Abstract
Background: Catheter-related infection (CRI) is a major complication in patients undergoing hemodialysis. The lack of high-throughput research on catheter-related microbiota makes it difficult to predict the occurrence of CRI. Thus, this study aimed to delineate the microbial structure and diversity landscape of hemodialysis catheter tips among patients during the perioperative period of kidney transplantation (KTx) and provide insights into predicting the occurrence of CRI.
Methods: Forty patients at the Department of Transplantation undergoing hemodialysis catheter removal were prospectively included. Samples, including catheter tip, catheter outlet skin swab, catheter blood, peripheral blood, oropharynx swab, and midstream urine, from the separate pre- and post-KTx groups were collected and analyzed using metagenomic next-generation sequencing (mNGS). All the catheter tips and blood samples were cultured conventionally.
Results: The positive detection rates for bacteria using mNGS and traditional culture were 97.09% (200/206) and 2.65% (3/113), respectively. Low antibiotic-sensitivity biofilms with colonized bacteria were detected at the catheter tip. In asymptomatic patients, no statistically significant difference was observed in the catheter tip microbial composition and diversity between the pre- and post-KTx group. The catheter tip microbial composition and diversity were associated with fasting blood glucose levels. Microorganisms at the catheter tip most likely originated from catheter outlet skin and peripheral blood.
Conclusions: The long-term colonization microbiota at the catheter tip is in a relatively stable state and is not readily influenced by KTx. It does not act as the source of infection in all CRIs, but could reflect hematogenous infection to some extent.
Keywords: Hemodialysis catheter-related infection, catheter tip, perioperative period of kidney transplantation, biofilm, metagenome next-generation sequencing
1. Introduction
Central venous catheter (CVC) is commonly used for vascular access during hemodialysis in patients with end-stage renal disease [1, 2]. Approximately 83.3% of patients initiated hemodialysis with CVCs in 2020, and 16.8% of these remained in use 12 months after hemodialysis [3]. One of the major clinical complications of indwelling hemodialysis catheter, i.e. catheter-related infection (CRI), is the second leading cause of death in patients undergoing hemodialysis following cardiovascular events [2, 4]. CVC contributes to a 1.5-to 3-fold increased risk of death from infectious causes and a 2-to 3-fold increased risk of death from all-cause [4]. In patients carrying hemodialysis catheters during the perioperative period of kidney transplantation (KTx), the prevention and early diagnosis of CRI is particularly important owing to the intensive use of immunosuppressants [5]. Some studies suggest that biofilms are closely associated with the occurrence and development of CRI [4]. However, the impact of intensive immunosuppressants during the perioperative period of KTx on the hemodialysis catheter biofilms, and the association between biofilm changes and the occurrence and development of CRI, remain underexplored.
Currently, the CRI diagnosis primarily relies on traditional culture, immunological detection, and polymerase chain reaction (PCR) [6]. However, traditional culture is limited by long wait times, low pathogen coverage, and insufficient positive detection rate. Although PCR testing is faster than traditional culture, the need for designing specific primer sets makes identification of mixed or rare infections difficult, and often leads to a lack of accurate clinical information [6, 7]. The interior of the catheter is relatively clean, and thus high-sensitivity detection methods are needed for the detection of catheter-related microbiota. Currently, next-generation sequencing (NGS) mainly includes three applications in clinical microbiology laboratories, namely whole-genome sequencing, targeted NGS and metagenomics NGS (mNGS), which are used extensively in precision medicine, especially for the identification of difficult-to-culture, atypical, and rare pathogens [8–11]. Among them, mNGS, known as agnostic or unbiased NGS, has emerged as a promising single and universal pathogen (i.e. bacteria, fungi, parasites, and viruses) detection method for allowing pan-nucleic acid detection directly from patient samples [6]. It has the advantages of a short detection cycle, wide coverage and high sensitivity. Multiple studies have confirmed the importance of mNGS in detecting pathogens and reducing the empirical treatment time in the absence of any evidence of pathogenicity [12–14]. Previous studies have mostly focused on the diagnostic value of mNGS in catheter-related bloodstream infections [6], with few on the composition and clinical significance of the colonization microbiota in catheters.
Therefore, this study aimed to delineate the microbial structure and diversity landscape of the hemodialysis catheter tip in dialysis patients during the perioperative period of KTx, bridge the gap in the normal colonization microbial database of hemodialysis catheters, improve the diagnostic specificity of mNGS for hemodialysis catheter samples, and provide new insights for prevention of CRIs.
2. Materials and methods
2.1. Patients and study design and biological samples collection
This study was approved by the Ethics Committee of Nanfang Hospital, Southern Medical University (NFEC-2023-105). Each patient voluntarily participated in the study and signed a written informed consent. All kidney allografts were from donation after brain death (DBD) donors. No organs were obtained from prisoners.
Inclusion criteria: 1. Patients undergoing internal jugular vein/femoral vein hemodialysis catheter removal or replacement from May 2020 to April 2022 at the Department of Transplantation, Nanfang Hospital, Southern Medical University, including those who routinely replace hemodialysis catheters before KTx and those who remove hemodialysis catheters after KTx due to no longer clinically indicated; 2. Patients who underwent hemodialysis catheter removal due to suspected CRI. CRI is defined as: 1. No obvious source of bloodstream infection other than the catheter; 2. The same microorganism is isolated from both peripheral blood and catheter tip after excluding other sources of infection; 3. Clinical symptoms of infection (such as fever, redness of the exit site of the catheter, etc.) improve after removal of the catheter [6, 15].
