Abstract
Background:
Antimicrobial resistance is a global health threat. To slow resistance and preserve antibiotics, stewardship interventions are increasingly promoted and mandated. Urine cultures are the most common microbiological test in the outpatient setting. Contamination most likely occurs during urine collection from surrounding vaginal, perineal, and epidermal flora. Sample contamination can lead to incorrect diagnosis, unnecessary or inappropriate treatment, poor patient outcomes, and higher costs. Therefore, ensuring proper collection of urinary samples serves as a prime diagnostic stewardship target, one that international nursing societies increasingly endorse as an opportunity for nurse involvement.
Objectives:
Determine the prevalence, predictors, and antibiotic prescribing associated with contaminated urine cultures in primary care clinics.
Design:
Cross-sectional study
Setting:
Two adult safety-net clinics in Houston, Texas
Participants:
1265 clinical encounters among 1114 primary care patients
Methods:
We reviewed charts from office visits among patients who had a urine culture ordered between November 2018-March 2020. Patient demographics, culture results and prescription orders were captured for each visit. Culture results were defined as no growth, contaminated (i.e., mixed flora, non-uropathogens, or ≥3 bacteria isolated), or low-count (102-105 colony forming units (CFU)/mL) or high-count (>105 CFU/mL) uropathogen-positive. We performed multinomial logistic regression to identify predictors independently associated with contaminated cultures.
Results:
Our study evaluated 1265 cultures from 1114 patients that were primarily female (84%), of Hispanic/Latino (74.4%) or Black/African American (18.9%) race/ethnicity with a mean age of 43 years. Out of 1265 urine cultures, 264 (20.9%) had no growth, 694 (54.9%) were contaminated, 159 (12.6%) were low-count positive, and 148 (11.7%) were high-count positive. Female gender, pregnancy, and obesity were associated with contaminated cultures (multinomial adjusted odds ratios: 15.89, 14.34, 1.93, respectively; 95% confidence intervals: 10.25-24.61, 8.03-25.61, 1.32-2.81, respectively). Antibiotic prescribing was significantly higher among symptomatic patients with contaminated cultures compared to those with no growth.
Conclusion:
Urine culture contamination occurred frequently in our clinics, and obesity, female sex and pregnancy were independent risk factors for contamination. The association of pregnancy and contamination is particularly concerning as pregnant females are routinely screened and treated for asymptomatic bacteriuria in the United States. Culture contamination may obscure underlying uropathogens, leading to pyelonephritis or potential neonatal infection if untreated. Conversely, overtreatment of false positive bacteriuria could lead to adverse effects from antibiotics and increased risk for antibiotic resistance. As nurses play a prominent role in patient education, diagnostic stewardship interventions may want to utilize nurses’ educational capabilities to improve urine culture collection.
Keywords: Urine specimen collection, microbial contamination, antimicrobial stewardship, primary health care, outpatients, urinary tract infections, bacteriuria
Tweetable abstract:
55% of urine cultures collected in primary care clinics were contaminated, revealing a major opportunity for nurse-driven diagnostic stewardship interventions
1. Background
Antimicrobial resistance is one of the most serious threats to global health (Centers for Disease Control and Prevention, 2019, World Health Organization, 2019). In response to rising rates of resistance, the field of antimicrobial stewardship is rapidly expanding to include a multidisciplinary, team approach (Gotterson et al., 2021). Nurses are uniquely positioned to augment antibiotic stewardship because they have direct roles in patient care and quality improvement. Strategies to encourage nurse involvement in antibiotic stewardship have been endorsed internationally via nursing societies, infection prevention centers, and government health agencies (Australian Commission on Safety and Quality in Health Care (ACSQHC), 2018, American Nursing Association and Centers for Disease Control and Prevention, 2017, Edwards et al., 2011, International Council of Nurses, 2017, Ladenheim, 2018). One of the practical, feasible and impactful areas for nurse engagement in stewardship is ensuring a proper, midstream clean-catch technique is followed by patients when a urinary specimen for culture is ordered (American Nursing Association and Centers for Disease Control and Prevention, 2017, Carter et al., 2018). Due to the widespread nature of urinary tract infections, nurse involvement in diagnostic stewardship interventions could mitigate risks of inappropriate prescribing on a large scale. Further, this recommendation received overwhelming support by nurses, nurse managers and infection preventionists in a 2018 qualitative study (Carter et al., 2018).
Urinary tract infections serve as one of the most common causes of bacterial infections globally, impacting an estimated 150 million individuals annually (Ozturk and Murt, 2020). Due to their ubiquity, this corresponds to high volumes of urine cultures ordered, especially within the outpatient setting. For example, in 2016, the United States Centers for Disease Control and Prevention reported approximately 16 million urine cultures were ordered in the outpatient setting (Rui and Okeyode, 2016). Outpatient urine culture ordering also surpassed the amount ordered within emergency departments in the same year by 25% (Rui et al., 2016). This places urine cultures as the most common microbiological study in the outpatient setting, double that of throat cultures (Rui and Okeyode, 2016).
Although clinicians appropriately treat many urinary tract infections empirically based on clinical signs and symptoms, a urine culture is indicated for complicated, recurrent, or urinary tract infections refractory to treatment (Anger et al., 2019, Gupta et al., 2011). With the rise of resistant pathogens, urine cultures give clinicians critical information to tailor therapy based on uropathogen susceptibly (Fauci and Marston, 2014). In addition, clinical guidelines published by the Infectious Disease Society of America, European Association of Urology and the Canadian Task Force on Preventive Health Care support obtaining a urine culture in pregnant patients to screen for asymptomatic bacteriuria (Bonkat et al., 2018, Moore et al., 2018, Nicolle et al., 2019).
Unfortunately, interpretation of urine cultures can be complicated by contamination (Lough et al., 2019). Contamination can occur prior to sample processing during the collection process from surrounding vaginal, perineal and epidermal skin flora (LaRocco et al., 2016). Further, improper storage (at room temperature without a preservative) prior to processing can amplify bacterial counts and lead to contamination (LaRocco et al., 2016). Proper collection techniques, including using a midstream clean-catch method with cleansing for adults, along with storage in boric acid or refrigeration for up to 24 hours in lieu of immediate processing, is supported by evidence from a recent, systematic review and meta-analysis (LaRocco et al., 2016). Studies have uncovered that contaminated cultures disrupt timely care, waste resources with repeated testing, undermine efforts to effectively treat patients, and increase inappropriate use of antibiotics (Burd and Kehl, 2011, Eley et al., 2016, Lough et al., 2019). In addition, securing a properly collected, clean-catch urine specimen has implications for appropriate treatment in patients with more severe or recurrent infections and among pregnant women where undue antibiotic treatment secondary to a contaminated culture could incur negative impacts on the fetus (Bookstaver et al., 2015). Therefore, obtaining a properly collected specimen serves as a key point governing antibiotic prescribing, which can enhance efforts to reduce resistance and negative outcomes from inappropriate treatment (Abbo and Hooton, 2014, Magill et al., 2014).
