Abstract
Background:
While consuming a Mediterranean-style diet (MSD) among pregnant women is expected to affect offspring neurodevelopment, the current evidence is limited. This prospective birth cohort study aimed to explore the association of maternal MSD with neurodevelopmental disabilities (NDD) in offspring, especially among children born to mothers with overweight or obesity (OWO) and/or diabetes mellitus (DM) since they have a higher risk for oxidative stress and immune/metabolic disturbances.
Methods:
We analyzed data from a subgroup of mother–child dyads enrolled in the Boston Birth Cohort. Maternal dietary information (via food frequency questionnaires, Food frequency questionnaires [FFQ]) and sociodemographic information were obtained via in-person interviews within 24 to 72 hours postpartum. Maternal clinical information and child diagnosis of NDD including autism, attention-deficit/hyperactivity disorder (ADHD), and other developmental disabilities (DD) were extracted from medical records. A Mediterranean-style diet score (MSDS) was calculated using the FFQ. The association of maternal MSDS with NDD, autism, ADHD, and other DD was evaluated using multivariable logistic regression models adjusted for pertinent covariates.
Results:
This study included 3153 mother–child pairs, from which we identified diagnoses of 1362 (43.2%) NDD, including 123 (3.9%) case of autism, 445 (14.1%) ADHD, and 794 (25.2%) other DD. In the overall sample, women with a higher maternal MSDS (per standard deviation increase) were less likely to have offspring with NDD (adjusted odds ratio [OR]: 0.904, 95% confidence interval [CI]: 0.817–1.000; P value: 0.049). Using MSDS quintile 1 as the reference, being in the combined group of quintiles 3–5 was associated with a 26% lower likelihood of NDD (adjusted OR: 0.738, 95% CI: 0.572–0.951; P value: 0.019). When stratified by mothers with OWO/DM vs. without OWO/DM, the association between maternal MSDS and offspring NDD was greater in children born to mothers with OWO/DM.
Conclusions:
In this prospective birth cohort, a higher maternal MSDS was associated with a lower likelihood of NDD in the offspring. Furthermore, this association of maternal MSDS with offspring NDD was greater in children born to women with OWO/DM. More studies are needed to replicate the findings and further analyze NDD subgroups and explore underlying molecular pathways.
Keywords: Diet, Neurodevelopmental disabilities, Autism, Attention-deficit/hyperactivity disorder, Pregnancy, Clinical nutrition
Introduction
Maternal nutrition during pregnancy is a critical factor in the neurodevelopment of the offspring. Fetal brain development is rapid and requires high levels of energy, protein, essential fatty acids, and key micronutrients.[1] A maternal diet that is deficient in essential nutrients or severely imbalanced may affect fetal genetic programming and expression and lead to an inflammatory response that can affect brain development.[2] Evidence based on animal, epidemiological, and genetic studies suggests that immune dysregulation affects brain development and is associated with many neurodevelopmental disabilities (NDD) such as autism and attention-deficit/hyperactivity disorder (ADHD).[3,4] In addition to maternal nutritional status, evidence also showed that maternal overweight/obesity (OWO) and diabetes mellitus (DM) may increase the likelihood of NDD among offspring through immune and metabolic dysregulation.[5–8]
There are two acknowledged models for how society views disabilities, including the medical model and the social model. Traditionally, the medical model views disability as a defect within the individual and that these defects should be cured, fixed, and/or eliminated, while the social model values the collective richness and diversity that disabilities bring and calls for an end to discrimination and oppression against disabled people through education, accommodation, and universal design.[9] Efforts to shift from the traditional medical model to the social model helps bring attention to the unconscious bias of the medical model and awareness of the need to advocate for full inclusion and equality of all people within society.
In recognition of the valid and unique experience of each disabled person, there is no universal language to describe disabilities. In this study, we used the term neurodevelopmental disabilities to describe individuals with diagnoses of autism, ADHD, and other neurodevelopmental disabilities and to emphasize the potential barriers they face as identified in the social model of disability.
The prevalence of NDD in children is over 15% worldwide.[10] Results from the National Health Interview Survey (NHIS) indicated that from 2015 to 2017, the prevalence of NDD among US children aged 3 to 17 years was 17.8%.[11] About 1 in 44 US children aged 8 years were autistic in 2018[12]; and approximately 6 million US children (9.8%) aged 3 to 17 years were diagnosed with ADHD from 2016 to 2019.[13] The underlying reasons for the rising diagnoses of NDD are not completely understood, while increased awareness of NDD and more screening efforts may have contributed to the increase.
A Mediterranean-style diet (MSD), which is characterized by a moderate daily intake of grains, vegetables, fruits, low-fat dairy products, olive oil, nuts, and wine, weekly intake of fish and legumes, and limited meat intake, is considered to be a healthy dietary pattern that has long been considered to have various positive health outcomes.[14–19] Adherence to a MSD has been associated with methylation changes in inflammation-related genes[20] and the generation of antioxidant and anti-inflammatory effects.[21] A MSD has also been associated with improved cognition in adults,[22] reductions in cardiovascular/chronic heart disease mortality and morbidity,[23] improvement of components of metabolic syndrome,[24] and decreased incidence of DM.[25] A multicenter randomized trial showed that a simple, individualized MSD during pregnancy has the potential to reduce gestational weight gain and the risk of gestational diabetes.[26] Many studies have explored the association of maternal dietary patterns with birth outcomes.[27] However, despite the potential of a MSD to improve maternal inflammatory status and to affect the neurodevelopment of the offspring, studies of the effects of maternal MSD on NDD have been limited. Most previous studies have only focused on the effects of the consumption of specific food groups or micronutrients on offspring neurodevelopment, though a broader understanding of maternal dietary patterns could provide a more accurate assessment of offspring nutritional status. In this study, we used a prospective birth cohort to explore the association of maternal MSD with NDD in offspring, especially in groups of women at high risk of metabolic disorders including OWO and/or DM, to better understand the potential association of maternal dietary patterns with offspring neurological development.
Methods
Study population
This study included 3153 mother–child pairs, a subset of the Boston Birth Cohort (BBC) recruited at birth at Boston Medical Center (BMC) from 1998 to 2018, who had at least one postnatal follow-up. The BBC was initially designed as a molecular epidemiologic study of the determinants of low birthweight (LBW) and preterm birth (PTB).[28] The BBC enrolled mothers who delivered a singleton live birth. For every PTB and/or LBW infant, two term and appropriate for gestation age (AGA) birthweight infants were enrolled. Exclusion criteria for enrollment included multiple-gestation pregnancy, pregnancies resulting from in vitro fertilization, PTB due to maternal trauma, and newborns with major birth defects.
Mothers provided written informed consent within 24 to 72 hours of delivery and were interviewed by trained research staff using a standardized questionnaire. Clinical information and birth outcomes were abstracted from electronic medical records (EMRs). Children who continued to seek postnatal care at BMC were followed. The study was approved by the Institutional Review Boards of Johns Hopkins Bloomberg School of Public Health and Boston Medical Center.
Diagnosis of neurodevelopmental disabilities
We defined five NDD groups based on physician diagnoses as documented in EMRs. The EMRs for BBC participants consist of all primary care and subspecialty visits at BMC since January 2004. Both International Classification of Diseases-9 (ICD-9) codes (before October 1, 2015) and ICD-10 codes (after October 1, 2015) were used to document the primary and secondary diagnoses at each visit. Cases were defined as follows: children ever diagnosed with autism (ICD-9: 299.0-299.91; ICD-10: F84.0-F84.9) constituted the autism group; children ever diagnosed with ADHD (ICD-9: 314.0-314.9; ICD-10: F90.0-F90.9) but without autism constituted the ADHD group; children ever diagnosed with dyslexia, dyscalculia, dyspraxia, or developmental delays (ICD-9: 315.0-315.9; ICD-10: F80.0-F81.89, F88-F89, R48.0, H93.25) but without autism and ADHD constituted the other developmental disabilities (other DD) group; children ever diagnosed with autism, ADHD, or other DD constituted the NDD group; and children without any of the aforementioned diagnoses in their EMRs were assigned to the typically developing (TD) group.
Definition of Mediterranean-style diet score
Among the 3165 mother–child pairs, dietary data were available for 3153 mothers. Food frequency questionnaires (FFQ) [Supplementary Table S1, http://links.lww.com/PN9/A27] administered at the postpartum interview included questions about the mother’s weekly intake of multiple foods during pregnancy. The foods ultimately selected for analysis were green vegetables, orange vegetables, fruits, meat, fish, eggs, beans, rice, dairy, and wheat (pasta, bread, and cereal). Questions regarding other foods including shellfish, soy, seeds, peanuts, nuts, and juice consumption were added as an update to the FFQ and therefore were not considered during the analysis. The detailed method for calculating the Mediterranean-style diet score (MSDS) has been described previously.[23] Briefly, there are different frequencies for every selected food group consumed weekly, including 6 to 7 days, 3 to 5 days, 1 to 2 days, and none. To calculate the MSDS, we assigned a score to each selected food group based on days per week that the selected food groups were consumed (0 = none; 1 = <1 day; 2 = 1–2 days; 3 = 3–5 days; 4 = 6–7 days). Based on a MSD characterized by little to no meat intake, all selected food groups were positively scored except for meat.[29] For data analysis, in addition to analyzing the MSDS as a continuous variable, we identified and compared quintiles of the MSDS with the first quintile (ie, lowest MSDS) as the reference.