Before catheter removal in the procedure room, the catheter outlet skin swab with a diameter of 2 cm was taken, and 10 mL of catheter blood (discharging the initial 5 mL of blood) and peripheral blood from the right arm vein were respectively collected using cfDNA vacuum collection tubes. The catheter was directly removed under aseptic conditions, and completed using a no-touch technique with sterile gloves and gowns. After completely removing the catheter, the distal 4 cm of the catheter tip was cut and divided into two parts along the long axis by sterile surgical scissors, and immersed in 10 mL of sterile saline for examination. The midstream urine was taken on the morning of the day of catheter removal and an oropharynx swab was simultaneously collected. The above samples are processed for DNA extraction within 24 h and stored at −80 °C. mNGS is performed on the same batch of samples within one working day, and catheter blood, peripheral blood, and catheter tip are simultaneously subjected to traditional culture.
The patients’ baseline demographics (such as age, sex, home address, and body mass index, etc.), date and location of the last catheterization, antibiotic usage, laboratory test indicators (such as white blood cell count, neutrophil count, platelet count, hemoglobin, serum creatinine, and C-reactive protein, etc.) on the day of catheter removal, history of kidney disease, comorbidities, and transplant-related information (including the immunosuppression induction regimen, maintenance immunosuppression regimen, and delayed graft function) of KTx recipients were collected.
2.2. DNA extraction and library construction
Cell-free DNA from blood samples was extracted using the QIAamp Circulating Nucleic Acid Kit (Qiagen, Germany) and urine DNA was extracted using the TIANamp Magnetic DNA Kit (Tiangen, China) according to the manufacturer’s instructions. The catheter tip samples soaking in saline solution, as well as the catheter outlet skin and oropharynx swabs placed in PBS, were vortexed thoroughly at 2800 rpm for 15 s. Then the saline and PBS wash were collected to extract DNA using a magnetic bead method kit developed by a third-party testing agency (Nanjing Dinfectome Technology Inc., Nanjing, China). The quantity and quality of DNA were assessed using the Qubit (Thermo Fisher Scientific, USA) and NanoDrop (Thermo Fisher Scientific, USA), respectively, and more than 3 ng of total DNA was required for quality control. The DNA library was prepared using the KAPA Hyper Prep Kit (KAPA Biosystems, USA) in accordance with the manufacturer’s protocol. The quality controll was conducted using the Agilent 2100 system (Agilent Technologies, Santa Clara, USA).
2.3. Sequencing and data analysis
DNA libraries were sequenced on the Illumina NextSeq 550Dx (Illumina, USA) using a 75-cycle single-end index sequencing kit. Raw sequencing data was filtered by bcl2fastq2, and high-quality sequencing data were generated using Trimmomatic by removing low-quality reads, adapter contamination, and duplicated and shot (length < 36 bp) reads. Human host sequences were subtracted by mapping to the human reference genome (hs37d5) using bowtie2. Reads that could not be mapped to the human genome were retained and aligned with the microorganism genome database for microbial identification by Kraken, and for species abundance estimating by Bracken. The microorganism genome database contained genomes or scaffolds of bacteria, fungi, viruses and parasites (download from GenBank release 238, ftp://ftp.ncbi.nlm.nih.gov/genomes/genbank/).
2.4. Metagenome and interpretation
Functional metagenome profiling was calculated using HUMAnN2 standard workflow with default settings. Tables of UniRef90 gene family and MetaCyc pathway abundances in units of reads per kilobase (RPK) were normalised to relative abundance using the humann2_renorm_table command of HUMAnN2. Tables of normalized gene family were converted into Gene Ontology (GO) and KEGG Orthology terms (KO) using humann2_regroup_table command of HUMAnN2. Then, normalized KO were mapped to KEGG pathways and KEGG modules using MinPath, as provided with the HUMAnN2.
2.5. Statistical analysis
Statistical analysis was performed by R software (v4.0.1). Alpha diversity was estimated by the Shannon index based on the taxonomic profile of each sample and beta diversity was assessed by the Bray-Curtis measure. Pre- and post-operative samples from pre- and post-KTx groups, which were separate patient populations selected at different time points, were compared by using Wilcoxon rank sum test and visualized by principal coordinate analysis (PCoA) plot. PERMANOVA was performed by the R package “vegan” to analyze Bray-Curtis distance in different groups. Differential relative abundance of taxonomic groups at the genus level among groups was tested by using Kruskal-Wallis rank sum test (R package “kruskal.test”). The genera with mean relative abundances greater than 1% and penetrance greater than 40% among all samples were compared. Spearman’s correlations between clinical features and the relative abundances of genera were calculated by the R package “cor.test”, and FDR correction was adopted to adjust all p values. Statistically significant differences in the relative abundance of microbe, GO function, KEGG pathways and MetaCyc pathways among groups were assessed by the linear discriminant analysis of effect size (LEfSe) analysis. Mean with standard diviation and median with interquartile range (IQR) values were described statistically, and the significance of the mean difference between groups was tested by t-test for continuous variables. Chi-square or Fisher’s exact tests were used to evaluate the significance of the sensitivity difference between different groups for non-matched samples.
3. Results
3.1. Sample and patient characteristics
Overall, 40 patients who underwent hemodialysis catheter removal during hospitalization were enrolled in the study, with 21 (52.50%) males and 19 (47.50%) females. Among them, 18 patients underwent catheter replacement before KTx, with a median duration of 35 (IQR: 14–69) days from the last catheter placement and a median time of 7 (IQR: 3–15) days to KTx, and four cases were suspected of CRI. Further, 22 patients underwent hemodialysis catheter removal after KTx, with a median duration of 10 (IQR: 6–11) days from KTx to catheter removal, and two cases were suspected of CRI. Patients with suspected CRI and those who underwent KTx received antibiotics. Patients after KTx were also treated with a combination of immunosuppression induction regimen with "basiliximab + methylprednisolone" and maintenance immunosuppression regimen with "tacrolimus + mycophenolate + prednisone". Other clinical information and laboratory findings on the day of catheter removal are presented in Table 1.