The most recent study on the prevalence of urine culture contamination among adults from outpatient clinics and emergency departments in North America was conducted in 2005. This study showed a large variation in contamination among the 127 laboratories studied, as the upper and lower 10th percentiles of contamination were 0.8% and 42%, while the median contamination level was 15% (Bekeris et al., 2008). The scale of the study provides a good representation of contamination nationally; however, this study was performed almost two decades ago and included self-reported survey data (Bekeris et al., 2008). A more recent study performed in Ireland in 2018 found high levels of urine culture contamination (46.7%) among pregnant females seeking antenatal care and identified that higher body mass index increased the risk of contamination (O’Leary et al., 2020). However, this study was limited to a population of pregnant females. The most recent retrospective study on contamination was performed in 2020 and found similarly high levels of contamination to O’Leary and colleagues during their baseline period (43.9%). They also identified older age and female sex were associated with contamination. However, this study was conducted in a urology-specific outpatient setting (Whelan et al., 2022).
To our knowledge, there have been no studies evaluating the prevalence of urine culture contamination in primary care clinics in the United States within the last decade. Furthermore, factors associated with urine specimen contamination in the primary care setting have not been well-explored in the literature. To fill this void in understanding and design informed interventions to reduce contamination, the objectives of this study were to investigate the prevalence of contaminated cultures, identify risk factors associated with contaminated cultures, and describe antibiotic prescribing by culture status based on the presence or absence of urinary tract infection symptoms in the primary care setting.
2. Methods
2.1. Study Population, Inclusion and Exclusion Criteria
We conducted this study in two safety-net, primary care clinics; one serves as a teaching clinic with residents and attending physicians (Clinic A), while the other has only attending physicians (Clinic B). Clinics were located in traditionally underserved areas and catered to a racially and ethnically diverse population in a metropolitan setting. Clinics A and B both provide continuity of care services and are not same-day clinics (appointments are required). They also have similar levels of indigent patients (range: 62%-65%). Clinic A differs from B in that it serves a higher percentage of Hispanic/Latinx patients (66%) compared to Clinic B (43%), as well as patients that report Spanish as their primary language (Clinic A: 54%, Clinic B: 34%). Clinic B serves a larger percentage of patients that are Black or African American (48%) compared to Clinic A (17%).
We included visits from patients aged 18 years and older when a urine culture was ordered for the following indications: infection of the genitourinary tract infection, pyelonephritis, and signs or symptoms associated with the latter diagnoses, as well as asymptomatic bacteriuria in pregnancy. Supplemental File 1 lists the diagnosis titles and codes we used to extract the above culture indications. We excluded visits for patients with an indwelling urinary catheter but did not exclude patient visits if they had been on any prior antibiotics.
2.2. Urine Culture Processing Details
Each clinic had identical urine collection procedures and samples were processed in the same, central laboratory. Laboratories also reported culture results at both clinics using identical formats. When urine cultures were ordered onsite, nursing staff primarily provided verbal instructions on collection based on their training and knowledge; however, if patients reported to the laboratory for sample submission with a stand-alone order, the laboratory technician gave standardized, written instructions on how to collect a clean-catch sample. Written instructions were also posted in the bathroom in English, Spanish and Vietnamese (Supplemental File 2).
For sample processing, urine samples were refrigerated at 4°C until put into a preservative. Alternatively, if samples were not refrigerated, they were put into a preservative collection device before transport to the laboratory for culture, per the Infectious Disease Society of America laboratory guidelines (Miller et al., 2018). In terms of quality control for specimen transport, a sensor was embedded within each container housing urine specimens, which constantly monitored and logged the temperature. This system transmitted that data to a web-based system for real-time monitoring and specimen tracking. The central laboratory workflow abided by the College of American Pathologists, Clinical Laboratory Improvements Amendments, and the Infectious Disease Society of America laboratory guidelines.
2.3. Study Design
We conducted a cross-sectional study by retrospectively reviewing all primary care clinic visits wherein a urine culture was ordered for the above-named indications between November 2018 and March 2020. During manual review of each encounter, we extracted the following: patient demographics, medical history, recent hospitalizations, visit-specific documented symptoms, urine culture results, and antibiotics prescribed between the visit and up to two weeks post-clinic appointment. Urinalysis results were not extracted. Listed comorbidities were used to calculate an individual’s Elixhauser score (Elixhauser et al., 1998, Li et al., 2008, Moore et al., 2017). Both males and females were included in the study; however, females were divided into pregnant and non-pregnant subcategories based on their pregnancy status at the visit.
One study coordinator performed the data extraction from the electronic health record, and charts were reviewed twice to ensure we captured any documentation of antibiotic treatment. The coordinator had a master’s degree in epidemiology and was a doctoral student in epidemiology during the study period. The coordinator extracted data directly from provider notes using a standardized form that has been previously used for clinical trials. She received extensive training on the definitions cited within the form and if questions arose, consultation of two medical doctors was sought: one a primary care physician and informaticist and the other an internal medicine resident physician. We did not calculate interrater reliability as all measures/outcomes were objective and simply copied from the chart. With regards to classification of contamination, we used the laboratory standards for contamination and consulted two infectious disease physicians and the microbiology director when assessing the classification of commensal bacteria as contamination.
2.4. Culture result determination
Culture results with one or two distinct uropathogen(s) > 105 colony forming units (CFU)/mL were defined as a ‘high-count positive’ and those between 102 and 105 CFU/mL were defined as ‘low-count positives.’ We considered the following organisms as uropathogens: Escherichia coli (E. coli), Group B streptococci, Klebsiella species, Proteus mirabilis, Pseudomonas aeruginosa, Candida species, Enterococcus species, Citrobacter species, Staphylococcus saprophyticus, Enterobacter cloacae, and Staphylococcus aureus (Tabibian et al., 2008). Results returned as ‘mixed flora’, growth of non-uropathogens (e.g., Gardnerella vaginalis and non-speciated Streptococcus or Staphylococcus organisms), or growth of > 2 uropathogens were considered ‘contaminated.’ Lastly, cultures without any detectable growth were identified as ‘no-growth’ (Wilson and Gaido, 2004).
2.5. Antibiotic treatment classification
For antibiotic treatment classification, we considered the following drugs identified in the medical record as urinary tract infection-relevant: amoxicillin with clavulanic acid, cefazolin, cefepime, cefpodoxime, ceftriaxone, cephalexin, ciprofloxacin, fosfomycin, levofloxacin, nitrofurantoin, and trimethoprim-sulfamethoxazole. As physicians could prescribe drugs empirically or culture informed, we included drugs prescribed at the time of the visit and up to 7 days post-visit. Patients were considered ‘symptomatic’ if their diagnosis or clinical note indicated the presence of dysuria, urinary frequency, urgency, hematuria, chills, fever, flank pain, or costovertebral angle tenderness. As urinary tract infection-relevant treatment is largely driven by the presence of symptoms, we evaluated prescribing among symptomatic patients and asymptomatic patients (excluding asymptomatic pregnant females) separately, by culture result. As we could not determine the exact day that a clinician had access to culture data, we were unable to clearly distinguish between an empirical prescribing period versus a culture-based prescribing period, so results reflect all antibiotics prescribed from the visit and 7 days post visit.
2.6. Statistical Analysis
To calculate the sample size for determining the prevalence of urine culture contamination, we assumed a contamination prevalence of 42%, which was based on the upper 90th percentile of contamination identified by Bekeris and colleagues (Bekeris et al., 2008). We utilized this study as it represented the most recent, comprehensive study conducted on urine culture contamination among ambulatory patients in the United States during the study design period. The required sample size was 375 patients assuming 0.05 precision (Pourhoseingholi et al., 2013). We included a larger sample to perform multivariable, multinomial logistic regression analysis on the predictors of contamination and uropathogen-positive cultures.
