Abstract
Severe rhabdomyolysis frequently results in acute kidney injury (AKI) due to myoglobin accumulation with the need of kidney replacement therapy (KRT). The present study investigated whether the application of Cytosorb® (CS) led to an increased rate of kidney recovery in patients with KRT due to severe rhabdomyolysis. Adult patients with a myoglobin-concentration >10,000 ng/ml and KRT were included from 2014 to 2021. Exclusion criteria were chronic kidney disease and CS-treatment before study inclusion. Groups 1 and 2 were defined as KRT with and without CS, respectively. The primary outcome parameter was independence from KRT after 30 days. Propensity score (PS) matching was performed (predictors: myoglobin, SAPS-II, and age), and the chi2-test was used. 35 pairings could be matched (mean age: 57 vs. 56 years; mean myoglobin: 27,218 vs. 26,872 ng/ml; mean SAPS-II: 77 vs. 76). The probability of kidney recovery was significantly (p = .04) higher in group 1 (31.4 vs. 11.4%, mean difference: 20.0%, odds ratio (OR): 3.6). Considering patients who survived 30 days, kidney recovery was also significantly (p = .03) higher in patients treated with CS (61.1 vs. 23.5%, mean difference: 37.6%, OR: 5.1). In conclusion, the use of CS might positively affect renal recovery in patients with severe rhabdomyolysis. A prospective randomized controlled trial is needed to confirm this hypothesis.
Keywords: Rhabdomyolysis, myoglobin, kidney replacement therapy, blood purification, kidney recovery, cytosorb®
Introduction
Rhabdomyolysis occurs frequently in intensive care unit (ICU) patients, resulting in elevated myoglobin and creatinekinase (CK) concentrations in the blood [1]. There are different causes for the disintegration of the skeletal muscle, e.g., polytrauma, sepsis, hemorrhagic shock, or medication toxicity [2,3]. The accumulation of myoglobin can lead to an acute kidney injury (AKI) up to the requirement of dialysis. High volume turnover as well as forced alkaline diuresis might be two opportunities to prevent patients of the necessity of kidney replacement therapy (KRT) [4]. However, both methods are only objective when diuresis is preserved.
The degradation of CK occurs by organ-independent degradation in patients’ blood. In contrast, myoglobin is excreted renally [5]. The elevation of myoglobin can lead to kidney function impairment and kidney parenchymal damage through various pathophysiological mechanisms and its concentration correlates with the development of AKI [6]. Myoglobin accumulation in patients with kidney impairment can result in potentially permanent kidney failure due to the damage of the tubular epithelial cells in the kidney [7]. The problem is that myoglobin clearance decreases rapidly in patients with anuric kidney failure, but its extracorporeal elimination with standard dialysis membranes is not effective due to the high molecular weight of myoglobin of approximately 17 kDa [8].
Various methods for sufficient extracorporeal myoglobin elimination have been introduced into clinical routine in recent years. On the one hand, high cutoff (HCO) dialyzers and high permeability dialysis membranes offer the opportunity to remove medium-sized molecules such as myoglobin [9,10]. Its intermittent use seems to be preferable in this context [11]. Similarly, plasmapheresis and continuous veno-venous hemofiltration (CVVH) can be used to achieve myoglobin elimination [12,13].
Another method for forced extracorporeal myoglobin elimination is the use of the cytokine adsorber Cytosorb® (CS) (CytoSorbents Europe, Berlin, Germany). It was initially approved for the adsorption of cytokines but has also been licensed for the removal of myoglobin since 2019 [14]. Especially hydrophobic substances with a molecular weight of <60 kDa are bound to small beads, and a large adsorption capacity seems possible due to the presence of a surface area of 45,000 m2 [14]. In addition to case reports [15,16], an analysis from our group demonstrated a reduction in myoglobin in patients’ blood after the application of CS [17].
It is unclear for any of the above-mentioned procedures whether their use only affect laboratory parameters or if they improve patients’ outcome. In particular, kidney recovery from dialysis through the use of CS has not yet been investigated. To address this question, the effect of CS application on the primary outcome variable ‘kidney recovery at day 30’ – defined as independence from intermittent or continuous KRT following dialysis-requiring AKI – was investigated in patients with severe rhabdomyolysis using a propensity score (PS) matching analysis.
Materials and methods
Study setting
This was a monocentric, PS matching study, investigating the effect of CS therapy in critically ill patients with severe rhabdomyolysis. Patients treated between 2014 and 2021 at the anesthesiologic ICUs at LMU hospital in Munich were included. CS therapy was initiated or avoided according to the responsible physicians. Data from two clinical trials were evaluated to investigate the primary outcome variable ‘kidney recovery at day 30’. The local institutional review board approved the two studies (registration numbers 20-477 and 21-236, NCT04913298). Study one (20-477) was a retrospective analysis with the intention to evaluate myoglobin concentration in patients with rhabdomyolysis and renal failure. Study two (21-236) was a prospective trial to evaluate the adsorption capacity of CS in patients with severe rhabdomyolysis.
Laboratory measurements and data collection
All laboratory parameters were determined at the Institute of Laboratory Medicine, LMU Munich, using routine instruments and assays. For data evaluation, demographic data, clinical, and laboratory variables were collected from the laboratory and patient information system.