Table 1.
Clinical characteristics of patients.
| Total (n = 40) | Before KT (n = 18) | After KT (n = 22) | t value | p value | |
|---|---|---|---|---|---|
| Patient demographic | |||||
| Age (mean ± SD) | 39.75 ± 12.19 | 37.83 ± 10.11 | 41.32 ± 13.47 | 0.885 | 0.382 |
| Male, n (%) | 21 (52.50%) | 10 (55.56%) | 11 (50.00%) | — | 0.761 |
| BMI | 20.60 ± 3.42 | 20.46 ± 3.60 | 20.77 ± 3.42 | 0.290 | 0.773 |
| Insertion site of CVC | |||||
| Insertion with CVC | 40 (100.00%) | 18 (45.00%) | 22 (55%) | — | — |
| Internal jugular vein, n (%) | 34 (85.00%) | 12 (66.67%) | 22 (100.00%) | — | 0.005 |
| Femoral vein, n (%) | 6 (15.00%) | 6 (33.33%) | 0 (0.00%) | — | 0.005 |
| Duration of catheterization, days (mean ± SD) | 115.9 ± 157.42 | 91.83 ± 161.71 | 135.59 ± 151.00 | 0.861 | 0.395 |
| ISD use, n (%) | 22 (55.00%) | 0 (0.00%) | 22 (100.00%) | — | — |
| Duration from ISD use to catheter removal, days (mean ± SD) | 14.00 ± 14.35 | — | 14.00 ± 14.35 | — | — |
| Suspected infection, n (%) | 6 (15.00%) | 4 (22.22%) | 2 (9.09%) | — | 0.381 |
| Antibiotic use, n (%) | 26 (65.00%) | 4 (22.22%) | 22 (100.00%) | — | <0.001 |
| Days hospitalized, days (IQR) | 15 (3-59) | 7 (3-29) | 15.5 (13-59) | 3.063 | 0.004 |
| Laboratory parameters | |||||
| WBC, (3.5–9.5) ×10^9/L | 8.57 ± 3.94 | 7.06 ± 2.07 | 9.81 ± 4.63 | 2.273 | 0.029 |
| NEU, (1.8–6.3) ×10^9/L | 6.14 ± 3.20 | 4.75 ± 1.86 | 7.28 ± 3.60 | 2.633 | 0.012 |
| RBC, Male (4.3–5.8) ×10^9/L, Female (3.8–5.1) ×10^9/L | 3.61 ± 0.75 | 3.39 ± 0.71 | 3.80 ± 0.74 | 1.729 | 0.092 |
| PLT, (125–350) ×10^9/L | 215.23 ± 63.81 | 220.39 ± 67.20 | 211.00 ± 60.57 | 0.452 | 0.654 |
| HGB, Male (130–175) g/L, female (115–150) g/L | 103.15 ± 17.53 | 95 ± 17.92 | 109.82 ± 14.04 | 2.858 | 0.007 |
| CRP, (0–6) mg/L | 11.34 ± 21.66 | 16.08 ± 29.59 | 7.34 ± 9.62 | 1.179 | 0.247 |
| Scr, Male (57–97) μmol/L, female (41–81) μmol/L | 215.20 ± 134.95 | — | 215.20 ± 134.95 | — | — |
| DGF | 5 (12.50%) | — | 5 (22.73%) | — | — |
| Underlying diseases | |||||
| Diabetes mellitus, n (%) | 5 (12.50%) | 1 (5.56%) | 4 (18.18%) | — | 0.356 |
| Chronic hepatitis/liver cirrhosis, n (%) | 8 (20.00%) | 4 (22.22%) | 4 (18.18%) | — | 1.000 |
| Hypertension, n (%) | 30 (75.00%) | 15 (83.33%) | 15 (68.18%) | — | 0.465 |
KT: kidney transplantation; BMI: Body Mass Index; CVC: Central venous catheter; SD: standard deviation; ISD: immunosuppressive drugs; IQR: interquartile range; WBC: white blood cell; NEU: Neutrophils; WBC: red blood cell; PLT: platelet; HGB: hemoglobin; Scr: serum creatinine; CRP: C-reactive protein; DGF: delayed graft function.
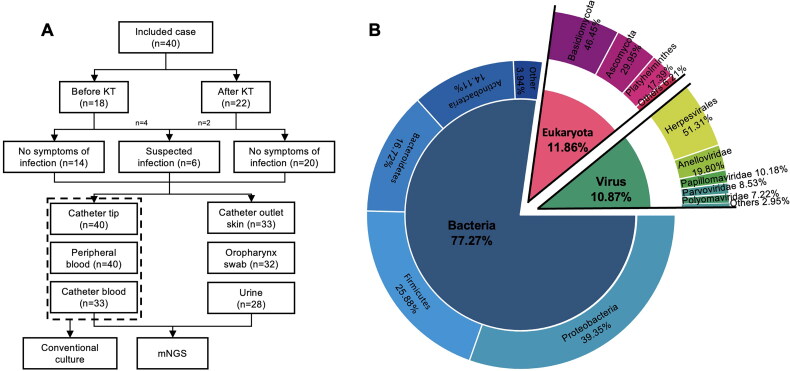
A total of 206 clinical samples were collected, including 40 catheter tip samples, 40 peripheral blood samples, 33 catheter blood samples, 33 catheter outlet skin swabs (30 from the neck and three from the groin), 32 oropharyngeal swabs, and 28 midstream urine samples (Figure 1). All the above samples were tested using mNGS. The catheter tip, peripheral blood and catheter blood samples were simultaneously cultured conventionally. Among the 206 samples, at least one pathogenic microorganism was detected in the other samples using mNGS (97.09%, 200/206), except in two catheter tip samples, three peripheral blood samples and one catheter blood sample. Among the 113 samples tested using traditional culture, only 3 samples (2.65%, 3/113) obtained from the catheter tip, catheter blood and peripheral blood of one patient with clinical indication of CRI were positive.
