Abstract
Background and aim
Plasma fibrinogen has been proven to be significantly associated with cardiovascular mortality in patients undergoing peritoneal dialysis (PD). The study aimed to investigate the role of fibrinogen in left ventricular (LV) remodeling and functions in patients on PD, and explore risk factors related to high fibrinogen level.
Methods
From February 2008 to July 2018, adult patients on regular PD for at least 1 month were recruited and followed up for two years. Correlation analysis was performed to explore the fibrinogen level and echocardiography measurements. Pathogenic factors correlated to the left ventricular hypertrophy (LVH) progression were explored by logistic regression models and the role of fibrinogen in it was verified by receiver operating characteristic (ROC) curves. Linear regression models were conducted to identify factors associated with fibrinogen level.
Results
A total of 278 patients undergoing PD (168 males, 60.4%) were recruited. Patients were trisected according to fibrinogen levels at baseline. Mean wall thickness (MWT), relative wall thickness (RWT), and left ventricular mass index (LVMI) were positively associated with fibrinogen level while E/A ratio was negatively associated with it. Multivariate logistic regression and ROC curve showed that fibrinogen was an independent risk factor for LVH progression. Multivariate linear regression analysis identified age, total cholesterol (CHO), fasting blood glucose (FBG), and high-sensitivity C-reactive protein (hsCRP) were significantly related to plasma fibrinogen level.
Conclusions
An elevated fibrinogen level was independently associated with LVH progression in patients undergoing PD. Older age, higher level of FBG, CHO, and hsCRP were risk factors for elevated plasma fibrinogen level.
Keywords: Cardiovascular risks, fibrinogen, inflammation, left ventricular hypertrophy, peritoneal dialysis
Introduction
Patients with end-stage renal disease (ESRD) undergoing dialysis more often have cardiovascular disorders such as hypertension, left ventricular (LV) remodeling, vascular stiffness, etc. [1]. These patients also suffer from a much higher cardiovascular mortality [2]. Plasma fibrinogen, a molecule playing an important role in the coagulation, is considered as an established predictor of cardiovascular events in the general population. It is reported that an increase in concentration of fibrinogen from 250 to 350 mg/dL is associated with an 80% increase in coronary risk [3].
In the general population, plasma fibrinogen level has been linked to LV hypertrophy (LVH) [4], which is more common among patients undergoing peritoneal dialysis (PD). According to statistics, the prevalence of LVH in patients on hemodialysis or PD is approximately 75% [5]. Recently, plasma fibrinogen level has been proven to be significantly associated with an increased risk of cardiovascular events and all-cause mortality in patients on PD [6,7]. Of note hyperfibrinogenemia is common among patients treated with dialysis, especially in those on PD. Establishing whether fibrinogen is correlated to cardiovascular abnormalities in patients on PD is an important issue for it might contribute to cardiovascular risk stratification in these patients. According to previous studies, physiological response such as fluid overload, higher systemic inflammation status in patients on PD could double the risk of LV dysfunction [8]. Assessing whether fibrinogen is related to LV remodeling independently of these risk factors might help to clarify the pathophysiological link between this molecule and LVH. Nevertheless, few studies have explored the relationship between plasma fibrinogen level and LV remodeling among patients on PD directly.
Therefore, we aimed to investigate the relationship of plasma fibrinogen level with LV structure and function, and to explore risks factors related to high plasma fibrinogen level in patients undergoing PD in this study.
Patients and methods
Patients and study design
It was designed as a prospective observational study. The specific inclusion and exclusion criteria were as follows:
The inclusion criteria: (1) from February 2008 to July 2018, patients on regular PD for at least 1 month at Huashan Hospital, Fudan University, China; (2) patients aged 18 years and above; (3) patients can be regularly followed up; (4) written informed consent was given.
The exclusion criteria: (1) patients exhibited an ongoing infection, neoplasia, an acute cardiovascular disease or were clinically unstable with a life expectancy of less than 6 months; (2) patients with consciousness disorder or mental disorders.
All participants were closely followed up for two years. The study was approved by the Ethics Committee of Huashan Hospital at Fudan University (KY2010-296).
Clinical and laboratory measurements
Demographic characteristics including age, sex, body mass index (BMI), history of diabetes, history of smoking, and medication status (use of statins and hypotensive drugs) were recorded at recruitment. Blood pressure, laboratory parameters, and dialysis-related parameters were also obtained at baseline and collected semiannually. Blood sampling was always performed after fasting for at least 8 h and all samples were processed at the Department of Laboratory Medicine of Huashan Hospital, Fudan University. The laboratory data include hemoglobin, iron metabolism indexes, albumin, calcium, phosphorus, intact parathyroid hormone (iPTH), lipid metabolism parameters, plasma fibrinogen, N-terminal pro-brain natriuretic peptide (NT-proBNP), and high-sensitivity C-reactive protein (hsCRP). The PD-related data include normalized protein catabolic rate (nPCR), total Kt/V, residual Kt/V, total clearance of creatinine (Ccr), residual Ccr, glucose absorption, and volume of dialysate. Dialysis adequacy was evaluated using Baxter PD Adequest 2.0 software (Baxter Healthcare Corporation, Deerfield, IL). The time-average values of all these indexes during the follow up were calculated.
Echocardiography
Cardiac function and structure were accessed using a specialized ultrasound system (HD15 S5-1 1–5 MHz). All measurement data were read and confirmed by a registered diagnostic cardiac sonographer according to the recommendations of the American Society of Echocardiography (ASE) [9]. Standard transthoracic echocardiogram was performed in each participant at enrollment and followed up yearly. Echocardiographic data include left ventricular end-diastolic diameter (LVDd), interventricular septum thickness (IVST), posterior wall thickness (PWT) during diastole, left ventricular ejection fraction (LVEF), and E/A ratio. Left ventricular mass (LVM) was calculated based on the Devereux formula and indexed to height2.7 (LVMI) [10]. Mean wall thickness (MWT) and relative wall thickness (RWT), as two indexes of the LV geometric pattern, were calculated by the standard formula as follows: MWT = (PWT + IVST)/2; RWT = 2*PWT/LVDd [11]. LVH progression was defined as a growth rate of LVMI ≥ 5% in two years [12].