Study population
All patients with a myoglobin serum concentration > 10,000 ng/ml were screened retrospectively for study inclusion. Inclusion criteria were age > 18 years and the use of continuous kidney replacement therapy (KRT) in association to severe rhabdomyolysis. KRT was initiated by the attending physicians due to the AKI classification of the KDIGO [18]. All patients had an acute kidney injury stage 2 or 3, which was defined by both the creatinine clearance and the urinary output of the patient. High-flux dialysis (Fresenius Ultraflux® AV 1000S, surface area 1.4 m2, CVVHD CiCa® or CVVHDF MultiBic® as indicated by the physicians) was used. Exclusion criteria were the necessity of KRT before ICU admission, chronic kidney disease > G3a (KDIGO–classification) before ICU admission [19], and CS treatment or ongoing KRT >24 h before study inclusion. Patients were divided into two groups: group 1 included patients with KRT (high-flux dialysis) and CS treatment and group 2 included patients with KRT (high-flux dialysis) but without CS therapy. The day of study inclusion (d0) was in both groups <24 h after initiation of CKRT and therefore equally for all patients. Patients allocated to group 1 got the first CS treatment at d0 (within 24 h after starting CKRT). Based on the clinical standard, patients were initially (∼2014–2017) treated infrequently with CS to reduce myoglobin, as it was not an established therapeutic modality at that time. In the course, the therapy was established in routine and most patients with severe rhabdomyolysis were treated with CS. The indication to start CS treatment was a myoglobin serum concentration >10,000 ng/ml. CS was installed post-dialyzer in the CKRT-circuit for 12–24 h per treatment session. The median duration of treatment were two days (IQR 2–3 d) and a median of 3 (IQR 2–4) CS were used per patient. The primary outcome measure was ‘kidney recovery at day 30’, which was defined as the independence of intermittent or continuous KRT on day 30, lasting for at least 7 d [20].
Statistical analysis
Statistical analysis were performed using IBM SPSS statistics (Version 26.0., IBM Corp., Armonk, NY, USA). Differences in baseline parameters in both groups were detected using the chi2–test (nominal variables) or the Welch–test (other variables). PS matching (1:1) was performed to compare both groups. The Simplified Acute Physiology Score (SAPS) II, age, and myoglobin serum concentrations were used as predictors in the PS matching, as these parameters previously showed an effect on the recovery of kidney function or patients’ outcome [21–23]. The adjustment tolerance was <0.05, and the nearest neighbor method was used. The standardized difference ‘d’ (d = (mean A – mean B)/pooled standard deviation of both groups) was <10% after matching as a quality criterion [24]. The effect of CS treatment on kidney recovery on day 30 was investigated using the chi2–test. Differences in the myoglobin kinetics were detected using the Wilcoxon-test with associated samples.
Results
Selection of the study population
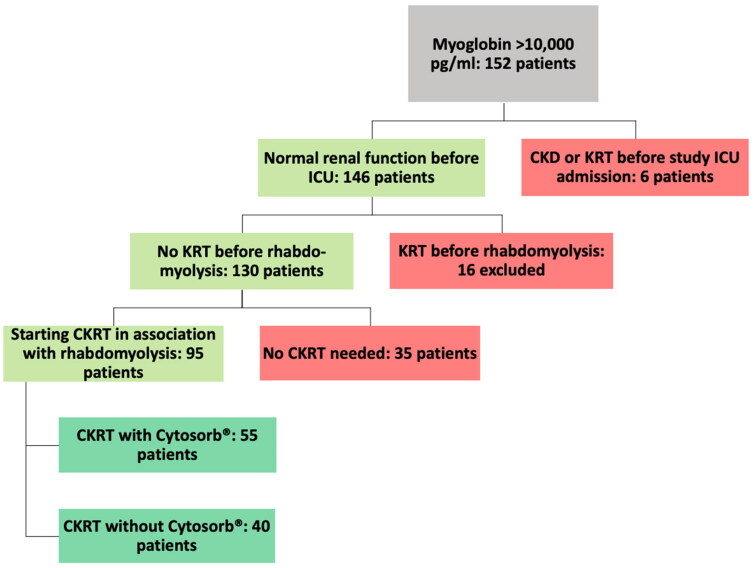
Figure 1 illustrates the selection of the study population based on the above-mentioned inclusion and exclusion criteria.
Figure 1.
Selection of the study population. ICU: intensive care unit; CKD: chronic kidney disease; ®KRT: (continuous) kidney replacement therapy
Demographic and clinical data
Table 1 shows the patient characteristics and laboratory measurements of the primary study population before PS matching.
Table 1.
Patient characteristics before PS matching.
| Group 1: n (%) or mean [SD] or median {IQR} | Group 2: n (%) or mean [SD] or median {IQR} | p-value (Welch /chi2-test) | |
|---|---|---|---|
| Number of patients | 55 (100) | 40 (100) | – |
| Age (years) | 54 [16] | 59 [18] | .21 |
| Male | 42 (76.4) | 27 (67.5) | .34 |
| BMI (kg/m2) | 27.1 [6.8] | 28.1 [6.1] | .48 |
| ECMO therapy d0 | 16 (30.1) | 11 (27.5) | .72 |
| SAPS II d0 | 78 [16] | 74 [18] | .21 |
| SAPS II d0 | 78 {70, 88} | 76 {63, 86} | |
| Mortality day 30 | 28 (50.9) | 22 (55.0) | .70 |
| Kidney recovery day 30 | 13 (26.6) | 4 (10.0) | .07 |
| Myoglobin d0 (ng/ml) | 38,042 [43,776] | 25,657 [19,876] | .08 |
| Myoglobin d0 (ng/ml) | 22,578 {12,391, 30,089} | 18,814 {13,030, 28,396} | |
| CK d0 (U/l) | 14,720 [28,270] | 7194 [11,411] | .07 |
| CK d0 (U/l) | 3806 {2024, 12,607} | 3818 {1687, 7644} |
BMI: body mass index; ECMO: extracorporeal membraneoxygenation; ICU: intensive care unit; SAPS: simplified acute physiology score; CK: creatine kinase; d0: study enrollment day.