Definition of covariates
This study carefully considered and controlled for prenatal and perinatal factors that are known or suspected to affect the likelihood of offspring NDD. Covariates used for adjustment in the model included race and ethnicity,[30] education,[31] marital status,[32] maternal age,[33] alcohol use,[34] ever smoking,[7] DM,[5] preeclampsia,[35] pre-pregnancy body mass index (BMI),[5] parity,[36] plasma folate and vitamin B12 levels,[37] child sex,[38] PTB,[33] and LBW.[33] Since sugar intake (from added sugars vs. naturally occurring sugars (ie, fruits)) is also an important component of dietary assessment, we also included soft drink consumption as a covariate.
The self-identified race and ethnicity of the study groups was categorized into four groups: non-Hispanic Black (African American and Haitian), non-Hispanic White, Hispanic, and Other (Cape Verdean, Asian, Pacific Islander, More than one race, and Other race). Education was categorized into three groups: lower than high school, high school, and higher than high school. Marital status was categorized into two groups: married and other (divorced, separated, single, and widowed). Parity (number of prior live births) was categorized into 0 and >0. Maternal age in years was used as a continuous variable. Alcohol consumption was defined as a binary variable categorized as ever or never used alcohol anytime from 6 months before pregnancy to delivery. Smoking status during pregnancy was defined as a binary variable categorized as ever or never smoked anytime from 6 months before pregnancy to delivery. Soft drink consumption was categorized into ≤1 cup per month and >1 cup per month during pregnancy. Pre-pregnancy BMI was categorized into overweight/obesity (BMI ≥25 kg/m2) and normal weight/underweight (BMI <25 kg/m2). DM was categorized into no DM and preexisting DM/gestational DM. Preeclampsia was categorized into no preeclampsia, mild preeclampsia, and severe preeclampsia. Child sex was defined as sex assigned at birth (male, female). PTB was defined as delivery at less than 37 completed weeks of gestation. LBW was defined as a birthweight under 2500 g. Plasma folate (nmol/L) was measured using chemiluminescent immunoassay with diagnostic kits (Shenzhen New Industries Biomedical Engineering Co., Ltd., Shenzhen, China) and plasma B12 (pmol/L) was measured using the Beckman Coulter ACCESS Immunoassay System (Beckman-Coulter Canada, Mississauga, Canada) using a MAGLUMI 2000 Analyzer.
We performed simple imputation for covariates with missing values with proportions not exceeding 5.5% of the total sample size. For categorical variables, we imputed the missing values to the group with the highest frequency; and for continuous variables, such as BMI, we imputed missing values based on race-specific mean values. Although annual household income is also a potentially relevant factor in the development of NDD, it was not considered in this study due to the proportion of missing values higher than 10.0%.
Statistical analysis
Descriptive statistics were performed for comparisons of the covariates across quintiles of MSDS. The normality assumption of continuous variables was tested using histograms and the Shapiro–Wilk test (rejected null hypothesis of normality if P < 0.05). Normally distributed continuous variables were reported as means and standard deviation (SD). Nonnormally distributed continuous variables were reported as medians and interquartile ranges (IQRs). Categorical variables were reported as numbers and percentages. The Kruskal-Wallis test was performed to compare continuous variables. Pearson’s chi-squared test/Fisher’s exact test was used to compare categorical variables. The maternal and child characteristics of the study children by the five groups (NDD, autism, ADHD, other DD, and TD) were also compared using Pearson’s chi-squared test/Fisher’s exact test and the Kruskal-Wallis test for categorical and continuous variables, respectively.
The maternal MSDSs included a small number of outliers [Supplementary Figure S1, http://links.lww.com/PN9/A27]. Considering that data from women with very low or high MSDSs may be informative, a MSDS at the 0.5% level (MSDS = 12) was assigned to individuals with MSDSs below 12 and a MSDS at the 99.5% level (MSDS = 35) was assigned to individuals with MSDSs above 35. Following this process, we analyzed maternal MSDS first as a categorical variable based on quintiles (ie, quintile 1: ≤21, 2: 21–24, 3: 24–26, 4: 26–28, 5: >28) using the lowest quintile as the reference, and second as a continuous variable to test for a linear trend. Due to fluctuations in the data, we also combined quintile groups 3 through 5 for additional analysis. In the overall sample, unadjusted and adjusted logistic regression models were performed to explore the association of maternal MSDS with offspring NDD, autism, ADHD, and other DD. For adjusted logistic regression analyses, two stages of adjustment were used. In model A, we adjusted for MSDS (continuous or categorical), race and ethnicity, education, maternal age, parity, marriage status, pre-pregnancy BMI, DM, preeclampsia, smoking, alcohol consumption, soft drink consumption, child sex, LBW, and PTB. In model B, we additionally adjusted for plasma folate and vitamin B12 levels. We removed individuals from the analyses if they were missing data for any covariate included in the regression model.
Since consumption of a MSD is considered to generate antioxidant and anti-inflammatory effects, we might expect any beneficial effects on child neurodevelopment to be greatest among offspring of mothers with OWO/DM, since they are at higher risk for oxidative stress and immune or metabolic disturbances.[5,6,8] Due to the proven combined effects of OWO and DM on offspring NDD,[5] we combined maternal OWO and DM into a binary variable (without OWO or DM vs. with OWO and/or DM) for further analyses. We stratified the analyses of OWO/DM status (dichotomized) to explore the potential effect modification of maternal OWO/DM on the association between MSDS and offspring NDD. Among women with OWO/DM, we further analyzed the association of MSDS with the NDD subgroups (autism, ADHD, and other DD). To avoid potential bias due to the MSDS outliers, we performed sensitivity analysis after removing women with MSDSs lower than 12 and higher than 35. We also performed sensitivity analyses stratified by maternal smoking, PTB, and LBW. All statistical analyses were performed using R (version 4.2.1).
Results
Population characteristics
A total of 3153 mother–child pairs were included for analyses. The baseline characteristics of mothers (n = 3153) were compared between the MSDS quintiles (Table 1). Mothers in quintile 1 (lowest MSDS; n = 637) were more likely to be younger, non-Hispanic White, unmarried (divorced, separated, widowed, and single), and to have fewer years of educational attainment, to have consumed >1 cup of soft drinks per month during pregnancy, and to have lower plasma folate levels overall compared to those in any other quintile. These mothers were also more likely to have smoked or consumed alcohol anytime from 6 months before pregnancy to delivery. The frequency of the mothers’ food group intake stratified by MSDS quintiles is shown in Supplementary Table S2, http://links.lww.com/PN9/A27.
Table 1.
Baseline characteristics of mothers in a subset of the Boston Birth Cohort, stratified by MSDS quintiles.