Descriptive statistics were performed for demographic and clinical characteristics stratified by culture result category (no growth, contaminated, and low-count and high-count positive). Age (in years) was treated as a quantitative, continuous variable and all other variables were categorical in nature. Patient demographics, symptoms, comorbidities, and clinic sites were evaluated for their association with contaminated and positive culture results using an unadjusted, multinomial logistic regression model. Based on a previous study conducted among women with antenatal clinic visits that showed higher body mass index was associated with contamination, we included this factor in our analysis. Our choice of potential predictors of contamination were based on previously published studies (Bekeris et al., 2008, O’Leary et al., 2020). All factors with a P-value ≤ 0.20 from univariate comparisons were incorporated within a final, adjusted multinomial model to determine the independent relationships of each potential risk factor with contaminated and positive culture results as compared to no growth cultures as the reference. Of note, low and high-count positive cultures were combined into one uropathogen-positive category in this phase of the analysis. In cases of categorical variables with more than 2 categories, the entire variable was included if 1 or more of the categories had a P-value ≤ 0.20.
Due to our outcome looking specifically at urine culture results, the dataset contained several repeat patients for different encounters. These repeat patients could have potentially interrupted the independent observation hypothesis and was addressed by using a clustered robust standard errors technique in our model. Multinomial adjusted odd ratios (maORs) from the multinomial regression model were reported with their corresponding 95% confidence intervals (CI), and respective P-values (alpha of 0.05). We evaluated differences in treatment status by culture outcome by using Pearson’s chi-square test or Fisher’s exact test when observations were less than five. Data was analyzed using R statistical software (version 3.6.1) in the RStudio environment, while using the ‘nnet’ package for multinomial logistic regression modelling (Ripley and Venables, 2020).
2.7. Ethical Considerations
The Baylor College of Medicine institutional review board approved this study (protocol number: H-43369). A waiver of consent and authorization was granted to conduct the chart reviews for data extraction.
3. Results
A total of 1265 urine cultures from all eligible patients with a qualifying diagnostic code (Supplemental File 1) and completed urine culture were analyzed between the two clinics. These cultures were obtained from 1114 distinct individuals seen in the clinic with a range of 1 to 4 urine cultures during the study time period (mean=1.1, standard deviation (sd) =0.4). Eighty-nine percent of patients (n=992) accounted for a single culture, while 96 patients had 2 cultures, and 23 had 3 or more cultures during the study period. The study population consisted predominantly of Hispanic or Latinx (74.4%) patients, followed by Black or African American (18.9%), White (3.2%) and patients of ‘other’ race/ethnicity; the majority of patients were also born outside the United States (68.1%). The mean age of the study population was 43.2 years-old (sd = 15.8) and had a high female predominance (n = 1062, 84%), of whom 36.8% were pregnant (n = 391). Twenty-one percent of cultures had no growth (95% CI: 18.7%-23.2%), 54.9% were contaminated (95% CI: 52.1%-57.6%), and 24.3% were uropathogen-positive (95% CI: 22.0%-26.7%); among uropathogen-positive cultures, 12.6% and 11.7% had low and high-counts, respectively (95% CIs: 10.9%-14.5% and 10.1%-13.6%, respectively) (Figure 1). Among pregnant females, 20.2% (95% CI: 11.7%-18.7%) had uropathogen-positive cultures. Patients had a median Elixhauser score of 0 (interquartile range = −1 to 3) and obesity was the most common comorbidity (n=431, 34.1%).
Figure 1.
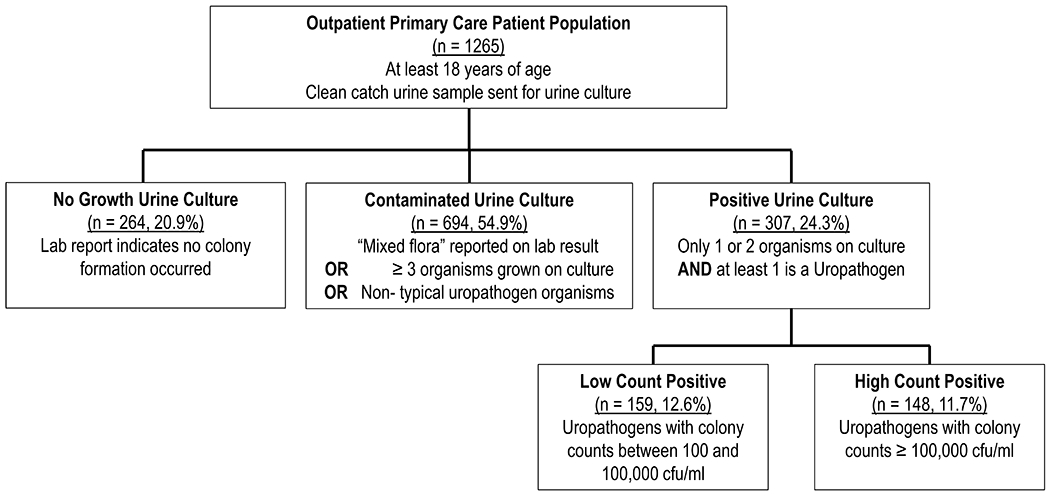
Study Population and Distribution of Culture Results. Detected uropathogens included: Escherichia coli, Group B streptococci, Klebsiella species, Proteus mirabilis, Candida species, Enterococcus species, Citrobacter species, Staphylococcus saprophyticus, Enterobacter cloacae, and Staphylococcus aureus.
Patients with contaminated cultures were on average younger (40.4) than patients in the other three groups (no growth = 45.8, low-count positive= 46.3, and high-count positive = 48.5) (Table 1). Similarly, a higher proportion of pregnant women had contaminated cultures (39.5%) compared to no growth (22.3%), low-count (18.2%), and high-count uropathogen-positive cultures (19.6%). Patients with contaminated cultures tended to have fewer comorbidities, reflected by an Elixhauser score of 0.99, compared to scores of 1.43, 2.12, and 2.57 in the no growth, low-count, and high-count uropathogen-positive groups, respectively. Distribution of racial and ethnic groups, country of birth, and clinic location were largely similar across culture result categories. Urinary tract infection-related symptoms were more prevalent in low and high-count uropathogen-positive culture groups (93.7% and 93.2%, respectively), as compared to no growth and contaminated culture groups (83% and 87.5%, respectively).
Table 1.
