Abstract
Purpose
The purpose of this study was to determine if control observers can be used as surrogates to predict visual acuity (VA) of patients with Down syndrome (DS).
Methods
Thirty adults with DS were enrolled in a clinical trial testing three refraction types: clinical refraction and two using wavefront aberration measures to optimize the metrics pupil fraction tessellated (PFSt) and visual Strehl ratio (VSX). Monocular VA was obtained through habitual refractions and each experimental refraction type. Five controls without DS viewed acuity charts simulating the retinal image produced when the corrections for each DS eye are worn, performing VA and scoring image quality of each chart. Group median VA (DS versus controls) were compared for each refraction type, and control image quality scores were compared to corresponding VA across refraction types.
Results
Median VA for participants with DS ranged from 0.46 logMAR (interquartile range [IQR] = 0.32 to 0.54) with habitual correction to 0.36 logMAR (IQR = 0.28 to 0.54) with VSX, whereas controls ranged from 0.37 logMAR (IQR = 0.29 to 0.42) with habitual correction to 0.01 logMAR (IQR = −0.02 to 0.05) with VSX. Overall image quality scores were best for PFSt and VSX and showed a strong linear relationship with control VA (r = −0.91, P < 0.001), and a lesser correlation with DS VA (r = −0.33, P < 0.001).
Conclusions
Using surrogate observers to judge image quality simulations of eyes with DS did not predict actual VA, suggesting additional, non-optical factors may be limiting VA in individuals with DS.
Translational Relevance
Findings may guide clinical refraction practices for patients with DS.
Keywords: down syndrome (DS), visual acuity (VA), refraction, wavefront aberrations
Introduction
Individuals with Down syndrome (DS) have unique visual systems with increased prevalence of high refractive errors, strabismus, and nystagmus,1–5 which can impede their visual function and quality of life. Those with DS tend to have reduced visual acuity (VA) compared to age-matched controls even with best clinical spectacle correction.2,6,7 Along with high refractive errors, children with DS have been found to have greater magnitudes of higher order aberrations compared to age-matched controls which in turn degrades their central optical quality without apparent internal compensation from the optical system.8 Recent efforts have been made to produce optimized spectacle corrections that take this population's high magnitudes of hyperopia or myopia, oblique astigmatism,2,9 and elevated higher-order wavefront aberrations8 into account as a way to improve VA. Although sphero-cylindrical lenses cannot fully compensate for higher-order aberrations, the strategies used to identify optimized spectacle corrections seek to identify corrections that minimize their negative effects.
Investigation of wavefront aberration correction has resulted in the utilization of various image quality metrics10 to help estimate visual performance.11 Two metrics that have a strong association to VA are pupil fraction tessellated (PFSt) and visual Strehl ratio in the spatial domain (VSX).12–14 Identifying refractions that maximize these metrics have been shown to improve aspects of blur, contrast, and ghosting in retinal image quality as judged by control observers viewing simulated acuity charts.15 A recent clinical trial dispensed these metric optimized corrections to individuals with DS and compared VA outcomes between patients’ habitual corrections to an expert-derived prescription and to prescriptions designed to optimize PFSt and VSX. The trial found that metric-optimized prescriptions produced equal acuity gains to the expert-derived prescriptions, and even performed better in some individuals with DS.16,17
Although the participants in the clinical trial were able to complete VA testing, this is not always the case for individuals with DS. Intellectual disability in this population can often limit participation in vision testing, as well as limit subjective feedback about the visual experience with various spectacle corrections. Thus, it would be useful to develop methods by which alternative observers could judge the image quality of a given correction on behalf of a patient with DS. The goal of the current study is to utilize the VA data from the individuals with DS who participated in the clinical trial to determine if control observers without DS can be used as a surrogate to predict the VA experienced by patients with DS wearing different refraction types. If a surrogate observer can accurately predict resultant VA improvement from a simulated retinal image for a given correction, the refraction process for patients with DS may be improved to produce optimal spectacle prescriptions that can best serve this population.
