Abstract
Differentiation between Mycobacterium tuberculosis and M. avium is helpful for the treatment of disseminated mycobacterial infection in AIDS patients. This can traditionally be done by time-consuming biochemical tests or with Accuprobe. Previously, PCR restriction enzyme analysis (PCR-REA) of the 16S-23S rRNA gene spacer was shown to be able to identify a limited number of strains of Mycobacterium. In this study the method was improved by using more specific primers and was tested with 50 clinical isolates of M. tuberculosis and 65 clinical isolates of M. avium complex. Probes specific to the spacers of M. tuberculosis and M. avium were also tested. Both M. tuberculosis and M. avium could be reliably identified either by PCR-REA or by PCR-hybridization, with the results completely agreeing with those obtained by biochemical tests and with the Accuprobe, respectively. The method may therefore be useful as an alternative in-house method for identification of the bacteria.
AIDS is a major cause of morbidity and mortality in both developed and developing countries. In general, Mycobacterium tuberculosis is a major mycobacterial infection among AIDS patients in developing countries, while M. avium is more commonly found in developed countries (7). Both mycobacterial species can cause disseminated disease in AIDS patients. Although disseminated M. avium infection is uncommon among AIDS patients in Africa (11), a recent study in Thailand revealed that both disseminated M. avium and M. tuberculosis infections were common (20). It is therefore helpful if clinical laboratories can rapidly differentiate between these two species once a mycobacterium is isolated from the blood.
Identification of mycobacterial species is usually done by time-consuming biochemical tests or with Accuprobe, which is rather expensive. Several other molecular genetic methods have also been reported. These include amplification of species-specific sequences (2, 5, 18, 27, 31), PCR amplification and restriction enzyme analysis (PCR-REA) (1, 17, 19, 24–26, 28), hybridization with species-specific oligonucleotide probes with or without prior DNA amplification (3, 6, 13), and nucleic acid sequence determination (15, 16, 23).
Recently, we reported a method for differentiating mycobacterial species by PCR-REA of 16S-23S rRNA gene spacer sequences (17). However, the primers used in that study were not specific to mycobacteria and the number of M. avium isolates was small. We therefore further improved the method by using more specific primers and tested it for its ability to differentiate between M. tuberculosis and M. avium. DNA oligonucleotide probes specific to M. tuberculosis and M. avium at the 16S-23S rRNA gene spacer were also developed and tested.
MATERIALS AND METHODS
Bacteria.
A total of 170 isolates of mycobacteria were tested. These included the H37Rv and H37Ra strains, as well as 50 clinical isolates of M. tuberculosis, 44 clinical isolates of M. avium, 10 clinical isolates of M. intracellulare, 11 clinical isolates of unclassified M. avium complex (MAC), and 53 isolates of other mycobacteria. These were 14 isolates of M. gordonae; 12 isolates of M. kansasii; 6 isolates of M. scrofulaceum; 2 isolates of M. flavescens; 1 isolate each of M. bovis, M. bovis BCG, M. africanum, M. marinum, M. xenopi, M. aurum, M. duvalii, M. vaccae, M. phlei, M. chelonae, and M. neolactis; 6 isolates of M. fortuitum; and 2 isolates of M. smegmatis. Each clinical isolate was from a different patient. All M. tuberculosis isolates were from sputum, while all MAC isolates were from blood. PCR-REA of the 16S-23S rRNA gene spacer of all the mycobacterial isolates other than M. tuberculosis and MAC was studied previously (17).
M. tuberculosis was identified by colonial morphology and biochemical tests. All isolates of M. tuberculosis also contained the insertion sequence IS6110, which is found almost exclusively in the members of the M. tuberculosis complex, as determined by Southern hybridization (29). All 65 isolates belonging to MAC were identified with the MAC-specific Accuprobe (GenProbe Inc., San Diego, Calif.). The MAC isolates were further identified with the M. avium- and M. intracellulare-specific Accuprobe. Forty-four isolates were hybridized by the M. avium-specific probe. Ten isolates were hybridized by the M. intracellulare-specific probe, while the other 11 isolates were not hybridized by either probe and were referred to as unclassified MAC.