Statistical analysis
Data were presented as mean values ± standard deviation or median (interquartile range) for continuous variables and numbers (percentage) for categorical variables. Comparisons among groups were made by one-way/two-way analysis of variance (ANOVA), the Kruskal–Wallis test, or Chi-squared test, as appropriate. Correlational analyses were conducted to test the relationship between plasma fibrinogen level and LV geometry and function. Logistic regression analysis was performed to explore the role of fibrinogen in LVH-progression and receiver operating characteristic (ROC) curve was used to explore its predictive power for LVH-progression. Univariate and multivariate linear regression analyses were conducted to identify risk factors for a high level of plasma fibrinogen.
Statistical analysis was performed with SPSS 22.0 (SPSS, Inc., Chicago, IL). All statistical tests were two-sided and p values less than .05 were considered statistically significant.
Results
Characteristics of study population
A total of 278 patients (168 males, 60.4%) were included in our study with a mean age of 55.86 ± 16.54 years and a mean BMI of 23.72 ± 3.81 kg/m2. Patients were trisected according to plasma fibrinogen levels (Table 1). Plasma fibrinogen level (median 4.02 g/L, interquartile range 3.40–4.63 g/L) was above the upper limit of the normal range (cutoff >3.50 g/L) in the majority of the patients (n = 187, 67.3%). Patients with higher plasma fibrinogen levels were older, had longer PD duration and higher BMI, apolipoprotein B (ApoB), and hsCRP with a higher use rate of statins but lower nPCR.
Table 1.
Clinical characteristics of participants with different plasma fibrinogen levels.
| Total | Plasma fibrinogen level |
||||
|---|---|---|---|---|---|
| I tertile (n = 91) (≤3.5 g/L) | II tertile (n = 93) (3.5–4.4 g/L) | III tertile (n = 94) (≥4.4 g/L) | p Value | ||
| Age | 55.86 ± 16.54 | 47.16 ± 15.47 | 58.15 ± 16.16 | 63.76 ± 13.25 | <.001 |
| Male, n (%) | 168 (60.4%) | 56 (61.5%) | 51 (54.8%) | 52 (55.3%) | .540 |
| BMI (kg/m2) | 23.72 ± 3.81 | 22.67 ± 3.55 | 23.95 ± 4.00 | 24.57 ± 3.72 | .004 |
| History of smoking | 55 (19.8%) | 17 (18.7%) | 20 (21.5%) | 16 (17.0%) | .754 |
| History of diabetes | 68 (24.5%) | 33 (36.3%) | 41 (44.1%) | 32 (34.0%) | .006 |
| Use of statins | 165 (60.4%) | 44 (48.4%) | 56 (60.2%) | 62 (66.0%) | .001 |
| Use of hypotensive drugs | 258 (94.5%) | 85 (93.4%) | 87 (93.5%) | 77 (81.9%) | .948 |
| SBP (mmHg) | 130 (120, 141) | 127 (112, 140) | 130 (120, 141.5) | 131 (120, 140.5) | .684 |
| DBP (mmHg) | 80 (70, 90) | 80 (70, 90) | 80 (70, 90) | 80 (70, 85) | .121 |
| MAP | 97.44 ± 14.4 | 97.42 ± 16.04 | 97.87 ± 13.31 | 95.73 ± 13.23 | .586 |
| Dialysis-related variables | |||||
| CAPD, n (%) | 238 (85.6%) | 83 (91.2%) | 79 (94.9%) | 76 (80.9%) | .349 |
| PD duration (months) | 25.07 (23.92, 25.43) | 24.38 (23.89, 27.07) | 24.80 (23.92, 30.88) | 25.02 (23.85, 47.24) | .021 |
| nPCR (g/kg/24 h) | 0.88 (0.77, 1.03) | 0.96 (0.83, 1.08) | 0.88 (0.77, 1.01) | 0.81 (0.73, 0.94) | <.001 |
| Total Ccr (L/week) | 53.79 (47.34, 68.88) | 54.93 (47.87, 72.60) | 53.50 (46.61, 67.85) | 52.78 (46.89, 64.99) | .178 |
| Residual renal Ccr (L/week) | 9.90 (0, 33.14) | 12.42 (0.00, 38.74) | 14.51 (0, 33.05) | 6.42 (0, 26.17) | .145 |
| Total Kt/V | 1.79 (1.59, 2.03) | 1.82 (1.59, 2.05) | 1.82 (1.59, 2.02) | 1.78 (1.61, 2.02) | .320 |
| Residual renal Kt/V | 0.18 (0, 0.54) | 0.29 (0.00, 0.90) | 0.25 (0.00, 0.57) | 0.10 (0.00, 0.49) | .145 |
