Abstract
Introduction
Coagulation disorders play a key role in chronic kidney disease, and the formation or elevation of plasma D-dimer levels reflects activation of the coagulation system. However, its relationship with the severity and progression of kidney disease in IgA nephropathy (IgAN) remains unclear.
Methods
We assessed 1818 patients with IgAN diagnosed between 2002 and 2019 at the First Affiliated Hospital, Zhejiang University School of Medicine. Plasma D-dimer levels were measured at the time of the renal biopsy. The association between plasma D-dimer levels and kidney disease progression events, defined as a 50% decline in eGFR and end-stage kidney disease (ESKD), was tested using restricted cubic splines and Cox proportional hazard models.
Results
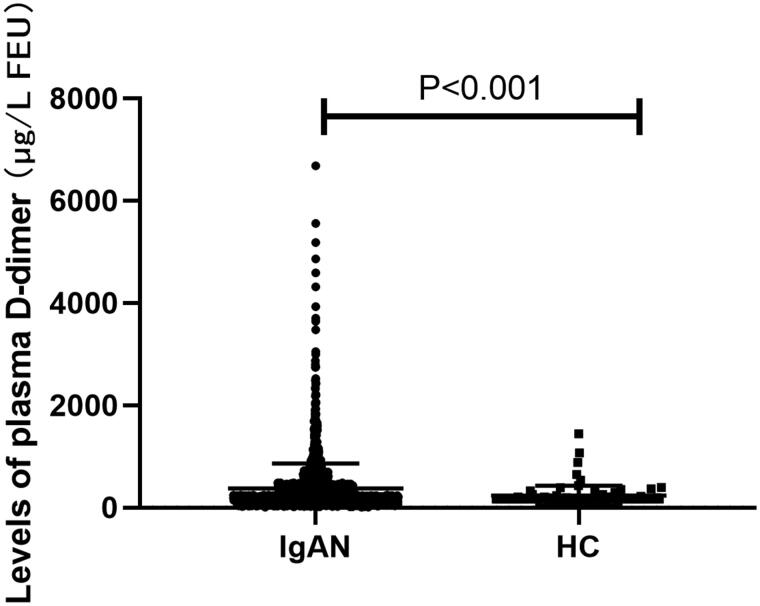
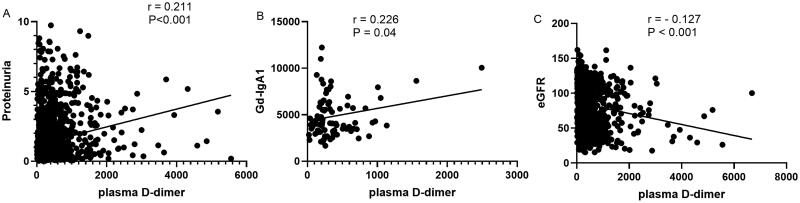
The median plasma D-dimer level was 220 (170–388.5) µg/L FEU, which was significantly higher than healthy controls 170 (170–202) µg/L FEU. Plasma D-dimer levels were positively correlated with proteinuria (r = 0.211, p < 0.001) and serum galactose-deficient IgA1 (r = 0.226, p = 0.004) and negatively correlated with eGFR (r=-0.127, p < 0.001) and Oxford T (p < 0.001) and C (p = 0.004) scores. After a median follow-up of 25.67 (13.03–47.44) months, 126 (6.93%) patients experienced composite kidney disease progression events. Higher plasma D-dimer levels were associated with an increased risk of kidney disease progression events (hazard ratio, 1.73; 95% confidence interval [95% CI], 1.40–2.23) per ln-transformed plasma D-dimer (p < 0.001), after adjustment for sex, age, proteinuria, Mean arterial pressure (MAP) and Oxford classification scores. In reference to the first tertile of plasma D-dimer, hazard ratios were 1.48 (95% CI, 0.76–2.88) for the second tertile, 3.03 (95% CI, 1.58–5.82) for the third tertile.
Conclusions
High plasma D-dimer levels were associated with the progression of kidney disease severity in IgA nephropathy.
Keywords: D-dimer, Coagulation disorders, IgA nephropathy, risk factor, kidney disease progression
Introduction
IgA nephropathy is one of the most common primary glomerular diseases worldwide, and the leading cause of kidney failure requiring kidney replacement therapy [1–3]. The clinical presentation of in IgAN ranges from a benign incidental condition to progressive kidney failure. The progression of IgA nephropathy is monitored by the presence of proteinuria, hypertension, changes in glomerular filtration rate (GFR), and Oxford classification MEST-C score [4–8]. In addition, persistent hematuria and hyperphosphatemia are associated with kidney disease progression in patients with IgA nephropathy [1,9–11].
In recent years, numerous studies have established that coagulation disorders play a key role in chronic kidney disease, especially in those with venous thrombosis [12–16]. In a recent study including IgA nephropathy with arteriolar microangiopathic (MA) lesions, among all rare gene variants, 31(33.7%) patients belonged to the coagulation pathway. we suspected that coagulation system activation may be involved in the pathogenesis of IgA nephropathy [17]. Plasma D-dimer is a plasmin-derived soluble degradation product of cross-linked fibrin [18,19], and its formation or elevation reflects activation of the coagulation and fibrinolytic systems. Plasma D-dimer was associated with microalbuminuria [20], and raised plasma D-dimer levels were a risk factor for the development of cardiovascular complications and atherothrombosis in patients with diabetes [21,22]. Some studies have established that plasma D-dimer levels are significantly higher in patients with chronic kidney disease (CKD) [23,24]; as CKD advances, D-dimer levels are higher [25]. However, its role in the progression of kidney diseas remains unclear.