Twenty-six isolates of other bacteria were also studied. These comprised an isolate each of Escherichia coli, Corynebacterium diphtheriae, Staphylococcus aureus, Staphylococcus epidermidis, Staphylococcus pyogenes, the viridans group of Streptococcus, Klebsiella pneumoniae, Neisseria sicca, Neisseria meningitidis, Burkholderia pseudomallei, Actinomyces spp., and Streptomyces spp.; two isolates of Rhodococcus spp.; five isolates of Nocardia asteroides; five isolates of Nocardia farcinica; and two isolates of Nocardia spp.
DNA primers and probes.
PCR was done with a single pair of primers, primers 16SC and 23SG, whose sequences were selected from the sequences that are shared by most mycobacteria but that are different from those of most other bacteria in the 3′ end of the 16S rRNA gene and the 5′ end of the 23S rRNA gene, respectively. The sequence of the 17-mer 16SC (5′-TCGAAGGTGGGATCGGC-3′) is identical to a sequence in the 16S rRNA gene 63 bp upstream of the spacers of most mycobacteria except M. asiaticum. M. asiaticum has the base A instead of C at the 14th position of the primer. The sequence is, however, shared by Nocardia asteroides. The sequence of the 18-mer, 23SG (5′-GCGCCCTTAGACACTTAC-3′), is completely complementary to a sequence in the 23S rRNA gene 2 bp downstream from the spacers of M. tuberculosis complex, M. kansasii, and M. gastri. It has a single-base mismatch (G instead of T) with the corresponding sequences of M. avium, M. paratuberculosis, and M. phlei at the 10th base of the primer.
Two probes, probes MT232 and MV222, were tested in this study. MT232 (5′-CGGTGGCGTGTTCTTTGTGCAATA) is a 24-mer oligonucleotide whose sequence is identical to that of the 16S-23S rRNA gene spacer of M. tuberculosis at positions 232 to 255 from the 5′ end of the spacer. MV222 (5′-GGTCTTCGTGGCCGGCGTTCA-3′) is a 21-mer oligonucleotide whose sequence is identical to that of the spacer of M. avium at positions 222 to 242 from the 5′ end of the spacer. The sequence of the probe is identical to those of sequevars Mav-A, Mav-C, and Mav-D of M. avium and has a single base different from Mav-B at the seventh base, which is G instead of C (8, 10). The sequence of the probe is different from those of all other mycobacteria.
Preparation of chromosomal DNA and mycobacterial cell lysate, PCR, and REA.
Preparation of chromosomal DNA and mycobacterial cell lysate, PCR amplification, and REA were done as described previously (17) except for the use of 16SC and 23SG as primers and the change of the annealing temperature to 62°C. Amplification of M. tuberculosis and M. avium complex was done with cell lysate as the template, while amplification of the other bacteria was done with purified chromosomal DNA as the template.
DNA probe labeling.
MT232 and MV222 probes were labeled with the DIG-Oligonucleotide 3′-End Labeling Kit (Boehringer Mannheim, Mannheim, Germany) as described by the manufacturer. The probes were finally dissolved in 20 μl of TE (Tris-EDTA) buffer and were stored at −20°C until use.
Dot blot hybridization.
A total of 2 μl of the amplified DNA was denatured by adding an equal volume of 3 N NaOH. Two microliters of the mixture was dotted onto a nylon membrane (Sigma Chemical Company, St. Louis, Mo.), and the membrane was incubated at 80°C for 2 h. The membrane was prehybridized with a solution containing 5× SSC (1× SSC is 0.15 M NaCl plus 0.015 M sodium citrate), 2% blocking reagent (Boehringer Mannheim), 0.02% sodium dodecyl sulfate (SDS), and 0.1% N-laurylsarcosine at 42°C for 1 h. The prehybridization solution was then discarded. The hybridization solution, which was of the same composition as the prehybridization solution, together with 100 pmol of a digoxigenin-labeled probe, was added. Hybridization was done for 2 h at 42°C. The membrane was then washed twice for 5 min each time in 2× SSC–0.1% SDS at room temperature and then twice for 15 min each time in 0.1× SSC–0.1% SDS at 42°C. The membrane was detected with anti-digoxigenin-alkaline phosphatase-Fab fragments (Boehringer Mannheim) as described by the manufacturer.
RESULTS
DNA amplification and PCR-REA.
Only DNA and cell lysates from Mycobacterium strains could be amplified by the 16SC and 23SG primers, and except for those from three isolates of Nocardia asterioides, none from other bacteria could be amplified. The amplified products of each of the Nocardia isolates contained one amplified fragment with a length of either 430 or 530 bp.