Distribution of Demographic Features by Culture Result Type
| No Growth n =264 |
Contaminated Cultures n = 694 |
Low-Count Positive Culturesa n = 159 |
High-Count Positive Culturesb n = 148 |
|||||
|---|---|---|---|---|---|---|---|---|
|
| ||||||||
| Avg. | Sd. | Avg. | Sd. | Avg. | Sd. | Avg. | Sd. | |
|
|
||||||||
| Age (years) | 45.8 | 15.7 | 40.4 | 15.4 | 46.3 | 14.6 | 48.5 | 16.6 |
| Med. | IQR | Med. | IQR | Med. | IQR | Med. | IQR | |
|
|
||||||||
| Elixhauser Score | 0 | −1, 3 | 0 | −1, 2 | 0 | 0,4 | 0 | −1, 5 |
| Gender/Pregnancy | No. | % | No. | % | No. | % | No. | % |
|
|
||||||||
| Male | 134 | 50.8 | 45 | 6.5 | 15 | 9.4 | 9 | 6.1 |
| Female non-pregnant | 71 | 26.9 | 375 | 54.0 | 115 | 72.3 | 110 | 74.3 |
| Female pregnant | 59 | 22.3 | 274 | 39.5 | 29 | 18.2 | 29 | 19.6 |
| Race and Ethnicity | ||||||||
| White | 8 | 3.0 | 14 | 2.0 | 7 | 4.4 | 11 | 7.4 |
| African American | 56 | 21.2 | 134 | 19.3 | 27 | 17.0 | 23 | 15.5 |
| Latino/Hispanic | 186 | 70.5 | 524 | 75.5 | 121 | 76.1 | 110 | 74.3 |
| Other | 14 | 5.3 | 22 | 3.2 | 4 | 2.5 | 4 | 2.7 |
| Country of Birth | ||||||||
| US | 83 | 31.4 | 220 | 31.7 | 52 | 32.7 | 48 | 32.4 |
| Outside US | 181 | 68.6 | 474 | 68.3 | 107 | 67.3 | 100 | 67.6 |
| Clinic | ||||||||
| Clinic A | 190 | 72.0 | 529 | 76.2 | 133 | 83.6 | 103 | 69.6 |
| Clinic B | 74 | 28.0 | 165 | 23.8 | 26 | 16.4 | 45 | 30.4 |
| UTI Symptoms | ||||||||
| None | 45 | 17.0 | 87 | 12.5 | 10 | 6.3 | 10 | 6.8 |
| Documented | 219 | 83.0 | 607 | 87.5 | 149 | 93.7 | 138 | 93.2 |
| Comorbidities | ||||||||
| Hypertension | 76 | 28.8 | 145 | 20.9 | 53 | 33.3 | 55 | 37.2 |
| Diabetes | 52 | 19.7 | 112 | 16.1 | 44 | 27.7 | 50 | 33.8 |
| Obesity | 58 | 22.0 | 249 | 35.9 | 69 | 43.4 | 55 | 37.2 |
| Anemia | 13 | 4.9 | 48 | 6.9 | 11 | 6.9 | 12 | 8.1 |
Low-count positive CFU/mL: 102-105
High-count positive CFU/mL: ≥ 105
Avg.-average, No.-number, Sd.-standard deviation, Med.-median, IQR-interquartile range, UTI-urinary tract infection
3.1. Contaminated Culture Risk Factors
In the unadjusted model, contaminated cultures were more common in younger patients, obese individuals, pregnant and non-pregnant women (multinomial odds ratios (mOR): 0.98, 1.99, 14.08, and 15.77; P-value < 0.001) (Table 2). In the adjusted, multinomial logistic regression model, age was no longer a significant factor, meanwhile non-pregnant females, pregnant females, and individuals with obesity had statistically significantly higher odds of having a contaminated culture by 15.89 (95% CI: 10.25-24.61), 14.34 (95% CI: 8.03-25.61), and 1.93 (95% CI: 1.32-2.81) times, respectively.
Table 2.
Risk Factors for Contaminated Urine Cultures
| Unadjusted Model | Adjusted Model | |||||
|---|---|---|---|---|---|---|
| mOR | 95% CI | P-value | maOR | 95% CI | P-value | |
| Age (years), continuous | 0.98 | 0.97-0.99 | <0.001 | 1.00 | 0.98-1.01 | 0.53 |
| Gender and Pregnancy | ||||||
| Male | reference | reference | ||||
| Female Non-Pregnant | 15.77 | 10.33-24.06 | <0.001 | 15.89 | 10.25-24.61 | <0.001 |
| Female Pregnant | 14.08 | 8.93-22.22 | <0.001 | 14.34 | 8.03-25.61 | <0.001 |
| Race and Ethnicity | ||||||
| White | reference | reference | ||||
| Hispanic | 1.55 | 0.64-3.77 | 0.33 | 0.95 | 0.33-2.74 | 0.92 |
| African American | 1.36 | 0.54-3.44 | 0.51 | 1.03 | 0.34-3.11 | 0.96 |
| Other | 0.90 | 0.30-2.69 | 0.84 | 0.93 | 0.25-3.46 | 0.91 |
| Country of Birth | ||||||
| US | reference | -- | -- | -- | ||
| Outside US | 0.96 | 0.71-1.30 | 0.79 | -- | -- | -- |
| Clinic | ||||||
| Clinic A | reference | reference | ||||
| Clinic B | 0.85 | 0.61-1.17 | 0.31 | 1.08 | 0.67-1.72 | 0.76 |
| UTI Symptoms | ||||||
| Asymptomatic | reference | reference | ||||
| Symptomatic | 1.37 | 0.92-2.03 | 0.12 | 1.25 | 0.74-2.10 | 0.40 |
| Comorbidities | ||||||
| Hypertension | 0.66 | 0.48-0.92 | 0.01 | 1.27 | 0.79-2.04 | 0.33 |
| Diabetes | 0.79 | 0.55-1.14 | 0.20 | 0.92 | 0.58-1.45 | 0.71 |
| Obesity | 1.99 | 1.43-2.76 | <0.001 | 1.93 | 1.32-2.81 | <0.001 |
| Anemia | 1.43 | 0.74-2.61 | 0.31 | -- | -- | -- |
mOR-multinomial odds ratio, CI-confidence interval, maOR- multinomial adjusted odds ratio, UTI-urinary tract infection. Bold text indicates a significant P-value (<0.05).
3.2. Uropathogen-Positive Culture Risk Factors
In the unadjusted model, non-pregnant females (mOR: 16.89), pregnant females (mOR: 4.00), presence of urinary tract infection-related symptoms (mOR: 2.53), and patients with diabetes (mOR:1.83) or obesity (mOR: 2.40) had statistically significant increased odds of having a positive culture for a uropathogen (Table 3). Similar to the predictors of contaminated cultures, the adjusted model showed non-pregnant females (maOR: 20.26, 95% CI: 11.17-33.08), pregnant females (maOR: 5.57, 95% CI: 2.69-11.52), and patients with obesity (maOR: 2.15, 95% CI: 1.41-3.26) had higher odds of having a uropathogen-positive urine culture. Additionally, the presence of urinary tract infection-related symptoms was also associated with an increased likelihood of a uropathogen-positive urine culture after adjustment (maOR of 2.94, 95% CI: 1.51-5.72).
Table 3.