In this study, we aimed to explore the association between plasma D-dimer levels, disease severity, and kidney disease progression in patients with IgAN.
Materials and methods
Study population
All biopsy-proven IgA nephropathy patients were diagnosed between 2002 and 2019 at our hospital. All patients were follow-up 2–6 months regularly according to the patients’ disease condition. Patients were excluded who presented at younger than 16 years of old, with incomplete clinical data and ESRD at baseline, with a history of infection and AKI at the time of renal biopsy or had less than 6 months follow-up. Those with secondary IgA nephropathy, such as IgA vasculitis, rheumatic disease, liver cirrhosis, systemic lupus were also excluded. A total of 1818 IgA nephropathy patients were included. In addition, 90 sex-, age-, and geographically-matched healthy individuals were enrolled as healthy controls.
The diagnosis of IgA nephropathy was based on the presence of dominant IgA deposition in the mesangial area by immunofluorescence. In addition, blood samples were collected from 83 participants for the measurement of galactose-deficient IgA1.
The study was conducted in accordance with the principles of the Declaration of Helsinki and approved by the local ethics committees.
Data collection and pathologic manifestations
Clinical manifestations at the time of kidney biopsy, including sex, age, 24-h urine protein excretion, systolic/diastolic pressure, serum creatine level, and plasma D-dimer levels were collected from the medical records. Coagulation function and D-dimer levels are examined before renal needle biopsy in all patients. The Oxford classification was used to evaluate the pathologic lesions [7].
Baseline was defined as the time of renal biopsy. Hypertension was defined as systolic blood pressure ≥140 mm Hg and/or diastolic blood pressure ≥90 mmHg or use of antihypertensive medication. The mean arterial pressure (MAP) was calculated as the sum of one-third of the pulse pressure and diastolic blood pressure. Estimated glomerular filtration rate (eGFR) was calculated using the Chronic Kidney Disease Epidemiology Collaboration (CKD-EPI) equation [26]. For survival analyses, the composite endpoint was defined as a 50% eGFR decline, end-stage kidney disease (ESKD), or death (whichever occurred first). Follow-up ended when patients reached the composite endpoint. ESKD was defined as eGFR < 15 mL/min/1.73 m2 or the need for kidney replacement therapy (including hemodialysis, peritoneal dialysis, or kidney transplantation).
Measurement of serum galactose-deficient IgA1
The levels of serum galactose-deficient IgA1 (Gd-IgA1) were measured using the KM55 mAb according to the manufacture’s instruction (Immuno-Biological Laboratories, Fujioka, Japan).
Statistical analyses
Normally distributed variables are expressed as mean ± standard deviation (SDs) and compared using the t-test; non-normally distributed variables are presented as median and interquartile range (IQR) and compared using the Kruskal-Wallis test. Categorical data are presented as percentages or frequencies and were tested using the chi-square test. The relationship between plasma D-dimer levels and the risk of composite kidney disease progression events wase estimated using Cox proportional hazards models. The adjustment factors handled in Cox models such as age, MAP and Ln transformed proteinuria were expressed as continuous variable. Cumulative incidence function methods were used to evaluate the association of plasma D-dimer and kidney disease progression. To obtain a complete picture of the relationship between plasma D-dimer levels and composite kidney disease progression events, restricted cubic splines were used to model plasma D-dimer levels after multivariable adjustment. Statistical analysis was performed using SPSS (version 24.0; SPSS, Chicago, IL, USA) and Stata version 14.0 (Statacorp, College Station, TX). Statistical significance was set at p < 0.05.
Results
Clinical characteristics
The clinical characteristics of the study population are summarized in Table 1. The study included 956 (52.59%) men with a mean age of 38.61 ± 12.20 years. At the time of kidney biopsy, the mean MAP was 95.02 ± 14.02 mmHg, the average eGFR was 83.87 ± 29.24 mL/min per 1.73m2, the initial proteinuria excretion was 0.94 (IQR, 0.49–1.82) g/d. and the median plasma D-dimer level was 220 (IQR, 170–388.5) µg/L FEU, significantly higher than healthy controls 170 (170–202) µg/L FEU (Figure 1). After a median follow-up of 25.67 months (IQR, 13.03–47.44), 126 (6.27%) patients reached the composite kidney disease progression events, including 92 (5.06%) patients with kidney failure and 114 (6.27%) patients with 50% eGFR decline. There was no patient died during follow-up.
Table 1.
Clinical characteristics of patients with IgA nephropathy.