| N (%) | MSDS quintiles | |||||
|---|---|---|---|---|---|---|
| Baseline characteristics | Total | Quintile 1 | Quintile 2 | Quintile 3 | Quintile 4 | Quintile 5 |
| No. | 3153 | 637 | 760 | 696 | 529 | 531 |
| Maternal age (years)* | 28 (23–33) | 27 (22–32) | 28 (23–33) | 28 (23–33) | 29 (24–33) | 30 (25–35) |
| Race and ethnicity* | ||||||
| Non-Hispanic Black† | 1849 (58.6) | 376 (59.0) | 437 (57.5) | 408 (58.6) | 310 (58.6) | 318 (59.9) |
| Non-Hispanic White | 229 (7.3) | 73 (11.5) | 56 (7.4) | 45 (6.5) | 31 (5.9) | 24 (4.5) |
| Hispanic | 702 (22.3) | 129 (20.3) | 176 (23.2) | 171 (24.6) | 123 (23.3) | 103 (19.4) |
| Other‡ | 373 (11.8) | 59 (9.3) | 91 (12.0) | 72 (10.3) | 65 (12.3) | 86 (16.2) |
| Education§ | ||||||
| Less than HS | 133 (4.2) | 27 (4.2) | 27 (3.6) | 39 (5.6) | 16 (3.0) | 24 (4.5) |
| HS or equivalent | 1904 (60.4) | 415 (65.1) | 474 (62.4) | 407 (58.5) | 317 (59.9) | 291 (54.8) |
| Greater than HS | 1116 (35.4) | 195 (30.6) | 259 (34.1) | 250 (35.9) | 196 (37.1) | 216 (40.7) |
| Marriage status* | ||||||
| Married | 1029 (32.6) | 149 (23.4) | 202 (26.6) | 248 (35.6) | 201 (38.0) | 229 (43.1) |
| Other | 2124 (67.4) | 488 (76.6) | 558 (73.4) | 448 (64.4) | 328 (62.0) | 302 (56.9) |
| Pre-pregnancy BMI | ||||||
| <25 kg/m2 | 1438 (45.6) | 277 (43.5) | 365 (48.0) | 313 (45.0) | 237 (44.8) | 246 (46.3) |
| ≥25 kg/m2 | 1715 (54.4) | 360 (56.5) | 395 (52.0) | 383 (55.0) | 292 (55.2) | 285 (53.7) |
| Parity | ||||||
| 0 | 1350 (42.8) | 285 (44.7) | 325 (46.3) | 310 (44.5) | 221 (41.8) | 209 (39.4) |
| >0 | 1803 (57.2) | 352 (55.3) | 435 (53.7) | 386 (55.5) | 308 (58.2) | 322 (60.6) |
| Diabetes | ||||||
| Yes | 344 (10.9) | 81 (12.7) | 79 (10.4) | 75 (10.8) | 53 (10.0) | 56 (10.5) |
| No | 2809 (89.1) | 556 (87.3) | 681 (89.6) | 621 (89.2) | 476 (90.0) | 475 (89.5) |
| Preeclampsia | ||||||
| Mild | 128 (4.1) | 21 (3.3) | 28 (3.7) | 31 (4.5) | 32 (6.0) | 16 (3.0) |
| Severe | 225 (7.1) | 52 (8.2) | 52 (6.8) | 45 (6.5) | 33 (6.2) | 43 (8.1) |
| No | 2800 (88.8) | 564 (88.5) | 680 (89.5) | 620 (89.1) | 464 (87.7) | 472 (88.9) |
| Ever smoking* | ||||||
| Yes | 580 (18.4) | 197 (30.9) | 146 (19.2) | 113 (16.2) | 70 (13.2) | 54 (10.2) |
| No | 2573 (81.6) | 440 (69.1) | 614 (80.8) | 583 (83.8) | 459 (86.8) | 477 (89.8) |
| Alcohol use§ | ||||||
| Yes | 251 (8.0) | 62 (9.7) | 76 (10.0) | 37 (5.3) | 33 (6.2) | 43 (8.1) |
| No | 2902 (92.0) | 575 (90.3) | 684 (90.0) | 659 (94.7) | 496 (93.8) | 488 (91.9) |
| Soft drink consumption* | ||||||
| ≤1 cup per month | 1390 (44.1) | 223 (35.0) | 351 (46.2) | 324 (46.6) | 234 (44.2) | 258 (48.6) |
| >1 cup per month | 1763 (55.9) | 414 (65.0) | 409 (53.8) | 372 (53.4) | 295 (55.8) | 273 (51.4) |
| Folate (nmol/L)§∥ | 31 (20–44) | 28 (19–41) | 32 (22–46) | 31 (20–43) | 31 (20–45) | 32 (21–49) |
| Vitamin B12 (pmol/L)∥ | 263 (214–323) | 262 (219–318) | 269 (213–329) | 257 (210–323) | 265 (221–311) | 261 (208–336) |
p values derived using the χ2 test or Kruskal-Wallis test. Percentages may not add up to 100% due to rounding.
p value <0.001.
Includes those who self-identified as African American and Haitian.
Includes those who self-identified as non-Hispanic Asian, Cape Verdean, Pacific Islander, more than one race, and other race.
p value ≤0.05.
Included 2068 mothers. BMI, body mass index; HS, high school; MSDS, Mediterranean-style diet score.
Of the 3153 children, we identified 1362 (43.2%) children with NDD, including 123 (3.9%) autistic children, 445 (14.1%) children with ADHD, 794 (25.2%) children with other DD, and 1791 (56.8%) children without any aforementioned NDD diagnoses in their EMRs who were defined as TD. The characteristics of each NDD group are shown in Supplementary Table S3, http://links.lww.com/PN9/A27. Compared with children defined as TD, children with NDD were more likely to be boys, have low birthweight, and to be born preterm; and their mothers were more likely to self-report as non-Hispanic Black, have a lower MSDS, have pre-pregnancy OWO, and smoked cigarettes anytime from 6 months before pregnancy to delivery. Similarly, compared with children defined as TD, autistic children and children with ADHD or other DD were also more likely to be boys, have low birthweight, and be born preterm. The mothers of autistic children were more likely to be older and to have DM. The mothers of children with ADHD were more likely to have lower education, to be unmarried (divorced, separated, widowed, or single), to have pre-pregnancy OWO, to have smoked cigarettes anytime from 6 months before pregnancy to delivery, and to have a lower level of plasma folate. The mothers of children with other DD were more likely to be older, to self-report as non-Hispanic Black, to have pre-pregnancy OWO, and to have experienced mild/severe preeclampsia.
Associations between maternal MSDS and NDD, autism, ADHD, and other DD
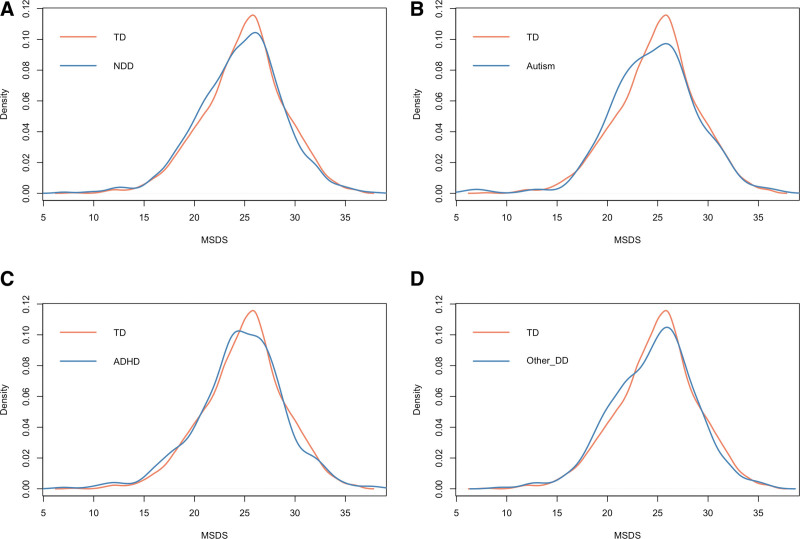
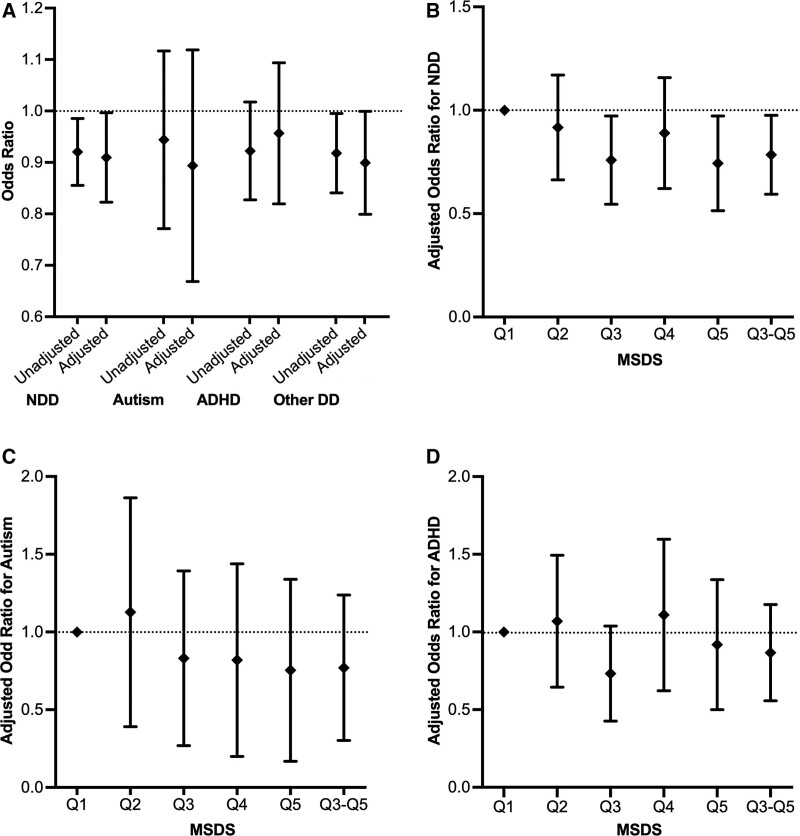
Compared to the mothers of children in the TD group, those with children in the NDD, autism, ADHD, and other DD groups were more likely to have lower MSDSs (Figure 1). As shown in Table 2 and Figure 2, the unadjusted logistic regression models showed that a high maternal MSDS (per SD increase) was associated with lower odds of offspring NDD (unadjusted odds ratio [OR]: 0.919, 95% confidence interval [CI]: 0.856–0.986; P value: 0.019) and other DD (unadjusted OR: 0.916, 95% CI: 0.842–0.996; P value:0.040). Using MSDS quintile 1 as the reference, having a maternal MSDS in quintile 3 (unadjusted OR: 0.770, 95% CI: 0.620–0.957; P value:0.019), quintile 5 (unadjusted OR: 0.750, 95% CI: 0.594–0.947; P value:0.016), or the combined group of quintiles 3 to 5 (unadjusted OR: 0.806, 95% CI: 0.671–0.967; P value:0.020) was associated with lower odds of offspring NDD; and having a maternal MSDS in quintile 5 (unadjusted OR: 0.748 95% CI: 0.567–0.984; P value:0.039) or the combined group of quintiles 3 to 5 (unadjusted OR: 0.803, 95% CI: 0.649–0.995; P value:0.044) was associated with lower odds of offspring other DD. Maternal MSDSs (whether analyzed as a categorical or continuous variable) were not associated with autism or ADHD in the offspring.