Figure 1.
(A) Analysis workflow for the 206 total samples in the study. (B) The pie chart presents the proportion of bacteria, fungi and viruses after the normalization of the microbial sequences of 206 samples. Phylum-level proportions were used to represent the distribution of bacteria and fungi, family-level proportions were used to represent the distribution of viruses.
3.2. Core microbiota composition across different sampling sites
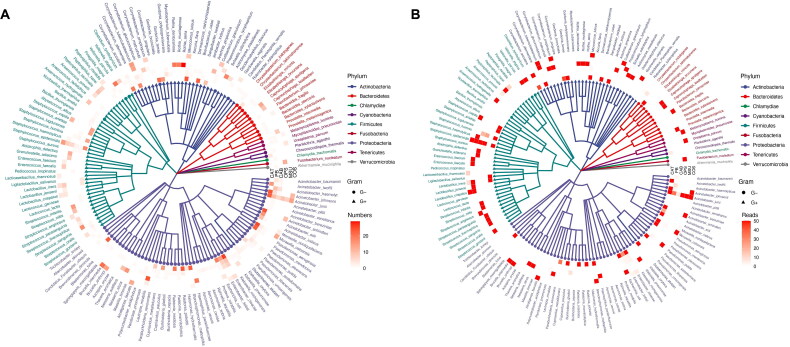
Through mNGS, a total of 21.22 million high-quality microbial reads were detected from 206 clinical samples, including 17.8 million bacteria, 1.87 million fungi, and 1.54 million viruses. Overall, 1,545 bacteria, 226 fungi, and 57 viruses were identified. Figure 2 presents the phylogenetic composition, detection frequency, and reads of the dominant bacterial species (relative abundance >10%) in the six types of samples. The average bacterial reads of catheter tip, catheter blood, peripheral blood, catheter outlet skin swab, oropharyngeal swab, and midsection urine samples were 16 954.18 ± 38 204.57, 116.82 ± 317.93, 75.55 ± 193.13, 69 180.48 ± 164 786.25, 390 619.69 ± 523 535.78, and 83 567.914 ± 3 886, respectively. Significant differences were observed in the bacterial reads between oropharynx swabs and other types of samples (p < 0.001), while no statistically significant differences between other sample types (p > 0.050). Proteobacteria, Firmicutes, Actinobacteria, and Bacteroidetes were the most frequently detected phyla, accounting for 96.18% of all detected bacteria and 95.01% of all bacterial reads. At the species level, the microbial detection frequency of the catheter tip and catheter outlet skin swab samples were the highest, with an average frequency of 116.88 ± 138.51 and 75.76 ± 134.01, respectively. The microbial detection frequency of the catheter blood and peripheral blood samples were the lowest, with an average frequency of 3.27 ± 2.96 and 4.38 ± 5.09, respectively. The average detection frequency in oropharyngeal swabs and midstream urine samples were 48.47 ± 32.60 and 25.39 ± 25.20, respectively. The bacterial detection frequency on the catheter tip was significantly different from those in catheter blood (p < 0.001), peripheral blood (p < 0.001), oropharyngeal swab (p = 0.009), and midstream urine (p < 0.001). The bacterial detection frequencies in the catheter outlet skin swab were significantly different from those of catheter blood (p = 0.007) and peripheral blood (p = 0.005). No statistically significant differences (p > 0.050) were observed in the frequencies of bacterial detection among the remaining samples. Except for the urine samples, all other samples had high detection frequencies of gram-negative bacteria (43.73%, 3 940/9 000).
Figure 2.
Phylogenetic composition of the microbiota across six different sampling sites. The phylogenetic tree reveals the dominant species of each sample type (relative abundance > 10%). Gram-negative bacteria are represented by circular lines, while gram-positive bacteria are represented by triangular lines. The circles from the innermost to the outer represent samples represent the catheter tip, peripheral blood, catheter blood, oropharyngeal swab, midstream urine, and catheter outlet skin swab, respectively. The colored squares indicate the detection frequency (A) and reads (B) of dominant bacterial species in each sample type.
Six patients were suspected of CRI after excluding other sources of infection based on symptoms including recurrent fever and skin redness around the catheter outlet. The catheter tip samples from the suspected CRI group had more dominant microorganisms than those from the asymptomatic group. Among the six patients with suspected CRI, three had consistent high-abundance pathogens in the catheter tip, catheter blood, and peripheral blood samples. One of these patients was infected with Staphylococcus aureus, while the other two were infected with Primate erythroparvovirus 1. The other three cases had high-abundance microorganisms in the catheter tip, including Pseudomonas azotoformans, Cupriavidus pauculus, Corynebacterium tuberculostearicum, and Escherichia coli.
3.3. Effect of immunosuppressants and antibiotics on microbiota in the catheter tip
Overall, 1 298 bacteria, 194 fungi, and 24 viruses were detected in the catheter tips. Proteobacteria, Actinobacteria, Firmicutes, and Bacteroidetes were the most frequently detected catheter tip organisms both in the pre- and post-KTx groups, accounting for 97.30% (4 549/4 675) of the total organisms detected. The majority of detected bacteria were gram-negative bacteria (43.36%, 2 027/4 675). Eukaryota was dominated by Ascomycota (65.51%, 397/606) (Figure 2).