| Characteristics | Total Cohort | Tertiles of plasma D-dimer (µg/L FEU) |
P | ||
|---|---|---|---|---|---|
| 1st tertile ≤170 |
2nd tertile 171-337 |
3rd tertile ≥338 |
|||
| N | 1818 | 689 | 565 | 564 | |
| Male, n % | 956 (52.59) | 277(40.20) | 330(58.41) | 349(61.88) | <0.001 |
| Age, yr, mean ± s.d. | 38.61 ± 12.20 | 36.77 ± 11.14 | 38.51 ± 11.78 | 40.96 ± 13.45 | <0.001 |
| MAP, mmHg, mean ± s.d. | 95.02 ± 14.02 | 95.19 ± 13.77 | 95.30 ± 14.38 | 94.56 ± 13.95 | 0.624 |
| Proteinuria, g/d, median, IQR | 0.94 (0.49 to 1.82) | 0.76(0.41 to 1.40) | 0.89(0.49 to 1.54) | 1.35(0.67 to 2.79) | <0.001 |
| eGFR, mL/min/1.73 m2, mean ± s.d. | 83.87 ± 29.24 | 86.45 ± 28.06 | 84.98 ± 27.35 | 78.22 ± 31.05 | <0.001 |
| Serum albumin, g/L | 38.99 ± 6.17 | 40.67 ± 5.61 | 39.55 ± 5.00 | 36.39 ± 6.99 | <0.001 |
| Plasma D-dimer µg/L FEU, median, IQR | 220 (170 to388.5) | 170.00 (111.00 to 170.00) | 236.00(204.00to 282.00) | 567.50(411.00to 897.00) | <0.001 |
| Plasma APTT, s | 27.69 ± 4.24 | 27.82 ± 3.86 | 27.57 ± 3.95 | 27.67 ± 4.88 | 0.683 |
| Plasma PT, s | 11.17 ± 0.95 | 11.17 ± 0.92 | 11.15 ± 0.79 | 11.19 ± 1.12 | 0.785 |
| CKD stages, n (%) | |||||
| 1 | 839 (46.15) | 343(49.78) | 273(48.32) | 223(39.54) | 0.001 |
| 2 | 540(29.70) | 209(30.33) | 176(31.15) | 155(27.48) | 0.362 |
| 3 | 383(21.07) | 123(17.85) | 101(17.88) | 159(28.19) | <0.001 |
| 4 | 56(3.08) | 14(2.03) | 15(2.65) | 27(4.79) | 0.015 |
| Oxford classification, n (%) | |||||
| M1 | 251(13.81) | 122(17.71) | 74(13.10) | 55 (9.75) | <0.001 |
| E1 | 122(6.71) | 45(6.53) | 28(4.96) | 49(8.69) | 0.042 |
| S1 | 1205(66.28) | 442(64.15) | 399(70.62) | 364(64.54) | 0.031 |
| T1-T2 | 189(10.40) | 44(6.39) | 64(11.33) | 81(14.36) | <0.001 |
| C1-C2 | 849(46.70) | 287(41.65) | 266(47.08) | 296(52.48) | 0.001 |
| Follow-up and Outcome | |||||
| Follow-up duration, mo, median, IQR | 25.67 (13.03-47.44) | 27.32(13.48-48.88) | 23.23(12.58-41.18) | 24.40(12.43-42.90) | 0.003 |
| 50% eGFR decline, % | 114 (6.27) | 42(6.10) | 27(4.78) | 45(7.98) | 0.083 |
| Kidney failure, % | 92 (5.06) | 32(4.64) | 19(3.36) | 41(7.27) | 0.009 |
| Composite outcome, % | 126 (6.93) | 47(6.82) | 28(4.96) | 51(9.04) | 0.026 |
Note: Values for continuous variables are expressed as mean ± standard deviation or median [interquartile ranges]; counts (percentages) are used for categorical variables. Composite outcome was defined as a 50% decrease in eGFR or kidney failure. M, mesangial hypercellularity; E1, endocapillary hypercellularity; S, segmental glomerulosclerosis/adhesion; T, severity of tubular atrophy/interstitial fibrosis; C, presence of crescent; APTT, activated partial thromboplastin time; PT, prothrombin time.
Figure 1.
Prevalence of plasma D-dimer in IgA nephropathy (IgAN) and healthy controls (HC).
Association of plasma D-dimer with the severity of IgA nephropathy
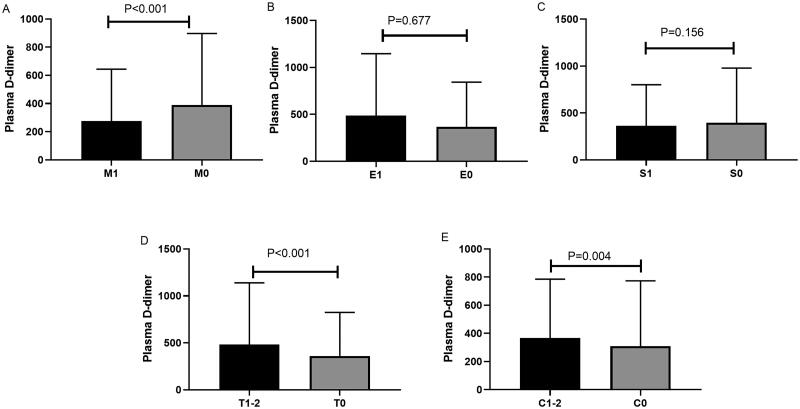
Correlation of plasma D-dimer levels with clinical and histological findings at the time of kidney biopsy was performed. Patients with higher levels of plasma D-dimer showed higher levels of proteinuria, higher prevalence of CKD stage 3–4 and lower eGFR as shown in Table 1. The incidence of kidney failure was higher in patients with higher plasma D-dimer levels. For histologic lesions, patients showed higher plasma D-dimer levels in T1-2 and C1-2 scores compared than in T0 and C0 (Figure 2). We also analyzed the correlation between plasma D-dimer levels and proteinuria, serum galactose-deficient IgA1(Gd-IgA1) levels, and eGFR. As shown in Figure 3, plasma D-dimer levels were positively correlated with proteinuria (r = 0.211, p < 0.001) (Figure 3A) and Gd-IgA1 (r = 0.226, p = 0.04) (Figure 3B) and negatively correlated with eGFR (r= − 0.127, p < 0.001) (Figure 3C). However, the correlation coefficient r was very low.
Figure 2.