Figure 1:
MSDS distribution between participants categorized as TD vs. with NDD (A). MSDS distribution between those categorized as TD vs. with autism (B). MSDS distribution between those categorized as TD vs. with ADHD (C). MSDS distribution between those categorized as TD vs. with other DD (D). ADHD, attention-deficit/hyperactivity disorder; DD, developmental disabilities; MSDS, Mediterranean-style diet score; NDD, neurodevelopmental disabilities; TD, typically developing.
Table 2.
Unadjusted and adjusted associations of maternal Mediterranean-style diet score with NDD, autism, ADHD, and other DD in offspring.
| Characteristics | TD | NDD | Autism | ADHD | Other DD | ||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| n | n | OR (95%CI) | P | n | OR (95%CI) | P | n | OR (95%CI) | P | n | OR (95%CI) | P | |
| MSDS (per 1 SD increase) | |||||||||||||
| Unadjusted | 1791 | 1362 | 0.919 (0.856–0.986) | 0.019 | 123 | 0.933 (0.777–1.122) | 0.459 | 445 | 0.919 (0.829–1.019) | 0.109 | 794 | 0.916 (0.842–0.996) | 0.040 |
| Model A* | 1791 | 1362 | 0.939 (0.870–1.012) | 0.099 | 123 | 0.926 (0.757–1.133) | 0.451 | 445 | 0.998 (0.892–1.117) | 0.969 | 794 | 0.918 (0.840–1.003) | 0.058 |
| Model B† | 1146 | 922 | 0.907 (0.824–0.998) | 0.045 | 86 | 0.874 (0.679–1.128) | 0.299 | 300 | 0.950 (0.823–1.097) | 0.483 | 536 | 0.896 (0.801–1.001) | 0.053 |
| MSDS quintiles | |||||||||||||
| Unadjusted | |||||||||||||
| Q1 | 339 | 298 | Ref | 28 | Ref | 93 | Ref | 177 | Ref | ||||
| Q2 | 424 | 336 | 0.901 (0.730–1.113) | 0.336 | 31 | 0.885 (0.520–1.512) | 0.652 | 119 | 1.023 (0.754–1.392) | 0.884 | 186 | 0.840 (0.654–1.070) | 0.173 |
| Q3 | 415 | 281 | 0.770 (0.620–0.957) | 0.019 | 25 | 0.729 (0.415–1.275) | 0.268 | 86 | 0.755 (0.545–1.047) | 0.092 | 170 | 0.785 (0.608–1.012) | 0.062 |
| Q4 | 293 | 236 | 0.916 (0.727–1.155) | 0.459 | 20 | 0.826 (0.450–1.491) | 0.530 | 80 | 0.995 (0.709–1.394) | 0.978 | 136 | 0.889 (0.676–1.167) | 0.398 |
| Q5 | 320 | 211 | 0.750 (0.594–0.947) | 0.016 | 19 | 0.719 (0.389–1.304) | 0.283 | 67 | 0.763 (0.537–1.080) | 0.129 | 125 | 0.748 (0.567–0.984) | 0.039 |
| Q3–Q5‡ | 1028 | 728 | 0.806 (0.671–0.967) | 0.020 | 64 | 0.754 (0.480–1.211) | 0.229 | 233 | 0.826 (0.632–1.086) | 0.166 | 431 | 0.803 (0.649–0.995) | 0.044 |
| Model A* | |||||||||||||
| Q1 | 339 | 298 | Ref | 28 | Ref | 93 | Ref | 177 | Ref | ||||
| Q2 | 424 | 336 | 0.952 (0.763–1.188) | 0.664 | 31 | 0.842 (0.480–1.482) | 0.547 | 119 | 1.156 (0.836–1.601) | 0.382 | 186 | 0.859 (0.663–1.113) | 0.249 |
| Q3 | 415 | 281 | 0.799 (0.636–1.004) | 0.054 | 25 | 0.650 (0.358–1.172) | 0.152 | 86 | 0.842 (0.596–1.190) | 0.330 | 170 | 0.776 (0.595–1.011) | 0.061 |
| Q4 | 293 | 236 | 0.995 (0.779–1.271) | 0.969 | 20 | 0.892 (0.469–1.671) | 0.722 | 80 | 1.232 (0.858–1.769) | 0.258 | 136 | 0.934 (0.703–1.240) | 0.637 |
| Q5 | 320 | 211 | 0.818 (0.638–1.048) | 0.112 | 19 | 0.724 (0.374–1.375) | 0.327 | 67 | 1.022 (0.701–1.487) | 0.909 | 125 | 0.765 (0.572–1.021) | 0.070 |
| Q3–Q5‡ | 1028 | 728 | 0.860 (0.708–1.044) | 0.127 | 64 | 0.734 (0.451–1.220) | 0.221 | 233 | 1.000 (0.750–1.341) | 0.999 | 431 | 0.817 (0.653–1.025) | 0.079 |
| Model B† | |||||||||||||
| Q1 | 195 | 194 | Ref | 17 | Ref | 62 | Ref | 115 | Ref | ||||
| Q2 | 272 | 230 | 0.894 (0.676–1.181) | 0.430 | 25 | 0.961 (0.488–1.932) | 0.910 | 81 | 1.012 (0.676–1.520) | 0.953 | 124 | 0.795 (0.574–1.102) | 0.168 |
| Q3 | 272 | 202 | 0.739 (0.556–0.982) | 0.037 | 21 | 0.702 (0.344–1.446) | 0.331 | 60 | 0.690 (0.449–1.057) | 0.088 | 121 | 0.721 (0.518–1.003) | 0.052 |
| Q4 | 201 | 159 | 0.863 (0.636–1.170) | 0.343 | 12 | 0.668 (0.289–1.500) | 0.333 | 53 | 1.038 (0.661–1.629) | 0.872 | 94 | 0.842 (0.592–1.195) | 0.336 |
| Q5 | 206 | 137 | 0.720 (0.527–0.983) | 0.039 | 11 | 0.608 (0.255–1.398) | 0.247 | 44 | 0.856 (0.534–1.364) | 0.514 | 82 | 0.682 (0.474–0.978) | 0.038 |
| Q3–Q5‡ | 679 | 498 | 0.769 (0.602–0.983) | 0.036 | 44 | 0.668 (0.361–1.280) | 0.209 | 157 | 0.828 (0.578–1.194) | 0.306 | 297 | 0.745 (0.562–0.989) | 0.041 |
Controlled for MSDS (continuous or categorical), race and ethnicity, education, maternal age, parity, marriage status, pre-pregnancy BMI, DM, preeclampsia, smoking and alcohol consumption anytime from 6 months before pregnancy to delivery, soft drinks consumption, child sex, low birthweight, and preterm birth.
Additionally controlled for plasma folate and vitamin B12 levels.
Combined group includes those in quintiles 3, 4, and 5. ADHD, attention-deficit/hyperactivity disorder; BMI, body mass index; CI, confidence interval; DD, developmental disabilities; DM, diabetes mellitus; MSDS, Mediterranean-style diet score; NDD, neurodevelopmental disabilities; OR, odds ratio; other DD, other developmental disabilities; TD, typically developing.
Figure 2:
Unadjusted and adjusted ORs for the association of offspring NDD, autism, ADHD, and other DD with MSDSs (A). Adjusted ORs for the association of offspring NDD with MSDSs (B). Adjusted ORs for the association of offspring Autism with MSDSs (C). Adjusted ORs for the association of offspring ADHD with MSDSs (D). ADHD, attention-deficit/hyperactivity disorder; DD, developmental disabilities; MSDS, Mediterranean-style diet score; NDD, neurodevelopmental disabilities; OR, odds ratios.
After controlling for covariates (model B), a high maternal MSDS (per SD increase) was still associated with lower odds of offspring NDD (adjusted OR: 0.907, 95% CI: 0.824–0.998; P value: 0.045). Using maternal MSDS quintile 1 as the reference, in model B, having a maternal MSDS in quintile 3 (adjusted OR: 0.739, 95% CI: 0.556–0.982; P value: 0.037), quintile 5 (adjusted OR: 0.720, 95% CI: 0.527–0.983; P value: 0.039) or in the combined group of quintiles 3 to 5 (adjusted OR: 0.769, 95% CI: 0.602–0.983; P value: 0.036) was associated with lower odds of offspring NDD; while having a maternal MSDS in quintile 5 (adjusted OR: 0.682, 95% CI: 0.474–0.978; P value: 0.038), or in the combined group of quintiles 3 to 5 (adjusted OR: 0.745, 95% CI: 0.562–0.989; P value:0.041) was associated with lower odds of offspring other DD. Again, maternal MSDSs (whether analyzed as a categorical or continuous variable) were not associated with autism or ADHD in the offspring.