| Glucose absorption (g/24 h) | 86.62 (68.23, 116.25) | 79.8 (66.24, 109.63) | 81.99 (66.97, 110.35) | 99.07 (72.72, 121.93) | .170 |
| Volume of dialysate (per 1000 mL/24 h) | 8.04 (6.10, 9.02) | 6.60 (6.04, 8.85) | 6.83 (6.16, 9.04) | 8.43 (6.06, 9.03) | .185 |
| Biochemical parameters | |||||
| Fibrinogen (g/L) | 3.98 ± 1.15 | 2.88 ± 0.30 | 3.72 ± 0.27 | 5.18 ± 1.00 | <.001 |
| Hemoglobin (g/L) | 106.1 ± 17.2 | 103.43 ± 17.64 | 109.39 ± 16.33 | 105.42 ± 17.36 | .059 |
| Serum iron (μmol/L) | 12.35 (9.70, 16.10) | 13.60 (10.50, 16.95) | 13.20 (10.40, 17.35) | 10.85 (8.05, 13.85) | .031 |
| TSAT (%) | 31 (24, 39) | 33 (25, 43) | 32 (25.5, 41) | 26 (20, 32) | <.001 |
| Ferritin (ng/mL) | 210.9 (128.0, 305.0) | 241.0 (133.5, 339) | 193.2 (115.5, 277.5) | 209.5 (125.6, 332.5) | .09 |
| Albumin (g/L) | 36 (32, 39) | 36 (36, 41) | 37 (33, 40) | 35 (31, 38) | .074 |
| Calcium (mmol/L) | 2.25 ± 0.26 | 2.25 ± 0.31 | 2.28 ± 0.21 | 2.23 ± 0.25 | .349 |
| Phosphate (mmol/L) | 1.53 ± 0.43 | 1.60 ± 0.47 | 1.50 ± 0.40 | 1.49 ± 0.44 | .177 |
| iPTH (pg/mL) | 267.2 (167.0, 431.5) | 263.1 (164.0, 401.5) | 297 (187.0, 467.0) | 250.9 (159.08, 430.5) | .886 |
| CHO (mmol/L) | 4.34 ± 1.03 | 4.03 (3.39, 4.78) | 4.15 (3.72, 5.02) | 4.28 (3.66, 5.05) | .178 |
| TG (mmol/L) | 2.17 (1.66, 2.70) | 1.48 (1.11, 2.35) | 1.79 (1.23, 2.57) | 1.92 (1.19, 2.75) | .312 |
| LDL (mmol/L) | 1.72 (1.15, 2.55) | 2.04 (1.51, 2.69) | 2.22 (1.73, 2.71) | 2.13 (1.80, 2.60) | .510 |
| HDL (mmol/L) | 0.89 (0.75, 1.15) | 0.89 (0.75, 1.10) | 0.91 (0.75, 1.16) | 0.85 (0.74, 1.17) | .420 |
| ApoA1 (g/L) | 0.99 (0.87, 1.17) | 1.00 (0.88, 1.12) | 0.98 (0.89, 1.20) | 0.95 (0.83, 1.15) | .383 |
| ApoB (g/L) | 0.66 (0.56, 0.78) | 0.60 (0.53, 0.71) | 0.65 (0.59, 0.78) | 0.69 (0.61, 0.82) | .030 |
| NT-proBNP (per 1000 pg/mL) | 2.15 (0.78, 6.73) | 1.61 (0.63, 6.91) | 2.01 (0.77, 4.71) | 3.39 (1.19, 8.70) | .287 |
| hsCRP (mg/L) | 1.07 (0.44, 4.52) | 0.46 (0.25, 0.83) | 0.96 (0.50, 2.21) | 5.46 (2.05, 10.20) | <.001 |
| Echocardiographic measurements | |||||
| LVDd (cm) | 4.80 (4.40, 5.20) | 4.88 ± 0.64 | 4.83 ± 0.59 | 4.86 ± 0.71 | .866 |
| LVPW (cm) | 1.10 (0.90, 1.20) | 1.0 (0.9, 1.2) | 1.0 (0.9, 1.2) | 1.1 (1.0, 1.2) | .113 |
| IVST (cm) | 1.20 (1.00, 1.30) | 1.1 (0.9, 1.2) | 1.2 (1.0, 1.3) | 1.2 (1.1, 1.3) | .002 |
| MWT (cm) | 1.10 (0.95, 1.20) | 1.05 (0.90, 1.20) | 1.10 (0.95, 1.20) | 1.15 (1.00, 1.25) | .015 |
| RWT (cm) | 0.43 (0.38, 0.40) | 0.42 (0.38, 0.47) | 0.42 (0.38, 0.48) | 0.43 (0.39, 0.50) | .096 |
| LVMI (g.m–2.7) | 53.89 (43.89, 66.47) | 51.39 (41.58, 62.52) | 53.87 (43.59, 65.30) | 57.90 (46.23, 70.99) | .046 |
| LVEF (%) | 66.24 ± 7.07 | 68.77 ± 6.29 | 66.55 ± 7.07 | 65.81 ± 7.80 | .762 |
| E/A | 0.69 (0.58, 0.84) | 0.75 (0.65, 0.88) | 0.66 (0.57, 0.78) | 0.65 (0.50, 0.72) | .001 |
SBP: systolic blood pressure; DBP: diastolic blood pressure; MAP: mean arterial pressure; BMI: body mass index; Kt/V: urea clearance; Ccr: creatinine clearance; CAPD: continuous ambulatory peritoneal dialysis; TSAT: transferrin saturation; iPTH: intact parathyroid hormone; CHO: cholesterol; TG: triglyceride; LDL: low-density lipoprotein; HDL: high-density lipoprotein; ApoA1: apolipoprotein A1; ApoB: apolipoprotein B; NT-proBNP: N-terminal pro-brain natriuretic peptide; hsCRP: high-sensitivity C-reactive protein; LVDd: left ventricular end-diastolic diameter; IVST: interventricular septum thickness; PWT: posterior wall thickness; LVEF: left ventricular ejection fraction; MWT: mean wall thickness; RWT: relative wall thickness.