Risk Factors for Positive Urine Cultures from Multinomial Modela
| Unadjusted Model | Adjusted Model | |||||
|---|---|---|---|---|---|---|
| mOR | 95% CI | P-value | maOR | 95% CI | P-value | |
| Age (years), continuous | 1.01 | 0.99-1.02 | 0.08 | 1.01 | (0.99-1.02) | 0.61 |
| Gender and Pregnancy | ||||||
| Male | reference | reference | ||||
| Female Non-Pregnant | 16.89 | 10.07-28.08 | <0.001 | 20.26 | (11.17-33.08) | <0.001 |
| Female Pregnant | 4.00 | (2.22-7.20) | <0.001 | 5.57 | (2.69-11.52) | <0.001 |
| Race and Ethnicity | ||||||
| White | reference | reference | ||||
| Hispanic | 0.49 | (0.21-1.15) | 0.10 | 0.50 | (0.17-1.42) | 0.19 |
| African American | 0.39 | (0.16-0.99) | 0.05 | 0.41 | (0.13-1.26) | 0.12 |
| Other | 0.25 | (0.08-0.84) | 0.03 | 0.31 | (0.07-1.32) | 0.11 |
| Country of Birth | ||||||
| US | reference | -- | -- | -- | ||
| Outside US | 0.86 | (0.60-1.23) | 0.41 | -- | -- | -- |
| Clinic | ||||||
| Clinic A | reference | reference | ||||
| Clinic B | 0.94 | (0.64-1.39) | 0.77 | 0.93 | (0.55-1.58) | 0.79 |
| UTI Symptoms | ||||||
| Asymptomatic | reference | reference | ||||
| Symptomatic | 2.53 | (1.44-4.43) | 0.001 | 2.94 | (1.51-5.72) | 0.002 |
| Comorbidities | ||||||
| Hypertension | 1.42 | (0.99-2.04) | 0.10 | 1.48 | (0.88-2.49) | 0.14 |
| Diabetes | 1.83 | (1.24-2.71) | 0.002 | 1.43 | (0.88-2.34) | 0.15 |
| Obesity | 2.40 | (1.66-3.49) | <0.001 | 2.15 | (1.41-3.26) | <0.001 |
| Anemia | 1.40 | (0.69-2.85) | 0.35 | -- | -- | -- |
mOR- multinomial odds ratio, CI-confidence interval, maOR- multinomial adjusted odds ratio, UTI-urinary tract infection
3.3. Antibiotic Prescribing by Culture Status
Overall, 344 urinary tract infection-relevant antibiotics were prescribed on the day of the visit and up to 7 days post-visit. The most common agents prescribed were Infectious Disease Society of America designated first-line agents for uncomplicated urinary tract infection—nitrofurantoin (n=122, 35.5%) and trimethoprim-sulfamethoxazole (n=97, 28.2%). Fluoroquinolones (n=74, 21.5%) and cephalosporins (n=47, 13.7%) were the third and fourth most common drugs classes ordered. The majority of antibiotics (n=231) were prescribed within the first three days, or likely by empirical means. There were no statistically significant changes in prescribing between the early (days 0 – 2) and later periods (days 3 – 7), although nitrofurantoin and cephalexin prescribing increased between the two periods by 10% (P-value = 0.12) and 6% (P-value = 0.28), respectively, while trimethoprim-sulfamethoxazole and ciprofloxacin prescribing decreased by 8% (P-value = 0.17) and 5% (P-value = 0.33), respectively.
As urinary tract infection treatment is largely driven by the presence of symptoms, we evaluated prescribing among symptomatic patients and asymptomatic patients separately, by culture status (Figure 2). Among symptomatic patients, 86.7% (95% CI: 79.2%-91.8%, n=98) of patients with high-count uropathogen-positive cultures were treated compared to 58.4% (95: CI: 49.6% to 66.7%, n=73) of patients with low-count uropathogen-positive cultures, which was statistically significant (P-value <0.001). This was also the case when comparing treatment between symptomatic patients with contaminated cultures (22.9%, 95% CI: 18.8%- 27.6%, n=79) to those with no growth cultures (11.6%, 95% CI: 7.5%-17.4%, n=19) (P-value = 0.004). In terms of asymptomatic patients with any culture result, only nine patients (5.9%, 95% CI: 2.9%-11.3%) were treated; however, 25% (95% CI: 11.2%-46.9%, n=5) of 20 patients with asymptomatic bacteriuria (high- and low-count uropathogen-positive cultures) were treated. Patients with asymptomatic bacteriuria had statistically significantly higher treatment than asymptomatic patients with contaminated or no growth cultures (P-value = 0.002). Among asymptomatic pregnant females that had contaminated cultures (n=262), 3.8% (95% CI: 2.1%-6.9%, n=19) received antibiotic treatment, similar to those that had no growth cultures (3.6%, 95% CI: 0.6%-13.6%, n=2) (P-value = 1.00).
Figure 2.
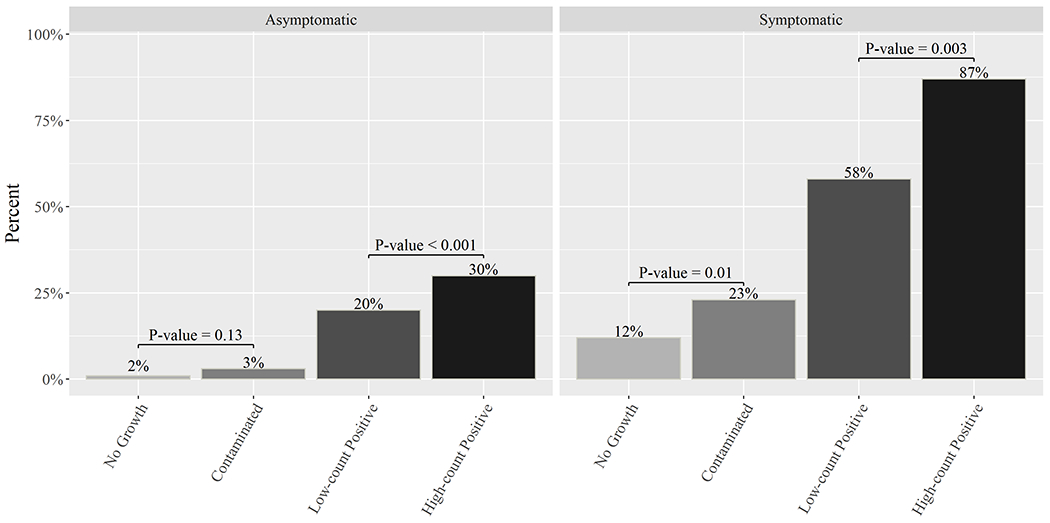
Antibiotic Treatment by Symptom Status and Culture Result Category. Asymptomatic pregnant females were excluded from this analysis. Low-count positive included uropathogens between 102-105 CFU/mL and high-count positives included uropathogens > 105 CFU/mL.
4. Discussion
Our study detected an overall contamination prevalence of 54.9% among 1254 visits from 1114 patients that sought care for suspected urinary tract infection, or that were screened for asymptomatic bacteriuria at safety-net, primary care clinics in a large metropolitan area. Female sex, pregnancy, and obesity were independently associated with both urine culture contamination, and having a uropathogen-positive urine culture; urinary tract infection symptoms were also found to be associated with having a uropathogen-positive culture. Treatment was statistically significantly higher among both symptomatic and asymptomatic patients with high-count uropathogen-positive cultures compared to those with low-count uropathogen-positive cultures. However, antibiotic prescribing was only statistically significantly higher among symptomatic patients with contaminated cultures compared to those with negative cultures.
The 54.9% contamination level detected in our study surpasses levels from the majority studies included in a national survey of laboratories conducted in 2005, but appears more comparable to contamination levels found in more recent studies. For example, in the former study that analyzed consecutive urine cultures from outpatients across 127 laboratories in the United States and Canada, the median contamination rate was 15%, with the lower and upper 10th percentile of labs exhibiting 0.8% and 42% contamination, respectively (Bekeris et al., 2008). This places our clinics among sites with the highest levels of contamination (Bekeris et al., 2008). However, a more recent study performed in 2020 found a urine culture contamination level of 46.2%—more in line with our findings (Whelan et al., 2022). When comparing our contamination levels to results from a 2020 cross-sectional study performed among pregnant women seeking antenatal care in Ireland, pregnant women in our study had higher levels of contamination (70.1%) than what was found among O’Leary and colleagues (46.7%). As both studies had the same levels of obesity, this suggests additional factors may be contributing to urine culture contamination in our population.