Associations of maternal MSDS with NDD stratified by maternal OWO/DM
We then stratified the analyses of maternal MSDS and offspring NDD by maternal OWO/DM status (Table 3). Among women with OWO and/or DM, after controlling for covariates (model B), a high maternal MSDS (per SD increase) was associated with lower odds of offspring NDD (adjusted OR: 0.840, 95% CI: 0.739–0.953; P value: 0.007). Using quintile 1 as the reference, having a maternal MSDS in quintile 3 (adjusted OR: 0.604, 95% CI: 0.415–0.877; P value: 0.008), quintile 5 (adjusted OR: 0.543, 95% CI: 0.358–0.820; P value: 0.004), or the combined group of quintiles 3 to 5 (adjusted OR: 0.643, 95% CI: 0.464-–.888; P value: 0.008) was associated with lower odds of offspring NDD. Among women with normal or underweight pre-pregnancy BMI who did not have DM, maternal MSDS was not associated with offspring NDD. We performed an interaction analysis to evaluate the effect of maternal OWO/DM on the association between maternal MSDS and offspring NDD. The results showed that among women with MSDSs in quintile 5, the association of maternal MSDS and offspring NDD was modified by maternal OWO/DM status (P = 0.036).
Table 3.
Adjusted associations between maternal MSDS and NDD in offspring stratified by maternal OWO/DM.
| Characteristics | OWO and/or DM | No OWO or DM | |||||||
|---|---|---|---|---|---|---|---|---|---|
| Participants | NDD | Participants | NDD | ||||||
| TD | n | OR (95% CI) | P | TD | n | OR (95% CI) | P | P interaction* | |
| MSDS (per 1 SD increase) | |||||||||
| Model A † | 974 | 818 | 0.908 (0.823–1.001) | 0.053 | 817 | 544 | 0.978 (0.868–1.103) | 0.717 | 0.226 |
| Model B ‡ | 617 | 568 | 0.840 (0.739–0.953) | 0.007 | 529 | 354 | 0.983 (0.846–1.143) | 0.823 | 0.065 |
| MSDS quintiles | |||||||||
| Model A† | |||||||||
| Q1 | 192 | 186 | Ref | 147 | 112 | Ref | |||
| Q2 | 220 | 195 | 0.951 (0.710–1.275) | 0.738 | 204 | 141 | 0.940 (0.668–1.324) | 0.724 | 0.984 |
| Q3 | 230 | 171 | 0.763 (0.566–1.026) | 0.074 | 185 | 110 | 0.827 (0.577–1.185) | 0.301 | 0.664 |
| Q4 | 156 | 146 | 1.025 (0.745–1.411) | 0.880 | 137 | 90 | 0.933 (0.635–1.368) | 0.722 | 0.798 |
| Q5 | 176 | 120 | 0.737 (0.531–1.021) | 0.067 | 144 | 91 | 0.922 (0.626–1.355) | 0.679 | 0.251 |
| Q3–Q5§ | 562 | 437 | 0.826 (0.642–1.064) | 0.138 | 466 | 291 | 0.886 (0.652–1.205) | 0.437 | 0.587 |
| Model B‡ | |||||||||
| Q1 | 99 | 125 | Ref | 96 | 69 | Ref | |||
| Q2 | 140 | 135 | 0.780 (0.536–1.132) | 0.192 | 132 | 95 | 1.036 (0.674–1.597) | 0.871 | 0.399 |
| Q3 | 157 | 127 | 0.604 (0.415–0.877) | 0.008 | 115 | 75 | 0.916 (0.583–1.440) | 0.705 | 0.130 |
| Q4 | 104 | 103 | 0.819 (0.547–1.225) | 0.332 | 97 | 56 | 0.839 (0.517–1.358) | 0.475 | 0.880 |
| Q5 | 117 | 78 | 0.543 (0.358–0.820) | 0.004 | 89 | 59 | 1.002 (0.616–1.627) | 0.993 | 0.036 |
| Q3–Q5§ | 378 | 308 | 0.643 (0.464–0.888) | 0.008 | 301 | 190 | 0.915 (0.622–1.350) | 0.652 | 0.123 |
Based on Model B, treating maternal OWO/DM as a binary variable (OWO and/or no OWO or DM) and the MSDS as either a categorical or continuous variable.
Controlled for MSDS (continuous or categorical), race and ethnicity, education, maternal age, parity, marriage status, preeclampsia, smoking and alcohol consumption anytime from 6 months before pregnancy to delivery, soft drink consumption, child sex, low birthweight, and preterm birth.
Additionally controlled for plasma folate and vitamin B12 levels.
Combined group includes those in quintiles 3, 4, and 5. CI, confidence interval; DM, diabetes mellitus; MSDS, Mediterranean-style diet score; NDD, neurodevelopmental disabilities; OR, odds ratio; OWO, overweight or obesity; TD, typically developing.
Association of maternal MSDS with autism, ADHD, and other DD among women with OWO/DM
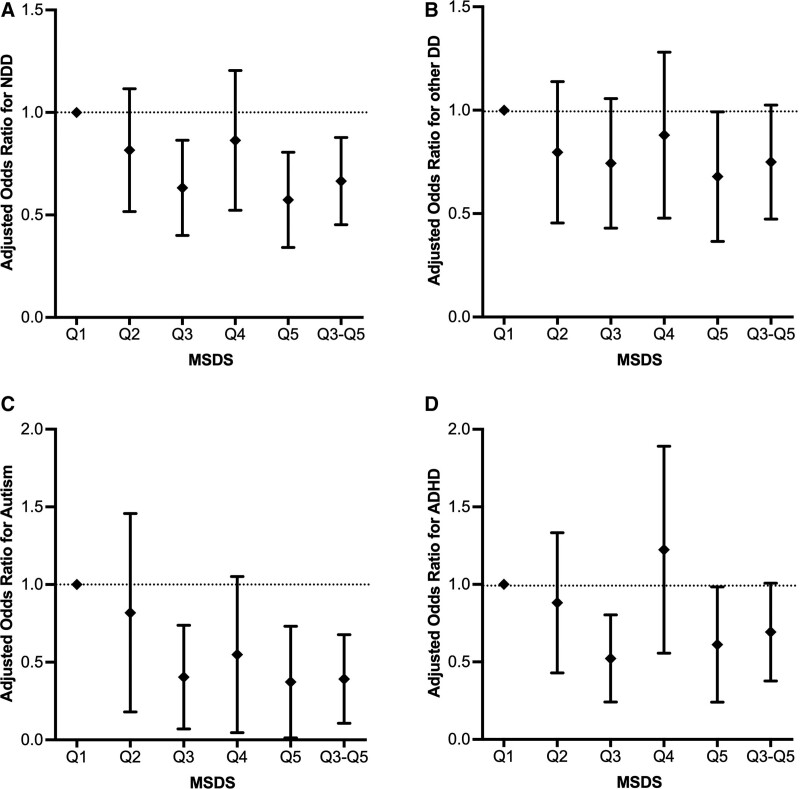
We also analyzed the association of maternal MSDS with offspring autism, ADHD, and other DD among women with OWO/DM [Supplementary Table S4, http://links.lww.com/PN9/A27 and Figure 3]. After controlling for covariates (model B), a high maternal MSDS (per SD increase) was associated with lower odds of offspring autism (adjusted OR: 0.658, 95% CI: 0.472–0.913; P value: 0.013). Using quintile 1 as the reference, having a maternal MSDS in quintile 3 (adjusted OR: 0.314, 95% CI: 0.124–0.773; P value: 0.012), quintile 5 (adjusted OR: 0.265, 95% CI: 0.079–0.773; P value: 0.020), or in the combined group of quintiles 3–5 (adjusted OR: 0.321, 95% CI: 0.149–0.706; P value: 0.004) was associated with decreased odds of offspring autism. Using quintile 1 as the reference, having a maternal MSDS in quintile 3 (adjusted OR: 0.473, 95% CI: 0.269–0.825; P value: 0.009) was associated with decreased odds of offspring ADHD. There was no association between maternal MSDS and offspring other DD among women with OWO/DM. We also conducted the same analysis among women without OWO or DM, and no association of maternal MSDS with offspring autism or ADHD was found [Supplementary Table S5, http://links.lww.com/PN9/A27].
Figure 3:
Adjusted ORs for the association of offspring NDD with MSDSs among women with OWO/DM (A). Adjusted ORs for the association of offspring other DD with MSDSs among women with OWO/DM (B). Adjusted ORs for the association of offspring autism with MSDSs among women with OWO/DM (C). Adjusted ORs for the association of offspring ADHD with MSDSs among women with OWO/DM (D). DM, diabetes mellitus; MSDS, Mediterranean-style diet score; NDD, neurodevelopmental disabilities; OR, odds ratios; OWO, overweight/obesity.