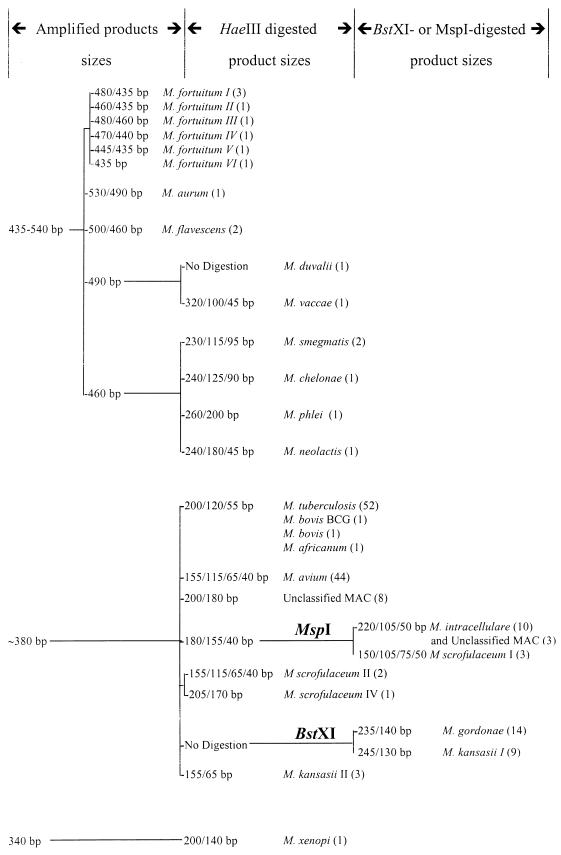
The rapidly growing mycobacteria had one or two amplified fragments, while the slowly growing mycobacteria had only one fragment. The species of the slowly growing mycobacteria could be identified by digesting the amplified products first with HaeIII and then, if necessary, with either MspI or BstXI. The dendrogram showing the results of the PCR-REA is essentially similar to the one reported previously (17) except for the length of the digested fragments, as shown in Fig. 1. This method could not differentiate between the members of the M. tuberculosis complex.
FIG. 1.
Dendrogram for differentiating Mycobacterium species by amplified 16S-23S rRNA gene length, with 16SC and 23SG as primers, and REA of the amplified products. The numbers of isolates are indicated in parentheses.
The amplified products of all 52 isolates of M. tuberculosis had the same length (about 380 bp). The HaeIII-digested amplified products of all of the isolates contained three fragments of 200, 120, and 55 bp. The PCR-REA pattern was shared by all members of the M. tuberculosis complex.
The amplified products of all 44 isolates of M. avium had the same length (about 380 bp). The HaeIII-digested amplified products of all isolates contained four fragments of 155, 115, 65, and 40 bp. The PCR-REA pattern was unique to M. avium.
The amplified products of all 10 isolates of M. intracellulare had the same length (about 380 bp). The HaeIII-digested amplified products of all isolates contained three fragments of 180, 155, and 40 bp, which is similar to the pattern for some isolates of M. scrofulaceum. When digested with MspI, the amplified products of all M. intracellulare isolates contained three fragments of 220, 105, and 50 bp, thus differing from those of M. scrofulaceum.
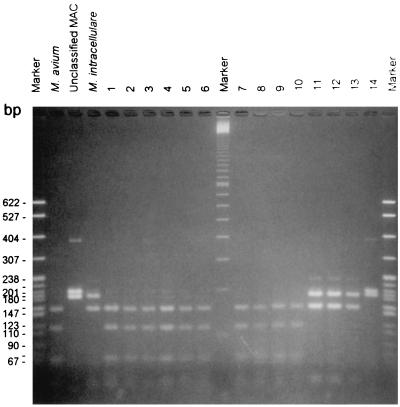
However, the PCR-REA pattern was not unique to M. intracellulare because three isolates of unclassified MAC isolates also exhibited the same HaeIII and MspI digestion patterns. These three isolates also had the same BstXI digestion patterns as M. intracellulare. The other eight isolates of unclassified MAC isolates all had HaeIII-digested amplified products of 200 and 180 bp but had the same MspI and BstXI digestion patterns as M. intracellulare. The HaeIII-digested products of some of the members of MAC are shown in Fig. 2.
FIG. 2.
