Abstract
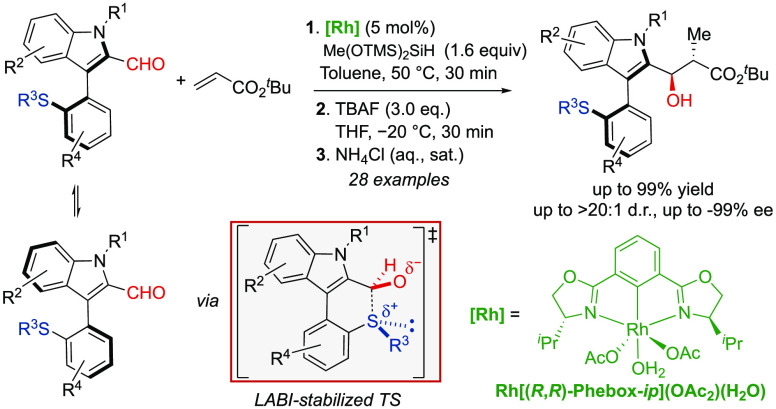
A highly enantio- and diastereoselective dynamic kinetic resolution (DKR) of configurationally labile 3-aryl indole-2-carbaldehydes is described. The DKR proceeds via a Rh-catalyzed intermolecular asymmetric reductive aldol reaction with acrylate esters, with simultaneous generation of three stereogenic elements. The strategy relies on the labilization of the stereogenic axis that takes place thanks to a transient Lewis acid–base interaction (LABI) between the formyl group and a thioether moiety strategically located at the ortho′ position. The atropisomeric indole products present a high degree of functionalization and can be further converted to a series of axially chiral derivatives, thereby expanding their potential application in drug discovery and asymmetric catalysis.
Keywords: asymmetric catalysis, axial chirality, 3-aryl indoles, dynamic kinetic resolution, rhodium
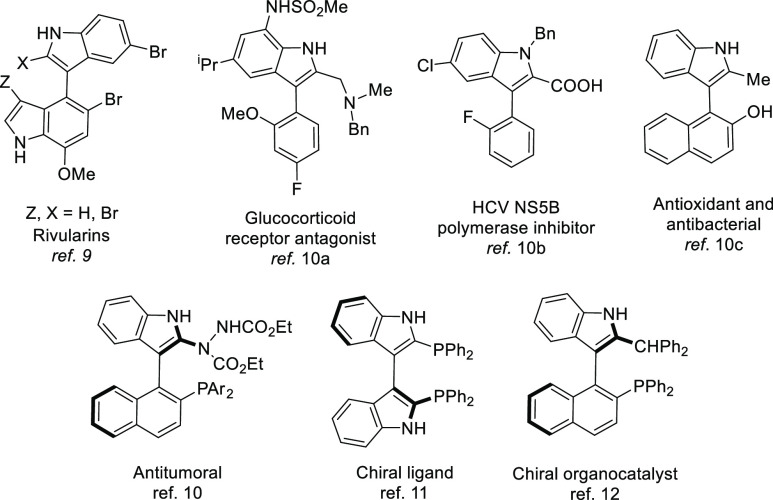
Enantioenriched axially chiral compounds constitute important scaffolds present in natural products,1 drug discovery,2 and material science.3 Furthermore, axially chiral skeletons widely exist in privileged chiral ligands4 and organocatalysts5 that play crucial roles in the preparation of chiral compounds. Despite the large development of catalytic asymmetric methodologies for the synthesis of these motives, most prepared axially chiral scaffolds consist of biaryls based on six-membered rings.6 In contrast, the construction of biaryl compounds based on five-membered rings has been less explored and is more challenging, as the large spatial distance between the ortho substituents of both aryl rings requires higher degrees of steric constraints to stabilize the stereogenic axis.7 An outstanding member of this family of atropisomers comprises C3 chiral indole derivatives and analogues.8 Their core structure is present in a number of natural products,9 bioactive compounds10 and chiral ligands,11 and organocatalysts12 (Figure 1). Furthermore, the indole ring has a unique reactivity that can serve to change the electron density, modulate the steric congestion, and allow participation as a hydrogen bond donor.13 Because of the interest in this class of compounds, a variety of catalytic enantioselective methods have recently been reported for their synthesis, including atroposelective arylation reactions,14 de novo construction of the indole ring,15 atroposelective desymmetrization based on C–H functionalizations,10d,16 and central-to-axial chirality transfer processes.17 Despite these developments, limitations exist regarding structural variability and the strategic introduction of functional groups. Additionally, the catalytic asymmetric synthesis of atropisomers bearing multiple stereogenic elements is underdeveloped. Therefore, novel synthetic strategies for the preparation of axially chiral aryl indole scaffolds are still needed.
Figure 1.
Selected 3-aryl indole frameworks.
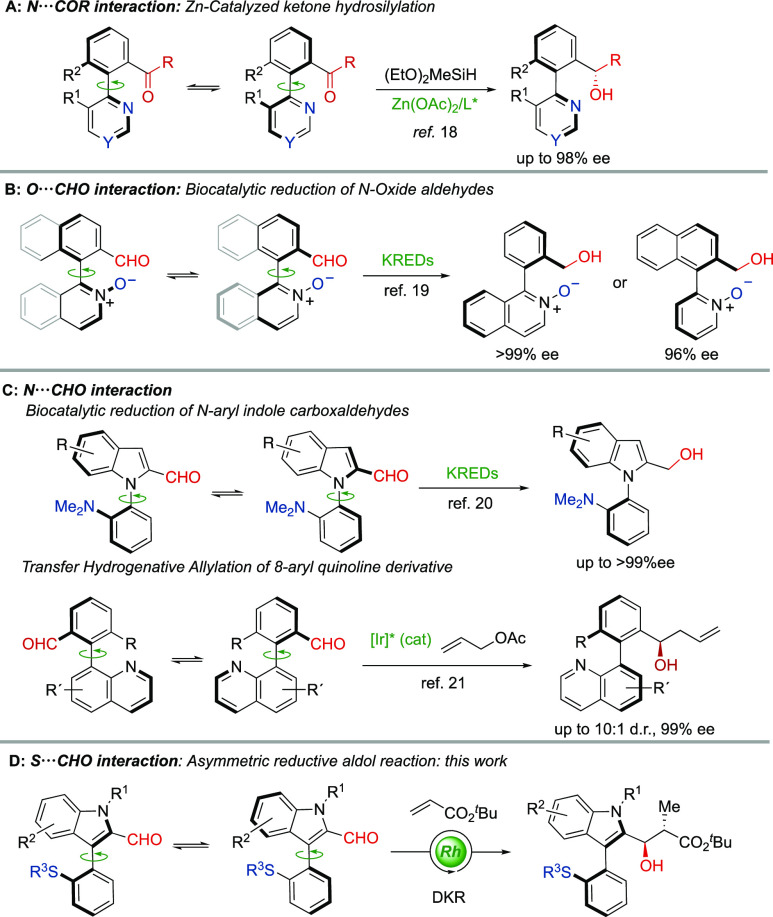
In the frame of our interest in atroposelective catalysis, we recently developed a dynamic kinetic resolution (DKR) strategy for the synthesis of axially chiral biaryls on the basis of the labilization of the stereogenic axis facilitated by transient Lewis acid–base interaction (LABI) between functional groups strategically located in the molecule. Such interaction overrides the steric repulsion in the transition structure and acts as a “lubricant” that substantially reduces the rotational barrier in the substrate. The axis could then be stabilized by any asymmetric transformation in which the interaction is disabled, thereby enabling a DKR scenario. This strategy was first demonstrated in the atroposelective zinc-catalyzed hydrosilylation of heterobiaryl ketones to obtain enantioenriched carbinols with central and axial chirality (Scheme 1A).18 A related DKR strategy was also reported by Clayden and Turner for the biocatalytic asymmetric reduction of heterobiaryl aldehydes, which showed a similar bonding interaction between a N-oxide and an aldehyde (Scheme 1B).19 More recently, LABI N···CHO interactions have been exploited for the biocatalytic dynamic resolution of N-aryl indoles20 and the allylation of 8-aryl quinoline derivatives21 (Scheme 1C).
