoring using intracranial EEG recordings only. J Neural Eng. 2022;19(2):026057. [DOI] [PubMed] [Google Scholar]
- 20.Azeem A, von Ellenrieder N, Hall J, Dubeau F, Frauscher B, Gotman J. Interictal spike networks predict surgical outcome in patients with drug-resistant focal epilepsy. Ann Clin Transl Neurol. 2021;8(6):1212–23. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Khoo HM, von Ellenrieder N, Zazubovits N, He D, Dubeau F, Gotman J. The spike onset zone: the region where epileptic spikes start and from where they propagate. Neurology. 2018;91(7):e666–74. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.King-Stephens D, Mirro E, Weber PB, Laxer KD, Van Ness PC, Salanova V, et al. Lateralization of mesial temporal lobe epilepsy with chronic ambulatory electrocorticography. Epilepsia. 2015;56(6):959–67. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Jacobs J, Zijlmans M, Zelmann R, Chatillon C-E, Hall J, Olivier A, et al. High-frequency electroencephalographic oscillations correlate with outcome of epilepsy surgery. Ann Neurol. 2010;67(2):209–20. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Fedele T, Burnos S, Boran E, Krayenbühl N, Hilfiker P, Grunwald T, et al. Resection of high frequency oscillations predicts seizure outcome in the individual patient. Sci Rep. 2017;7:13836. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Nevalainen P, von Ellenrieder N, Klimeš P, Dubeau F, Frauscher B, Gotman J. Association of fast ripples on intracranial EEG and outcomes after epilepsy surgery. Neurology. 2020;95(16):e2 235–45. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.vant Klooster MA, van Klink NEC, WJEM Z, FSS L, Zelmann R, Ferrier CH, et al. Tailoring epilepsy surgery with fast ripples in the intraoperative electrocorticogram. Ann Neurol. 2017;81(5):664–76. [DOI] [PubMed] [Google Scholar]
- 27.Jacobs J, Zijlmans M. HFO to measure seizure propensity and improve prognostication in patients with epilepsy. Epilepsy Curr. 2020;20(6):338–47. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Cai Z, Sohrabpour A, Jiang H, Ye S, Joseph B, Brinkmann BH, et al. Noninvasive high-frequency oscillations riding spikes delineates epileptogenic sources. Proc Natl Acad Sci U S A. 2021;118(17):e2011130118. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Cserpan D, Rosch R, Lo Biundo SP, Sarnthein J, Ramantani G. Variation of scalp EEG high frequency oscillation rate with sleep stage and time spent in sleep in patients with pediatric epilepsy. Clin Neurophysiol. 2022;135:117–25. [DOI] [PubMed] [Google Scholar]
- 30.Cserpan D, Gennari A, Gaito L, Lo Biundo SP, Tuura R, Sarnthein J, et al. Scalp HFO rates are higher for larger lesions. Epilepsia Open. 2022;7(3):496–503. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Cserpan D, Gennari A, Gaito L, Lo Biundo SP, Tuura R, Sarnthein J, et al. Scalp HFO rates decrease after successful epilepsy surgery and are not impacted by the skull defect resulting from craniotomy. Sci Rep. 2022;12(1):1301. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Cserpan D, Boran E, Lo Biundo SP, Rosch R, Sarnthein J, Ramantani G. Scalp high-frequency oscillation rates are higher in younger children. Brain Commun. 2021;3(2):fcab052. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Boran E, Sarnthein J, Krayenbühl N, Ramantani G, Fedele T. High-frequency oscillations in scalp EEG mirror seizure frequency in pediatric focal epilepsy. Sci Rep. 2019;9(1):16560. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Zweiphenning W, van’t Klooster MA, van Klink NEC, Leijten FSS, Ferrier CH, Gebbink T, et al. Intraoperative electrocorticography using high-frequency oscillations or spikes to tailor epilepsy surgery in The Netherlands (the HFO trial): a randomised, single-blind, adaptive non-inferiority trial. Lancet Neurol. 