In asymptomatic patients, the microbial reads of catheter tips in the pre- (n = 14) and post-KTx group (n = 20) were 17 578.71 ± 30 920.07 and 18 022.80 ± 50 546.98 (p > 0.050), respectively corresponding to 100.14 ± 93.15 and 104.45 ± 116.54 bacterial strains, respectively (p > 0.050). Enterobacter cloacae (9/14), Achromobacter xylosoxidans (9/14), Acinetobacter schindleri (9/14), Moraxella osloensis (8/14), Micrococcus luteus (8/14), Acinetobacter johnsonii (8/14), Massilia timonae (8/14), Pseudomonas aeruginosa (8/14), Brevundimonas diminuta (8/14), Acinetobacter baumannii (8/14), Stenotrophomonas maltophilia (8/14), Acinetobacter pittii (8/14), and Candida parapsilosis (8/14) were the most frequently detected bacteria in the pre-KTx group. Enterobacter cloacae (13/20), Ralstonia pickettii (13/20), Moraxella osloensis (12/20), Micrococcus luteus (12/20), Acinetobacter johnsonii (12/20), Brevibacterium casei (12/20), Kocuria palustris (12/20), Acinetobacter junii (12/20), Malassezia restricta (12/20), and Corynebacterium accolens (12/20) were the most frequently detected bacteria in the post-KTx group. A total of 60 strains of dominant bacteria were detected in the two groups, with 19 and 41 strains, respectively, and the majority were gram-negative bacteria (55.00%, 33/60). High-frequency (detection frequency > 50.00%) dominant bacteria detected in the two groups included Micrococcus luteus, Brevibacterium casei, Moraxella osloensis and Pseudomonas aeruginosa, whereas their common dominant bacteria included Acinetobacter indicus, Cupriavidus pauculus, Janibacter indicus, Pseudomonas azotoformans and Staphylococcus capitis. In the post-KTx group, patients were routinely treated with cefoperazone sodium sulbactam sodium, caspofungin, and metronidazole for infection prophylaxis within 7 days after KTx. The coverage rate of their antibacterial spectrum against the dominant catheter tip and blood bacteria was only 23.33%. Moreover, microorganisms in the antibacterial spectrum were still detected at the catheter tip, with an average abundance of 18.08%. No statistically significant differences were observed in microbial composition, alpha diversity, or beta diversity between the pre- and post-KTx groups (p > 0.050).
3.4. Microbiota composition in correlation to clinical characteristics
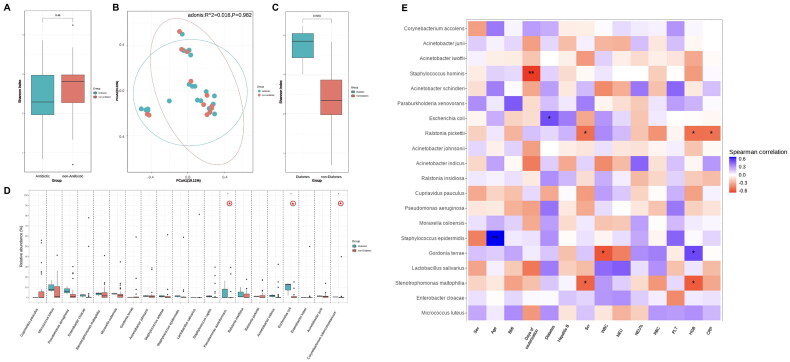
Microbial analysis of the catheter tip revealed that the microbial composition diversity was significantly higher in the diabetic group than in the non-diabetic group (p < 0.010). Pseudomonas azotoformans, Corynebacterium tuberculostearicum, and Escherichia coli strains were more abundant in the diabetic group than those in the non-diabetic group (p = 0.037, p = 0.046, p = 0.012) (Figure 3(D)). The species composition, alpha diversity, and beta diversity of the catheter tip microbiota had no significant correlation with underlying diseases, including hepatitis B virus infection and hypertension. In the post-KTx group, serum creatinine negatively correlated with the relative abundance of Stenotrophomonas maltophilia (R = −0.55, p = 0.027), and Ralstonia pickettii (R = −0.52, p = 0.041); no significant correlation was observed between delayed graft function and the species composition, alpha diversity, and beta diversity of the catheter tip microbiota. In both the pre- and post-KTx groups, hemoglobin levels negatively correlated with the relative abundance of Stenotrophomonas maltophilia (R= −0.58, p = 0.019) and Ralstonia pickettii (R = −0.52, p = 0.040); C-reactive protein levels negatively correlated with the relative abundance of Ralstonia pickettii (R = −0.51, p = 0.045); the duration of dialysis catheter placement negatively correlated with the relative abundance of Staphylococcus hominis (R = −0.66, p < 0.010); hemoglobin levels positively correlated with the relative abundance of Gordonia terrae (R = 0.54, p = 0.030); and white blood cell count negatively correlated with the relative abundance of Gordonia terrae (R = −0.60, p = 0.015) (Figure 3(E)).
Figure 3.
Microbiota composition in correlation to clinical characteristics. Alpha diversity (A) and beta diversity (B) analysis results between the immunosuppressant/antibiotic-treated group and non-immunosuppressant/antibiotic-treated group. Alpha diversity (C) and analysis of species difference (D) between the diabetes mellitus group and non-diabetes mellitus group. (E) Heat map representing the correlation between catheter tip microbiota and clinical characteristics. The top 20 bacterial species with the highest relative abundance were displayed on the vertical axis, and correlation analysis was conducted with clinical indicators on the horizontal axis. The gradient and similarity of colors reflect the differences and similarities in the composition of multiple samples at various classification levels. *p <0.050, **p <0.010.