Scheme 1. Atropisomerization via Lewis Acid/Base Interactions.
However, axially chiral biaryls bearing thioether moieties are rare in the literature compared with phosphine or amine analogues22 but hold appealing structures for their application in asymmetric catalysis23 and feature interesting bioactivities.24 With this motivation, we wondered whether a thioether as the Lewis base and a carbonyl group as a weak Lewis acid would be a competent Lewis pair able to promote the labilization of a stereogenic axis on the basis of the discussed strategy. According to a recent report,22i however, trisubstituted biphenyl derivatives bearing CHO and SMe groups in ortho,ortho′ positions exhibit configurational stability, which suggests that the steric repulsion dominates the LABI SMe···CHO interaction in this system. However, we speculated whether the repulsive/attractive force balance could be reverted in a sterically more relaxed heterobiaryl scaffold featuring a five-membered ring.7a Considering that the introduction of a thioether moiety in functionalized axially chiral 3-aryl indoles could open new avenues for not only chiral ligands23 but also drug candidates,24 we decided to explore the suitability of the LABI-based DKR strategy to the resolution of trisubstituted 3-aryl indole scaffolds 1 bearing an aldehyde and a thioether groups at ortho, ortho′ positions (Scheme 1D).
The HPLC analysis of N-benzyl-3-[2-(methylthio)phenyl]-1H-indole-2-carbaldehyde 1a, chosen as a model substrate, showed a unique narrow peak on a variety of chiral stationary phases, thereby pointing to its configurational lability in solution. Additionally, DFT computational studies at the ωB97XD/def2tzvp/smd=toluene level of theory were conducted to estimate the barrier of racemization in the 3-aryl indole system and, for the sake of comparison, in the aforementioned biphenyl derivatives. Transition structures TSAcis and TSBcis were located for the 3-aryl indole system A and the biaryl analogue B, respectively (Figure 2). A barrier of 24.1 kcal mol–1 was calculated for the racemization of A, which demonstrates that this system is stable enough to detect atropisomers, but at ambient temperature and above, the racemization rate enables a dynamic kinetic resolution.25 In sharp contrast, the barrier of 37.9 kcal mol–1 calculated for B grants its configurational stability with a half-life (t1/2) of 2 × 107 years at 25 °C. At first sight, these values are surprisingly high in comparison with those calculated for similar transition structures featuring O (N-oxide, 18.4 kcal mol–1)19 or N (NMe2, 24.2 kcal mol–1)20 basic functionalities. Reasonably, however, the barrier correlates well with the interaction distance (1.96 Å for O···CHO,19 2.15 Å for N···CHO,20 and 2.64 Å for S···CHO), which causes severe distortions in TSBcis. Among the two possible transition structures for the racemization, those facing the carbonyl group and the sulfur atom are the most stable in both cases (by 10.4 and 6.6 kcal mol–1 for A and B, respectively) because of the designed noncovalent interaction between the sulfur atom and the carbonyl carbon. The key role of the LABI interaction was further demonstrated by replacing the formyl group in A or B by a methyl group, which resulted in much higher barriers of racemization (for details, see the Supporting Information). Two relative configurations are possible for these transition structures, depending on the orientation of the methyl group with respect to the carbonyl group. Interestingly, those structures with a cis orientation (TSAcis and TSBcis) feature a CH···O hydrogen bond that provides a significant extra stabilization with respect to the trans isomers TSAtrans and TSBtrans (1.3 and 1.0 kcal mol–1 for A and B, respectively). This interaction can be rationalized by considering the enhanced basicity of the oxygen and the enhanced acidity of the methyl group in the transition state as a consequence of the developing negative and positive charges in the oxygen and sulfur atoms, respectively. All these interactions were confirmed by a noncovalent interactions (NCI) analysis.
Figure 2.
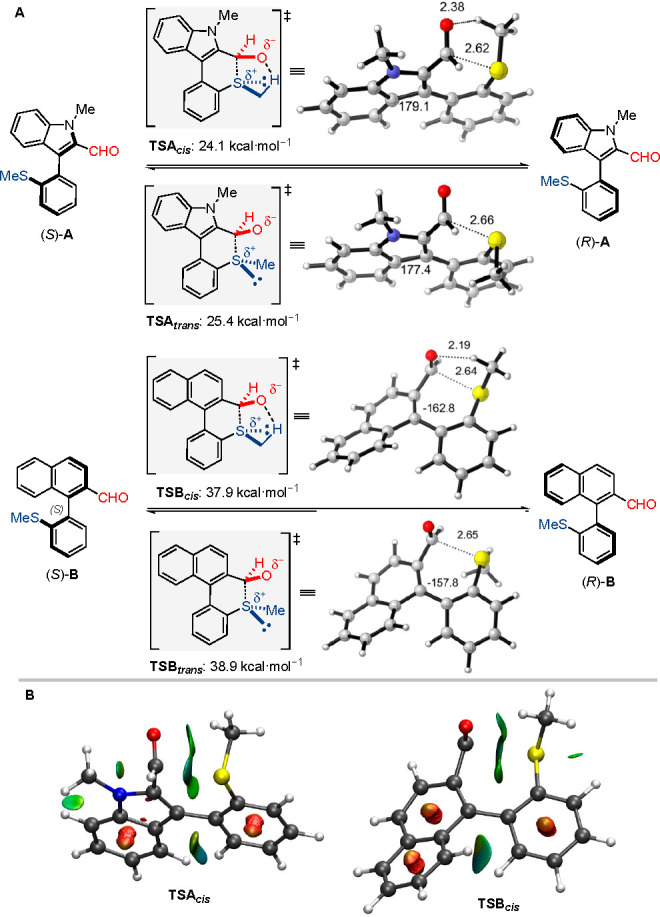
(A) Transition structures for the racemization of 3-aryl indole A and biaryl B. (B) NCI analysis. Thin, delocalized green surfaces indicate van der Waals interactions. Small, lenticular, and bluish surfaces indicate strong interactions. Steric clashes are shown as red isosurfaces.
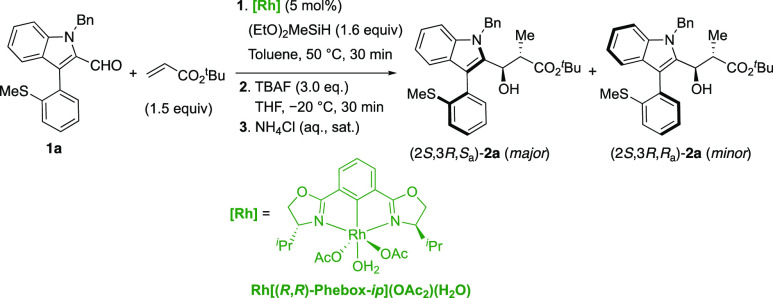
On this basis, we envisioned quaternization of the carbonyl group of indole derivatives 1 (e.g., by nucleophilic addition) to destroy its Lewis acidic character, thereby increasing the rotational barrier in the resulting product to make it configurationally stable. Among the countless possible catalytic enantioselective transformations, we selected the rhodium-catalyzed reductive aldol reaction as an appealing option that provides densely functionalized products.26 The designed atroposelective version of this transformation represents a major challenge for two main reasons: First, an exquisite level of stereocontrol is required because the reaction could potentially afford up to eight different stereoisomers derived from the simultaneous installation of two stereocenters and a stereogenic axis. Second, conditions have to be found to avoid catalyst deactivation or undesirable side reactions caused by coordination of the thioether functional group in the substrate. Inspired by the Nishiyama’s enantioselective reductive coupling of α,β-unsaturated esters catalyzed by chiral rhodium(bisoxazolinylphenyl) complexes [Rh(Phebox)],26a we first examined the reaction of 1a with tert-butyl acrylate (1.5 equiv) in toluene using 6 mol % of catalyst. The desired coupling product 2a was efficiently formed in 98% conversion and 90% ee with a 6:1 diastereoselectivity (Table 1, entry 1). A significant influence by the solvent on reactivity and stereoselectivity was found (entries 2–5 and Supporting Information), with toluene still giving the best results. No benefits were observed at lower temperatures: dr and ee values were essentially maintained at 40 or 30 °C (entries 6 and 7), but longer reaction times were required and a moderate conversion was observed in the latter case. A systematic study of hydrosilanes (entries 8–14 and Supporting Information) revealed that sterically hindered Me(OTMS)2SiH is the optimal reagent, which affords excellent 99% ee with full conversion and 9:1 diastereoselectivity (entry 14). Et3SiH was also found to perform well and afforded a similar diastereoselectivity, although at the expense of conversion and enantioselectivity (entry 13). The reaction proceeds with an excellent anti selectivity and, remarkably, the absolute (2S, 3R) configuration of the newly created stereocenters suggests that the sulfenyl group of the substrate causes no interferences with the catalytic cycle and the stereochemical model.26f This statement applies also to the minor diastereomers and epimers in the stereogenic axis.