HaeIII-digested PCR products of some of the members of MAC. The markers on both sides are plasmid pBR322 digested with MspI. The marker in the middle is the 100-bp ladder. Lanes 1 to 10, M. avium; lanes 11 to 13, M. intracellulare; lane 14, unclassified MAC. The species of the samples in the other three lanes are labeled.
Hybridization with digoxigenin-labeled MT232 probe.
The amplified products of the M. tuberculosis complex isolates as well as a representative isolate of each species of other bacteria were dot blotted onto a nylon filter and were hybridized with the digoxigenin-labeled MT232 oligonucleotide probe. The probe could hybridize only to the amplified products of M. tuberculosis complex isolates, not to those of any other species of bacteria, including all MAC isolates. The dot-blotted PCR products of all 50 clinical M. tuberculosis isolates, as well as the H37Rv and H37Ra strains, could be hybridized by MT232 (data not shown).
Hybridization with digoxigenin-labeled MV222 probe.
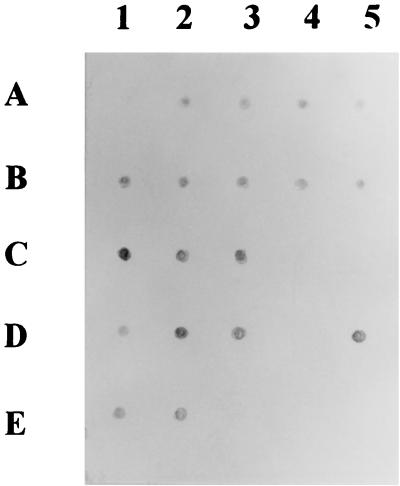
The amplified products of all M. avium isolates as well as a representative isolate of each species of other bacteria were dot blotted onto a nylon filter and were hybridized with the digoxigenin-labeled MV222 oligonucleotide probe. The MV222 probe could hybridize to the amplified products of the M. avium isolates but not to those of any other species of bacteria including M. intracellulare and unclassified MAC isolates. The dot-blotted PCR products of all 44 clinical M. avium isolates could be hybridized by MV222, as shown in Fig. 3.
FIG. 3.
Dot blot hybridization of the amplified products of some isolates belonging to the M. avium complex. A1, amplified product of M. tuberculosis H37Rv; C4, C5, and D4, amplified products of unclassified MAC; E3 to E5, amplified products of M. intracellulare.
DISCUSSION
Several DNA regions have been used as targets for the identification of Mycobacterium species. These include hsp65 (6, 19, 23, 26), dnaJ (24), 16S rRNA gene (1, 2, 15, 16, 18, 25, 28), and the 16S-23S rRNA gene spacer (8–10, 12, 13, 17). M. malmoense could be identified with a probe specific to the 16S-23S rRNA gene spacer (13). M. tuberculosis could be identified by PCR with primers specific to the spacer region (12) or sequencing of the amplified spacers (9), while members of MAC could be identified by sequencing of the spacer (8, 10).
This study revealed that M. tuberculosis complex and M. avium could be readily and reliably differentiated from each other by analysis of the 16S-23S rRNA gene spacer. Differentiation could be achieved either by PCR-REA with the HaeIII enzyme or PCR and hybridization with the MT232 and MV222 probes.
Intraspecies variation of the spacer of M. avium was well documented, with five different sequences being deposited in GenBank (8, 10). All five sequevars were predicted to have the same HaeIII digestion products as those of all 44 clinical isolates tested in this study. The MV222 probe was originally designed from an area of the spacer shared by most M. avium isolates but not by other mycobacteria, with one sequevar, Mav-B, having a base different from that of the probe. While the test of the probe was ongoing, a new sequevar (Mav-E) of M. avium was described. The sequence of the spacer of the sequevar is two bases different from the sequence of the probe at positions 7 and 8, being TA instead of CG (GenBank accession no. Z46422). Although the sequevar distribution of M. avium in Thailand is unknown, the MV222 probe was able to hybridize to all M. avium isolates in this study.
The results of the identification of M. intracellulare by PCR-REA of the spacer did not completely agree with the Accuprobe results because three isolates of unclassified MAC were identified as M. intracellulare by PCR-REA. Since none of the seven sequevars of unclassified MAC, whose spacer sequences were deposited in GenBank, should have HaeIII digestion products identical to those of M. intracellulare, these isolates might belong to a novel sequevar of unclassified MAC. However, it was also possible that these three isolates might be more properly classified as M. intracellulare since the taxonomic status of unclassified MAC is still unclear. Most of the unclassified MAC strains belong to serotypes 22 to 24 and 26 to 28, which are regarded by some as M. intracellulare (14).