Propensity score matching
PS matching was performed as described in the methods section. 35 pairs were successfully matched due to the abovementioned criteria. Table 2 shows the patient characteristics after PS matching, the dialysis modality in both groups, and the reasons for the admission to the ICU. The standardized difference for the matched parameters age, SAPS II d0, and myoglobin d0 was 6.2, 5.6, and 4.3%, respectively. All patients were critically ill (mean SAPS II 76), frequently treated with ECMO-therapy (approximately 27%), and had a high 30-day mortality.
Table 2.
Patient characteristics, dialysis modality, and reasons for the admission to the ICU after PS matching.
| Group 1: n (%) or mean [SD] | Group 2: n (%) or mean [SD] | p-value (Welch/chi2-test) | |
|---|---|---|---|
| Patient characteristics | |||
| Number of patients | 35 (100) | 35 (100) | – |
| Age (years) | 57 [15] | 56 [17] | .70 |
| Male | 26 (74.3) | 23 (65.7) | .43 |
| BMI (kg/m2) | 27.6 [7.0] | 28.0 [6.3] | .81 |
| ECMO therapy d0 | 9 (25.7) | 10 (28.6) | .79 |
| SAPS II d0 | 77 [17] | 76 [17] | .81 |
| Mortality day 30 | 17 (48.6) | 18 (51.4) | .81 |
| Kidney recovery day 30 | 11 (31.4) | 4 (11.4) | .04 |
| Dialysis modalities | |||
| Citrat/Heparin | 12 (34.3)/23 (65.7) | 19 (54.3)/16 (45.7) | .23 |
| Blood flow (ml/min) | 150 [68] | 130 [56] | .46 |
| Dialysate + (substituate) flow (ml/kg/h) | 36 [18] | 32 [13] | .29 |
| CVVHD/CVVHDF | 12 (34.3)/23 (65.7) | 19 (54.3)/16 (45.7) | .23 |
| Reasons for admission to the ICU | |||
| Acute respiratory distress syndrome | 9 (25.7) | 10 (28.6) | |
| Polytrauma | 5 (14.3) | 4 (11.4) | |
| Septic shock | 10 (28.6) | 7 (20.0) | |
| Solid organ trans-plantation (lung or liver) | 4 (11.4) | 9 (25.7) | |
| Acute respiratory failure | 3 (8.6) | 0 (0.0) | |
| Aortic surgery | 2 (5.7) | 5 (14.3) | |
| Intoxication | 2 (5.7) | 0 (0.0) | |
BMI: body mass index; ECMO: extracorporeal membrane oxygenation; CK: creatin-kinase; SAPS: simplified acute physiology score; d0: study enrollment day; CVVHD(F): continuous venovenous hemodialysis/hemodiafiltration.
Following Table 3 illustrates the main cause of rhabdomyolysis and the cause of death in both groups
Table 3.
Main cause of rhabdomyolysis and death in both groups.
| Group 1: n (%) | Group 2: n (%) | p-value (chi2-test) | |
|---|---|---|---|
| Main cause of rhabdomyolysis | |||
| Polytrauma | 5 (14.3) | 4 (11.4) | .72 |
| - Mortality | 0 (0.0) | 1 (25.0) | |
| - Independence of dialysis | 3 (60.0) | 2 (50.0) | |
| Viral infection (COVID-19 or influenza) | 6 (17.1) | 7 (20.0) | .76 |
| - Mortality | 4 (66.7) | 4 (57.1) | |
| - Independence of dialysis | 0 (0.0) | 0 (0.0) | |
| Intestinal ischemia | 4 (11.4) | 3 (8.6) | .69 |
| - Mortality | 3 (75.0) | 2 (66.6) | |
| - Independence of dialysis | 1 (25.0) | 0 (0.0) | |
| Intoxication/toxic side effect | 3 (8.6) | 2 (5.7) | .65 |
| - Mortality | 0 (0.0) | 1 (50.0) | |
| - Independence of dialysis | 1 (33.3) | 0 (0.0) | |
| Post-resuscitation | 2 (5.7) | 3 (8.6) | .65 |
| - Mortality | 2 (100) | 2 (66.6) | |
| - Independence of dialysis | 0 (0.0) | 0 (0.0) | |
| Arterial hypoperfusion e.g., compartment | 4 (11.4) | 6 (17.1) | .50 |
| Mortality | 2 (50.0) | 3 (50.0) | |
| Independence of dialysis | 1 (25.0) | 1 (16.7) | |
| hypoxic respiratory failure | 5 (14.3) | 6 (17.1) | .75 |
| - Mortality | 3 (60.0) | 4 (66.6) | |
| - Independence of dialysis | 2 (40.0) | 0 (0.0) | |
| Unclear | 6 (17.1) | 4 (11.4) | .50 |
| - Mortality | 2 (33.3) | 1 (25.0) | |
| - Independence of dialysis | 3 (50.0) | 1 (25.0) | |
| Cause of death | |||
| Liver failure | 5 (29.4) | 4 (22.2) | .72 |
| Multi organ failure | 5 (29.4) | 5 (27.8) | 1.00 |
| Cardiac failure | 2 (11.8) | 4 (22.2) | .40 |
| Septic shock | 2 (11.8) | 2 (11.1) | 1.00 |
| Cerebral bleeding | 3 (17.6) | 3 (16.7) | 1.00 |
Comparison of the matched study population
Table 4 shows different laboratory parameters in both groups.