Table 1. Variation of Reaction Parametersa.
| entry | variation of standard conditions | conversion (%)b | drb | ee (%)c |
|---|---|---|---|---|
| 1 | none | 98 | 6:1 | 90 |
| 2 | in 1,4-dioxane | 89 | 7:2 | 89 |
| 3 | in THF | 91 | 7:2 | 89 |
| 4 | in DMSO | 79 | 3:1 | 67 |
| 5 | in MeCN | 58 | 5:2 | 83 |
| 6 | T = 40 °Cd | 97 | 6:1 | 90 |
| 7 | T = 30 °Ce | 45 | 7:1 | 91 |
| 8 | with Me3SiH | <5 | n.d. | n.d. |
| 9 | with (EtO)3SiH | 40 | 5:1 | 70 |
| 10 | with Me2PhSiH | 72 | 6:1 | 10 |
| 11 | with (MeO)3SiH | 60 | 5:1 | 75 |
| 12 | with PMHS | 78 | 7:3 | 85 |
| 13 | with Et3SiH | 75 | 9:1 | 95 |
| 14 | with Me(OTMS)2SiH | 98 | 9:1 | 99 |
Conditions: 0.1 mmol of 1a.
Determined by 1H NMR.
Determined by HPLC analysis on chiral stationary phases.
Reaction time of 1.5 h.
Reaction time of 4.5 h.
With the optimal conditions in hand, we next investigated the substrate scope of the methodology (Scheme 2). A wide range of 2-formyl aryl indoles smoothly reacted with tert-butyl acrylate to deliver the corresponding axially chiral products (2a–2z) in good to excellent yields and diastereomeric ratios (up to 99% yield, >20:1 dr) and with excellent enantioselectivities (up to 99% ee). We first investigated the effect of aryl substituents at the benzyl group. Both electron-withdrawing (e.g., Br, CF3, and OCF3; 1b–1d, 1o, 1p), and electron-donating groups (1q, 1r) were tolerated and afforded the corresponding products in good yields with high selectivity. Other nitrogen substituents, including methyl and allyl groups, were also amenable to give the desired products in good yields and similar enantiocontrol (2e, 2f, 2s). Different aryl substitutions on the indole ring were also examined. A series of functional groups, such as halogen (1j–1l and 1v–1y), benzyl (2i and 2u), and methyl ethers (1g, 1h, and 1t), could be well tolerated under the optimal reaction conditions. The excellent enantiocontrol was unperturbed by C-5 or C-6 substitution at the indole ring. It is worth noting that near perfect diastereoselectivities (>20:1 dr) were regularly observed in the reaction of substrates containing ethylsufinyl and benzylsulfinyl substituents (2m–2z) while maintaining high yields and excellent enantioselectivities.27 Aldehyde 1a was also made to react with n-butyl acrylate under the optimized conditions to afford the expected aldol product 2aa in good yield but with decreased diastereo- and enantioselectivity. This result highlights the importance of the bulkier tert-butyl ester to afford a highly selective DKR process. Conversely, products 2ab and 2ac featuring benzothiophenyl and dibenzothiophenyl rings at the bottom fragment of the heterobiaryl skeleton were obtained in excellent yields and enantioselectivity but were configurationally labile. The wider SCorthoCipso angles associated with the benzothiophene ring facilitate the rotation about the stereogenic axis in these compounds. Thus, the observed 1.7:1 and 1.2:1 diastereomeric ratios for 2ab and 2ac, respectively, correspond to the relative stability (conformational equilibrium) of the resulting atropisomers.
Scheme 2. Reaction Scope.
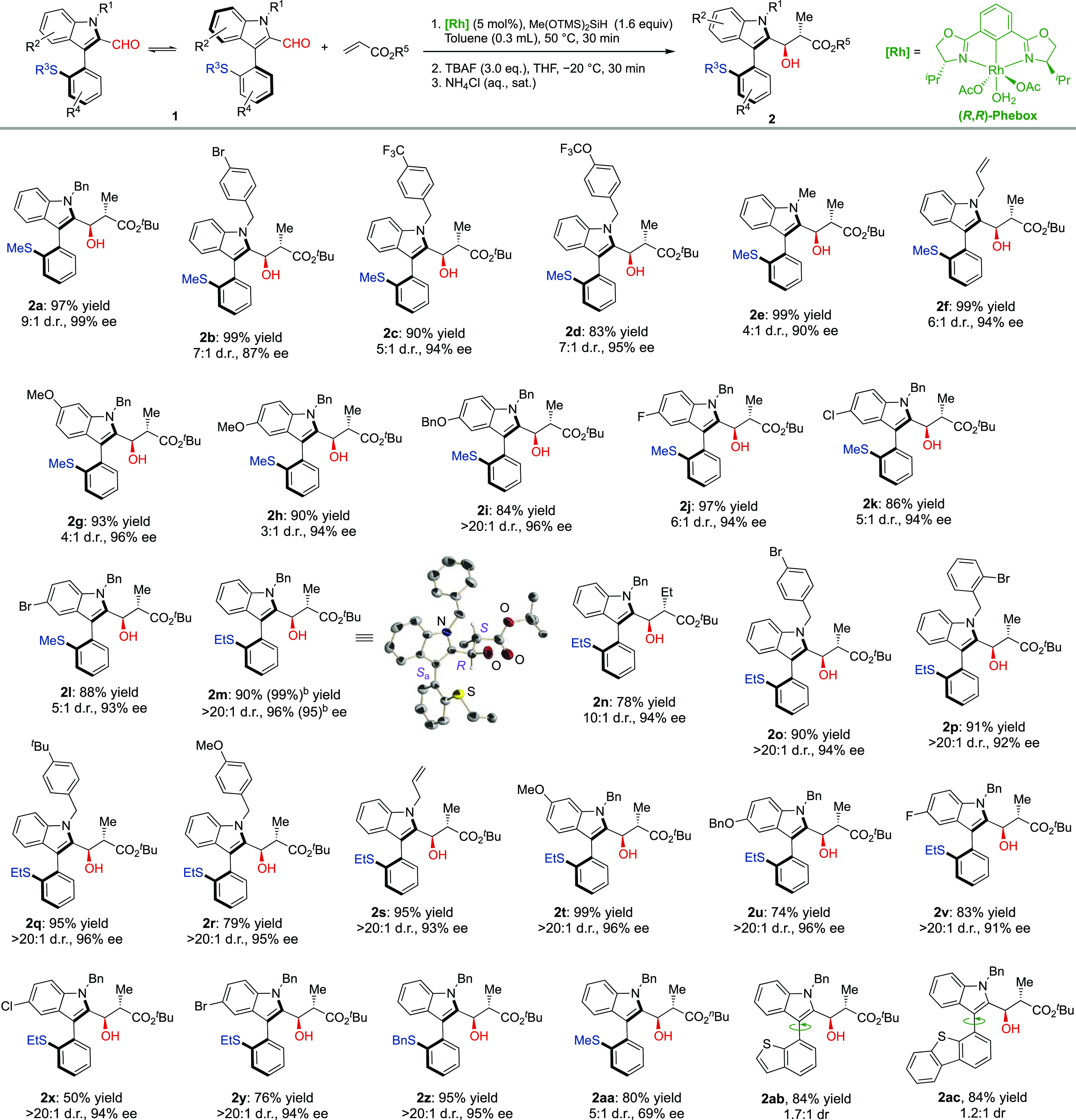
Reactions performed at 0.1 mmol scale in 0.3 mL of toluene. Compounds 1a–w (1 equiv), tert-butyl acrylate (1.5 equiv). Isolated yields after chromatography. HPLC on chiral stationary phases determined the ee values.