2022;21(11):982–93. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Gliske SV, Irwin ZT, Chestek C, Hegeman GL, Brinkmann B, Sagher O, et al. Variability in the location of high frequency oscillations during prolonged intracranial EEG recordings. Nat Commun. 2018;9(1):2155. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Jacobs J, Wu JY, Perucca P, Zelmann R, Mader M, Dubeau F, et al. Removing high-frequency oscillations: a prospective multicenter study on seizure outcome. Neurology. 2018;91(11):e1040–52. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Roehri N, Pizzo F, Lagarde S, Lambert I, Nica A, McGonigal A, et al. High-frequency oscillations are not better biomarkers of epileptogenic tissues than spikes. Ann Neurol. 2018;83(1):84–97. [DOI] [PubMed] [Google Scholar]
- 38.Dimakopoulos V, Mégevand P, Boran E, Momjian S, Seeck M, Vulliémoz S, et al. Blinded study: prospectively defined high-frequency oscillations predict seizure outcome in individual patients. Brain Commun. 2021;3(3):fcab209. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Fedele T, Ramantani G, Sarnthein J. High frequency oscillations as markers of epileptogenic tissue – end of the party? Clin Neurophysiol. 2019;130(5):624–6. [DOI] [PubMed] [Google Scholar]
- 40.Chen Z, Maturana MI, Burkitt AN, Cook MJ, Grayden DB. High-frequency oscillations in epilepsy: what have we learned and what needs to be addressed. Neurology. 2021;96(9):439–48. [DOI] [PubMed] [Google Scholar]
- 41.Boran E, Ramantani G, Krayenbühl N, Schreiber M, König K, Fedele T, et al. High-density ECoG improves the detection of high frequency oscillations that predict seizure outcome. Clin Neurophysiol. 2019;130(10):1882–8. [DOI] [PubMed] [Google Scholar]
- 42.Dimakopoulos V, Gotman J, Stacey W, von Ellenrieder N, Jacobs J, Papadelis C, et al. Protocol for multicentre comparison of interictal high-frequency oscillations as a predictor of seizure freedom. Brain Commun. 2022;4(3):fcac151. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Chen X, Yin W, Chen S, Zhang W, Li H, Kuang H, et al. Loss of PIGK function causes severe infantile encephalopathy and extensive neuronal apoptosis. Hum Genet. 2021;140(5):791–803. [DOI] [PubMed] [Google Scholar]
- 44.von Ellenrieder N, Dubeau F, Gotman J, Frauscher B. Physiological and pathological high-frequency oscillations have distinct sleep-homeostatic properties. NeuroImage Clin. 2017;14:566–73. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Brázdil M, Pail M, Halámek J, Plešinger F, Cimbálník J, Roman R, et al. Very high-frequency oscillations: novel biomarkers of the epileptogenic zone. Ann Neurol. 2017;82(2):299–310. [DOI] [PubMed] [Google Scholar]
- 46.Burnos S, Frauscher B, Zelmann R, Haegelen C, Sarnthein J, Gotman J. The morphology of high frequency oscillations (HFO) does not improve delineating the epileptogenic zone. Clin Neurophysiol. 2016;127(4):2140–8. [DOI] [PubMed] [Google Scholar]
- 47.Remakanthakurup Sindhu K, Staba R, Lopour BA. Trends in the use of automated algorithms for the detection of high-frequency oscillations associated with human epilepsy. Epilepsia. 2020;61(8):1553–69. [DOI] [PubMed] [Google Scholar]
- 48.Burnos S, Hilfiker P, Sürücü O, Scholkmann F, Krayenbühl N, Grunwald T, et al. Human intracranial high frequency oscillations (HFOs) detected by automatic time-frequency analysis. PLoS One. 2014;9(4):e94381. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Gliske SV, Irwin ZT, Davis KA, Sahaya K, Chestek C, Stacey WC. Universal automated high frequency oscillation detector for real-time, long term EEG. Clin Neurophysiol. 2016;127(2):1057–66. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Sharifshazileh M, Burelo K, Sarnthein J, Indiveri G. An electronic neuromorphic system for real-time detection of high frequency oscillations (HFO) in intracranial EEG. Nat Commun. 