Table 4.
Laboratory parameters in both groups.
| Group 1: mean [SD] | Group 2: mean [SD] | p-value (Welch/chi2-test) | |
|---|---|---|---|
| Myoglobin d0 (ng/ml) | 27,218 [22,573] | 26,872 [20,582] | .95 |
| Myoglobin d1 (ng/ml) | 25,704 [28,237] | 29,032 [26,685] | |
| Myoglobin d2 (ng/ml) | 19,646 [19,035] | 29,323 [26,685] | |
| Myoglobin d6 (ng/ml) | 4530 [3856] | 5382 [5568] | |
| CK d0 (U/l) | 9683 [11,697] | 7856 [11,868] | .53 |
| CK d1 (U/l) | 12,499 [16,566] | 9610 [13,636] | |
| CK d2 (U/l) | 10,189 [15,752] | 5880 [10,137] | |
| Lactate d0 (mmol/l) | 6.2 [5.3] | 6.1 [6.9] | .97 |
| Creatinine d0 (mg/dl) | 2.3 [1.6] | 2.3 [1.5] | .89 |
| Urea d0 (mg/dl) | 87 [45] | 89 [44] | .84 |
| Sodium d0 (mg/dl) | 4.5 [0.7] | 4.9 [0.8] | .03 |
| Bicarbonate d0 (mmol/l) | 21.3 [5.5] | 22.3 [4.7] | .45 |
| Potassium d0 (mmol/l) | 4.6 [0.5] | 5.0 [0.9] | .02 |
| Phosphate (mg/dl) | 4.9 [1.8] | 4.7 [1.6] | .33 |
| eGFR (CKD-EPI) d0 (ml/min) | 44 [27] | 43 [27] | .85 |
| eGFR (CKD-EPI) d30 in patients without dialysis (ml/min) | 68 [43] | 52 [22] | .29 |
| Residual diuresis d0 (ml/24 h) | 400 [660] | 440 [730] | .80 |
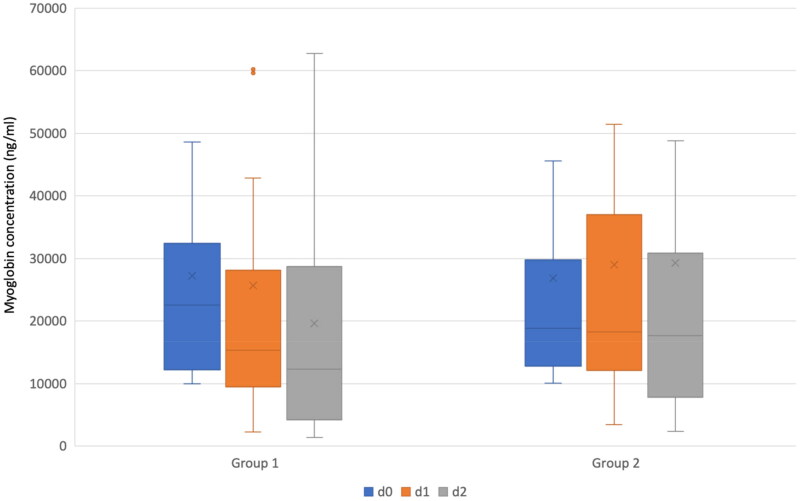
The median myoglobin at d0 and d1 in patients allocated to group 1 was 22,578 (IQR: 12,391–30,089) and 15,373 (IQR: 9808–27,336) ng/ml with a significant reduction (p < .01). A significant decrease was also observed between d0 and d2 (median myoglobin d2 12,357 ng/ml (IQR: 4385–26,884); p = .03). The median myoglobin at d0 and d1 in patients allocated to group 2 was 18,814 (IQR: 13,030–28,396) and 18,261 (IQR 12,293–36,633) ng/ml with no significant reduction. There was also no significant decrease between d0 and d2 (median myoglobin d2 17,586 ng/ml, (IQR: 7985–30,265)). The median (IQR) CK in patients allocated to group 1 was on days 0, 1 and 2 3806 (2024–12,607), 5719 (2779–15,033), and 4400 (1605–9718) U/l with no significant change during the time. Similar results were observed in patients allocated to group 2 with a median (IQR) CK on days 0, 1, and 2 of 3818 (1687–7644), 4894 (1741–9058), and 2452 (1332–4232) with no significant change between days 0 and 1, but a significant reduction between day 1 and 2 (p < .01). Following Figure 2 illustrates the myoglobin concentration on day 0, 1, and 2 in both groups as box plots.
Figure 2.
Myoglobin serum concentration (ng/ml) on days 0, 1, and 2 in patients treated with cytosorb® (group 1) and those without cytosorb® application (group 2). Group 1 included patients treated with Cytosorb® and group 2 without Cytosorb® application. The boxes of the boxplots represent the interquartile-range (IQR) and the line the median. Whiskers were limited to 1.5 times the IQR. The cross represents the mean. Blue boxplots represent day 0 (d0), orange ones day 1 (d1), and grey ones day 2 (d2).