Compared to the biochemical tests, amplification of the 16S-23S rRNA gene spacer is more rapid and less laborious. The major drawback is its inability to differentiate between members of the M. tuberculosis complex, since all members of the M. tuberculosis complex were shown to have identical 16S-23S rRNA gene spacer sequences (9, 12). This drawback is also shared by PCR-REA of other genes such as the 16S rRNA gene (1, 25, 28), hsp65 (19, 26), and dnaJ (24). Most M. tuberculosis-specific probes including Accuprobe also could not differentiate between members of the M. tuberculosis complex (3, 6). At present, molecular genetic methods for differentiation of members of the complex rely on the amplification of DNA fragments specific to each species such as mtp40 (4, 30) or studies of single base differences in pncA (pyrazinamidase-nicotinamidase) (21) or oxyR (22) genes.
Identification of Mycobacterium species by PCR amplification of the 16S-23S rRNA gene spacer can be accomplished within 1 to 2 days. Compared to hybridization with Accuprobe, which can identify the bacterial species within a few hours, the PCR method is slower and slightly more laborious. However, the method is fairly cheap. In Thailand the cost per test by the PCR method is about 40 to 50% of the cost of the Accuprobe. The PCR-REA is advantageous because it can differentiate between M. tuberculosis and M. avium in a single test. PCR and hybridization with the species-specific probes may be useful when several isolates suspected of belonging to the same species are tested simultaneously. At present, the PCR method can reliably amplify 20 pg of mycobacterial DNA, which is equal to the amount of DNA present in about 4,000 or more mycobacterial cells.
For identifying M. tuberculosis and M. avium, the results of both PCR-REA and PCR-hybridization with MT232 and MV222 completely agreed with each other. The results of identification of M. tuberculosis by analysis of the 16S-23S rRNA gene spacer were also in complete agreement with those of the biochemical tests, while the results of identification of M. avium by analysis of the 16S-23S rRNA gene spacer were superimposable on the results of identification with the Accuprobe. PCR amplification of the 16S-23S rRNA gene spacer may therefore be useful as an alternative in-house method for the identification of M. tuberculosis and M. avium.
ACKNOWLEDGMENTS
We thank Roongnapa Prachaktam, Faculty of Medicine Ramathibodi Hospital, Mahidol University, and Suchart Panjaisri, Faculty of Associated Medical Science, Chiangmai University, for providing us with some bacterial samples. We also thank Charnnarong Sudsamart for technical assistance.
This work has been supported by the National Center for Genetic Engineering and Biotechnology, Ministry of Science, Bangkok, Thailand.
REFERENCES
- 1.Avaniss-Aghajani E, Jones K, Holtzman A, Aronson T, Glover N, Boian M, Froman S, Brunk C F. Molecular technique for rapid identification of mycobacteria. J Clin Microbiol. 1996;34:98–102. doi: 10.1128/jcm.34.1.98-102.1996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Chen Z H, Butler W R, Baumstark B R, Ahearn D G. Identification and differentiation of Mycobacterium avium and M. intracellulare by PCR. J Clin Microbiol. 1996;34:1267–1269. doi: 10.1128/jcm.34.5.1267-1269.1996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.De Beenhouwer H, Liang Z, de Rijk P, Van Eekeren C, Portaels F. Detection and identification of mycobacteria by DNA amplification and oligonucleotide-specific capture plate hybridization. J Clin Microbiol. 1995;33:2994–2998. doi: 10.1128/jcm.33.11.2994-2998.1995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Del Portillo P, Thomas M C, Martinez E, Maranon C, Valladares B, Patarroyo M E, Carlos Lopez M. Multiprimer PCR system for differential identification of mycobacteria in clinical samples. J Clin Microbiol. 