2021;12(1):3095. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Stovall T, Hunt B, Glynn S, Stacey WC, Gliske SV. Interictal high frequency background activity as a biomarker of epileptogenic tissue. Brain Commun. 2021;3(3):fcab188. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Markello RD, Hansen JY, Liu Z-Q, Bazinet V, Shafiei G, Suárez LE, et al. Neuromaps: structural and functional interpretation of brain maps. Nat Methods. 2022;19(11):1472–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Groppe DM, Bickel S, Keller CJ, Jain SK, Hwang ST, Harden C, et al. Dominant frequencies of resting human brain activity as measured by the electrocorticogram. Neuroimage. 2013;79:223–33. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Frauscher B, von Ellenrieder N, Zelmann R, Doležalová I, Minotti L, Olivier A, et al. Atlas of the normal intracranial electroencephalogram: neurophysiological awake activity in different cortical areas. Brain. 2018;141(4):1130–44. [DOI] [PubMed] [Google Scholar]
- 55.Taylor PN, Papasavvas CA, Owen TW, Schroeder GM, Hutchings FE, Chowdhury FA, et al. Normative brain mapping of interictal intracranial EEG to localize epileptogenic tissue. Brain. 2022;145(3):939–49. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Bernabei JM, Sinha N, Arnold TC, Conrad E, Ong I, Pattnaik AR, et al. Normative intracranial EEG maps epileptogenic tissues in focal epilepsy. Brain. 2022;145(6):1949–61. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Freestone DR, Kuhlmann L, Grayden DB, Burkitt AN, Lai A, Nelson TS, et al. Electrical probing of cortical excitability in patients with epilepsy. Epilepsy Behav. 2011;22(Suppl 1):S110–8. [DOI] [PubMed] [Google Scholar]
- 58.Kalitzin S, Velis D, Suffczynski P, Parra J, da Silva FL. Electrical brain-stimulation paradigm for estimating the seizure onset site and the time to ictal transition in temporal lobe epilepsy. Clin Neurophysiol. 2005;116(3):718–28. [DOI] [PubMed] [Google Scholar]
- 59.Premoli I, Castellanos N, Rivolta D, Belardinelli P, Bajo R, Zipser C, et al. TMS-EEG signatures of GABAergic neurotransmission in the human cortex. J Neurosci. 2014;34(16):5603–12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Premoli I, Biondi A, Carlesso S, Rivolta D, Richardson MP. Lamotrigine and levetiracetam exert a similar modulation of TMS-evoked EEG potentials. Epilepsia. 2017;58(1):42–50. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Mouthaan BE, vant Klooster MA, Keizer D, Hebbink GJ, Leijten FSS, Ferrier CH, et al. Single pulse electrical stimulation to identify epileptogenic cortex: clinical information obtained from early evoked responses. Clin Neurophysiol. 2016;127(2):1088–98. [DOI] [PubMed] [Google Scholar]
- 62.Iwasaki M, Enatsu R, Matsumoto R, Novak E, Thankappen B, Piao Z, et al. Accentuated cortico-cortical evoked potentials in neocortical epilepsy in areas of ictal onset. Epileptic Disord. 2010;12(4):292–302. [DOI] [PubMed] [Google Scholar]
- 63.Guo Z-H, Zhao B-T, Toprani S, Hu W-H, Zhang C, Wang X, et al. Epileptogenic network of focal epilepsies mapped with cortico-cortical evoked potentials. Clin Neurophysiol. 2020;131(11):2657–66. [DOI] [PubMed] [Google Scholar]
- 64.Smith RJ, Hays MA, Kamali G, Coogan C, Crone NE, Kang JY, et al. Stimulating native seizures with neural resonance: a new approach to localize the seizure onset zone. Brain. 2022;145(11):3886–900. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Valentín A, Alarcón G, Honavar M, García Seoane JJ, Selway RP, Polkey CE, et al. Single pulse electrical stimulation for identification of structural abnormalities and prediction of seizure outcome after epilepsy surgery: a prospective study. Lancet Neurol. 2005;4(11):718–26. [DOI] [PubMed] [Google Scholar]