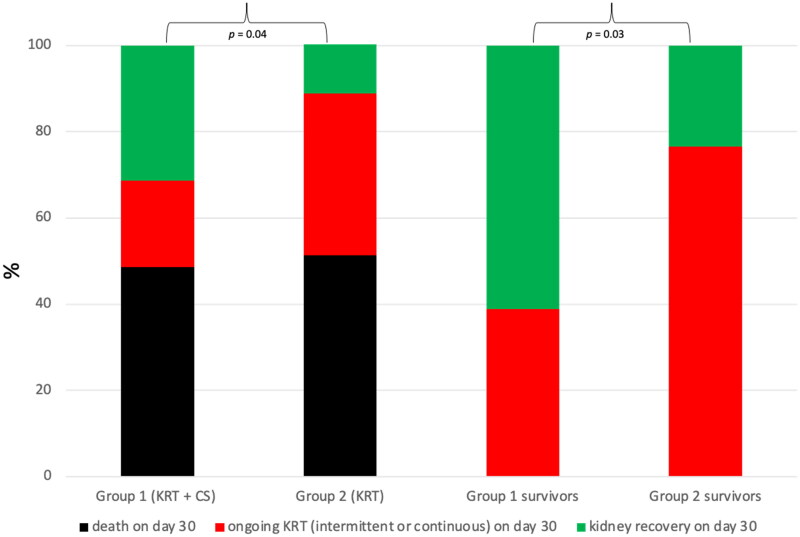
Kidney recovery on day 30 was observed in 31.4 and 11.4% of patients allocated to groups 1 and 2, respectively. It was significantly more frequent in patients allocated to group 1 compared to group 2 (p = .04, odds ratio (OR): 3.6, 95% confidence interval: 1.1–11.6). The mean risk reduction was 20.0%, and the number needed to treat was five. Termination of KRT was not observed in any of the patients who died prior to day 30. Following Table 5 shows different parameters that might affect kidney recovery in patients who survived until day 30 (group 1: 18 patients, group 2: 17 patients).
Table 5.
Parameters of kidney recovery in survivors until day 30.
| Group 1: mean [SD] | Group 2: mean [SD] | p-value (Welch-test) | |
|---|---|---|---|
| Duration until kidney recovery (days) | 13.2 [7.3] | 13.8 [7.9] | 0.46 |
| eGFR (CKD-EPI) d0 (ml/min) | 47 [28] | 48 [29] | 0.48 |
| eGFR (CKD-EPI) d7 (ml/min) | 66 [45] | 61 [40] | 0.38 |
| eGFR (CKD-EPI) d14 (ml/min) | 69 [38] | 63 [43] | 0.66 |
| eGFR (CKD-EPI) d30 (ml/min) | 83 [41] | 74 [47] | 0.28 |
| Duration of inotropes (days) | 20 [9] | 19 [11] | 0.32 |
| Duration of mechanical ventilation (days) | 17 [12] | 22 [11] | 0.09 |
Based on those patients who survived 30 days after study inclusion, kidney recovery with and without CS was 61.1 and 23.5%, respectively. It was also significantly more frequent in patients allocated to group 1 compared to group 2 (p = .03, OR: 5.1, 95% confidence interval: 1.1–9.6). The mean risk reduction was 37.5%, and the number needed to treat was 2.7. The following Figure 3 illustrates the percentage of patients with kidney recovery, ongoing KRT, and death on day 30 in both groups.
Figure 3.
The proportion of patients (%) with kidney recovery, ongoing KRT or death on day 30. KRT: kidney replacement therapy; CS: Cytosorb®, kidney recovery was defined as independence of KRT on day 30, which then lasts for at least seven days. Kidney recovery was significantly more often observed in patients treated with dialysis + CS compared to dialysis alone.
Discussion
Severe rhabdomyolysis leads to the release of myoglobin and CK into the blood [25]. The need of KRT due to severe rhabdomyolysis is associated with an increased mortality in critically ill patients and its therapy remains challenging for responsible health-care professionals [26,27]. Candela et al. were able to show that the renal outcome in these patients correlates strongly with the myoglobin serum concentration [28]. If KRT is needed due to an anuric kidney failure, extracorporeal myoglobin elimination might be an approach to attempt the recovery of kidney function and to abbreviate the duration of continuous and intermittent KRT [29,30]. Therefore, dialyzers with larger cutoff values have been used successfully for several years [31,32], but data regarding efficacy in terms of patient outcome are completely lacking.
The presented study examined the effects of CS in critically ill patients with severe rhabdomyolysis and the necessity of continuous KRT on kidney recovery defined as independence from intermittent or continuous KRT on day 30. There was no significant difference in the baseline parameters of all patients (Table 1) before PS matching. Nevertheless, PS matching of the two groups was performed to minimize the influence of confounders and to achieve a higher degree of concordance of the data. Three predictors were used for matching: SAPS II as a predictor for the severity of the disease, patients’ age as a predictor to estimate the recovery from the underlying disease, and myoglobin as a predictor for the severity of rhabdomyolysis and renal toxicity. CK was not included, as it neither affects kidney function nor is eliminated renally or by CS. The studied population was extremely ill with a high SAPS II and 30-day mortality. However, severe rhabdomyolysis with the indication for CKRT affects severely ill patients and high mortality rates of up to 59% are described [33,34]. Furthermore, the different causes of rhabdomyolysis are typical for ICU patients. In particular, viral infections with influenza or COVID-19 are associated with severe rhabdomyolysis [34].