Sensitivity analyses
After removing women with MSDSs lower than 12 or higher than 35, the associations of maternal MSDS with offspring NDD, autism, ADHD, and other DD were consistent with the results of the main analyses described above [Supplementary Table S6, http://links.lww.com/PN9/A27]. We also stratified the analyses of maternal MSDS and offspring NDD by maternal cigarette smoking anytime from 6 months before pregnancy to delivery [Supplementary Table S7, http://links.lww.com/PN9/A27], PTB [Supplementary Table S8, http://links.lww.com/PN9/A27], and LBW [Supplementary Table S9, http://links.lww.com/PN9/A27]. The results indicated that having higher maternal MSDS was consistently associated with lower odds of offspring NDD among women who reported being nonsmokers, and among women who delivered full-term births and AGA-weight infants. Conversely, possibly due to sample size limitations, we did not identify an association between maternal MSDS and offspring NDD among women who reported being smokers or among women who delivered PTB and LBW infants.
Discussion
In this prospective birth cohort, after controlling for several key potential confounders, a high maternal MSDS showed a lower likelihood of overall NDD and other DD in offspring, while there was no association of maternal MSDS with autism or ADHD in the offspring. In confirmation of our hypothesis, among women with OWO and/or DM, a high maternal MSDS also showed a lower likelihood of NDD in the offspring and this association was greater than that in women without OWO and/or DM. Among women with OWO and/or DM, a high maternal MSDS was also associated with a lower likelihood of autism in the offspring.
Several previous studies have explored the association of maternal dietary patterns with the neurodevelopment of their offspring.[39–43] A longitudinal study published in 2018 from the United Kingdom showed that pregnant women with a diet rich in fruits and vegetables were more likely to have children (aged 8 years) with higher average IQs.[39] Findings from a birth cohort in China including 1178 mother–child pairs also indicated that high maternal intake of dietary fiber and high-quality protein during mid and late-pregnancy was associated with better gross motor and receptive communication development in offspring aged 1 year.[40] An Australian longitudinal study on women’s health including 1554 mother–child dyads indicated that a higher maternal healthy eating index (HEI) in pre-pregnancy was associated with a lower likelihood behavioral disorders in the offspring.[41] Similarly, a French cohort including 1242 mother–child pairs showed that a maternal “low healthy diet” (characterized by low intake of fruits, vegetables, fish, and whole grains) was associated with trajectories of greater ADHD symptomology among the study children.[42] Furthermore, the UK longitudinal study mentioned above showed that a prenatal high-fat and high-sugar diet was positively associated with ADHD symptoms in 83 adolescents with early-onset persistent conduct problems, but not in 81 adolescents with low conduct problems.[43] Although there are differences in maternal dietary measurements, outcome assessment methods, and the results of related studies, a meta-analysis including 18 studies with 63,861 participants showed that better maternal diet quality (fish intake, Omega-6/Omega-3 fatty acid ratio, saturated fat intake, or dietary fiber were used as proxies) during pregnancy had a small association with child neurodevelopment.[44]
Few studies have explored the potential association of maternal MSD with offspring NDD, and the limited available evidence has been inconclusive. Findings from the investigation of another US cohort including 1580 mother–child pairs showed that a higher maternal MSDS was associated with better visual-spatial skills in offspring in early childhood and better intelligence and executive function at mid-childhood.[45] A Dutch cohort study including 3104 child–mother pairs showed that maternal low adherence to a MSD was associated with an increased likelihood of inattention and aggression in their children.[46] In a US neonatal epigenetic study including 325 children, higher perinatal adherence to a MSD was associated with a lower likelihood of autism in the offspring.[47] However, a recent study based on two prospective longitudinal cohorts of more than 800 pregnant women in the US did not find strong evidence that consumption of a MSD among the women was associated with autism in offspring.[48] In our study, a high maternal MSDS was associated with a lower likelihood of overall NDD and other DD in the offspring. Although there was heterogeneity in the NDD and other DD groups, including different categories of neurodevelopmental disabilities, which made the results hard to explain, the results still indicated that a high maternal MSDS might have an influence on neurodevelopment in offspring. The underlying mechanisms should be further explored.
In recent years, there has been increasing evidence of the impact of gene-environment interactions on NDD in offspring.[4] The maternal diet may influence offspring neurodevelopment by affecting epigenetic methylation modifications, microbiome, oxidative stress, and immune response.[49–51] Some essential properties of the MSD have been shown to affect offspring neurodevelopment. For instance, several studies have linked a higher intake of fish (a major dietary source of polyunsaturated fatty acids), which is a characteristic of the MSD, in pregnant women with neurodevelopment in offspring, although recommendations for fish consumption during pregnancy should also consider the potential negative effects of mercury exposure.[52] Polyunsaturated fatty acids may affect the proliferation and differentiation of neural stem cells and the regulation of microglia and neuroinflammation.[53] Some animal and human studies have also indicated that dietary fiber intake, which is high in the MSD, modulates fetal and early postnatal offspring neurodevelopment by affecting maternal gut microbiome diversity.[54] Fruits and vegetables, which are key food groups of the MSD, are rich in many essential vitamins, including vitamins A, B, and D, which may impact offspring neurodevelopment.[55]
Our results showed that older mothers were more likely to have autistic children or children with other DD, while further analyses did not show interaction effects between maternal age and MSDS on autism and other DD. In the overall sample, after additionally controlling for maternal plasma folate and vitamin B12 levels, a high maternal MSDS was associated with a lower likelihood of NDD among offspring. However, the correlation analysis did not show a significant correlation between maternal MSDS and plasma folate and/or vitamin B12 levels [Supplementary Material, http://links.lww.com/PN9/A27]. We speculate that during pregnancy, women were more likely to use multivitamins or prenatal vitamin supplements regardless of MSD adherence; however, we did not ask them about nutritional guidance from medical providers, including recommendations for consumption of an MSD. Future studies should assess the role of dietary recommendations especially since there is a current lack of clinical nutrition education for physicians.[56,57]
Maternal OWO/DM status has been associated with a higher likelihood of NDD in offspring.[5,58] DNA methylation modifications of immune genes were found in the cord blood of newborns whose mothers were exposed to OWO/DM, with persistent epigenetic alterations into adulthood.[8] In our study, maternal MSD adherence showed a greater association with NDD in offspring of mothers with OWO/DM compared to mothers without OWO/DM. Among women with the highest MSDSs (quintile 5), the association of MSDS with offspring NDD was modified by maternal OWO/DM status. There was no association of maternal MSDS with autism or ADHD in offspring in the overall sample and in women without OWO or DM, while among women with OWO and/or DM, the results indicated that a high maternal MSDS was associated with a lower likelihood of autism in offspring and having a maternal MSDS in quintile 3 was associated with a lower likelihood of ADHD in the offspring. The underlying mechanisms for how a higher maternal MSDS can impact the effects of OWO/DM on offspring neurodevelopment still require further research; however, due to its associated antioxidant and anti-inflammatory properties, maternal MSD adherence may reduce the likelihood of NDD in offspring by alleviating the inflammatory response and affecting epigenetic methylation modifications in women with OWO/DM.[17,49] Therefore, women with low MSDSs, especially women with OWO/DM, as indicated by our findings, are more likely to have children with NDD. Identifying these women would prompt early screening for NDD in their offspring as well as the provision of needed social supports for both mothers and children. Furthermore, due to the rising prevalence of obesity and diabetes among women of childbearing age, dietary interventions such as the MSD may prove to be a potential protective measure against a range of adverse health outcomes among women.
This study had several strengths. Our study was built on a relatively large sample size and the data collection was conducted by professionally trained research personnel based on standardized protocols. All the study children were delivered and received pediatric care at a single medical center with a uniform EMR system. We identified consistent and significant associations between maternal MSDSs and offspring NDD in the overall sample. We also adjusted for several pertinent sociodemographic variables in the analyses to control for potential confounding.
Our study population, a subset of the BBC, included a high proportion of self-reported non-Hispanic Black women and women with low educational attainment. This is noteworthy in the sense that the findings from this study help to fill in important data gaps for this traditionally understudied population; however, caution is needed to generalize our study’s findings to groups with different characteristics.
This study also had some limitations. Information about prenatal maternal diet intake was based on postpartum maternal self-report with possible recall bias and social desirability bias. Diet was assessed according to the frequency of intake of different food groups rather than quantity of consumption; and we did not collect information regarding food quality, preparation methods, and/or access to diverse foods. More information on multiple social and environmental factors that affect women’s food choices should be collected in future studies. Food groups and MSD scores were not adjusted for energy intake. However, considering that some women may still have poor dietary habits despite having high MSDSs, we also adjusted for soft drink consumption since it reflects consumption of added sugars in the diet. In addition, this study only considered maternal MSDS and did not adjust for postnatal dietary data for the study children. Maternal MSDSs may be associated with childhood postnatal dietary data, which would affect NDD and thus mediate the observed association between maternal MSDS and NDD in the offspring. We recommend that future studies should collect childhood dietary data to explore potential associations.