Methods
This study was approved by the University of Houston Committee for the Protection of Human Subjects and adhered to the Declaration of Helsinki. Parental permission was obtained from the parent or legal guardian of all participants with DS, followed by participant's assent. All control participants provided informed consent. As described in previous publications,16,17 30 adults with DS at least 18 years of age with no ocular conditions, such as corneal scarring, cataracts, strabismic or anisometropic amblyopia, or nystagmus, who were also able to be dilated and fixate for several seconds for imaging, were recruited. Participants were recruited from the University of Houston's University Eye Institute, local DS organizations, and by word-of-mouth. The study was listed on clinicaltrials.gov (NCT03367793) in accordance with National Institutes of Health (NIH) policy and the primary outcome has previously been reported.17 For the purposes of the present study, five healthy young adults without DS were later recruited from the University of Houston College of Optometry faculty, staff, and students as control participants.
Participants With Down Syndrome
An initial study visit comprised of a comprehensive eye examination was performed by a single investigator who is a licensed pediatric optometrist with over 35 years of experience examining children and individuals with special needs. Measures of VA at distance, assessments of binocular vision and ocular health, measurement of pupil diameter in dim and dark lighting conditions, and determination of the patient’s eligibility were performed. Eligible participants were dilated with 1% tropicamide and 2.5% phenylephrine (separated by 4 to 6 minutes), followed by measurements of Zeiss Atlas topography (Carl Zeiss Meditec, Inc., Jena, Germany) and COAS-HD wavefront aberrometry (Johnson & Johnson, Santa Ana, CA, USA).
Habitual corrections were determined for each participant with lensometry of presenting spectacles (n = 21), or considered plano for those who presented without habitual corrections (n = 9). Clinical refractions were determined through the examiner's best clinical judgment using any combination of clinical techniques, such as autorefraction (both dry and wet), retinoscopy (both dry and wet), and subjective refraction.
At the end of the initial study visit, three to five wavefront images per eye were re-sized to the individual eye's average pupil diameter in dim illumination, and the resultant images were averaged using a custom program (Spectacle Sweep, University of Houston College of Optometry Core Programming Module, Houston, TX, USA) using MATLAB (MathWorks, Natick, MA, USA). Spectacle Sweep was then used to apply refractions over a search range of over 20,000 sphero-cylindrical combinations in 0.25 D sphere and cylinder steps surrounding the patient's habitual correction for the entire range of cylindrical axes in 1-degree steps. For each refraction, values of image quality metrics PFSt and VSX, previously identified metrics that provide top performing refractions for eyes from both typical individuals and those with either keratoconus or DS,10,12,18 were calculated. The calculated metric value for each refraction was sorted and the single refractions associated with the best values of PFSt and VSX, respectively, were determined for each eye and made into two separate pairs of spectacles. All three experimental correction types – clinical, PFSt, and VSX – were fabricated in an identical frame selected by the patient. Randomization of treatment order and eye testing order prior to subsequent study visits was performed for all participants, ensuring five participants received each of the six randomization orderings.
A second study visit included measurements of VA with the participant's habitual correction and all three experimental spectacle prescriptions upon initial dispense. Participants read logMAR-style acuity charts one line at a time that were either composed of five letter lines in the British Standard (D, E, F, H, N, P, R, U, V, and Z) or a restricted set (H, O, T, and V) with one repeated letter per line, along with a matching card if needed, depending on the cognitive ability of the participant. Participants read the chart monocularly according to the randomization of eye testing order for the habitual correction first, then for each experimental correction in the predetermined randomized order. The largest line (0.8 logMAR) was presented first, continuing line by line until the participant made five total mistakes. Final logMAR acuity was scored letter-by-letter such that each letter was equal to 0.02 logMAR, and total score of correct letters (number of correct letters times 0.02) was subtracted from 0.9 logMAR. One study participant was unable to complete the monocular VA testing at the initial dispense visit due to difficulty with cooperation, and thus monocular VA results from subsequent study visits after the dispense of each refraction type (2 months between visits) were used for that participant in this analysis.