Reaction performed at 1.2 mmol scale.
The absolute (2S,3R,Sa) configuration of product 2m was determined by X-ray crystallographic analysis.28 The absolute (2S,3R,Ra) configuration of the minor diastereomer was determined after thermal equilibration: heating of the enantiopure major (2S,3R,Sa)-2m epimer at 80 °C for 12 h in toluene afforded a 2:1 mixture of (Sa/Ra) atropoisomers.29 The absolute configuration of other products 2 was assigned by analogy and assuming a uniform reaction pathway.
Importantly, the catalyst loading could be reduced to 2 mol % when the reaction of 1m was performed at 1.2 mol scale, which improved the isolated yield of the product while maintaining the stereochemical efficiency (>20:1 dr and 96% ee).
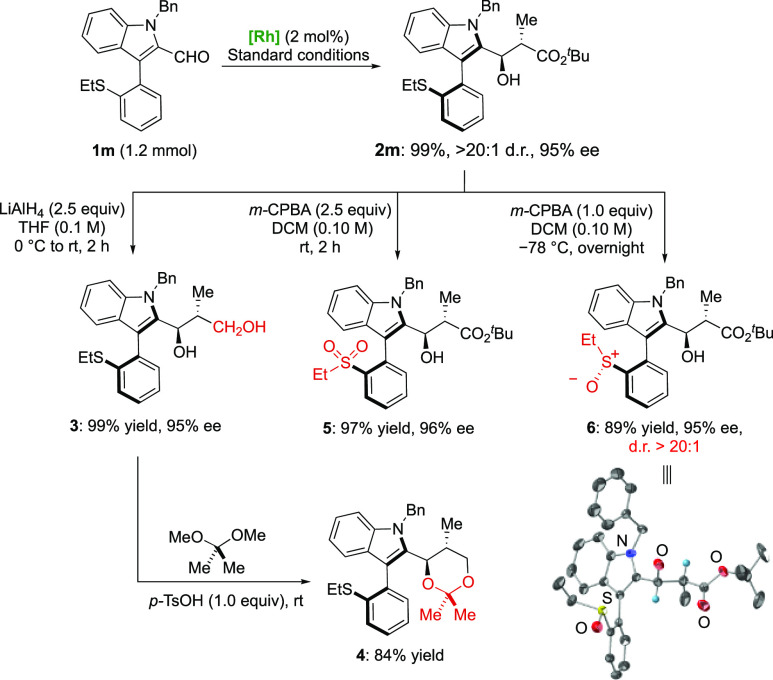
Further derivatizations were performed using ethyl thioether derivative 2m as a representative example (Scheme 3). The carboxylate group could be reduced using LiAlH4 to afford diol 3 in 99% yield with 95% ee. This product was further transformed into acetal derivative 4 in 84% yield. The thioether group could also be oxidized to the corresponding sulfoxide derivative 6(28) with excellent diastereoselectivity (>20:1 dr) using 1.0 equiv of m-CPBA at −78 °C. The oxidation to the sulfone 5 was also achieved in 97% yield by increasing the amount of m-CPBA (2.5 equiv) and performing the reaction at room temperature. Importantly, both oxidation reactions proceeded without noticeable racemization.
Scheme 3. Representative Transformations from 2m.
In summary, the labilization of trisubstituted ortho′-sulfenyl 3-aryl indole 2-carbaldehydes by means of an attractive SR···CHO LABI interaction can be exploited to perform a Rh-catalyzed dynamic kinetic resolution via reductive aldol reaction with t-butyl acrylate. The reaction proceeds with the generation of two stereocenters and a stereogenic axis to afford densely functionalized, axially chiral indole derivatives in good to excellent yields and high diastereo- and enantioselectivities.
Acknowledgments
We thank the Spanish Ministerio de Ciencia e Innovación (Grants PID2019-106358GB-C21, PID2019-106358GB-C22, PID2019-104090RB-100, contracts RYC-2017-22294 for V. H. and fellowship FPU18/02677 for C.R.-F.), European funding (ERDF), and Junta de Andalucía (Grants P18-FR-3531, P18-FR-644, US-1262867, and US-1260906) and the Regional Government of Aragón (Grupos 17R-34). The authors thank the resources from the supercomputers “Memento” and “Cierzo” and technical expertise and assistance provided by BIFI-ZCAM (Universidad de Zaragoza, Spain).
Supporting Information Available
The Supporting Information is available free of charge at https://pubs.acs.org/doi/10.1021/acscatal.3c03422.
Author Contributions
The manuscript was written through contributions of all authors. All authors have given approval to the final version of the manuscript.
The authors declare no competing financial interest.
Supplementary Material
References
- Bringmann G.; Gulder T.; Gulder T. A. M.; Breuning M. Atroposelective Total Synthesis of Axially Chiral Biaryl Natural Products. Chem. Rev. 2011, 111, 563–639. 10.1021/cr100155e. [DOI] [PubMed] [Google Scholar]
- a Toenjes S. T.; Gustafson J. L. Atropisomerism in Medicinal Chemistry: Challenges and Opportunities. Future Med. Chem. 2018, 10, 409–422. 10.4155/fmc-2017-0152. [DOI] [PMC free article] [PubMed] [Google Scholar]; b Clayden J.; Moran W. J.; Edwards P. J.; LaPlante S. R. The Challenge of Atropisomerism in Drug Discovery. Angew. Chem., Int. Ed. 2009, 48, 6398–6401. 10.1002/anie.200901719. [DOI] [PubMed] [Google Scholar]; c Smyth J. E.; Butler N. M.; Keller P. A. A Twist of Nature-The Significance of Atropisomers in Biological Systems. Nat. Prod. Rep. 2015, 32, 1562–1583. 10.1039/C4NP00121D. [DOI] [PubMed] [Google Scholar]
- a Dotsevi G.; Sogah Y.; Cram D. J. Total Chromatographic Optical Resolution of α-Amino Acid and Ester Salts through Chiral Recognition by a Host Covalently Bound to Polystyrene Resin. J. Am. Chem. Soc. 1976, 98, 3038–3041. 10.1021/ja00426a073. [DOI] [PubMed] [Google Scholar]; b Takaishi K.; Yasui M.; Ema T. Binaphthyl-Bipyridyl Cyclic Dyads as a Chiroptical Switch. J. Am. Chem. Soc. 2018, 140, 5334–5338. 10.1021/jacs.8b01860. [DOI] [PubMed] [Google Scholar]; c Li Q.; Green L.; Venkataraman N.; Shiyanovskaya I.; Khan A.; Urbas A.; Doane J. W. Reversible Photoswitchable Axially Chiral Dopants with High Helical Twisting Power. J. Am. Chem. Soc. 2007, 129, 12908–12909. 10.1021/ja0747573. [DOI] [PubMed] [Google Scholar]
- a Privileged Chiral Ligands and Catalysts; Zhou Q.-L., Ed.; Wiley-VCH: Weinheim, Germany, 2011. [Google Scholar]; b Li Y.-M.; Kwong F.-Y.; Yu W.-Y.; Chan A. S.C. Recent Advances in Developing New Axially Chiral Phosphine Ligands for Asymmetric Catalysis. Coord. Chem. Rev. 2007, 251, 2119. 10.1016/j.ccr.2007.07.020. [DOI] [Google Scholar]; c Chen Y.; Yekta S.; Yudin A. K. Modified BINOL Ligands in Asymmetric Catalysis. Chem. Rev. 2003, 103, 3155. 10.1021/cr020025b. [DOI] [PubMed] [Google Scholar]
- a Parmar D.; Sugiono E.; Raja S.; Rueping M. Complete Field Guide to Asymmetric BINOL-Phosphate Derived Brønsted Acid and Metal Catalysis: History and Classification by Mode of Activation; Brønsted Acidity, Hydrogen Bonding, Ion Pairing, and Metal Phosphates. Chem. Rev. 2014, 114, 9047. 10.1021/cr5001496. [DOI] [PubMed] [Google Scholar]; b Min C.; Seidel D. Asymmetric Brønsted Acid Catalysis with Chiral Carboxylic Acids. Chem. Soc. Rev. 2017, 46, 5889. 10.1039/C6CS00239K. [DOI] [PubMed] [Google Scholar]