Plasma fibrinogen, LV mass, and function
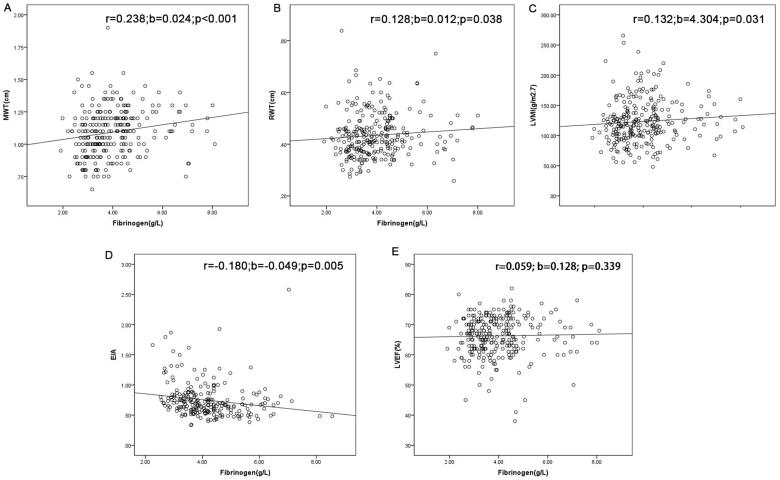
As shown in Table 1, patients in the third tertile had a higher MWT, RWT, and LVMI while E/A ratio was lower in these patients. No difference was observed on LVEF among three groups. On correlation analysis, plasma fibrinogen was directly related to MWT (r = 0.238, b = 0.024, p < .001), RWT (r = 0.128, b = 0.012, p = .038), LVMI (r = 0.132, b = 4.304, p = .031), and E/A ratio (r = –0.180, b = –0.049, p = .005) (Figure 1).
Figure 1.
Relationship between plasma fibrinogen level and mean wall thickness (MWT) (A), relative wall thickness (RWT) (B), left ventricular mass index (LVMI) (C), E/A (D), and left ventricular ejection fraction (LVEF) (E). Data are Pearson’s product moment correlation coefficient (r), regression coefficients (b), and p value.
The role of fibrinogen in LVH progression
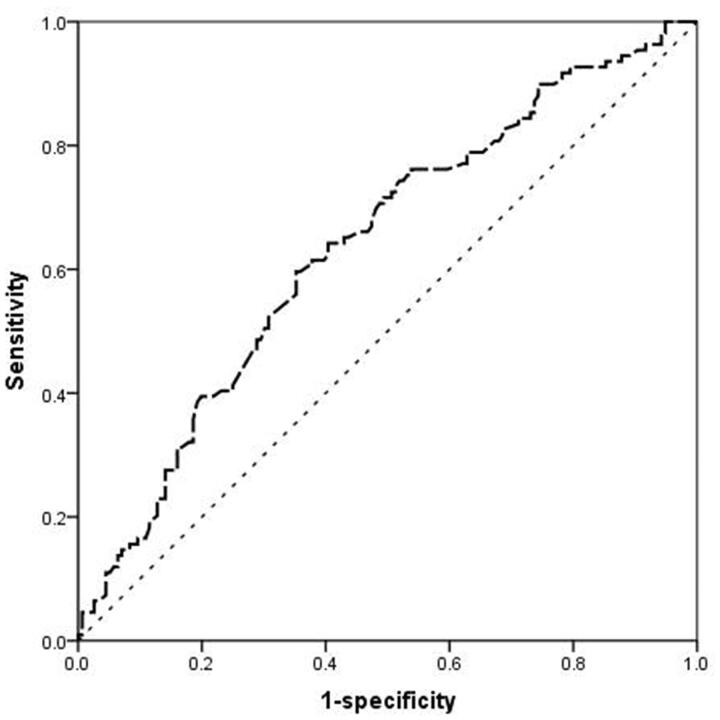
A total of 112 patients were detected to have LVH progression (Table 2). It was more prevalent in patients with fibrinogen level above 3.5 g/L (n = 86, 76.8%) than those in the first (n = 26, 28.6%) tertile (p < .001). Univariate and multivariate binary logistic regression models identified that fibrinogen, mean artery pressure (MAP), hemoglobin, and NT-proBNP were independent risk factors for LVH progression. The multivariate analysis in Table 3 showed that patients in the third tertile had a risk for LV progression that was 1.738 (95%CI: 1.231–2.453) fold higher than those in the first tertile (p = .002). The predictive power of fibrinogen for LVH progression was analyzed by ROC curves. The area under the curve (AUC) value was 0.637 (p < .001). The optimal cutoff of plasma fibrinogen level for predicting LVH progression was 3.94 g/L with a sensitivity of 59.60% and a specificity of 64.7% (Figure 2).
Table 2.
Clinical characteristics of peritoneal dialysis patients with and without LVH-progression.
| Without LVH-progression N = 166 |
With LVH-progression N = 112 |
p Value | |
|---|---|---|---|
| Demographics | |||
| Age | 54.88 ± 16.92 | 57.32 ± 15.91 | .228 |
| Male, n (%) | 96 (57.8%) | 72 (64.3%) | .280 |
| BMI (kg/m2) | 23.62 ± 3.65 | 23.85 ± 4.04 | .623 |
| PD duration (months) | 24.67 (23.89, 30.49) | 24.90 (23.89, 36.40) | .498 |
| History of smoking | 33 (19.9%) | 22 (19.6%) | .961 |
| History of diabetes | 40 (24.1%) | 28 (25.0%) | .864 |
| Use of statins | 92 (57.1%) | 73 (65.2%) | .182 |
| Use of hypotensive drugs | 151 (93.8%) | 107 (95.5%) | .533 |
| SBP (mmHg) | 129 (118, 140) | 140 (120, 150) | .005 |
| DBP (mmHg) | 80 (70, 88) | 80 (70, 90) | .117 |
| MAP (mmHg) | 95.63 ± 12.83 | 100.05 ± 16.11 | .012 |
| LVMI (cm) | 50.17 ± 14.33 | 65.34 ± 20.13 | <.001 |
| LVIDd (cm) | 4.70 (4.30, 5.10) | 5.05 (4.65, 5.50) | <.001 |
| PWT (cm) | 1.00 (0.90, 1.10) | 1.10 (1.00, 1.20) | <.001 |
| IVST (cm) | 1.10 (0.90, 1.20) | 1.20 (1.10, 1.30) | <.001 |
| LVEF (%) | 66.0 (63.0, 72.0) | 66.0 (61.5, 71.0) | .347 |
| E/A | 0.68 (0.57, 0.84) | 0.69 (0.61, 0.84) | .632 |
| Dialysis-related variables | |||
| nPCR (g/kg/24 h) | 0.87 (0.75, 1.04) | 0.90 (0.79, 1.02) | .529 |
| Total Ccr (L/week) | 54.54 (47.38, 68.94) | 52.22 (47.07, 66.80) | .423 |
| Residual renal Ccr (L/week) | 11.11 (0.00, 34.87) | 8.74 (0.00, 28.80) | .979 |
| Total Kt/V | 1.82 (1.62, 2.08) | 1.75 (1.54, 1.95) | .066 |
| Residual renal Kt/V | 0.20 (0, 0.71) | 0.16 (0, 0.54) | .977 |
| Glucose absorption (g/24 h) | 85.35 (67.18, 113.35) | 87.70 (69.6, 119.14) | .444 |