1996;34:324–328. doi: 10.1128/jcm.34.2.324-328.1996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Devallois A, Picardeau M, Goh K S, Sola C, Vincent V, Rastogi N. Comparative evaluation of PCR and commercial DNA probes for detection and identification to species level of Mycobacterium avium and Mycobacterium intracellulare. J Clin Microbiol. 1996;34:2756–2759. doi: 10.1128/jcm.34.11.2756-2759.1996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Fiss E H, Chehab F F, Brooks G F. DNA amplification and reverse dot blot hybridization for detection and identification of mycobacteria to the species level in the clinical laboratory. J Clin Microbiol. 1992;30:1220–1224. doi: 10.1128/jcm.30.5.1220-1224.1992. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.French A L, Benator D A, Gordin F M. Nontuberculous mycobacterial infections. Med Clin N Am. 1997;81:361–379. doi: 10.1016/s0025-7125(05)70522-8. [DOI] [PubMed] [Google Scholar]
- 8.Frothingham R, Wilson K H. Sequence-based differentiation of strains in the Mycobacterium avium complex. J Bacteriol. 1993;175:2818–2825. doi: 10.1128/jb.175.10.2818-2825.1993. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Frothingham R, Hills H G, Wilson K H. Extensive DNA sequence conservation throughout the Mycobacterium tuberculosis complex. J Clin Microbiol. 1994;32:1639–1643. doi: 10.1128/jcm.32.7.1639-1643.1994. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Frothingham R, Wilson K H. Molecular phylogeny of the Mycobacterium avium complex demonstrates clinically meaningful division. J Infect Dis. 1994;169:305–312. doi: 10.1093/infdis/169.2.305. [DOI] [PubMed] [Google Scholar]
- 11.Gilks C F, Brindle R J, Mwachari C, Batchelor B, Bwayo J, Kimari J, Arbeit R D, von Reyn C F. Disseminated Mycobacterium avium infection among HIV-infected patients in Kenya. J Acquired Immune Defic Syndr Hum Retrovirol. 1995;8:195–198. [PubMed] [Google Scholar]
- 12.Glennon M, Smith T, Cormican M, Noone D, Barry T, Maher M, Dawson M, Gilmartin J J, Gannon F. The ribosomal intergenic spacer region: a target for the PCR based diagnosis of tuberculosis. Tubercle Lung Dis. 1994;75:353–360. doi: 10.1016/0962-8479(94)90081-7. [DOI] [PubMed] [Google Scholar]
- 13.Glennon M, Cormican M G, Ni Riain U, Heiginbothom M, Gannon F, Smith T. A Mycobacterium malmoense-specific DNA probe from the 16S/23S rRNA intergenic spacer region. Mol Cell Probes. 1996;10:337–345. doi: 10.1006/mcpr.1996.0046. [DOI] [PubMed] [Google Scholar]
- 14.Grange J M. Mycobacteria and human diseases. 2nd ed. London, United Kingdom: Arnold; 1996. pp. 47–49. [Google Scholar]
- 15.Hughes M S, Skuce R A, Beck L A, Neill S D. Identification of mycobacteria from animals by restriction enzyme analysis and direct DNA cycle sequencing of polymerase chain reaction-amplified 16S rRNA gene sequences. J Clin Microbiol. 1993;31:3216–3222. doi: 10.1128/jcm.31.12.3216-3222.1993. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Kirschner P, Springer B, Vogel U, Meier A, Wrede A, Kiekenbeck M, Bange F C, Bottger E C. Genotypic identification of mycobacteria by nucleic acid sequence determination: report of a 2-year experience in a clinical laboratory. J Clin Microbiol. 1993;31:2882–2889. doi: 10.1128/jcm.31.11.2882-2889.1993. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Lappayawichit P, Rienthong S, Rienthong D, Chuchottaworn C, Chaiprasert A, Panbangred W, Saringcarinkul H, Palittapongarnpim P. Differentiation of Mycobacterium species by restriction enzyme analysis of amplified 16S-23S ribosomal DNA spacer sequences. Tubercle Lung Dis. 1996;77:257–263. doi: 10.1016/s0962-8479(96)90010-6. [DOI] [PubMed] [Google Scholar]
- 18.Oggioni M R, Fattorini L, Li B, De Milito A, Zazzi M, Pozzi G, Orefici G, Valensin P E. Identification of Mycobacterium tuberculosis complex, Mycobacterium avium and Mycobacterium intracellulare by selective nested polymerase chain reaction. Mol Cell Probes. 1995;9:321–326. doi: 10.1016/s0890-8508(95)91604-0. [DOI] [PubMed] [Google Scholar]