Comparison of plasma D-dimer levels among IgA nephropathy patients with different Oxford classifications (A, M1 vs M0; B, E1 vs E0; C, S1 vs S0; D, T1-2 vs T0; E, C1-2 vs C0). M, mesangial hypercellularity; E1, endocapillary hypercellularity; S, segmental glomerulosclerosis/adhesion; T, severity of tubular atrophy/interstitial fibrosis; C, presence of crescent.
Figure 3.
Correlation between plasma D-dimer, proteinuria, galactose-deficient IgA1 (Gd-IgA1) and estimated glomerular filtration rate (eGFR). Plasma D-dimer was positively correlated with (A) proteinuria and (B) Gd-IgA1 (n = 83), and negatively correlated with (C) eGFR.
Association of plasma D-dimer levels with kidney disease progression
Patients were divided into three groups according to the levels of plasma D-dimer distribution. Group 1: plasma D-dimer ≤ 170 µg/L FEU, n = 689; Group 2, 171–337 µg/L FEU, n = 565; Group 3, ≥339 µg/L FEU, n = 564. The characteristics of participants were presented as Table 1.
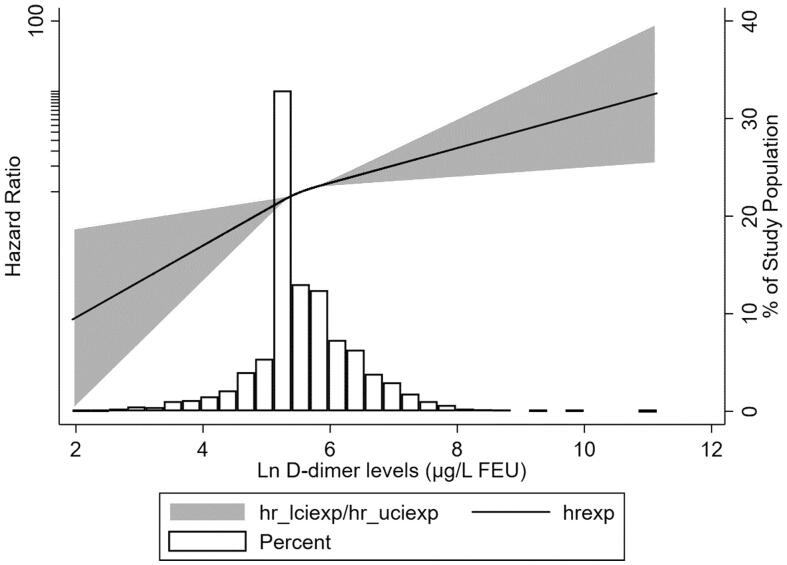
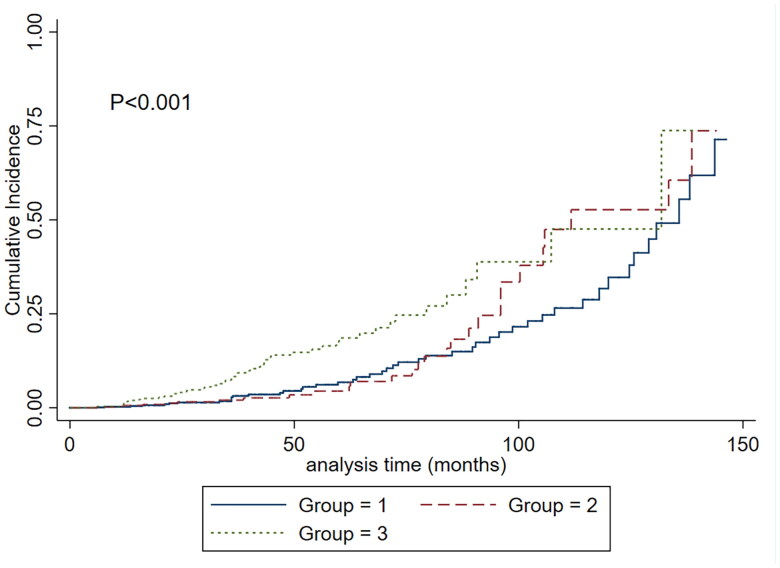
To assess the association between plasma D-dimer levels and kidney disease progression, we modeled plasma D-dimer levels as a continuous variable by using restricted cubic splines. As shown in Figure 4, the risk of kidney disease progression was greater with higher levels of plasma D-dimer. Cumulative incidence function methods demonstrated that patients with lower plasma D-dimer had lower incidence of the composite renal outcome (p < 0.001) (Figure 5). In the Cox proportional hazards model, plasma D-dimer level was significantly associated with an increased risk of composite kidney disease progression events with a hazard ratio [HR] of 1.61 (95% confidence interval [CI], 1.24–2.08; p < 0.001) per ln-transformed (plasma D-dimer) (Table 2). Compared to the first tertile of plasma D-dimer, higher plasma D-dimer level was an independent risk factor for the composite kidney disease progression event after adjusted traditional risk factors, including sex, age, 24-h urine protein excretion, MAP, eGFR and Oxford classification (MEST-C scores), the HRs were 1.25(0.65–2.40) in the second tertile, 2.54(1.38–4.66) in the third tertile (p-value for the trend =0.003).
Figure 4.
The association between plasma D-dimer levels and adjusted hazard ratios of composite kidney progression outcome. Three knots at the 25th, 50th, and 75th percentiles were used to model the association between plasma D-dimer and composite kidney progression outcome, using restricted cubic splines. Model was adjusted for sex, age, proteinuria, MAP, eGFR and Oxford classification (MEST-C scores). the reference value was set at the medians. The histogram represents the distribution of plasma D-dimer in patients with IgA nephropathy; the solid line represents the estimated hazard ratio; the shaded area represents the 95% confidence interval.