We found female sex, pregnancy, and obesity were independent risk factors for contamination, with female and pregnancy having the strongest effects. In addition, to the best of our knowledge, our study is the first to identify the independent association of pregnancy on urine culture contamination in an adjusted analysis. Pregnancy and these other predictors (female sex, obesity) make sense physically, as body habitus and anatomical differences may impede urine collection, while the surrounding vaginal tissue adjacent to the urethra may make females more prone to urine contamination with vaginal microbiota. O’Leary and colleagues echoed our finding that obesity increased the risk of contamination as they identified that higher body mass index increased the odds of contamination by 1.08 (95% CI: 1.04-1.13) among pregnant females (O’Leary et al., 2020). In addition, in a previous study, the rates of urine culture contamination were higher in females compared to males in an unadjusted analysis (Bekeris et al., 2008) and a more recent study found females had significantly higher odds of contamination in an adjusted analysis (Whelan et al., 2022). In this latter study performed in urology clinics, age was also found to be significantly associated with higher odds of contamination (Whelan et al., 2022). In our univariate analysis, however, we found the inverse. Since being a pregnant female had a strong effect on the odds of contamination, and pregnant females were significantly younger than females as well as males, this may have overshadowed any effects of age on contamination. Thus, differing findings between age and contamination in our study and Whelan et al. may be due to differences in patient populations as Whelan et al. had an older population (median age 62, IQR: 46-78) with unknown pregnancy levels, while our patients had a median age of 41 (IQR: 30-56) and higher levels of pregnancy (31%).
Similarly, positive cultures were also associated with female gender, pregnancy, and obesity. As expected, the presence of urinary tract infection symptoms was also associated with uropathogen-positive urine cultures. We found that positive results could be divided into two clinically meaningful categories: cultures with low- and high-colony counts. Likewise, we found antibiotic treatment was statistically significantly higher among high-growth uropathogen-positive cultures compared to low-growth uropathogen-positive cultures among both symptomatic and asymptomatic patients. Studies focusing on patients with asymptomatic bacteriuria have also identified that cultures with higher organism count triggered clinicians to treat patients (Flokas et al., 2017). Thus, regardless of symptoms, it appears that organism count drives clinician behavior.
In addition to finding substantial levels of contamination, we also found that 22.9% of symptomatic patients with contaminated cultures were treated with antibiotics, which was significantly higher than patients with negative cultures. In contrast, only 3.8% of asymptomatic pregnant females with contaminated cultures were treated. Contamination could trigger unnecessary treatment, which could lead to adverse effects from antibiotics on patients, the fetus (in pregnant women), and increase the risk of resistant bacteria emergence. Furthermore, if the contaminated culture only contained commensal bacteria, further workup for other causes of the urinary symptoms may not be pursued. On the other hand, contaminated cultures could obscure an underlying uropathogen, causing undertreatment in pregnant patients with asymptomatic bacteriuria, which could lead to pyelonephritis or preterm labor (Nicolle et al., 2019). For example, if a pregnant woman has bladder colonization with E. coli, but the urine becomes contaminated with two other organisms from the skin, that culture would be discarded without further laboratory workup. Thus, the E. coli would not be identified by the laboratory, and the pregnant woman would not be treated for true E. coli bacteriuria. Therefore, her untreated asymptomatic bacteriuria would put her at higher risk for pyelonephritis and preterm labor (Nicolle et al., 2019).
In 2019, the Joint Commission accreditation body announced new requirements for all ambulatory clinics to begin measuring and improving antibiotic stewardship practices (Joint-Commission, 2019). This requires identifying specific areas to be addressed, establishing an antibiotic stewardship goal annually, using evidence-based practice guidelines, providing educational material to practitioners, and collecting relevant data. As urinary tract infections are the most common condition associated with antibiotic use, our study reveals a high impact area that can be addressed with a stewardship intervention. Notably, the results from our study show that reducing contaminated cultures could be instrumental in improving quality metrics and potentially reducing inappropriate antibiotic prescriptions.
Mitigating urine culture contamination has been a critical obstacle in other antibiotic stewardship studies, highlighting the continued need for better instruction and facilitation of clean-catch samples across various clinical settings (Jacob et al., 2018, Maher et al., 2017). Based on a study by Frazee and colleagues performed in an emergency department setting, nurses rarely gave clear instructions to patients on clean-catch urine collection, if at all (Frazee et al., 2012). We believe that our high rate of urine culture contamination is due to similar problems, but needs further investigation.
This part of the collection process is also a particular area where nurse-driven leadership and intervention can play a crucial role. Previously failed interventions to ameliorate urine culture contamination did not involve nurses, nor were these interventions patient-centered, as they were designed without patient input (Jacob et al., 2018, Maher et al., 2017). Future interventions for preventing urine culture contamination could involve a nurse-driven, patient-centered approach using a digital aide to impart clear instructions in the patient’s native language, coupled with visual reinforcement. The intervention would be both nurse-driven and patient-centered, as it would involve obtaining feedback through usability testing by a group of clinic nurses and patients to assess the video’s acceptability and appropriateness for patients and nurses. We could also capture the nurse’s input on the feasibility on implementing it within their workflow. As nurses play a direct role in patient interaction for obtaining urine cultures, nurse involvement appears as a natural fit. Therefore, future stewardship interventions should consider involving this integral member of the healthcare team when shaping an intervention.
4.1. Limitations
Our study has certain strengths and limitations. Our study population may not be representative of the overall United States population. However, this study benefits from its large size and manual extraction of detailed clinical information for each urine culture-associated visit. The strength of our study is that we reviewed all urine culture orders during the study period. During manual review, in addition to flagging and recording information on pre-specified variables, we examined free-text fields in the clinical notes for pertinent symptoms that may have been overlooked. However, clinical documentation may not always accurately reflect clinical care in its entirety. Documentation that was entered incorrectly or in an atypical format may not have been captured, while some patient data could be lacking due to omission. We do not believe this would cause any differential misclassification.
For calculating percent treatment by culture status, although we captured antibiotic data up to 7 days post-visit, we assumed that empiric prescribing based on symptoms may account for treatment in many cases. Due to the limited nature of our retrospective data extraction, ascertainment of whether urinary symptoms or urine culture results influenced prescribing was difficult; however, this area should be further investigated in tandem with evaluating the appropriateness of urine culture ordering based on evidence-based guidelines.
In terms of analyzing quality control during the pre-analytic phase of urine culture processing, we had information on this for the transport phase, but we did not directly assess the time between collection and refrigeration, which could amplify bacterial counts. However, a sophisticated system was in place to monitor container temperature between transport from clinics to the central laboratory for processing.