Control Observers
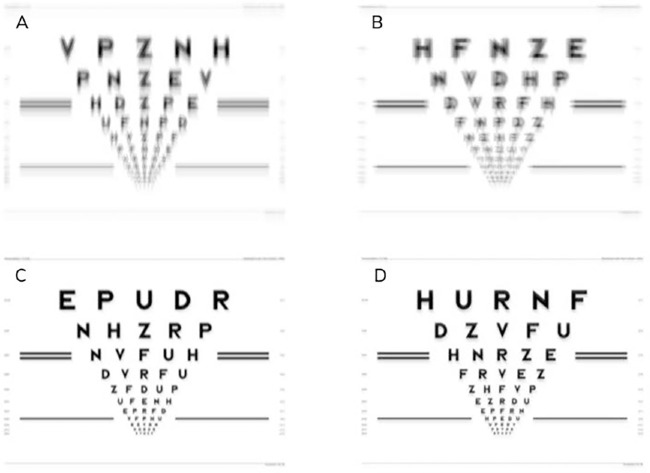
After the participants with DS completed the study visits, a group of 5 control observers who did not have DS and had a best corrected VA of 20/20 (or better) were recruited. For each of the four correction types for each participant with DS, acuity charts were generated with Image Simulation software (Sarver and Associates, Cookeville, TN, USA) by convolving a clear chart with the point spread function determined from the residual wavefront error calculated with the Spectacle Sweep program.10,15 The generated logMAR-style acuity charts simulated the predicted retinal image for each eye (2 eyes) of each participant with DS (30 participants) in the presence of each of the 4 correction types, resulting in 240 total acuity charts produced (Fig. 1).
Figure 1.
Simulated acuity charts generated from residual wavefront error for the left eye of Participant DS_007 for habitual correction (A), clinical correction (B), PFSt correction (C), and VSX correction (D).
Each control observer viewed sets of 60 simulated charts in random order with a clear chart inserted in the randomization to serve as a baseline for each set that was viewed. The largest line for acuity testing was 0.7 logMAR because more lines were needed toward the bottom of the chart to properly measure VA, especially on the clear chart where 0.0 or −0.1 logMAR acuity was expected. Therefore, acuity was calculated similarly to that for the group with DS using the equation (0.8 – [number of correct letters times 0.02]). All acuity charts were viewed monocularly through a unit magnification telescope with a 3 mm aperture and best spectacle correction in place after dilation with 1% tropicamide and 2.5% phenylephrine.10 This testing paradigm was designed to minimize the impact of the observer's own aberrations and refractive error on the visual experience. Controls read letters on each chart until they had a total of five misses and logMAR acuity was scored the same as participants with DS. In addition to performing VA, control observers provided subjective rankings regarding the quality of each chart which included an overall quality score (0 to 100, and 100 = best),15 and three subscale scores for each of perceived ghosting (position offset), perceived blur, and perceived contrast (scored 1 to 10, where 1 = best quality).15 The subjective rankings were used to identify aspects of image quality related to VA performance.
Data Analysis
Statistical analysis was performed using SAS version 9.4 (Cary, NC, USA). Descriptive tables of median and interquartile ranges (IQR = 25th and 75th percentiles) were calculated for the acuities obtained from participants with DS and the control observers for each eye and refraction type. An initial Bland-Altman19 analysis comparing the differences between acuity for participants with DS and controls versus the acuity of participants with DS was conducted; however, a strong linear relationship was observed with worsening acuity of participants with DS and thus failing one of the assumptions for Bland-Altman analysis. To explore the relationship between overall image quality score and VA, linear correlations were performed for both control observers and participants with DS. Last, mean subscale scores assigned by control observers were compared across refraction types using repeated measures analysis of variance (ANOVA) with Tukey's method to adjust for multiple comparisons.20
Results
Thirty participants with DS were randomized into the study, with an average age of 29 ± 10 years (range = 18–52 years) and an even distribution of men and women (women = 15). Strabismus was present in nine participants (7 eso deviations and 2 exo deviations), with seven of these participants having an alternating strabismus which reduced the risk of strabismic amblyopia. The range of refractive errors based on dry auto refractor readings was large (−15.25 to +6.00 D sphere) with all but 2 participants having at least one eye with −1.00 D cylinder power or more (range = −7.25 to −0.25 D cylinder). There were 23 myopic eyes, 21 hyperopic eyes, 15 eyes with mixed astigmatism, and 1 eye with emmetropia (emmetropia defined as both principal meridians falling between −0.50 and +1.00 D with less than 0.50 D of cylinder).