| Volume of dialysate (per 1000 mL/24 h) | 8.08 (6.11, 9.07) | 7.94 (6.06, 8.90) | .702 |
| Biochemical parameters | |||
| Hemoglobin (g/L) | 108.5 ± 15.4 | 102.8 ± 19.1 | .007 |
| Serum iron (μmol/L) | 13.05 (10.1, 16.95) | 11.6 (9.10, 15.0) | .054 |
| TSAT (%) | 32.0 (25.0, 41.0) | 28.0 (21.5, 36.0) | .005 |
| Ferritin (ng/mL) | 229.5 (152.0, 326.6) | 179.5 (106.8, 295.0) | .011 |
| Albumin (g/L) | 36.3 ± 5.6 | 35.6 ± 5.4 | .426 |
| Calcium (mmol/L) | 2.25 ± 0.28 | 2.24 ± 0.22 | .715 |
| Phosphate (mmol/L) | 1.49 ± 0.42 | 1.60 ± 0.04 | .037 |
| iPTH (per 10 pg/mL) | 266.6 (153.0, 428.0) | 269.5 (183.0, 435.5) | .352 |
| FBG (mmol/L) | 5.1 (4.6, 6.2) | 5.3 (4.9, 6.1) | .815 |
| Serum insulin (mU/L) | 13.2 (8.7, 19.1) | 11.6 (7.9, 16.6) | .068 |
| CHO (mmol/L) | 4.34 ± 1.13 | 4.34 ± 0.88 | .975 |
| LDL (mmol/L) | 2.09 (1.58, 2.62) | 2.24 (1.80, 2.71) | .100 |
| TG (mmol/L) | 1.84 (1.27, 2.78) | 1.57 (1.06, 2.24) | .024 |
| HDL (mmol/L) | 0.89 (0.75, 1.10) | 0.91 (0.76, 1.23) | .196 |
| ApoA1 (g/L) | 0.99 (0.87, 1.17) | 0.98 (0.86, 1,19) | .909 |
| ApoB (g/L) | 0.64 (0.56, 0.78) | 0.68 (0.57, 0.76) | .432 |
| NT-proBNP (per 1000 pg/mL) | 1.63 (0.63, 4.66) | 3.51 (1.39, 12.95) | <.001 |
| hsCRP (mg/L) | 0.94 (0.41, 3.57) | 1.31 (0.51, 4.83) | .182 |
BMI: body mass index; SBP: systolic blood pressure; DBP: diastolic blood pressure; MAP: mean arterial pressure; LVMI: left ventricular mass index; LVDd: left ventricular end-diastolic diameter; IVST: interventricular septum thickness; PWT: posterior wall thickness; LVEF: left ventricular ejection fraction; MWT: mean wall thickness; RWT: relative wall thickness; nPCR: normalized protein catabolic rate; Ccr: creatinine clearance; Kt/V: urea clearance; TSAT: transferrin saturation; iPTH: intact parathyroid hormone; FBG: fasting blood glucose; CHO: cholesterol; TG: triglyceride; LDL: low-density lipoprotein; HDL: high-density lipoprotein; ApoA1: apolipoprotein A1; ApoB: apolipoprotein B; NT-proBNP: N-terminal pro-brain natriuretic peptide; hsCRP: high-sensitivity C-reactive protein.
Table 3.
Multiple logistic regression analysis of LVH-progression.
| Units of increase | OR (95%CI) | p Value | |
|---|---|---|---|
| Plasma fibrinogen level | |||
| Tertile I | ≤3.5 g/L | 1a | |
| Tertile II | 3.5–4.4 g/L | 1.756 (0.883,3.492) | .108 |
| Tertile III | ≥4.4 g/L | 1.738 (1.231–2.453) | .002 |
| Hemoglobin | 1 g/L | 0.92 (0.966–0.999) | .041 |
| Ferritin | 1 ng/mL | 0.997 (0.995–0.999) | .008 |
| NT-proBNP | 1000 pg/mL | 1.046 (1.015–1.079) | .004 |
| MAP | 1 mmHg | 1.010 (0.990–1.031) | .265 |
| Phosphate | 1 mmol/L | 1.269 (0.645–2.497) | .270 |
| TG | 1 mmol/L | 0.951 (0.788–1.149) | .284 |
| HDL | 1 mmol/L | 1.811 (0.741–4,426) | .106 |
NT-proBNP: N-terminal pro-brain natriuretic peptide; MAP: mean arterial pressure; TG: triglyceride; HDL: high-density lipoprotein.
Reference group.
Figure 2.
Receiver operating characteristic (ROC) curves of the fibrinogen level for predicting LVH-progression. The area under the curve (AUC) value was 0.637 (p < .001).
Independent risk factors associated with plasma fibrinogen levels
According to univariate linear regression results, age, sex, BMI, PD duration, history of diabetes, albumin, fasting blood glucose (FBG), total cholesterol (CHO), ApoB, hsCRP, nPCR, and residual renal Kt/V were correlated to plasma fibrinogen level and were added into multivariate linear regression model for adjustment. It turned out that age, FBG, CHO, and hsCRP were independent risk factors for high level of plasma fibrinogen (Table 4).
Table 4.