Lastly, we did not gather data on additional predictors that could be of importance which include the training level among medical personnel that collect urine cultures, staff-to-patient ratios, and health literacy and language barriers among patients. For example, we did not collect data on the percent of nurses compared to medical assistants that perform the task of instructing patients on urine culture collection. Staff workload could also serve as a potential factor; clinics that have lower staff-to-patient ratios in general or on certain days may have less time to give detailed instructions, or there could be delayed processing of cultures, which could increase the level of contamination in a sample. In addition, language barriers and health literacy could reduce understanding and uptake of instructions given to patients. As we retrospectively extracted visit-associated data, we were not able to collect this information, as it would have to be measured via a survey or interview. Therefore, future directions may include analyzing the relationship between urine culture contamination and staff training levels, the effect of staff-to-patient ratios, urine culture workload per clinic, and language barriers and health literacy among patients.
5. Conclusion
In conclusion, we detected significant levels of urine culture contamination in a primary care, safety-net setting and identified pregnancy, female sex and obesity as independent risk factors for contamination. Antibiotic treatment in patients with contaminated cultures could represent overtreatment, which can lead to resistance or adverse drug outcomes in patients, or undertreatment in pregnancy if the contamination obscures a uropathogen. Considering the sheer volume of cultures collected in the outpatient setting, reducing urine culture contamination is an excellent diagnostic stewardship target. International nursing societies support incorporating nurses as part of the antibiotic stewardship team; further the Centers for Disease Control and Prevention and the American Nursing Association highlight the role of nurses in ensuring aseptic collection of urine for culture. As nurses have direct patient interaction during primary care visits, stewardship interventions should consider incorporating their skills, knowledge, and educational capacity to shape interventions to reduce contamination and improve patient outcomes.
Supplementary Material
What is already known
The prevalence of urine culture contamination has varied widely from 0.8% to 42% (upper and lower 10th percentiles) based on a national study of ambulatory patients in North America in 2005, whilst high levels (~46%) have been found among pregnant females and urology clinic patients.
Epidemiological studies have found specimen refrigeration was associated with lower levels of urine culture contamination, while higher body mass index, female sex and older age have been associated with higher contamination.
What this paper adds
We detected concerning levels of contamination (55%) among patients who submitted urine specimens for culture in primary care, safety-net clinics within a public healthcare system.
We found obesity, female sex, and pregnancy were strongly associated with contamination and demonstrated prescribing practices differed significantly depending on symptoms and culture results
Substantial urine culture contamination and antibiotic prescribing in patients with contaminated cultures provides an opportunity for nurse-driven diagnostic stewardship.
Acknowledgements:
George Germanos, MD, MPH assisted with early study conceptualization and feedback on the project.
Funding Sources:
This study was supported by the National Institute of Allergy and Infectious Diseases of the National Institutes of Health under Award Number UM1AI104681. MVK and MH are supported in part by the Health Resources and Services Administration, an agency of the U.S. Department of Health and Human Services (grant number T32 HP1003). This work is supported by VA HSR&D IIR 16-025 (to BWT), I01 RX002595 from VA RR&D (to BWT), IK2 CX001981 from VA CSR&D (to AC), and by the Center for Innovations in Quality, Effectiveness and Safety (CIN 13-413) at the Michael E. DeBakey VA Medical Center, Houston, Texas, USA. The content is solely the responsibility of the authors and does not necessarily represent the official views of the National Institutes of Health or the U.S. Department of Veterans Affairs.
Conflicts of Interest:
Drs. AC, LG, and BWT report grants from, the U.S. Department of Veterans Affairs Health Services Research & Development Service and the Agency for Healthcare Research and Quality.
Data Availability:
De-identified data available upon request.
References
- Australian Commission on Safety and Quality in Health Care (ACSQHC), 2018. Chapter 12: Role of nurses, midwives and infection control practitioners in antimicrobial stewardship. In: Antimicrobial Stewardship in Australian Health Care, ACSQHC, Sydney. [Google Scholar]
- Abbo LM, Hooton TM, 2014. Antimicrobial Stewardship and Urinary Tract Infections. Antibiot Basel Switz 3 (2), 174–192. [DOI] [PMC free article] [PubMed] [Google Scholar]
- American Nursing Association and Centers for Disease Control and Prevention, 2017. Redefining the antibiotic stewardship team: Recommendations from the American Nurses Association/Centers for Disease Control and Prevention Workgroup on the role of registered nurses in hospital antibiotic stewardship practices. JAC Antimicrob Resist. (2632-1823 (Electronic)). [DOI] [PMC free article] [PubMed] [Google Scholar]
- Anger J, Lee U, Ackerman AL, Chou R, Chughtai B, Clemens JQ, Hickling D, Kapoor A, Kenton KS, Kaufman MR, Rondanina MA, Stapleton A, Stothers L, Chai TC, 2019. Recurrent Uncomplicated Urinary Tract Infections in Women: AUA/CUA/SUFU Guideline. J Urol 202 (2), 282–289. [DOI] [PubMed] [Google Scholar]
- Bekeris LG, Jones BA, Walsh MK, Wagar EA, 2008. Urine culture contamination: a College of American Pathologists Q-Probes study of 127 laboratories. Arch Pathol Lab Med 132 (6), 913–917. [DOI] [PubMed] [Google Scholar]
- Bonkat G, Pickard R, Bartoletti R, Cai T, Bruyere F, Geerlings SE, Koves B, Wagenlehner F, 2018. EAU Guidelines on Urological Infections. In, EAU Guidelines. EAU Guidelines Office, Arnhem, Netherlands. [Google Scholar]
- Bookstaver PB, Bland CM, Griffin B, Stover KR, Eiland LS, McLaughlin M, 2015. A Review of Antibiotic Use in Pregnancy. Pharmacotherapy: The Journal of Human Pharmacology and Drug Therapy 35 (11), 1052–1062. [DOI] [PubMed] [Google Scholar]
- Burd EM, Kehl KS, 2011. A Critical Appraisal of the Role of the Clinical Microbiology Laboratory in the Diagnosis of Urinary Tract Infections. (0095-1137 (Print)).
- Carter EJ, Greendyke WG, Furuya EY, Srinivasan A, Shelley AN, Bothra A, Saiman L, Larson EL, 2018. Exploring the nurses’ role in antibiotic stewardship: A multisite qualitative study of nurses and infection preventionists. (1527-3296 (Electronic)). [DOI] [PMC free article] [PubMed]
- Centeres for Disease Control and Prevention, 2019. Antibiotic Resistance Threats in the United States, 2019., Atlanta, GA. [Google Scholar]
- Edwards R, Drumright L Fau - Kiernan M, Kiernan M Fau - Holmes A, Holmes A, 2011. Covering more Territory to Fight Resistance: Considering Nurses’ Role in Antimicrobial Stewardship. (1757-1774 (Print)). [DOI] [PMC free article] [PubMed]
- Eley R, Judge C, Knight L, Dimeski G, Sinnott M, 2016. Illustrations reduce contamination of midstream urine samples in the emergency department. (1472-4146 (Electronic)). [DOI] [PubMed]
- Elixhauser A, Steiner C, Harris DR, Coffey RM, 1998. Comorbidity measures for use with administrative data. Med Care 36 (1), 8–27. [DOI] [PubMed] [Google Scholar]
- Fauci AS, Marston l.D., 2014. The perpetual challenge of antimicrobial resistance. JAMA 311 (18), 1853–1854. [DOI] [PubMed] [Google Scholar]
- Flokas ME, Andreatos N, Alevizakos M, Kalbasi A, Onur P, Mylonakis E, 2017. Inappropriate Management of Asymptomatic Patients With Positive Urine Cultures: A Systematic Review and Meta-analysis. Open Forum Infect Dis 4 (4), ofx207. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Frazee BW, Frausto K Fau - Cisse B, Cisse B Fau - White DEA, White De Fau - Alter H, Alter H, 2012. Urine collection in the emergency department: what really happens in there? (1936-900X (Print)). [DOI] [PMC free article] [PubMed]
- Gotterson F, Buising K, Manias E, 2021. Nurse role and contribution to antimicrobial stewardship: An integrative review. Int J Nurs Stud 117, 103787. [DOI] [PubMed] [Google Scholar]
- Gupta K, Hooton TM, Naber KG, Wullt B, Colgan R, Miller LG, Moran GJ, Nicolle LE, Raz R, Schaeffer AJ, Soper DE, Infectious Diseases Society of, A., European Society for, M., Infectious, D., 2011. International clinical practice guidelines for the treatment of acute uncomplicated cystitis and pyelonephritis in women: A 2010 update by the Infectious Diseases Society of America and the European Society for Microbiology and Infectious Diseases. Clin Infect Dis 52 (5), e103–120. [DOI] [PubMed] [Google Scholar]
- International Council of Nurses, 2017. Position Statement on Antimicrobial Resistance. International Council of Nurses, Geneva. [Google Scholar]
- Jacob MS, Kulie P, Benedict C, Ordoobadi AJ, Sikka N, Steinmetz E, McCarthy ML, 2018. Use of a midstream clean catch mobile application did not lower urine contamination rates in an ED. Am J Emerg Med 36 (1), 61–65. [DOI] [PubMed] [Google Scholar]
- Joint-Commission, 2019. R3 Report: Antimicrobial Stewardship in Ambulatory Health Care.