Conclusions
In this prospective birth cohort, a higher maternal MSDS was associated with a lower likelihood of NDD in offspring. This association of maternal MSD with offspring NDD was greater in children born to women with OWO/DM. More studies are needed to replicate these findings and further analyze the NDD subgroups, and explore potential underlying molecular pathways. Based on our findings, women of childbearing age should be educated before and during pregnancy about the potential effects of consuming a MSD on the neurodevelopment of their offspring. Consuming a MSD may also reduce maternal OWO and DM, which may affect maternal health. To support this recommendation, we advocate for an increased emphasis on clinical nutrition in medical training, particularly for those who care for women of childbearing age and pregnant women, but also more generally for any medical provider who cares for individuals with OWO and DM. Considering that food choices are affected by multiple individual, social, and environmental factors, it is recommended to increase access to MSD food choices at the community level. We also advocate for identifying women with low MSDSs to prompt early screening of NDD in offspring and to provide early social supports to mothers and children.
Acknowledgments
The authors wish to thank the study participants in the BBC, the nursing staff at labor and delivery of the Boston Medical Center, as well as the field team for their contributions to the Boston Birth Cohort.
Funding
The Boston Birth Cohort (the parent study) was supported in part by the National Institutes of Health (NIH) grants (2R01HD041702, R01HD086013, R01HD098232, R21AI154233, R01ES031272, R03HD096136, R01ES031521, and U01 ES034983); and by the Health Resources and Services Administration (HRSA) of the U.S. Department of Health and Human Services (HHS) (UT7MC45949). This information or content and conclusions are those of the authors and should not be construed as the official position or policy of, nor should any endorsements be inferred by the funding agencies.
Author Contributions
XC analyzed the data, drafted the initial manuscript, and revised the manuscript. SG and XW conceptualized the study. XW acquired funding. XW, GW, XH, CP, MA supervised field data collection. All the authors critically reviewed and revised the manuscript and approved the final manuscript. XC, SG, and XW agreed to be accountable for all aspects of the work. All authors have read and approved the manuscript.
Conflicts of Interest
The authors report no conflicts of interest.
Supplementary Material
Footnotes
Supplemental Digital Content is available for this article.
How to cite this article: Che X, Gross SM, Wang G, Hong X, Pearson C, Bartell T, Wang X. Impact of consuming a Mediterranean-style diet during pregnancy on neurodevelopmental disabilities in offspring: results from the Boston Birth Cohort. Precis Nutr 2023;2(3):e00047. doi: 10.1097/PN9.0000000000000047
The data, data dictionary, and analytical programs used in this study are not currently available to the public. However, they can be made available upon reasonable request and after the review and approval of the Institutional Review Boards. The source code to replicate the results will be provided upon request.
References
- [1].Cusick SE, Georgieff MK. The role of nutrition in brain development: the golden opportunity of the “first 1000 days.” J Pediatr 2016;175:16–21. doi:10.1016/j.jpeds.2016.05.013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [2].Bordeleau M, Fernández de Cossío L, Chakravarty MM, et al. From maternal diet to neurodevelopmental disorders: a story of neuroinflammation. Front Cell Neurosci 2021;14:612705. doi:10.3389/fncel.2020.612705. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [3].Jyonouchi H, Geng L, Streck DL, et al. Children with autism spectrum disorders (ASD) who exhibit chronic gastrointestinal (GI) symptoms and marked fluctuation of behavioral symptoms exhibit distinct innate immune abnormalities and transcriptional profiles of peripheral blood (PB) monocytes. J Neuroimmunol 2011;238(1–2):73–80. doi:10.1016/j.jneuroim.2011.07.001. [DOI] [PubMed] [Google Scholar]
- [4].Han VX, Patel S, Jones HF, et al. Maternal immune activation and neuroinflammation in human neurodevelopmental disorders. Nat Rev Neurol 2021;17(9):564–579. doi:10.1038/s41582-021-00530-8. [DOI] [PubMed] [Google Scholar]
- [5].Li M, Fallin MD, Riley A, et al. The association of maternal obesity and diabetes with autism and other developmental disabilities. Pediatrics 2016;137(2):e20152206. doi:10.1542/peds.2015-2206. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [6].Bilbo SD, Tsang V. Enduring consequences of maternal obesity for brain inflammation and behavior of offspring. FASEB J 2010;24(6):2104–2115. doi:10.1096/fj.09-144014. [DOI] [PubMed] [Google Scholar]
- [7].Tran NQV, Miyake K. Neurodevelopmental disorders and environmental toxicants: epigenetics as an underlying mechanism. Int J Genomics 2017;2017:7526592. doi:10.1155/2017/7526592. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [8].Han VX, Patel S, Jones HF, et al. Maternal acute and chronic inflammation in pregnancy is associated with common neurodevelopmental disorders: a systematic review. Transl Psychiatry 2021;11(1):71. doi:10.1038/s41398-021-01198-w. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [9].Bunbury S. Unconscious bias and the medical model: How the social model may hold the key to transformative thinking about disability discrimination. Int J Discrim 2019;19(1):26–47. doi:10.1177/1358229118820742. [Google Scholar]
- [10].Romero-Ayuso D. Future challenges in research in children with neurodevelopmental disorders. Children (Basel) 2021;8(5):328. doi:10.3390/children8050328. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [11].Zablotsky B, Black LI, Maenner MJ, et al. Prevalence and trends of developmental disabilities among children in the United States: 2009–2017. Pediatrics 2019;144(4):e20190811. doi:10.1542/peds.2019-0811. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [12].Maenner MJ, Shaw KA, Bakian AV, et al. Prevalence and characteristics of autism spectrum disorder among children aged 8 years—autism and developmental disabilities monitoring network, 11 sites, United States, 2018. MMWR Morb Mortal Wkly Rep 2021;70(11):1. doi:10.15585/mmwr.ss7011a1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [13].Danielson ML, Holbrook JR, Bitsko RH, et al. State-level estimates of the prevalence of parent -reported ADHD diagnosis and treatment among US children and adolescents, 2016 to 2019. J Atten Disord 2022;26(13):1685–1697. doi:10.1177/10870547221099961. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [14].Martinez-Lacoba R, Pardo-Garcia I, Amo-Saus E, et al. Mediterranean diet and health outcomes: a systematic meta-review. Eur J Public Health 2018;28(5):955–961. doi:10.1093/eurpub/cky113. [DOI] [PubMed] [Google Scholar]
- [15].Bach-Faig A, Berry EM, Lairon D, et al. ; Mediterranean Diet Foundation Expert Group. Mediterranean diet pyramid today. Science and cultural updates. Public Health Nutr 2011;14(12A):2274–2284. doi:10.1017/S1368980011002515. [DOI] [PubMed] [Google Scholar]
- [16].Bonaccio M, Iacoviello L, de Gaetano G; Moli-Sani Investigators. The Mediterranean diet: the reasons for a success. Thromb Res 2012;129:401–404. doi:10.1016/j.thromres.2011.10.018. [DOI] [PubMed] [Google Scholar]
- [17].Tektonidis TG, Åkesson A, Gigante B, et al. A Mediterranean diet and risk of myocardial infarction, heart failure and stroke: a population-based cohort study. Atherosclerosis 2015;243:93–98. doi:10.1016/j.atherosclerosis.2015.08.039. [DOI] [PubMed] [Google Scholar]
- [18].Stefler D, Malyutina S, Kubinova R, et al. Mediterranean diet score and total and cardiovascular mortality in Eastern Europe: the HAPIEE study. Eur J Nutr 2017;56:421–429. doi:10.1007/s00394-015-1092-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [19].Bonaccio M, Di Castelnuovo A, Costanzo S, et al. ; MOLI-SANI study Investigators. Adherence to the traditional Mediterranean diet and mortality in subjects with diabetes. Prospective results from the MOLI-SANI study. Eur J Prev Cardiol 2016;23:400–407. doi:10.1177/2047487315569409. [DOI] [PubMed] [Google Scholar]
- [20].Arpon A, Riezu-Boj JI, Milagro FI, et al. Adherence to Mediterranean diet is associated with methylation changes in inflammation-related genes in peripheral blood cells. J Physiol Biochem 2016;73(3):445–455. doi:10.1007/s13105-017-0552-6. [DOI] [PubMed] [Google Scholar]
- [21].Casas R, Sacanella E, Estruch R. The immune protective effect of the Mediterranean diet against chronic low-grade inflammatory diseases. Endocr Metab Immune Disord Drug Targets 2014;14(4):245–254. doi:10.2174/1871530314666140922153350. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [22].Petersson SD, Philippou E. Mediterranean diet, cognitive function, and dementia: a systematic review of the evidence. Adv Nutr 2016;7(5):889–904. doi:10.3945/an.116.012138. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [23].Martínez-González MA, Gea A, Ruiz-Canela M. The Mediterranean diet and cardiovascular health: a critical review. Circ Res 2019;124(5):779–798. doi:10.1161/CIRCRESAHA.118.313348. [DOI] [PubMed] [Google Scholar]