- a Lassaletta J. M.Atropisomerism and Axial Chirality, 1st ed.; World Scientific: Hackensack, NJ, 2019. [Google Scholar]; Selected reviews:; b Zhang D.; Wang Q. Palladium Catalyzed Asymmetric Suzuki-Miyaura Coupling Reactions to Axially Chiral Biaryl Compounds: Chiral Ligands and Recent Advances. Coord. Chem. Rev. 2015, 286, 1–16. 10.1016/j.ccr.2014.11.011. [DOI] [Google Scholar]; c Loxq P.; Manoury E.; Poli R.; Deydier E.; Labande A. Synthesis of Axially Chiral Biaryl Compounds by Asymmetric Catalytic Reactions with Transition Metals. Coord. Chem. Rev. 2016, 308, 131–190. 10.1016/j.ccr.2015.07.006. [DOI] [Google Scholar]; d Ma G.; Sibi M. P. Catalytic Kinetic Resolution of Biaryl Compounds. Chem.—Eur. J. 2015, 21, 11644–11657. 10.1002/chem.201500869. [DOI] [PubMed] [Google Scholar]; e Wencel-Delord J.; Panossian A.; Leroux F. R.; Colobert F. Recent Advances and New Concepts for the Synthesis of Axially Stereoenriched Biaryls. Chem. Soc. Rev. 2015, 44, 3418–3430. 10.1039/C5CS00012B. [DOI] [PubMed] [Google Scholar]; f Zilate B.; Castrogiovanni A.; Sparr C. Catalyst-Controlled Stereoselective Synthesis of Atropisomers. ACS Catal. 2018, 8, 2981–2988. 10.1021/acscatal.7b04337. [DOI] [Google Scholar]; g Wang Y.-B.; Tan B. Construction of Axially Chiral Compounds via Asymmetric Organocatalysis. Acc. Chem. Res. 2018, 51, 534–547. 10.1021/acs.accounts.7b00602. [DOI] [PubMed] [Google Scholar]; h Liao G.; Zhou T.; Yao Q.-J.; Shi B.-F. Recent Advances in the Synthesis of Axially Chiral Biaryls via Transition Metal-catalysed Asymmetric C–H Functionalization. Chem. Commun. 2019, 55, 8514–8523. 10.1039/C9CC03967H. [DOI] [PubMed] [Google Scholar]; I Cheng D.-J.; Shao Y.-D. Advances in the Catalytic Asymmetric Synthesis of Atropisomeric Hexatomic N-Heterobiaryls. Adv. Synth. Catal. 2020, 362, 3081–3099. 10.1002/adsc.202000354. [DOI] [Google Scholar]; j Carmona J. A.; Rodríguez-Franco C.; Fernández R.; Hornillos V.; Lassaletta J. M. Atroposelective transformation of axially chiral (hetero)biaryls. From desymmetrization to modern resolution strategies. Chem. Soc. Rev. 2021, 50, 2968–2983. 10.1039/D0CS00870B. [DOI] [PubMed] [Google Scholar]; k Cheng J. K.; Xiang S.-H.; Li S.; Ye L.; Tan B. Recent Advances in Catalytic Asymmetric Construction of Atropisomers. Chem. Rev. 2021, 121, 4805–4902. 10.1021/acs.chemrev.0c01306. [DOI] [PubMed] [Google Scholar]
- a Bonne D.; Rodriguez J. A Bird’s Eye View of Atropisomers Featuring a Five-Membered Ring. Eur. J. Org. Chem. 2018, 2018, 2417–2431. 10.1002/ejoc.201800078. [DOI] [Google Scholar]; b Bonne D.; Rodriguez J. Enantioselective Syntheses of Atropisomers Featuring a Five-Membered Ring. Chem. Commun. 2017, 53, 12385–12393. 10.1039/C7CC06863H. [DOI] [PubMed] [Google Scholar]; c Kumarasamy E.; Raghunathan R.; Sibi M. K.; Sivaguru J. Nonbiaryl and Heterobiaryl Atropisomers: Molecular Templates with Promise for Atropselective Chemical Transformations. Chem. Rev. 2015, 115, 11239–11300. 10.1021/acs.chemrev.5b00136. [DOI] [PubMed] [Google Scholar]; d Corti V.; Bertuzzi G. Organocatalytic Asymmetric Methodologies towards the Synthesis of Atropisomeric N-Heterocycles. Synthesis 2020, 52, 2450–2468. 10.1055/s-0040-1707814. [DOI] [Google Scholar]; e He X.-L.; Wang C.; Wen Y.-W.; Wang Z.; Qian S. Recent Advances in Catalytic Atroposelective Construction of Pentatomic Heterobiaryl Scaffolds. ChemCatChem. 2021, 13, 3547–3564. 10.1002/cctc.202100539. [DOI] [Google Scholar]; f Rodríguez-Salamanca P.; Fernández R.; Hornillos V.; Lassaletta J. M. Asymmetric Synthesis of Axially Chiral C–N Atropisomers. Chem.—Eur. J. 2022, 28, e202104442 10.1002/chem.202104442. [DOI] [PMC free article] [PubMed] [Google Scholar]; g Zhang H.-H.; Shi F. Organocatalytic Atroposelective Synthesis of Indole Derivatives Bearing Axial Chirality: Strategies and Applications. Acc. Chem. Res. 2022, 55, 2562–2580. 10.1021/acs.accounts.2c00465. [DOI] [PubMed] [Google Scholar]; h Sheng F.-T.; Yang S.; Wu S.-F.; Zhang Y.-C.; Shi F. Catalytic Asymmetric Synthesis of Axially Chiral 3,3′-Bisindoles by Direct Coupling of Indole Rings. Chin. J. Chem. 2022, 40, 2151–2160. 10.1002/cjoc.202200327. [DOI] [Google Scholar]
- a Li T.-Z.; Liu S.-J.; Tan W.; Shi F. Catalytic Asymmetric Construction of Axially Chiral Indole-Based Frameworks: An Emerging Area. Chem.—Eur. J. 2020, 26, 15779–15792. 10.1002/chem.202001397. [DOI] [PubMed] [Google Scholar]; b Zhang Y.-C.; Jiang F.; Shi F. Organocatalytic Asymmetric Synthesis of Indole-Based Chiral Heterocycles: Strategies, Reactions, and Outreach. Acc. Chem. Res. 2020, 53, 425–446. 10.1021/acs.accounts.9b00549. [DOI] [PubMed] [Google Scholar]; c Corti V.; Bertuzzi G. Organocatalytic Asymmetric Methodologies towards the Synthesis of Atropisomeric N-Heterocycles. Synthesis 2020, 52, 2450–2468. 10.1055/s-0040-1707814. [DOI] [Google Scholar]
- a Norton R. S.; Wells R. J. A Series of Chiral Polybrominated Biindoles from the Marine Blue-Green Alga Rivularia Firma. Application of Carbon-13 NMR Spin-Lattice Relaxation Data and Carbon-13-Proton Coupling Constants to Structure Elucidation. J. Am. Chem. Soc. 1982, 104, 3628–3635. 10.1021/ja00377a014. [DOI] [Google Scholar]; b Maehr H.; Smallheer J. Total Syntheses of Rivularins D1 and D3. J. Am. Chem. Soc. 1985, 107, 2943–2945. 10.1021/ja00296a018. [DOI] [Google Scholar]