Group Median Acuity by Refraction Type
For participants with DS, habitual corrections resulted in the worst VA with a median logMAR acuity of 0.46 (IQR = 0.32 to 0.54) for the right eye (OD) and 0.40 (IQR = 0.28 to 0.62) for the left eye (OS), whereas VSX corrections produced the best VA, with a median logMAR of 0.42 (IQR = 0.28 to 0.50) for OD and 0.36 (IQR = 0.28 to 0.54) for OS (see the Table). Similarly, simulated charts from habitual corrections resulted in the worst VA for control observers, with a median logMAR of 0.37 (IQR = 0.32 to 0.54) for OD and 0.30 (IQR = 0.19 to 0.44) for OS. Charts simulated from VSX corrections resulted in the better acuity for controls with a median logMAR of 0.01 (IQR = −0.01 to 0.08) for the OD and 0.01 (IQR = −0.02 to 0.05) for the OS (see the Table). However, the difference in acuity between habitual corrections and VSX corrections was minimal in participants with DS (group median improvement = 0.04 logMAR, or 2 letters) in comparison to the large improvements seen in control observer performance (approximately 3 full lines).
Table.
Median and Interquartile Range For Visual Acuity of Participants With DS and Control Observers Per Correction Type
| Participants With DS (n = 30) | Control Observersa (n = 5) | ||||||
|---|---|---|---|---|---|---|---|
| Eye | Refraction | Median | 25th Pctl | 75th Pctl | Median | 25th Pctl | 75th Pctl |
| OD | Clinical | 0.44 | 0.28 | 0.52 | 0.31 | 0.22 | 0.40 |
| Habitual | 0.46 | 0.32 | 0.54 | 0.37 | 0.29 | 0.42 | |
| PFSt | 0.42 | 0.34 | 0.54 | 0.08 | 0.02 | 0.12 | |
| VSX | 0.42 | 0.28 | 0.50 | 0.01 | −0.01 | 0.08 | |
| OS | Clinical | 0.39 | 0.22 | 0.52 | 0.28 | 0.09 | 0.38 |
| Habitual | 0.40 | 0.28 | 0.62 | 0.30 | 0.19 | 0.44 | |
| PFSt | 0.42 | 0.26 | 0.52 | 0.06 | −0.00 | 0.11 | |
| VSX | 0.36 | 0.28 | 0.54 | 0.01 | −0.02 | 0.05 | |
Average of 5 controls.
Visual acuity measures are listed in logMAR.
Pctl, percentile.
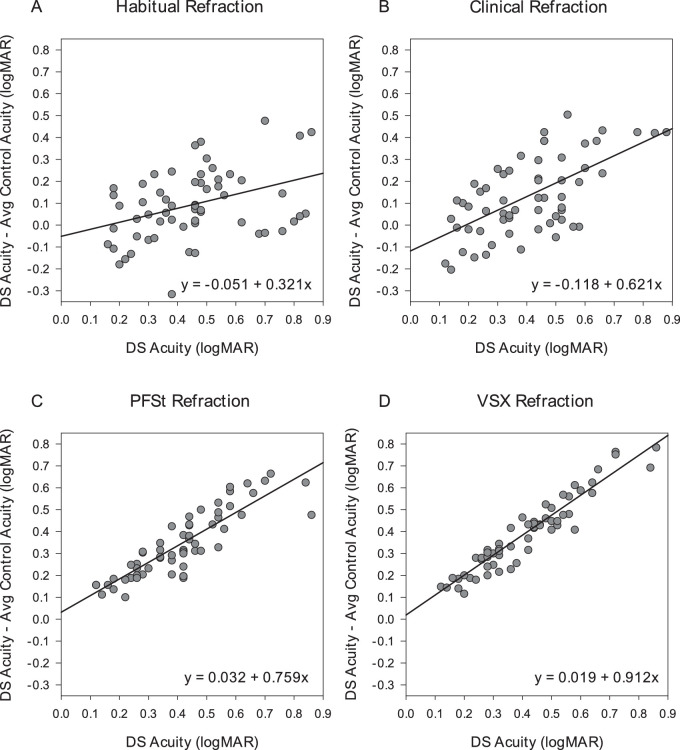
Plots of Agreement for Visual Acuity Measures
In Figure 2, plots of agreement indicate a linear relationship between actual VA of participants with DS and the difference between DS and average control participant VA. Participants with DS and worse acuity showed greater differences in VA from control observers, with this trend most evident for the metric-optimized corrections (PFSt and VSX). In other words, the actual acuity of the participants with DS was worse than that predicted by the control observers.
Figure 2.