Thirty-five pairs were successfully matched with no relevant baseline differences (Table 2), demonstrating that the PS matching worked well. PS matching can be regarded as the gold standard to compare two groups in a retrospective dataset and it is regularly used for this purpose [24,35]. In the matched population, the probability of kidney recovery, defined as the end of KRT on day 30, was found to be significantly more likely with CS than without. The mean relative risk reduction was 20% and the number needed to treat was five. These new results do not exist for CS or for any other procedure that eliminates myoglobin so far. Furthermore, a significant decrease of myoglobin in the blood on days 1 and 2 was observed in patients treated with CS, whereas no significant change was detected in those with standard care (CVVHD or CVVHDF).
Considering only those patients who survived 30 days after study enrollment (group 1: 18 patients; group 2: 17 patients), a significantly more frequent recovery of kidney function was also observed in patients treated with CS. The mean risk reduction of ongoing dialysis-requiring kidney failure was 38%, corresponding to the number needed to treat of 2.7. However, no significant difference in 30-day mortality was observed between both groups (48 vs. 51%). This can be explained as all patients had other serious medical conditions and not only severe rhabdomyolysis (e.g., acute respiratory distress syndrome or solid organ transplantation). Treatment and prognosis of these diseases are likely unaffected using CS. However, if patients finally survive the severe disease, it is of high relevance whether chronic kidney failure requiring dialysis develops or not. Our results indicate that this risk might be reduced using CS. When using unspecific adsorption procedures such as CS, it must be noted that various substances can be adsorbed. In example: while there is no evidence of a relevant meropenem adsorption by CS [36], it was recently shown that more than 500 mg vancomycin are adsorbed in one CS treatment session [37]. Thus, it remains unclear whether it was the adsorption of myoglobin alone that contributed to a faster recovery of renal function or whether the adsorption of other metabolites by CS may also have contributed.
Kidney recovery is enormously important as well at the ICU as after surviving the critical disease. On the one hand, the venous catheter for dialysis is a source of infection that can lead to sepsis [38]. In addition, ongoing kidney failure requiring KRT is associated with an increased risk of cardiovascular complications and death [39]. Furthermore, the patients’ quality of life, which is significantly reduced by ongoing KRT, must be considered [40]. Last, ongoing dialysis treatment causes high costs and can lead to kidney transplantation, which is additionally associated with lifelong immunosuppression, resulting in an increased rate of infections. As myoglobin has direct toxic effects, extracorporeal removal seems reasonable [41]. Indeed, not only CS but also HCO dialyzers or the use of CVVH with high blood flow are suitable for this purpose [31]. Whether and which method has the best outcome is not clear yet and no clear treatment strategy can be given.
Due to the retrospective and monocentric study approach, the present work has several limitations. Although PS matching allows for the comparison of two populations, it is not comparable to a randomized controlled trial. This should follow in the future to further investigate these new findings in a larger population. Furthermore, the population of 70 patients is relatively small, which limits the power of the study. However, it must be noted that no data concerning this research question are available to date. In addition, the observation period was limited to 30 days; it is unclear whether there was recovery from dialysis in some patients later. Moreover, the cause of the necessity of CKRT is often multifactorial in critically ill patients. This could have implications for the results of the study, though the underlying diseases of the patients were similarly distributed in both groups. Finally, CS was used in routine clinical practice and, thus, not under controlled study conditions. The number and duration of CS treatments was at the discretion of the attending physicians. Again, a prospective study would be helpful in this purpose.
Conclusions
In conclusion, the use of CS might positively affect renal recovery in patients with severe rhabdomyolysis and dialysis-requiring acute kidney injury. The gained findings are suitable to establish the hypothesis that the application of CS might be beneficial in those patients. A prospective randomized controlled trial is needed to confirm this hypothesis.
Funding Statement
This work was funded by institutional sources. Furthermore, CS got financial support my Else Kröner-Fresenius-Stiftung (2021_EKEA.101). UL acknowledges the funding of research by the Munich Clinician-Scientist Program.
Author contributions
Conceptualization, Christina Scharf; Data curation, Uwe Liebchen and Nils Maciuga; Methodology, Caroline Gräfe, Uwe Liebchen and Michael Paal; Project administration, Antonia Greimel; Software, Michael Irlbeck; Supervision, Mathias Bruegel and Michael Zoller; Validation, Mathias Bruegel and Lorenz Weidhase; Visualization, Caroline Gräfe; Writing – original draft, Christina Scharf; Writing – review & editing, Caroline Gräfe, Uwe Liebchen, Lorenz Weidhase and Michael Paal.
Institutional review board statement
Ethical approval was obtained from the ethical review committee of the Ludwig-Maximilians-Universität (registration number 20-477 and 21-236). Written informed consent to participate was obtained from the patients or their legal representatives for the trial 21-236 (not necessary for 20-477 due to a retrospective study approach). The study have been performed in accordance with the Declaration of Helsinki.
Consent form
Informed consent was obtained from all subjects involved in the study if necessary due to the ethics approval.
Disclosure statement
No potential conflict of interest was reported by the author(s).
Data availability statement
All data have been included to the manuscript.