- a Luz J. G.; Carson M. W.; Condon B.; Clawson D.; Pustilnik A.; Kohlman D. T.; Barr R. J.; Bean J. S.; Dill M. J.; Sindelar D. K.; Maletic M.; Coghlan M. Indole Glucocorticoid Receptor Antagonists Active in a Model of Dyslipidemia Act via a Unique Association with an Agonist Binding Site. J. Med. Chem. 2015, 58, 6607–6618. 10.1021/acs.jmedchem.5b00736. [DOI] [PubMed] [Google Scholar]; b Anilkumar G. N.; Lesburg C. A.; Selyutin O.; Rosenblum S. B.; Zeng Q.; Jiang Y.; Chan T.-Y.; Pu H.; Vaccaro H.; Wang L.; Bennett F.; Chen K.-X.; Duca J.; Gavalas S.; Huang Y.; Pinto P.; Sannigrahi M.; Velazquez F.; Venkatraman S.; Vibulbhan B.; Agrawal S.; Butkiewicz N.; Feld B.; Ferrari E.; He Z.; Jiang C.-K.; Palermo R. E.; Mcmonagle P.; Huang H.-C.; Shih N.-Y.; Njoroge G.; Kozlowski J. A. I. Novel HCV NS5B polymerase inhibitors: Discovery of indole 2-carboxylic acids with C3-heterocycles. Bioorg. Med. Chem. Lett. 2011, 21, 5336–5341. 10.1016/j.bmcl.2011.07.021. [DOI] [PubMed] [Google Scholar]; c Sharma K.; Baral E. R.; Akhtar M. S.; Lee Y. R.; Kim S. H.; Wee Y.-J. 3-Naphthylindoles as new promising candidate antioxidant, antibacterial, and antibiofilm agents. Res. Chem. Intermed. 2017, 43, 2387–2399. 10.1007/s11164-016-2768-4. [DOI] [Google Scholar]; d Jiang F.; Chen K.-W.; Wu P.; Zhang Y.-C.; Jiao Y.; Shi F. A Strategy for Synthesizing Axially Chiral Naphthyl-Indoles: Catalytic Asymmetric Addition Reactions of Racemic Substrates. Angew. Chem., Int. Ed. 2019, 58, 15104–15110. 10.1002/anie.201908279. [DOI] [PubMed] [Google Scholar]; e Surgenor R. R.; Liu X.; Keenlyside M. J. H.; Myers W.; Smith M. D. Enantioselective synthesis of atropisomeric indoles via iron-catalysed oxidative cross-coupling. Nat. Chem. 2023, 15, 357–365. 10.1038/s41557-022-01095-9. [DOI] [PubMed] [Google Scholar]
- Berens U.; Brown J. M.; Long J.; Selke R. Synthesis and resolution of 2,2′-bis-diphenylphosphino [3,3′]biindolyl; a new atropisomeric ligand for transition metal catalysis. Tetrahedron: Asymmetry 1996, 7, 285–292. 10.1016/0957-4166(95)00447-5. [DOI] [Google Scholar]
- Jiang F.; Luo G.-Z.; Zhu Z.-Q.; Wang C.-S.; Mei G.-J.; Shi F. Application of Naphthylindole-Derived Phosphines as Organocatalysts in [4 + 1] Cyclizations of o-Quinone Methides with Morita–Baylis–Hillman Carbonates. J. Org. Chem. 2018, 83, 10060–10069. 10.1021/acs.joc.8b01390. [DOI] [PubMed] [Google Scholar]
- Bandini M.; Eichholzer A. Catalytic functionalization of indoles in a new dimension. Angew. Chem., Int. Ed. 2009, 48, 9608–9644. 10.1002/anie.200901843. [DOI] [PubMed] [Google Scholar]
- Selected examples:; a Zhang H.-H.; Wang C.-S.; Li C.; Mei G.-J.; Li Y.; Shi F. Design and Enantioselective Construction of Axially Chiral NaphthylIndole Skeletons. Angew. Chem., Int. Ed. 2017, 56, 116–121. 10.1002/anie.201608150. [DOI] [PubMed] [Google Scholar]; b Qi L. W.; Mao J. H.; Zhang J.; Tan B. Organocatalytic Asymmetric Arylation of Indoles Enabled by Azo Groups. Nat. Chem. 2018, 10, 58–64. 10.1038/nchem.2866. [DOI] [PubMed] [Google Scholar]; c Lu D.-L.; Chen Y.-H.; Xiang S.-H.; Yu P.; Tan B.; Li S. Atroposelective Construction of Arylindoles by Chiral Phosphoric Acid-Catalyzed Cross-Coupling of Indoles and Quinones. Org. Lett. 2019, 21, 6000–6004. 10.1021/acs.orglett.9b02143. [DOI] [PubMed] [Google Scholar]; d Liu J.-Y.; Yang X.-C.; Liu Z.; Luo Y.-C.; Lu H.; Gu Y.-C.; Fang R.; Xu P.-F. An Atropo-enantioselective Synthesis of Benzo Linked Axially Chiral Indoles via Hydrogen-Bond Catalysis. Org. Lett. 2019, 21, 5219–5224. 10.1021/acs.orglett.9b01828. [DOI] [PubMed] [Google Scholar]; e Xi C. C.; Zhao X. J.; Tian J. M.; Chen Z. M.; Zhang K.; Zhang F. M.; Tu Y. Q.; Dong J. W. Atroposelective Synthesis of Axially Chiral 3-Arylindoles by Copper-Catalyzed Asymmetric Cross-Coupling of Indoles with Quinones and Naphthoquinones. Org. Lett. 2020, 22, 4995–5000. 10.1021/acs.orglett.0c01558. [DOI] [PubMed] [Google Scholar]; f Chen Y.-H.; Li H.-H.; Zhang X.; Xiang S.-H.; Li S.; Tan B. Organocatalytic Enantioselective Synthesis of Atropisomeric Aryl-p-Quinones: Platform Molecules for Diversity-Oriented Synthesis of Biaryldiols. Angew. Chem., Int. Ed. 2020, 59, 11374–11378. 10.1002/anie.202004671. [DOI] [PubMed] [Google Scholar]; g Xi C. C.; Zhao X. J.; Tian J. M.; Chen Z. M.; Zhang K.; Zhang F. M.; Tu Y. Q.; Dong J. W. Atroposelective Synthesis of Axially Chiral 3-Arylindoles by Copper-Catalyzed Asymmetric Cross-Coupling of Indoles with Quinones and Naphthoquinones. Org. Lett. 2020, 22, 4995–5000. 10.1021/acs.orglett.0c01558. [DOI] [PubMed] [Google Scholar]; h Liang H.; Zhu G.; Pu X.; Qiu L. Copper-Catalyzed Enantioselective C–H Arylation between 2-Arylindoles and Hypervalent Iodine Reagents. Org. Lett. 2021, 23, 9246–9250. 10.1021/acs.orglett.1c03596. [DOI] [PubMed] [Google Scholar]; i Surgenor R. R.; Liu X.; Keenlyside M. J. H.; Myers W.; Smith M. D. Enantioselective synthesis of atropisomeric indoles via iron-catalysed oxidative cross-coupling. Nat. Chem. 2023, 15, 357–365. 10.1038/s41557-022-01095-9. [DOI] [PubMed] [Google Scholar]