- 66.Davis TS, Rolston JD, Bollo RJ, House PA. Delayed high-frequency suppression after automated single-pulse electrical stimulation identifies the seizure onset zone in patients with refractory epilepsy. Clin Neurophysiol. 2018;129(11):2466–74. [DOI] [PubMed] [Google Scholar]
- 67.Cuello Oderiz C, von Ellenrieder N, Dubeau F, Eisenberg A, Gotman J, Hall J, et al. Association of Cortical Stimulation-Induced Seizure with Surgical Outcome in patients with focal drug-resistant epilepsy. JAMA Neurol. 2019;76(9):1070–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Baud MO, Ramantani G. Seizure remissions and cycles in pediatric focal epilepsy. Dev Med Child Neurol. 2022;65:308–9. [DOI] [PubMed] [Google Scholar]
- 69.Saito Y, Sugai K, Iwasaki M, Atobe M, Sato N, Kakita A, et al. Periodic cycles of seizure clustering and suppression in children with epilepsy strongly suggest focal cortical dysplasia. Dev Med Child Neurol. 2022;65:431–6. [DOI] [PubMed] [Google Scholar]
- 70.Spencer DD, Gerrard JL, Zaveri HP. The roles of surgery and technology in understanding focal epilepsy and its comorbidities. Lancet Neurol. 2018;17(4):373–82. [DOI] [PubMed] [Google Scholar]
- 71.Bartolomei F, Lagarde S, Wendling F, McGonigal A, Jirsa V, Guye M, et al. Defining epileptogenic networks: contribution of SEEG and signal analysis. Epilepsia. 2017;58(7):1131–47. [DOI] [PubMed] [Google Scholar]
- 72.Panzica F, Varotto G, Rotondi F, Spreafico R, Franceschetti S. Identification of the epileptogenic zone from stereo-EEG signals: a connectivity-graph theory approach. Front Neurol. 2013;4:175. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.Makhalova J, Medina Villalon S, Wang H, Giusiano B, Woodman M, Bénar C, et al. Virtual epileptic patient brain modeling: relationships with seizure onset and surgical outcome. Epilepsia. 2022;63(8):1942–55. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74.Balatskaya A, Roehri N, Lagarde S, Pizzo F, Medina S, Wendling F, et al. The “connectivity epileptogenicity index “ (cEI), a method for mapping the different seizure onset patterns in StereoElectroEncephalography recorded seizures. Clin Neurophysiol. 2020;131(8):1947–55. [DOI] [PubMed] [Google Scholar]
- 75.Shah P, Ashourvan A, Mikhail F, Pines A, Kini L, Shinohara RT, et al. Local structural connectivity directs seizure spread in focal epilepsy [internet]. Biorxiv. 2018. 10.1101/406793 [DOI] [Google Scholar]
- 76.Li A, Chennuri B, Subramanian S, Yaffe R, Gliske S, Stacey W, et al. Using network analysis to localize the epileptogenic zone from invasive EEG recordings in intractable focal epilepsy. Netw Neurosci. 2018;2(2):218–40. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77.Li Y-H, Ye X-L, Liu Q-Q, Mao J-W, Liang P-J, Xu J-W, et al. Localization of epileptogenic zone based on graph analysis of stereo-EEG. Epilepsy Res. 2016;128:149–57. [DOI] [PubMed] [Google Scholar]
- 78.Burns SP, Santaniello S, Yaffe RB, Jouny CC, Crone NE, Bergey GK, et al. Network dynamics of the brain and influence of the epileptic seizure onset zone. Proc Natl Acad Sci U S A. 2014;111(49):E5321–30. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79.Yaffe R, Burns S, Gale J, Park H-J, Bulacio J, Gonzalez-Martinez J, et al. Brain state evolution during seizure and under anesthesia: a network-based analysis of stereotaxic eeg activity in drug-resistant epilepsy patients. Annu Int Conf IEEE Eng Med Biol Soc. 2012;2012:5158–61. [DOI] [PubMed] [Google Scholar]
- 80.Yaffe RB, Borger P, Megevand P, Groppe DM, Kramer MA, Chu CJ, et al. Physiology of functional and effective networks in epilepsy. Clin Neurophysiol. 2015;126(2):227–36. [DOI] [PubMed] [Google Scholar]