Figure 5.
Cumulative incidence of the composite kidney disease progression and according to plasma D-dimer. The time zero was kidney biopsy. Group 1: the first tertile, plasma D-dimer ≤ 170µg/L FEU; second tertile, 171-337µg/L FEU; third tertile, ≥339 µg/L FEU.
Table 2.
Association of plasma D-dimer levels with the composite kidney disease progression outcome.
| Hazard Ratio, 95% Confidence Interval and P value |
||||
|---|---|---|---|---|
| Unadjusted | Model 1 | Model 2 | Model 3 | |
| Composite kidney disease progression events per ln (plasma D-dimer) | 1.61(1.35-1.92) < 0.001 |
1.59(1.34-1.90) < 0.001 |
1.41(1.17-1.71) <0.001 |
1.61(1.24-2.08) <0.001 |
| Plasma D-dimer tertiles | ||||
| 1 | 1 (Reference) | 1 (Reference) | 1 (Reference) | 1 (Reference) |
| 2 | 1.13 (0.71-1.81) 0.608 |
1.15 (0.71-1.84) 0.577 |
1.20(0.71-2.03) 0.507 |
1.25(0.65-2.40) 0.501 |
| 3 | 2.51(1.66-3.78) <0.001 |
2.48 (1.63-3.77) <0.001 |
2.05 (1.29-3.27) 0.003 |
2.54 (1.38-4.66) 0.003 |
| P for trend | <0.001 | <0.001 | <0.001 | 0.003 |
CKD progression events were a 50% decrease in the eGFR or kidney failure.
Model 1 was adjusted for sex and age and sex was expressed as a dichotomous variable.
Model 2 was adjusted for the covariates in Model 1: mean arterial pressure, log-transformed proteinuria.
Model 3 was adjusted for covariates in Model 2 and Oxford classification (MEST-C scores).
Sensitivity analyses
In addition, we evaluated the role of plasma D-dimer level and initial proteinuria. A total of 1765 patients had baseline proteinuria. Patients were divided into two groups according to baseline proteinuria: protein excretion > 1.0 g/d and protein excretion ≤1.0 g/d. 23 (2.45%) patients in the protein excretion ≤1.0 g/d group (937) and 96 (11.59%) patients in the protein excretion > 1.0 g/d group (828) reached the composite outcome (p < 0.001). After adjustment for traditional risk factors, Ln-transformed Plasma D-dimer level was an independent risk factor for composite kidney disease progression events in protein excretion > 1.0 g/d group (HR, 1.54; 95% CI 1.16–2.05; p = 0.003) and was not associated with poor renal outcome in the protein excretion ≤ 1.0 g/d group (HR, 2.07; 95% CI 0.99–4.28; p = 0.05). The interaction between proteinuria and plasma D-dimer groups was analyzed; proteinuria and plasma D-dimer had no significant interactions with kidney disease progression (p for interaction =0.159).
We also explored the role of plasma D-dimer in kidney disease progression in subgroups of patients with eGFR < 60 mL/min/1.73 m2 and eGFR ≥ 60 mL/min/1.73 m2. We found that after adjustment for sex, age, proteinuria, MAP, and Oxford classification, high plasma D-dimer levels were associated with poor renal outcome in patients with eGFR < 60 mL/min/1.73 m2, (HR, 1.902; 95% CI 1.297–2.790; p = 0.001). A further subgroup analysis was conducted according to eGFR 30–45 and 45–60, we found that the HR for composite renal outcome was 3.76(1.27–11.17) for patients with eGFR 30–45 mL/min/1.73 m2, and 2.32(1.15–4.70) for those with eGFR 45–60 mL/min/1.73 m2. However, in those with eGFR ≥ 60 mL/min/1.73 m2, Plasma D-dimer level was not a risk factor for kidney disease progression events (HR, 1.437; 95% CI 0.987–2.091; p = 0.058). An interaction analysis was performed, and no significant multiplicative interactions for kidney disease progressio were found between plasma D-dimer levels and eGFR.
Discussion
Coagulation abnormalities are common in chronic kidney disease. Thrombotic complications caused by hypercoagulability are one of the most common causes of death among patients with chronic kidney disease and are one of the difficulties in renal replacement therapy [12,13,23,27,28]. In a recent study, in patients with IgAN-MA, 13 (30.2%) gene variants in coagulation pathway genes were identified in 35 patients with IgAN-MA lesions [17], which suggested that abnormal activation of the coagulation system may be involved in the pathogenesis of IgA nephropathy. Plasma D-dimer level reflects the activation of the coagulation system and is commonly used as a sensitive indicator of thrombotic conditions. However, the role of plasma D-dimer in kidney disease progression remains unclear, especially in patients without thrombosis. In this retrospective cohort study with 1818 participants with IgA nephropathy, we found that elevated plasma D-dimer levels were associated with kidney disease progression, especially in those with proteinuria > 1.0 g/d and eGFR < 60 mL/min/1.73 m2.