Plots of agreement between visual acuity outcome of participants with DS and difference between control and DS visual acuity outcomes for habitual correction (A), clinical correction (B), PSFt correction (C), and VSX correction (D). The comparisons are shown as plots of difference versus mean with the best-fit regression line rather than lines delineating the 95% limits of agreement.
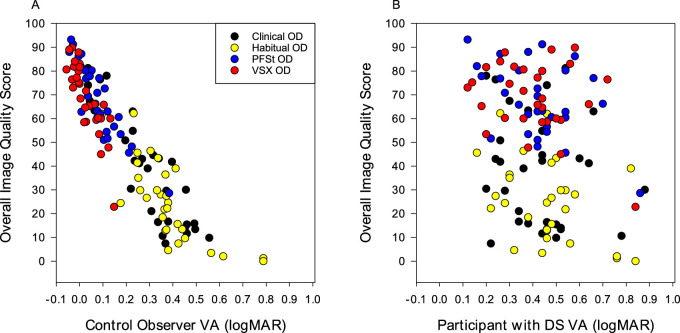
Correlation of Image Quality Score and Visual Acuity
The overall quality score of each acuity chart was significantly correlated with the VA of the control observers such that a better VA corresponded to a better overall quality score (Fig. 3A; r = −0.91, P < 0.001). Conversely, there is more scatter and a weaker correlation (r = −0.33, P < 0.001) when comparing the overall quality score given by control observers and the VA of participants with DS (Fig. 3B), indicating that control observer quality score was not a great predictor of actual VA for participants with DS.
Figure 3.
Mean control observer overall quality score versus visual acuity of control observers (A) (r = −0.91, P < 0.001) and versus visual acuity of participants with DS (B) (r = −0.33, P < 0.001). Overall quality score ranges from 0 to 100, with 100 = best quality. Data reported for the right eye only for simplicity, the left eye results showed similar pattern. Legend on (A) is applicable to both plots.
Image Quality Subscale Scores by Refraction Type
Figure 4 shows the distribution of subscale scores assigned by the control observers for position offset (see Fig. 4A), blur (see Fig. 4B), and contrast (see Fig. 4C) by refraction type. For each refraction type, the mean scores for each subscale were all significantly different across the four refraction types (adjusted P ≤ 0.028), with the metric-optimized refractions (PFSt and VSX) receiving the best average scores for all three subscores of quality (see Fig. 4).
Figure 4.
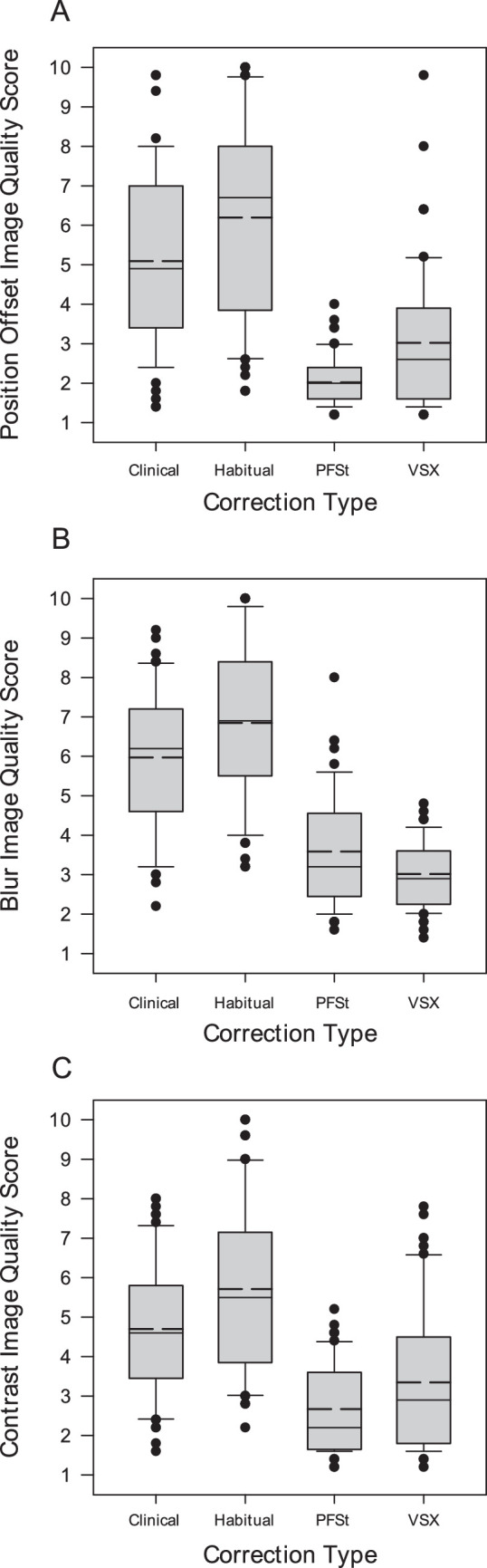
Adjusted mean subscale scores by refraction type for position offset (A), blur (B), and contrast (C). Subscale scores range from 1 to 10, with 1 = best. Solid line is the median score, and the dashed line is the mean score.