3.5. Characterization of microbiota in catheter tip from the other samples
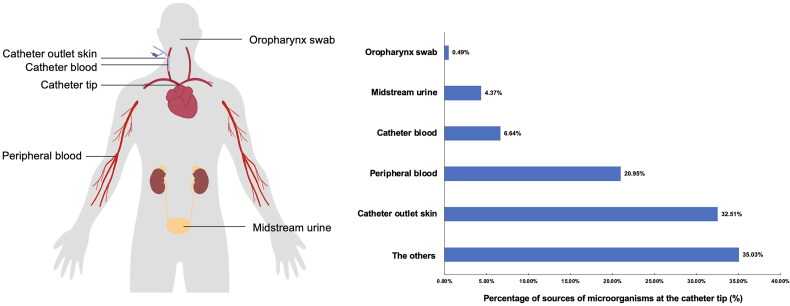
This study revealed the presence of colonization microorganisms in deep-vein hemodialysis catheters of asymptomatic patients and characterized the catheter biofilm flora. To explore the possible sources of microorganisms at the catheter tip, a Bayesian Algorithm Traceability Analysis was used to trace the source of the microorganisms detected from the catheter outlet skin swabs, oropharyngeal swabs, catheter blood, peripheral blood, and midstream urine samples, with microorganisms at the catheter tip considered as the target samples. Catheter outlet skin swabs were predicted to be one of the main sources of microorganisms at the catheter tip (32.51%), followed by occasional retention of microorganisms in the peripheral (20.95%) and catheter blood (6.64%) (Figure 4). Moreover, approximately 35.03% of the microorganisms did not belong to any of these sources.
Figure 4.
Tracing of microorganisms in catheter tip from the other samples.
4. Discussion
The incidence of CVC-related infections is much higher than those of autogenous arteriovenous and artificial vascular fistulas, and often causes catheter-related bacteremia, sepsis, and severe migratory infections, including infective endocarditis, osteomyelitis, joint abscess, and epidural abscess [16–18]. Previous studies have suggested that biofilm growth on indwelling catheters may be associated with the occurrence and development of CRI [4]. In this study, asymptomatic patients harbored abundant microorganisms, including dominant species with biofilm-forming ability, which involves irreversible adherence of microorganisms to device surfaces, followed by slow replication creating a self-sustaining bacterial community protected by a self-produced extracellular polysaccharide matrix [19, 20]. Examples of such organisms include Micrococcus luteus, Pseudomonas aeruginosa, and Stenotrophomonas maltophilia at the catheter tips [21–24]. Moreover, these microorganisms are difficult to detect using traditional culture methods.
At present, the lack of a normal colonization microbial database of hemodialysis catheters makes it difficult to rapidly and accurately distinguish between normal colonization and infection, which is inconducive for clinical diagnosis [25–27]. In this study, 80.00% of the participants were patients during the perioperative period of KTx with no clinical infection symptoms, thus, the mNGS results would serve as a suitable supplement to compensate for the lack of normal colonization microbial database of hemodialysis catheters in the KTx group [6]. In addition, a highly curated database is necessary to reduce false negative or false positive results in high-throughput sequencing. The data of this study help to improve the diagnostic specificity of mNGS for hemodialysis catheter samples. Our study revealed inter-species heterogeneity among catheter tip microbiota in a noninfectious state; however, the community was relatively stable. The catheter tip and lumen are non-sterile and harbor a stable biofilm composed of microorganisms, which can originate from the catheter outlet skin and peripheral blood and reach a stable state, based on the patient’s physiological condition.
The inter-species heterogeneity of the catheter tip microbiota may be associated with the source of microorganisms and the physiological conditions of the patients [6, 28]. To identify the source of microorganisms at the catheter tip, we collected samples from multiple sites including the catheter outlet skin, oropharynx, and peripheral blood [6, 28]. The results revealed that the catheter tip microbiota mainly originated from the catheter outlet skin, followed by those from peripheral blood. Based on this conclusion and the high cost of mNGS, we propose different sample retention patterns for the KTx group carrying hemodialysis catheters under different infection states, with a focus on preserving the catheter tip, catheter outlet skin, and peripheral blood samples. For groups without infection symptoms, the catheter tip and catheter outlet skin samples may be reserved for DNA extraction. If unexplained clinical infection symptoms occur early after catheter removal (within one month) and clinical samples culture is negative, then the retained DNA samples and corresponding clinical samples should be sent for NGS, providing clues for identifying the infection-causing pathogen and future course of treatment. For groups with clinically suspected infection symptoms, the catheter tip, catheter outlet skin and peripheral blood samples may be reserved for DNA extraction. If effective antibiotic treatment can be discerned based on the traditional detection of the catheter tip/peripheral blood, then the retained DNA samples need not be sent for NGS. Otherwise, reserved DNA samples should be sent for NGS, to prevent compound or notorious infections.
In this study, we collected hospital environmental samples for NGS testing from 80.00% (32/40) of patients. The mNGS results showed that there was no statistical difference in microbial diversity among environmental samples from different patients, and there was no significant correlation with the microbial composition of the catheter tip. Based on the dynamic network of microbial dispersal and considering that the hospital ward would undergo 12 h of routine ultraviolet disinfection before patient entry, we hypothesized that the unknown source of catheter tip microbiota most likely originated from long-term residential community environments (such as home and workplace) rather than short-term stay in the ward environment [29]. After disseminating from the public environment to the hosts’ skin, these microorganisms may not adapt well to the skin environment and are not established in microbial community networks. Thus after a brief presence on the catheter outlet skin, they further spread to the catheter tip and stably colonize at the catheter tip. Moreover, the patient’s physiological state including age and levels of fasting blood glucose, serum creatinine, hemoglobin, and inflammatory responses (C-reactive protein), can also affect colonization of the microbiota.