Plasma D-dimer levels were significantly elevated in both patients with diabetic nephropathy and nondiabetic chronic renal failure when compared with healthy participants. Patients with acute kidney failure had markedly elevated plasma D-dimer levels [24]. Huang et al. reported that the levels of plasma D-dimer were higher in CKD patients than healthy controls, and grossly elevated in AKI patients in a study including 95 CKD patients and 20 healthy controls [23]. In another study of 382 participants with essential hypertension, 168 (44%) patients with impaired renal function had significantly greater levels of plasma D-dimer than those with normal renal function, and plasma D-dimer levels were correlated with renal function independent of blood pressure, urinary protein excretion and other traditional confounders [29]. In another study of 12 patients with IgA nephropathy, the authors also found that plasma D-dimer levels were increased compared to healthy controls [30]. Compared with previous studies, this large cohort study established a correlation between plasma D-dimer levels and the risk of kidney disease progression; the risk increased with higher plasma D-dimer levels (Figure 4). Importantly, we found that plasma D-dimer levels were positively correlated with proteinuria and negatively correlated with eGFR in IgAN, however, the correlation coefficient r was very low. Thus, the findings still need further confirmation. We also found that the HR for composite renal outcome was 3.76(1.27–11.17) for patients with eGFR 30–45 mL/min/1.73 m2, and 2.32(1.15–4.70) for those with eGFR 45–60 mL/min/1.73 m2. D-dimer may be a marker of low eGFR rather than a predictor of further worsening. Patients with severe Oxford T and C showed higher plasma D-dimer levels, suggesting that they may contribute to the progression of glomerulonephritis in IgA nephropathy.
The plasma D-dimer level is a sensitive indicator for predicting venous thrombosis in patients with CKD [31–33]. In patients with IgA nephropathy, venous thromboembolism and thrombosis in kidney tissue are uncommon, and it is likely that elevated plasma D-dimer levels may be due to the primary event leading to kidney injury and progression of kidney disease, not necessarily to thrombosis occurring within the kidney itself in IgA nephropathy, which is consistent with previous assumptions [24]. Interestingly, in our study, plasma D-dimer levels were positively correlated with serum galactose-deficient IgA1 levels. Elevated galactose-deficient IgA1 levels were correlated with an increased risk of kidney disease progression in patients with IgA nephropathy [34]. D-dimer was detected in glomeruli in patients with IgA nephropathy [35], we hypothesized that the deposition of Gd-IgA1 in glomeruli might activate the coagulation system of renal tissue, or serum Gd-IgA1 and D-dimer form circulating immune complexes then deposited in glomeruli, aggravating renal damage in IgA nephropathy. We also found that the plasma D-dimer level was higher in patients with C1-2 lesions than those with C0 lesions, we hypothesize that the plasma D-dimer may be involved in the crescent formation in IgA nephropathy. Which requires need further investigation.
Plasma D-dimer levels are commonly used to predict coagulation system activation in clinical practice. Various coagulation factors have been observed in the glomeruli of renal biopsy specimens and circulation in patients with CKD. Liu et al. found that the important indicators of the coagulation system intact XFb and Factor V were colocalized in the glomerular mesangium in active IgA nephropathy, and the deposition of factor V was correlated with the degree of disease activity and exerted procoagulant activity in IgA nephropathy [36]. Activated partial thromboplastin time (APTT) and shorter prothrombin time (PT) are associated with Oxford T lesions in IgA nephropathy [37]. The coagulation system is activated in the serum of both diabetic and nondiabetic progressive kidney failure [38]. An experimental animal study indicated that activation of the coagulation system was associated with fibrosis throµgh the formation of microthrombi, which could disrupt localized blood flow and ischemia [39]. Further studies have established that anticoagulation can reduce renal fibrosis in a murine model of type 1 diabetes murine model [40]. These results indicate that activation of the coagulation system may play an important role in the pathogenesis and development of kidney disease, and anticoagulation therapy may improve the outcome of patients with CKD. An early study showed that anticoagulation therapy using heparin-warfarin combined with dipyridamole was not effective in improving clinical and pathological damage in patients with IgA nephropathy [41]. Combination treatment including prednisolone and mizoribine with warfarin and dipyridamole was better at reducing proteinuria in patients with severe childhood IgA nephropathy than in those without warfarin and dipyridamole [42]. Anticoagulants may be promising adjuncts in targeting IgA nephropathy, and future studies are warranted to evaluate whether anticoagulation treatment improves kidney outcomes.
To the best of our knowlege, this is the largest study to explore the association between coagulation and kidney disease progression. In this large cohort study, we found that higher plasma D-dimer levels were a moderate risk factor for the progression of kidney disease. The strengths of this study are the large sample size and the large number of kidney failure events, which provided good research power to assess the association of plasma D-dimer with hard endpoints, which is independent of established clinical predictors. The limitations were as follows: The short-term follow-up with a small number of kidney failure events, and the participants were from a single center; The protein excretion was recorded at the time of kidney biopsy, the use of steroids and other immunosuppressants and reninangiotensin-aldosterone system inhibitors (RAASi) was recorded during follow-up according to patients’ disease condition, because > 90% of patients received steroids and other immunosuppressants and RAASi therapy in our center, Then use of immunosuppressive and RAASi were not included into analysis in this study. Thus, the results require further confirmation.
In conclusion, in this large-sample study, we demonstrated an association between plasma D-dimer levels and kidney disease progression in patients with IgA nephropathy. The risk of kidney disease progression was greater with higher plasma D-dimer levels.
Acknowledgements
We thank the patients who participated in this study.
Funding Statement
This work was supported by a grant from the National Natural Science Foundation of China (82200781).
Disclosure statement
The authors declare they have no other relevant finical interests.