Discussion
The goal of the current study was to determine if control observers could be used as a surrogate to predict acuity of patients with DS wearing different correction types. Although the comparisons of overall group median performance (see the Table) might have indicated a trend of habitual corrections performing worst and metric-optimized corrections performing best, the control observers had acuity gains for the metric-optimized corrections that were substantially larger than the actual gains observed in the participants with DS. This is further illustrated in the plots of agreement (see Fig. 2) that showed a growing discrepancy between VA of those with DS and control observers as the level of acuity in the participants with DS worsened. These findings show a poor predictive ability of control observers to identify VA in adults with DS for different refractive corrections. It should be noted that for the nine individuals with DS presenting unaided, some had been recommended glasses in the past, although they had not worn them consistently, and thus the habitual performance may have been further reduced due to the presence of uncorrected refractive error. However, this should not have impacted the ability to compare the VA of the individuals with DS to the control observers because the charts generated for the control observers were based upon the retinal image quality of the participants with DS for each viewing condition.
Although there was poor agreement between the visual performance of control observers and participants with DS, the VA and image quality scores from the control observers did follow the expected trend of improvement with the metric optimized corrections. Control observers showed a nearly three line VA gain and large image quality improvements with PFSt and VSX compared to habitual and clinical refractions; specifically reduced position offset, reduced blur, and increased contrast. Despite these large improvements in VA and image quality, the same strong relationship between image quality and VA in the participants with DS was not observed.
Due to the poor predictive ability of control observer performance, it is reasonable to assume that some factor other than optical quality is responsible for reduced VA in those with DS. For the PFSt and VSX corrections, there is very little spread in the data as acuity decreases for participants with DS (see Figs. 2C, 2D), suggesting that the factor responsible for the difference in acuity between those with DS and control observers is consistent across all participants. Although this study excluded participants with monocular amblyopia (from anisometropia or unilateral strabismus), bilateral amblyopia may be a factor that limited the acuity gains observed. A study of 213 children and adults with DS (average age = 17.2 ± 4.8 years) and 184 age and gender-matched controls without DS (average age = 17.2 ± 4.4 years) showed that amblyopia, defined as 2 lines or greater inter-ocular difference in best corrected VA in the absence of pathology, was detected in 36.3% of the participants with DS compared to only 3.8% in the control group.21
In the present study, some adults with DS likely had poor spectacle correction adherence from a young age that resulted in decreased retinal image quality amblyogenic in nature. Even in individuals who were wearing spectacles from a young age, we know from past studies that typical spectacles do not correct for the elevated higher order aberrations seen in this group, and thus amblyopia is likely to have developed from persistent poor image quality from one or both eyes. Little et al.22 found multi-line improvements in VA in children with DS when tested with interferometry that bypasses the optics of the eye. This level of improvement is similar to what we would have predicted to see based on the control observer performance; however, our patients with DS were adults and had longer duration over which amblyopia could develop. Although the participants enrolled in the clinical trial wore each treatment type for 2 months, analyzing comparisons of control observer VA and 2 month adapted VA in participants with DS did not result in any improvement in predictive ability, suggesting if amblyopia is present, it was not improved with longer spectacle wear. However, 2 months of spectacle wear in an adult with amblyopia would not be expected to result in meaningful acuity improvement.
Other potential factors that could be limiting the VA of those with DS include sensory deficits in the retina and visual cortex. A recent study using spectral domain OCT demonstrated that those patients with DS showed higher prevalence of foveal hypoplasia and overall thickening of inner retinal layers compared to age-matched controls.23 Alternative measures of acuity that bypass the optics of the visual system include visual evoked potentials (VEP)24 and Vernier acuity,25 and studies utilizing these techniques showed a reduction in acuity at higher levels of visual processing in those with DS compared to controls. Whereas these studies have demonstrated sensory deficits, their role in reduced VA for this population must be explored further.