- Selected examples:; a Lu S.; Ong J.-Y.; Yang H.; Poh S. B.; Liew X.; Seow C. S. D.; Wong M. W.; Zhao Y. Diastereo- and Atroposelective Synthesis of Bridged Biaryls Bearing an Eight-Membered Lactone through an Organocatalytic Cascade. J. Am. Chem. Soc. 2019, 141, 17062–17067. 10.1021/jacs.9b08510. [DOI] [PubMed] [Google Scholar]; b Tian M.; Bai D.; Zheng G.; Chang J.; Li X. Rh(III)-Catalyzed Asymmetric Synthesis of Axially Chiral Biindolyls by Merging C-H Activation and Nucleophilic Cyclization. J. Am. Chem. Soc. 2019, 141, 9527–9532. 10.1021/jacs.9b04711. [DOI] [PubMed] [Google Scholar]; c He X.-L.; Zhao H.- R.; Song X.; Jiang B.; Du W.; Chen Y.-C. Asymmetric Barton-Zard Reaction to Access 3-Pyrrole-Containing Axially Chiral Skeletons. ACS Catal. 2019, 9, 4374–4381. 10.1021/acscatal.9b00767. [DOI] [Google Scholar]; d Li X. J.; Zhao L. J.; Qi Z. S.; Li X. Construction of Atropisomeric 3-Arylindoles via Enantioselective Cacchi Reaction. Org. Lett. 2021, 23, 5901–5905. 10.1021/acs.orglett.1c02012. [DOI] [PubMed] [Google Scholar]; e Wang C.-S.; Wei L.; Fu C.; Wang X.-H.; Wang C.-J. Asymmetric Synthesis of Axially Chiral Naphthyl-C3-indoles via a Palladium-Catalyzed Cacchi Reaction. Org. Lett. 2021, 23, 7401–7406. 10.1021/acs.orglett.1c02574. [DOI] [PubMed] [Google Scholar]; f Yang H.; Sun H. R.; He R. Q.; Yu L.; Hu W.; Chen J.; Yang S.; Zhang G. G.; Zhou L. Organocatalytic cycloaddition of alkynylindoles with azonaphthalenes for atroposelective construction of indole-based biaryls. Nat. Commun. 2022, 13, 632. 10.1038/s41467-022-28211-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Selected examples:; a He C.; Hou M.; Zhu Z.; Gu Z. Enantioselective Synthesis of Indole-Based Biaryl Atropisomers via Palladium-Catalyzed Dynamic Kinetic Intramolecular C–H Cyclization. ACS Catal. 2017, 7, 5316–5320. 10.1021/acscatal.7b01855. [DOI] [Google Scholar]; b Ma C.; Jiang F.; Sheng F.-T.; Jiao Y.; Mei G.-J.; Shi F. Design and Catalytic Asymmetric Construction of Axially Chiral 3,3′-Bisindole Skeletons. Angew. Chem., Int. Ed. 2019, 58, 3014–3020. 10.1002/anie.201811177. [DOI] [PubMed] [Google Scholar]; c Sheng F.-T.; Li Z.-M.; Zhang Y.-Z.; Sun L.-X.; Zhang Y.- C.; Tan W.; Shi F. Atroposelective Synthesis of 3,3′-Bisindoles Bearing Axial and Central Chirality: Using Isatin-Derived Imines as Electrophiles. Chin. J. Chem. 2020, 38, 583–589. 10.1002/cjoc.202000022. [DOI] [Google Scholar]; d Chen K.-W.; Wang Z.-S.; Wu P.; Yan X.-Y.; Zhang S.; Zhang Y.-C.; Shi F. Catalytic Asymmetric Synthesis of 3,3′-Bisindoles Bearing Single Axial Chirality. J. Org. Chem. 2020, 85, 10152–10166. 10.1021/acs.joc.0c01528. [DOI] [PubMed] [Google Scholar]; e Yuan X.; Wu X.; Peng F.; Yang H.; Zhu C.; Fu H. Organocatalytic asymmetric synthesis of arylindolyl indolin-3-ones with both axial and central chirality. Chem. Commun. 2020, 56, 12648–12651. 10.1039/D0CC05432A. [DOI] [PubMed] [Google Scholar]
- Bisag G. D.; Pecorari D.; Mazzanti A.; Bernardi L.; Fochi M.; Bencivenni G.; Bertuzzi G.; Corti V. Central-to-Axial Chirality Conversion Approach Designed on Organocatalytic Enantioselective Povarov Cycloadditions: First Access to Configurationally Stable Indole-Quinoline Atropisomers. Chem. - Eur. J. 2019, 25, 15694–15701. 10.1002/chem.201904213. [DOI] [PubMed] [Google Scholar]
- Hornillos V.; Carmona J. A.; Ros A.; Iglesias-Sigüenza J.; López-Serrano J.; Fernández R.; Lassaletta J. M. Dynamic Kinetic Resolution of Heterobiaryl Ketones by Zinc-Catalyzed Asymmetric Hydrosilylation. Angew. Chem., Int. Ed. 2018, 57, 3777–3781. 10.1002/anie.201713200. [DOI] [PubMed] [Google Scholar]
- Staniland S.; Adams R. W.; McDouall J. J. W.; Maffucci I.; Contini A.; Grainger D. M.; Turner N. J.; Clayden J. Biocatalytic Dynamic Kinetic Resolution for the Synthesis of Atropisomeric Biaryl-Oxide Lewis Base Catalysts. Angew. Chem., Int. Ed. 2016, 55, 10755–10759. 10.1002/anie.201605486. [DOI] [PubMed] [Google Scholar]
- Rodríguez-Salamanca P.; de Gonzalo G.; Carmona J. A.; López-Serrano J.; Iglesias-Sigüenza J.; Fernández R.; Lassaletta J. M.; Hornillos V. Biocatalytic Atroposelective Synthesis of Axially Chiral N-Arylindoles via Dynamic Kinetic Resolution. ACS Catal. 2023, 13, 659–664. 10.1021/acscatal.2c06175. [DOI] [Google Scholar]
- Carmona J. A.; Rodríguez-Salamanca P.; Fernández R.; Lassaletta J. M.; Hornillos V. Dynamic Kinetic Resolution of 2-(Quinolin-8-yl)Benzaldehydes: Atroposelective Iridium-Catalyzed. Angew. Chem., Int. Ed. 2023, 62, e202306981 10.1002/anie.202306981. [DOI] [PubMed] [Google Scholar]
- a Shimada T.; Cho Y.-H.; Hayashi T. Nickel-Catalyzed Asymmetric Grignard Cross-Coupling of Dinaphthothiophene Giving Axially Chiral 1,1′-Binaphthyls. J. Am. Chem. Soc. 2002, 124, 13396–13397. 10.1021/ja0282588. [DOI] [PubMed] [Google Scholar]; b Armstrong R. J.; Smith M. D. Catalytic Enantioselective Synthesis of Atropisomeric Biaryls: A Cation-Directed Nucleophilic Aromatic Substitution Reaction. Angew. Chem., Int. Ed. 2014, 53, 12822–12826. 10.1002/anie.201408205. [DOI] [PubMed] [Google Scholar]; c Cardenas M. M.; Toenjes S. T.; Nalbandian C. J.; Gustafson J. L. Enantioselective Synthesis of Pyrrolopyrimidine Scaffolds through Cation-Directed Nucleophilic Aromatic Substitution. Org. Lett. 2018, 20, 2037–2041. 10.1021/acs.orglett.8b00579. [DOI] [PMC free article] [PubMed] [Google Scholar]; d Maddox S. M.; Dawson G. A.; Rochester N. C.; Ayonon A. B.; Moore C. E.; Rheingold A. L.; Gustafson J. L. Enantioselective Synthesis of Biaryl Atropisomers via the Addition of Thiophenols into Aryl-Naphthoquinones. ACS Catal. 2018, 8, 5443–5447. 10.1021/acscatal.8b00906. [DOI] [PMC free article] [PubMed] [Google Scholar]; e Hou M.; Deng R.; Gu Z. Cu-Catalyzed Enantioselective Atropisomer Synthesis via Thiolative Ring Opening of Five-Membered Cyclic Diaryliodoniums. Org. Lett. 2018, 20, 5779–5783. 10.1021/acs.orglett.8b02477. [DOI] [PubMed] [Google Scholar]; f Liang Y.; Ji J.; Zhang X.; Jiang Q.; Luo J.; Zhao X. Enantioselective Construction of Axially Chiral Amino Sulfide Vinyl Arenes by Chiral Sulfide-Catalyzed Electrophilic Carbothiolation of Alkynes. Angew. Chem., Int. Ed. 2020, 59, 4959–4964. 10.1002/anie.201915470. [DOI] [PubMed] [Google Scholar]; g Cardenas M. M.; Saputra M. A.; Gordon D. A.; Sanchez A. N.; Yamamoto N.; Gustafson J. L. Catalytic Atroposelective Dynamic Kinetic Resolutions and Kinetic Resolutions towards 3-Arylquinolines via SNAr. Chem. Commun. 2021, 57, 10087–10090. 10.1039/D1CC04335H. [DOI] [PMC free article] [PubMed] [Google Scholar]; h Li Y.; Liou Y.-C.; Chen X.; Ackermann L. Thioether-Enabled Palladium-Catalyzed Atroposelective C–H Olefination for N–C and C–C Axial Chirality. Chem. Sci. 2022, 13, 4088–4094. 10.1039/D2SC00748G. [DOI] [PMC free article] [PubMed] [Google Scholar]; i Liao G.; Zhang T.; Jin L.; Wang B.-J.; Xu C.-K.; Lan Y.; Zhao Y.; Shi B.-F. Experimental and Computational Studies on the Directing Ability of Chalcogenoethers in Palladium-Catalyzed Atroposelective C–H Olefination and Allylation. Angew. Chem., Int. Ed. 2022, 61, e202115221 10.1002/anie.202115221. [DOI] [PubMed] [Google Scholar]