References
- 1.Yu G, Cheng J, Jiang Y, et al. . Serum phosphorus and calcium levels, and kidney disease progression in immunoglobulin a nephropathy. Clin Kidney J. 2021;14:1–8. doi: 10.1093/ckj/sfab002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.McGrogan A, Franssen CF, de Vries CS.. The incidence of primary glomerulonephritis worldwide: a systematic review of the literature. Nephrol Dial Transplant. 2011;26:414–430. doi: 10.1093/ndt/gfq665. [DOI] [PubMed] [Google Scholar]
- 3.Lai KN, Tang SC, Schena FP, et al. . IgA nephropathy. Nat Rev Dis Primers. 2016;2:16001. doi: 10.1038/nrdp.2016.1. [DOI] [PubMed] [Google Scholar]
- 4.Barbour SJ, Espino-Hernandez G, Reich HN, et al. . The MEST score provides earlier risk prediction in lgA nephropathy. Kidney Int. 2016;89:167–175. doi: 10.1038/ki.2015.322. [DOI] [PubMed] [Google Scholar]
- 5.Berthoux F, Mohey H, Laurent B, et al. . Predicting the risk for dialysis or death in IgA nephropathy. J Am Soc Nephrol. 2011;22:752–761. doi: 10.1681/ASN.2010040355. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Li X, Liu Y, Lv J, et al. . Progression of IgA nephropathy under current therapy regimen in a Chinese population. Clin J Am Soc Nephrol. 2014;9:484–489. doi: 10.2215/CJN.01990213. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Trimarchi H, Barratt J, Cattran DC, et al. . Oxford classification of IgA nephropathy 2016: an update from the IgA Nephropathy Classification Working Group. Kidney Int. 2017;91:1014–1021. doi: 10.1016/j.kint.2017.02.003. [DOI] [PubMed] [Google Scholar]
- 8.Goto M, Wakai K, Kawamura T, et al. . A scoring system to predict renal outcome in IgA nephropathy: a nationwide 10-year prospective cohort study. Nephrol Dial Transplant. 2009;24:3068–3074. doi: 10.1093/ndt/gfp273. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Sevillano AM, Gutiérrez E, et al. . Remission of hematuria improves renal survival in IgA nephropathy. J Am Soc Nephrol. 2017;28:3089–3099. doi: 10.1681/ASN.2017010108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Yu GZ, Guo L, Dong JF, et al. . Persistent hematuria and kidney disease progression in IgA nephropathy: a cohort study. Am J Kidney Dis. 2020;76:90–99. doi: 10.1053/j.ajkd.2019.11.008. [DOI] [PubMed] [Google Scholar]
- 11.Da J, Xie X, Wolf M, et al. . Serum phosphorus and progression of CKD and mortality: a meta-analysis of cohort studies. Am J Kidney Dis. 2015;66:258–265. doi: 10.1053/j.ajkd.2015.01.009. [DOI] [PubMed] [Google Scholar]
- 12.Wattanakit K, Cushman M, Stehman-Breen C, et al. . Chronic kidney disease increases risk for venous thromboembolism. J Am Soc Nephrol. 2008;19:135–140. doi: 10.1681/ASN.2007030308. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Liang CC, Wang SM, Kuo HL, et al. . Upper gastrointestinal bleeding in patients with CKD. Clin J Am Soc Nephrol. 2014;9:1354–1359. doi: 10.2215/CJN.09260913. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Tran L, Pannier B, Lacolley P, et al. . A case-control study indicates that coagulation imbalance is associated with arteriosclerosis and markers of endothelial dysfunction in kidney failure. Kidney Int. 2021;99:1162–1172. doi: 10.1016/j.kint.2020.12.011. [DOI] [PubMed] [Google Scholar]
- 15.Lutz J, Menke J, Sollinger D, et al. . Haemostasis in chronic kidney disease. Nephrol Dial Transplant. 2014;29:29–40. doi: 10.1093/ndt/gft209. [DOI] [PubMed] [Google Scholar]
- 16.Khan F, Tritschler T, Kahn SR, et al. . Venous thromboembolism. Lancet. 2021;398:64–77. doi: 10.1016/S0140-6736(20)32658-1. [DOI] [PubMed] [Google Scholar]
- 17.Li J, Guo L, Shi S, et al. . The role of complement in microangiopathic lesions of IgA nephropathy. Kidney Int Rep. 2022;7:1219–1228. doi: 10.1016/j.ekir.2022.03.028. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Adam SS, Key NS, Greenberg CS.. D-dimer antigen: current concepts and future prospects. Blood. 2009;113:2878–2887. doi: 10.1182/blood-2008-06-165845. [DOI] [PubMed] [Google Scholar]
- 19.Weitz JI, Fredenburgh JC, Eikelboom JW.. A test in context: D-Dimer. J Am Coll Cardiol. 2017;70:2411–2420. doi: 10.1016/j.jacc.2017.09.024. [DOI] [PubMed] [Google Scholar]
- 20.Wakabayashi I, Masuda H.. Association of D-dimer with microalbuminuria in patients with type 2 diabetes mellitus. J Thromb Thrombolysis. 2009;27:29–35. doi: 10.1007/s11239-007-0155-0. [DOI] [PubMed] [Google Scholar]
- 21.Nwose EU, Richards RS, Jelinek HF, et al. . D-dimer identifies stages in the progression of diabetes mellitus from family history of diabetes to cardiovascular complications. Pathology. 2007;39:252–257. doi: 10.1080/00313020701230658. [DOI] [PubMed] [Google Scholar]
- 22.Soares AL, Rosário PW, Borges MA, et al. . PAI-1 and D-dimer in type 2 diabetic women with asymptomatic macrovascular disease assessed by carotid Doppler. Clin Appl Thromb Hemost. 2010;16:204–208. doi: 10.1177/1076029609334626. [DOI] [PubMed] [Google Scholar]