- Ladenheim D, 2018. Role of nurses in supporting antimicrobial stewardship. Nursing Standard (2047-9018 (Electronic)). [DOI] [PubMed] [Google Scholar]
- LaRocco MT, Franek J, Leibach EK, Weissfeld AS, Kraft CS, Sautter RL, Baselski V, Rodahl D, Peterson EJ, Cornish NE, 2016. Effectiveness of Preanalytic Practices on Contamination and Diagnostic Accuracy of Urine Cultures: a Laboratory Medicine Best Practices Systematic Review and Meta-analysis. (1098-6618 (Electronic)). [DOI] [PMC free article] [PubMed] [Google Scholar]
- Li B, Evans D, Faris P, Dean S, Quan H, 2008. Risk adjustment performance of Charlson and Elixhauser comorbidities in ICD-9 and ICD-10 administrative databases. BMC Health Serv Res 8, 12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lough ME, Shradar E, Hsieh C, Hedlin H, 2019. Contamination in Adult Midstream Clean-Catch Urine Cultures in the Emergency Department: A Randomized Controlled Trial. J Emerg Nurs (1527-2966 (Electronic)). [DOI] [PubMed] [Google Scholar]
- Magill SS, Edwards JR, Beldavs ZG, Dumyati G, Janelle SJ, Kainer MA, Lynfield R, Nadle J, Neuhauser MM, Ray SM, Richards K, Rodriguez R, Thompson DL, Fridkin SK, 2014. Prevalence of antimicrobial use in US acute care hospitals, May-September 2011. JAMA (1538-3598 (Electronic)). [DOI] [PMC free article] [PubMed] [Google Scholar]
- Maher PJ, Brown AEC, Gatewood MO, 2017. The Effect of Written Posted Instructions on Collection of Clean-Catch Urine Specimens in the Emergency Department. J Emerg Med 52 (5), 639–644. [DOI] [PubMed] [Google Scholar]
- Miller JM, Binnicker MJ, Campbell S, Carroll KC, Chapin KC, Gilligan PH, Gonzalez MD, Jerris RC, Kehl SC, Patel R, Pritt BS, Richter SS, Robinson-Dunn B, Schwartzman JD, Snyder JW, Telford S 3rd, Theel ES, Thomson RB Jr., Weinstein MP, Yao JD, 2018. A Guide to Utilization of the Microbiology Laboratory for Diagnosis of Infectious Diseases: 2018 Update by the Infectious Diseases Society of America and the American Society for Microbiology. Clin Infect Dis 67 (6), 813–816. [DOI] [PubMed] [Google Scholar]
- Moore A, Doull M, Grad R, Groulx S, Pottie K, Tonelli M, Courage S, Garcia AJ, Thombs BD, 2018. Recommendations on screening for asymptomatic bacteriuria in pregnancy. Canadian Medical Association Journal 190 (27), E823. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Moore BJ, White S, Washington R, Coenen N, Elixhauser A, 2017. Identifying Increased Risk of Readmission and In-hospital Mortality Using Hospital Administrative Data: The AHRQ Elixhauser Comorbidity Index. Med Care 55 (7), 698–705. [DOI] [PubMed] [Google Scholar]
- Nicolle LE, Gupta K, Bradley SF, Colgan R, DeMuri GP, Drekonja D, Eckert LO, Geerlings SE, Köves B, Hooton TM, Juthani-Mehta M, Knight SL, Saint S, Schaeffer AJ, Trautner B, Wullt B, Siemieniuk R, 2019. Clinical Practice Guideline for the Management of Asymptomatic Bacteriuria: 2019 Update by the Infectious Diseases Society of America. Clin Infect Dis 68 (10), 1611–1615. [DOI] [PubMed] [Google Scholar]
- O’Leary BD, Armstrong FM, Byrne S, Talento AF, O’Coigligh S, 2020. The prevalence of positive urine dipstick testing and urine culture in the asymptomatic pregnant woman: A cross-sectional study. (1872-7654 (Electronic)). [DOI] [PubMed] [Google Scholar]
- Ozturk R, Murt A, 2020. Epidemiology of urological infections: a global burden. World J Urol 38 (11), 2669–2679. [DOI] [PubMed] [Google Scholar]
- Pourhoseingholi MA, Vahedi M, Rahimzadeh M, 2013. Sample size calculation in medical studies. Gastroenterol Hepatol Bed Bench 6 (1), 14–17. [PMC free article] [PubMed] [Google Scholar]
- Ripley B, Venables W, 2020. Nnet Package: Feed-Forward Neural Networks and Multinomial Log-Linear Models.
- Rui P, Kang K, Ashmann JJ, 2016. National Ambulatory Medical Care Survey: 2016 Emergency Department Summary Tables.
- Rui P, Okeyode T, 2016. National Ambulatory Medical Care Survey: 2016 National Summary Tables.
- Tabibian JH, Gornbein J, Heidari A, Dien SL, Lau VH, Chahal P, Churchill BM, Haake DA, 2008. Uropathogens and host characteristics. J Clin Microbiol 46 (12), 3980–3986. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Whelan PS, Nelson A, Kim CJ, Tabib C, Preminger GM, Turner NA, Lipkin M, Advani SD, 2022. Investigating risk factors for urine culture contamination in outpatient clinics: A new avenue for diagnostic stewardship. Antimicrobial Stewardship & Healthcare Epidemiology 2 (1), e29. [DOI] [PMC free article] [PubMed] [Google Scholar]
- World Health Organization, 2019. Ten threats to global health in 2019. World Health Organization. [Google Scholar]
- Wilson ML, Gaido L, 2004. Laboratory diagnosis of urinary tract infections in adult patients. Clin Infect Dis 38 (8), 1150–1158. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Data Availability Statement
De-identified data available upon request.