- a Gladiali S.; Dore A.; Fabbri D. Novel heterobidentate ligands for asymmetric catalysis: Synthesis and rhodium-catalysed reactions of S-alkyl (R)-2-diphenylphosphino-1,1′-binaphthyl-2′-thiol. Tetrahedron: Asymmetry 1994, 5, 1143–1146. 10.1016/0957-4166(94)80141-X. [DOI] [Google Scholar]; b Kang J.; Yu S. H.; Kim J. I.; Cho H. G. Catalytic Asymmetric Allylic Alkylation with A Novel P-S Bidentate Ligand. Bull. Korean Chem. Soc. 1995, 16, 439–443. 10.5012/bkcs.1995.16.5.439. [DOI] [Google Scholar]; c Gladiali S.; Medici S.; Pirri G.; Pulacchini S.; Fabbri S. BINAPS - An axially chiral P,S-heterodonor ligand for asymmetric catalysis based on binaphthalene backbone. Can. J. Chem. 2001, 79, 670–678. 10.1139/v01-041. [DOI] [Google Scholar]; d Zhang W.; Shi M. Axially chiral P,S-heterodonor ligands with a binaphthalene framework for palladium-catalyzed asymmetric allylic substitutions: experimental investigation on the reversal of enantioselectivity between different alkyl groups on sulfur atom. Tetrahedron: Asymmetry 2004, 15, 3467–3476. 10.1016/j.tetasy.2004.09.027. [DOI] [Google Scholar]; e Hoshi T.; Sasaki K.; Sato S.; Ishii Y.; Suzuki T.; Hagiwara H. Highly Enantioselective Pd-Catalyzed Allylic Alkylation of Indoles Using Sulfur-MOP Ligand. Org. Lett. 2011, 13, 932–935. 10.1021/ol102977b. [DOI] [PubMed] [Google Scholar]; f Berthelot-Bréhier A.; Panossian A.; Colobert F.; Leroux F. R. Atroposelective synthesis of axially chiral P,S-ligands based on arynes. Org. Chem. Front. 2015, 2, 634–644. 10.1039/C5QO00067J. [DOI] [Google Scholar]
- a Fleischmann R.; Kerr B.; Yeh L. T.; Suster M.; Shen Z. C.; Polvent E.; Hingorani V.; Quart B.; Manhard K.; Miner J. N.; Baumgartner S. Pharmacodynamic, pharmacokinetic and tolerability evaluation of concomitant administration of lesinurad and febuxostat in gout patients with hyperuricaemia. Rheumatology 2014, 53, 2167–2174. 10.1093/rheumatology/ket487. [DOI] [PubMed] [Google Scholar]; b Hoy S. M. Lesinurad: First Global Approval. Drugs 2016, 76, 509–516. 10.1007/s40265-016-0550-y. [DOI] [PubMed] [Google Scholar]; c Wang J.; Zeng W.; Li S.; Shen L.; Gu Z.; Zhang Y.; Li J.; Chen S.; Jia X. Discovery and Assessment of Atropisomers of (±)-Lesinurad. ACS Med. Chem. Lett. 2017, 8, 299–303. 10.1021/acsmedchemlett.6b00465. [DOI] [PMC free article] [PubMed] [Google Scholar]
- The value of the barrier accounts for a half-life (t1/2) of ∼15 h at 25 °C (35 min at 50 °C). It must be also taken into account, however, that enhanced electrophilicity of the carbonyl group by interaction with acidic species in the reaction media is expected to decrease the calculated racemization barrier.
- a Nishiyama H.; Shiomi T.; Tsuchiya Y.; Matsuda I. High Performance of Rh(Phebox) Catalysts in Asymmetric Reductive Aldol Reaction: High Anti-Selectivity. J. Am. Chem. Soc. 2005, 127, 6972–6973. 10.1021/ja050698m. [DOI] [PubMed] [Google Scholar]; b Ito J.; Shiomi T.; Nishiyama H. Efficient Preparation of New Rhodium- and Iridium-[Bis(oxazo-linyl)-3,5-dimethylphenyl] Complexes by C–H Bond Activation: Applications in Asymmetric Synthesis. Adv. Synth. Catal. 2006, 348, 1235–1240. 10.1002/adsc.200606049. [DOI] [Google Scholar]; c Shiomi T.; Ito J.; Yamamoto Y.; Nishiyama H. 4-Substituted-Phenyl(bisoxazoline)-Rhodium Complexes: Efficiency in the Catalytic Asymmetric Reductive Aldol Reaction. Eur. J. Org. Chem. 2006, 2006, 5594–5600. 10.1002/ejoc.200600722. [DOI] [Google Scholar]; d Shiomi T.; Nishiyama H. Intermolecular Asymmetric Reductive Aldol Reaction of Ketones as Acceptors Promoted by Chiral Rh(Phebox) Catalyst. Org. Lett. 2007, 9, 1651–1654. 10.1021/ol070251d. [DOI] [PubMed] [Google Scholar]; e Hashimoto T.; Shiomi T.; Ito J.; Nishiyama H. Asymmetric synthesis of α-chiral dihydrocinnamates by catalytic reductive aldol coupling and subsequent dehydroxylation. Tetrahedron 2007, 63, 12883–12887. 10.1016/j.tet.2007.10.055. [DOI] [Google Scholar]; f Yang Y.-F.; Shi T.; Zhang X.-H.; Tang Z.-X.; Wen Z.-Y.; Quan J.-M.; Wu Y.-D. Theoretical Studies on the Mechanism and Stereoselectivity of Rh(Phebox)-Catalyzed Asymmetric Reductive Aldol Reaction. Org. Biomol. Chem. 2011, 9, 5845–5855. 10.1039/c1ob05501a. [DOI] [PubMed] [Google Scholar]
- According to previous mechanistic studies, the reaction takes place through a highly congested rhodium intermediate (ref (26f)). We, therefore, assume that the stereochemical outcome of the reaction should be highly dependent on steric factors
- CCDC 2254164 and 2272283 contain the supplementary crystallographic data for 2m and 6, respectively. These data can be obtained free of charge from The Cambridge Crystallographic Data Centre
- The same mixture was also obtained from the enantioenriched minor product, isolated in small amounts from the reaction performed at 1.2 mmol scale
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.