For noninfectious perioperative kidney transplant recipients carrying hemodialysis CVC, KTx has no significant impact on the stability of the microbial community on the hemodialysis catheter tip. No significant differences were observed in the composition and diversity of the microbial community at the hemodialysis catheter tip between the pre- and post-KTx groups under noninfectious states. Previous studies have reported that in patients with normal allograft function (without the need for resuming dialysis) post-surgery, the incidence of CRIs in recipients undergoing maintenance immunosuppressive therapy is not higher than that in patients undergoing hemodialysis before transplantation or after loss of kidney graft function [5]. In this study, antibiotics, such as cefoperazone sodium sulbactam sodium, caspofungin, and metronidazole, at conventional therapeutic concentrations, did not significantly affect the composition and diversity of catheter tip microbiota, and a stable microbial community at the catheter tip did not promote the development of CRI. Thus, the catheter tip microbiota is in a relatively stable state, not easily influenced by KT (p > 0.050), which could reflect hematogenous infection to some extent. Specifically, the long-term colonized microbiota at the catheter tip does not act as the source of infection in all CRIs, while the detected dominant species can be actual hematogenous pathogens that have destroyed the stability of the normal catheter tip microbiota.
Our study had some limitations that warrant discussion. Since all three positive traditional culture samples were from the same patient, it is statistically impossible to conduct a significance test on the correlation between the traditional culture results and the abundance of mNGS strains. Subsequent research with a larger sample size of the suspected CRI group can be conducted to establish a direct correlation test between the traditional culture results and the abundance of mNGS strains. Meanwhile, insufficient sample size in the diabetes group may have led to a conclusion bias and limited analysis in this study. Follow-up studies with a larger sample size of the diabetes group may elucidate the effect of blood glucose concentration on the composition of relevant microbiota in patients during the perioperative period of KTx. Due to limitations of sample size and sampling methods, the specific mechanisms of catheter tip biofilm formation, further tracing of microbes of unknown sources, and the predictive and monitoring value of biofilm status for the occurrence and development of CRI were not characterized in the study and need to be further explored.
In conclusion, to our knowledge, this is the first study to investigate the hemodialysis catheter tip microbiota during the perioperative period of KTx using mNGS. The mNGS results would serve as a suitable supplement to compensate for the deficiency of normal colonization microbial databases for the hemodialysis catheter in the KTx group. This information may provide new insights for preventing CRIs during the perioperative period of KTx.
Acknowledgments
ZYY, YCW and YM participated in research design. ZYY and YNL participated in the writing of the paper. HJD, JX, YCW and YM performed critical revision of the manuscript for important intellectual content. WLZ, RFX, ZTW and WFD participated in the performance of the research. YM took charge of obtaining funding. ZYY and MZ performed statistical analysis. YM and HJD supervised the study. All authorship read and approved the final version.
Funding Statement
This study is funded by National Natural Science Foundation of China (Grant No.82270784, 82070770), Natural Science Foundation of Guangdong Province (Grant No.2023A1515012276), and Youth Medical Innovation and Practice Research Program of Guangzhou (Grant No. 2023QNYXZD002).
Disclosure statement
No potential conflict of interest was reported by the author(s).
References
- 1.Sheng KX, Zhang P, Li JW, et al. Comparative efficacy and safety of lock solutions for the prevention of catheter-related complications including infectious and bleeding events in adult haemodialysis patients: a systematic review and network meta-analysis. Clin Microbiol Infect. 2020;26(5):1–9. doi: 10.1016/j.cmi.2019.12.003. [DOI] [PubMed] [Google Scholar]
- 2.Ravani P, Palmer SC, Oliver MJ, et al. Associations between hemodialysis access type and clinical outcomes: a systematic review. J Am Soc Nephrol. 2013;24(3):465–473. doi: 10.1681/ASN.2012070643. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.US Renal Data System . 2022. Annual Data Report. Volume 2: End Stage Renal Disease. Available at: https://www.niddk.nih.gov/about-niddk/strategic-plans-reports/usrds.