This study had several limitations. First, this study involved only adults with DS, most of whom were 30 years of age or older. This limits the generalizability of the study to children with DS. Second, amblyopia is more challenging to treat in adults with spectacle correction; therefore, if amblyopia was a limiting factor in the VA of the participants with DS, we would not expect large VA gains with optimized correction in an older population. Third, this study excluded individuals that have nystagmus, which is observed in approximately 12% to 18% of the DS population.3 This exclusion also limits the generalizability of the study results to the DS population. Further work is needed to determine if predicting VA in children with DS would be more successful.
In conclusion, control observers were poor predictors of visual performance for those with DS, although VA and image quality did improve markedly with metric-optimized corrections for the control observers. It is important to note that although all individuals with DS may not be capable of participation in VA tasks and other subjective testing, our participants were testable. Further work is needed to improve eye examination methods to allow for participation from individuals with intellectual disabilities, including improved ways to obtain subjective feedback about their vision.
Acknowledgments
The authors thank Hope Queener for developing the Spectacle Sweep software used in this study and Ayeswarya Ravikumar for generating the simulated charts used for visual acuity testing.
Supported by the National Institutes of Health (NIH) R01 EY024590 (H.A.), and NIH P30 EY07551 (L.F.).
Disclosure: L.V. Schneider, None; G.L. Mitchell, None; J.D. Marsack, None; H.A. Anderson, None
References
- 1. da Cunha RP, Moreira JB.. Ocular findings in Down's syndrome. Am J Ophthalmol. 1996; 122(2): 236–244. PubMed PMID: 8694092. [DOI] [PubMed] [Google Scholar]
- 2. Gardiner PA. Visual defects in cases of Down's syndrome and in other mentally handicapped children. Br J Ophthalmol. 1967; 51(7): 469–474. PubMed PMID: 4226447; PMCID: PMC506427. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. Wagner RS, Caputo AR, Reynolds RD.. Nystagmus in Down's syndrome. Ophthalmology. 1990; 97(11): 1439–1444. PubMed PMID: 2147744. [DOI] [PubMed] [Google Scholar]
- 4. Haugen OH, Hovding G.. Strabismus and binocular function in children with Down syndrome. A population-based, longitudinal study. Acta Ophthalmol Scand. 2001; 79(2): 133–139. PubMed PMID: 11284750. [DOI] [PubMed] [Google Scholar]
- 5. Cregg M, Woodhouse JM, Stewart RE, et al. Development of refractive error and strabismus in children with Down syndrome. Invest Ophthalmol Vis Sci. 2003; 44(3): 1023–1030. PubMed PMID: 12601024. [DOI] [PubMed] [Google Scholar]
- 6. Woodhouse JM, Pakeman VH, Cregg M, et al. Refractive errors in young children with Down syndrome. Optom Vis Sci. 1997; 74(10): 844–851. PubMed PMID: 9383798. [DOI] [PubMed] [Google Scholar]
- 7. Courage ML, Adams RJ, Reyno S, Kwa PG.. Visual acuity in infants and children with Down syndrome. Dev Med Child Neurol. 1994; 36(7): 586–593. PubMed PMID: 8034120. [DOI] [PubMed] [Google Scholar]
- 8. McCullough SJ, Little JA, Saunders KJ.. Higher order aberrations in children with Down syndrome. Invest Ophthalmol Vis Sci. 2013; 54(2): 1527–1535. Epub 20130228. PubMed PMID: 23361511. [DOI] [PubMed] [Google Scholar]
- 9. Berk AT, Saatci AO, Ercal MD, Tunc M, Ergin M.. Ocular findings in 55 patients with Down's syndrome. Ophthalmic Genet. 1996; 17(1): 15–19. Epub 1996/03/01. PubMed PMID: 8740693. [DOI] [PubMed] [Google Scholar]