References
- 1.Zimmerman JL, Shen MC.. Rhabdomyolysis. Chest. 2013;144(3):1–8. doi: 10.1378/chest.12-2016. [DOI] [PubMed] [Google Scholar]
- 2.Sousa A, Paiva JA, Fonseca S, et al. Rhabdomyolysis: risk factors and incidence in polytrauma patients in the absence of major disasters. Eur J Trauma Emerg Surg. 2013;39(2):131–137. doi: 10.1007/s00068-012-0233-7. [DOI] [PubMed] [Google Scholar]
- 3.Kolovou G, Cokkinos P, Bilianou H, et al. Non-traumatic and non-drug-induced rhabdomyolysis. Arch Med Sci Atheroscler Dis. 2019;4(1):e252–e263. doi: 10.5114/amsad.2019.90152. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Brown CVR, Rhee P, Chan L, et al. Preventing renal failure in patients with rhabdomyolysis: do bicarbonate and mannitol make a difference? J Trauma. 2004;56(6):1191–1196. doi: 10.1097/01.ta.0000130761.78627.10. [DOI] [PubMed] [Google Scholar]
- 5.Knochel JP. Rhabdomyolysis and myoglobinuria. Annu Rev Med. 1982;33(1):435–443. doi: 10.1146/annurev.me.33.020182.002251. [DOI] [PubMed] [Google Scholar]
- 6.El-Abdellati E, Eyselbergs M, Sirimsi H, et al. An observational study on rhabdomyolysis in the intensive care unit. Exploring its risk factors and main complication: acute kidney injury. Ann Intensive Care. 2013;3(1):8. doi: 10.1186/2110-5820-3-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Petejova N, Martinek A.. Acute kidney injury due to rhabdomyolysis and renal replacement therapy: a critical review. Crit Care. 2014;18(3):224. doi: 10.1186/cc13897. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Stefanovic V, Bogicevic M, Mitic M.. Myoglobin elimination in end stage kidney disease patients on renal replacement treatment. Int J Artif Organs. 1993;16(9):659–661. [PubMed] [Google Scholar]
- 9.Sorrentino SA, Kielstein JT, Lukasz A, et al. High permeability dialysis membrane allows effective removal of myoglobin in acute kidney injury resulting from rhabdomyolysis. Crit Care Med. 2011;39(1):184–186. doi: 10.1097/CCM.0b013e3181feb7f0. [DOI] [PubMed] [Google Scholar]
- 10.Heyne N, Guthoff M, Krieger J, et al. High cut-off renal replacement therapy for removal of myoglobin in severe rhabdomyolysis and acute kidney injury: a case series. Nephron Clin Pract. 2012;121(3–4):c159–c164. doi: 10.1159/000343564. [DOI] [PubMed] [Google Scholar]
- 11.Suzuki S, Moriyama K, Hara Y, et al. Comparison of myoglobin clearance in three types of blood purification modalities. Ther Apher Dial. 2021;25(4):401–406. doi: 10.1111/1744-9987.13657. [DOI] [PubMed] [Google Scholar]
- 12.Huang RSP, Tholpady A, Wahed A, et al. Therapeutic plasmapheresis and red blood cell exchange in a sickle cell trait patient with rhabdomyolysis. J Clin Apher. 2012;27(6):342–345. doi: 10.1002/jca.21247. [DOI] [PubMed] [Google Scholar]
- 13.Zhang L, Kang Y, Fu P, et al. Myoglobin clearance by continuous venous-venous haemofiltration in rhabdomyolysis with acute kidney injury: a case series. Injury. 2012;43(5):619–623. doi: 10.1016/j.injury.2010.08.031. [DOI] [PubMed] [Google Scholar]
- 14.Poli EC, Rimmele T, Schneider AG.. Hemoadsorption with CytoSorb(R). Intensive Care Med. 2019;45(2):236–239. doi: 10.1007/s00134-018-5464-6. [DOI] [PubMed] [Google Scholar]
- 15.Dilken O, Ince C, van der Hoven B, et al. Successful reduction of creatine kinase and myoglobin levels in severe rhabdomyolysis using extracorporeal blood purification (Cyt®rb(R)). Blood Purif. 2020;49(6):743–747. doi: 10.1159/000505899. [DOI] [PubMed] [Google Scholar]
- 16.Padiyar S, Deokar A, Birajdar S, et al. Cytosorb for management of acute kidney injury due to rhabdomyolysis in a child. Indian Pediatr. 2019;56(11):974–976. doi: 10.1007/s13312-019-1661-9. [DOI] [PubMed] [Google Scholar]
- 17.Scharf C, Liebchen U, Paal M, et al. Blood purification with a cytokine adsorber for the elimination of myoglobin in critically ill patients with severe rhabdomyolysis. Crit Care. 2021;25(1):41. doi: 10.1186/s13054-021-03468-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Ad-Hoc Working Group Of E, et al. European renal best practice (ERBP) position statement on the kidney disease improving global outcomes (KDIGO) clinical practice guidelines on acute kidney injury: part 1: definitions, conservative management and contrast-induced nephropathy. Nephrol Dial Transplant. 2012;27(12):4263–4272. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Andrassy KM. Comm’nts on ‘KDIGO 2012 clinical practice guideline for the evaluation and management of chronic kidney disease. Kidney Int. 2013;84(3):622–623. doi: 10.1038/ki.2013.243. [DOI] [PubMed] [Google Scholar]
- 20.Stads S, Kant KM, de Jong MFC, et al. Predictors of short-term successful discontinuation of continuous renal replacement therapy: results from a prospective multicentre study. BMC Nephrol. 2019;20(1):129. doi: 10.1186/s12882-019-1327-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Forni LG, Darmon M, Ostermann M, et al. Renal recovery after acute kidney injury. Intensive Care Med. 2017;43(6):855–866. doi: 10.1007/s00134-017-4809-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Le Gall JR, Lemeshow S, Saulnier F.. A new simplified acute physiology score (SAPS II) based on a European/North American multicenter study. JAMA. 1993;270(24):2957–2963. doi: 10.1001/jama.1993.03510240069035. [DOI] [PubMed] [Google Scholar]