- 4.Almeida BM, Moreno DH, Vasconcelos V, et al. Interventions for treating catheter-related bloodstream infections in people receiving maintenance haemodialysis. Cochrane Database Syst Rev. 2022;4(4):CD013554. doi: 10.1002/14651858.CD013554.pub2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Woodside KJ, Schirm ZW, Noon KA, et al. Fever, infection, and rejection after kidney transplant failure. Transplantation. 2014;97(6):648–653. doi: 10.1097/01.TP.0000437558.75574.9c. [DOI] [PubMed] [Google Scholar]
- 6.Okuda KI, Yoshii Y, Yamada S, et al. Detection of bacterial DNA from Central venous catheter removed from patients by next generation sequencing: a preliminary clinical study. Ann Clin Microbiol Antimicrob. 2018;17(1):44. doi: 10.1186/s12941-018-0297-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Yang A, Chen C, Hu Y, et al. Application of metagenomic Next-Generation sequencing (mNGS) using bronchoalveolar lavage fluid (BALF) in diagnosing pneumonia of children. Microbiol Spectr. 2022;10(5):e148822. doi: 10.1128/spectrum.01488-22. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Li N, Cai Q, Miao Q, et al. High-Throughput metagenomics for identification of pathogens in the clinical settings. Small Methods. 2021;5(1):2000792. doi: 10.1002/smtd.202000792. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Wang Y, Liu Y, Deng W, et al. Viral integration in BK polyomavirus-associated urothelial carcinoma in renal transplant recipients: multistage carcinogenesis revealed by next-generation virome capture sequencing. Oncogene. 2020;39(35):5734–5742. doi: 10.1038/s41388-020-01398-6. [DOI] [PubMed] [Google Scholar]
- 10.Yan S, Wang Y, Liu Y, et al. Coexistence of BKPyV lytic infection and viral integration in the development of BKPyV diseases after renal transplantation. Am J Transplant. 2021;21(S4):427–428.34551194 [Google Scholar]
- 11.Wang Y, Yan S, Liu Y, et al. Dynamic viral integration patterns actively participate in the progression of BK polyomavirus-associated diseases after renal transplantation. Am J Transplant. 2023. Online ahead of print]. doi: 10.1016/j.ajt.2023.07.014. [DOI] [PubMed] [Google Scholar]
- 12.Yan Z, Deng W, Wang Y, et al. Case report: malacoplakia due to E. coli with Cryptococcus albidus infection of a transplanted kidney in a patient with recurrent urinary tract infection. Front Med. 2021;8:721145. doi: 10.3389/fmed.2021.721145. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Wang Y, Mo X, Zhang J, et al. Clinical features of Talaromyces marneffei infection in HIV-positive and HIV-negative individuals: a retrospective study in Southern China. Med Mycol. 2023;61(8):myad083. [DOI] [PubMed] [Google Scholar]
- 14.Zhang Y, Cui P, Zhang HC, et al. Clinical application and evaluation of metagenomic next-generation sequencing in suspected adult Central nervous system infection. J Transl Med. 2020;18(1):199. doi: 10.1186/s12967-020-02360-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Mermel LA, Allon M, Bouza E, et al. Clinical practice guidelines for the diagnosis and management of intravascular catheter-related infection: 2009 update by the infectious diseases society of america. Clin Infect Dis. 2009;49(1):1–45. doi: 10.1086/599376. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.El-Hennawy AS, Frolova E, Romney WA.. Sodium bicarbonate catheter lock solution reduces hemodialysis catheter loss due to catheter-related thrombosis and blood stream infection: an open-label clinical trial. Nephrol Dial Transplant. 2019;34(10):1739–1745. doi: 10.1093/ndt/gfy388. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Balikci E, Yilmaz B, Tahmasebifar A, et al. Surface modification strategies for hemodialysis catheters to prevent catheter-related infections: a review. J Biomed Mater Res B Appl Biomater. 2021;109(3):314–327. doi: 10.1002/jbm.b.34701. [DOI] [PubMed] [Google Scholar]
- 18.Donati G, Spazzoli A, Croci CA, et al. Bloodstream infections and patient survival with tunneled-cuffed catheters for hemodialysis: a single-center observational study. Int J Artif Organs. 2020;43(12):767–773. doi: 10.1177/0391398820917148. [DOI] [PubMed] [Google Scholar]
- 19.Donlan RM. Biofilm formation: a clinically relevant microbiological process. Clin Infect Dis. 2001;33(8):1387–1392. doi: 10.1086/322972. [DOI] [PubMed] [Google Scholar]
- 20.Arciola CR, Campoccia D, Montanaro L.. Implant infections: adhesion, biofilm formation and immune evasion. Nat Rev Microbiol. 2018;16(7):397–409. doi: 10.1038/s41579-018-0019-y. [DOI] [PubMed] [Google Scholar]
- 21.Encinas N, Yang CY, Geyer F, et al. Submicrometer-sized roughness suppresses bacteria adhesion. ACS Appl Mater Interfaces. 2020;12(19):21192–21200. doi: 10.1021/acsami.9b22621. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Celiksoy V, Moses RL, Sloan AJ, et al. Synergistic in vitro antimicrobial activity of pomegranate rind extract and zinc (II) against Micrococcus luteus under planktonic and biofilm conditions. Pharmaceutics. 2021;13(6):851. doi: 10.3390/pharmaceutics13060851. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Martínez E, Campos-Gómez J.. Oxylipins produced by Pseudomonas aeruginosa promote biofilm formation and virulence. Nat Commun. 2016;7(1):13823. doi: 10.1038/ncomms13823. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Brooke JS. Advances in the microbiology of Stenotrophomonas maltophilia. Clin Microbiol Rev. 2021;34(3):e3019. doi: 10.1128/CMR.00030-19. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Wilson MR, Sample HA, Zorn KC, et al. Clinical metagenomic sequencing for diagnosis of meningitis and encephalitis. N Engl J Med. 2019;380(24):2327–2340. doi: 10.1056/NEJMoa1803396. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Boolchandani M, D’Souza AW, Dantas G.. Sequencing-based methods and resources to study antimicrobial resistance. Nat Rev Genet. 2019;20(6):356–370. doi: 10.1038/s41576-019-0108-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Mounier R, Kapandji N, Gricourt G, et al. Assessment of bacterial colonization of intracranial pressure transducers: a prospective study. Neurocrit Care. 2021; Jun34(3):814–824. doi: 10.1007/s12028-020-01096-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Zanwar S, Jain P, Gokarn A, et al. Antibiotic lock therapy for salvage of tunneled Central venous catheters with catheter colonization and catheter-related bloodstream infection. Transpl Infect Dis. 2019;21(1):e13017. doi: 10.1111/tid.13017. [DOI] [PubMed] [Google Scholar]
- 29.Wilkins D, Tong X, Leung M, et al. Diurnal variation in the human skin microbiome affects accuracy of forensic microbiome matching. Microbiome. 2021; 9(1):129. doi: 10.1186/s40168-021-01082-1. [DOI] [PMC free article] [PubMed] [Google Scholar]