Univariate and multivariate linear regression analyses for factors related to plasma fibrinogen level.
| Univariate analysis |
Multivariate analysis |
|||||
|---|---|---|---|---|---|---|
| β | 95%CI | p | β | 95%CI | p | |
| Age | 0.024 | 0.017–0.032 | <.001 | 0.018 | 0.011–0.024 | <.001 |
| Sex | 0.062 | −0.201 to 0.324 | .643 | |||
| PD duration | 0.008 | 0.003–0014 | .004 | |||
| History of diabetes | 0.315 | 0.019–0.611 | .037 | |||
| BMI (kg/m2) | 0.038 | 0.005–0.072 | .024 | |||
| Use of statins | 0.417 | 0.159–0.674 | .002 | |||
| Albumin (g/L) | −0.019 | −0.036 to 0.002 | .031 | |||
| FBG (mmol/L) | 0.159 | 0.092–0.226 | <.001 | 0.092 | 0.034–0.151 | .002 |
| CHO (mmol/L) | 0.144 | 0.014–0.273 | .030 | 0.136 | 0.028–0.244 | .013 |
| ApoB(g/L) | 0.992 | 0.235–1.749 | .010 | |||
| hsCRP (mg/L) | 0.074 | 0.056–0.092 | <.001 | 0.061 | 0.045–0.078 | <.001 |
| nPCR (g/kg/24 h) | −1.059 | −1.626 to 0.492 | <.001 | |||
| Residual renal Kt/V | −0.274 | −0.513 to 0.034 | .025 | |||
BMI: body mass index; FBG: fasting blood glucose; CHO: cholesterol; ApoB: apolipoprotein B; hsCRP: high-sensitivity C-reactive protein; nPCR: normalized protein catabolic rate.
Data are expressed as regression coefficient (β) and p value.
Discussion
In this study, we followed two-year laboratory and echocardiographic data in patients undergoing PD and explored the relationship between plasma fibrinogen level and LV structure and function. The results showed that an elevated plasma fibrinogen level was directly related to higher MWT, RWT, and LVMI. The elevated fibrinogen level was an independent risk factor for LVH regression. The findings indicated that plasma fibrinogen level could be a valuable marker for LV-remodeling monitoring. Meanwhile, we investigated risk factors associated with high fibrinogen level, it turned out that age, CHO, FBG, and hsCRP were significantly related to plasma fibrinogen level.
Fibrinogen, also known as coagulation factor I, is a large plasma glycoprotein with a molecular mass of 340 kDa and plays an important part in primary and secondary hemostasis [13]. Levels of fibrinogen that are too high or too low may lead to a high risk of thrombosis and bleeding, respectively. It is reported that PD alters serum concentrations of several molecules and one example is plasma fibrinogen [14]. Several measurements showed that plasma fibrinogen levels increased in patients on long-term PD therapy. In Tekin et al.’s study on patients initiated PD therapy, fibrinogen level was elevated in 80.9% participants [15]. In our study, plasma fibrinogen levels in 67.3% patients were beyond the upper limit value. The phenomenon can be explained as follows: first, fibrinogen synthesis is strongly stimulated with the loss of albumin in the peritoneal dialysate and accumulation of free fatty acids in the blood [16]. Second, the clearance of fibrinogen by the kidneys is weakened with the loss of renal function [17,18]. Third, long-term and continuous exposure to high glucose-based dialysate can cause metabolic syndrome including insulin resistance and dyslipidemia, leading to prothrombotic tendency. Therefore, though hyperfibrinogenemia and prothrombotic profile are also common in patients on HD, they tend to be more severe in patients on PD [19,20].
In the general population, fibrinogen was considered as an established predictor of cardiovascular events with hyperfibrinogenemia commonly found in patients with cardiovascular disease such as atherosclerosis, arterial stiffness, and calcification [21,22]. Patients with ESRD, especially those on PD, are not a homogeneous cohort. For the coagulation profile of these individuals is not completely the same, the relationship between fibrinogen and cardiovascular risks could be complicated and controversial. Besides, patients undergoing PD are always complicated with anemia, fluid overload, micro-inflammation, and metabolic disorders. Interactions among these factors may mask the effect of fibrinogen level alone on the prognosis of patients undergoing PD. Recently, Yu et al. found that an elevated plasma fibrinogen level was significantly associated with an increased risk of CV and all-cause mortality in patients undergoing PD. The relationship was nonlinear, exhibiting approximate J-shaped curves [6]. Xia et al. found that patients undergoing PD with a high albumin to fibrinogen ratio had reduced all-cause and cardiovascular mortality [7]. In our study, we found that plasma fibrinogen was an independent risk factor for LV remodeling in patients undergoing PD. The results to some extent further validated the adverse role of fibrinogen in detail.
LV remodeling is an adaptive process aiming to minimize ventricular wall stress. Hypertension was considered as a major factor in the pathogenesis of LVH in ESRD [23]. Nevertheless, hypertension only in part accounts for a raised LV mass. Our study revealed that plasma fibrinogen level, ferritin, anemia, and fluid overload also had an important influence on this process. Previous studies revealed that iron deficiency anemia was related to the higher risk of LVH in the general population [24,25]. Consistent with the conclusion above, our study showed that lower ferritin and hemoglobin levels were independently related to LVH progression. On the one hand, anemia can be a primary cause of LV remodeling through its induction of an increase in cardiac output. On the other hand, iron is involved in multiple important physiological processes related to inflammation and cell growth including transport, storage of oxygen by hemoglobin, energy production, oxidative phosphorylation, etc. [26]. Iron deficiency might influence the growth of cardiac myocytes. Therefore, iron deficiency anemia is an important risk factor for LVH progression in patients on PD. It is reported that ApoB is inversely associated with LVH in patients undergoing PD [27]. However, we did not find the relationship between lipid metabolism parameters and LVH progression. The possible reasons can be explained as follows: first, participants in our study were only followed up for 2 years, the influence of dyslipidemia has not been emanated yet. Second, the effect of dyslipidemia might be diluted by active use of statins in our center.