- 23.Huang MJ, Wei RB, Wang Y, et al. . Blood coagulation system in patients with chronic kidney disease: a prospective observational study. BMJ Open. 2017;7:e014294. doi: 10.1136/bmjopen-2016-014294. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Gordge MP, Faint RW, Rylance PB, et al. . Plasma D dimer: a useful marker of fibrin breakdown in renal failure. Thromb Haemost. 1989;61(03):522–525. doi: 10.1055/s-0038-1646627. [DOI] [PubMed] [Google Scholar]
- 25.Irish A. Cardiovascular disease, fibrinogen and the acute phase response: associations with lipids and blood pressure in patients with chronic renal disease. Atherosclerosis. 1998;137:133–139. doi: 10.1016/s0021-9150(97)00273-6. [DOI] [PubMed] [Google Scholar]
- 26.Stevens LA, Claybon MA, Schmid CH, et al. . Evaluation of the Chronic Kidney Disease Epidemiology Collaboration equation for estimating the glomerular filtration rate in multiple ethnicities. Kidney Int. 2011;79:555–562. doi: 10.1038/ki.2010.462. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Foley RN, Parfrey PS, Sarnak MJ.. Clinical epidemiology of cardiovascular disease in chronic renal disease. Am J Kidney Dis. 1998;32:S112–S119. doi: 10.1053/ajkd.1998.v32.pm9820470. [DOI] [PubMed] [Google Scholar]
- 28.Floege J, Barbour SJ, Cattran DC, et al. . Management and treatment of glomerular diseases (part 1): conclusions from a Kidney Disease: Improving Global Outcomes (KDIGO) controversies conference. Kidney Int. 2019;95(2):268–280. doi: 10.1016/j.kint.2018.10.018. [DOI] [PubMed] [Google Scholar]
- 29.Catena C, Zingaro L, Casaccio D, et al. . Abnormalities of coagulation in hypertensive patients with reduced creatinine clearance. Am J Med. 2000;109:556–561. doi: 10.1016/s0002-9343(00)00567-2. [DOI] [PubMed] [Google Scholar]
- 30.Sagripanti A, Cupisti A, Giannessi D, et al. . The use of picotamide in nephropathy with mesangial IgA deposits. The effect on thromboxane generation. Clin Ter. 1993;142:47–52. [PubMed] [Google Scholar]
- 31.Pulivarthi S, Gurram MK.. Effectiveness of d-dimer as a screening test for venous thromboembolism: an update. N Am J Med Sci. 2014;6:491–499. doi: 10.4103/1947-2714.143278. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Koch V, Biener M, Müller-Hennessen M, et al. . Diagnostic performance of D-dimer in predicting venous thromboembolism and acute aortic dissection. Eur Heart J Acute Cardiovasc Care. 2020:2048872620907322. doi: 10.1177/2048872620907322. [DOI] [PubMed] [Google Scholar]
- 33.Karny-Epstein N, Abuhasira R, Grossman A.. Current use of D-dimer for the exclusion of venous thrombosis in hospitalized patients. Sci Rep. 2022;12:12376. doi: 10.1038/s41598-022-16515-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Chen P, Yu G, Zhang X, et al. . Plasma galactose-deficient IgA1 and C3 and CKD progression in IgA nephropathy. Clin J Am Soc Nephrol. 2019;14(10):1458–1465. doi: 10.2215/CJN.13711118. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Ono T, Kanatsu K, Ueda S, et al. . Detection of the antigenicity of the d-dimer of cross linked fibrin in the glomerulus by plasmin treatment. Kidney Int. 1994;46(1):260–265. doi: 10.1038/ki.1994.268. [DOI] [PubMed] [Google Scholar]
- 36.Liu N, Ono T, Suyama K, et al. . Mesangial factor V expression colocalized with fibrin deposition in IgA nephropathy. Kidney Int. 2000;58(2):598–606. doi: 10.1046/j.1523-1755.2000.00206.x. [DOI] [PubMed] [Google Scholar]
- 37.Xia M, Liu D, Peng L, et al. . Coagulation parameters are associated with the prognosis of immunoglobulin a nephropathy: a retrospective study. BMC Nephrol. 2020;21(1):447. doi: 10.1186/s12882-020-02111-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Gordge MP, Leaker BR, Rylance PB, et al. . Haemostatic activation and proteinuria as factors in the progression of chronic renal failure. Nephrol Dial Transplant. 1991;6(1):21–26. doi: 10.1093/ndt/6.1.21. [DOI] [PubMed] [Google Scholar]
- 39.Pant A, Kopec AK, Luyendyk JP.. Role of the blood coagulation cascade in hepatic fibrosis. Am J Physiol Gastrointest Liver Physiol. 2018;315(2):G171–g176. doi: 10.1152/ajpgi.00402.2017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Zheng Z, Ma T, Lian X, et al. . Clopidogrel reduces fibronectin accumulation and improves diabetes-induced renal fibrosis. Int J Biol Sci. 2019;15(1):239–252. doi: 10.7150/ijbs.29063. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Yoshikawa N, Ito H, Sakai T, et al. . A controlled trial of combined therapy for newly diagnosed severe childhood IgA nephropathy. The Japanese Pediatric IgA Nephropathy Treatment Study Group. J Am Soc Nephrol. 1999;10(1):101–109. doi: 10.1681/ASN.V101101. [DOI] [PubMed] [Google Scholar]
- 42.Shima Y, Nakanishi K, Kaku Y, et al. . Combination therapy with or without warfarin and dipyridamole for severe childhood IgA nephropathy: an RCT. Pediatr Nephrol. 2018;33(11):2103–2112. doi: 10.1007/s00467-018-4011-6. [DOI] [PubMed] [Google Scholar]