- 23.Schmitt R, Coca S, Kanbay M, et al. Recovery of kidney function after acute kidney injury in the elderly: a systematic review and meta-analysis. Am J Kidney Dis. 2008;52(2):262–271. doi: 10.1053/j.ajkd.2008.03.005. [DOI] [PubMed] [Google Scholar]
- 24.Benedetto U, Head SJ, Angelini GD, et al. Statistical primer: propensity score matching and its alternatives. Eur J Cardiothorac Surg. 2018;53(6):1112–1117. doi: 10.1093/ejcts/ezy167. [DOI] [PubMed] [Google Scholar]
- 25.Cote DR, Fuentes E, Elsayes AH, et al. A “crush” course on rhabdomyolysis: risk stratification and clinical management update for the perioperative clinician. J Anesth. 2020;34(4):585–598. doi: 10.1007/s00540-020-02792-w. [DOI] [PubMed] [Google Scholar]
- 26.Mikkelsen TS, Toft P.. Prognostic value, kinetics and effect of CVVHDF on serum of the myoglobin and creatine kinase in critically ill patients with rhabdomyolysis. Acta Anaesthesiol Scand. 2005;49(6):859–864. doi: 10.1111/j.1399-6576.2005.00577.x. [DOI] [PubMed] [Google Scholar]
- 27.Hoste EAJ, Bagshaw SM, Bellomo R, et al. Epidemiology of acute kidney injury in critically ill patients: the multinational AKI-EPI study. Intensive Care Med. 2015;41(8):1411–1423. doi: 10.1007/s00134-015-3934-7. [DOI] [PubMed] [Google Scholar]
- 28.Candela N, Silva S, Georges B, et al. Short- and long-term renal outcomes following severe rhabdomyolysis: a French multicenter retrospective study of 387 patients. Ann Intensive Care. 2020;10(1):27. doi: 10.1186/s13613-020-0645-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Masakane I, Sakurai K.. Current approaches to Middle molecule removal: room for innovation. Nephrol Dial Transplant. 2018;33(Suppl 3):iii12–iii21. doi: 10.1093/ndt/gfy224. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Ronco C. Extracorporeal therapies in acute rhabdomyolysis and myoglobin clearance. Crit Care. 2005;9(2):141–142. doi: 10.1186/cc3055. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Weidhase L, de Fallois J, Haußig E, et al. Myoglobin clearance with continuous veno-venous hemodialysis using high cutoff dialyzer versus continuous veno-venous hemodiafiltration using high-flux dialyzer: a prospective randomized controlled trial. Crit Care. 2020;24(1):644. doi: 10.1186/s13054-020-03366-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Premru V, Kovač J, Buturović-Ponikvar J, et al. Some kinetic considerations in high cut-off hemodiafiltration for acute myoglobinuric renal failure. Ther Apher Dial. 2013;17(4):396–401. doi: 10.1111/1744-9987.12085. [DOI] [PubMed] [Google Scholar]
- 33.de Meijer AR, Fikkers BG, de Keijzer MH, et al. Serum creatine kinase as predictor of clinical course in rhabdomyolysis: a 5-year intensive care survey. Intensive Care Med. 2003;29(7):1121–1125. doi: 10.1007/s00134-003-1800-5. [DOI] [PubMed] [Google Scholar]
- 34.Mlynarska E, et al. Rhabdomyolysis-Induced AKI (RIAKI) including the role of COVID-19. Int J Mol Sci. 2022;23(15):8251. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Lenis D, Nguyen TQ, Dong N, et al. It’s all about balance: propensity score matching in the context of complex survey data. Biostatistics. 2019;20(1):147–163. doi: 10.1093/biostatistics/kxx063. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Liebchen U, Scharf C, Zoller M, et al. No clinically relevant removal of meropenem by cytokine adsorber C®Sorb((R)) in critically ill patients with sepsis or septic shock. Intensive Care Med. 2021;47(11):1332–1333. doi: 10.1007/s00134-021-06487-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Scharf C, Weinelt F, Schroeder I, et al. Does the cytokine adsorbe®ytoSorb((R)) reduce vancomycin exposure in critically ill patients with sepsis or Septic shock? a prospective observational study. Ann Intensive Care. 2022;12(1):44. doi: 10.1186/s13613-022-01017-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Souweine B, Liotier J, Heng AE, et al. Catheter colonization in acute renal failure patients: comparison of Central venous and dialysis catheters. Am J Kidney Dis. 2006;47(5):879–887. doi: 10.1053/j.ajkd.2006.01.023. [DOI] [PubMed] [Google Scholar]
- 39.Pannu N, James M, Hemmelgarn B, et al. Association between AKI, recovery of renal function, and long-term outcomes after hospital discharge. Clin J Am Soc Nephrol. 2013;8(2):194–202. doi: 10.2215/CJN.06480612. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Vijayan A, Abdel-Rahman EM, Liu KD, et al. Recovery after critical illness and acute kidney injury. Clin J Am Soc Nephrol. 2021;16(10):1601–1609. doi: 10.2215/CJN.19601220. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Panizo N, Rubio-Navarro A, Amaro-Villalobos JM, et al. Molecular mechanisms and novel therapeutic approaches to rhabdomyolysis-induced acute kidney injury. Kidney Blood Press Res. 2015;40(5):520–532. doi: 10.1159/000368528. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
All data have been included to the manuscript.