In patients on chronic hemodialysis, fibrinogen was related to LV concentric hypertrophy and systolic dysfunction independently of a large series of traditional and non-traditional risk factors [11]. Similar to previous findings, we also found that the relationship between LV remodeling and plasma fibrinogen level was largely independent of traditional risk factors. It was reported that fibrinogen could participate directly in vascular injury in several ways. It plays an important part in increased coagulation activity, increased plasma viscosity, platelet aggregation, and vascular endothelial dysfunction, whereas its degradation products are mitogenic for vascular smooth muscle cells [14]. Kerlin et al. has clarified the direct effect of fibrinogen on the blood vessels [28]. We found that age, FBG, CHO, and hsCRP were independently associated with plasma fibrinogen level. Actually, fibrinogen also plays a key role in the inflammatory process, for instance, promoting the synthesis of proinflammatory cytokines in the peripheral blood environment [29]. Same with CRP, fibrinogen is also an acute-phase reactant reflecting a state of inflammation [30]. It is reasonable that fibrinogen level could be higher in patients older, with dyslipidemia or poor glycemic control because of a worse inflammatory environment. Consistent with our results, Zou et al. found that patients with higher fibrinogen were older and more frequently had diabetes [31]. In other words, the plasma fibrinogen level has been shown to be positively correlated with microinflammation. Therefore, we proposed that the fibrinogen level in patients with a hyper-inflammatory state be close monitored.
Our study has several limitations. First, the study was a single-center study with a relatively small sample size. Further multi-center research and larger sample size are required to confirm the findings based on the strict sample size calculation. Second, the transthoracic echocardiogram was not performed and read by the same technician. The inter-personal variation in assessing LV structure cannot be avoided. Finally, the observational nature of the study did not allow us to explore whether better glucose or lipid control could reduce plasma fibrinogen level and slow LVH progression. The association between plasma fibrinogen levels and LV remodeling was not causal.
In summary, elevated plasma fibrinogen level was an independent risk factor for LVH-progression in patients on PD. Age, FBG, CHO, and hsCRP were significantly associated with fibrinogen level. The findings provided new insights into cardiovascular prognostic impact of elevated fibrinogen levels among patients undergoing PD. Further studies are needed to explore interventions on reducing fibrinogen levels and whether it could improve LV remodeling in patients undergoing PD.
Acknowledgements
The study was submitted to the American Society of Nephrology (ASN) Kidney week 2022, November 3–6 held in Orlando, FL. It was accepted for poster presentation (FR-PO466, https://asn.scientificposters.com/epsSearchASN.cfm).
Funding Statement
This study was supported by the National Natural Science Foundation of China (Grant Numbers 81670692, 81930120, and 81520108006).
Author contributions
Tongying Zhu designed the study. Yun Chen is the major contributors in writing the manuscript and Chuanming Hao reviewed it. Xiaolin Ge and Da Shang interpreted the patient data. Shuqi Dai and Qionghong Xie made analysis. All authors read and approved the final manuscript.
Disclosure statement
The authors have no conflicts of interest to declare.
References
- 1.Cheng X, Nayyar S, Wang M, et al. . Mortality rates among prevalent hemodialysis patients in Beijing: a comparison with USRDS data. Nephrol Dial Transplant. 2013;28(3):1–8. doi: 10.1093/ndt/gfs326. [DOI] [PubMed] [Google Scholar]
- 2.Coresh J, Longenecker JC, Miller ER, et al. . Epidemiology of cardiovascular risk factors in chronic renal disease. J Am Soc Nephrol. 1998;9(12 Suppl.):S24–S30. [PubMed] [Google Scholar]
- 3.Danesh J, Collins R, Appleby P, et al. . Association of fibrinogen, C-reactive protein, albumin, or leukocyte count with coronary heart disease: meta-analyses of prospective studies. JAMA. 1998;279(18):1477–1482. doi: 10.1001/jama.279.18.1477. [DOI] [PubMed] [Google Scholar]
- 4.Dervisoglu E, Kozdag G, Etiler N, et al. . Association of glomerular filtration rate and inflammation with left ventricular hypertrophy in chronic kidney disease patients. Hippokratia. 2012;16(2):137–142. [PMC free article] [PubMed] [Google Scholar]
- 5.Park M, Hsu CY, Li Y, et al. . Associations between kidney function and subclinical cardiac abnormalities in CKD. J Am Soc Nephrol. 2012;23(10):1725–1734. doi: 10.1681/ASN.2012020145. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Yu J, Lin T, Huang N, et al. . Plasma fibrinogen and mortality in patients undergoing peritoneal dialysis: a prospective cohort study. BMC Nephrol. 2020;21(1):349. doi: 10.1186/s12882-020-01984-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Xia W, Kuang M, Li C, et al. . Prognostic significance of the albumin to fibrinogen ratio in peritoneal dialysis patients. Front Med. 2022;9:820281. doi: 10.3389/fmed.2022.820281. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Lee JK, Lin HH, Tsai CT, et al. . Differential association of proinflammatory cytokines with left ventricular diastolic dysfunction in subjects with and without continuous ambulatory peritoneal dialysis. Nutr Metab Cardiovasc Dis. 2012;22(11):974–980. doi: 10.1016/j.numecd.2011.01.001. [DOI] [PubMed] [Google Scholar]