- 81.Khambhati AN, Davis KA, Lucas TH, Litt B, Bassett DS. Virtual cortical resection reveals push-pull network control preceding seizure evolution. Neuron. 2016;91(5):1170–82. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 82.Khambhati AN, Davis KA, Oommen BS, Chen SH, Lucas TH, Litt B, et al. Dynamic network drivers of seizure generation, propagation and termination in human neocortical epilepsy. PLoS Comput Biol. 2015;11(12):e1004608. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 83.Bassett DS, Sporns O. Network neuroscience. Nat Neurosci. 2017;20(3):353–64. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 84.Abreu R, Leal A, Figueiredo P. Identification of epileptic brain states by dynamic functional connectivity analysis of simultaneous EEG-fMRI: a dictionary learning approach. Sci Rep. 2019;9(1):638. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 85.Wang Y, Sinha N, Schroeder GM, Ramaraju S, McEvoy AW, Miserocchi A, et al. Interictal intracranial electroencephalography for predicting surgical success: the importance of space and time. Epilepsia. 2020;61(7):1417–26. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 86.Ogata K. Modern control engineering. Vol 5. Upper Saddle River, NJ: Prentice Hall; 2010. [Google Scholar]
- 87.Gunnarsdottir KM, Li A, Smith RJ, Kang J-Y, Korzeniewska A, Crone NE, et al. Source-sink connectivity: a novel interictal EEG marker for seizure localization. Brain. 2022;145(11): 3901–15. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 88.Wilke C, van Drongelen W, Kohrman M, He B. Neocortical seizure foci localization by means of a directed transfer function method. Epilepsia. 2010;51(4):564–72. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 89.Kamali G, Smith RJ, Hays M, Coogan C, Crone NE, Kang JY, et al. Transfer function models for the localization of seizure onset zone from Cortico-cortical evoked potentials. Front Neurol. 2020;11:579961. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 90.Swiderski B, Osowski S, Cichocki A, Rysz A. Single-class SVM and directed transfer function approach to the localization of the region containing epileptic focus. Neurocomputing. 2009;72(7):1575–83. [Google Scholar]
- 91.Franaszczuk PJ, Bergey GK. Application of the directed transfer function method to mesial and lateral onset temporal lobe seizures. Brain Topogr. 1998;11(1):13–21. [DOI] [PubMed] [Google Scholar]
- 92.Li A, Huynh C, Fitzgerald Z, Cajigas I, Brusko D, Jagid J, et al. Neural fragility as an EEG marker of the seizure onset zone. Nat Neurosci. 2021;24(10):1465–74. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 93.Engel J Jr, Pitkänen A, Loeb JA, Edward Dudek F, Bertram EH III, Cole AJ, et al. Epilepsy biomarkers. Epilepsia. 2013;54(s4):61–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 94.FDA-NIH Biomarker Working Group. BEST (Biomarkers, EndpointS, and other tools) resource [Internet]. Silver Spring, Bethesda: Food and Drug Administration (US); National Institutes of Health (US); 2016. [PubMed] [Google Scholar]
- 95.Wagenaar N, van den Berk DJM, Lemmers PMA, van der Aa NE, Dudink J, van Bel F, et al. Brain activity and cerebral oxygenation after perinatal arterial ischemic stroke are associated with neurodevelopment. Stroke. 2019;50(10):2668–76. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 96.Assistance Publique Hopitaux De Marseille. Improving EPilepsy Surgery Management and progNOsis Using Virtual Epileptic Patient Software (VEP) [Internet]. clinicaltrials.gov; 2022. [cited 2023]. Report No.: NCT03643016. https://clinicaltrials.gov/ct2/show/NCT03643016 [Google Scholar]
- 97.Astner-Rohracher A, Zimmermann G, Avigdor T, Abdallah C, Barot N, Brázdil M, et al. Development and validation of the 5-SENSE score to predict focality of the seizure-onset zone as assessed by Stereoelectroencephalography. JAMA Neurol. 2022;79(1):70–9. [DOI] [PMC free article] [PubMed] [Google Scholar]