- 19.Plikaytis B B, Plikaytis B D, Yakrus M A, Butler W R, Woodley C L, Silcox V A, Shinnick T M. Differentiation of slowly growing Mycobacterium species, including Mycobacterium tuberculosis, by gene amplification and restriction fragment length polymorphism analysis. J Clin Microbiol. 1992;30:1815–1822. doi: 10.1128/jcm.30.7.1815-1822.1992. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Sathapatayavong B, Tansupasawatdikul S, Kantiphong P, Pornchaipoolthavee S, Chuchottaworn C. Program and abstracts of the 20th International Congress of Chemotherapy. 1997. Prevalence of disseminated M.A.C. in Thai AIDS patients, abstr. 5281; p. 200. [Google Scholar]
- 21.Scorpio A, Collins D, Whipple D, Cave D, Bates J, Zhang Y. Rapid differentiation of bovine and human tubercle bacilli based on a characteristic mutation in the bovine pyrazinamidase gene. J Clin Microbiol. 1997;35:106–110. doi: 10.1128/jcm.35.1.106-110.1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Sreevatsan S, Escalante P, Pan X, Gillies II D A, Siddiqui S, Khalaf C N, Kreiswirth B N, Bifani P, Adams L G, Ficht T, Perumaalla V S, Cave M D, van Embden J D, Musser J M. Identification of a polymorphic nucleotide in oxyR specific for Mycobacterium bovis. J Clin Microbiol. 1996;34:2007–2010. doi: 10.1128/jcm.34.8.2007-2010.1996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Swanson D S, Pan X, Musser J M. Identification and subspecific differentiation of Mycobacterium scrofulaceum by automated sequencing of a region of the gene (hsp65) encoding a 65-kilodalton heat shock protein. J Clin Microbiol. 1996;34:3151–3159. doi: 10.1128/jcm.34.12.3151-3159.1996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Takewaki S, Okuzumi K, Manabe I, Tanimura M, Miyamura K, Nakahara K, Yazaki Y, Ohkubo A, Nagai R. Nucleotide sequence comparison of the mycobacterial dnaJ gene and PCR-restriction fragment length polymorphism analysis for identification of mycobacterial species. Int J Syst Bacteriol. 1994;44:159–166. doi: 10.1099/00207713-44-1-159. [DOI] [PubMed] [Google Scholar]
- 25.Taylor T B, Patterson C, Hale Y, Safranek W W. Routine use of PCR-restriction fragment length polymorphism analysis for identification of mycobacteria growing in liquid media. J Clin Microbiol. 1997;35:79–85. doi: 10.1128/jcm.35.1.79-85.1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Telenti A, Marchesi F, Balz M, Bally F, Bottger E C, Bodmer T. Rapid identification of mycobacteria to the species level by polymerase chain reaction and restriction enzyme analysis. J Clin Microbiol. 1993;31:175–178. doi: 10.1128/jcm.31.2.175-178.1993. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Thierry D, Matsiota-Bernard P, Nauciel C, Guesdon J L. Comparison of polymerase chain reaction and non-radioactive hybridization techniques for the identification of Mycobacterium avium strains. Mol Cell Probes. 1994;8:469–471. doi: 10.1006/mcpr.1994.1067. [DOI] [PubMed] [Google Scholar]
- 28.Vaneechoutte M, De Beenhouwer H, Claeys G, Verschraegen G, De Rouck A, Paepe N, Elaichouni A, Portaels F. Identification of Mycobacterium species by using amplified ribosomal DNA restriction analysis. J Clin Microbiol. 1993;31:2061–2065. doi: 10.1128/jcm.31.8.2061-2065.1993. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.van Embden J D, Cave M D, Crawford J T, Dale J W, Eisenach K D, Gicquel B, Hermans P, Martin C, McAdam R, Shinnick T M. Strain identification of Mycobacterium tuberculosis by DNA fingerprinting: recommendations for a standardized methodology. J Clin Microbiol. 1993;31:406–409. doi: 10.1128/jcm.31.2.406-409.1993. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Weil A, Plikaytis B B, Butler W R, Woodley C L, Shinnick T M. The mtp40 gene is not present in all strains of Mycobacterium tuberculosis. J Clin Microbiol. 1996;34:2309–2311. doi: 10.1128/jcm.34.9.2309-2311.1996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Yamazaki T, Nakamura R M. Identification of Mycobacterium intracellulare by a polymerase chain reaction using species-specific primers. Tubercle Lung Dis. 1995;76:330–335. doi: 10.1016/s0962-8479(05)80032-2. [DOI] [PubMed] [Google Scholar]