- 9.Marwick TH, Gillebert TC, Aurigemma G, et al. . Recommendations on the use of echocardiography in adult hypertension: a report from the European Association of Cardiovascular Imaging (EACVI) and the American Society of Echocardiography (ASE). J Am Soc Echocardiogr. 2015;28(7):727–754. doi: 10.1016/j.echo.2015.05.002. [DOI] [PubMed] [Google Scholar]
- 10.Devereux RB, Alonso DR, Lutas EM, et al. . Echocardiographic assessment of left ventricular hypertrophy: comparison to necropsy findings. Am J Cardiol. 1986;57(6):450–458. doi: 10.1016/0002-9149(86)90771-x. [DOI] [PubMed] [Google Scholar]
- 11.Zoccali C, Benedetto FA, Mallamaci F, et al. . Fibrinogen, inflammation and concentric left ventricular hypertrophy in chronic renal failure. Eur J Clin Invest. 2003;33(7):561–566. doi: 10.1046/j.1365-2362.2003.01169.x. [DOI] [PubMed] [Google Scholar]
- 12.Chen Y, Dai S, Ge X, et al. . Prognostic values of left ventricular mass index progression in incident peritoneal dialysis patients: a prospective cohort study. BMC Nephrol. 2022;23(1):200. doi: 10.1186/s12882-022-02831-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Mosesson MW. Fibrinogen and fibrin structure and functions. J Thromb Haemost. 2005;3(8):1894–1904. doi: 10.1111/j.1538-7836.2005.01365.x. [DOI] [PubMed] [Google Scholar]
- 14.Goldwasser P, Feldman JG, Emiru M, et al. . Effect of dialysis modality on plasma fibrinogen concentration: a meta-analysis. Am J Kidney Dis. 2004;44(6):941–949. doi: 10.1053/j.ajkd.2004.08.019. [DOI] [PubMed] [Google Scholar]
- 15.Tekin IO, Pocan B, Borazan A, et al. . Positive correlation of CRP and fibrinogen levels as cardiovascular risk factors in early stage of continuous ambulatory peritoneal dialysis patients. Ren Fail. 2008;30(2):219–225. doi: 10.1080/08860220701813350. [DOI] [PubMed] [Google Scholar]
- 16.Prinsen BH, Rabelink TJ, Beutler JJ, et al. . Increased albumin and fibrinogen synthesis rate in patients with chronic renal failure. Kidney Int. 2003;64(4):1495–1504. doi: 10.1046/j.1523-1755.2003.00211.x. [DOI] [PubMed] [Google Scholar]
- 17.Schlieper G, Hess K, Floege J, et al. . The vulnerable patient with chronic kidney disease. Nephrol Dial Transplant. 2016;31(3):382–390. doi: 10.1093/ndt/gfv041. [DOI] [PubMed] [Google Scholar]
- 18.Roberts MA, Hare DL, Ratnaike S, et al. . Cardiovascular biomarkers in CKD: pathophysiology and implications for clinical management of cardiac disease. Am J Kidney Dis. 2006;48(3):341–360. doi: 10.1053/j.ajkd.2006.06.005. [DOI] [PubMed] [Google Scholar]
- 19.Brophy DF, Carl DE, Mohammed BM, et al. . Differences in coagulation between hemodialysis and peritoneal dialysis. Perit Dial Int. 2014;34(1):33–40. doi: 10.3747/pdi.2013.00036. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Gackler A, Rohn H, Lisman T, et al. . Evaluation of hemostasis in patients with end-stage renal disease. PLOS One. 2019;14(2):e212237. doi: 10.1371/journal.pone.0212237. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Mauriello A, Sangiorgi G, Palmieri G, et al. . Hyperfibrinogenemia is associated with specific histocytological composition and complications of atherosclerotic carotid plaques in patients affected by transient ischemic attacks. Circulation. 2000;101(7):744–750. doi: 10.1161/01.cir.101.7.744. [DOI] [PubMed] [Google Scholar]
- 22.Palmieri V, Celentano A, Roman MJ, et al. . Fibrinogen and preclinical echocardiographic target organ damage: the strong heart study. Hypertension. 2001;38(5):1068–1074. doi: 10.1161/hy1101.095335. [DOI] [PubMed] [Google Scholar]
- 23.Middleton RJ, Parfrey PS, Foley RN.. Left ventricular hypertrophy in the renal patient. J Am Soc Nephrol. 2001;12(5):1079–1084. doi: 10.1681/ASN.V1251079. [DOI] [PubMed] [Google Scholar]
- 24.Chen Y, Wan J, Xia H, et al. . Total iron binding capacity (TIBC) is a potential biomarker of left ventricular remodelling for patients with iron deficiency anaemia. BMC Cardiovasc Disord. 2020;20(1):4. doi: 10.1186/s12872-019-01320-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Zhou Q, Shen J, Liu Y, et al. . Assessment of left ventricular systolic function in patients with iron deficiency anemia by three-dimensional speckle-tracking echocardiography. Anatol J Cardiol. 2017;18(3):194–199. doi: 10.14744/AnatolJCardiol.2017.7694. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Sutil-Vega M, Rizzo M, Martínez-Rubio A.. Anemia and iron deficiency in heart failure: a review of echocardiographic features. Echocardiography. 2019;36(3):585–594. doi: 10.1111/echo.14271. [DOI] [PubMed] [Google Scholar]
- 27.Ye M, Liu Y, Wang H, et al. . Serum apolipoprotein B is inversely associated with eccentric left ventricular hypertrophy in peritoneal dialysis patients. Int Urol Nephrol. 2018;50(1):155–165. doi: 10.1007/s11255-017-1737-1. [DOI] [PubMed] [Google Scholar]
- 28.Kerlin B, Cooley BC, Isermann BH, et al. . Cause–effect relation between hyperfibrinogenemia and vascular disease. Blood. 2004;103(5):1728–1734. doi: 10.1182/blood-2003-08-2886. [DOI] [PubMed] [Google Scholar]
- 29.Gobel K, Eichler S, Wiendl H, et al. . The coagulation factors fibrinogen, thrombin, and factor XII in inflammatory disorders—a systematic review. Front Immunol. 2018;9:1731. doi: 10.3389/fimmu.2018.01731. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Luyendyk JP, Schoenecker JG, Flick MJ.. The multifaceted role of fibrinogen in tissue injury and inflammation. Blood. 2019;133(6):511–520. doi: 10.1182/blood-2018-07-818211. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Zou Y, Zhu Z, Zhou J, et al. . Fibrinogen/albumin ratio: a more powerful prognostic index for patients with end-stage renal disease. Eur J Clin Invest. 2020;2020:e13266. doi: 10.1111/eci.13266. [DOI] [PubMed] [Google Scholar]