- [24].Gantenbein KV, Kanaka-Gantenbein C. Mediterranean diet as an antioxidant: the impact on metabolic health and overall wellbeing. Nutrients 2021;13(6):1951. doi:10.3390/nu13061951. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [25].Koloverou E, Esposito K, Giugliano D, et al. The effect of Mediterranean diet on the development of type 2 diabetes mellitus: a meta-analysis of 10 prospective studies and 136,846 participants. Metabolism 2014;63(7):903–911. doi:10.1016/j.metabol.2014.04.010. [DOI] [PubMed] [Google Scholar]
- [26].Al Wattar BH, Dodds J, Placzek A, et al. Mediterranean-style diet in pregnant women with metabolic risk factors (ESTEEM): a pragmatic multicentre randomised trial. PLoS Med 2019;16(7):e1002857. doi:10.1371/journal.pmed.1002857. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [27].Chia AR, Chen LW, Lai JS, et al. Maternal dietary patterns and birth outcomes: a systematic review and meta-analysis. Adv Nutr 2019;10(4):685–695. doi:10.1093/advances/nmy123. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [28].Pearson C, Bartell T, Wang G, et al. Boston Birth Cohort profile: rationale and study design. Precis Nutr 2022;1(2):e00011. doi:10.1097/PN9.0000000000000011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [29].Rhee DK, Ji Y, Hong X, et al. Mediterranean-style diet and birth outcomes in an urban, multiethnic, and low-income US population. Nutrients 2021;13(4):1188. doi:10.3390/nu13041188. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [30].Abdullahi I, Wong K, Bebbington K, et al. Diagnosis of autism spectrum disorder according to maternal-race ethnicity and country of birth: a register-based study. J Autism Dev Disord 2019;49(9):3611–3624. doi:10.1007/s10803-019-04068-z. [DOI] [PubMed] [Google Scholar]
- [31].Russell AE, Ford T, Williams R, et al. The association between socioeconomic disadvantage and attention deficit/hyperactivity disorder (ADHD): a systematic review. Child Psychiatry Hum Dev 2016;47:440–458. doi:10.1007/s10578-015-0578-3. [DOI] [PubMed] [Google Scholar]
- [32].Kim MJ, Park I, Lim MH, et al. Prevalence of attention-deficit/hyperactivity disorder and its comorbidity among Korean children in a community population. J Korean Med Sci 2017;32(3):401–406. doi:10.3346/jkms.2017.32.3.401. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [33].Carlsson T, Molander F, Taylor MJ, et al. Early environmental risk factors for neurodevelopmental disorders–a systematic review of twin and sibling studies. Dev Psychopathol 2021;33(4):1448–1495. doi:10.1017/S0954579420000620. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [34].Landgren M, Svensson L, Strömland K, et al. Prenatal alcohol exposure and neurodevelopmental disorders in children adopted from eastern Europe. Pediatrics 2010;125(5):e1178–e1185. doi:10.1542/peds.2009-0712. [DOI] [PubMed] [Google Scholar]
- [35].Barron A, McCarthy CM, O’Keeffe GW. Preeclampsia and neurodevelopmental outcomes: potential pathogenic roles for inflammation and oxidative stress? Mol Neurobiol 2021;58(6):2734–2756. doi:10.1007/s12035-021-02290-4. [DOI] [PubMed] [Google Scholar]
- [36].Cheslack-Postava K, Jokiranta E, Suominen A, et al. Variation by diagnostic subtype in risk for autism spectrum disorders associated with maternal parity among Finnish births. Paediatr Perinat Epidemiol 2014;28(1):58–66. doi:10.1111/ppe.12094. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [37].Raghavan R, Riley AW, Volk H, et al. Maternal multivitamin intake, plasma folate and vitamin B12 levels and autism spectrum disorder risk in offspring. Paediatr Perinat Epidemiol 2018;32(1):100–111. doi:10.1111/ppe.12414. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [38].May T, Adesina I, McGillivray J, et al. Sex differences in neurodevelopmental disorders. Dev Med Child Neurol 2019;32(4):622–626. doi:10.1097/WCO.0000000000000714. [DOI] [PubMed] [Google Scholar]
- [39].Freitas-Vilela AA, Pearson RM, Emmett P, et al. Maternal dietary patterns during pregnancy and intelligence quotients in the offspring at 8 years of age: Findings from the ALSPAC cohort. Matern Child Nutr 2018;14(1):e12431. doi:10.1111/mcn.12431. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [40].Lv S, Qin R, Jiang Y, et al. Association of maternal dietary patterns during gestation and offspring neurodevelopment. Nutrients 2022;14(4):730. doi:10.3390/nu14040730. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [41].Gete DG, Waller M, Mishra GD. Pre-pregnancy diet quality and its association with offspring behavioral problems. Eur J Nutr 2021;60:503–515. doi:10.1007/s00394-020-02264-7. [DOI] [PubMed] [Google Scholar]
- [42].Galéra C, Heude B, Forhan A, et al. ; EDEN Mother-Child Cohort Study Group. Prenatal diet and children’s trajectories of hyperactivity–inattention and conduct problems from 3 to 8 years: the EDEN mother–child cohort. J Child Psychol Psychiatry 2018;59(9):1003–1011. doi:10.1111/jcpp.12898. [DOI] [PubMed] [Google Scholar]
- [43].Rijlaarsdam J, Cecil CA, Walton E, et al. Prenatal unhealthy diet, insulin-like growth factor 2 gene (IGF 2) methylation, and attention deficit hyperactivity disorder symptoms in youth with early-onset conduct problems. J Child Psychol Psychiatry 2017;58(1):19–27. doi:10.1111/jcpp.12589. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [44].Borge TC, Aase H, Brantsæter AL, et al. The importance of maternal diet quality during pregnancy on cognitive and behavioural outcomes in children: a systematic review and meta-analysis. BMJ Open 2017;7(9):e016777. doi:10.1136/bmjopen-2017-016777. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [45].Mahmassani HA, Switkowski KM, Scott TM, et al. Maternal diet quality during pregnancy and child cognition and behavior in a US cohort. Am J Clin Nutr 2022;115(1):128–141. doi:10.1093/ajcn/nqab325. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [46].Steenweg-de Graaff J, Tiemeier H, Steegers-Theunissen RP, et al. Maternal dietary patterns during pregnancy and child internalising and externalising problems. The Generation R Study. Clin Nutr 2014;33(1):115–121. doi:10.1016/j.clnu.2013.03.002. [DOI] [PubMed] [Google Scholar]
- [47].House JS, Mendez M, Maguire RL, et al. Periconceptional maternal Mediterranean diet is associated with favorable offspring behaviors and altered CpG methylation of imprinted genes. Front Cell Dev Biol 2018;6:107. doi:10.3389/fcell.2018.00107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [48].Vecchione R, Wang S, Rando J, et al. Maternal dietary patterns during pregnancy and child autism-related traits: results from two US cohorts. Nutrients 2022;14(13):2729. doi:10.3390/nu14132729. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [49].Banik A, Kandilya D, Ramya S, et al. Maternal factors that induce epigenetic changes contribute to neurological disorders in offspring. Genes (Basel) 2017;8(6):150. doi:10.3390/genes8060150. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [50].Renz H, Holt PG, Inouye M, et al. An exposome perspective: early-life events and immune development in a changing world. J Allergy Clin Immunol 2017;140(1):24–40. doi:10.1016/j.jaci.2017.05.015. [DOI] [PubMed] [Google Scholar]
- [51].O’Neil A, Itsiopoulos C, Skouteris H, et al. Preventing mental health problems in offspring by targeting dietary intake of pregnant women. BMC Med 2014;12:208. doi:10.1186/s12916-014-0208-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [52].Ryan AS, Astwood JD, Gautier S, et al. Effects of long-chain polyunsaturated fatty acid supplementation on neurodevelopment in childhood: a review of human studies. Prostaglandins Leukot Essent Fatty Acids 2010;82(4–6):305–314. doi:10.1016/j.plefa.2010.02.007. [DOI] [PubMed] [Google Scholar]
- [53].Martinat M, Rossitto M, Di Miceli M, et al. Perinatal dietary polyunsaturated fatty acids in brain development, role in neurodevelopmental disorders. Nutrients 2021;13(4):1185. doi:10.3390/nu13041185. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [54].Di Gesù CM, Matz LM, Buffington SA. Diet-induced dysbiosis of the maternal gut microbiome in early life programming of neurodevelopmental disorders. Neurosci Res 2021;168:3–19. doi:10.1016/j.neures.2021.05.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [55].Tengeler AC, Kozicz T, Kiliaan AJ. Relationship between diet, the gut microbiota, and brain function. Nutr Rev 2018;76(8):603–617. doi:10.1093/nutrit/nuy016. [DOI] [PubMed] [Google Scholar]
- [56].Vetter ML, Herring SJ, Sood M, et al. What do resident physicians know about nutrition? An evaluation of attitudes, self-perceived proficiency and knowledge. J Am Coll Nutr 2008;27(2):287–298. doi:10.1080/07315724.2008.10719702. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [57].Devries S, Dalen JE, Eisenberg DM, et al. A deficiency of nutrition education in medical training. Am J Med 2014;127(9):804–806. doi:10.1016/j.amjmed.2014.04.003. [DOI] [PubMed] [Google Scholar]
- [58].Sanchez CE, Barry C, Sabhlok A, et al. Maternal pre-pregnancy obesity and child neurodevelopmental outcomes: a meta-analysis. Obes Rev 2018;19(4):464–484. doi:10.1111/obr.12643. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.