- 10. Ravikumar A, Benoit JS, Marsack JD, Anderson HA.. Image quality metric derived refractions predicted to improve visual acuity beyond habitual refraction for patients with Down syndrome. Transl Vis Sci Technol. 2019; 8(3): 20. Epub 20190520. PubMed PMID: 31157125; PMCID: PMC6532430. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Cheng X, Thibos LN, Bradley A.. Estimating visual quality from wavefront aberration measurements. J Refract Surg. 2003; 19(5): S579–S584. Epub 2003/10/02. PubMed PMID: 14518747. [DOI] [PubMed] [Google Scholar]
- 12. Cheng X, Bradley A, Thibos LN.. Predicting subjective judgment of best focus with objective image quality metrics. J Vis. 2004; 4(4): 310–321. Epub 20040423. PubMed PMID: 15134478. [DOI] [PubMed] [Google Scholar]
- 13. Hastings GD, Marsack JD, Nguyen LC, Cheng H, Applegate RA.. Is an objective refraction optimised using the visual Strehl ratio better than a subjective refraction? Ophthalmic Physiol Opt. 2017; 37(3): 317–325. Epub 20170330. PubMed PMID: 28370389; PMCID: PMC5469359. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Applegate RA, Marsack JD, Ramos R, Sarver EJ.. Interaction between aberrations to improve or reduce visual performance. J Cataract Refract Surg. 2003; 29(8): 1487–1495. PubMed PMID: 12954294. [DOI] [PubMed] [Google Scholar]
- 15. Benoit JS, Ravikumar A, Marsack JD, Anderson HA.. Understanding the impact of individual perceived image quality features on visual performance. Transl Vis Sci Technol. 2020; 9(5): 7. Epub 20200415. PubMed PMID: 32821479; PMCID: PMC7401969. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. Anderson HA, Benoit JS, Marsack JD, et al. A randomized trial of objective spectacle prescriptions for adults with Down syndrome: baseline data and methods. Optom Vis Sci. 2021; 98(1): 88–99. PubMed PMID: 33394936; PMCID: PMC7789324. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. Anderson HA, Marsack JD, Benoit JS, Manny RE, Fern KD. Visual acuity outcomes in a randomized trial of wavefront metric-optimized refractions in adults with Down syndrome. Optom Vis Sci. 2022; 99(1): 58–66. PubMed PMID: 34882603; PMCID: PMC8720070. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. Ravikumar A, Benoit JS, Morrison KB, Marsack JD, Anderson HA.. Repeatability of monocular acuity testing in adults with and without Down syndrome. Optom Vis Sci. 2018; 95(3): 202–211. PubMed PMID: 29461409; PMCID: PMC5822740. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. Bland JM, Altman DG.. Statistical methods for assessing agreement between two methods of clinical measurement. Lancet. 1986; 1(8476): 307–310. PubMed PMID: 2868172. [PubMed] [Google Scholar]
- 20. Tukey JW, Brillinger DR, Cox DR, Braun HI.. The collected works of John W. Tukey. Belmont, CA: Wadsworth Advanced Books & Software; 1984. [Google Scholar]
- 21. Hashemi H, Mehravaran S, Asgari S, Dehghanian Nasrabadi F. Refractive and vision status in Down syndrome: a comparative study. Turk J Ophthalmol. 2021; 51(4): 199–205. PubMed PMID: 34461695; PMCID: PMC8411285. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22. Little JA, Woodhouse JM, Lauritzen JS, Saunders KJ.. The impact of optical factors on resolution acuity in children with Down syndrome. Invest Ophthalmol Vis Sci. 2007; 48(9): 3995–4001. PubMed PMID: 17724178. [DOI] [PubMed] [Google Scholar]
- 23. Nicholson R, Osborne D, Fairhead L, Beed L, Hill CM, Lee H.. Segmentation of the foveal and parafoveal retinal architecture using handheld spectral-domain optical coherence tomography in children with Down syndrome. Eye (Lond). 2022; 36(5): 963–968. Epub 20220110. PubMed PMID: 35001092; PMCID: PMC9046253. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24. John FM, Bromham NR, Woodhouse JM, Candy TR.. Spatial vision deficits in infants and children with Down syndrome. Invest Ophthalmol Vis Sci. 2004; 45(5): 1566–1572. PubMed PMID: 15111616. [DOI] [PubMed] [Google Scholar]
- 25. Little JA, Woodhouse JM, Lauritzen JS, Saunders KJ.. Vernier acuity in Down syndrome. Invest Ophthalmol Vis Sci. 2009; 50(2): 567–572. Epub 20080929. PubMed PMID: 18824732. [DOI] [PubMed] [Google Scholar]